Abstract
Almost all life histories are phenotypically plastic: that is, life-history traits such as timing of breeding, family size or the investment in individual offspring vary with some aspect of the environment, such as temperature or food availability. One approach to understanding this phenotypic plasticity from an evolutionary point of view is to extend the optimality approach to the range of environments experienced by the organism. This approach attempts to understand the value of particular traits in terms of the selection pressures that act on them either directly or owing to trade-offs due to resource allocation and other factors such as predation risk. Because these selection pressures will between environments, the predicted optimal phenotype will too. The relationship expressing the optimal phenotype for different environments is the optimal reaction norm and describes the optimal phenotypic plasticity. However, this view of phenotypic plasticity ignores the fact that the reaction norm must be underlain by some sort of control system: cues about the environment must be collected by sense organs, integrated into a decision about the appropriate life history, and a message sent to the relevant organs to implement that decision. In multicellular animals, this control mechanism is the neuroendocrine system. The central question that this paper addresses is whether the control system affects the reaction norm that evolves. This might happen in two different ways: first, the control system will create constraints on the evolution of reaction norms if it cannot be configured to produce the optimal reaction norm and second, the control system will create additional selection pressures on reaction norms if the neuroendocrine system is costly. If either of these happens, a full understanding of the way in which selection shapes reaction norms must include details of the neuroendocrine control system. This paper presents the conceptual framework needed to explain what is meant by a constraint or cost being created by the neuroendocrine system and discusses the extent to which this occurs and some possible examples. The purpose of doing this is to encourage endocrinologists to take a fresh look at neuroendocrine mechanisms and help identify the properties of the system and situations in which these generate constraints and costs that impinge on the evolution of phenotypic plasticity.
Keywords: reaction norms, causal mechanisms, phenotypic approach, optimality models, trade-offs
1. Introduction
Historically, endocrinologists and evolutionary ecologists have taken very different approaches to understanding life histories. As early as 1938, Baker used a life-history trait—timing of breeding in birds—to make the distinction between what he called ‘proximate’ and ‘ultimate’ explanations, which later became two out of Tinbergen's (1963) four ‘why's’, ‘causation’ and ‘function’ (‘survival value’). Endocrinologists have been primarily interested in the former kinds of question: unravelling the chain of causation from the perception of the environment, via the workings of the neuroendocrine system, to the production of a particular morphological, physiological or behavioural phenotype. Advances in molecular biology, and in particular genomics, have contributed to breath-taking progress in recent years, revealing a dazzling array of exquisite detail. Evolutionary ecologists, on the other hand, have been concerned with the second kind of question: instead of the causal mechanisms, they have attempted to understand how selection pressures favour one particular life history over another, and in doing so to provide an evolutionary explanation for the diversity of life histories in the living world—and thus an understanding of how life histories might respond to changes in selection pressures, including those currently occurring at breakneck speed through anthropogenic environmental change.
While both endocrinologists and evolutionary ecologists acknowledge that their own discipline would be illuminated by insights from the other discipline, their disparate interests have led to an increasing conceptual rift that is a barrier to communication. Eventually, the understanding of endocrinologists (and others) of the causal mechanisms by which phenotypes are generated from genotypes will be integrated with that of evolutionary ecologists about how various selection pressures determine fitness in relation to phenotype (and that of geneticists of the rules of inheritance) to produce a complete mechanistic and functional explanation of evolutionary change. There are some encouraging initiatives towards this goal (see Zera et al. 2007), but for the time being progress is likely to be made piecemeal, by building bridges from either side of the gulf, and this paper is an attempt to do this. The question in the title essentially asks what needs to be known about neuroendocrinological mechanisms to understand the evolution of certain ecological traits. The answer to this question is of considerable interest to evolutionary ecologists, but it is the endocrinologists who have the knowledge that will underpin the answers. My aim in writing this paper is to explain what kinds of properties of neuroendocrinological control systems are of interest to ecologists, and why, and thereby promote communication between the two disciplines.
(a) Why are life histories interesting to evolutionary ecologists?
Life-history traits are traits related to the amount or rate, timing or location of events (particularly reproduction and death) during the life cycle. For example, clutch size is an example of the amount of reproduction, laying date of the timing of reproduction and natal dispersal (the net movement between birth and first reproduction) of a trait affecting the location of reproduction. Life-history traits are of interest to ecologists for two reasons: population ecologists are interested in them because they determine population size and how it varies through time. Evolutionary ecologists are interested in them because reproduction and survival rates together determine the genetic fitness of an organism. As a result of being strongly related to fitness, life-history traits are generally under considerable selection. Evolutionary ecologists are interested in how the diversity of life-history traits in the living world can be explained in terms of the differences in selection pressures acting on different species.
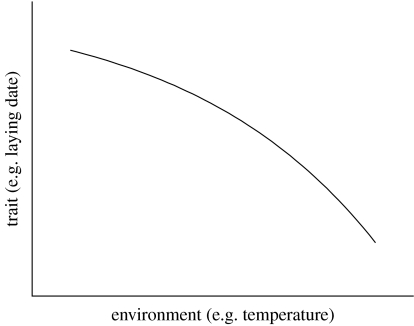
One of the other key characteristics of life-history traits is that they often show ‘phenotypic plasticity’—that is, a single genotype produces a different phenotype in different environments (Pigilucci 2001). A good example of this is that many temperate bird species breed earlier in warmer springs (e.g. Visser et al. 2003). The phenotypic plasticity of a single genotype can be described by a ‘reaction norm’—the relationship between the trait (in this case, a life-history trait) and the environment. In the case of great tit (Parus major) laying dates, the reaction norm is the relationship between laying date and temperature (figure 1).
Figure 1.
A reaction norm. Reaction norms describe the phenotypic plasticity of traits—how the value of the trait produced by a given genotype varies in relationship to one or more environmental variables. For example, temperate birds of many species breed earlier in warmer springs.
There are a number of important general points to be made about reaction norms: first, different genotypes produce reaction norms that may differ in slope, intercept or shape. If the reaction norm was perfectly flat (i.e. the slope was zero), the same phenotype would be produced across different environments. For example, individual bar-tailed godwits Limosa limosa baueri are remarkably consistent year-to-year in the timing of their migration (Battley 2006). Thus, reaction norms can describe the full range of phenotypic plasticity from fixed traits, like timing of migration in bar-tailed godwits, to traits which show strong phenotypic plasticity. Second, evolution of reaction norms proceeds by changes in gene frequency as a result of selection in the same way as for fixed (non-phenotypically plastic) traits. The only difference is that instead of a gene, or genes, for, say, laying date changing in frequency, it is a gene, or genes, that determine the shape of the reaction norm that does so. Third, although single environmental variables may have a strong effect on the expression of traits, it is likely that multiple environmental traits will be involved in phenotypic plasticity. For example, food availability might have a direct effect on the laying date in addition to that of temperature. This could be shown graphically in a three-dimensional figure, with temperature and food availability as the horizontal axes, and the reaction norm plotted as a surface showing the laying date (on the vertical axis) produced at each combination of temperature and food availability. The figures in this paper only show a single environmental variable affecting a trait for the sake of visual simplicity and because, from a theoretical point of view, the general principle is the same irrespective of the number of environmental traits involved. Finally, ‘environment’ is used here in a very wide sense to include aspects of the (extended) phenotype of the individual such as the level of its fat reserves and whether it currently has a territory. For example, laying date might be affected by temperature and the level of fat reserves, or by fat reserves alone.
One of the consequences of the strong links of life histories with population dynamics and fitness, and their tendency to be phenotypically plastic is that they are key traits in understanding how organisms will react to environmental change: changed environments will produce changed expression of life-history traits that are phenotypically plastic; changed environments impose changed selection pressures and will cause evolution of life-history traits (including of their reaction norms); and the changes in life-history traits will in turn affect the population dynamics and whether the species will persist or go extinct in the new environment. Understanding how species react to environmental change has become critical given current rates of anthropogenic change.
(b) Phenotypic plasticity and neuroendocrine control systems
Phenotypic plasticity implies, by definition, that the value of a particular trait varies with some aspect of the environment. This in turn implies that the organism must contain some system embodied in its physiology and gene expression that collects information from the environment, uses that information to ‘decide’ what value of the trait will be produced, and signals the decision within the body to the site where it will be implemented. Information may be collected not only by specially evolved sense organs but also by something as simple as the effect of temperature on the rate of a chemical reaction (de Wilde 1978). In multicellular animals, the integration of the information to make a decision and signalling of the decision to the relevant tissue are carried out by the neuroendocrine system. The neuroendocrine system can therefore be thought of as the control system that instructs the production system embodied by the rest of the organism. In this paper, I use the term neuroendocrine control system broadly to include transduction (the processing of environmental information), signalling (actual secretion of hormone or hormones) and reception of the signal (hormone receptors).
Factories provide an analogy for this division into a control system and a production system. The production system might take in raw materials and use them to produce various basic components that are used in varying proportions on different production lines to produce a range of finished products. It is, in principle, possible for all this to occur without the intervention of a control system: all the processes could be set to run at fixed rates determined so that raw materials and basic components are sent in the right proportions to different production lines, each of which has been equipped with the right machinery and manpower. In practice, raw materials will vary in availability, as will the demand for different end-products. As a result, factories also have control systems that integrate information about the supply of raw materials and the demand for end-products, and send instructions to different parts of the factory to divert raw materials and components between, and speed up or slow down—or start up or shut down—the various processes.
2. Endocrinological and ecological perspectives
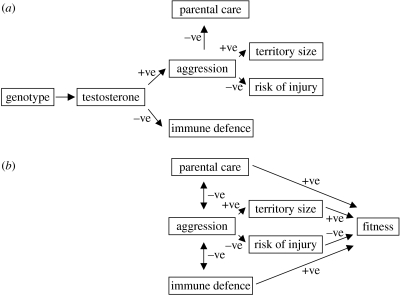
Endocrinologists and evolutionary ecologists are often interested in the same traits, but from different perspectives. Neuroendocrinologists are interested in the causal mechanisms by which phenotypes are generated (ontogeny) and then orchestrated within their life cycles. In thinking about a specific hormone—for example, testosterone (figure 2a)—neuroendocrinologists are interested in the mechanisms by which hormones affect physiology, morphology and behaviour. Some of these effects may be direct (as is implied in figure 2a for aggression and immune defence), and in some cases indirect (e.g. the effects on parental care, territory size and the risk of injury), but all of these effects are generally regarded as having been caused by testosterone (Wingfield et al. 2001 is an exception). By the same token, testosterone is also regarded as the cause of the resultant correlations between the phenotypic traits—for example, a negative correlation between aggression and immune defence.
Figure 2.
Relationships between traits from different perspectives. (a) A neuroendocrinological perspective: neuroendocrinologists are interested in the causal mechanisms by which the phenotypes are generated. Correlations between variables (e.g. the negative correlation between aggression and immune defence) are seen as the consequence of single hormones either directly or indirectly having multiple effects. (b) An ecological perspective: ecologists are interested in how selection acts on traits, either directly on the trait or indirectly through selection on traits that are causally linked. Correlations between traits are seen as the consequence of trade-offs (two-headed arrows in the figure) resulting from functional constraints. (For example, the trade-off between aggression and immune defence may be caused by the allocation of energy.) (−ve indicates a negative relationship, and +ve, a positive relationship). The purpose of this figure is to contrast neuroendocrinological and ecological views of the causal links between traits. For the sake of clarity, some known effects of testosterone, e.g. sexual behaviour, have been omitted.
In contrast, evolutionary ecologists are most interested in how life history and other traits have been shaped by selection. Evolutionary ecologists use both genetic and phenotypic approaches to model evolution. In this paper, I focus on the phenotypic approach (e.g. figure 2b). In this kind of approach, selection can act in two ways: either directly on a trait through its effects on fitness (e.g. increased immune defence will increase survival, and therefore fitness) or indirectly through selection on other traits to which the focal trait is causally linked (e.g. spending more energy on immune defence means that there is less energy to spend on aggression). Such ‘trade-offs’ are the result of functional constraints, which may be either physiological or ecological in origin. Physiological constraints include the principle of allocation: time, energy and raw materials can only be spent once. Ecological trade-offs often arise through exposure to predators, parasites and pathogens. For example, many animals are more exposed to predators when foraging. As a result, there is a trade-off between food intake and predation risk. Information about selection pressures and trade-offs can be used to predict the ‘optimal’ phenotype. This is the phenotype which maximizes fitness and is therefore the one that is expected to evolve under natural selection. As an example, laying date in birds is shaped by selection pressures acting through the current offspring and through the future reproductive prospects of the parents (Daan & Tinbergen 1997). Selection on current offspring production generally favours earlier breeding: depending on the species, this can be because food for the chicks is more available early in the nestling period, and because early fledging gives juveniles more time before the winter to learn to forage efficiently, moult and establish territories. However, the second selection pressure—through the parents' future reproduction—may act in the opposite direction: individuals starting breeding earlier may end up in poorer condition because food is less available to meet the energy demands of laying earlier in spring. This might reduce overwinter survival, and hence their expected number of offspring over the remainder of their lifespan. The result of such opposing selection pressures is that the number of surviving young a parent produces over its entire lifespan (lifetime reproductive success or fitness) will be maximized at an intermediate optimal laying date.
Neither of the above approaches is intrinsically better. Instead, they are useful for asking different kinds of questions about the same biological traits. The mechanistic approach is useful in understanding causal mechanisms, and the phenotypic evolutionary approach to understanding how the interactions between organisms and their environment give rise to selection pressures and trade-offs. The question asked in this paper is about how neuroendocrine mechanisms act as selection pressures on phenotypic plasticity. An answer to this question requires viewing endocrinological facts from an evolutionary perspective. This paper is therefore an invitation to endocrinologists to look at their knowledge of neuroendocrine systems from a novel viewpoint—that of an evolutionary ecologist. Of course, other questions are most readily answered from other viewpoints, and in any overall synthesis the two approaches are complementary.
(a) Reaction norms from endocrinological and ecological perspectives
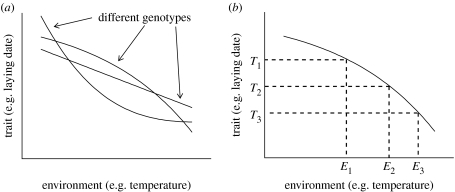
From a neuroendocrinological perspective, a reaction norm is determined by the causal mechanisms embodied by the neuroendocrine system. The genes underlying the neuroendocrine system will determine the way in which the different neurological and hormonal components are connected and interact with each other. This, in turn, will determine what the output—for example, the laying date—will be when there is a given input—for example, a given ambient temperature. If any gene underlying the neuroendocrine control of laying date is changed, the way in which the components of the system are connected or interact is changed, and the same ambient temperature may give rise to a different laying date—in other words, the reaction norm will be changed (figure 3a).
Figure 3.
Reaction norms from different perspectives. (a) A neuroendocrinological perspective: the neuroendocrinological system integrates information and signals to the rest of the organism. The genotype determines how the neuroendocrinological system is configured, and hence the resulting relationship between the trait value and the environment. Different genotypes therefore produce different reaction norms. (b) An ecological perspective: Selection pressures and functional constraints vary between environments, so that the optimal values of traits (T1, T2, T3, …) vary across environments (E1, E2, E3, …). The optimal reaction norm represents the optimal trait values across a range of environments and is the reaction norm that would give the organism highest fitness.
From an ecological point of view, reaction norms are the evolutionary result of selection pressures on a trait varying in different environments. Optimality models predict the optimal phenotype for the specific selection pressures and trade-offs that are included in the model, but selection pressures acting on life-history traits often vary with environmental conditions. For example, higher ambient temperature may increase the availability of food earlier in the season (for instance, by speeding up the development of insect prey). When this happens, the optimal trait value will vary with the relevant environmental variable; for example, optimal laying date will be earlier at higher ambient temperatures. By applying the optimality approach for each of a range of environmental conditions, we can, in principle, construct the optimal reaction norm (figure 3b).
We thus have two views of reaction norms: the first is a neuroendocrinological view which gives us the range of possible reaction norms that can be generated by the neuroendocrine system. The second is an ecological view which tells us how selection will act on those alternative reaction norms. How should we combine these two views? If the neuroendocrine system can costlessly produce the optimal reaction norm, this is the one that we would expect natural selection to pick out from among the array of possible reaction norms. In this case, information about the neuroendocrine system will not enlarge our understanding of how reaction norms have been shaped by selection. Conversely, if the neuroendocrine system cannot costlessly generate the optimal reaction norm, we will need to know at least some details about the neuroendocrine system in order to understand the evolution of phenotypic plasticity. This identifies the two kinds of things we need to know to answer the question posed by this paper: first, whether the neuroendocrine system creates constraints—that is, whether it can be configured to generate the optimal reaction norm; second, whether the neuroendocrine system generates costs—that is, whether it creates selection on the reaction norm. These two questions are considered in the following two sections. These sections give a number of examples, but these are meant as illustrations of the kinds of properties that will create costs and constraints, and not intended as a comprehensive review.
3. Does the neuroendocrine system create constraints?
One of the striking features of neuroendocrine systems is that single hormones may have effects on many different aspects of behaviour, physiology and morphology. For example, testosterone has an influence on a wide range of characters, from some primary and secondary sex characteristics, through effects on muscle and aggression, to immunosuppression and oncogenic effects (Wingfield et al. 2001). The multiplicity of action of single hormones suggests that the extent to which different traits can be controlled independently by the neuroendocrine system might be limited. This view suggests that the neuroendocrine control system cannot necessarily be configured to produce the optimal reaction norm and may therefore act as a constraint on phenotypic plasticity.
However, before accepting that multiple effects of single hormones are a constraint generated by the neuroendocrine system (including hormone receptors), we should look first at other explanations for such multiple effects. The first of these is that there are constraints, but that these come from outside the neuroendocrine control system: functional constraints giving rise to trade-offs would result in hormones that affect one trait inevitably affecting the other trait involved in the trade-off. For example, a hormone that acts as a signal to increase the amount of aggression (and the amount of energy that it consumes) must, other things being equal, take energy away from other activities such as the immune system. In this case, it is the functional constraint (energy allocation) that creates the trade-off, while the hormone mediates the same trade-off. In other words, the hormone acts as part of the ‘switch’ which determines how much of the energy is allocated to aggression. It could be argued that such cases could be detected by detailed investigation of the molecular mechanisms: only one of the traits (in this case aggression) would respond directly to the hormone, and immunosuppression would instead result from energy starvation. This argument, however, does not take into account the most efficient way for the immune system to reduce activity: better to shut it down in an orderly fashion that allows remaining resources to be used to maximum effect than to starve it into inactivity. We might therefore expect the immune system to evolve a response to signals that predict an imminent shortage of resources: in other words, the immune system would evolve to react directly to the testosterone signal (as indeed, it does; Nelson et al. 2002). In the factory analogy, we would expect that when the control system diverted components, energy or manpower from a production line, it would also signal a reduction in production on the resource-deprived line to a level that could be maintained with the reduced resources. A demonstration that a negative relationship between two traits is caused by signalling is therefore not sufficient to exclude the possibility of an underlying trade-off (Lessells & Colegrave 2001). Thus relying on whether causal effects are direct or not, as suggested by Wingfield et al. (2001), may not safely discriminate constraints imposed by the neuroendocrine system from other constraints.
A second explanation for multiple effects of a single hormone are that two traits have a more than additive effect on fitness, or put another way, that there is selection for the two traits to be coordinated. The simplest way of achieving this is for the both to respond to the same hormone. Hormone-controlled behaviour abounds with such examples (Adkins-Regan 2005): for instance, coordination will generally be selected between mating behaviour, including the suppression of aggression towards potential mates, and the availability of gametes to be fertilized. A specific example of this occurs in golden hamsters (Mesocricetus auratus), where sex steroids in oestrous females suppress aggression towards males (Floody 1983). Other examples of behaviours that may be coordinated by responding to common hormone signals are activities like aggression or reproduction and the availability of energy for associated metabolism, and signalling behaviour and the morphology to produce the signal (Hews & Quinn 2003). Coordination of different activities is therefore another explanation for multiple direct effects of single hormones that does not imply a constraint created by the hormonal control system.
Convincing examples of multiple effects of hormones that cannot be contributed to trade-offs in the production system or selection for coordination are scarce. If such multiple effects generate a relationship between traits with antagonistic effects on fitness, there will be a trade-off between the two traits. Unlike trade-offs that are caused by functional constraints, in this case, hormones create, rather than merely mediate, the trade-off. One possible example of a trade-off being created by the multiple effects of a hormone is the oestrogen-dependent anaemia shown during egg-laying in birds (Williams et al. 2004), which is reduced by blocking oestrogen receptors during egg production (Wagner et al. submitted). These results suggest that as well as stimulating vitellogenesis during egg-laying, oestrogen suppresses erythropoiesis (red blood cell formation), resulting in anaemia. However, these results do not rule out the possibility that the anaemia is adaptive in freeing up the products of haemoglobin breakdown which may function as antioxidants or in egg shell pigments.
Examples of hormones being constrained to have multiple effects are probably rare because independent control of these effects can be achieved in a number of ways. These include varying the number and responsiveness of hormone receptors in different tissues and restricting the active form of hormones to the desired target sites by the use of specific hormone-binding proteins in the blood, conversion of biologically inert circulating signals to active forms at the target site, or local synthesis of hormones at the target site (Wingfield et al. 2001; Hau 2007). Taken overall, there seem to be plenty of mechanisms by which different activities can be independently controlled by the neuroendocrine control system and a paucity of evidence that phenotypic plasticity is constrained by the ways in which the neuroendocrine control system can be configured. However, we have little idea of how common this really is, and a need therefore for endocrinologists to investigate how widespread such constraints are (Partridge et al. 2005).
Whereas the neuroendocrine control system does not appear to strongly constrain the endpoint of evolution of reaction norms, it may increase the time taken to reach that endpoint. This is because genes underlying the neuroendocrine system (including cellular responses) tend to have multiple effects and gene products to work in cascades, so that any change to the system tends to have negative as well as positive effects, and local adaptive peaks may be separated from higher peaks by intervening valleys. One specific case where the neuroendocrine system may slow the rate of evolution is when the traits that hormones produce have antagonistic effects on fitness in the two sexes. If these hormones are controlled by the same underlying genes, there will be a negative genetic correlation between fitness of the two sexes and selection on each sex holds back adaptation of the other. For example, testosterone implants in dark-eyed juncos Junco hyemalis suggest that selection favours an increase in testosterone levels over observed levels in males, but may select against an increase at the same time of year in females (Ketterson et al. 2005; Reed et al. 2006). The existence of sexually antagonistic traits will select for modifier genes for sex-limited expression (Fisher 1958), but the rate at which they evolve may be slow (Lande 1980, 1987). The question of whether neuroendocrine systems are an important source of evolutionary inertia is an important one owing to its implications for responses to environmental change and is dealt with in far greater detail by Adkins-Regan (2008).
4. Does the neuroendocrine system create costs?
The second way in which neuroendocrine control systems may influence the evolution of phenotypic plasticity is by creating costs. Using the factory analogy, given information about the effects on production of reallocating raw materials or components, power or manpower between production lines (trade-offs), an economist should be able to predict the optimal pattern of production (that which would maximize profitability) by the different production lines given the current costs of raw materials, power and manpower, and market value of the end-products of the factory. We would then expect the factory owner to install a control system that implemented these patterns of production, and which might, for example, consist of a computer system that calculated the optimal pattern of production at any time and an electronic system to signal instructions to the machinery and workforce. This control system, however, would invoke additional costs—for its purchase, maintenance and running. With these costs taken into account, profitability might no longer be maximized by the same pattern of production as when these costs were ignored—it might instead pay to have less precise regulation, or not regulate some processes at all. In other words, the optimal pattern of production would be changed if there were costs to the control system. In the same way, the optimal reaction norm may be changed if there are fitness costs created by the neuroendocrine control system (including hormone receptors). If this is the case, costs created by the neuroendocrine system are acting as a selection pressure on phenotypic plasticity.
Which costs should be included in the cost of the neuroendocrine control system? An endocrinologist thinking in terms of causal mechanisms would include the costs of any of the direct or indirect effects of a hormone as costs of the hormone. Thus the costs of testosterone would include reduced parental care, reduced immune defence and increased risk of injury (Wingfield et al. 2001; Adkins-Regan 2005). However, attributing all negative effects on fitness to testosterone does not take into account the conceptual division into a production system and a control system: some costs of testosterone may originate in the production system (through functional constraints) rather than in the control system. The optimal reaction norm calculated by an evolutionary ecologist takes into account how selection acts on the production system, including costs arising through trade-offs due to functional constraints, but does not include the costs of the control system. This provides a conceptual tool that enables costs of the neuroendocrine system to be identified: if the actual neuroendocrine system were replaced with an (imaginary) costless control system that made decisions and signalled instructions to the rest of the organism, which costs would still exist? Those costs that remain are costs of the production system, while those that disappear are costs of the neuroendocrine control system. In the case of testosterone, if the costless control system signalled for an increase in aggression, there would still be a cost of injury (because it is the aggression not the testosterone that increases this risk). There would also probably be a cost in terms of reduced parental care (because aggression will probably reduce the amount of parental care through time and energy allocation trade-offs). Thus neither of these is a cost of the control system. On the other hand, any costs of synthesizing testosterone and maintaining the response system would disappear with an imaginary costless control system. These, then, are a cost of the control system. Finally, there are some costs where we do not yet know whether they are costs of the control or production system. This applies to the effect of testosterone on immune defence, where the cause of immunosuppresson is as yet unclear: on the one hand, it could be the result of a trade-off based on the allocation of energy or raw materials and on the other, it could be due to interference of testosterone molecules with some component of the immune system producing an effect akin to toxicity. Only the latter cost would disappear with a costless control system and should therefore be counted as a cost of the control system.
Costs can be invoked in a variety of ways from energy consumption to risk of predation. As a result, costs are measured in a variety of units including the amount of raw materials or energy consumed, loss of reproductive success, mortality or interference with the functioning of molecular machinery. This raises the questions whether all of these costs should be included and what common currency should be used. The answer lies in the fact that we are ultimately interested in whether the neuroendocrine system acts as a selection pressure on phenotypic plasticity. Selection occurs on a trait when the fitness of individuals varies with the value of that trait. Thus we are interested in any costs that eventually impact on fitness (survival or reproduction), and fitness is the common currency by which the relative impact of different kinds of cost should be judged.
The following is a list of some ways in which the neuroendocrine control system may create fitness costs acting as selection pressures on reaction norms. It is not intended as a comprehensive review of evidence for or against the existence of particular costs. Instead it has a twofold purpose: first, to stimulate endocrinologists to think broadly in identifying possible costs of neuroendocrine control by providing a wide array of (possible) examples of ways in which fitness costs may be invoked; and second, to illustrate the distinction between costs that do—or do not—originate from the neuroendocrine control system. This latter distinction is not important to endocrinologists when thinking solely about causal mechanisms, but is important when thinking about how natural selection acts on phenotypic plasticity (§2).
(a) Costs of hormone synthesis
The potential cost of the neuroendocrine system that springs most readily to mind is the cost of hormone synthesis (Adkins-Regan 2005). The precursor of steroid hormones, cholesterol, is abundantly available, so the costs of their production are probably mainly in the cost of the enzymes involved in the synthesis of steroids (including the synthesis of cholesterol from acetyl-CoA). Neuropeptides probably have similar costs to other proteins of about the same size (although their pre–pro forms are often much larger). In general, hormones do not contain rare elements, although iodine limits the production of thyroid hormones. Energetic costs may be translated into fitness cost because energy must either be diverted from other activities or obtained from additional foraging (taking time and possibly exposing the animal to increased predation risk). Likewise, the need for rare elements may require animals to devote foraging time to specific food items that are rich in the relevant chemicals. However, because hormones are generally produced in tiny amounts, these fitness costs are likely to be close to negligible, but actual data are lacking.
(b) Other costs of running/maintenance of the neuroendocrine system
Although the costs of hormone synthesis may be small, this may not be the case for the maintenance of neuroendocrine tissues. Brain tissue has high energy consumption, so there may be energetic costs to the information processing part of the neuroendocrine control system (e.g. Jacobs 1996). The mass (bulk and weight) of neuroendocrine tissues may also invoke costs—increasing the energetic costs of locomotion, and reducing manoeuvrability and hence increasing predation risk—especially in flying organisms, such as birds. Lastly, the miniscule quantities in which most hormones are produced must require sophisticated detection and response systems, although the extent to which this sophistication incurs costs in terms of fitness is unknown.
(c) Basal metabolic rate/energy consumption
Although it is not clear how taxonomically widespread the effects are, steroid and other hormones (e.g. thyroid hormones) increase metabolic rate in some species: for example, testosterone increases metabolic rate in male house sparrows Passer domesticus (Buchanan et al. 2001), and testosterone and oestradiol increase oxygen consumption in a lizard (Chalcides ocellatus; Al Sadon et al. 1990). Here, however, we need to be careful whether the fitness costs that increased energy consumption invoke should be regarded as costs of the neuroendocrine system. Instead, increased basal metabolic rate may be selected because it increases the effectiveness of other behaviours (such as increased foraging or aggression) that the hormone switches on, or increased energy consumption may be a direct consequence of the behaviour. In these cases, the fitness costs of increased energy consumption would not be a selection pressure created by the neuroendocrine system.
(d) Toxicity
Toxicity is about the most direct fitness cost imaginable. Several hormones are known to have toxic effects: oestrogen administered systemically may be lethal and glucocorticoids are highly oxidized molecules that tend to be toxic and mutagenic (Adkins-Regan 2005). This may not be a problem at normal physiological levels that are transient, but may become problematic if secretion is maintained at a high rate for long periods (Wingfield et al. 2001).
(e) Immune suppression
The immunosuppressive effects of hormones, particularly of glucocorticoids, have been known for several decades (Munck et al. 1984), but have received more attention from evolutionary biologists since the suggestion that the immunosuppressive effects of testosterone might be the fitness cost responsible for maintaining the honesty of male sexual advertisement (Folstad & Karter 1992). There is continuing debate as to whether testosterone has a generally immunosuppressive effect and whether other hormones might have larger effects (Owen-Ashley et al. 2004; Roberts et al. 2004). However, the more important question here is whether any such costs can be regarded as having been created by the neuroendocrine control system. This would be the case if the hormone in question interfered with the functioning of the immune system in a manner akin to toxicity. On the other hand, immunosuppression might be the result of an energy allocation trade-off, and result from decreased availability of energy for immunocompetence because it has been diverted to activity metabolism (Wikelski & Ricklefs 2001). In this case, the cost of immunosuppression is generated by that activity and not by the neuroendocrine system itself. Distinguishing these two kinds of possibility will in practice be difficult: even a demonstration that the relevant hormone interacts directly with the immune system would not distinguish these possibilities. This is because, given the existence of an energy allocation trade-off, downregulating the immune system in response to the hormone might be an adaptive evolved response freeing up energy for the other hormone-dependent activities (Lessells & Colegrave 2001).
(f) Revealing information to predators and competitors
Hormones not only require precursors for their synthesis, but also will eventually be broken down into other compounds and excreted in the urine or other substances. Detection of these metabolites by conspecifics or heterospecifics may impose fitness costs to these hormones. One possible example occurs in female deer mice Peromyscus maniculatus, whose odour is dependent on excreted steroid metabolites, and who are more vulnerable to predation during oestrus than non-oestrus (Cushing 1985). At first sight, the example seems to suggest that the use of steroids to regulate reproductive physiology carries a cost in terms of increased predation risk. However, the same odour that attracts weasels also attracts males. An alternative interpretation in terms of selection is that the odour is selected for its mate-attraction function, and that increased predation is the result of an ecological trade-off. In the former case, the risk of predation is a cost of the neuroendocrine system. In the second case, it is not. (The same increase in predation might occur if a totally different compound was used for mate attraction.) Excreted metabolites might also be a way in which information is ‘leaked’ to other individuals. For example, such odours might give away that an individual was in poor condition or not physiologically ready to fight. Individuals will generally be selected to hide such information from competitors, so a lack of corresponding fitness gain (such as mate attraction) would imply that the cost originates from the neuroendocrine system.
(g) Oncogenesis
In mammals, oestrogen increases the risk of breast cancer, possibly by increasing the rate of cell division and therefore the chance of an oncogenic mutation occurring (Feigelson & Henderson 1996). Whether this should be regarded as a cost of the neuroendocrine system is unclear. The increased cancer risk might, as suggested, reflect a trade-off with the benefits of increased growth in breast tissue. Alternatively, the molecular processes involved in switching on and off gene expression might promote oncogenic mutations, so that the increased cancer risk would not only be a cost of modulating gene expression (not strictly a cost of neuroendocrine control) but also acting as a selection pressure on reaction norms. Finally, the specific hormone involved might in some way increase oncogenic mutations. Only this last possibility would represent a cost created by the neuroendocrine control system.
(h) Via trade-offs created by multiple effects of hormones
Many of the cases in which hormones have effects on traits with antagonistic effects on fitness are probably underlain by trade-offs due to functional constraints (Lessells & Colegrave 2001). In other words, the hormone mediates, but does not create, the trade-off. However, the neuroendocrine system might be constrained so that trade-offs are created by single hormones having multiple effects. One possible example is the effects of oestrogen on vitellogenesis and erythropoeisis in female birds during egg-laying (see §3). If this example is true, low haematocrit (and its consequences) would be a cost of egg-laying that arises from the neuroendocrine control system.
5. Discussion
The primary aim of this paper is to ask which properties of neuroendocrine control systems may influence the endpoint of evolution of phenotypic plasticity. The answer to this question is when the neuroendocrine system creates constraints or costs (selection pressures) on reaction norms. It is worth reminding ourselves that the control system involves three major components—transduction (the processing of environmental information), signalling (actual secretion of hormone or hormones) and the target cell receptor system. Each component, or a combination of them, could be targets for investigations on possible constraints or costs. The purpose of this paper is to stimulate those with detailed knowledge of the causal mechanisms involved in neuroendocrine control to think of these mechanisms in terms of constraints and costs, and thereby identify specific features of the neuroendocrine control system that act in this way. The examples of constraints and costs given in this paper are therefore not intended to be comprehensive, but to illustrate the kinds of information that may be relevant. The examples suggest that the costs or constraints created by the neuroendocrine control system are modest (although it must inevitably generate some costs, such as those of the maintenance of neuroendocrine tissue (including hormone receptors) and synthesis of hormones). However, this is not a definitive conclusion. Instead, I hope that it will be a stimulus to endocrinologists to think about which properties of neuroendocrine systems might act as costs and constraints on the evolution of phenotypic plasticity. Conversely, the central message of this paper is that, when thinking about this question, it is important to distinguish costs and constraints that arise from the neuroendocrine system from those that arise from the production system (through functional constraints). When this distinction is made, the costs and constraints that can be attributed to the neuroendocrine control system are only a subset of the list suggested by thinking of the effects of the neuroendocrine system in terms of mechanistic causation.
From an evolutionary perspective, it would not be surprising if there are rather few costs or constraints of the control system, because natural selection is expected to minimize these in the same way that factory owners are expected to choose control systems for their factory that can calculate and implement the optimal pattern of production without incurring large costs. Nevertheless, we can ask the question whether there are specific properties of the neuroendocrine system that are more likely to generate costs and constraints. One possible example is that it may be more difficult to reduce costs of information processing than signalling. This is because the information processing part of the system is selected to produce a specific output when it receives a specific input (in other words there is an optimal reaction norm determined by selection pressures and trade-offs), whereas the link between a hormonal signal and its meaning is to some extent arbitrary. Natural selection can therefore choose molecules to act as hormonal signals that do not have unnecessary costs, so hormones that are toxic, carcinogenic or mutagenic raise the question why these costs were not avoided by the use of a different molecule to carry the signal.
This paper uses a conceptual division between production and control systems to identify which negative effects on fitness should be regarded as costs of neuroendocrine control. The discussion above (§4) of possible examples of costs of neuroendocrine control emphasizes that it is not always easy in practice to say whether a known cost such as immunosuppression is a cost of neuroendocrine control. Is there a general method by which this can be done? The least that can be said is that there are two techniques which will not be helpful: the first is phenotypic engineering (Ketterson et al. 1996), in which experimental treatment with hormones is used to induce phenotypic change. This technique is useful in genetic evolutionary approaches because it measures nearly all the costs (those of hormone synthesis are not included) of a change in hormone levels whether these originate from functional constraints or are costs of neuroendocrine control. It thus tells us how selection would act on a novel genetic variant that brought about such a change in hormone levels. Clearly though, this makes these kinds of manipulations uninformative when we are interested in partitioning costs into those that are, or are not, due to neuroendocrine control. The second technique that is not helpful to the central question here is investigating the mode of action of the hormone to determine whether it acts directly on the trait in question—in other words whether the hormone is acting as a signal. This is because multiple signalling effects may not be the result of a constraint on the neuroendocrine system, but evolved responses that are selected by the existence of trade-offs or the fitness benefits of coordinating activities (§3). Thus the separation into direct and indirect effects of hormones (Wingfield et al. 2001) does not correspond with costs that are, or are not, created by the neuroendocrine control system as a whole.
Given the difficulty of discriminating costs that are created by the neuroendocrine system in practice, does this conceptual framework have any value? I believe it does, in focusing attention on the possibility that costs that originate, in terms of causal mechanism, from the neuroendocrine system (e.g. immune suppression in response to testosterone), may not have been created by costs of hormonal control but instead from functional constraints (in the example, from a time or energy allocation trade-off). In such circumstances, if the goal is elucidating the selection pressures that have led to traits and reaction norms, detailed investigation of the neuroendocrine system will not add to our understanding. Attempting to apply the conceptual framework will likewise identify situations where we do not understand how selection pressures are generated: is the immunosuppressive effect of testosterone really due to an energy allocation trade-off or is it a cost of the neuroendocrine system?
Lastly, the aim of this paper is to understand where knowledge of neuroendocrine control mechanisms is essential in answering an evolutionarily ecological question. Hopefully, it may also stimulate evolutionary thinking about properties of the neuroendocrine system—for example, why hormones with toxic, carcinogenic or mutagenic properties are ever found—so that any increased communication that it stimulates is beneficial to researchers from both disciplines.
Acknowledgments
I would like to thank Marcel Visser, Tony Williams and John Wingfield, and the other organizers and participants in the ESF/NSERC/NSF networks integrating endocrinological and ecological thinking in avian reproduction. In particular, I would like to thank Liz Adkins-Regan and Ellen Ketterson for forcing me to be clearer about what I mean and for earlier discussions on the subject of this paper. Barbara Helm, Marcel Visser, Tony Williams, John Wingfield and an anonymous referee made helpful comments on an earlier version of this paper.
Footnotes
One contribution of 12 to a Theme Issue ‘Integration of ecology and endocrinology in avian reproduction: a new synthesis’.
References
- Adkins-Regan E. Princeton University Press; Princeton, NJ: 2005. Hormones and animal social behavior. [Google Scholar]
- Adkins-Regan E. Do hormonal control systems produce evolutionary inertia? Phil. Trans. R. Soc. B. 2008;363:1599–1609. doi: 10.1098/rstb.2007.0005. doi:10.1098/rstb.2007.0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Sadon M.K, el Banna A.A, Ibrahim M.M, Abdo M.N, al Rasheid K.A. Effect of gonadal steroid hormones on the metabolic rate of the cold-acclimatized gonadectomized male and female Chalcides ocellatus (Forkal) Gen. Comp. Endocrinol. 1990;80:345–348. doi: 10.1016/0016-6480(90)90182-l. doi:10.1016/0016-6480(90)90182-L [DOI] [PubMed] [Google Scholar]
- Baker J.R. The evolution of breeding seasons. In: de Beer G.R, editor. Evolution: essays on aspects of functional biology. Clarendon Press; Oxford, UK: 1938. pp. 161–177. [Google Scholar]
- Battley P.F. Consistent annual schedules in a migratory shorebird. Biol. Lett. 2006;2:517–520. doi: 10.1098/rsbl.2006.0535. doi:10.1098/rsbl.2006.0535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K.L, Evans M.R, Goldsmith A.R, Bryant D.M, Rowe L.V. Testosterone influences basal metabolic rate in male house sparrows: a new cost of dominance signalling? Proc. R. Soc. B. 2001;268:1337–1344. doi: 10.1098/rspb.2001.1669. doi:10.1098/rspb.2001.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing B.S. Estrous mice and vulnerability to weasel predation. Ecology. 1985;66:1976–1978. doi:10.2307/2937393 [Google Scholar]
- Daan, S. Tinbergen, J.M. 1997 Adaptation of life histories. In Behavioural ecology (eds J.R. Krebs & N.B. Davies), pp. 311–333, 4th edn. Oxford, UK: Blackwell.
- de Wilde J. Seasonal states and endocrine levels in insects. In: Assenmacher I, Farner D.S, editors. Environmental endocrinology. Springer; Berlin, Germany: 1978. pp. 10–19. [Google Scholar]
- Feigelson H.S, Henderson B.E. Estrogens and breast cancer. Carcinogenesis. 1996;17:2279–2284. doi: 10.1093/carcin/17.11.2279. doi:10.1093/carcin/17.11.2279 [DOI] [PubMed] [Google Scholar]
- Fisher R.A. Dover Press; New York, NY: 1958. The genetical theory of natural selection. [Google Scholar]
- Folstad I, Karter A.J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–662. doi:10.1086/285346 [Google Scholar]
- Floody O.R. Hormones and aggression in female mammals. In: Svare B.B, editor. Hormones and aggressive behavior. Academic Press; San Diego, CA: 1983. pp. 39–89. [Google Scholar]
- Hau M. Regulation of male traits by testosterone: implications for the evolution of vertebrate life histories. BioEssays. 2007;29:133–144. doi: 10.1002/bies.20524. doi:10.1002/bies.20524 [DOI] [PubMed] [Google Scholar]
- Hews D.K, Quinn V.S. Endocrinology of species differences in sexually dichromatic signals: using the organization and activation model in a pheyolgenetic framework. In: Fox S.F, McCoy J.K, Baird T.A, editors. Lizard social behavior. John Hopkins University Press; Baltimore, MD: 2003. pp. 235–277. [Google Scholar]
- Jacobs L.F. Sexual selection and the brain. Trends Ecol. Evol. 1996;11:82–86. doi: 10.1016/0169-5347(96)81048-2. doi:10.1016/0169-5347(96)81048-2 [DOI] [PubMed] [Google Scholar]
- Ketterson E.D, Nolan V, Jr, Cauthorn M.J, Parker P.G, Ziegenfus C. Phenotypic engineering: using hormones to explore the mechanistic and functional bases of phenotypic variation in nature. Ibis. 1996;138:70–86. [Google Scholar]
- Ketterson E.D, Nolan V, Jr, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 2005;166(Suppl. 5):S585–S598. doi: 10.1086/444602. doi:10.1086/444602 [DOI] [PubMed] [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. doi:10.2307/2407393 [DOI] [PubMed] [Google Scholar]
- Lande R. Genetic correlations between the sexes in the evolution of sexual dimorphism and mating preferences. In: Bradbury J, Andersson M.B, editors. Sexual selection: testing the alternatives. Wiley; Chichester, UK: 1987. pp. 83–94. [Google Scholar]
- Lessells K, Colegrave N. Molecular signals or the loi de balancement? Trends Ecol. Evol. 2001;16:284–285. doi: 10.1016/s0169-5347(00)02032-2. doi:10.1016/S0169-5347(01)02162-0 [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre P.M, Holbrook N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Demas G.E, Klein S.L, Kriegsfeld L.J. Cambridge University Press; Cambridge, UK: 2002. Seasonal patterns of stress, immune function, & disease. [Google Scholar]
- Owen-Asley N.T, Hasselquist D, Wingfield J.C. Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am. Nat. 2004;64:490–505. doi: 10.1086/423714. doi:10.1086/423714 [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers D.J. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. doi:10.1016/j.cell.2005.01.026 [DOI] [PubMed] [Google Scholar]
- Pigilucci M. John Hopkins University Press; Baltimore, MD: 2001. Phenotypic plasticity. [Google Scholar]
- Reed W.L, Clark M.E, Parker P.C, Raouf S.A, Arguedas N, Monk D.S, Snajdr E, Nolan V, Jr, Ketterson E.D. Physiological effects on demography. Am. Nat. 2006;167:667–683. doi: 10.1086/503054. doi:10.1086/503054 [DOI] [PubMed] [Google Scholar]
- Roberts M.L, Buchanan K.L, Evans M.R. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. doi:10.1016/j.anbehav.2004.05.001 [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Z. Tierpsychol. 1963;20:410–433. [Google Scholar]
- Visser M.E, et al. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. B. 2003;270:367–372. doi: 10.1098/rspb.2002.2244. doi:10.1098/rspb.2002.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, E. C., Prevolsek, J. S., Wynne-Edwards, K. E. & Williams, T. D. Submitted. Anemia associated with egg production is an estrogen-dependent and repeatable trait. [DOI] [PubMed]
- Wikelski M, Ricklefs R.E. The physiology of life histories. Trends Ecol. Evol. 2001;16:479–481. doi:10.1016/S0169-5347(01)02279-0 [Google Scholar]
- Williams T.D, Challenger W.O, Christians J.K, Evanson M, Love O, Vezina F. What causes the decrease in haematocrit during egg production? Funct. Ecol. 2004;18:330–336. doi:10.1111/j.0269-8463.2004.00829.x [Google Scholar]
- Wingfield J.C, Lynn S.E, Soma K.K. Avoiding the “costs” of testosterone: ecological bases of hormone–behavior interactions. Brain Behav. Ecol. 2001;57:239–251. doi: 10.1159/000047243. doi:10.1159/000047243 [DOI] [PubMed] [Google Scholar]
- Zera A.J, Harshman L.G, Williams T.D. Evolutionary endocrinology: the developing synthesis between endocrinology and evolutionary genetics. Ann. Rev. Ecol. Evol. Syst. 2007;38:793–817. [Google Scholar]