Abstract
Investigations of the cellular and molecular mechanisms of physiology and behaviour have generally avoided attempts to explain individual differences. The goal has rather been to discover general processes. However, understanding the causes of individual variation in many phenomena of interest to avian eco-physiologists will require a consideration of such mechanisms. For example, in birds, changes in plasma concentrations of steroid hormones are important in the activation of social behaviours related to reproduction and aggression. Attempts to explain individual variation in these behaviours as a function of variation in plasma hormone concentrations have generally failed. Cellular variables related to the effectiveness of steroid hormone have been useful in some cases. Steroid hormone target sensitivity can be affected by variables such as metabolizing enzyme activity, hormone receptor expression as well as receptor cofactor expression. At present, no general theory has emerged that might provide a clear guidance when trying to explain individual variability in birds or in any other group of vertebrates. One strategy is to learn from studies of large units of intraspecific variation such as population or sex differences to provide ideas about variables that might be important in explaining individual variation. This approach along with the use of newly developed molecular genetic tools represents a promising avenue for avian eco-physiologists to pursue.
Keywords: steroid hormones, sex differences, oestrogen receptor
1. Introduction: fundamental issues related to the hormonal regulation of behaviour and other phenotypic traits. How do hormones exert their effects on behaviour and physiology?
Hormones are one type of chemical messenger that exert marked effects on physiology and behaviour. They are released into the blood and can act simultaneously on many different target tissues including the brain. As such, it is reasonable to hypothesize that variation in hormone concentrations in the blood might explain individual variation in behaviour and physiology. However, attempts at relating individual variation in hormone concentrations with variation in behaviour have generally failed (Crews 1998; Adkins-Regan 2005). In this review, we argue that knowledge about cellular and molecular aspects of target tissues is essential for one to develop a comprehensive understanding of individual variation in hormone-regulated traits. Although measurements of variation in plasma hormone concentrations will continue to be useful for our understanding of hormone–behaviour interrelationships, investigations focused on cellular/molecular variables that influence hormone efficacy at target tissues will provide an important new dimension in our thinking about this problem. We will rely primarily on examples concerning the effects of sex steroid hormones on reproductive behaviours in birds owing to the wealth of data available on species in this taxon ranging from field studies to cellular/molecular mechanistic investigations (Ball & Balthazart 2002; Wingfield & Silverin 2002). Studies in other taxa notably reptiles such as the leopard gecko (Eublepharis macularius), where the system of temperature-dependent sex determination can lead to pronounced individual variation in sexual behaviour, have also been promising candidate species for the study of individual differences in hormone–behaviour relationships (Crews 1998). A detailed discussion of other vertebrate groups is beyond the scope of this paper.
Early investigators trying to link hormones with behavioural effects purposely selected behaviours that exhibited an extreme pattern of hormone dependence in order to establish the fact that hormones can actually exert marked behavioural effects (Feder 1984). Studies tended to focus on group effects comparing, for example, subjects in which a critical endocrine gland had been removed with subjects that had the gland removed but were additionally administered a replacement hormone treatment (Berthold 1849). There was a realization, even at early stages in this field, that individual differences in behaviour might not be explained by variation in the hormone concentrations in the blood. For example, Grunt & Young (1952) classified male guinea-pigs into groups of high, medium and low sexual activity based on behavioural tests with females. When castrated, all the males exhibited substantial reductions in their rate of sexual activity. When tested with similar replacement doses of testosterone though, they segregated into the same three separate groups of subjects displaying again high, medium and low frequencies of sexual behaviour. This was one of the first systematic studies suggesting that properties of the target tissue are more important in explaining individual variation than plasma concentrations per se. These studies, which even pre-dated the ability to measure steroid hormones reliably in the blood, pointed towards the target of steroid hormone action as being important in understanding individual differences.
The targets of hormone action that control modifications in behaviour can be logically divided into at least three modules. Hormones can modulate sensory inputs, they can act directly on the central nervous system or they can regulate effector systems. There is evidence for all three types of effects (Pfaff et al. 2004; Adkins-Regan 2005). It is important to note that hormones do not cause behaviours directly nor do they elicit behaviours in a simple stimulus–response relationship. Rather they change the probability and intensity of a behavioural response to a particular stimulus situation (Pfaff et al. 2004). The effects of steroid hormones on peripheral auditory tuning (Sisneros et al. 2004) or on olfactory sensitivity (Pfaff & Pfaffmann 1969) as well as on peripheral effector systems have been identified in specific cases and play a key role in the control of reproductive behaviours. They are, however, not the rule, and most attention is now focused on the brain. Hormones can induce prominent shifts in neural processing, which result in an individual exhibiting qualitative differences in their response to a stimulus such as a sexually attractive mating partner. Endogenous changes in response to a constant stimulus are often referred to as changes in motivation, and this is indeed one of the most dramatic ways steroid hormones can modify behaviour (Pfaff 1999). With the discovery of steroid hormone receptors, one could map the location of candidate brain regions where hormones might act (Morrell & Pfaff 1978). These data were then combined with methods such as lesions and the stereotaxic implantation of crystalline steroids in localized areas of the brain to identify brain sites involved in specific behaviours (Barfield 1971; Balthazart & Surlemont 1990). The combined data provided a focus for investigations of target properties in specific brain regions involved in particular behaviours. These findings thus form a foundation for studies of the cellular basis of individual differences in the behavioural response to steroid hormones. In this review, we first consider attempts to relate plasma concentrations of steroids to variation in behaviour and then elucidate how the problem of individual variation in the response to hormonal stimulation could be attacked by investigating cellular/molecular properties of target tissues in the brain. This research programme is still in its infancy. One way to guide it in a profitable direction is to learn from the results of more advanced studies that have analysed other forms of intraspecific variation, e.g. sex differences in the effects of hormones on behaviour. Insight of this sort needs to be dealt with cautiously though, as sex and gender differences represent an example of a group difference that may not always be based on the same mechanisms as individual differences that can exhibit a wide range in variation. However, when appropriate, comparisons will be made between studies investigating the mechanisms of individual and sex differences in the hormonal control of behaviour.
2. Plasma concentrations of sex steroids and the control of individual differences in behaviour
Initial studies in birds correlated changes in the timing of reproductive behaviour with plasma sex steroid concentrations. As expected, clear positive correlations between these two variables were detected based on sampling across the annual breeding cycle of temperate zone species. Copulatory behaviour frequency in male ducks (Anas platyrhynchos), for example, is well correlated with changes in plasma testosterone concentrations, with both variables peaking in April and then rapidly decreasing when the female begins to incubate the eggs (Balthazart & Hendrick 1976).
Unexpectedly, attempts to correlate, at a specific time point, individual plasma testosterone concentrations with measures of male sexual behaviour produced extremely disappointing results. For example, there was no relationship between individual variations in sexual behaviours (grabbing female neck feathers, ‘pumping’, mounting and actual copulations) and plasma testosterone in a large group of 30 male domestic ducks (Balthazart et al. 1977), despite the fact that all these behaviours had been shown to be testosterone-dependent by the castration–replacement approach (Balthazart & Stevens 1975; Balthazart & Hendrick 1979; Deviche 1979).
Similarly, in a study of agonistic behaviour displayed by male Japanese quail (Coturnix japonica) during brief dyadic encounters of a round-robin tournament, it was found that the aggressive or submissive behaviour was not related to plasma testosterone concentrations, except in the early phases of the tournament before the agonistic associations were established (Ramenofsky 1984). Once the social relationships had stabilized, concentrations of testosterone in winners declined to levels seen in losers and the qualitatively different behaviours displayed by winners and losers were no longer related to their circulating testosterone concentrations (Ramenofsky 1984). This study therefore offered a partial explanation for the lack of correlation between individual differences and plasma hormone concentrations, as correlations were only found during periods of social instability. Other studies have failed to find such correlations. For example, the amount of sexual behaviour and social displays exhibited by castrated ducks injected with a standard dose of testosterone propionate is significantly correlated with precastration activity (Deviche 1979). Similar results were observed in zebra finches (Taeniopygia guttata; Arnold 1975) and ring doves (Streptopelia risoria). In these species, quantitative individual differences in courtship behaviour are maintained after castration and standardized androgen replacement therapy (Hutchison 1970, 1971; for examples concerning quail behaviour, see also Balthazart et al. (1979) and Tsutsui & Ishii (1981); for additional examples in both mammals and birds, see also Balthazart (1983) and Wingfield & Ramenofsky (1985)).
Published studies indicate that this phenomenon appears to be quite widespread. Correlations are observed only during the period of fast changes in behaviour. These correlations reflect the asynchrony of behavioural changes between individuals rather than true correlations between individual differences in hormones and behaviour.
This lack of correlation was similarly detected in genetic selection studies. Systematic bidirectional selection for high and low mating behaviour has resulted in lines of chicken and quail that consistently display different frequencies of mating behaviour during standardized tests. In both species, treatment of castrated males with similar doses of exogenous testosterone induced sexual activity that remained significantly more frequent in the high than in the low mating line (McCollom et al. 1971; Cunningham et al. 1977).
Although it was later established that plasma testosterone concentrations are higher in the high than in the low mating line of chicken (Benoff et al. 1978), these data suggest that individual differences in sexual activity are not caused by limiting circulating testosterone concentrations, since treating all subjects with a same dose re-established these differences after castration. A limiting factor other than the plasma steroid concentration presumably has to be invoked.
3. Different models of the relationship between plasma hormone levels and behaviour
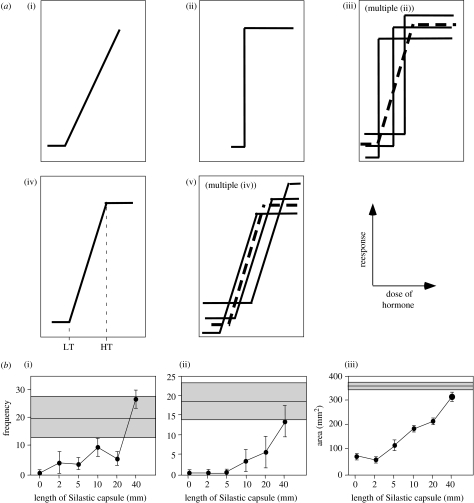
Based on a theoretical review of hormone influence on sex-specific traits (Hews & Moore 1997), it has been argued that the lack of any correlation between individual variation in plasma steroid concentrations and behaviour could be a direct consequence of the nature of the dose–response relationship between hormone concentration and behaviour (Adkins-Regan 2005). The concept is that for many hormone-dependent, non-behavioural characters, there is a graded dose–response relationship between the concentration of steroid available and the morphological or behavioural response (figure 1a, model (i)). This would only happen occasionally for behaviour but instead, in most cases, a step function would be seen: behaviour would be absent below a certain threshold of hormone concentration and present above that threshold; once the threshold is reached, behaviour would occur at similar levels regardless of the dose (figure 1a, model (ii)). At the population level, the accumulation of individual variations in threshold would provide the impression of a graded response, but this gradation would in fact not exist in any subject (figure 1a, model (iii)).
Figure 1.
Relationships between hormone concentrations and activation of hormone-dependent traits. (a) Theoretical models of the relationships between hormone levels and biological responses. At the individual level, the relationship can be represented by various functions such as (i) dose-dependent relationship, (ii) threshold response or (iv) dose-dependent relationship with a LT and a HT corresponding to the maximal level of activation. Both models (ii) and (iv) should result at the population level in similar dose–response relationships (respectively (iii) and (v)). Models (i)–(iii) are adapted from Adkins-Regan (2005) and (iv) and (v) are new interpretations proposed here. We do not consider here the case of inverted U-shape curves also considered by Adkins-Regan. (b) Experimental data illustrating the effects of increasing doses of exogenous testosterone (expressed by the length of the Silastic capsules implanted in castrated males) on the activation of two types of reproductive behaviour, (i) mount attempts and (ii) crowing, and on the growth of (iii) the cloacal gland, an androgen-dependent structure. Both behavioural and morphological responses demonstrate a dose–response relationship with the amounts of testosterone supplied to the subjects. The grey areas indicate the mean±s.e. of corresponding data observed during the same experiment in sexually mature gonadally intact males. Adapted from data in Balthazart et al. (1990).
We would like to suggest an alternative explanation to this phenomenon. We see no a priori reason why the activation of behaviour by steroids would be a quantal phenomenon while morphological and physiological effects of the hormone would be more quantitative in nature. We argue that sex steroids can activate behaviour in a graded manner but that this dose–response relationship is only observed within certain concentration limits (figure 1a, model (iv)). There is a low threshold (LT; figure 1a(iv)) below which no behaviour is observed and a high threshold (HT) above which no further increase in behavioural performance can be induced by increasing the dose of the hormone. Between these two points, it is quite plausible that a dose–response relationship would occur. The slope of this line or curve (its exact nature is unclear at present) may be very steep but is not necessarily vertical. Experiments in which animals are treated with increasing doses of steroids will, according to this model, produce a graded response only if the different doses fall in the range between LT and HT and behaviour is measured on an appropriate ordinal scale rather than based on a nominal scheme.
In support of this contention, we observed, during several dose–response studies in quail performed over the years, that both behavioural (attempts to mount the female or crowing occurrence frequency) and morphological variables (e.g. size of the androgen-dependent cloacal gland) were stimulated in a graded fashion by increasing the doses of testosterone (e.g. Balthazart et al. 1990; figure 1b). It is important to note that these dose–response studies were carried out with different groups of subjects being treated with the different doses of the hormone. The observed dose–response relationship described at the population level could thus reflect the superposition of multiple individual dose–responses as well as the addition of multiple threshold functions (figure 1a, models (iii) and (v)). The critical test needed to discriminate between these models would consist in performing a dose–response experiment in a single group of subjects, with each subject being its own control for different doses of the hormone. But even in this case, as correctly pointed out by Hews & Moore, changes in time of the critical threshold could obscure the results (Hews & Moore 1997).
Other approaches, based largely on field observations, have been taken to this problem of examining interrelationships among hormones, behaviour and morphological traits (e.g. Wingfield et al. 1990). When considering birds in the natural environment, many environmental factors (e.g. weather, population density, predator prevalence) can interact with an individual's circulating hormone concentration to affect the expression of behaviour. Therefore from this perspective, correlations between variation in hormone concentration and behaviour are not to be expected. The pattern of hormone secretion in a range of environmental situations may be the key hormonal variable to measure when considering individual differences rather than hormone–behaviour correlations per se. For example, the relationships between testosterone and aggression, as discussed, based on the ideas associated with the ‘challenge hypothesis’ suggest that increases in testosterone will not always be observed in all individuals encountering aggressive situations but rather will be limited by the particular mating system and seasonal state of the individual (Wingfield et al. 1990; Goymann et al. 2007). But even with this view, the efficacy of the hormonal response will be influenced by target tissue properties. In this review, we focus on cellular/molecular aspects of target tissue properties in relation to hormone–behaviour relationships.
4. Plasma testosterone concentrations are usually higher than the minimal requirements for behavioural activation
Dose–response experiments coupled with measurement of circulating concentration of testosterone established by Silastic implants of various sizes greatly helped to clarify the relationships between spontaneously occurring testosterone concentration and behavioural activation. For example, when castrated rats are treated with Silastic implants filled with testosterone, which have sizes ranging from 2 to 60 mm, mating behaviour is restored to precastration levels in most subjects with implants of 2–5 mm in length, which establish circulating concentration of testosterone that are only 10–20% of the values observed in gonadally intact sexually active males (0.2–0.3 versus 2–3 ng ml−1; Damassa et al. 1977). In agreement with the theoretical model proposed in §3, there was a graded reaction to increasing testosterone treatments in the low range of the doses that were used (implants of 2 or 5 mm in length), but, at higher doses, no such relationship could be observed. This experiment clearly demonstrated that plasma concentrations in intact males are substantially above the critical threshold for activating behaviour and possibly occur owing to the physiological requirement for high testosterone levels to sustain spermatogenesis (Turek et al. 1976; Desjardins & Turek 1977). In a normal population of untreated subjects, plasma testosterone concentration is clearly not a limiting factor for behavioural activation, and therefore there is no reason to expect a correlation between individual differences in behaviour and testosterone concentrations.
5. Individual differences in properties of endocrine target tissues regulating behaviour
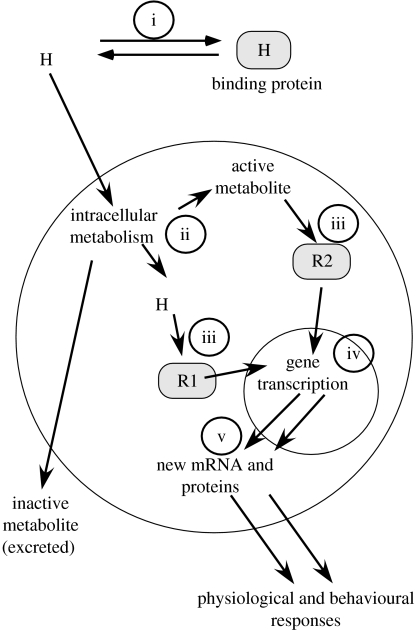
Having realized that plasma concentrations of steroids could not explain individual differences in behaviours, behavioural endocrinologists began exploring alternative possibilities. It is widely assumed that most (but not all; see Balthazart & Ball 2006) behavioural effects of sex steroids are mediated by the interaction of the steroid with its intracellular cognate receptors, which, when occupied, dimerize, associate with the DNA and enhance the transcription of specific steroid-sensitive genes (McEwen & Alves 1999; McEwen 2001). Any of these steps in hormonal action could therefore be specifically regulated in individual subjects and create a differential response to a same concentration of circulating hormone (figure 2).
Figure 2.
Schematic of the sequential steps in sex steroid action on cellular responses resulting in the activation of reproductive behaviour. (i) Circulating hormones (H) such as steroids in the plasma are associated in a reversible manner with transport proteins. (ii) When they enter their target cells, sex steroids are potentially metabolized into behaviourally active or inactive metabolites. (iii) Active metabolites will then bind to specific receptors, which will then undergo a number of transformations leading to their association with (iv) specific sites in the DNA. The occupied receptors will at this level serve as transcription factors resulting in a change in transcription of specific genes and their translation into (v) proteins that will ultimately affect behaviour expression. Each of these steps is potentially regulated by a variety of environmental or endocrine factors and could contribute to the modulation of individual differences in behaviour.
These multiple possibilities have been investigated to some degree and positive results have been obtained in a number of cases. Some prototypical examples are provided in this section and §6.
Steroids also exert some of their effects on brain and behaviour by non-genomic mechanisms that involve the interaction with the neuronal membrane and/or with intracellular signalling cascades. These effects that are not mediated by the binding to intracellular receptors and the resulting changes in gene transcription have been shown to modify the electrical activity of neurons, their metabolism and, probably as a result, the overt behaviour of the subjects. They have been best documented for oestrogens, in particular oestrogens derived from testosterone aromatization in the brain (see Balthazart & Ball (2006) and Cornil et al. (2006) for recent reviews on his topic). Non-genomic effects have unfortunately never been studied in relationship with individual differences in behaviour, and such effects will therefore not be discussed further here except to observe that the experimental analysis of these effects probably represents a valuable topic for future research.
(a) Steroid-binding proteins in the plasma
Because they are highly lipophilic, steroids do not easily dissolve in the blood. To achieve sufficient concentrations, they must therefore be associated with a carrier protein to which they bind in a reversible manner. Only free steroids can enter their target cells and exert physiological effects. As a consequence, (individual) variation in carrier proteins could obscure the relationship between behaviour and total steroid concentration in the plasma. Most assays are indeed performed after the extraction with organic solvent of the steroids from the plasma, which releases the steroid from its carrier. These assays therefore measure the sum of free and bound steroids.
The limiting role of carrier proteins in the expression of physiological effects of steroids has been well documented for corticosterone in birds. In the plasma, corticosterone, which circulates in much higher concentration than sex steroids, is reversibly associated with corticosterone-binding globulin (CBG) and, in multiple experimental situations, it has been demonstrated that CBG limits corticosterone action (Hammond 1995, 2002; Deviche et al. 2001; Breuner & Orchinik 2002; Breuner et al. 2006).
CBG does not bind sex steroids with high affinity (Deviche et al. 2001), and the low affinity of this binding suggests that it probably does not play a major physiological role. Another protein, the sex hormone-binding globulin (SHBG), has been identified in the blood of some mammals, although its presence in the plasma of any avian species has still not been confirmed to this date. The protein is expressed in the brain of representative species of all classes of vertebrates (Wang et al. 1990; Herbert et al. 2003, 2005) and the transcript for SHBG has been detected in the brain of zebra fish and sea bass using in situ hybridization and PCR (Solange Miguel-Queralt and Geoffrey L. Hammond, University of British Columbia 2005–2006, unpublished data). Based on indirect evidence, it seems that SHBG, if present in birds, would not play a major role in limiting testosterone entry and action in the brain. It has been demonstrated in quail lines selected for high and low levels of mating behaviour that following identical treatment with radioactive testosterone, similar amounts of accumulated radioactivity (the sum of testosterone and its metabolites, see §5b) are seen in the brain of the two lines. This suggests that the low copulatory activity in the low mating line is not due to testosterone being sequestered by a carrier protein in the blood (Cohen-Parsons et al. 1983). The expression of SHBG within brain cells (either neurons or glia) could be a way to limit the availability of steroid in a specific brain region. Therefore, even if one has evidence for accumulation of radioactive testosterone in a brain region, the steroid might not be readily available to the steroid receptor.
(b) Intracellular metabolism
After entering their target cells, but before binding to their intracellular receptors, sex steroids are exposed to a variety of metabolizing enzymes that can transform them into other behaviourally active steroids or inactive/less active metabolites. This intracellular metabolism has been described for all sex steroids including androgens, oestrogens and progestagens (see Balthazart & Schumacher (1985) and Balthazart (1989) for reviews of this topic focusing mostly on birds) but has been best studied and appears physiologically most relevant in the case of the androgen testosterone. In birds, as in mammals, testosterone can be transformed into an oestrogen, such as 17β-oestradiol (E2) by aromatase, or into 5α-dihydrotestosterone (DHT), a process catalysed by 5α-reductase. A substantial fraction of the behavioural effects of testosterone are actually mediated by the action at the cellular level of these two metabolites (E2 and DHT). In birds, testosterone can also be 5β-reduced, which produces a suite of metabolites that have little or no behavioural activity (see Balthazart (1983, 1989) for a more comprehensive review).
It is therefore immediately obvious that the observed behavioural effects of a given amount of testosterone could be regulated by the activity of the enzymes that transform this steroid into active versus inactive metabolites. The idea that this mechanism could contribute to explaining individual differences in behaviour was originally tested in a study of rats by Dessi-Fulgheri et al., who demonstrated that individual differences in fighting behaviour of rats are not correlated with their plasma testosterone or oestradiol concentrations but are directly related to the rate of transformation of testosterone into E2 in the brain (Dessi-Fulgheri et al. 1976).
Intrigued by this observation, we performed during the 1980s a number of experiments assessing the relationship of the brain metabolism of testosterone with individual differences in sexual and aggressive behaviours of male quail, but also with the well-established sex difference in behavioural responses to testosterone in that same species (castrated males produce an active copulatory behaviour when treated with exogenous testosterone but ovariectomized females never respond to such a treatment (Adkins 1975; Balthazart et al. 1983).
In a first study, we analysed the frequency of sexual behaviour displayed by 20 male quail during standardized encounters with sexually mature females. We also quantified aggressive behaviour directed towards other males, plasma concentration of testosterone and the activity of two testosterone reductases (5α and 5β) in the preoptic area–hypothalamus (HPOA). No relationship could be detected between any of the behavioural measures and plasma testosterone, but aggressive interactions were more frequent in birds exhibiting low 5β-reductase activity in their HPOA and high production of androstenedione (catalysed by the 17β-hydroxysteroid dehydrogenase; Balthazart et al. 1979).
During two additional experiments under slightly different conditions, we again assessed the same dependent variables in two large groups (n=21 and 16) of sexually mature male quail. Once again, no significant correlation between plasma testosterone and any aspect of behaviour was detected, but several significant correlations between behaviour and 5β-reductase activity were observed in both experiments (Delville et al. 1984). The direction of these correlations was, however, opposite in the two experiments, making an interpretation of these data difficult based on available evidence: if 5β-reductase inactivates testosterone and testosterone activates behaviour, then a negative correlation should be observed between individual variation of these two variables, as shown in two of the three experiments reported in these papers (Balthazart et al. 1979; Delville et al. 1984). Taken together, these data suggest the existence of relationships between the expression of aggressive behaviour and 5β-reductase activity in the HPOA, but the reason(s) explaining the reversal of this relationship in one of the three experiments remains unclear (see Balthazart & Schumacher (1985) for a more detailed presentation and discussion).
In many avian (and mammalian species), the aromatization of testosterone into an oestrogen plays a key, limiting role in the activation of male sexual behaviour (see Balthazart (1989) and Balthazart et al. (2004) for reviews highlighting avian studies). We found in Japanese quail that aromatase activity in the HPOA is significantly higher in males than in females and the enzymatic activity, which had decreased to basal levels in both sexes after gonadectomy, was differentially induced (higher activity in males than in females) by a similar treatment with testosterone in both sexes (Schumacher & Balthazart 1986). This suggested that the sex difference affecting the activation by testosterone of male-typical copulatory behaviours (probably the most prominent form of individual difference within a same species) could be the result of a sexually differentiated aromatase activity, with females producing less of the behaviourally active metabolite in critical brain areas. This sex difference in enzyme activity, though it may contribute to explaining the differential responsiveness of males and females to testosterone, is, however, not sufficient to explain the difference by itself. If ovariectomized females are treated with exogenous oestradiol, a procedure that should bypass the enzymatic sex difference, they still fail to display male-typical copulatory behaviour (Schumacher & Balthazart 1983).
Similarly, differences in testosterone aromatization in the brain do not seem to explain the differences in copulatory behaviour between the high and low mating lines of quail and chickens. Injection of identical amounts of tritiated testosterone resulted in a similar, nearly identical accumulation of tritiated oestrogens in the brain of males from these two mating lines, suggesting that the behavioural difference between lines is not caused by a lower aromatization rate in the low mating line (Cohen-Parsons et al. 1983; Van Krey et al. 1983).
Likewise, brain aromatase activity is not decreased in non-copulating rats when compared with active subjects (Portillo et al. 2007). Other factors, downstream in the action of testosterone on its target neurons, should therefore be invoked to explain individual differences in behaviour.
(c) Sex steroid receptor density
The next logical step in sex steroid action involves the binding of the hormone to its cognate receptor. The density of these receptors could also obviously be a limiting factor for the activation of behaviour since they are expressed in very low densities (less than a hundred femtomoles per milligram protein; see Blaustein & Olster 1989). This possibility is difficult to assess in the context of individual differences since no study has, to our knowledge, attempted to correlate individual levels of sexual or aggressive behaviour with the brain concentrations of the sex steroid receptors supposed to mediate the activation of these behaviours. A search in the ISI Web of Knowledge as of May 2007 retrieves 17429 entries for ‘steroid receptors’ but only 31 of these refer to ‘individual differences’ and none are concerned with behaviour (they mostly relate to biomedical studies of individual differences in disease susceptibility).
In addition, it seems fairly well established that sex differences in behaviour are explained, at least in part, by the differential expression of sex steroid receptors in key areas of the brain. The best-documented case probably concerns sex differences in singing behaviour of oscine species living in the temperate zone. The production of song in most of these species is more frequent or even sometimes exclusively observed in males (Arnold 1992). This behavioural difference corresponds with a sex difference in the size of brain regions (song control nuclei) mediating vocal production, in particular the nuclei HVC (used as a proper name; see Reiner et al. 2004) and the nucleus robustus arcopallialis (RA; MacDougall-Shackleton & Ball 1999). Both HVC and RA express high densities of androgen (AR) and/or oestrogen receptors (ER) of the alpha subtype (ERα; Ball et al. 2002). Testosterone and its brain metabolite E2 are clearly implicated in the control of singing in oscines (Harding et al. 1983, 1988; Walters & Harding 1988), but the specific site of their action and the role played by these steroids in HVC or RA remains somewhat unclear at present (e.g. see Ball et al. (2003, 2004) for discussion). Given the large sex difference in volume of the song nuclei in many species, the number of steroid receptors should be significantly greater in males than in females. In zebra finches where only males sing, even taking into account the sex difference in nucleus volume, there are still a lower proportion of cells expressing ER in the female HVC and another nucleus, lateral part of the magnocellular nucleus of the anterior nidopallium (lMAN), per unit tissue than in the corresponding male nucleus (Arnold & Saltiel 1979). In canaries, both sexes sing, though males sing a more complex song than females. In this species, there is not a sex difference in ER per unit tissue because there is a sex difference in HVC volume with greater numbers of cells expressing ER in males compared with females (Brenowitz & Arnold 1992). This study and other comparative studies have led Brenowitz and colleagues to hypothesize that the complexity of song produced is related to the total number of hormone-sensitive cells present in the song nuclei (Brenowitz & Arnold 1992). A second more recently proposed hypothesis, based on studies of oestrogenic metabolites of testosterone and the quality of song activated, is that ER in HVC is directly related to the ability to produce complex syllables preferred by females (Fusani & Gahr 2006). Both of these hypotheses require further testing.
In Japanese quail, several studies have also identified sex differences in brain sex steroid receptors. In vivo autoradiographic studies have demonstrated that following injection of tritiated E2, males accumulate more radioactivity than females in the nucleus taeniae of the amygdala (Watson & Adkins-Regan 1989), suggesting higher numbers of ERs. Subsequent immunocytochemical studies specifically analysing and quantifying the distribution of ERα-immunoreactive cells in the quail brain failed to confirm this difference but did not provide any explanation for the discrepancy (e.g. biological variation, differences in technical approach; Balthazart et al. 1989). In 1996, a second form of ER, called ERβ to distinguish it from the previously known ERα, was identified in mammals (Kuiper et al. 1996), and soon thereafter cloned and found to be expressed also in avian species (Bernard et al. 1999; Foidart et al. 1999). We recently completed an extensive survey by radioactive in situ hybridization of the comparative expression in the male and female quail brains of ERα, ERβ and ARs. Multiple sex differences in expression were identified (Voigt et al. 2007), but, in particular, a significantly higher expression in males than in females was detected for ERβ in nucleus taeniae (no sex difference in ERα or AR was present in this nucleus). These data therefore strongly suggest that the originally reported higher accumulation in males of tritiated E2 in this nucleus was due to a denser expression of ERβ but not ERα. Although the nucleus taeniae of the amygdala is obviously implicated in the control of various aspects of sexual and possibly other behaviours (Phillips 1964; Maley 1969; Thompson et al. 1998; Cheng et al. 1999; Absil et al. 2002), it is, at this point, difficult to relate the sex difference in ERβ expression to the control of any specific behaviour and, for the purpose of the present review, to predict whether such differences in receptor expression could contribute to explaining individual differences in behaviour.
6. Other aspects of steroid action: co-regulators of transcription and phosphorylations
Binding of the steroid to its receptor initiates a cascade of intracellular biochemical events including receptor phosphorylation, dimerization and translocation to the nucleus, which will result in the association of the occupied receptor with steroid-specific binding sites on the DNA (Tsai & O'Malley 1994). The receptors then act as transcription factors and regulate the expression of specific genes (e.g. neurotransmitter receptors), and this will ultimately result in changes in behaviour. Any of these steps could of course be the target of specific regulations that would be individually specific and would then contribute to explaining individual differences in behaviour. This proposition has not been specifically tested so far, but it is already clear that many steps in the process schematically summarized above are the object of very specific controls. This has been best established in a limited number of mammalian species (mice, rats and humans), but exploratory studies already indicate that similar control mechanisms are also present in birds. The analysis of steroid receptor co-regulators represents an enlightening example here.
A few years ago, a new family of proteins was discovered, which controls the association and transcription efficacy of intracellular nuclear receptors, a group of proteins that includes all sex steroid receptors (e.g. McKenna et al. 1999; McKenna & O'Malley 2002). These co-regulators markedly increase (receptor co-activators) or decrease (receptor co-repressors) transcriptional activity induced by the nuclear receptors, and thus play a very important role in the control of the activity of various signalling processes including sex steroid hormones action. At least two of these receptor co-activators have now been cloned or identified in birds: the steroid receptor co-activator-1 (SRC-1) and the cyclic AMP response element-binding protein (CBP; Auger et al. 2002; Charlier et al. 2002). They are expressed in particularly high densities in sex steroid-sensitive areas that are known to play a critical role in the activation by steroids of reproductive behaviour such as male copulatory behaviour in quail (Charlier et al. 2002) or singing in songbirds (Auger et al. 2002; Charlier et al. 2003). It is now clearly established, at least for SRC-1, that the expression of this co-activator is not constitutive but rather is regulated by a variety of factors including the endocrine condition or sex of the subject, the stress it recently experienced or even the time of the day (Charlier et al. 2006). Blocking the expression of SRC-1 by intracerebroventricular injection of antisense oligonucleotides partially blocked the activation in male quail of copulatory behaviour by testosterone, thereby confirming the significant role played by this protein in the control of behaviour (Charlier et al. 2005). Whether SRC-1 is implicated in the regulation of individual differences in behaviour remains unknown at present.
SRC-1 is only one protein among a large family of co-regulators that includes at least 30 members at present (McKenna & O'Malley 2000), and the control of co-regulator expression and action is only one among multiple mechanisms that could regulate the transcriptional activity of sex steroid receptors. Other steps such as the dimerization of the receptors or their translocation to the nucleus or events taking place at the post-transcriptional level (mRNA editing and translation of mRNA into protein) could similarly modulate the causal link between steroid entry into its behaviourally important brain target(s) and the expression of behaviour. Understanding the control of individual differences in behaviour at this level will be a daunting task but fortunately a few alternative approaches to this goal have been emerging in recent years, such as the analysis of single nucleotide polymorphism or of the epigenetic regulation of DNA transcription following methylation or acetylation of associated histones.
7. Conclusion: in the absence of detailed studies of individual differences how might we study such variables at the cellular/molecular level?
The general problem of individual differences in behaviour relates to the fact that ultimately at some level every individual is unique. Although the functioning of the nervous system exhibits many generalities as a function of population or species, it is also the case that each individual has a unique set of experiences that shapes the nervous system so that each member of a population has its own peculiar memories and predispositions that influence how a male/female responds to events in their life. Understanding the mechanistic physiological basis of such variability is in its infancy. There is a robust field trying to understand the causes of personality variation (Nettle 2006). Here animal studies of groups that exhibit patterns of behaviour such as reliably seeking novelty or reliably engaging in risk avoidance have clearly found that among birds and other taxa, there are definitely systematic coping strategies that constitute a form of intraspecific variation akin to what is described as human personality variation (Groothuis & Carere 2005). With the use of the candidate gene approach, investigators are starting to find variation in gene expression that corresponds with differences in these personality types (Fidler et al. 2007). One issue to keep in mind when contemplating the mechanistic basis of individual variation in the response to hormones is that more general variation in neurophysiology or connectivity related to a coping strategy could be as important in modulating the effectiveness of hormones as variables directly involved in hormone action. It is also the case though that ontogenetic variation in hormonal exposure that can be regulated by epigenetic factors could be one of the major sources of nervous system variation that limits the actions of hormones on behaviour (Carere & Balthazart 2007). In the absence of a clear theory about variables that might explain individual variation in the response to hormones, how might one proceed? One proposal is to first identify variables that explain intraspecific variation in the responsiveness to hormones. Sex difference in reactivity to hormones is one obvious case. For example, males will respond to testosterone treatment in a qualitatively different way than females (Ball & Balthazart 2002). Even though the causes of such variation have been investigated for many years, the exact basis of such differences is still poorly understood (Balthazart et al. 1996) and more work along those lines is clearly needed. With the advent of genomic methods being applied to birds (e.g. Clayton 2004), one can also envision being able to identify clusters of genes that vary with phenotypes associated with variation in hormone action. The identification of these genes could then guide research to a specific aspect of steroid hormone action or to other steroid-independent mechanisms that are causally involved in the control of individual differences. This approach in conjunction with the appropriate behavioural studies directed towards a causal analysis of hormone action could prove to be one promising method by which to identify the mechanistic basis of variation in the response to hormones.
Collaborations with evolutionary biologists and ecologists can also be helpful in this regard. Their thinking about reaction norms and other measures of intraspecific phenotypic variation could profitably be applied to ideas about the mechanisms that modulate hormone effectiveness. Such collaborations would reveal the full potential of integrating laboratory–field approaches to the study of individual differences.
Acknowledgments
The preparation of this review and the experimental work described in it was supported by grants from the National Institutes of Health (R01 MH50388 and NS 35467) to G.F.B. and the Belgian FRFC (2.4562.05) to J.B. We thank Tony Williams, John Wingfield and two anonymous reviewers for their helpful comments on the manuscript.
Footnotes
One contribution of 12 to a Theme Issue ‘Integration of ecology and endocrinology in avian reproduction: a new synthesis’.
References
- Absil P, Braquenier J.B, Balthazart J, Ball G.F. Effects of lesions of nucleus taeniae on appetitive and consummatory aspects of male sexual behavior in Japanese quail. Brain Behav. Evol. 2002;60:13–35. doi: 10.1159/000064119. doi:10.1159/000064119 [DOI] [PubMed] [Google Scholar]
- Adkins E.K. Hormonal basis of sexual differentiation in the Japanese quail. J. Comp. Physiol. Psychol. 1975;89:61–71. doi: 10.1037/h0076406. doi:10.1037/h0076406 [DOI] [PubMed] [Google Scholar]
- Adkins-Regan E. Princeton University Press; Princeton, NJ: 2005. Hormones and animal social behavior. [Google Scholar]
- Arnold A.P. The effects of castration and androgen replacement on song courtship, and aggression in zebra finches (Poephila guttata) J. Exp. Zool. 1975;191:309–326. doi: 10.1002/jez.1401910302. doi:10.1002/jez.1401910302 [DOI] [PubMed] [Google Scholar]
- Arnold A.P. Developmental plasticity in neural circuits controlling birdsong: sexual differentiation and the neural basis of learning. J. Neurobiol. 1992;23:1506–1528. doi: 10.1002/neu.480231010. doi:10.1002/neu.480231010 [DOI] [PubMed] [Google Scholar]
- Arnold A.P, Saltiel A. Sexual difference in pattern of hormone accumulation in the brain of a song bird. Science. 1979;205:702–705. doi: 10.1126/science.205.4407.702. doi:10.1126/science.205.4407.702 [DOI] [PubMed] [Google Scholar]
- Auger C.J, Bentley G.E, Auger A.P, Ramamurthy M, Ball G.F. Expression of cAMP response element binding protein-binding protein in the song control system and hypothalamus of adult european starlings (Sturnus vulgaris) J. Neuroendocrinol. 2002;14:805–813. doi: 10.1046/j.1365-2826.2002.00842.x. doi:10.1046/j.1365-2826.2002.00842.x [DOI] [PubMed] [Google Scholar]
- Ball G.F, Balthazart J. Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. vol. 2. Academic Press; San Diego, CA: 2002. pp. 649–798. [Google Scholar]
- Ball G.F, Riters L.V, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: multiple sites of action of sex steroid hormones. Front. Neuroendocrinol. 2002;23:137–178. doi: 10.1006/frne.2002.0230. doi:10.1006/frne.2002.0230 [DOI] [PubMed] [Google Scholar]
- Ball G.F, Castelino C.B, Maney D.L, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Ann. NY Acad. Sci. 2003;1007:211–231. doi: 10.1196/annals.1286.021. doi:10.1196/annals.1286.021 [DOI] [PubMed] [Google Scholar]
- Ball G.F, Auger C.J, Bernard D.J, Charlier T.D, Sartor J.J, Riters L.V, Balthazart J. Seasonal plasticity in the song control system. Multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann. NY Acad. Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. doi:10.1196/annals.1298.043 [DOI] [PubMed] [Google Scholar]
- Balthazart J. Hormonal correlates of behavior. In: Farner D.S, King J.R, Parkes K.C, editors. Avian biology. vol. VII. Academic Press; New York, NY: 1983. pp. 221–365. [Google Scholar]
- Balthazart J. Steroid metabolism and the activation of social behavior. In: Balthazart J, editor. Advances in comparative and environmental physiology. vol. 3. Springer; Berlin, Germany: 1989. pp. 105–159. [Google Scholar]
- Balthazart J, Ball G.F. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. doi:10.1016/j.tins.2006.03.004 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Hendrick J.C. Annual variation in reproductive behavior, testosterone, and plasma FSH levels in the Rouen duck, Anas platyrhynchos. Gen. Comp. Endocrinol. 1976;28:171–183. doi: 10.1016/0016-6480(76)90169-6. doi:10.1016/0016-6480(76)90169-6 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Hendrick J.C. Effects of exogenous gonadotropic and steroid hormones on the social behaviour and gonadal maturation of male domestic ducks. Arch. Int. Physiol. Biochem. 1979;87:741–761. doi: 10.3109/13813457909070533. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M. In Neurobiology, current comparative approaches. Springer; Berlin, Germany: 1985. Role of testosterone metabolism in the activation of sexual behaviour in birds; pp. 121–140. [Google Scholar]
- Balthazart J, Stevens M. Effects of testosterone propionate on the social behaviour of groups of male comestic ducklings Anas platyrhynchos L. Anim. Behav. 1975;23:926–931. doi: 10.1016/0003-3472(75)90116-5. doi:10.1016/0003-3472(75)90116-5 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Surlemont C. Androgen and estrogen action in the preoptic area and activation of copulatory behavior in quail. Physiol. Behav. 1990;48:599–609. doi: 10.1016/0031-9384(90)90198-d. doi:10.1016/0031-9384(90)90198-D [DOI] [PubMed] [Google Scholar]
- Balthazart J, Deviche P, Hendrick J.C. Effects of exogenous hormones on the reproductive behaviour of adult male domestic ducks. II Correlation with morphology and hormone plasma levels. Behav. Process. 1977;2:147–161. doi: 10.1016/0376-6357(77)90017-1. doi:10.1016/0376-6357(77)90017-1 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Massa R, Negri-Cesi P. Photoperiodic control of testosterone metabolism, plasma gonadotrophins, cloacal gland growth, and reproductive behavior in the Japanese quail. Gen. Comp. Endocrinol. 1979;39:222–235. doi: 10.1016/0016-6480(79)90227-2. doi:10.1016/0016-6480(79)90227-2 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Schumacher M, Ottinger M.A. Sexual differences in the Japanese quail: behavior, morphology and intracellular metabolism of testosterone. Gen. Comp. Endocrinol. 1983;51:191–207. doi: 10.1016/0016-6480(83)90072-2. doi:10.1016/0016-6480(83)90072-2 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Gahr M, Surlemont C. Distribution of estrogen receptors in the brain of the Japanese quail: an immunocytochemical study. Brain Res. 1989;501:205–214. doi: 10.1016/0006-8993(89)90638-0. doi:10.1016/0006-8993(89)90638-0 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Hendrick J.C. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol. Behav. 1990;47:83–94. doi: 10.1016/0031-9384(90)90045-6. doi:10.1016/0031-9384(90)90045-6 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Tlemçani O, Ball G.F. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm. Behav. 1996;30:627–661. doi: 10.1006/hbeh.1996.0066. doi:10.1006/hbeh.1996.0066 [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil C.A, Ball G.F. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol. Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. doi:10.1016/j.physbeh.2004.08.025 [DOI] [PubMed] [Google Scholar]
- Barfield R.J. Activation of sexual and aggressive behavior by androgen implantation into the male ring dove brain. Endocrinology. 1971;89:1470–1476. doi: 10.1210/endo-89-6-1470. [DOI] [PubMed] [Google Scholar]
- Benoff F.H, Siegel P.B, Van Krey H.P. Testosterone determinations in lines of chickens selected for differential mating frequency. Horm. Behav. 1978;10:246–250. doi: 10.1016/0018-506x(78)90068-5. doi:10.1016/0018-506X(78)90068-5 [DOI] [PubMed] [Google Scholar]
- Bernard D.J, Bentley G.E, Balthazart J, Turek F.W, Ball G.F. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. doi:10.1210/en.140.10.4633 [DOI] [PubMed] [Google Scholar]
- Berthold A.A. Transplantation der Hoden. Arch. Anat. Physiol. 1849;16:42–46. [Google Scholar]
- Blaustein J.D, Olster D.H. Gonadal steroid hormone receptors and social behaviors. In: Balthazart J, editor. Advances in comparative and environmental physiology. vol. 3. Springer; Berlin, Germany: 1989. pp. 31–104. [Google Scholar]
- Brenowitz E.A, Arnold A.P. Hormone accumulation in song regions of the canary brain. J. Neurobiol. 1992;23:871–880. doi: 10.1002/neu.480230708. doi:10.1002/neu.480230708 [DOI] [PubMed] [Google Scholar]
- Breuner C.W, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. doi:10.1677/joe.0.1750099 [DOI] [PubMed] [Google Scholar]
- Breuner C.W, Lynn S.E, Julian G.E, Cornelius J.M, Heidinger B.J, Love O.P, Sprague R.S, Wada H, Whitman B.A. Plasma-binding globulins and acute stress response. Horm. Metab. Res. 2006;38:260–268. doi: 10.1055/s-2006-925347. doi:10.1055/s-2006-925347 [DOI] [PubMed] [Google Scholar]
- Carere C, Balthazart J. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol. Metab. 2007;18:73–80. doi: 10.1016/j.tem.2007.01.003. doi:10.1016/j.tem.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Charlier T.D, Lakaye B, Ball G.F, Balthazart J. Steroid receptor coactivator SRC-1 exhibits high expression in steroid-sensitive brain areas regulating reproductive behaviors in the quail brain. Neuroendocrinology. 2002;76:297–315. doi: 10.1159/000066624. doi:10.1159/000066624 [DOI] [PubMed] [Google Scholar]
- Charlier T.D, Balthazart J, Ball G.F. Sex differences in the distribution of the steroid receptor coactivator SRC-1 in the song control nuclei of male and female canaries. Brain Res. 2003;959:263–274. doi: 10.1016/s0006-8993(02)03758-7. doi:10.1016/S0006-8993(02)03758-7 [DOI] [PubMed] [Google Scholar]
- Charlier T.D, Ball G.F, Balthazart J. Inhibition of steroid receptor coactivator-1 blocks estrogen and androgen action on male sex behavior and associated brain plasticity. J. Neurosci. 2005;25:906–913. doi: 10.1523/JNEUROSCI.3533-04.2005. doi:10.1523/JNEUROSCI.3533-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier T.D, Ball G.F, Balthazart J. Plasticity in the expression of the steroid receptor coactivator 1 in the Japanese quail brain: effect of sex, testosterone, stress and time of the day. Neuroscience. 2006;140:1381–1394. doi: 10.1016/j.neuroscience.2006.03.002. doi:10.1016/j.neuroscience.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Cheng M.F, Chaiken M, Zuo M, Miller H. Nucleus taenia of the amygdala of birds: anatomical and functional studies in ring doves (Streptopelia risoria) and European starlings (Sturnus vulgaris) Brain Behav. Evol. 1999;53:243–270. doi: 10.1159/000006597. doi:10.1159/000006597 [DOI] [PubMed] [Google Scholar]
- Clayton D.F. Songbird genomics: methods, mechanisms, opportunities, and pitfalls. Ann. NY Acad. Sci. 2004;1016:45–60. doi: 10.1196/annals.1298.028. doi:10.1196/annals.1298.028 [DOI] [PubMed] [Google Scholar]
- Cohen-Parsons M, Van Krey H.P, Siegel P.B. In vivo aromatization of [3H] testosterone in high and low mating lines of Japanese quail. Horm. Behav. 1983;17:316–323. doi: 10.1016/0018-506x(83)90031-4. doi:10.1016/0018-506X(83)90031-4 [DOI] [PubMed] [Google Scholar]
- Cornil C.A, Ball G.F, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. doi:10.1016/j.brainres.2006.07.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. On the organization of individual difference in sexual behavior. Am. Zool. 1998;38:118–132. [Google Scholar]
- Cunningham D.L, Siegel P.B, Van Krey H.P. Androgen influence on mating behavior in selected lines of Japanese quail. Horm. Behav. 1977;8:166–174. doi: 10.1016/0018-506x(77)90033-2. doi:10.1016/0018-506X(77)90033-2 [DOI] [PubMed] [Google Scholar]
- Damassa D.A, Smith E.R, Tennent B, Davidson J.M. The relationship between circulating testosterone levels and male sexual behavior in rats. Horm. Behav. 1977;8:275–286. doi: 10.1016/0018-506x(77)90002-2. doi:10.1016/0018-506X(77)90002-2 [DOI] [PubMed] [Google Scholar]
- Delville Y, Hendrick J.C, Sulon J, Balthazart J. Testosterone metabolism and testosterone-dependent characteristics in Japanese quail. Physiol. Behav. 1984;33:817–823. doi: 10.1016/0031-9384(84)90053-2. doi:10.1016/0031-9384(84)90053-2 [DOI] [PubMed] [Google Scholar]
- Desjardins C, Turek F.W. Effects of testosterone on spermatogenesis and luteinizing hormone release in Japanese quail. Gen. Comp. Endocrinol. 1977;33:293–303. doi: 10.1016/0016-6480(77)90253-2. doi:10.1016/0016-6480(77)90253-2 [DOI] [PubMed] [Google Scholar]
- Dessi-Fulgheri F, Lucarini N, Lupo di Prisco C. Relationships between testosterone metabolism in the brain, other endocrine variables and intermale aggression in mice. Aggr. Behav. 1976;2:223–231. doi:10.1002/1098-2337(1976)2:3<223::AID-AB2480020307>3.0.CO;2-0 [Google Scholar]
- Deviche P. Behavioral effects of castration and tesosterone propionate replacement combined with ACTH in the male domestic duck (Anas platyrhynchos L.) J. Exp. Zool. 1979;207:471–480. doi:10.1002/jez.1402070315 [Google Scholar]
- Deviche P, Breuner C, Orchinik M. Testosterone, corticosterone, and photoperiod interact to regulate plasma levels of binding globulin and free steroid hormone in dark-eyed juncos, Junco hyemalis. Gen. Comp. Endocrinol. 2001;122:67–77. doi: 10.1006/gcen.2001.7613. doi:10.1006/gcen.2001.7613 [DOI] [PubMed] [Google Scholar]
- Feder H.H. Hormones and sexual behavior. Ann. Rev. Psychol. 1984;1984:165–200. doi: 10.1146/annurev.ps.35.020184.001121. doi:10.1146/annurev.ps.35.020184.001121 [DOI] [PubMed] [Google Scholar]
- Fidler A.E, van Oers K, Drent P.J, Kuhn S, Meuller J.C, Kempaenaers B. Drd4 gene polymorphisms are associated with personality variation in a passerine bird. Proc. R. Soc. B. 2007;274:1685–1691. doi: 10.1098/rspb.2007.0337. doi:10.1098/rspb.2007.0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foidart A, Lakaye B, Grisar T, Ball G.F, Balthazart J. Estrogen receptor-beta in quail: cloning, tissue expression and neuroanatomical distribution. J. Neurobiol. 1999;40:327–342. doi:10.1002/(SICI)1097-4695(19990905)40:3<327::AID-NEU5>3.0.CO;2-L [PubMed] [Google Scholar]
- Fusani L, Gahr M. Hormonal influence on song structure and organization: the role of estrogen. Neuroscience. 2006;138:939–946. doi: 10.1016/j.neuroscience.2005.08.041. doi:10.1016/j.neuroscience.2005.08.041 [DOI] [PubMed] [Google Scholar]
- Goymann W, Landys M.M, Wingfield J.C. Distinguishing seasonal androgen responses from male–male androgen responsiveness—revisiting the challenge hypothesis. Horm. Behav. 2007;51:463–476. doi: 10.1016/j.yhbeh.2007.01.007. doi:10.1016/j.yhbeh.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G, Carere C. Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 2005;29:137–150. doi: 10.1016/j.neubiorev.2004.06.010. doi:10.1016/j.neubiorev.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Grunt J.A, Young W.C. Differential reactivity of individuals and the response of the male guinea pig to testosterone propionate. Endocrinology. 1952;51:237–248. doi: 10.1210/endo-51-3-237. [DOI] [PubMed] [Google Scholar]
- Hammond G.L. Potential function of plasma steroid-binding proteins. Trends Endocrinol. Metab. 1995;6:298–304. doi: 10.1016/1043-2760(95)00162-x. doi:10.1016/1043-2760(95)00162-X [DOI] [PubMed] [Google Scholar]
- Hammond G.L. Access of reproductive steroids to target tissues. Obstet. Gynecol. Clin. North Am. 2002;29:411–423. doi: 10.1016/s0889-8545(02)00008-6. doi:10.1016/S0889-8545(02)00008-6 [DOI] [PubMed] [Google Scholar]
- Harding C.F, Sheridan K, Walters M.J. Hormonal specificity and activation of sexual behavior in male zebra finches. Horm. Behav. 1983;17:111–133. doi: 10.1016/0018-506x(83)90021-1. doi:10.1016/0018-506X(83)90021-1 [DOI] [PubMed] [Google Scholar]
- Harding C.F, Walters M.J, Collado D, Sheridan K. Hormonal specificity and activation of social behavior in male red-winged blackbirds. Horm. Behav. 1988;22:402–418. doi: 10.1016/0018-506x(88)90011-6. doi:10.1016/0018-506X(88)90011-6 [DOI] [PubMed] [Google Scholar]
- Herbert Z, Pollak E, Caldwell J.D, Jirikowski G.F. Subcellular localization of sex hormone-binding globulin/SHBG in rat hypothalamus and pituitary. Abstr. Soc. Neurosci. 2003:29. [Google Scholar]
- Herbert Z, Gothe S, Caldwell J.D, Bernstein H.G, Melle C, Von Eggeling F, Lewis J, Jirikowski G.F. Identification of sex hormone-binding globulin in the human hypothalamus. Neuroendocrinology. 2005;81:287–293. doi: 10.1159/000088170. doi:10.1159/000088170 [DOI] [PubMed] [Google Scholar]
- Hews D.K, Moore M.C. Hormones and sex-specific traits: critical questions. In: Beckage N.E, editor. Parasites and pathogens: effects on host hormones and behavior. International Thomas Publisher; New York, NY: 1997. pp. 277–292. [Google Scholar]
- Hutchison J.B. Differential effects of testosterone and oestradiol on male courtship behaviour in the Barbary dove (Streptopelia risoria) Anim. Behav. 1970;18:41–51. doi: 10.1016/0003-3472(70)90068-0. doi:10.1016/0003-3472(70)90068-0 [DOI] [PubMed] [Google Scholar]
- Hutchison J.B. Effects of hypothalamic implants of gonadal steroids on courtship behaviour in Barbary doves (Streptopelia risoria) J. Endocrinol. 1971;50:97–113. doi: 10.1677/joe.0.0500097. [DOI] [PubMed] [Google Scholar]
- Kuiper G.G.J.M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J.Å. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. doi:10.1073/pnas.93.12.5925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall-Shackleton S.A, Ball G.F. Comparative studies of sex differences in the song-control system of songbirds. Trends Neurosci. 1999;22:432–436. doi: 10.1016/s0166-2236(99)01434-4. doi:10.1016/S0166-2236(99)01434-4 [DOI] [PubMed] [Google Scholar]
- Maley M.J. Electrical stimulation of agonistic behavior in the mallard. Behaviour. 1969;34:138–160. [Google Scholar]
- McCollom R.E, Siegel P.B, Van Krey H.P. Responses to androgen in lines of chickens selected for mating behavior. Horm. Behav. 1971;2:31–42. doi:10.1016/0018-506X(71)90035-3 [Google Scholar]
- McEwen B.S. Genome and hormones: gender differences in physiology—invited review: estrogens effects on the brain: multiple sites and molecular mechanisms. J. Appl. Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- McEwen B.S, Alves S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. doi:10.1210/er.20.3.279 [DOI] [PubMed] [Google Scholar]
- McKenna N.J, O'Malley B.W. From ligand to response: generating diversity in nuclear receptor coregulator function. J. Steroid Biochem. Mol. Biol. 2000;74:351–356. doi: 10.1016/s0960-0760(00)00112-6. doi:10.1016/S0960-0760(00)00112-6 [DOI] [PubMed] [Google Scholar]
- McKenna N.J, O'Malley B.W. Minireview: nuclear receptor coactivators—an update. Endocrinology. 2002;143:2461–2465. doi: 10.1210/endo.143.7.8892. doi:10.1210/en.143.7.2461 [DOI] [PubMed] [Google Scholar]
- McKenna N.J, Lanz R.B, O'Malley B.W. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. doi:10.1210/er.20.3.321 [DOI] [PubMed] [Google Scholar]
- Morrell J.I, Pfaff D.W. A neuroendocrine approach to brain function: localization of sex steroid concentrating cells in vertebrate brains. Am. Zool. 1978;18:447–460. [Google Scholar]
- Nettle D. The evolution of personality variation in humans and other animals. Am. Psychol. 2006;61:622–631. doi: 10.1037/0003-066X.61.6.622. doi:10.1037/0003-066X.61.6.622 [DOI] [PubMed] [Google Scholar]
- Pfaff D.W. The MIT Press; Cambridge, MA: 1999. Drive. Neurobiological and molecular mechanisms of sexual motivation. [Google Scholar]
- Pfaff D.W, Pfaffmann C. Olfactory and hormonal influences on the basal forebrain of the male rat. Brain Res. 1969;15:137–156. doi: 10.1016/0006-8993(69)90315-1. doi:10.1016/0006-8993(69)90315-1 [DOI] [PubMed] [Google Scholar]
- Pfaff D.W, Phillips M.I, Rubin R.T. Elsevier; Amsterdam, The Netherlands: 2004. Principles of hormone/behavior relations. [Google Scholar]
- Phillips R.E. Wildness in the mallard duck. Effects of brain lesions and stimulation on escape behavior and reproduction. J. Comp. Neurol. 1964;122:139–155. doi: 10.1002/cne.901220202. doi:10.1002/cne.901220202 [DOI] [PubMed] [Google Scholar]
- Portillo W, Castillo C.G, Retana-Marquez S, Roselli C.E, Paredes R.G. Neuronal activity of aromatase enzyme in non-copulating male rats. J. Neuroendocrinol. 2007;19:139–141. doi: 10.1111/j.1365-2826.2006.01513.x. doi:10.1111/j.1365-2826.2006.01513.x [DOI] [PubMed] [Google Scholar]
- Ramenofsky M. Agonistic behaviour and endogenous plasma hormones in male japanese quail. Anim. Behav. 1984;32:698–708. doi:10.1016/S0003-3472(84)80145-1 [Google Scholar]
- Reiner A.D, et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. doi:10.1002/cne.20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. The effects of testosterone and its metabolites on sexual behavior and morphology in male and female Japanese quail. Physiol. Behav. 1983;30:335–339. doi: 10.1016/0031-9384(83)90135-x. doi:10.1016/0031-9384(83)90135-X [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Testosterone-induced brain aromatase is sexually dimorphic. Brain Res. 1986;370:285–293. doi: 10.1016/0006-8993(86)90483-x. doi:10.1016/0006-8993(86)90483-X [DOI] [PubMed] [Google Scholar]
- Sisneros J.A, Forlano P.M, Dietcher D.L, Bass A.H. Steroid-dependent plasticity leads to adaptive coupling of sender and receiver. Science. 2004;305:404–407. doi: 10.1126/science.1097218. doi:10.1126/science.1097218 [DOI] [PubMed] [Google Scholar]
- Thompson R.R, Goodson J.L, Ruscio M.G, Adkins-Regan E. Role of the archistriatal nucleus taeniae in the sexual behavior of male Japanese quail (Coturnix japonica): a comparison of function with the medial nucleus of the amygdala in mammals. Brain Behav. Evol. 1998;51:215–229. doi: 10.1159/000006539. doi:10.1159/000006539 [DOI] [PubMed] [Google Scholar]
- Tsai M.J, O'Malley B.W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. doi:10.1146/annurev.bi.63.070194.002315 [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Ishii S. Effects of sex steroids on agressive behavior of adult male japanese quail. Gen. Comp. Endocrinol. 1981;44:480–486. doi: 10.1016/0016-6480(81)90336-1. doi:10.1016/0016-6480(81)90336-1 [DOI] [PubMed] [Google Scholar]
- Turek F.W, Desjardins C, Menaker M. Antigonadal and progonadal effects of testosterone in male house sparrows. Gen. Comp. Endocrinol. 1976;28:395–402. doi: 10.1016/0016-6480(76)90147-7. doi:10.1016/0016-6480(76)90147-7 [DOI] [PubMed] [Google Scholar]
- Van Krey H.P, Siegel P.B, Balander R.J, Benoff F.H. Testosterone aromatization in high and low mating lines of Gallinaceous birds. Physiol. Behav. 1983;31:153–157. doi: 10.1016/0031-9384(83)90112-9. doi:10.1016/0031-9384(83)90112-9 [DOI] [PubMed] [Google Scholar]
- Voigt, C., Ball, G.F. & Balthazart, J. 2007 Sex differences in the expression of sex steroid receptors mRNA in the quail brain, Abst. 209.21/HHH26 of the Soc. for Neurosci. Meeting. [DOI] [PMC free article] [PubMed]
- Walters M.J, Harding C.F. The effects of an aromatization inhibitor on the reproductive behavior of male zebra finches. Horm. Behav. 1988;22:207–218. doi: 10.1016/0018-506x(88)90067-0. doi:10.1016/0018-506X(88)90067-0 [DOI] [PubMed] [Google Scholar]
- Wang Y.M, Bayliss D.A, Millhorn D.E, Petrusz P, Joseph D.R. The androgen-binding protein gene is expressed in male and female rat brain. Endocrinology. 1990;127:3124–3130. doi: 10.1210/endo-127-6-3124. [DOI] [PubMed] [Google Scholar]
- Watson J.T, Adkins-Regan E. Neuroanatomical localization of sex steroid-concentrating cells in the Japanese quail (Coturnix japonica): autoradiography with [3H]-testosterone, [3H]-estradiol, and [3H]-dihydrotestosterone. Neuroendocrinology. 1989;49:51–64. doi: 10.1159/000125091. [DOI] [PubMed] [Google Scholar]
- Wingfield J, Ramenofsky M. Testosterone and aggressive behaviour during the reproductive cycle of male birds. In: Balthazart J, editor. Comparative neurobiology. Springer; Berlin, Germany: 1985. pp. 93–104. [Google Scholar]
- Wingfield J.C, Silverin B. Ecophysiological studies of hormone–behavior relations in birds. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. vol. 2. Elservier; Amsterdam, The Netherlands: 2002. pp. 587–648. [Google Scholar]
- Wingfield J.C, Hegner R.E, Dufty A.M, Ball G.F. The challenge hypothesis—theoretical implications for patterns of testosterone secretion, mating systems and breeding strategies. Am. Nat. 1990;136:829–846. doi:10.1086/285134 [Google Scholar]