Abstract
The relative homogeneity of pelagic environments has been regarded as the reason for the absence of hybrid zones for hybridizing planktonic Daphnia (Crustacea: Cladocera); occasional dominance of interspecific hybrids over parental species was explained by their temporal superiority in fluctuating environments. However, water bodies with spatially varying environmental conditions might facilitate the formation of hybrid zones in plankton. We studied the distribution of species and hybrids of the Daphnia longispina complex in 11 canyon-shaped reservoirs, localities characterized by horizontal environmental gradients (particularly of food supply and size-selective predation); we also analysed patterns of carapace size and fecundity among coexisting taxa. Spatial distribution of taxa agreed with their ecological characteristics; those showing different affinities along longitudinal reservoir profiles differed in size according to the presumed fish predation gradient. Only hybrids of Daphnia galeata with Daphnia cucullata and D. longispina (=hyalina) were recorded. The latter two species preferred opposite ends of gradients, such spatial segregation probably explaining the absence of their hybrids. Distributional patterns were relatively stable in two consecutive summers, apart from a substantial decline of D. galeata×cucullata in the second year. The observed pattern of a hybrid-dominated zone in intermediate conditions suggests that local Daphnia hybrid zones may indeed form within reservoirs.
Keywords: interspecific hybridization, Daphnia longispina complex, hybrid zones, canyon-shaped reservoirs, ITS-RFLP, allozyme electrophoresis
1. Introduction
The reproductive mode of cladocerans, cyclical parthenogenesis, makes this crustacean group an interesting model for ecological and evolutionary studies. The ability to switch between parthenogenetic reproduction during favourable conditions and sexual reproduction associated with the formation of diapausing stages (‘resting eggs’), and the possibility to grow clonal lineages in laboratory conditions, and therefore to disentangle the effects of environmental factors, genetic background and phenotypic variation, makes especially members of the genus Daphnia (Crustacea: Cladocera: Anomopoda) favourite study organisms.
Hybridization between different Daphnia species has been documented in several species groups (Schwenk & Spaak 1995), with the resulting reproductive modes of hybrids ranging from parthenogenetically reproducing hybrids unable to form diapausing eggs (Hebert & Finston 1996), obligate parthenogens, often polyploid, producing asexual resting eggs (e.g. Hebert et al. 1989a; Dufresne & Hebert 1994; Hebert & Finston 1996) to hybrids capable of sexual reproduction and backcrossing with the parental taxa, as observed within the Daphnia longispina complex. Hybridizing species belonging to this complex (Daphnia galeata Sars, Daphnia cucullata Sars and the pelagic form of D. longispina (O. F. Müller)=D. hyalina Leydig; for taxonomy and nomenclature, see Petrusek et al. 2008) are ecologically particularly important, as they are among the most common Daphnia species inhabiting large European permanent water bodies. The occurrence of their hybrids has been reported from many natural as well as man-made lakes (e.g. Hebert et al. 1989b; Seda et al. 2007b; Wolinska et al. 2007).
Despite potential introgression and occasional hybrid dominance, parental species within the D. longispina complex remain genetically distinct, as interspecific hybrids exhibit various aspects of hybrid breakdown (Keller et al. 2007), such as lower hatching and survival rate (Schwenk et al. 2001) and lower sexual reproductive success (Keller & Spaak 2004). It has been therefore proposed that reproductive isolation effectively exists among hybridizing species within the complex (Keller et al. 2007). Hybrids are nevertheless fully competitive with parental species when reproducing parthenogenetically, and due to asexual reproduction may at least temporarily avoid hybrid breakdown. Some Daphnia interspecific hybrids may exhibit superior population rates of increase under certain conditions, which has been shown in both the laboratory and field conditions. This phenomenon has been attributed to heterosis (Repka et al. 1999) or a combination of advantageous traits of both parental species (Spaak & Hoekstra 1995; Declerck & De Meester 2003). As a result, hybrids may occasionally be more abundant than the parental species (e.g. Spaak & Hoekstra 1993; Seda et al. 2007b).
Unlike in terrestrial habitats, which often exhibit various gradients in factors affecting the distribution of taxa and often facilitating the presence of interspecific hybrids in intermediate conditions (i.e. in spatially restricted hybrid zones; Arnold 1997), lakes and ponds tend to offer much more homogeneous conditions for plankton, and ecologically different pelagic environments are usually isolated from each other in island-like nature (Schwenk & Spaak 1995). As the space used by parental taxa and hybrids typically overlaps in the whole lake area, the coexistence of hybrids and parental species of Daphnia in the pelagic environment within any particular lake may not be appropriately modelled by hybrid zone scenarios.
The temporal hybrid superiority hypothesis (Spaak & Hoekstra 1995) has been proposed to explain the often observed hybrid dominance within localities by the fluctuation of environmental conditions that may temporarily favour hybrid genotypes. The most important factors affecting the presence of species and hybrids of the D. longispina complex seem to be size-selective fish predation pressure and food level, as it is the case for pelagic Daphnia in general (Gliwicz 2003). However, other factors such as parasites (Wolinska et al. 2006, 2007) or food quality (Seidendorf et al. 2007) may also strongly affect the patterns of species coexistence.
To study the impact of variation in environmental factors affecting the success of the parental taxa and hybrids, three potential approaches are available. First, zooplankton communities from various isolated water bodies with differing ecological conditions may be compared (e.g. Keller 2007). Second, it might be possible to track long-term changes in community structure in localities with substantial temporal variation or gradual changes in key environmental factors (such as fish stock or trophic level), e.g. by combining historical samples and data with a palaeogenetic analysis of resting eggs from the sediment (Jankowski & Straile 2003; N. Brede, C. Sandrock, D. Straile, P. Spaak, T. Jankowski, B. Streit & K. Schwenk 2007, unpublished data).
Third, we may examine the variation in the spatial distribution of the parental and hybrid taxa within water bodies in which substantial environmental gradients exist. Vertical gradients are characteristic of thermally stratified water bodies such as temperate lakes (figure 1). Hybridizing Daphnia taxa may indeed show different spatial distributions within these gradients (Weider & Stich 1992; Seda et al. 2007b), and intraspecific genetic differentiation on a vertical scale has been documented as well (Seda et al. 2007a). The vertical segregation of taxa may be caused by variable selection pressures in different depths or by individual spatial preferences. However, pelagic daphnids often exhibit diel vertical migrations across the thermocline (e.g. King & Miracle 1995; Spaak et al. 2004), showing that vertical temperature and oxygen gradients on a relatively small spatial scale do not necessarily form substantial barriers for individual movement.
Figure 1.
Schematic of key environmental gradients in canyon-shaped reservoirs affecting the zooplankton community. (a) Horizontal gradients: water inflow brings in the limiting nutrients that stimulate phytoplankton growth in the upstream reservoir regions. For grazers, food quantity (algal biomass, often expressed as chlorophyll a content) as well as quality (C : P ratio) is more favourable in the upstream part; however, this region is also more attractive for planktivorous fishes, size-selective predators preferring larger zooplankton prey. (b) Vertical gradients in thermally stratified water bodies include, among other factors, changes in temperature, light intensity (i.e. visibility to predators), oxygen concentration, food quantity and predation pressure. While temperature and light always decrease with increasing depth, some parameters such as oxygen or chlorophyll concentration may show heterogeneous profiles. (Outline, temperature and oxygen profiles are of a typical canyon-shaped reservoir, Vír, 8 km long and 58 m deep at the dam; values measured in July 2004. Arrows and stars in (a) indicate the direction of the water flow and our sampling stations, respectively; rectangles E, M and H in (b) indicate the extent of the epi-, meta- and hypolimnetic layers, respectively, sampled separately.)
Although water bodies with substantial internal horizontal gradients are apparently much rarer, they may be particularly interesting, as the dispersal of pelagic species is less limited by physical barriers and the uneven distribution of taxa in areas connected by water may suggest ‘barriers’ of ecological nature, i.e. differential selection forces. Our model systems, deep canyon-shaped reservoirs (figure 1), provide good conditions for studying this phenomenon. The well-defined main reservoir tributary as a point source of nutrient input, the unidirectional flow of water down the reservoir, occasional turbidity stress from upstream sites and significantly higher affinity of fishes to upstream locations are all significant environmental factors for the maintenance of strong heterogeneity of the selective forces responsible for the overall longitudinal heterogeneity of reservoir zooplankton (Urabe 1990).
In our recent study (Seda et al. 2007b), we demonstrated significant differences in the horizontal distribution of species and hybrids of the D. longispina complex within canyon-shaped reservoirs in Central Europe (Czech Republic). The presence of two or even three hybridizing species in a particular reservoir was common, but each taxon showed different patterns in relation to horizontal gradients. The spatial distribution of taxa was in agreement with their ecological characteristics (Gliwicz 1990; Flößner 2000): D. galeata was a ubiquitous species; D. cucullata was generally more common in the high-food and high-predation environment of upstream reservoir regions; and D. longispina had the opposite tendency. The distribution of hybrids of D. galeata with the remaining two species partly overlapped with their non-galeata parental species; the preference of D. galeata×cucullata hybrids for upstream regions was especially characteristic.
The substantial environmental gradient, together with the non-random distribution of parental taxa and zones with a preferential occurrence of hybrids, suggests that unlike in lakes studied so far, canyon-shaped reservoirs could offer favourable conditions for the emergence of local within-lake hybrid zones of zooplankters. This hypothesis may be supported by the partial overlap in the distribution of parental taxa with differing ecological requirements and the ongoing formation of hybrid genotypes, which usually only attain ecologically relevant abundances in certain parts of any given reservoir.
In this paper, we summarize our current knowledge of Daphnia hybridization in reservoirs, based on the data from two consecutive years. We discuss the patterns of taxon coexistence within environmental gradients and their temporal stability, and evaluate whether the distribution patterns re-form after the winter season, when Daphnia populations either disappear completely from the water column or at least experience severe bottlenecks. We also describe in detail a characteristic canyon-shaped reservoir with three hybridizing Daphnia species. Finally, we focus on the variation in the taxon body size and fecundity as population characteristics that probably contribute to the hybrid success, and are strongly influenced by the selection forces in reservoirs.
2. Material and methods
(a) Locality selection
To study the spatial distribution of taxa and genotypes within environmental gradients in reservoirs, it is important to ensure that local processes are crucial for shaping the observed patterns, rather than immigration or import of individuals. The primary selection criterion for our study localities was their canyon-like morphology (i.e. elongated shape along the longitudinal axis and increasing depth from the inflow regions towards the dam), which may result in longitudinal environmental gradients; however, we excluded those reservoirs in which import of zooplankton from upstream water bodies could strongly affect the Daphnia taxon composition in the inflow regions. Altogether, we sampled 11 reservoirs located in the Czech Republic, covering a substantial range of reservoir size, depth and retention time (table 1). Schematic outlines and locations of the studied reservoirs are shown in Seda et al. (2007b), where also additional details about localities are provided. We analysed the longitudinal and vertical distributions of Daphnia species and hybrids in samples from two consecutive summer seasons, collected once in July 2004 and once in July 2005.
Table 1.
Basic characteristics of the investigated reservoirs, their occupancy by taxa of the D. longispina complex and the number of analysed individuals (for allozymes, data for each year are shown separately; variation in numbers is caused by the occasional absence of Daphnia in the hypolimnion and/or scoring problems). (Species (D. galeata, D. cucullata and D. longispina) are abbreviated by the first letters of their species names; taxa composing at least 5% in any sample from the particular reservoir are listed in uppercase, accessorial taxa recorded in lower relative abundances are in lowercase. The asterisk indicates the locality where all ‘hybrid’ individuals were probably backcrosses or later-generation hybrids; cases where one of the parental species of a hybrid was not detected in a particular reservoir are marked by the hash symbol. More details about reservoirs (geographical position, altitude and age) can be found in Seda et al. 2007b.)
| reservoir | area (km2) | maximum depth (m) | length (km) | theoretical retention time (days) | taxa present | no. of individuals analysed by | ||
|---|---|---|---|---|---|---|---|---|
| 2004 | 2005 | allozymes (2004+2005) | ITS-RFLP (2004) | |||||
| Horka | 1.3 | 40 | 5 | 348 | L, G×L*,# | L, G×L*,# | 160+220 | n.a. |
| Kníničky | 2.3 | 19 | 5 | 27 | C, G, G×L, L | C, G, L, g×l | 177+218 | 145 |
| Římov | 2.1 | 44 | 9 | 89 | G, c, g×c, | G, c | 200+210 | 134 |
| Šance | 3 | 45 | 4 | 169 | G, G×L# | G, G×L# | 160+175 | 132 |
| Seč | 1.9 | 29 | 4 | 86 | C, G, g×c | C, G, g×c | 199+219 | 124 |
| Stanovice | 1.4 | 45 | 3.5 | 555 | G | G, g×l# | 199+200 | 163 |
| Trnávka | 0.8 | 17 | 3.5 | 27 | C, G, g×c | C, G | 200+219 | 124 |
| Vír | 2.1 | 58 | 8 | 166 | C, G, G×L, L | C, G, G×L, L | 200+258 | 141 |
| Vranov | 7.7 | 45 | 18 | 134 | C, G×C, G, G×L, L | C, G, G×L, L | 208+228 | 172 |
| Želivka | 14 | 49 | 29 | 604 | G, G×L, L | G, G×L, L, c | 200+199 | 130 |
| Žlutice | 1.5 | 20 | 3.8 | 135 | G, G×L, l | G, G×L, L | 200+215 | n.a. |
(b) Sample collection and processing
The methods of sample collection and processing, identical for both years, were described in detail in Seda et al. (2007b). At each sampling date, we collected zooplankton by vertical hauls of plankton nets (mesh size 170 μm) from three sampling stations along the reservoir main axis, which covered the longitudinal environmental gradients: upstream near the river inflow; in the centre of the reservoir; and at the deepest part of the reservoir near the dam (figures 1a and 3). Sampling at the downstream site also focused on the vertical spatial distribution in the stratified water body, therefore we collected separately zooplankton from the epi-, meta- and hypolimnion, using a closing net for deeper layers. Prior to sampling, we measured vertical profiles of temperature and dissolved oxygen to evaluate the extent of stratification and determine the borders of the epi-, meta- and hypolimnion (figure 1b). From each site/layer, we collected a quantitative sample preserved in 4% formaldehyde solution for the analysis of zooplankton abundance (to estimate the density of all Daphnia in the respective samples, regardless of their taxon composition) and an additional sample deep-frozen on site in liquid nitrogen for genetic analyses (to identify proportions of parental species and hybrid genotypes within the D. longispina complex).
Figure 3.
Spatial distribution of parental species and hybrids along horizontal and vertical gradients within the Vranov Reservoir in summer 2004 and 2005. Pie charts show the relative abundance of the taxa at each sampling station (absolute Daphnia abundances differed among sites; see also Seda et al. 2007b).
We genetically analysed approximately 40–50 randomly selected adult Daphnia females (if available) from each deep-frozen sample. Before processing, each individual was digitally photographed under the microscope for subsequent measuring of its carapace size, and the number of eggs/embryos in its brood chamber was counted.
We compared the results of two different molecular marker systems available for the discrimination of species and hybrids within the complex. Primarily, we used allozyme electrophoresis on cellulose acetate gels (Hebert & Beaton 1989), which has been successfully applied in a number of studies. Two allozyme loci were scored: sAAT, supernatant amino aspartate transferase (EC 2.6.1.1); AO, aldehyde oxidase (EC 1.2.3.1). It was shown that sAAT and AO can be used as diagnostic markers to discriminate among D. galeata, D. longispina (named D. hyalina in the respective papers) and D. cucullata, and to identify their hybrids (Wolf & Mort 1986; Gießler 1997a). Daphnia galeata is virtually fixed for the F (fast) and D. longispina for S (slow) alleles at both loci; D. cucullata is fixed for the S− (very slow) allele at sAAT. The alleged potential for the discrimination of all species using the locus AO (Gießler 1997a), however, could not be fully used. Apparently, at least in some cases, D. cucullata AO alleles may not be reliably differentiated from those of other species. In samples from the Czech Republic, bands of D. cucullata often overlapped with those of D. galeata; similarly, in a North German lake, D. cucullata could not be well differentiated at AO from D. longispina (Spaak et al. 2004).
Using the allozyme markers, we classified Daphnia individuals as belonging to parental species (carrying alleles typical of one species only) or as genotypes of hybrid origin (sharing alleles of two different species). Some individuals sharing alleles of D. galeata and D. longispina showed patterns clearly suggesting backcrossing or the formation of later-generation hybrids. However, as the relative frequencies of such genotypes were usually low, and two markers do not allow for reliable assignment to a particular hybrid class, we pooled them with apparent F1 hybrids for the purpose of this study.
Additionally, we used a DNA-based method—the restriction fragment length polymorphism (RFLP) of the nuclear ribosomal internal transcribed spacer (ITS)—to validate allozyme results on a subset of samples from 2004 (1265 individuals from 9 out of 11 studied reservoirs; table 1). This method, originally developed by Billiones et al. (2004), uses the restriction of a short part of ITS1, the 5.8S ribosomal RNA gene, and a large part of ITS2, to obtain species-specific fragment patterns that are additive in hybrid genotypes. The original ITS-RFLP protocol commonly suffered from a point mutation in D. galeata alleles, which caused their misidentification as belonging to D. cucullata; we therefore applied a newly developed alternative double-digest protocol (Skage et al. 2007) to circumvent this problem.
To allow direct comparison of allozyme and ITS-RFLP patterns, DNA was prepared from Daphnia homogenates used for the allozyme electrophoresis. A 2.5 μl aliquot of the homogenate was transferred to 30 μl of a solution containing H3 buffer and proteinase K (Schwenk et al. 1998) and incubated at 55°C for 6–10 hours; proteinase was subsequently inactivated by heating to 95°C for 10 min. The subsequent DNA amplification and restriction by overnight incubation with endonucleases MbiI and Eco52I (Fermentas) mostly followed the protocol by Skage et al. (2007). However, we used an alternative forward primer, ITS-F-New (5′-GGT AAC CGC TGA ACC TCC TTC-3′; Skage et al. 2007), which provides longer amplified fragments to reliably differentiate between D. galeata pattern and potentially uncut PCR products. The banding patterns were interpreted according to Skage et al. (2007); individuals combining fragments from two species were identified as hybrids even if bands of one species were more intense than those of another species. Occasional very weak bands were nevertheless not considered. Based on the fit between the two marker systems (see §3) and the morphology of studied individuals, allozyme data were further used for evaluating the distribution of species and hybrids in our study.
(c) Statistical analyses
Multivariate analyses of Daphnia spatial distribution were performed with the software package Canoco for Windows v. 4.5 (ter Braak & Šmilauer 2002). Original counts (number of individuals identified as respective species or hybrids) were log transformed, and standardization by sample norm was used to focus the analyses on the differences in the relative proportion of individual taxa. To summarize occurrence patterns of Daphnia taxa visually, principal component analysis (PCA) was used. To test for differences in the community composition along the horizontal profiles, partial redundancy analysis (RDA), using reservoir identity as a covariate, was performed. We pooled the data from the epi-, meta- and hypolimnion at the downstream sampling station for the analysis of horizontal distribution, weighing taxon abundances from different layers by the respective layer volume. The significance of the relationship between species composition and spatial gradient was tested using a model-based type of a Monte Carlo permutation test (ter Braak & Šmilauer 2002).
The contribution of individual factors (unique effects of locality, of longitudinal or vertical position and their interaction) was quantified using three different partial RDA models (Lepš & Šmilauer 2003). In two analyses, one of the two factors was used as the explanatory variable and the other as a covariate; in the third analysis, the interaction term was tested. The partial analyses were needed also for the two main effects due to the unbalanced nature of the sampling design.
Differences among individual taxa in the carapace size and fecundity of adult females were tested using a nested-design ANOVA model. This model included, besides the effect of taxon, also the fixed effect of habitat (epi-, hypo-, metalimnion, inflow and middle) and the random effects of reservoir identity and sampling year (nested within reservoir identity). The size of carapace and fecundity of adult females were log transformed to achieve homogeneity of variances.
Owing to the partially hierarchical nature of the ANOVA model, there was no straightforward way to perform multiple comparisons allowing the testing of differences among individual taxa. In addition, only some differences were of interest, as not all taxon pairs co-occurred in the same samples, nor did they all hybridize. Therefore, we tested the differences among D. cucullata, D. galeata and their hybrids and among D. longispina, D. galeata and their hybrids separately, by performing six pairwise comparisons followed by Holm's correction for simultaneous tests (Holm 1979).
3. Results
(a) Allozymes and ITS-RFLP: marker selection
Results of the two different nuclear marker sets used to identify taxa (ITS rDNA region and allozymes) were largely in agreement (85% of the 1265 individuals analysed by both methods), although not corresponding to each other completely. In most reservoirs, the choice of marker would not substantially affect the patterns of spatial distribution of parental species and hybrids. Disagreements in identification between allozyme and ITS-RFLP patterns were almost always observed for individuals from sites where species in question co-occurred and hybridization was common. A notable exception was the Stanovice Reservoir, in which only D. galeata was detected by allozymes in 2004 but 19% of the individuals showed ITS-RFLP patterns characteristic for D. galeata×longispina hybrids. Morphological characteristics of most of these individuals agreed with the allozyme identification, which was subsequently confirmed by the detailed analysis of 12 microsatellite loci classifying all analysed individuals as D. galeata (Š. Ruthová, A. Petrusek, J. Seda, A. Thielsch, N. Brede & K. Schwenk 2008, unpublished data). This prompted us to use allozyme markers for primary taxon identification, although occasional differences between the two marker systems indicated that a certain proportion of introgressed individuals remained undetected and the extent of hybridization may have been underestimated.
(b) Patterns of taxon coexistence and their temporal stability
All the three parental Daphnia species (D. galeata, D. longispina and D. cucullata) occurred commonly in the investigated set of reservoirs (table 1), and all three actually co-occurred in three of them. Out of the three potential interspecific hybrids, however, we recorded only two; hybrids D. cucullata×longispina were never observed. Daphnia galeata×longispina strongly dominated over D. galeata×cucullata in number of individuals as well as localities occupied (656 over 51 individuals out of 4464 analysed; seven versus four localities). In three reservoirs with the presence of hybrid genotypes, we did not record one of their parental species (table 1). In one of these, the Horka Reservoir, we recorded only D. longispina and hybrid genotypes with D. galeata; the allozyme patterns (sAAT heterozygous and AO homozygous for the D. longispina marker), however, suggested that these individuals did not represent the F1 hybrid generation but later-generation hybrids or backcrosses with D. longispina.
The taxon composition of the D. longispina complex significantly differed among localities (RDA, p=0.001, pseudo-F-statistic 10.832). The results of the PCA of the same data (figure 2) show that the 11 sampled reservoirs can be divided into three groups based on the presence of particular Daphnia species and hybrids. Four reservoirs in the upper half of the ordination diagram are characterized by high proportions of D. cucullata in at least some samples (typically upstream). The isolated position of the Horka Reservoir at the right edge reflects the fact that D. galeata was completely absent from this locality (the only case among the investigated reservoirs), and only D. longispina and hybrid or backcrossed genotypes were present. The remaining six reservoirs contained a substantial proportion of D. galeata, with D. cucullata being rare or absent. The positions of sampling sites within reservoirs in figure 2 (grey squares) indicates the general tendency of D. cucullata and its hybrids to occur more at upstream sites, and the opposite tendency of D. longispina and its hybrids. The overall Daphnia taxon composition within reservoirs was very similar in 2004 and 2005 samples (table 1; figure 2). However, we observed a sharp decline in the presence of D. galeata×cucullata hybrids, which were not recorded in 2005 in three out of four reservoirs in which they had been present a year before.
Figure 2.
PCA of the Daphnia taxonomic composition in 11 analysed reservoirs, based on the data from the three sampling stations along the longitudinal reservoir axes from both 2004 and 2005 (data from different depth layers at the dam were combined together). Black triangles, reservoirs; grey squares, sampling stations; open circles, different seasons. Taxa of the D. longispina complex are indicated by arrows. The first two principal axes explain 82% of the total variation.
To compare the extent of variability in the Daphnia taxon composition along both the horizontal and vertical gradients with the variation among localities, we decomposed the total compositional variance in an ANOVA style, using partial RDA methods (table 2). In both the cases, differences in the species composition among localities explained about two-thirds of the overall variation. The differences in the Daphnia taxon composition along the longitudinal and vertical directions explained, respectively, 23 and 16% of the variability remaining after correction for differences among reservoirs; the effects of position along the gradient as well as the interactions ‘sampling station–locality’ were always statistically significant. The residual variance, which includes the effect of season, was substantially lower for the longitudinal gradient, showing that the horizontal differentiation in the taxon composition was more stable than the vertical structure at the dam.
Table 2.
Hierarchical decomposition of the variance in the taxon composition within the D. longispina complex along longitudinal and vertical gradients in the reservoirs. (Numbers in parentheses give percentages after correcting for the variation in the community composition among reservoirs.)
| effect | longitudinal gradient (upstream, middle and dam) | vertical gradient (epi-, meta- and hypolimnion) | ||||
|---|---|---|---|---|---|---|
| % variability | d.f. | p | % variability | d.f. | p | |
| locality | 67.1 | 10 | 0.001 | 64.2 | 10 | 0.001 |
| position along the gradient | 7.6 (23.1) | 2 | 0.001 | 5.6 (15.6) | 2 | 0.003 |
| interaction of locality–position | 16.2 (49.2) | 20 | 0.002 | 14.2 (39.7) | 20 | 0.030 |
| residuals (including the effect of year) | 9.1 (27.7) | 33 | — | 16.0 (44.7) | 33 | — |
Apart from summarizing variability over all taxa and localities, we tested whether individual taxa exhibit significant differences in their relative abundance along both the gradients. In the longitudinal direction, the strongest response was observed for D. galeata×longispina hybrids, occurring significantly more often in the downstream locations (F=9.859, p<0.001); other two significantly responding taxa were D. longispina (F=5.496, p=0.008) and D. cucullata (F=4.803, p=0.013), the former apparently preferring the downstream and the latter the upstream sites. In reservoirs where both of these two parental species were found, they only very rarely co-occurred at the same sampling sites; if so, either one or both of them were in minor proportion (less than 5% of all Daphnia in the sample). Out of the three taxa commonly occurring at the downstream locations (D. galeata, D. longispina and their hybrids), a significant response to vertical differentiation was found for both the parental species: D. galeata typically dominating in the epilimnion and D. longispina being more common in the deeper layers (F=12.284, p<0.001 and F=7.405, p=0.002, respectively).
(c) Hybridization in the Vranov Reservoir
The Vranov Reservoir, 18 km long, up to 45 m deep, and with a theoretical retention time of 134 days, is the second longest locality in our dataset, and is the only one in which we found all the three parental species and the two hybrids. The patterns observed at this locality may be used as a characteristic example of the effect of both longitudinal and vertical environmental gradients on the Daphnia taxon composition, and on the body size and fecundity of the respective taxa. The distribution pattern of all taxa in the two seasons, shown in figure 3, illustrates not only the differing characteristics of taxon spatial distribution but also the temporal dynamics of such patterns. In 2004, the interspecific hybrids occupied an intermediate position between their parental species. However, we observed remarkable changes in the occurrence of hybrids between the two seasons. On the one hand, D. galeata×cucullata, which was common in the upstream and especially central part of the reservoir in 2004 (being actually the most common taxon at the middle sampling station), completely disappeared in 2005; on the other hand, the relative abundance of D. galeata×longispina increased in the second year.
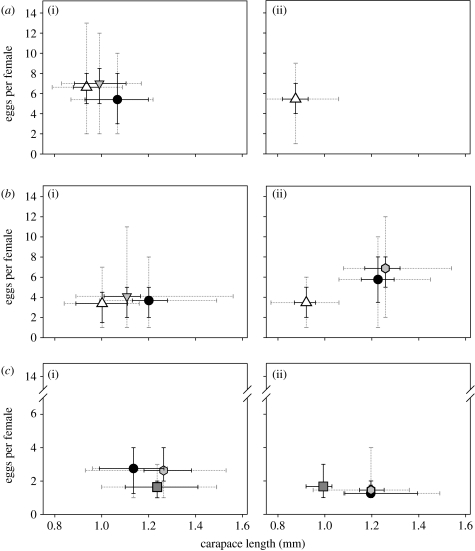
The patterns in Daphnia size and fecundity along the horizontal axis of the Vranov Reservoir (figure 4) reflected the environmental gradients. We observed a gradual decrease in the carapace size and increase in Daphnia brood size towards the upstream region, this pattern being consistent in both years; both the sampling station and the taxon had significant effects on size and fecundity (ANOVA, p<10−5 in all cases). The D. galeata×cucullata hybrids were intermediate and significantly differed from their parental taxa in size (adjusted p<0.002) but not in fecundity. The relationships among D. galeata×longispina hybrids and their parental species were different. The size of the three taxa did not differ significantly, and the patterns of fecundity varied depending on the sampling station and date (figure 4). However, in overall comparison, coexisting D. galeata and D. longispina did not differ in brood size, and hybrids actually had slightly but significantly higher fecundity (adjusted p<0.05) than either of the parental taxa.
Figure 4.
Relationship between carapace length and fecundity (egg number) of coexisting species and hybrids of the D. longispina complex in the Vranov Reservoir along its longitudinal gradient ((a) upstream, (b) middle and (c) dam: (i) 2004 and (ii) 2005). Taxa for which at least five individuals were genetically identified in the respective sample are included; data from both seasons are compared. Symbols, mean values (up triangle, D. cucullata; down triangle, D. galeata×cucullata; circle, D. galeata; hexagon, D. galeata×longispina; square, D. longispina); solid black lines, interquartile ranges; grey dotted lines, minimal and maximal values. Note that the y-axis for the dam samples differs from the rest.
(d) Taxon-specific differences in carapace size and fecundity
To check whether such patterns of body size and fecundity are consistent in other localities as well, we analysed differences in carapace and brood size between coexisting and spatially overlapping taxa within the two hybridizing species pairs (D. galeata–D. cucullata and D. galeata–D. longispina), using data from all samples (4544 individuals in total). A comparison of animals from the same sampling sites and dates demonstrated highly significant effects of taxon identity on carapace size (ANOVA, p<10−15) as well as on fecundity (p<10−13).
We observed highly significant differences in each pairwise comparison among D. cucullata, D. galeata and their hybrid: the carapace size increased from D. cucullata to hybrid and to D. galeata, with the corresponding adjusted p-values of 2.64×10−8 and 6.83×10−6, respectively. However, the differences in fecundity of these taxa were not significant. In the second hybridizing pair, the general patterns were different from those recorded in the Vranov Reservoir: the carapace size of D. galeata was significantly larger than in coexisting D. longispina or their hybrids (adjusted p<10−4); the difference between D. longispina and D. galeata×longispina hybrids was not significant (adjusted p=0.12). The fecundity of the three taxa followed the same trend: D. galeata had substantially more eggs/embryos than the two other taxa (adjusted p<2.4×10−4); the difference between D. longispina and D. galeata×longispina hybrids was non-significant (adjusted p=0.10).
4. Discussion
The taxon distribution patterns on longitudinal profiles observed in the studied reservoirs in 2004 (Seda et al. 2007b) remained relatively stable over two consecutive seasons, showing that spatially differentiated Daphnia communities re-formed after the winter bottleneck. Hybrids mostly coexisted with the parental taxa, suggesting that they are formed locally within reservoirs, as has been shown for lakes (Spaak 1997). This is also in agreement with the common presence of hybrid eggs in resting egg banks of the studied localities (I. Vaníčková, J. Seda & A. Petrusek 2007, unpublished data). In reservoirs where all the three parental species coexisted, differences in selection forces along horizontal gradients resulted in the almost complete spatial separation of two potentially hybridizing parental species (D. cucullata and D. longispina). This segregation, acting as an ecological reproductive barrier, was the most likely cause of the absence of D. cucullata×longispina hybrids in our samples. In general, the coexistence of these two species, which prefer opposite ends of predation and trophic gradients, is less likely than the coexistence of either of them with D. galeata. This may explain the relative scarcity or absence of D. cucullata×longispina hybrids in many lakes (e.g. Wolf & Mort 1986; Hebert et al. 1989b; Spaak 1997), although locally they may be more common (Gießler 1997b).
The numerical dominance of D. galeata×longispina over D. galeata×cucullata in our samples, however, is unlikely to be explained by the different degrees of coexistence of the two taxa. Both the parental species pairs co-occur, and while species of the former show different preferences on the vertical profile (figure 3; see also Seda et al. 2007b), this certainly does not prevent successful hybridization; on the contrary, D. galeata×longispina hybrids were common and apparently successful in reservoirs. Hybridization between D. galeata and D. cucullata is common as well; in a preliminary analysis of resting egg banks from those reservoirs where all the three parental species coexisted (I. Vaníčková, J. Seda & A. Petrusek 2007, unpublished data), hybrids with D. cucullata were actually found more frequently than those with D. longispina, and D. galeata×cucullata hybrid eggs were common in the resting egg bank of the Kníničky Reservoir, where such hybrids were apparently absent from the active population.
A reduced hatching success of hybrids (Schwenk et al. 2001; Keller et al. 2007) combined with the competition with parental species may partly explain this absence of hybrids in the water column. However, our long-term data on the clonal composition from the Římov Reservoir (J. Seda, J. Macháček, A. Petrusek & K. Schwenk 2007, unpublished data), as well as the temporal pattern of taxon dominance in the Vranov Reservoir (figure 3), suggest another factor contributing to the scarcity of D. galeata×cucullata hybrids in our samples: apparently, they are less likely to overwinter than D. galeata×longispina, and new hybrid genotypes have to be recruited from resting egg banks. The higher sensitivity of D. galeata×cucullata to winter conditions may be due to their smaller size and therefore lower efficiency under low food supply in winter, or because shallower parts of reservoirs, where they occur more often, provide a less suitable environment in winter and the bottlenecks in Daphnia populations are much more severe there than in the deeper parts (J. Seda, J. Macháček, A. Petrusek & K. Schwenk 2007, unpublished data). By contrast, at least some successful D. galeata×longispina clones probably survive in the deep lacustrine parts of reservoirs and contribute to the next season's community; the long-term survival of such hybrid genotypes has been documented in various European lakes (e.g. Spaak & Hoekstra 1993; Jankowski & Straile 2004). However, the apparently strong decline of D. galeata×cucullata hybrids in 2005 may have also been due to within-season fluctuations of taxon abundance (e.g. Keller & Spaak 2004), which would not be detected by our single summer sampling, or due to their distribution being restricted to the non-sampled regions of the studied localities.
It is important to note that the presence of substantial gradients in the Daphnia taxon composition did not depend on the reservoir flushing rate, although we would not expect them to develop in localities with very fast water flow. Gradients corresponding to species' ecological requirements were observed in reservoirs with a short retention time (average value below one month, e.g. Kníničky), in those with intermediate values (four to six months, e.g. Vranov and Vír) as well as in one with a retention time well over 1 year (Želivka; see table 1). Of all factors affecting the spatial heterogeneity of the Daphnia taxonomic composition, we presume the most crucial one to be the intensity of size-selective fish predation and different susceptibilities of different taxa to it (Spaak & Hoekstra 1997; Spaak & Boersma 2006). Fish predation pressure physically limits the size of Daphnia occurring in different parts of the water body, and only when the predator pressure is relaxed under a certain threshold does interspecific competition among larger taxa come into play. Fishes are preferentially present in the upstream reservoir regions (Vašek et al. 2004); this fact is reflected in the small size of Daphnia present there (figure 4). The tendencies in longitudinal arrangement of the taxa from the hybridizing pair D. galeata–cucullata agree well with significant differences in carapace size of the respective taxa, favouring the smallest Daphnia upstream.
The dominance of larger Daphnia further downstream corresponds with their higher filtering efficiency (Gliwicz 2003), small D. cucullata or its hybrids being probably outcompeted by larger taxa. Predation pressure may contribute not only to the absence of D. longispina and its hybrids in the upstream regions of eutrophic reservoirs but also to the vertical distribution of these taxa at the dam. Although the differences in size of coexisting taxa are rather low, hardly giving one taxon a selective advantage against predators, species and hybrids differ in predator susceptibility due to their differing vertical distribution in the stratified water column. Additionally, differences in life-history traits (Macháček & Seda 2007, 2008) certainly contribute to changes in the community and clonal composition; the responses to food quality and quantity nevertheless highly depend on individual genotypes even within taxa (Seidendorf et al. 2007). Fecundity of at least some hybrid clones may certainly exceed their coexisting parental species (figure 4); nevertheless, the brood size variation seems to be locality dependent—in Vranov, D. galeata×longispina hybrids exhibited higher fecundity than parental taxa, but, overall, such a pattern was not observed. This might be due to different genetic backgrounds of hybrids in different reservoirs, or due to their response to temporally or spatially varying environmental conditions (Spaak & Hoekstra 1995).
The existence of longitudinal environmental gradients in reservoirs may improve conditions for the presence of hybrids. Characteristics of interspecific hybrids, such as the intermediate size of D. galeata×cucullata, may promote their intermediate spatial distribution in comparison with the parental species in the way observed in the Vranov Reservoir. The presence of a clear hybrid-dominated zone on the horizontal axis of this reservoir suggests that hybrid dominance may not only be a temporal phenomenon, but also have a specific spatial aspect. We hypothesize that somewhere along reservoir axes, an environment favouring intermediate phenotypes may exist over most of the growing season. Areas of hybrid dominance would certainly be limited to only a certain section of the reservoir longitudinal profile, spatially more restricted than regions where parental taxa possibly coexist. Additionally, we presume that such zones may shift along the reservoir axis, depending on the stability of environmental gradients and the flow rate in the reservoir. Three sampling stations in our project only very crudely covered the longitudinal heterogeneity in reservoirs, so it is possible that denser sampling would reveal more areas where the relative abundance of hybrid genotypes exceed that of the parental species.
Hybrid zones in plankton, if developed, are definitely less stable and a much rarer phenomenon than those in the terrestrial environment. The three-dimensional nature of the pelagic environment allowing relatively free movement of zooplankters and passive dispersal due to wind-induced mixing of water masses and water currents tend to disrupt heterogeneities in the community composition necessary for forming such zones. For taxa entering diapause during their life cycle, such as cladocerans, the process is further complicated by the dispersal of the dormant stages (ephippia in Daphnia), which may be deposited in other parts of the water body than where they are formed; hybrid genotypes may therefore be hatching in conditions substantially different from those where the distribution of their parental species overlap. However, our observations suggest that despite all mixing, strong environmental gradients in reservoirs may exert selection pressures sufficient for hybrid zone formation.
The long-term hybrid presence in reservoirs may have important consequences on the gene flow among parental species during the induction of sexual reproduction, as zones of hybrid dominance may increase the likelihood of the production of later-generation hybrid or backcrossed genotypes. It is not unlikely that the reservoir environment, which offers suitable conditions to species adapted to strikingly different environments, is also suitable for introgression among species to a higher degree than in other, more homogeneous lakes. Although our data are not detailed enough to analyse the extent of introgression, it is worth noting that the disagreement between ITS-RFLP and the allozyme pattern was more often found for individuals from localities with more coexisting taxa, and that the fit between the identification obtained from ITS-RFLP and from other markers was lower in our studied Czech reservoirs than in other localities such as mountain or boreal lakes (Skage et al. 2007).
Patterns of introgression and maintenance of polymorphism of the ITS rDNA region are nevertheless complicated by the multi-copy character of this marker, and its concerted evolution through gene conversion (Arnheim 1983). At least in the Stanovice Reservoir, for which detailed microsatellite data were available (Š. Ruthová, A. Petrusek, J. Seda, A. Thielsch, N. Brede & K. Schwenk 2008, unpublished data), the apparently common ‘hybrid’ patterns detected by ITS-RFLP could not be due to contemporary local hybridization. In this case, the alleged longispina-specific ITS allele must had been introgressed into D. galeata genotypes earlier, and probably persisted in a certain proportion of ITS copies in the genome. Apparently, a detailed comparison of different marker systems, and the use of a wide array of polymorphic markers, preferably microsatellites (Brede et al. 2006), would be needed to reveal the fine-scale patterns of local introgression processes.
Acknowledgments
This study conformed to all relevant legislation of the Czech Republic.
Our study of reservoir Daphnia was funded by the Czech Science Foundation (project 206/04/0190), Grant Agencies of the Charles University (GAUK 114807) and of the Academy of Sciences of the Czech Republic (AVOZ 60170517), and the Czech Ministry of Education (MSM0021620828 and MSM6007665801). Kateřina Kolářová and Barbora Horová helped during allozyme analyses and Ivana Vaníčková provided results from resting egg banks. Comments from Derek Taylor and one anonymous reviewer helped to improve the manuscript, and David Hardekopf made language corrections. We appreciate also the support of State Water Authority managers during sampling.
Footnotes
One contribution of 16 to a Theme Issue ‘Hybridization in animals: extent, processes and evolutionary impact’.
References
- Arnheim N. Concerted evolution of multigene families. In: Nei M, Koehen R.K, editors. Evolution of genes and proteins. Sinauer; Sunderland, MA: 1983. pp. 38–61. [Google Scholar]
- Arnold M.L. Oxford University Press; New York, NY: 1997. Natural hybridization and evolution. [Google Scholar]
- Billiones R, Brehm M, Klee J, Schwenk K. Genetic identification of Hyalodaphnia species and interspecific hybrids. Hydrobiologia. 2004;526:43–53. doi:10.1023/B:HYDR.0000041615.65087.06 [Google Scholar]
- Brede N, Thielsch A, Sandrock C, Spaak P, Keller B, Streit B, Schwenk K. Microsatellite markers for European Daphnia. Mol. Ecol. Notes. 2006;6:536–539. doi:10.1111/j.1471-8286.2005.01218.x [Google Scholar]
- Declerck S, De Meester L. Impact of fish predation on coexisting Daphnia taxa: a partial test of the temporal hybrid superiority hypothesis. Hydrobiologia. 2003;500:83–94. doi:10.1023/A:1024656602248 [Google Scholar]
- Dufresne F, Hebert P.D.N. Hybridization and origins of polyploidy. Proc. R. Soc. B. 1994;258:141–146. doi:10.1098/rspb.1994.0154 [Google Scholar]
- Flößner D. Backhuys; Leiden, Germany: 2000. Die Haplopoda und Cladocera (ohne Bosminidae) Mitteleuropas. [Google Scholar]
- Gießler S. Analysis of reticulate relationships within the Daphnia longispina species complex. Allozyme phenotype and morphology. J. Evol. Biol. 1997a;10:87–105. doi:10.1046/j.1420-9101.1997.10010087.x [Google Scholar]
- Gießler S. Gene flow in the Daphnia longispina hybrid complex (Crustacea, Cladocera) inhabiting large lakes. Heredity. 1997b;79:231–241. [Google Scholar]
- Gliwicz Z.M. Food thresholds and body size in cladocerans. Nature. 1990;343:638–640. doi:10.1038/343638a0 [Google Scholar]
- Gliwicz Z.M. Between hazards of starvation and risk of predation: the ecology of offshore animals. In: Kinne O, editor. Excellence in ecology. Book 12. International Ecology Institute; Oldendorf/Luhe, Germany: 2003. [Google Scholar]
- Hebert P.D.N, Beaton M. Helena Laboratories; Beaumont, TX: 1989. Methodologies for allozyme analysis using cellulose acetate electrophoresis—a practical handbook. [Google Scholar]
- Hebert P.D.N, Finston T.L. A taxonomic reevaluation of North American Daphnia (Crustacea, Cladocera). 2. New species in the Daphnia pulex group from the South-Central United States and Mexico. Can. J. Zool. 1996;74:632–653. [Google Scholar]
- Hebert P.D.N, Beaton M.J, Schwartz S.S, Stanton D.J. Polyphyletic origins of asexuality in Daphnia pulex. I. Breeding-system variation and levels of clonal diversity. Evolution. 1989a;43:1004–1015. doi: 10.1111/j.1558-5646.1989.tb02546.x. doi:10.2307/2409581 [DOI] [PubMed] [Google Scholar]
- Hebert P.D.N, Schwartz S.S, Hrbáček J. Patterns of genotypic diversity in Czechoslovakian Daphnia. Heredity. 1989b;62:207–216. doi:10.1038/hdy.1989.30 [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Jankowski T, Straile D. A comparison of egg-bank and long-term plankton dynamics of two Daphnia species, D. hyalina and D. galeata: potentials and limits of reconstruction. Limnol. Oceanogr. 2003;48:1948–1955. [Google Scholar]
- Jankowski T, Straile D. Allochronic differentiation among Daphnia species, hybrids and backcrosses: the importance of sexual reproduction for population dynamics and genetic architecture. J. Evol. Biol. 2004;17:312–321. doi:10.1046/j.1420-9101.2003.00666.x [PubMed] [Google Scholar]
- Keller, B. 2007 Extent of hybridisation and reproductive isolation in Daphnia Doctoral thesis, Swiss Federal Institute of Technology, Zurich.
- Keller B, Spaak P. Nonrandom sexual reproduction and diapausing egg production in a Daphnia hybrid species complex. Limnol. Oceanogr. 2004;49:1393–1400. [Google Scholar]
- Keller B, Wolinska J, Tellenbach C, Spaak P. Reproductive isolation keeps hybridizing Daphnia species distinct. Limnol. Oceanogr. 2007;52:984–991. [Google Scholar]
- King C.E, Miracle M.R. Diel vertical migration by Daphnia longispina in a Spanish lake: genetic sources of distributional variation. Limnol. Oceanogr. 1995;40:226–231. [Google Scholar]
- Lepš J, Šmilauer P. Cambridge University Press; Cambridge, UK: 2003. Multivariate analysis of ecological data using Canoco. [Google Scholar]
- Macháček J, Seda J. Life history response of Daphnia galeata to heterogeneous conditions within a reservoir as determined in a cross-designed laboratory experiment. Aquat. Ecol. 2007;41:55–66. doi:10.1007/s10452-006-9045-3 [Google Scholar]
- Macháček J, Seda J. Diversity of Daphnia galeata life history traits in a vertically structured environment. J. Plankton Res. 2008;30:221–231. doi:10.1093/plankt/fbm100 [Google Scholar]
- Petrusek, A., Hobæk, A., Nilssen, J. P., Skage, M., Černý, M., Brede, N. & Schwenk, K. 2008 A taxonomic reappraisal of the European Daphnia longispina complex (Crustacea, Cladocera, Anomopoda). Zool. Scr (doi:10.1111/j.1463-6409.2008.00336.x)
- Repka S, Veselá S, Weber A, Schwenk K. Plasticity in filtering screens of Daphnia cucullata×galeata hybrids and parental species at two food concentrations. Oecologia. 1999;120:485–491. doi: 10.1007/s004420050881. doi:10.1007/s004420050881 [DOI] [PubMed] [Google Scholar]
- Schwenk K, Spaak P. Evolutionary and ecological consequences of interspecific hybridization in cladocerans. Experientia. 1995;51:465–481. doi:10.1007/BF02143199 [Google Scholar]
- Schwenk K, Sand A, Boersma M, Brehm M, Mader E, Offerhaus D, Spaak P. Genetic markers, genealogies and biogeographic patterns in the cladocera. Aquat. Ecol. 1998;32:37–51. doi:10.1023/A:1009939901198 [Google Scholar]
- Schwenk K, Bijl M, Menken S.B.J. Experimental interspecific hybridization in Daphnia. Hydrobiologia. 2001;442:67–73. doi:10.1023/A:1017594325506 [Google Scholar]
- Seda J, Kolářová K, Petrusek A, Macháček J. Daphnia galeata in the deep hypolimnion: spatial differentiation of a “typical epilimnetic” species. Hydrobiologia. 2007a;594:47–57. doi:10.1007/s10750-007-9075-4 [Google Scholar]
- Seda J, Petrusek A, Macháček J, Šmilauer P. Spatial distribution of the Daphnia longispina species complex and other planktonic crustaceans in the heterogeneous environment of canyon-shaped reservoirs. J. Plankton Res. 2007b;29:619–628. doi:10.1093/plankt/fbm044 [Google Scholar]
- Seidendorf B, Boersma M, Schwenk K. Evolutionary stoichiometry: the role of food quality for clonal differentiation and hybrid maintenance in a Daphnia species complex. Limnol. Oceanogr. 2007;52:385–394. [Google Scholar]
- Skage M, Hobæk A, Ruthová Š, Keller B, Petrusek A, Seda J, Spaak P. Intra-specific rDNA-ITS restriction site variation and an improved protocol to distinguish species and hybrids in the Daphnia longispina complex. Hydrobiologia. 2007;594:19–32. doi:10.1007/s10750-007-9090-5 [Google Scholar]
- Spaak P. Hybridization in the Daphnia galeata complex: are hybrids locally produced? Hydrobiologia. 1997;360:127–133. doi:10.1023/A:1003157117667 [Google Scholar]
- Spaak P, Boersma M. Predator mediated coexistence of hybrid and parental Daphnia taxa. Arch. Hydrobiol. 2006;167:55–76. doi:10.1127/0003-9136/2006/0167-0055 [Google Scholar]
- Spaak P, Hoekstra J.R. Clonal structure of the Daphnia population in Lake Maarsseveen: its implications for diel vertical migration. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1993;39:157–165. [Google Scholar]
- Spaak P, Hoekstra J.R. Life-history variation and the coexistence of a Daphnia hybrid with its parental species. Ecology. 1995;76:553–564. doi:10.2307/1941213 [Google Scholar]
- Spaak P, Hoekstra J.R. Fish predation on a Daphnia hybrid species complex: a factor explaining species coexistence? Limnol. Oceanogr. 1997;42:753–762. [Google Scholar]
- Spaak P, Denk A, Boersma M, Weider L.J. Spatial and temporal patterns of sexual reproduction in a hybrid Daphnia species complex. J. Plankton Res. 2004;26:625–635. doi:10.1093/plankt/fbh064 [Google Scholar]
- ter Braak, C. J. F. & Šmilauer, P. 2002 Canocoreference manual andCanoDrawfor Windows user's guide: software for canonical community ordination, version 4.5. Ithaca, NY: Microcomputer Power.
- Urabe J. Stable horizontal variation in the zooplankton community structure of a reservoir maintained by predation and competition. Limnol. Oceanogr. 1990;35:1703–1717. [Google Scholar]
- Vašek M, Kubečka J, Peterka J, Čech M, Draštík V, Hladík M, Prchalová M, Frouzová J. Longitudinal and vertical spatial gradients in the distribution of fish within a canyon-shaped reservoir. Int. Rev. Hydrobiol. 2004;89:352–362. doi:10.1002/iroh.200410734 [Google Scholar]
- Weider L.J, Stich H.B. Spatial and temporal heterogeneity of Daphnia in Lake Constance; intra- and interspecific comparisons. Limnol. Oceanogr. 1992;37:1327–1334. [Google Scholar]
- Wolf H.G, Mort M.A. Inter-specific hybridization underlies phenotypic variability in Daphnia populations. Oecologia. 1986;68:507–511. doi: 10.1007/BF00378763. doi:10.1007/BF00378763 [DOI] [PubMed] [Google Scholar]
- Wolinska J, Bittner K, Ebert D, Spaak P. The coexistence of hybrid and parental Daphnia: the role of parasites. Proc. R. Soc. B. 2006;273:1977–1983. doi: 10.1098/rspb.2006.3523. doi:10.1098/rspb.2006.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinska J, Keller B, Manca M, Spaak P. Parasite survey of a Daphnia hybrid complex: host-specificity and environment determine infection. J. Anim. Ecol. 2007;76:191–200. doi: 10.1111/j.1365-2656.2006.01177.x. doi:10.1111/j.1365-2656.2006.01177.x [DOI] [PubMed] [Google Scholar]