Abstract
Over the past decade, birds have proven to be excellent models to study hormone-mediated maternal effects in an evolutionary framework. Almost all these studies focus on the function of maternal steroid hormones for offspring development, but lack of knowledge about the underlying mechanisms hampers further progress. We discuss several hypotheses concerning these mechanisms, point out their relevance for ecological and evolutionary interpretations, and review the relevant data. We first examine whether maternal hormones can accumulate in the egg independently of changes in hormone concentrations in the maternal circulation. This is important for Darwinian selection and female physiological trade-offs, and possible mechanisms for hormone accumulation in the egg, which may differ among hormones, are reviewed. Although independent regulation of plasma and yolk concentrations of hormones is conceivable, the data are as yet inconclusive for ovarian hormones. Next, we discuss embryonic utilization of maternal steroids, since enzyme and receptor systems in the embryo may have coevolved with maternal effect mechanisms in the mother. We consider dose–response relationships and action pathways of androgens and argue that these considerations may help to explain the apparent lack of interference of maternal steroids with sexual differentiation. Finally, we discuss mechanisms underlying the pleiotropic actions of maternal steroids, since linked effects may influence the coevolution of parent and offspring traits, owing to their role in the mediation of physiological trade-offs. Possible mechanisms here are interactions with other hormonal systems in the embryo. We urge endocrinologists to embark on suggested mechanistic studies and behavioural ecologists to adjust their interpretations to accommodate the current knowledge of mechanisms.
Keywords: maternal effects, maternal hormones, birds, yolk steroids, hormone transfer, hormone action
1. Introduction
(a) Hormone-mediated maternal effects: the bird model
Maternal effects are epigenetic modifications of offspring phenotype caused by the environment provided by the mother during development (Mousseau & Fox 1998). Maternal effects were once viewed as pathological perturbations of rigid developmental programmes of gene expression (Gilbert 2001), caused by sub-optimal environments during development. Now, there is much more appreciation for adaptive phenotypic plasticity and the possibility that mothers may adjust the development and the phenotype of their offspring to environmental conditions to increase Darwinian fitness (Mousseau & Fox 1998). For two reasons, steroids may provide powerful maternal signals for adaptive modifications of offspring development in response to the environmental conditions experienced by the mother. They are integral regulatory signals in the cascade of programmed processes of development and differentiation that leads from genotype to phenotype (Arnold 2002). At the same time, these very same steroids are integral mediators of phenotypic responses by adults to environmental cues and change. Birds provide excellent models to test this hypothesis since their embryos develop outside the mother's body in relatively large eggs, facilitating descriptive and experimental studies (Groothuis et al. 2005).
Since the seminal report of systematic intra-clutch variation of testosterone in avian eggs (Schwabl 1993), the field of hormone-mediated maternal effects in birds has developed rapidly, focusing within an adaptive framework on the effects of yolk testosterone on post-natal growth and behaviour. The flourishing field is currently pushed forward mainly by behavioural ecology, which succeeded in changing the view of maternal effects as a disturbance of programmed development to that of adaptation. Functional and evolutionary aspects (Gil 2003; Groothuis et al. 2005; Müller et al. 2007b) and conceptual (Groothuis & von Engelhardt 2005) and methodological issues (Von Engelhardt & Groothuis 2005) were recently addressed. Therefore, we summarize only a few novel findings before discussing mechanisms.
Yolk testosterone varies with maternal parasite exposure (Tschirren et al. 2004; Gil et al. 2006) and influences natal dispersal distance (e.g. Tschirren et al. 2007), indicating consequences for parasite–host coevolution and population genetics. Sex differences in effects of yolk hormones (Love et al. 2005; Saino et al. 2005; Hayward et al. 2006; Rubolini et al. 2006a; Von Engelhardt et al. 2006; Sockman et al. in press) or in hormone concentrations in the eggs (Müller et al. 2002; Gilbert et al. 2005; Rutstein et al. 2005; Rutkowska & Badyaev 2008) open the possibility of maternal modifications of secondary or even primary sex ratios. Yolk androgen and glucocorticoid concentrations respond to artificial selection for behaviour (androgens: Gil & Faure 2007; Groothuis et al. submitted; corticosterone: Hayward et al. 2005), suggesting the mechanisms of hormone accumulation in the egg as targets for natural selection. Comparative studies show that, among passerine birds, yolk androgen concentrations correlate positively with rate of development (Gorman & Williams (2005); Gil et al. (2007), although in this dataset the correlation was lost after controlling for phylogeny; Schwabl et al. (2007); Martin & Schwabl (2008)) and offspring mortality by nest predation (Schwabl et al. 2007; Martin & Schwabl 2008) identifying selection pressures. Finally, oestrogens and stress and metabolic hormones are now being studied (oestradiol: Williams et al. 2004, 2005; corticosterone: Hayward & Wingfield 2003; Love et al. 2005; Rubolini et al. 2005; thyroid hormones: McNabb & Wilson 1997), a timely expansion of a so far myopic focus on the androgen testosterone. Although adaptive functional and evolutionary studies are rapidly proliferating (reviewed in Groothuis et al. 2005; see also Moreno-Rueda 2007; Müller et al. 2007b; Sockman et al. in press), a thorough knowledge of the mechanisms operating in the mother and the offspring is lacking.
(b) The quest for mechanistic studies and questions of relevance
Understanding mechanisms of hormone-mediated maternal effects is important. First, mechanisms operating in the mother (e.g. steroidogenesis and transmission of steroids into the oocyte) and the embryo (e.g. uptake of steroids and action of steroids in development and differentiation) determine and limit the adaptive potential and the scope for evolution by posing or relaxing physiological trade-offs. Functional studies often use language such as females ‘allocate’, ‘manipulate’ and ‘invest’ yolk hormones, yet we do not know how much ‘control’ females in fact have over the concentrations of their hormones in eggs and to what extent this might be costly. Second, knowledge about mechanisms can provide insights into hitherto unstudied developmental actions of hormones and the extent to which observed multiple effects of yolk steroids are tightly linked or independent of one another. At the same time, we feel that a full understanding of mechanisms can be obtained only by considering evolutionary forces behind their current expression.
Two important questions will be discussed in this paper: first, how do maternal hormones accumulate in the egg? Does the mother exert control over this process and is she able to regulate offspring hormone exposure without also influencing her own exposure to the same hormone? This is of crucial importance to answering the question of whether hormone transfer to the egg is costly for the mother by inducing physiological trade-offs, and how flexible she is in ‘allocating’ (if at all) doses of hormones to eggs. Second, how do these hormones affect the embryo? When do response pathways to maternal hormonal signals develop? Would embryos be able to modify their response to hormones they are exposed to by the mother, a relevant question in the framework of family conflict? Do yolk hormones interact with hormones produced by the embryo that mediate sexual differentiation, and if not, why? (Carere & Baltazart 2007). Are the target tissues of yolk steroids different from those for sexual differentiation or are they affected in a different way or at a different time? What are the dose–response relationships, a largely unexplored area essential for experimentation in ultimate studies? How are maternal hormonal effects integrated? Are pleiotropic effects due to developmental modification of a single or a few traits and therefore tightly linked to each other or are effects independent of one another, widening the playing field for maternal adjustment?
Most of these questions can be addressed only by speculation, but we hope that they spur new work in this rapidly growing field that presently lacks a sound mechanistic understanding. Below, we summarize studies that are relevant for these crucial questions, point out potential pitfalls and delineate approaches and strategies for future study.
2. Potential mechanisms
(a) How do steroids accumulate in eggs?
(i) Three alternative mechanisms and their consequences for adaptation
Concentrations of maternal steroids in the yolk may be a mere consequence and reflection of hormone production and secretion to regulate female physiological processes such as egg formation, reproduction-related behaviour (ovarian steroids) and energy homeostasis (corticosterone, thyroid hormones). We call this the physiological epiphenomenon hypothesis (PEH). It is the most parsimonious hypothesis that accounts for the lipophilic nature of steroids and predicts a positive correlation between concentrations of these hormones in mother and egg. This possibility imposes physiological trade-offs between optimal hormone exposure for offspring and mother. In this case, yolk hormones may be maladaptive, neutral, exaptative (Ketterson & Nolan 1999; Groothuis et al. 2005) or adaptive.
An alternative is regulation of the distribution of produced hormones between maternal circulation and the yolk of oocytes. We call this the flexible distribution hypothesis (FDH). This mechanism would give rise to trade-off between optimal hormone concentration in the egg and the mother. Yolk acting as a sink for very high levels of steroids in maternal circulation (Navara et al. 2006) is unlikely; this could be achieved by reducing hormone production that would not have potential detrimental effects on the embryo. In cases where maximum hormone production is restrained, the FDH would allow for elevated levels in eggs at the expense of hormone concentrations in the mother. The FDH can explain negative correlation between ovarian hormones in eggs and female plasma found in some studies (see below).
At the other extreme, hormone concentrations in eggs and maternal circulation may be regulated independently of each other by two mechanisms: one operating at the level of hormone production and another one at the level of distribution of the hormones to oocytic yolk or maternal circulation. In this case, selection has acted directly on the mechanisms that allow variation of hormone concentrations in yolk without interference with the female's own hormonal state. We call this the independent regulation hypothesis (IRH). It predicts absence of correlation between hormone concentrations in mother and egg. This mechanism would minimize physiological trade-offs and justify a view of hormone-mediated maternal effects as adaptations. Production of androgens and oestrogens in the follicle wall that surrounds each proliferating oocyte provide support for this hypothesis. However, other yolk hormones (e.g. corticosterone and thyroid hormones) are produced by distant glands and require transport in the female bloodstream to reach the oocyte. Independent regulation of the levels of these hormones in oocyte and maternal circulation seems therefore unlikely. We will first address locally versus distantly produced yolk hormones and then discuss possible partitioning mechanisms and evidence for the three hypotheses.
(ii) Sources of maternal egg hormones and their function in the mother
Steroids produced locally in the ovary
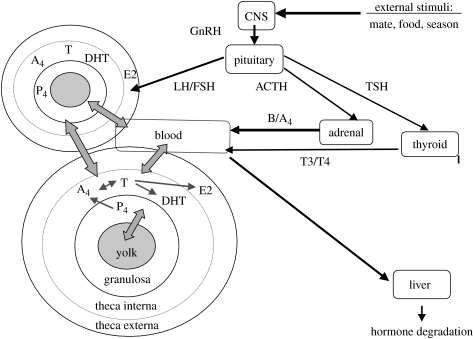
Except for species that lay only one egg per clutch, several ovarian oocytes proliferate (are yolked) simultaneously during the formation of a clutch; these ovarian oocytes are generally assumed to form a proliferation hierarchy (Johnson 2002). Unfortunately, most of the information on oocyte proliferation and maturation and follicular steroid production comes from poultry, selected for maximal egg production; therefore, we cannot necessarily assume that the processes are exactly the same in wild birds, and some evidence exists that this is not the case (Young & Badyaev 2004; Badyaev et al. 2005). Nevertheless, these studies reveal general principles of how ovarian steroids might accumulate in oocytes and how to study these processes. The main sources of ovarian steroids are the cell layers of the follicular wall that surrounds each growing oocyte. Progestins (P) are produced by the granulosa cells, closest to the oocyte, androgens (A) by the theca interna cells, the middle layer, and oestrogens (E) by the theca externa cells, the outer layer. These hormones are metabolites of a single synthesis pathway (P>A>E) that is compartmentalized among cell layers (e.g. Johnson 2002; figure 1). The pituitary hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), regulate follicular hormone production and follicular growth in response to information from the environment and the body. The follicle wall contains blood vessels that transport hormones and ‘feed’ the growing follicles.
Figure 1.
Diagram depicting hormone production and their accumulation in egg yolk in avian species. The two large circles represent two growing ovarian follicles, including the three steroidogenic layers (theca externa, theca interna and granulosa) in the follicle wall and the oocyte with egg yolk depicted as grey circles. The large rectangle represents a blood vessel and the wide gray arrows possible transport routes for hormones. Large black arrows depict environmental stimuli affecting the female brain (CNS and releasing hormones (e.g. GnRH)), stimulating the pituitary to release hormones (LH, FSH, ACTH and TSH) into the female circulation to regulate the production of peripheral hormones (B, corticosterone; P4, progesterone; A4, androstenedione; T, testosterone, E2, oestradiol; T3/T4, thyroid hormones; DHT, dihydrotestosterone). Small black arrows depict synthesis pathways of ovarian steroid hormones.
Each ovarian follicle in the hierarchy is assumed to undergo the same changes in steroid production during its proliferation. Briefly, a small follicle produces mainly oestrogens; a growing, intermediate follicle produces androgens; and a mature, pre-ovulatory follicle produces mainly progesterone (Johnson 2002). Androgens circulate in measurable concentrations in female blood in the majority of avian species and in many of them in significant concentrations. Androgens probably affect aggressive and sexual behaviour and expression of secondary sexual and nuptial traits, and are important as precursors for oestrogens (Staub & De Beer 1997; Ketterson et al. 2005). Circulating oestrogens regulate hepatic vitellogenesis and yolk deposition, oviduct proliferation and ovulation; they also contribute to nest building behaviour and receptivity (Johnson 2002). Circulating progesterone is associated with onset of broodiness (e.g. Cheng 1979; Sockman & Schwabl 2000). These local intra-ovarian and systemic signalling actions of ovarian steroids in the female have to be considered when proposing adaptive functions for their presence in the egg. Androgens produced by the adrenal glands (e.g. androstenedione) may contribute to yolk and circulating levels but the importance of this source in adult birds is as yet unclear.
Glucocorticoids and thyroid hormones
In contrast to androgens and oestrogens that are produced at the interface between female circulation and the oocytes, glucocorticoids and thyroid hormones are produced distantly in the adrenal and thyroid glands; therefore, they have to reach the ovum via the circulation. These hormones regulate adult energy homeostasis (corticosterone: e.g. Sapolsky et al. 2000; McEwen & Wingfield 2003; Landys et al. 2006; thyroid hormones: McNabb 1995, 2007). They could accumulate in yolk during follicle proliferation and also in albumin during its deposition in the oviduct after ovulation. The latter is indicated by presence of radio-labelled steroid hormones in albumin of eggs that were already ovulated at the time the female was injected with hormone (Von Engelhardt & Groothuis in preparation). Proteins that bind circulating steroids such as corticosterone and thyroid hormones (e.g. McNabb 2001; Breuner & Orchinik 2002) may influence the rate at which these hormones can enter the oocyte. These anatomical and physiological properties render the IRH for these hormones unlikely, potentially resulting in trade-off between their effects on the embryo and the mother.
(iii) Possible mechanisms for partitioning steroids between yolk and plasma
We propose the following potential mechanisms for FDH and IRH.
Regulation of follicle development. Maternal plasma levels of ovarian steroids probably reflect hormone production of all active follicles in their different stages of maturation, while steroid levels in the yolk of a particular egg are probably influenced mostly by hormone production in its own follicle wall. Therefore, the relative concentrations of a particular ovarian steroid in female plasma and yolk may not correlate tightly. In cases where the female, via the hypothalamus–pituitary–gonadal (HPG) axis, can facultatively regulate the number and rate of follicle maturation, for which some evidence exists (Young & Badyaev 2004), the IRH becomes possible.
Selective transport into oocyte or circulation. There is currently no evidence for selective transport mechanisms of steroids produced in the follicular wall into either the oocyte or the female plasma, although some evidence for a lipoprotein that transports steroid hormones exists (McNabb 2001). Moreover, prokaryotic and eukaryotic cells possess transport proteins (e.g. ATP-binding cassette proteins) that move a variety of chemicals including steroids across biological membranes (Kralli et al. 1995; Kralli & Yamamoto 1996; Petry et al. 2006). This cellular machinery could allow selective uptake of ovarian steroids into the oocyte or the female's circulation. Immunohistochemistry and tracing approaches might be helpful to address these questions.
Retention of passively diffused steroids in oocyte yolk. The lipid–lipoprotein matrix of yolk may facilitate high concentrations of steroids compared with blood plasma (see below), or yolk contains variable amounts of steroid-binding proteins providing a sink for steroids and a potential mechanism for FDH and IRH.
Differential metabolism of steroids in plasma versus yolk. Yolk may contain enzymes that convert steroids to other active or inactive metabolites. On the other hand, circulating steroids are rapidly degraded in the liver, perhaps at a much higher rate than in the yolk. This could explain the much higher concentrations of certain steroids in oocyte yolk than blood plasma (see below). If steroid metabolism in the yolk or liver can be regulated by the female, independent regulation of yolk and plasma concentrations becomes possible. So far, these processes have not been examined.
(iv) Evidence for the physiological epiphenomenon hypothesis, flexible distribution hypothesis and independent regulation hypothesis
Correlation of concentrations of steroids in female plasma and yolk
The three hypotheses predict a positive, negative or no relationship, respectively, between hormone concentrations in female circulation (at the time the yolk is deposited in the oocyte) and egg yolk. Below we review descriptive and experimental studies of this relationship, separately for gonadal and other steroids.
Establishing the relationship between plasma and yolk hormone concentrations is a technical challenge. Plasma steroid levels change rapidly due to environmental factors, maturation and ovulation of follicles, and onset of incubation. Yolk steroid levels vary among yolk layers of the same egg, reflecting differences in hormone production during maturation of the follicle (see above), and among eggs of the same clutch. Since the rapid yolk deposition phase of the egg occurs several days before it is laid, this time interval (that varies among species) has to be taken into account. As a consequence, lack of correlation between maternal and yolk hormone concentrations may result from the timing of blood hormone measurements in relation to egg formation rather than providing evidence for the IRH.
One way to deal with rapid fluctuations of plasma hormone concentrations in the mother is to analyse hormone concentrations in her faeces, a method that integrates hormone levels over several hours. However, this requires extensive validation as the concentrations of the target hormone in faeces are affected by their catabolism in the liver for excretion (Palme 2005). Analysing hormone concentrations in separate yolk layers in relation to faecal samples collected at the time when that particular yolk layer was deposited might be a promising avenue, especially when the proper timing of both samples is validated by, for example, feeding birds with different dyes at different times.
Descriptive data. To date, 10 studies of 7 different species investigated the relationship of concentrations of gonadal steroids in yolk and female circulation; two studies are available for corticosterone and one for thyroid hormones (table 1). Some studies analysed this relationship on the basis of individual females. Others compared the effect of an environmental manipulation on both female and yolk concentrations (group level). Some looked at the correlation between the changes in plasma hormone concentrations and yolk concentrations over the course of the laying of the eggs of a clutch. Most studies analysing at the group or laying sequence level measured hormone concentrations in female and egg in the same study, but some used a less powerful approach of measuring plasma and yolk hormone concentrations in separate studies. Finally, some studies measured hormone concentrations after completion of laying (onset of incubation), which is inappropriate since gonadal androgens and oestrogen rapidly decline during that phase (e.g. Schwabl 1996a; Sockman & Schwabl 1999; Badyaev et al. 2005).
Table 1.
Direction of correlation (positive, negative or absent, see column 1) between concentrations of a particular hormone (column 2: T, testosterone; E, oestradiol; A4, androstenedione; DHT, dihydrotestosterone; B, corticosterone; T4, thyroxine) in maternal plasma and egg yolks in 11 studies on several bird species (column 3: B-h., black-headed; B-b., black-backed). The type of data are indicated in column 5: individual females, correlation based on data from individual females and their eggs; group level, comparison of the effect of a manipulation (social challenge and food provisioning during egg laying) and artificial selection for adult plasma hormone levels on female plasma and yolk hormone concentrations; laying sequence, comparing the patterns of change in plasma and yolk over the laying sequence of a clutch. The last column refers to sampling protocol; see also text.
| correlation | hormone | species | reference | kind of data | remarks |
|---|---|---|---|---|---|
| + | T | domestic canary | Schwabl (1996a) | individual females | females: faecal samples |
| + | T | house finch | Badyaev et al. (2005) | individual females | eggs: yolk rings |
| 0 | T | house sparrow | Egbert (2006) | individual females | eggs: yolk rings |
| 0 | T | domestic canary | Marshall et al. (2005) | group level: social challenge | plasma and yolk in different studies |
| 0 | T | Eurasian starling | Pilz et al. (2003) and Williams et al. (2004) | group level: laying sequence | plasma and yolk in different studies |
| − | T | house sparrow | Mazuk et al. (2003) | group level: social challenge | plasma at incubation |
| − | T | eastern bluebird | Navara et al. (2006) | group level: social challenge | |
| + | T | B-h. gull | Groothuis & Eising (in preparation) | Individual females | plasma at start of incubation |
| − | A4, T, DHT | B-b. gull | Verboven et al. (2003) | group level: food provisioning | plasma at incubation |
| − | E | Eurasian starling | Williams et al. (2005) | individual females | small sample size |
| + | B | Japanese quail | Hayward et al. (2005) | group level: selection lines | |
| + | B | Eurasian starling | Love et al. (2005) | individual females | data of controls and E treated together |
| +/0 | T4 | Japanese quail | Wilson & McNabb (1997) | individual females | weakly positive |
For gonadal hormones, the results are clearly inconsistent, showing positive, negative and no correlations (table 1, first column). Even if one, based on the above considerations, only takes those studies into account that most likely would detect correlations (the first three studies in table 1 and those by Williams et al. (2005), Navara et al. (2006) and Groothuis et al. (submitted)), there is still no consistent picture. Even the two studies that took yolk layers into account produced different results (Badyaev et al. 2005; Egbert 2006). In sum, these data are inconclusive and do not allow us to reject any of the three hypotheses. The results for corticosterone and thyroxine are much more consistent. All the three studies found a positive correlation, in support of the PEH (table 1, last three studies), although the positive correlations for thyroxine in control and treatment groups are weak and perhaps not significant.
Finally, differences in steroid concentration between eggs containing a male or female embryo (see §1) have been taken as evidence for maternal control over deposition of hormones in the yolk. However, hormone accumulation occurs before sex determination by meiosis. Therefore, it is more likely that the sex of the egg is a consequence of hormone concentrations rather than yolk hormone concentrations being adjusted to the sex of the egg (see Badyaev et al. 2005).
Experimental data. Another approach that has been taken was to elevate female circulating hormone levels and monitor the hormone levels in the eggs. A single injection of radio-labelled testosterone led only to a minor fraction of it ending up in the egg yolk (Hackl et al. 2003). However, steroids are quickly degraded by the liver and a single injection may not simulate elevated hormone production in the female over the course of the proliferation of oocytes. Indeed, longer-lasting hormone treatment of the female by using hormone implants led to elevated hormone levels in the yolk in all studies published to date (five studies of gonadal hormones, two for corticosterone and one for thyroxine; table 2). However, while these approaches might be appropriate for distantly produced hormones (e.g. corticosterone and thyroid hormones), they have to be interpreted cautiously for ovarian steroids. Although they show that high hormone concentrations in maternal blood may lead to high hormone concentrations in the yolk, they do not account for the local production of the hormones in ovarian follicles, unless ovarian hormones are first released from the follicle wall into the circulation, which seems unlikely. Thus, at the moment, these results do not provide strong evidence for the PEH. However, a recent study stimulating ovarian steroidogenesis with gonadotrophin-releasing hormone (GnRH; Jawor et al. 2007) provides some evidence for the PEH. GnRH injections did not elevate plasma T unless a female was in the egg formation stage. In this stage, the magnitude of the GnRH-induced increase in plasma testosterone correlated positively with the concentration of testosterone in the yolk. This suggests that a stimulus that activates the HPG axis will simultaneously increase plasma and yolk testosterone. Interestingly, there was no correlation between absolute concentrations of testosterone in female plasma and its eggs, but only between the change in plasma testosterone in response to the GnRH challenge and yolk testosterone levels; this held true even when the (short-lasting) GnRH effect was induced days before egg laying. This may indicate that the responsiveness of the HPG axis, and not the plasma testosterone concentration, correlates with yolk testosterone levels. Perhaps, in the search for the relationship between female and yolk hormone concentrations, we should focus on within-female rather than among-female variation.
Table 2.
Direction of correlations between the effect of hormone treatment of the female on hormone concentrations in her plasma and the yolks of her eggs. (For legends, see table 1 and text.)
| correlation | hormone | species | reference | manipulation |
|---|---|---|---|---|
| + | T | dark-eyed junco | Clotfelter et al. (2004) | implantation |
| + | T | zebra finch | Rutkowska et al. (2005) | injections |
| + | T | dark-eyed junco | Jawor et al. (2007) | GnRH challenge |
| + | E | Japanese quail | Adkins-Regan et al. (1995) | implantation |
| + | E | Eurasian starling | Williams et al. (2005) | injections |
| ++ | B | Japanese quail | Hayward & Wingfield (2004) | implantation |
| + | B | Eurasian starling | Love et al. (2005) | implantation |
| + | T4 | Japanese quail | Wilson & McNabb (1997) | injections |
Differences in absolute and relative concentrations of steroids between plasma and yolk
Concentrations of androgens are generally much higher in yolk than female plasma. For technical reasons, yolk androgen concentrations are usually reported on per mass basis (pg mg−1 or ng g−1 yolk), while plasma concentrations are reported on per volume basis (pg ml−1 or ng ml−1). Ignoring, for simplicity, the slightly lower specific mass of yolk, yolk hormone concentrations in pg mg−1 are converted into ng ml−1. Among species, female plasma testosterone concentration during egg formation is 0.45 ng ml−1 on average (range 0.03–12.4; Ketterson et al. 2005), while yolk testosterone concentration is 18 ng ml−1 on average (range between 0.5 and 86 ng ml−1; Schwabl et al. 2007), which is approximately 40-fold higher. Thus, the yolk lipid/lipoprotein matrix (see above) seems to facilitate high concentrations of testosterone compared with blood. If the ‘binding’ capacity of the yolk matrix fluctuates independently of that of the maternal circulation, independent regulation becomes possible. In contrast to testosterone, corticosterone concentrations in yolk and plasma are similar at approximately 15 ng ml−1 (Love et al. 2005), perhaps because corticosterone-binding globulin in the plasma (Breuner & Orchinik 2002) either hampers uptake into the yolky follicle or facilitates equally high levels in plasma and yolk. Oestrogens, locally produced in the ovary, are rather low in their concentrations in the yolk compared with androgens. In the blood, on the other hand, oestrogen concentrations are high while androgen concentrations are low (Navara et al. 2006; Carere & Baltazart 2007), suggesting less retention in the yolk or transport into the circulation. Differences in lipid (yolk) and water (plasma) solubility do not explain these relative differences in androgen and oestrogen concentrations in yolk and plasma.
However, three alternative processes may account for the relatively low levels of oestradiol in the yolk. First, in contrast to androgens, oestradiol is produced in the cell layer of the follicle wall that is most distant from the oocyte. Second, oestradiol is produced in relatively high amounts by small immature follicles. This may result in elevated levels of oestradiol in plasma, but not in the yolk of the mature oocyte, since at the time of maximal yolk deposition oestradiol production in that follicle has decreased (Johnson 2002). A third possibility is different steroid metabolism in yolk and plasma (see above). Although androstenedione is the immediate precursor for testosterone and testosterone is the immediate precursor for 5α-dihydrotestosterone (DHT), concentrations of these three androgens are not always correlated within a species. Likewise, yolk oestradiol is not always correlated with its precursors, namely testosterone and androstenedione (e.g. Elf & Fivizzani 2002; Groothuis & Schwabl 2002; fig. 1, Tschirren et al. 2004). Moreover, comparative analyses of the three yolk androgens in eggs of passerine birds reveal a strong correlation of 5α-DHT with testosterone, but not of testosterone with androstenedione (Schwabl et al. 2007). This variation in correlations of concentrations of substrates and metabolites in the ovarian steroid synthesis pathway may suggest presently unknown processes of ovarian enzyme regulation that could allow for differential regulation of concentrations of androgens and oestrogens in the yolk. This regulation may occur in the follicle wall during steroid synthesis, affecting both plasma and yolk concentrations of steroids. Alternatively, regulation might occur in the yolky oocyte itself, affecting only yolk concentrations and thereby supporting the IRH. Studies of steroidogenic enzyme activity of ovarian follicles and yolk seem a promising research area to address this question.
Unequal hormone concentrations in yolk layers
Evidence against the PEH, ruling out circulation as a major source for yolk steroids, comes from the systematic distribution of steroids in yolk layers, e.g. high oestradiol concentrations in the centre, high androstenedione and testosterone concentrations in the middle and high progesterone concentrations in the peripheral layer of the yolk sphere (Lipar et al. 1999; Eising 2004; Hackl et al. 2003; H. Schwabl 1995, unpublished data). These unequal distribution patterns remain stable until incubation is underway and reflect the dynamics of follicular steroid production during maturation of the oocyte (see above). This suggests (i) mechanisms such as binding proteins that keep steroids from freely diffusing across the yolk matrix as well as out of the oocyte into the plasma and (ii) potential fluctuation in this binding capacity between yolk layers. Perhaps a binding protein, similar to testicular androgen-binding protein, or yolk lipoproteins themselves, traps steroids, maintains them in yolk layers and allows for high concentrations in the yolk compared with blood plasma. This could be studied by binding assays similar to those used for steroid binding proteins in plasma (Breuner & Orchinik 2002).
In the end, answers to the critical question of female regulation of yolk and plasma hormone concentrations to achieve adaptive maternal effects on the offspring and dampen potentially costly physiological trade-off are still lacking. Therefore, we urge researchers to refrain from the use of language such as yolk hormone allocation, hormonal manipulation and hormone deposition that suggests mechanisms that allow for such regulation. On the other hand, we hope to have shown that several regulation mechanisms are conceivable, offering ample opportunity for future molecular, cellular and physiological studies.
(b) How are effects on the offspring mediated?
(i) Embryonic utilization versus degradation of yolk steroids and its functional consequences
The concentrations of hormones in the yolk substantially decrease during early embryo development for unknown reasons (gonadal hormones: Elf & Fivizzani 2002; Eising et al. 2003; Eising 2004; Pilz et al. 2004; T. G. G. Groothuis 2006, unpublished data; H. Schwabl 1995, unpublished data; thyroid hormones: Wilson & McNabb 1997). Understanding the causes of these declines is important for functional explanations. A probable cause is water influx from albumin, diluting the yolk. This would not affect overall embryo hormone exposure. Hormones may degrade, meaning that hormone levels measured at laying are irrelevant for late-embryonic development. Hormones may be assimilated from yolk and used by the embryo. Since the decrease occurs before substantial embryo development, this is unlikely. Another possibility is the presence in yolk of metabolizing enzymes of maternal or embryonic origin. Indeed, genes of steroid-metabolizing enzymes are expressed already from embryonic day 2 in the chicken embryo (Bruggeman et al. 2002). If steroids are metabolized in the yolk, the embryo would be exposed to concentrations and types of hormones different from what we measure in freshly laid eggs. A further decrease later during incubation may well reflect embryonic utilization, but we do not understand how steroids are assimilated from the yolk, how and where they are metabolized, and when, where and how they act. Since the embryo proliferates and differentiates in a steroid environment from its inception, long before its own hormone secretion begins, there might be effects of these steroids that have so far not been considered by developmental endocrinology that focused on sexual differentiation during advanced embryo stages.
(ii) Embryonic enzymes and receptors and the possibility for coevolution
For maternal hormones in the egg to exert actions on the embryo, response pathways such as enzymes, receptors and intracellular transduction cascades must be in place. Knowledge of where and when these systems develop can help in ultimate approaches to hormone-mediated maternal effects. Do maternal hormones act on the embryo before the embryo produces hormones itself? Do individual embryos vary in the timing of the development of response pathways? Might responsiveness of embryos vary in ways that would allow selection to favour offspring at a cost to the mother (parent–offspring conflict; Müller et al. 2007b)? For example, embryos exposed to low levels of maternal testosterone may upregulate their hormone sensitivity in order to grow as fast as their siblings that are exposed to higher levels of maternal hormone. So far, studies of hormone transduction pathways in embryos are limited to sexual differentiation using a few model species such as the precocial galliforms Japanese quail and chicken, and the altricial passeriform zebra finch. Because these studies focus on sexually dimorphic structures and traits, little is known about distribution of enzymes and receptors in tissues other than those that become sexually differentiated. Yet, maternal steroids can affect a trait in both sexes or only in one sex. For example, in the house sparrow, testosterone injections into the yolk enhanced agonistic behaviour in both sexes, but plumage colour that is characteristic for males only in males. Treatment did not induce this male colouration in females (Strasser & Schwabl 2004; Partecke & Schwabl in preparation). In the American kestrel, androgen injections (a cocktail of A4 and T) into yolk blunted growth of male but not female nestlings (Sockman et al. in press). In the zebra finch, in contrast, yolk testosterone injections enhanced begging behaviour and growth in female but not in male nestlings (Von Engelhardt et al. 2006). What is causing such species and sex differences in responses to yolk androgens? Perhaps receptor distribution and timing of their expression differ, and such differences coevolved with exposure to maternal hormones. Furthermore, the early synthesis by the embryo of enzymes to metabolize steroids can be viewed as an adaptation to the presence of maternal hormones, if these enzymes play a functional role prior to the endogenous production of steroids by the embryo itself. Maternal yolk steroids may exert their early effects via non-genomic pathways prior to embryonic expression of classical steroid receptors, as has been demonstrated in adult birds (Balthazart & Ball 2006). Or, androgen receptors are already present in non-classical androgen targets at very early stages of development, given that both mouse blastocysts and embryonic stem cells express androgen receptors (Chang et al. 2006).
The impacts of yolk steroids during development probably differ between altricial and precocial species because many tissues and functions, including reproductive organs, differentiate and become functional in ovo in precocial birds, while in altricial birds they differentiate after hatching, when most of the steroid-laced yolk has been consumed.
Altricial species
Androgen and oestrogen receptors and aromatase mRNA are abundantly expressed in the brains of both sexes of canaries and zebra finches before hatching (Gahr et al. 1996; Gahr & Metzdorf 1997; Perlman & Arnold 2003; Perlman et al. 2003). In the zebra finch brain, one of the first androgen receptor expression domains is the hindbrain. From embryonic day 7 onwards, the newly formed hypoglossal motor nucleus (nXII) expresses androgen receptor mRNA in both sexes. From embryonic day 8 onwards, there is expression in the supraspinal motor nucleus (nSSp), which innervates neck muscles. By embryonic day 10, the syrinx contains androgen receptor mRNA. These structures are important for hatching and begging for food (Godsave et al. 2002) and thus, the neural substrates for hatching and begging behaviour, shown to be influenced by yolk testosterone (e.g. Schwabl 1996a,b; Lipar & Ketterson 2000; Von Engelhardt et al. 2006), express androgen receptors. However, we are not aware of any studies with altricial birds that looked for steroid receptors and enzymes at earlier stages and embryonic structures other than those reported above.
Precocial species
Embryos of precocial species express steroid-metabolizing enzymes and steroid receptors very early. For example, in the chicken embryo, mRNA for steroid-metabolizing enzymes such as p450scc, 3β-HSD, p450c17 and 17β-HSD are present in the undifferentiated gonads of both sexes by day 2 of incubation and oestrogen receptor mRNA occurs in the left female gonad on day 7 (Bruggeman et al. 2002). Thus, these enzymes may be active at a time when the embryo is exposed to maternal androgens in the yolk; they could convert yolk androgens such as androstenedione and testosterone into oestrogens, the ligands for the oestrogen receptors. Androgen receptors are expressed in various tissues of both sexes of chicken embryo, for example, brain, heart, liver, intestines and mesonephros at embryonic day 15; in the gonad, androgen receptor expression is dimorphic, detectable in females by embryonic day 7 (Katoh et al. 2006). These studies hint at potential targets for yolk androgens in embryos of both sexes, possibly explaining effects occurring in both sexes, and also demonstrating potential metabolism into oestrogens.
(iii) Why do yolk steroids apparently not interfere with sexual differentiation?
Oestrogens, produced by the differentiating and differentiated ovary, are an essential link in sexual differentiation of gonads, reproductive tracts, brain and behaviour, although the role of steroids in differentiation of the passerine song system is not clear (for review see Balthazart & Adkins-Regan (2002) and Carere & Baltazart (2007)). Although concentrations of maternal oestrogen in yolk are low, oestrogens could be derived from yolk androgens by embryonic aromatase. Yet, yolk androgens apparently do not interfere with proper sexual differentiation of gonads, reproductive tracts, brain and behaviour. Although thorough studies of testes, reproductive tracts and brains have not been conducted, androgen injections into eggs within the physiological range did not induce the development of ornaments typical for one sex in the other (e.g. Strasser & Schwabl 2004; K. Pfannkuche & T. G. G. Groothuis 2007, unpublished data; Von Engelhardt & Groothuis in preparation; Rubolini et al. 2006b). How can this discrepancy be understood? First, as suggested by Carere & Baltazart (2007), there may be differences in the window of embryonic sensitivity to maternal (present from the beginning of embryonic development) and embryonic steroids (not produced in a sex-specific way until the second week after the start of incubation in quail, duck and chicken (Tanabe et al. 1983, 1986; Ottinger et al. 2001; see also Carere & Baltazart (2007) for an excellent discussion on this point)). Second, the contrasting results between developmental hormone manipulation in the frameworks of maternal effects (no effect on sexual differentiation) and sexual differentiation (androgens and estrogens demasculinize and feminize genetically male embryos) might very well be explained by hormone dosage. The studies showing that egg injections of androgens demasculinize male sexual traits in birds (recent review by Carere & Baltazart (2007)) applied extremely high doses (milligram and microgram range; Wilson & Glick 1970; Adkins 1979; Sayag et al. 1991). These dosages exceed yolk androgen concentrations 10- to 1000-fold. Yet, lower dosages, for example, below 50 μg testosterone propionate (yielding approximately 1666 pg mg−1 yolk), were ineffective in demasculinizing male Japanese quail (Adkins 1979). Dosages of oestradiol benzoate and diethylstilbestrol that affected sexual differentiation in these studies were also extremely high, while maternal oestradiol levels in yolk are very low. Thus, the discrepancy between studies on effects of maternal hormones and those addressing sexual differentiation may be explained by dose alone. Moreover, in the zebra finch, exposure to androgens during early development does influence, but not disrupt, sexual differentiation of the song system (Arnold 2002). This could suggest maternal modification of, rather than interference with, the development of this sexually dimorphic system. Finally, interference with sexual differentiation is more likely in precocial than altricial species (Carere & Baltazart 2007) because the former start and complete sexual differentiation much earlier, during the phase of yolk utilization.
In the end, the indications of maternal androgens not acting according to the classical view of demasculinization and feminization may require relaxation of the concept of how steroid hormones influence phenotype and call for novel approaches to the role of hormones in development.
(iv) Hormone specificity and dose–response relationships
Studies often refer to yolk androgens as a whole, implying that androstenedione (A4), testosterone (T) and 5α-dihydrotestosterone (5α-DHT) have similar effects and act via the same pathway. 5α-DHT is the endpoint of the androgen synthesis pathway and cannot be converted to T or oestrogens and therefore can act only via the androgen receptor. A4 and T, in contrast, in addition of being ligands of the androgen receptor (Chen et al. 2004; Jasuja et al. 2005; Sonneveld et al. 2005, 2006), can be converted to oestrogens (Payne & Hales 2004). Therefore, they can potentially act through an oestrogen receptor pathway. Even when acting through the androgen receptor pathway, the three androgens have different affinities for the androgen receptor (5α-DHT>T≫A4). The androgen receptor's affinity for A4 is low (0.1% of 5α-DHT; Holterhus et al. 2002; Chen et al. 2004; Jasuja et al. 2005) and A4 has a relatively low biological activity. For example, the relative agonistic activity of A4 in cell-line based in vivo assays is 5.7% of 5α-DHT, while that of T is 14.6% of 5α-DHT (Sonneveld et al. 2005, 2006). Moreover, A4, T and 5α-DHT can induce different gene expression patterns via the androgen receptor pathway (Holterhus et al. 2002). Consequently, yolk A4, T and 5α-DHT can be expected to have different potencies in affecting developmental processes. This is, for example, emphasized by a stronger negative correlation between length of embryo period and yolk 5α-DHT, rather than T concentration among passerine birds (Schwabl et al. 2007; Martin & Schwabl 2008). Finally, A4 may serve as a precursor for T in the embryo, thereby contributing to the pool of the biologically more potent T or 5α-DHT (Groothuis & Schwabl 2002; Schwabl et al. 2007) if the conversion enzymes are present in the yolk or embryo. For these reasons, it is important to distinguish between steroid moieties, even ‘androgens’, and studies of effects of individual androgens as well as their mixtures, as they are present in yolks, are urgently needed.
It is often assumed that, within the physiological range of yolk hormone concentrations of a species, increased dosages cause greater effects, i.e. the relationship is linear. Yet, dose–response curves are often non-monotonic showing an inverted U-shape, with intermediate dosages having greater effects than either higher or lower doses (e.g. Sheehan et al. 1999; Welshons et al. 2003; Weltje et al. 2005). We are aware of only a few experimental studies that have used more than one dose of a hormone. For example, Navara et al. (2005) showed that a super-physiological dose of testosterone resulted in different effects on several traits (e.g. growth and mass, immune response) than a lower dose that was within the physiological range. A study by Norton & Wira (1977) showed that in ovo injections of a low dose of testosterone stimulated, while a high dose inhibited growth of the bursa of Fabricius, an organ crucial for the development of the avian immune system. A nonlinear dose–response relationship might set an upper limit to hormone-mediated maternal effects, i.e. mothers cannot necessarily elicit even greater responses in the embryo by further increasing hormone exposure. On the other hand, a nonlinear, inverted U-shaped relationship (with less effect at higher doses) might relax constraints, i.e. the mothers could produce large amounts of a hormone for regulation of their own functions with the resulting high exposure of the embryo having little additional physiological consequence; or, intermediate hormone secretion might influence the embryo with no effects on the mother. Finally, low doses of maternal hormones, as often found in first-laid eggs, do not necessarily have no effect on the offspring as studies of developmental endocrine disruption show that embryos are exquisitely sensitive to hormonal signals (Sheehan et al. 1999; Welshons et al. 2003). Clearly, dose–response studies and separating effects of different steroids are now necessary.
(v) Pleiotropic actions of yolk androgens and integration of the developmental phenotype
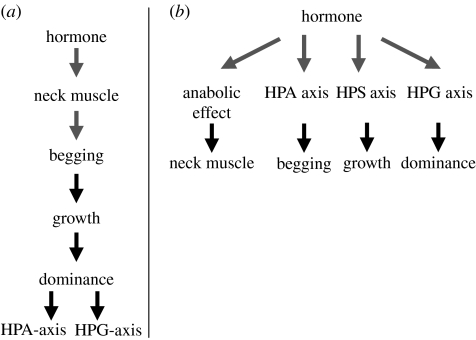
Maternal steroids affect multiple and diverse traits of the offspring in birds, for example, size and growth, immune and endocrine system, feather colouration and behaviour, some of which are expressed during development, others in adulthood, some sex-linked, others not (see Groothuis et al. 2005; and update in §1). Are these effects consequences of multiple singular actions of, for example, yolk testosterone on individual tissues such as bone, immune cells, feather follicles, neurons and muscle? Or, do they result from developmental actions on a common mediator that later interacts with multiple systems? A common mediator would allow much less scope for the mother to adjust specific offspring traits independently from other traits. For example, testosterone might affect early muscle growth, including the neck muscle (Lipar & Ketterson 2000) that facilitates begging (Schwabl & Lipar 2002), which in turn affects growth leading to long-lasting effects on dominance and immune function (see Monaghan 2008). Consequently, an effect of testosterone on growth would be indirect and depend on the extent of sibling competition and food availability. However, some studies detected growth differences without differences in begging behaviour (e.g. Pilz et al. 2004), suggesting a more direct effect of testosterone on growth (figure 2). In addition, embryonic exposure to maternal androgens increases the sensitivity to testosterone later in life (see below, Groothuis & Müller in preparation), suggesting that maternal androgens affect testosterone-mediated traits by changing hormone sensitivity, for example, by upregulation of androgen receptors. In a similar vein, the suppressive effect of yolk androgens on immune function has been interpreted as androgens causing reallocation of energy resources to growth at the expense of immune development (Groothuis et al. 2005), but such effects might occur directly via effects on cells of the immune system.
Figure 2.
Two conceptual alternatives for pleiotropic effects of a yolk hormone. (a) The hormone influences a single trait, resulting in faster early growth and indirect consequences for the function of other traits; (b) early exposure to the hormone influences multiple traits directly and independently from each other.
One obvious way in which yolk hormones could have pleiotropic effects is by influencing the production or action of a diversity of hormones early and later in development. For example, hormones of the HPG axis, such as testosterone, interact with the hypothalamus–pituitary–adrenal (HPA) axis (McCormick et al. 1998), the hypothalamus–pituitary–somatotrophic axis (HPS; growth hormone and IGF-I; e.g. Meinhardt & Ho 2006) and the immune system, possibly indirectly via the HPA axis (e.g. Martin 2000; Olsen & Kovac 2001; Owen-Ashley et al. 2004). Below, we briefly summarize what is known about effects of yolk steroids on these systems.
Hypothalamus–pituitary–gonadal axis
Yolk androgens may influence androgen-regulated traits later in life by changing androgen production (Müller et al. 2007a; but see Partecke & Schwabl in preparation) or by influencing sensitivity (numbers of androgen receptors; Benowitz-Fredericks et al. 2006), as in the case of sexual differentiation. This may explain why androgen-dependent traits that are expressed later in life, for example the black bib in the house sparrow or the nuptial plumage in gulls, are affected by androgen injections in ovo (Strasser & Schwabl 2004; Partecke & Schwabl in preparation; Eising et al. 2006). Treatment of juvenile gulls with testosterone led to a faster and higher expression level of social display behaviour in birds hatched from androgen-treated eggs than control eggs, indicating that in ovo exposure to testosterone increases the sensitivity to testosterone later in life (Groothuis & Müller in preparation).
Hypothalamus–pituitary–adrenal axis
Some evidence suggests that yolk androgens influence the secretion of glucocorticoids of nestlings and adults. For example, nestling corticosterone concentrations were higher in American kestrel chicks hatched from androgen-treated than from control eggs (Sockman & Schwabl 2000); similar results were obtained for Japanese quail (Daisley et al. 2005). In American kestrels, androgen-exposed chicks also showed reduced growth that may have indirectly led to their higher corticosterone levels or vice versa. Since chick begging behaviour, at least in the kittiwake, is under the regulation of corticosterone (Kitaysky et al. 2001), the effects of androgen injections in ovo on early begging behaviour may be mediated by altered HPA axis function. Studies with house sparrows treated with testosterone in the egg indicate an enhanced corticosterone stress response of adult females but not males, suggesting sex-specific effects on the HPA axis that carry over into adulthood (Partecke & Schwabl in preparation). Conversely, stressed mothers lay eggs with high yolk corticosterone concentrations (Hayward & Wingfield 2004; Saino et al. 2005) and exposure to corticosterone in the yolk enhances HPA function in Japanese quail (Hayward & Wingfield 2003).
Hypothalamus–pituitary–thyroid axis
We are unaware of any study that investigated effects of yolk androgens on the HPT axis. Thyroid hormones affect multiple aspects of avian development including growth, neural cell proliferation, development of thermoregulation, differentiation of cartilage, bone calcification and the formation of contractile proteins in skeletal muscle (Nunez 1984; Stockdale & Miller 1987; McNabb & Wilson 1997). Therefore, some of the effects of exposure to maternal androgens on body mass and growth might be mediated by interactions with the HPT axis.
Hypothalamus–pituitary–somatic axis
Sex steroids clearly affect this axis at two levels: central secretion of GH and peripheral responsiveness of IGF-1 secretion, the mediator of anabolic actions of GH (e.g. Meinhardt & Ho 2006). Thus, the potential exists for yolk androgens to modify this system. Corticosterone influences the ontogeny of adeno-hypophyseal GH-secreting cells (somatotrophs) in the chicken (Bossis & Porter 2000; Porter 2005) as well, possibly opening the door for effects of maternal corticosterone via this system (Love et al. 2005).
In sum, it needs to be shown how far the actions of androgens on multiple systems are coordinated and come as an integrated package resulting in trade-off of costs and benefits of the various effects, or whether these effects are independent of one another leaving much more flexibility for adaptive expression of maternal effects.
3. Conclusions and perspective
We hope to have shown that a deeper understanding of the mechanisms underlying hormone-mediated maternal effects in birds is indispensable for understanding the function and evolution of these effects. The field is at the moment dominated by the ultimate approach and much work is needed on the mechanisms by which hormones accumulate in the egg and how is regulated (or not), and how, when and where these maternal hormones affect embryo target tissues. This provides ample questions for interested physiologists to get at the underpinnings of hormone-mediated maternal effects. (i) What are the mechanisms by which maternal hormones accumulate in the egg and what is the degree of regulation over this process? Several mechanisms are conceivable and the analyses of the correlation between plasma and yolk concentration of a specific hormone might not be the best test to disentangle these mechanisms. (ii) What is the timing and location of expression of relevant enzymes and receptors in the embryo? Because enzymes and receptors have mainly been studied within the framework of sexual differentiation, there is little information about expression before the embryo starts to produce its own hormones but is already exposed to maternal hormones for several days. (iii) What are the pathways underlying the many pleiotropic effects of maternal hormones? Only after these mechanisms are better described, will we be able to comprehend the significance of maternal effects in evolution and selection. Therefore, we hope that this review stimulates endocrinologists to undertake such studies in the knowledge that they may have a large impact not only in their own field but also in the field behavioural and evolutionary ecology, which has provided us with an excellent, but perhaps somewhat unusual, animal model for physiologists to study hormone-mediated maternal effects. We hope to have shown behavioural ecologists how important the proximate approach is and to have encouraged a them to be more cautious in their interpretation of results and use of language that is currently not supported by a full knowledge of underlying mechanism.
Now that so many effects of maternal androgens on offspring development have been documented, the time is ripe to look at long-term and inter-generational effects, and to incorporate the expertise of neurobiologists and geneticists. Yolk, as a source of androgens for developing birds, is consumed mainly by the embryo and, within a few days after hatching, by the nestling. Yet, effects persist into later life, even into adulthood. Some might be indirect effects, such as a consequence of early hatching for the social position in sibling rivalry, others may be analogous to the classical organizational actions of steroids in differentiation between the sexes. Upregulation of hormone production in the adult by prenatal exposure to maternal hormones may be reflected in the eggs of the next generation resulting in transgenerational carry-over. Long-term effects may be mediated by changes in chromatin state, similar to the epigenetic mechanism by which the style of maternal care stably alters the expression of hippocampal glucocorticoid receptors in rats (e.g. Weaver et al. 2004). Finally, similar to developmental exposure to endocrine disruptors (Anway & Skinner 2006), maternal hormones might cause epigenetic modifications of the germ line that carry over into several generations. Study of such epigenetic and transgenerational actions of maternal hormones offer an exciting future perspective.
Acknowledgments
We thank John Wingfield, three anonymous reviewers, Nikolaus von Engelhardt, Jeremy Egbert and the Schwabl lab for comments and suggestions on the manuscript.
Footnotes
Both authors contributed equally to this work.
One contribution of 12 to a Theme Issue ‘Integration of ecology and endocrinology in avian reproduction: a new synthesis’.
References
- Adkins E.K. Effect of embryonic treatment with estradiol or testosterone on sexual differentiation of quail brain. Neuroendocrinology. 1979;29:178–185. doi: 10.1159/000122920. [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E, Ottinger M.A, Park J. Maternal transfer of estradiol to egg yolks alter sexual differentiation of avian offspring. J. Exp. Zool. 1995;271:466–470. doi:10.1002/jez.1402710608 [Google Scholar]
- Anway M.D, Skinner M.K. Epigenetic transgenerational actions of endocrine disruptors. Endocrinology. 2006;147:S43–S49. doi: 10.1210/en.2005-1058. doi:10.1210/en.2005-1058 [DOI] [PubMed] [Google Scholar]
- Arnold, A. P. 2002 Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In Hormones, brain, and behavior, vol. 4 (eds D. W. Pfaff, A. Arnold, A. Etgen, S. Fahrbach & R. Rubin), pp. 105–135. New York, NY: Academic Press.
- Badyaev A.V, Schwabl H, Young R.L, Duckworth R.A, Navara K, Parlow A.F. Adaptive sex differences in growth of pre-ovulation oocytes in a passerine bird. Proc. R. Soc. B. 2005;272:2165–2172. doi: 10.1098/rspb.2005.3194. doi:10.1098/rspb.2005.3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Adkins-Regan E. Sexual differentiation of brain and behaviour in birds. In: Pfaff D.W, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, brain and behavior. Academic Press; New York, NY: 2002. pp. 223–301. [Google Scholar]
- Balthazart J, Ball G.F. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. doi:10.1016/j.tins.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Benowitz-Fredericks, M., Kitaysky, A. & Meddle, S. 2006 Effects of elevated yolk steroids on steroid receptor and aromatase mRNA expression in the hatchling quail (Coturnix japonica) brain. In Poster E-bird Conf. Glasgow, November 2006
- Bossis I, Porter T.E. Onogeny of corticosterone-inducible growth hormone-secreting cells during chick embryo development. Endocrinology. 2000;141:2683–2690. doi: 10.1210/endo.141.7.7554. doi:10.1210/en.141.7.2683 [DOI] [PubMed] [Google Scholar]
- Breuner C.W, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. doi:10.1677/joe.0.1750099 [DOI] [PubMed] [Google Scholar]
- Bruggeman V, Van As P, Decuypere E. Developmental endocrinology of the reproductive axis in the chicken embryo. Comp. Biochem. Physiol. A. 2002;131:839–846. doi: 10.1016/s1095-6433(02)00022-3. doi:10.1016/S1095-6433(02)00022-3 [DOI] [PubMed] [Google Scholar]
- Carere C, Baltazart L. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol. Metab. 2007;18:73–80. doi: 10.1016/j.tem.2007.01.003. doi:10.1016/j.tem.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Chang C, Hsuuw Y, Huang F, Shyr C, Chang H.C, Kang H, Huang K. Androgenic and antiandrogenic effects and expression of androgen receptor in mouse embryonic stem cells. Fertil. Steril. 2006;85(Suppl. 1):1195–1203. doi: 10.1016/j.fertnstert.2005.11.031. doi:10.1016/j.fertnstert.2005.11.031 [DOI] [PubMed] [Google Scholar]
- Chen F, et al. Partial agonist/antagonist properties of androstenedione and 4-androsten-3β,17β-diol. J. Steroid Biochem. Mol. Biol. 2004;91:247–257. doi: 10.1016/j.jsbmb.2004.04.009. doi:10.1016/j.jsbmb.2004.04.009 [DOI] [PubMed] [Google Scholar]
- Cheng M.F. Progress and prospects in ring dove research: a personal view. Adv. Study Behav. 1979;9:97–129. [Google Scholar]
- Clotfelter E.D, O'Neal D.M, Gaudioso J.M, Casto J.M, Parker-Renga I.M, Snajdr E.A, Duffy D.L, Nolan V, Jr, Ketterson E.D. Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm. Behav. 2004;46:171–178. doi: 10.1016/j.yhbeh.2004.03.003. doi:10.1016/j.yhbeh.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Daisley J.N, Bromundt V, Möstl E, Kotrschal K. Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks Coturnix japonica. Horm. Behav. 2005;47:185–194. doi: 10.1016/j.yhbeh.2004.09.006. doi:10.1016/j.yhbeh.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Egbert, J. R. 2006 Behavioral and endocrine responses of female house sparrows to a social challenge: the relationship between plasma and yolk testosterone. Master's thesis, Washington State University, Washington, DC.
- Eising, C. 2004 Mother knows best? Costs and benefits of maternal hormone allocation in birds. Fig.10.2. PhD thesis, University of Groningen, The Netherlands.
- Eising C.M, Müller W, Dijkstra C, Groothuis T.G.G. Maternal androgens in egg yolks: relation with sex, incubation time and embryonic growth. Gen. Comp. Endocrinol. 2003;132:241–247. doi: 10.1016/s0016-6480(03)00090-x. doi:10.1016/S0016-6480(03)00090-X [DOI] [PubMed] [Google Scholar]
- Eising C.M, Muller W, Groothuis T.G.G. Avian mothers create different phenotypes by hormone deposition in their eggs. Biol. Lett. 2006;2:20–22. doi: 10.1098/rsbl.2005.0391. doi:10.1098/rsbl.2005.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elf P.K, Fivizzani A.J. Changes in sex steroid levels in yolks of the leghorn chicken, Gallus domesticus, during embryonic development. J. Exp. Biol. 2002;293:594–600. doi: 10.1002/jez.10169. [DOI] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res. Bull. 1997;44:509–517. doi: 10.1016/s0361-9230(97)00233-5. doi:10.1016/S0361-9230(97)00233-5 [DOI] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R, Aschenbrennner S. The ontogeny of the canary HVC revealed by the expression of androgen and estrogen receptors. NeuroReport. 1996;8:311–315. doi: 10.1097/00001756-199612200-00062. doi:10.1097/00001756-199612200-00062 [DOI] [PubMed] [Google Scholar]
- Gil D. Golden eggs: maternal manipulation of offspring phenotype by egg androgen in birds. Ardeola. 2003;50:281–294. [Google Scholar]
- Gil D, Faure J. Correlated response in yolk testosterone levels following divergent genetic selection for social behaviour in Japanese quail. J. Exp. Zool. 2007;307A:91–94. doi: 10.1002/jez.a.340. doi:10.1002/jez.a.340 [DOI] [PubMed] [Google Scholar]
- Gil D, Marzal A, De Lope F, Puerta M, Møller A.P. Female house martins (Delichon urbica) reduce egg androgen deposition in response to a challenge of their immune system. Behav. Ecol. Sociobiol. 2006;60:96–100. doi:10.1007/s00265-005-0145-1 [Google Scholar]
- Gil D, Biard C, Lacroix A, Spottiswoode C, Saino N, Puerta M, Moller A.P. Evolution of yolk androgens in birds: development, coloniality and sexual dichromatism. Am. Nat. 2007;169:802–819. doi: 10.1086/516652. doi:10.1086/516652 [DOI] [PubMed] [Google Scholar]
- Gilbert S.F. Ecological developmental biology. Developmental biology meets the real world. Dev. Biol. 2001;233:1–12. doi: 10.1006/dbio.2001.0210. doi:10.1006/dbio.2001.0210 [DOI] [PubMed] [Google Scholar]
- Gilbert L, Rutstein A.N, Hazon N, Graves J.A. Sex-biased investment in yolk androgens depends on female quality and laying order in zebra finches (Taeniopygia guttata) Naturwissenschaften. 2005;92:178–181. doi: 10.1007/s00114-004-0603-z. doi:10.1007/s00114-004-0603-z [DOI] [PubMed] [Google Scholar]
- Godsave S.F, Lohman R, Vloet R.P.M, Gahr M. Androgen receptors in the embryonic zebra finch hindbrain suggest a function for maternal androgens in perihatching survival. J. Comp. Neurol. 2002;453:57–70. doi: 10.1002/cne.10391. doi:10.1002/cne.10391 [DOI] [PubMed] [Google Scholar]
- Gorman K.B, Williams T.D. Correlated evolution of maternally derived yolk testosterone and early developmental traits in passerine birds. Biol. Lett. 2005;1:461–464. doi: 10.1098/rsbl.2005.0346. doi:10.1098/rsbl.2005.0346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis, T. G. G. & Eising, C. M. In preparation. Social stimulation and maternal hormone deposition in egg yolks: experimental evidence from black-headed gulls, Larus ridibundus L
- Groothuis, T. G. G. & Müller, W. In preparation. Embryonic exposure to testosterone enhances behavioural sensitivity to testosterone later in life: a study in gulls.
- Groothuis T.G.G, Schwabl H. Determinants of within and among clutch variation in levels of maternal hormones in black-headed gull eggs. Funct. Ecol. 2002;16:281–289. doi:10.1046/j.1365-2435.2002.00623.x [Google Scholar]
- Groothuis T.G.G, von Engelhardt N. Investigating maternal hormones in avian eggs: measurement, manipulation, and interpretation. Ann. N Y Acad. Sci. 2005;1046:168–180. doi: 10.1196/annals.1343.014. doi:10.1196/annals.1343.014 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Müller W, Von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. doi:10.1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Groothuis, T. G. G., Carere, C., Lipar, J., Drent, P. J. & Schwabl, H. Submitted. Selection on personality in a wild songbird affects egg maternal hormone levels turned to its effect on timing of reproduction. [DOI] [PMC free article] [PubMed]
- Hackl R, Bromundt V, Daisley J.M, Kotrschal K, Möstl E. Distribution and origin of steroid hormones in the yolk of Japanese quail eggs (Coturnix coturnix japonica) J. Comp. Physiol. B. 2003;173:327–331. doi: 10.1007/s00360-003-0339-7. doi:10.1007/s00360-003-0339-7 [DOI] [PubMed] [Google Scholar]
- Hayward L.S, Wingfield J.C. Maternal corticosterone slows chick growth and increases adult stress response. Integr. Comp. Biol. 2003;43:925. [Google Scholar]
- Hayward L.S, Wingfield J.C. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen. Comp. Endocrinol. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. doi:10.1016/j.ygcen.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Hayward L.S, Satterlee D.G, Wingfield J.C. Japanese quail selected for high plasma corticosterone response deposit high levels of corticosterone in their eggs. Physiol. Biochem. Zool. 2005;78:1026–1031. doi: 10.1086/432854. doi:10.1086/432854 [DOI] [PubMed] [Google Scholar]
- Hayward L.S, Richardson J.B, Grogan M.N, Wingfield J.C. Sex differences in the organizational effects of corticosterone in the egg yolk of Japanese quail. Gen. Comp. Endocrinol. 2006;146:144–148. doi: 10.1016/j.ygcen.2005.10.016. doi:10.1016/j.ygcen.2005.10.016 [DOI] [PubMed] [Google Scholar]
- Holterhus P.M, Piefke S, Hiort O. Anabolic steroids, testosterone-precursors and virilizing androgens induce distinct activation profiles of androgen responsive promoter constructs. J. Steroid Biochem. Mol. Biol. 2002;82:269–275. doi: 10.1016/s0960-0760(02)00220-0. doi:10.1016/S0960-0760(02)00220-0 [DOI] [PubMed] [Google Scholar]
- Jasuja R, et al. Δ-4-Androstene-3,17-dione binds androgen receptor, promotes myogenesis in vitro, and increases serum testosterone levels, fat-free mass, and muscle strength in hypogonadal males. J. Clin. Endocrinol. Metab. 2005;90:855–863. doi: 10.1210/jc.2004-1577. doi:10.1210/jc.2004-1577 [DOI] [PubMed] [Google Scholar]
- Jawor J.M, McGlothlin J.W, Casto J.M, Greives T.J, Snajdr E.A, Bentley G.E, Ketterson E.D. Testosterone response to GnRH in a female songbird varies with stage of reproduction: implications for adult behaviour and maternal effects. Funct. Ecol. 2007;21:761–775. doi:10.1111/j.1365-2435.2007.01280.x [Google Scholar]
- Johnson, A. L. 2002 Reproduction in the female. In Sturkie's avian physiology (ed. G. C. Whittow), pp. 569–591, 5th edn. San Diego, CA: Academic Press.
- Katoh H, Ogino Y, Yamada G. Cloning and expression of androgen receptor gene in chicken embryogenesis. FEBS Lett. 2006;580:1607–1615. doi: 10.1016/j.febslet.2006.01.093. doi:10.1016/j.febslet.2006.01.093 [DOI] [PubMed] [Google Scholar]
- Ketterson E, Nolan V., Jr Adaptation, exaptation, and constraint: a hormonal perspective. Am. Nat. 1999;154:S4–S25. doi: 10.1086/303280. doi:10.1086/303280 [DOI] [PubMed] [Google Scholar]
- Ketterson E.D, Nolan V, Jr, Sandell M. Testosterone in females: mediators of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 2005;166:S85–S89. doi: 10.1086/444602. doi:10.1086/444602 [DOI] [PubMed] [Google Scholar]
- Kitaysky A.S, Wingfield J.C, Piatt J.F. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 2001;12:619–625. doi:10.1093/beheco/12.5.619 [Google Scholar]
- Kralli A, Yamamoto K.R. An FK506-sensitice transporter selectively decreases intracellular levels and potency of steroid hormones. J. Biol. Chem. 1996;271:17 152–17 156. doi: 10.1074/jbc.271.29.17152. doi:10.1074/jbc.271.29.17152 [DOI] [PubMed] [Google Scholar]
- Kralli A, Bohen S.P, Yamamoto K.R. LEM1, an ATP-binding-cassette transporter, selectively modulates the biological potency of steroid hormones. Proc. Natl Acad. Sci. USA. 1995;92:4701–4705. doi: 10.1073/pnas.92.10.4701. doi:10.1073/pnas.92.10.4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landys M.M, Ramenofsky M, Wingfield J.C. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 2006;148:132–149. doi: 10.1016/j.ygcen.2006.02.013. doi:10.1016/j.ygcen.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Lipar J.L, Ketterson E.D. Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Aegelius phoeniceus. Proc. R. Soc. B. 2000;267:2005–2010. doi: 10.1098/rspb.2000.1242. doi:10.1098/rspb.2000.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipar J.L, Ketterson E.D, Nolan V, Jr, Casto J.M. Egg yolk layers vary in the concentration of steroid hormones in two avian species. Gen. Comp. Endocrinol. 1999;115:220–227. doi: 10.1006/gcen.1999.7296. doi:10.1006/gcen.1999.7296 [DOI] [PubMed] [Google Scholar]
- Love O.P, Chin E.H, Wynne-Edwards K.E, Williams T.D. Stress hormones: a link between maternal condition and sex-biased reproductive investment. Am. Nat. 2005;166:751–766. doi: 10.1086/497440. doi:10.1086/497440 [DOI] [PubMed] [Google Scholar]
- Marshall R.C, Leisler B, Catchpole C.K, Schwabl H. Male song quality affects circulating but not yolk steroid concentrations in female canaries (Serinus canaria) J. Exp. Biol. 2005;208:4593–4598. doi: 10.1242/jeb.01949. doi:10.1242/jeb.01949 [DOI] [PubMed] [Google Scholar]
- Martin J.T. Sexual dimorphism in immune function: the role of prenatal exposure to androgens and estrogens. Eur. J. Pharmacol. 2000;405:251–261. doi: 10.1016/s0014-2999(00)00557-4. doi:10.1016/S0014-2999(00)00557-4 [DOI] [PubMed] [Google Scholar]
- Martin T.M, Schwabl H. Variation in maternal effects and embryonic development among passerine bird species. Phil. Trans. R. Soc. B. 2008;363:1663–1674. doi: 10.1098/rstb.2007.0009. doi:10.1098/rstb.2007.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazuk J, Bonneaud C, Chastel O, Sorci G. Social environment affects female and egg testosterone levels in the house sparrow (Passer domesticus) Ecol. Lett. 2003;6:1084–1090. doi:10.1046/j.1461-0248.2003.00535.x [Google Scholar]
- McCormick C.M, Furey B.F, Child M, Sawyer M.J, Donohue S.M. Neonatal sex hormones have ‘organizational’ effects on the hypothalamic-pituitary-adrenal axis of male rats. Dev. Brain Res. 1998;105:295–307. doi: 10.1016/s0165-3806(97)00155-7. doi:10.1016/S0165-3806(97)00155-7 [DOI] [PubMed] [Google Scholar]
- McEwen B.S, Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. doi:10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- McNabb F.M.A. Thyroid hormones, their activation, degradation and effects on metabolism. J. Nutr. 1995;125:S1773–S1776. doi: 10.1093/jn/125.suppl_6.1773S. [DOI] [PubMed] [Google Scholar]
- McNabb F.M.A. Maternal thyroid hormones in avian eggs: potential for disruption of thyroid hormone deposition and effects on embryonic development. In: Dawson A, Chatuverdi C.M, editors. Avian endocrinology. Narosa Publishing House; New Delhi, India: 2001. pp. 219–230. [Google Scholar]
- McNabb F.M.A. The hypothalamic-pituitary-thyroid (HPT) axis in birds and its role in bird development and reproduction. Crit. Rev. Toxicol. 2007;37:163–193. doi: 10.1080/10408440601123552. doi:10.1080/10408440601123552 [DOI] [PubMed] [Google Scholar]
- McNabb F.M.A, Wilson C.A. Thyroid hormone deposition in avian eggs and effects on embryonic development. Am. Zool. 1997;37:553–560. [Google Scholar]
- Meinhardt U.J, Ho K.K.Y. Modulation of growth hormone action by sex steroids. Clin. Endocrinol. 2006;65:413–422. doi: 10.1111/j.1365-2265.2006.02676.x. doi:10.1111/j.1365-2265.2006.02676.x [DOI] [PubMed] [Google Scholar]
- Monaghan P. Early development, phenotypic development and environmental change. Phil. Trans. R. Soc. B. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. doi:10.1098/rstb.2007.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Rueda G. Yolk androgen deposition as a female tactic to manipulate paternal contribution. Behav. Ecol. 2007;18:496–498. doi:10.1093ar1106 [Google Scholar]
- Mousseau T.A, Fox C.W. Oxford University Press; Oxford, UK: 1998. Maternal effects as adaptations. [Google Scholar]
- Müller W, Eising C, Dijkstra C, Groothuis T.G.G. Sex differences in yolk hormones depend on maternal social status in Leghorn chickens (Gallus gallus domesticus) Proc. R. Soc. B. 2002;269:2249–2256. doi: 10.1098/rspb.2002.2159. doi:10.1098/rspb.2002.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W, Deptuch K, López-Rull I, Gil D. Elevated yolk androgen levels benefit offspring development in a between clutch context. Behav. Ecol. 2007a;18:929–936. doi:10.1093/beheco/arm060 [Google Scholar]
- Müller W, Lessells C, Korsten P, von Engelhardt N. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am. Nat. 2007b;169:E84–E96. doi: 10.1086/511962. doi:10.1086/511962 [DOI] [PubMed] [Google Scholar]
- Navara K.J, Hill G.E, Mendonca M.T. Variable effects of yolk androgens on growth, survival, and immunity in Eastern bluebird nestlings. Physiol. Biochem. Zool. 2005;78:570–578. doi: 10.1086/430689. doi:10.1086/430689 [DOI] [PubMed] [Google Scholar]
- Navara K.J, Siefferman L.M, Hill G.E, Mendonca M.T. Yolk androgens vary inversely to maternal androgens in Eastern bluebirds: an experimental study. Funct. Ecol. 2006;20:449–456. doi:10.1111/j.1365-2435.2006.01114.x [Google Scholar]
- Norton J.M, Wira C.R. Dose-related effects of the sex hormones and cortisol on the growth of the Bursa Fabricius in chick embryos. J. Steroid Biochem. 1977;8:985–987. doi: 10.1016/0022-4731(77)90197-2. doi:10.1016/0022-4731(77)90197-2 [DOI] [PubMed] [Google Scholar]
- Nunez J. Effects of thyroid hormones during brain differentiation. Mol. Cell Endocrinol. 1984;37:125–132. doi: 10.1016/0303-7207(84)90043-1. doi:10.1016/0303-7207(84)90043-1 [DOI] [PubMed] [Google Scholar]
- Olsen N.J, Kovac W.J. Effects of androgens on T and B lymphocyte development. Immunol. Res. 2001;23:281–288. doi: 10.1385/IR:23:2-3:281. doi:10.1385/IR:23:2-3:281 [DOI] [PubMed] [Google Scholar]
- Ottinger M.A, Oitts S, Abelnabi M.A. Steroid hormones during embryonic development in Japanese quail: plasma, gonadal and andrenal levels. Poult. Sci. 2001;80:795–799. doi: 10.1093/ps/80.6.795. [DOI] [PubMed] [Google Scholar]
- Owen-Ashley N, Hasselquist D, Wingfield J.C. Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am. Nat. 2004;164:490–505. doi: 10.1086/423714. doi:10.1086/423714 [DOI] [PubMed] [Google Scholar]
- Palme, R. 2005 Measuring fecal steroids—guidelines for practical application. Bird hormones and bird migrations: analyzing hormones in droppings and egg yolks and assessing adaptations in long-distance migration. Ann. N Y Acad. Sci.1046, 75–80. [DOI] [PubMed]
- Partecke, J. & Schwabl, H. In preparation. Long-term effects of yolk testosterone treatment in male and female house sparrows.
- Payne A.H, Hales D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. doi:10.1210/er.2003-0030 [DOI] [PubMed] [Google Scholar]
- Perlman W.R, Arnold A.P. Expression of estrogen receptor and aromatase mRNAs in embryonic and post-hatch zebra finch brain. J. Neurobiol. 2003;55:204–219. doi: 10.1002/neu.10190. doi:10.1002/neu.10190 [DOI] [PubMed] [Google Scholar]
- Perlman W.R, Ramachandan B, Arnold A.P. Expression of androgen recpetor mRNA in the late embryonic and early posthatch zebra finch. J. Comp. Neurol. 2003;455:513–530. doi: 10.1002/cne.10510. doi:10.1002/cne.10510 [DOI] [PubMed] [Google Scholar]
- Petry F, Ritz V, Meineke C, Kietzmann T, Schmitz-Salue C, Hirsch-Ernst K.I. Subcellular localization of rat Abca5, a rat ATP-binding-cassette transporter expressed in Leydig cells, and characterization of its splice variant apparently encoding a half-transporter. Biochem. J. 2006;393:79–87. doi: 10.1042/BJ20050808. doi:10.1042/BJ20050808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz K.M, Smith H.G, Sandell M.I, Schwabl H. Interfemale variation in egg yolk androgen allocation in the European starling: do high-quality females invest more? Anim. Behav. 2003;65:841–850. doi:10.1006/anbe.2003.2094 [Google Scholar]
- Pilz K.M, Quiroga M, Schwabl H, Adkins-Regan E. European starling chicks benefit from high yolk testosterone levels during a drought year. Horm. Behav. 2004;46:179–192. doi: 10.1016/j.yhbeh.2004.03.004. doi:10.1016/j.yhbeh.2004.03.004 [DOI] [PubMed] [Google Scholar]
- Porter T.E. Regulation of pituitary somatotroph differentiation by hormones of peripheral endocrine glands. Domest. Anim. Endocrinol. 2005;29:52–62. doi: 10.1016/j.domaniend.2005.04.004. doi:10.1016/j.domaniend.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Rubolini D, Romano M, Boncoraglio G, Ferrari R.P, Martinelli R, Galeotti P, Fasola M, Saino N. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm. Behav. 2005;47:592–605. doi: 10.1016/j.yhbeh.2005.01.006. doi:10.1016/j.yhbeh.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Rubolini D, Romano M, Martinelli R, Saino N. Effects of elevated yolk testosterone levels on survival, growth and immunity of male and female yellow-legged gull chicks. Behav. Ecol. Sociobiol. 2006a;59:344–352. doi:10.1007/s00265-005-0057-0 [Google Scholar]
- Rubolini D, Romano M, Martinelli R, Leoni B, Saino N. Effects of prenatal yolk androgens on armaments and ornaments of the ring-necked pheasant. Behav. Ecol. Sociobiol. 2006b;59:549–560. doi:10.1007/s00265-005-0080-1 [Google Scholar]
- Rutkowska J, Badyaev A. Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Phil. Trans. R. Soc. B. 2008;363:1675–1686. doi: 10.1098/rstb.2007.0006. doi:10.1098/rstb.2007.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowska J, Cichoń M, Puerta M, Gil D. Negative effects of elevated testosterone on female fecundity in zebra finches. Horm. Behav. 2005;47:585–591. doi: 10.1016/j.yhbeh.2004.12.006. doi:10.1016/j.yhbeh.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Rutstein A, Gilbert L, Slater P, Graves J. Sex-specific patterns of yolk androgen allocation depend on maternal diet in the zebra finch. Behav. Ecol. 2005;16:16–69. [Google Scholar]
- Saino N, Romano M, Ferrari R.P, Martinelli R, Møller A.P. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J. Exp. Zool. A Comp. Exp. Biol. 2005;303A:998–1006. doi: 10.1002/jez.a.224. doi:10.1002/jez.a.224 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M, Romero L.M, Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Sayag N, Robinzon B, Snapir N, Arnon E, Grimm V.E. The effects of embryonic treatments with gonadal hormones on sexually dimorphic behavior of chicks. Horm. Behav. 1991;25:137–153. doi: 10.1016/0018-506x(91)90047-l. doi:10.1016/0018-506X(91)90047-L [DOI] [PubMed] [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. doi:10.1073/pnas.90.24.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Environment modifies the testosterone levels of a female bird and its eggs. J. Exp. Zool. 1996a;276:157–163. doi: 10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N. doi:10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N [DOI] [PubMed] [Google Scholar]
- Schwabl H. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. 1996b;114A:271–276. doi: 10.1016/0300-9629(96)00009-6. doi:10.1016/0300-9629(96)00009-6 [DOI] [PubMed] [Google Scholar]
- Schwabl H, Lipar J. Hormonal regulation of begging behaviour. In: Wright J, Leonnard M.L, editors. Competition, corporation and communication. Kluwer Academic; Dordrecht, The Netherlands: 2002. pp. 221–244. [Google Scholar]
- Schwabl H, Palacios M.G, Martin T.M. Selection for rapid embryo development correlates with embryo exposure to maternal androgens among passerine birds. Am. Nat. 2007;170:196–206. doi: 10.1086/519397. doi:10.1086/519397 [DOI] [PubMed] [Google Scholar]
- Sheehan D.M, Willingham E, Gaylor D, Bergeron J.M, Crews D. No threshold dose for estradiol-induced sex reversal of turtle embryos: How little is too much? Environ. Health Perspect. 1999;107:155–159. doi: 10.1289/ehp.99107155. doi:10.2307/3434373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman K.W, Schwabl H. Yolk androgens reduce offspring survival. Proc. R. Soc. B. 2000;267:1451–1456. doi: 10.1098/rspb.2000.1163. doi:10.1098/rspb.2000.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman K.W, Schwabl H. Daily estradiol and progesterone levels relative to laying and onset of incubation in canaries. Gen. Comp. Endocrinol. 1999;114:257–268. doi: 10.1006/gcen.1999.7252. doi:10.1006/gcen.1999.7252 [DOI] [PubMed] [Google Scholar]
- Sockman K.W, Weiss J, Webster M.S, Talbot V, Schwabl H. Sex-specific effects of yolk androgens on growth of nestling American kestrels. Behav. Ecol. Socioecol. In press. doi:10.1007/s00265-007-0486-z [Google Scholar]
- Sonneveld E, Jansen H.J, Riteco J.A.C, Brouwer A, Van der Burg B. Development of androgen- and estrogen-response bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol. Sci. 2005;83:136–148. doi: 10.1093/toxsci/kfi005. doi:10.1093/toxsci/kfi005 [DOI] [PubMed] [Google Scholar]
- Sonneveld E, Riteco J.A.C, Jansen H.J, Pieterse B, Brouwer A, Schoonen W.G, Van der Burg B. Comparison of in vitro and in vivo screening models for androgenic and estrogenic activities. Toxicol. Sci. 2006;89:173–187. doi: 10.1093/toxsci/kfj009. doi:10.1093/toxsci/kfj009 [DOI] [PubMed] [Google Scholar]
- Staub N.L, De Beer M. The role of androgens in female vertebrates. Gen. Comp. Endocrinol. 1997;108:1–24. doi: 10.1006/gcen.1997.6962. doi:10.1006/gcen.1997.6962 [DOI] [PubMed] [Google Scholar]
- Stockdale F.E, Miller B.J. The cellular basis of myosin heavy chain isoform expression during development of avian skeletal muscles. Dev. Biol. 1987;123:1–9. doi: 10.1016/0012-1606(87)90420-9. doi:10.1016/0012-1606(87)90420-9 [DOI] [PubMed] [Google Scholar]
- Strasser R, Schwabl H. Yolk testosterone modifies the expression of sexually selected traits in sons. Behav. Ecol. Sociobiol. 2004;56:491–497. doi:10.1007/s00265-004-0810-9 [Google Scholar]
- Tanabe Y, Takashi Y, Nakamura T. Steroid hormone synthesis and sevretion by testis, ovary and adrenals of embryonic and post-embryonic ducks. Gen. Comp. Endocrinol. 1983;49:144–153. doi: 10.1016/0016-6480(83)90018-7. doi:10.1016/0016-6480(83)90018-7 [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Saito N, Nakamura T. Ontogenetic steroidgenesis by testis, ovary and adrenals of embryonic and postembryonic chickens. Gen. Comp. Endocrinol. 1986;63:456–463. doi: 10.1016/0016-6480(86)90146-2. doi:10.1016/0016-6480(86)90146-2 [DOI] [PubMed] [Google Scholar]
- Tschirren B, Richner H, Schwabl H. Ectoparasite-modulated deposition of maternal androgens in Great tit eggs. Proc. R. Soc. B. 2004;271:1371–1375. doi: 10.1098/rspb.2004.2730. doi:10.1098/rspb.2004.2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschirren B, Fitze P.S, Richner H. Maternal modulation of natal dispersal in a passerine bird: an adaptive strategy to cope with parasitism? Am. Nat. 2007;169:87–93. doi: 10.1086/509945. doi:10.1086/509945 [DOI] [PubMed] [Google Scholar]
- Verboven N, Monaghan P, Evans D.M, Schwabl H, Evans N, Whitelaw C, Nager R. Maternal condition, yolk androgens and offspring performance: a supplementary feeding experiment in the lesser black-backed gull (Larus fuscus) Proc. R. Soc. B. 2003;270:2223–2232. doi: 10.1098/rspb.2003.2496. doi:10.1098/rspb.2003.2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Engelhardt N, Groothuis T.G.G. Measuring steroid hormones in avian eggs. Ann. N Y Acad. Sci. 2005;1046:181–192. doi: 10.1196/annals.1343.015. doi:10.1196/annals.1343.015 [DOI] [PubMed] [Google Scholar]
- Von Engelhardt, N. & Groothuis, T. G. G. In preparation. Transfer to and metabolism of maternal steroid in avian eggs: studies with radio-labeled hormones.
- Von Engelhardt N, Carere C, Dijkstra C, Groothuis T.G.G. Sex specific effects of yolk testosterone on survival, begging, and growth of zebra finches. Proc. R. Soc. B. 2006;273:65–70. doi: 10.1098/rspb.2005.3274. doi:10.1098/rspb.2005.3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver I.C.G, Cervoni N, Champagne F.A, D'Alessio A.C, Sharma S, Seckl J.R, Dymov S, Szyf M, Meaney M.J. Epigenetic programming by maternal behavior. Nat. Genet. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Welshons W.V, Thayer K.A, Judy B.M, Taylor J.A, Curran E.M, Vom Saal F.S. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003;111:994–1006. doi: 10.1289/ehp.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltje L, Vom Saal F.S, Oehlmann J. Reproductive stimulation by low doses of xenoestrogens contrasts with the view of hormesis as an adaptive response. Hum. Exp. Toxicol. 2005;24:431–437. doi: 10.1191/0960327105ht551oa. doi:10.1191/0960327105ht551oa [DOI] [PubMed] [Google Scholar]
- Williams T.D, Kitaysky A.S, Vézina F. Individual variation in plasma estradiol-17β and androgen levels during egg formation in the European starling Sturnus vulgaris: implications for regulation of yolk steroids. Gen. Comp. Endocrinol. 2004;136:346–352. doi: 10.1016/j.ygcen.2004.01.010. doi:10.1016/j.ygcen.2004.01.010 [DOI] [PubMed] [Google Scholar]
- Williams T.D, Ames C.E, Kiparissis Y. Laying-sequence-specific variation in yolk oestrogen levels, and relationship to plasma oestrogen in female zebra finches (Taeniopygia guttata) Proc. R. Soc. B. 2005;272:173–177. doi: 10.1098/rspb.2004.2935. doi:10.1098/rspb.2004.2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J.A, Glick B. Onotgeny of mating behavior in the chicken. Am. J. Physiol. 1970;218:951–955. doi: 10.1152/ajplegacy.1970.218.4.951. [DOI] [PubMed] [Google Scholar]
- Wilson C.M, McNabb F.M.A. Maternal thyroid hormones in Japanese quail eggs and their influence on embryonic development. Gen. Comp. Endocrinol. 1997;107:153–165. doi: 10.1006/gcen.1997.6906. doi:10.1006/gcen.1997.6906 [DOI] [PubMed] [Google Scholar]
- Young R.L, Badyaev A.V. Evolution of sex-biased maternal effects in birds I: sex specific resource allocation among simultaneously growing follicles. J. Evol. Biol. 2004;17:1355–1366. doi: 10.1111/j.1420-9101.2004.00762.x. doi:10.1111/j.1420-9101.2004.00762.x [DOI] [PubMed] [Google Scholar]