Abstract
Clonal reproduction in vertebrates can always be traced back to hybridization events as all known unisexual vertebrates are hybrids between recognized species or genetically defined races. Interestingly, clonal vertebrates often also rely on interspecific matings for their reproduction because gynogenesis (sperm-dependent parthenogenesis) and hybridogenesis are common modes of propagation. While in most cases these hybridization events leave no hereditary traces in the offspring, occasionally the genome exclusion mechanism fails and either small parts of male genetic material remain inside the oocyte in the form of microchromosomes, or fusion of the sperm nucleus with the oocyte nucleus leads to polyploid individuals. In this review, we highlight the important role of hybridization for the origin and evolution of a unisexual hybrid: the Amazon molly, Poecilia formosa.
Keywords: Amazon molly, paternal introgression, microchromosome, B-chromosome, polyploidy, hybridization
1. Introduction
Hybridization is defined as the successful mating of individuals from two populations or groups of populations that are distinguishable on the basis of one or more heritable traits (Harrison 1993; Arnold 1997; Dowling & Secor 1997). Based on this definition, 40–70% of all allopolyploid plant species (Stace 1987) are believed to be of hybrid decent and a substantial proportion of animal species hybridizes (e.g. 9% of birds worldwide; Grant & Grant 1992). While botanists have already long ago acknowledged the important role of hybridization and DNA introgression (Arnold 1997), hybridization in animals was often assumed to be a reproductive mistake (Mayr 1963; Mallet 2005). This assessment may be due to the fact that hybridization in plants is thought to be much more common than that in animals (Mable 2004; Mallet 2005) and that in animals hybrids are often less fit than their parental species (Templeton 1981; Burke & Arnold 2001). Also, animal species groups differ considerably in hybridization rates. For instance, in passion flower butterflies 26% of the 73 species are known to hybridize with at least one other species, while in European mammals (200 species) the hybridization rate is approximately 6% (Mallet 2005).
Even though in animals the probability for an individual to mate with a heterospecific is low, the proportion of species that occasionally hybridize is high. This opens up the possibility that, by adding genetic variation and functional novelty, hybridization might be an important factor in the process of speciation in animals as well (Seehausen 2004). On the other hand, the combination of two different genotypes poses problems, most evidently for meiosis, because pairing of homologues will be more difficult to accomplish when the two parental genomes are more divergent. In plants, hybridization is often correlated with polyploidization (Chapman & Burke 2007). Plants show high tolerance against polyploidization probably because often they are capable of asexual reproduction. Asexual reproduction could be one reason explaining why successful hybridizations occur at a higher rate in plants than in animals (Schultz 1969; Mable 2004). Of course, the problem arises only if distantly related species hybridize, closely related species should not encounter problems during gamete formation.
Mammals and birds are particularly sensitive to polyploidy. Even partial aberrations from the regular diploid cell stage usually result in severe developmental defects (FitzPatrick et al. 2002) and are an important factor in cancer formation (Krämer & Ho 2001). Fishes, amphibians and reptiles on the other hand cope very well with polyploidy (Pandian & Koteeswaran 1998). In this group polyploidy is considered an important driving force in evolution as it increases the genetic material on which mutation and selection can act (Ohno 1970).
Interestingly, in many teleost fish species, hybrids can easily be produced between closely related species and may well occur under natural conditions (Salzburger et al. 2002). In fact, hybridization is reported frequently in many species of teleosts (Salzburger et al. 2002; Meyer et al. 2006) even though some fish species show elaborate mating behaviour and internal fertilization—two prezygotic isolation mechanisms that are, in general, assumed to prevent hybridization in other animals (Howard & Berlocher 1998; Mallet 1998; Mabry & Verrell 2004). The ready acceptance of foreign genomes in some species may be correlated to the fish's tolerance towards polyploidy (Dowling & DeMarais 1993; Leggatt & Iwama 2003).
Among fishes, amphibians and reptiles, the unisexual ‘species’ are special cases. Studying these exceptions to the ‘rule of sex’ can deepen our understanding of the origins and reasons for this rule (Vrijenhoek 1989). Unisexual vertebrates have, therefore, become model systems to study the evolution and benefits of sex. They are all derived from hybridization either between species or between populations or chromosomal races (Dawley 1989; Vrijenhoek et al. 1989). In hybrids, meiosis is often altered (Lampert et al. 2007), which in unisexual vertebrates leads to either the production of unreduced eggs (parthenogenesis, gynogenesis) or the transmission of only one particular set of chromosomes (hybridogenesis). Unisexual organisms reproduce clonally without recombination and should therefore be prone to the accumulation of deleterious mutations and limited in their ability to evolve (Muller 1932; Leslie & Vrijenhoek 1978; Spinella & Vrijenhoek 1982; Kondrashov 1988). They are often assessed as evolutionary ‘dead ends’ (Bell 1982). Many species, however, are rather abundant, show large ranges of geographical distribution and have shown to be evolutionary older than predicted from theoretical models (Quattro et al. 1992; Schartl et al. 1995b; Kearney et al. 2006).
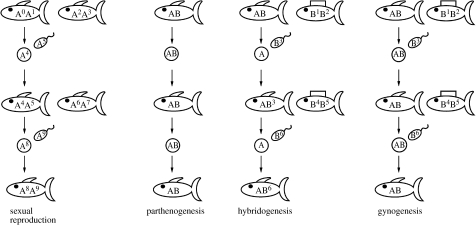
Reproductive modes vary from parthenogenesis in lizards to gynogenesis and hybridogenesis in teleost fish and amphibians (figure 1; Dawley 1989). In hybridogenesis, meiosis reduces the diploid oocyte to a haploid stage, selectively discarding the genetic material inherited from the father. Therefore, only maternal genetic material is transferred to the next generation. The paternal genetic material from the sperm complements the egg only for a single generation and is lost during the next cycle of meiosis (hemiclonal reproduction; Vrijenhoek et al. 1977). In gynogenesis, females produce unreduced eggs but need sperm from a closely related sexual species to trigger the onset of embryonic development. The sperm, however, does not fuse with the oocyte nucleus and the paternal genetic material does not contribute to the embryo (Hubbs & Hubbs 1932; Schartl et al. 1990; Turner et al. 1990). In very rare cases, however, this exclusion mechanism fails. Genetic material from the father is included into the oocyte nucleus and transferred to the following generations (paternal introgression; Nanda et al. 2007).
Figure 1.
Reproductive modes. Capital letters depict genomes—different letters indicate different species. Numbers (0–9) differentiate individual genomes derived from recombination. In sexual reproduction, both mating partners produce haploid germ cells that are unique due to recombination and result in highly variable offspring. In parthenogenesis, females produce unreduced diploid oocytes that develop into all female offspring that is genetically identical to its mother. In hybridogenesis, the female genome passes through generations unchanged while the male genome is exchanged every generation (hemiclonal). In gynogenesis or sperm-dependent parthenogenesis, females produce unreduced oocytes that develop into all female offspring. They need, however, sperm from a closely related sexual species to trigger the onset of embryonic development. The male genetic material does not contribute to the offspring’ genotypes. Reproduction in gynogenesis is truly clonal.
Both forms of unisexual reproduction in fishes require mating with males from closely related sexual species. Unisexual fish species, therefore, owe their origin to hybridization and in addition are in constant need of hybrid matings to ensure reproduction. Additional genetic material in unisexuals, derived from paternal introgression events, is the only trace of the constant hybrid matings and might possibly ensure the reproductive success of gynogenetic fishes (Schartl et al. 1995a).
In this review, we will summarize the available information on the initial hybridization process that led to the formation of the gynogenetic Amazon molly, Poecilia formosa. We will present the current knowledge about paternal introgressions in this species and its evolutionary impact and consequences. We suggest that hybridization leading to paternal introgression might be the key to understanding the evolutionary success and longevity of the Amazon molly and other unisexual vertebrates.
We will use the Amazon molly, P. formosa, as a model organism because it is among the most exhaustively studied unisexual vertebrates and an extensive amount of information about its hybrid origin as well as paternal introgression has been gathered.
2. Interspecific hybridization leading to the origin of the Amazon molly
The hybrid origin of P. formosa had been recognized very early after it was found to reproduce clonally. Hubbs & Hubbs (1932) realized that morphologically P. formosa is an almost exact intermediate between one species of the long-fin mollies, the sailfin molly (Poecilia latipinna), on one side and the short-fin mollies (then grouped all in one species: Poecilia sphenops) on the other side. In the P. sphenops species complex, geographical evidence from species distributions more specifically pointed to Poecilia mexicana as the most likely species involved in the hybridization event (Rezneat & Abramoff 1968). Mitochondrial DNA showed that P. mexicana was the female parent of P. formosa (Avise et al. 1991). Sequence analyses of nuclear genes and a phylogenetic analysis suggested a subspecies of P. mexicana, Poecilia mexicana limantouri to be the maternal ancestor of P. formosa (Turner et al. 1980a; Schartl et al. 1995b). Molecular evidence for the hybrid origin of P. formosa was also found with serum samples (Abramoff et al. 1968) and later confirmed by allozyme data (Turner et al. 1980b). Several more protein, allozyme and, more recently, neutral marker studies on P. formosa demonstrated molecular patterns characteristic for F1 hybrids and their fixed heterozygous state (Turner 1982; Tiedemann et al. 2005; Lampert et al. 2006).
Hybrid origin and a fixed heterozygous state have been found in many other unisexual vertebrates, e.g. the Squalius alburnoides complex (Alves et al. 2001), the Poeciliopsis complex (Schultz 1969), the Rana complex (Berger 1973) and the parthenogenetic Lacerta complex (Uzzell & Darevsky 1975; for a complete list see Dawley & Bogart (1989) and references therein), and have been attributed to the clonal reproduction mode in these animals. The fact, however, that the fixed heterozygous state is found in all successful clonally reproducing vertebrates suggests to most researchers that it might be evolutionarily advantageous. This idea will be discussed in more detail below.
(a) Evolutionary ecology of P. formosa
As with all gynogenetic species, P. formosa depends on the sperm of its host species and can therefore not occur outside their geographical range. It is sympatric with P. latipinna in its northernmost range and with P. m. limantouri in the northern part of Mexico. In the southernmost habitats, it is found together with Poecilia mexicana mexicana (Darnell & Abramoff 1968; Schlupp et al. 2002). The expansion of P. formosa within the hosts' habitats seems to be limited by physical barriers, most importantly marine currents and mountain ranges (Schlupp et al. 2002).
The co-occurrence of P. formosa with its closely related sexual parental species poses an evolutionary problem. Unisexual hybrids have the obvious advantage of not having to produce males. Therefore, newly arising all-female lineages should, all other things being equal, rapidly replace their bisexual ancestors and non-hybrid host species (Maynard Smith 1978). A total replacement of the parental or host species on a broad geographical scale, however, has never been observed (Vrijenhoek 1989). Several mechanisms might ensure the co-occurrence of unisexual species and their sexual ancestors. One hypothesis suggested by Vrijenhoek (1989) is niche partitioning. Since unisexual and sexual species should not be able to persist in the same ecological niche, he postulated that microhabitat selection might actually lead to ecological separation allowing a coexistence of sexual and asexual lineages (Schenck & Vrijenhoek 1989). Following his argument clonal lineages are well adapted to a rather narrow ecological niche, allowing them to compete with the sexual individuals that show a broader niche range. Sexuals should be more flexible in ecological niche use but due to the constant new combination of alleles produced with each new generation, they are expected to be less well adapted to specific conditions (Vrijenhoek 1994). So far, there are no empirical data available for P. formosa that can support this hypothesis. According to Schlupp and co-workers, P. formosa females form mixed shoals with the host species fish (Schlupp et al. 1991; Schlupp & Ryan 1996; as they do in Poeciliopsis; Schenck & Vrijenhoek 1989). However, unisexuals and sexuals may differ in food preferences or predator avoidance strategies, but this has yet to be tested.
Another possibility allowing the co-occurrence of unisexual species and their sexual ancestors is mate choice favouring non-hybrid genotypes (Coyne 1992). Mate choice is generally considered to be a common mechanism to prevent hybridization (Wirtz 1999). It is relevant for unisexual fishes because males are needed for gynogenetic and hybridogenetic reproductions, and access to mates therefore controls the reproductive success in unisexual fishes despite them being clonal (Moore & McKay 1971; Moore 1975). A male mating preference for conspecific females has been shown in both parental species of P. formosa (Schlupp et al. 1991; Schlupp & Ryan 1996). The incapability of unisexual fishes to take over mixed species populations may therefore be attributed to a fitness disadvantage due to lower female mating success and could be mediated by frequency-dependent selection, as P. formosa is unable to persist without the possibility to obtain sperm from a closely related species.
The coexistence of the unisexual hybrid females with sexual females of the pure host species can be regarded as a balanced polymorphism, where trade-offs and selective advantages over evolutionary times guarantee that both forms are present in constant proportions (Smith et al. 1993; Schlupp et al. 1994; Losey et al. 1997). Hybrid unisexuals have evolved behavioural adaptations and chemical cues to seduce host males and enforce copulations. The importance of chemical cues for attracting mates has been shown for P. formosa (Schlupp et al. 1991). Trade-offs such as an increase in parasite susceptibility in clonal species have been shown in Poeciliopsis (Lively et al. 1990; Leberg & Vrijenhoek 1994) and Cnemidophorus (Moritz et al. 1991), but have not been documented for P. formosa (Tobler & Schlupp 2005).
(b) Age of hybrid species
Despite the general assumption that unisexual reproduction in vertebrates is a dead end (Bell 1982) and that clonal species are short lived and of rather recent origin, several unisexual species have been found to be rather old. The parthenogenetic grasshopper Warramaba virgo was estimated to be ca 300 000 years old (Kearney et al. 2006). In unisexual fishes (Poeciliopsis complex), mtDNA variability was high indicating a rather ancient origin (Quattro et al. 1992). Similar results were found in parthenogenetic geckos (Moritz & Heideman 1993; Kearney et al. 2006). Recent analyses that included information from mitochondrial as well as nuclear DNA suggested that the initial hybridization event leading to the formation of P. formosa happened ca 280 000 years ago (Schartl et al. 1995b; Meyer et al. 2006). Estimating three generations per year (Hubbs & Hubbs 1932), this equals approximately 840 000 generations. It is therefore much older than expected from theoretical considerations that postulate clonal vertebrates to be short lived and predict a species lifespan of a maximum of a few thousand generations due to mutational meltdown (Lynch & Gabriel 1990). The evident success of this clonally reproducing species could, at least in part, be ascribed to the positive effects of occasional introgression of new genetic material (Schartl et al. 1995a; see below).
(c) Evolutionary consequences of hybrid unisexual reproduction
In the discussion about the advantages and disadvantages of unisexual reproduction, the presumably higher number of female offspring is often referred to as the main advantage of unisexual reproduction. The accumulation of deleterious mutations and the lack of genotypic variability of the offspring are mentioned as the main disadvantages of unisexual reproduction (Maynard Smith 1978, 1986). The latter two disadvantages have caused the general assumption that unisexual vertebrates are evolutionary dead ends and doomed to an early extinction (Bell 1982; Lynch 1984; Maynard Smith 1986). Interestingly, this view is somewhat congruent with the general valuation of the role of hybridization in the evolution of animals. Since unisexual vertebrates are hybrids, the negative perception of hybridization in animals may have contributed to the, in general, low confidence in the evolutionary potential of unisexual vertebrates. Hybrids, however, often show heterosis. Heterosis or hybrid vigour is defined as an increased performance of heterozygous offspring relative to its homozygous parents. It is best described and investigated in corn (Comings & MacMurray 2000; Springer & Stupar 2007) but is also documented in the natural populations of other plants (Hansson & Westerberg 2002) and animals (Hotz et al. 1999). The molecular basis of heterosis still remains unclear but two main hypotheses have survived the elaborate discussion of the topic (Springer & Stupar 2007). Firstly, dominance models assume that each homozygous line contains slightly deleterious mutations. A combination of two genomes with a different mutational history will result in a compensation of the deleterious alleles and benefit the hybrid. Secondly, the overdominance model on the other hand suggests that the combination of different alleles at a locus is superior to either parental homozygous combination of alleles therefore rendering an overall superior hybrid.
Unisexual vertebrates show a ‘frozen’ heterozygosity due to their extraordinary modes of reproduction, which means that the heterozygosity is maintained for as long as the clone is reproducing instead of being lost after the F1 generation. The potential benefit from this ‘conserved’ heterozygosity may (at least partially) outweigh the disadvantages of clonal reproduction. The advantage of hybrid vigour has been shown in hybridogenetic frogs (Hotz et al. 1999) but was so far never considered in studies about the gynogenetic Amazon molly.
3. Hybridization leading to the introgression of paternal genetic material
Paternal introgression, the inclusion of paternal DNA into the genome of otherwise clonally reproducing organisms, are the only genetic traces of the constant hybrid matings needed for the persistence of gynogenetic animals. Paternal introgression is of special interest to evolutionary biologists because it might be advantageous to the clonal species by adding to their genotypic variability and by buffering the organism from deleterious mutations (Schartl et al. 1995a; Archetti 2004). Organisms that cannot perform recombination should suffer from genetic decay, because deleterious mutations cannot be purged. Owing to the low genotypic variability in the offspring, they should also show slower evolutionary rates (Muller 1932; Kondrashov 1988).
(a) Forms of paternal introgression
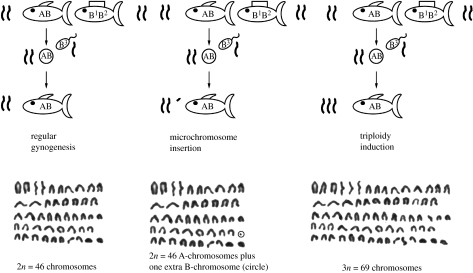
Two major forms of paternal introgression have been recognized: either small parts of the paternal genome remain in the oocyte (microchromosomes; Schartl et al. 1995a; Lamatsch et al. 2004) or the whole paternal genome fertilizes the egg leading to polyploid individuals (Rasch & Balsano 1974). In P. formosa, both forms of paternal introgression occur (figure 2) and can even co-occur in the same individual (Lamatsch 2001).
Figure 2.
Forms of paternal introgression. Schematic and examples of resulting karyotypes. While during regular gynogenesis no genetic material from the father is included into the genome, in very rare cases the exclusion mechanism can fail either leaving small pieces of genetic material inside the oocyte (microchromosome indicated by asterisk) or fertilizing the diploid egg leading to triploid individuals.
B-chromosomes (Bs)—in contrast to A-chromosomes that constitute the species-specific regular set of chromosomes in an organism—are supernumerary chromosomes, which are inherited in a non-Mendelian way (Trivers et al. 2004). They can arise within the same genome from duplicated or fragmented A-chromosomes or can be acquired during a hybridization event from foreign DNA that evolves into the supernumerary chromosome (Jones & Rees 1982; Camacho et al. 2000). Because Bs usually lack a homologous partner to pair with during meiosis they are distributed unevenly among the gametes, and individuals within a population may differ in the number of Bs they carry. Even though clonal species are less likely to acquire Bs, once Bs are present, they should be stably transferred due to the lack of recombination.
B-chromosomes have traditionally been classified as selfish genetic elements that decrease the fitness of the ‘host’ genome (Shaw & Hewitt 1990; Camacho et al. 2000); however, there are a few examples where Bs are beneficial to their hosts (Bougourd & Jones 1997). In the Amazon molly, wild animals containing supernumerary B-chromosomes have been found quite frequently in the Río Purificación/Río Soto la Marina river system (Lamatsch et al. 2004) where the species co-occurs with wild-type pigmented P. mexicana and in the laboratory where the fish are bred with black molly, but not in other areas of the range of the Amazon molly (Schlupp 2005). The B-chromosomes of P. formosa are very small and classified as microchromosomes. In P. formosa microchromosomes are stably inherited for several generations (Lamatsch et al. 2004; Nanda et al. 2007), which suggests a neutral or even beneficial evolutionary effect, even though a positive effect has so far not been demonstrated. B-chromosomes have not yet been documented in other clonal vertebrates. Most probably, this is due to the fact that owing to their small size they are hard to detect. Bs may therefore have been overlooked in several species and may be much more frequent than generally assumed.
While B-chromosomes have only recently attracted the attention of researchers, polyploidy has been observed much earlier and more frequently. The main effect of polyploidization is that the amount of DNA per organism increases drastically. Consequently, genes become redundant and redundancy due to gene duplication can be advantageous (Comai 2005) mainly because redundant genes are available for the evolution of new functions or for subfunction partitioning (two gene duplicates dividing the ancestral gene's functions) or can compensate for defect or malfunctioning alleles (Taylor & Raes 2004). Polyploidy is therefore thought to have a high positive impact on evolution by increasing the potential for speciation as well as adaptation (Ohno 1970).
Triploid and tetraploid individuals are reported for several fish species (Vasil'ev et al. 1989; Futami et al. 2005; Moghadam et al. 2005) and polyploidization seems to have played an exceptionally large role during evolution and speciation processes in this group (Le Comber & Smith 2004; Volff 2005). In fishes, polyploidy is often associated with unisexual reproduction (Mogie 1986); however, it is not clear whether it is the cause or the consequence (Schultz 1969, 1980; Mable 2004). Interestingly, hybridization is also very common in fishes and a positive correlation of hybridization and polyploidy can be observed (Le Comber & Smith 2004).
The majority of P. formosa populations is composed of diploid individuals. Triploid Amazon mollies were first reported in laboratory breeding stocks (Rasch et al. 1965; Schultz & Kallman 1968) and soon afterwards were also found in natural populations of the Soto la Marina river system and in the Río Guayalejo (Balsano et al. 1972; Turner et al. 1983). Triploidization of diploid lineages occurs rather frequently in laboratory breeding stocks. Approximately one in a thousand births of diploid mothers results in a triploid offspring (Nanda et al. 1995; Lamatsch et al. 2004). These newly emerging triploid females are all sterile (Schultz & Kallman 1968; Nanda et al. 1995), while triploid clones from the field reproduce gynogenetically (Turner et al. 1983). In P. formosa, triploids most probably have arisen only two times during the evolutionary history of the species (Schories et al. 2007). They show an unexpectedly low genotypic diversity and are most probably of recent origin (Lampert et al. 2005). Triploids in other unisexual species show higher levels of genotypic variability leading to the assumption that they are derived from several introgression events (Alves et al. 2001; Janko et al. 2003). Triploid unisexuals are never as variable as diploids except for the Phoxinus eos-neogaeus system where triploids are genetically more variable than diploids (Goddard et al. 1989) most probably because they arise de novo in each generation (Angers & Schlosser 2007). In Poeciliopsis, evidence for several independent origins of triploids comes from allozyme and mtDNA data but sequence variation within the different triploid lineages is always low (Mateos & Vrijenhoek 2005).
A higher level of introgression is represented by the first tetraploid P. formosa reported so far (Lampert et al. 2008). It was found to be an allotetraploid most probably derived from the fertilization of a triploid egg. Even though tetraploids are very rare (one tetraploid in 672 measured triploids) and have not yet been observed in the field, they might still have a considerable impact on the evolutionary ecology of P. formosa. In other unisexual species, tetraploidy occurs frequently and contributes considerably to the complexity of the reproductive system (Vasil'ev et al. 1989; Alves et al. 1999). Schultz (1969) even speculated that allotetraploids in unisexual vertebrates might lead to the evolution of new sexual species (Schultz 1969).
(b) Evolutionary effects of paternal introgressions
The majority of unisexually reproducing vertebrates are polyploid even though their ancestral species are sexually reproducing diploids (Vrijenhoek et al. 1989; Alves et al. 2001). Several hypotheses have been put forward to explain this phenomenon: (i) polyploidization might interrupt meiosis, therefore creating asexual lineages, (ii) selection against polyploidy in asexual lineages may be weak, and (iii) it might be advantageous for the asexuals to be polyploid (Mogie 1986). Even though in P. formosa diploid lineages also reproduce gynogenetically, we cannot quite discard the first of these hypotheses since diploid P. formosa might have evolved from triploids that lost a genome (Vrijenhoek 1989).
Despite elaborate research of different aspects of paternal introgression, it remains unclear if the additional genetic material might be advantageous to the carrier. We found that under laboratory conditions triploids have a reproductive disadvantage compared with diploids. Diploids out-competed triploids in only a few generations (Lamatsch et al. submitted). In the field, however, triploids constitute a stable part (5–16%) of the mixed populations in the Río Purificación (Lamatsch 2001) as well as the Río Guayalejo (Schories et al. 2007). This may indicate that the reproductive disadvantage shown in the laboratory is compensated by some beneficial effect of triploidy.
We hypothesized that a frequent introgression of male genetic material might enhance genotypic variability in the triploids, therefore, counteracting the costs associated with common genotypes (Red Queen hypothesis; Van Valen 1973). A higher genotypic variability, however, could not be found in triploids. In a microsatellite study of several hundreds of field-caught individuals, it was found that the triploid clones in one river system have a much lower genotypic variability than the diploids from the same system and are most probably of monophyletic and recent origin (Lampert et al. 2005).
Even though no evolutionary advantage of paternal introgression could be found on the population level in P. formosa, individuals might still benefit from the additional genetic material. The buffer against the accumulation of deleterious mutations that is potentially provided by the additional genetic material may be sufficient to counteract the disadvantages of being triploid. Triploids could also have a key function in creating starting points for mutation and/or may be stepping stones on the way to tetraploids (Lampert et al. 2008).
4. Conclusions
Until recently, hybrids were perceived as reproductive errors with low adaptive survival and reproductive potential (Mallet 2005). Only the increase in the number of studies reporting on animal hybrids during the last 20 years has sparked more scientific interest, and the impact of hybridization on evolutionary processes, particularly on speciation, is now discussed more frequently (Seehausen 2004; Mallet 2005). Similar to hybrid animals in general, unisexual vertebrates were for a long time dismissed as evolutionary dead ends and being doomed to extinction. Populations of unisexual vertebrates, however, are quite successful in terms of their geographical distribution and individual numbers and have been shown to exist for evolutionary time scales much longer than expected from theoretical models (Quattro et al. 1992; Schartl et al. 1995b; Kearney et al. 2006). A common feature of all unisexual vertebrates is their hybrid origin and the constant hybridization needed to maintain the species. Hybridization clearly is a key factor for understanding these extraordinary systems as heterosis and paternal introgression may be the most important factors contributing to the unexpected long-term evolutionary success of unisexual vertebrates.
Acknowledgments
We thank Robert C. Vrijenhoek and two anonymous reviewers for their valuable comments that improved the manuscript. The support of the Deutsche Forschungsgemeinschaft (SFB 567 Mechanismen der Interspezifischen Interaktion von Organismen) for the P. formosa project is gratefully acknowledged.
Footnotes
One contribution of 16 to a Theme Issue ‘Hybridization in animals: extent, processes and evolutionary impact’.
References
- Abramoff P, Rezneat M.D, Balsano J.S. Electrophoretic demonstration of the hybrid origin of the gynogentic teleost Poecila formosa. Am. Nat. 1968;102:555–558. doi:10.1086/282567 [Google Scholar]
- Alves M.J, Coelho M.M, Próspero M.I, Collares-Pereira M.J. Production of fertile unreduced sperm by hybrid males of the Rutilus alburnoides complex (Teleostei Cyprinidae): an alternative route to genome tetraploidization in unisexuals. Genetics. 1999;151:277–283. doi: 10.1093/genetics/151.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M.J, Coelho M.M, Collares-Pereira M.J. Evolution in action through hybridization and polyploidy in an Iberian freshwater fish: a genetic review. Genetica. 2001;111:375–385. doi: 10.1023/a:1013783029921. doi:10.1023/A:1013783029921 [DOI] [PubMed] [Google Scholar]
- Angers B, Schlosser I.J. The origin of Phoxinus eos-neogaeus unisexual hybrids. Mol. Ecol. 2007;16:4562–4571. doi: 10.1111/j.1365-294X.2007.03511.x. doi:10.1111/j.1365-294X.2007.03511.x [DOI] [PubMed] [Google Scholar]
- Archetti M. Recombination and loss of complementation: a more than two-fold cost for parthenogenesis. J. Evol. Biol. 2004;17:1084–1097. doi: 10.1111/j.1420-9101.2004.00745.x. doi:10.1111/j.1420-9101.2004.00745.x [DOI] [PubMed] [Google Scholar]
- Arnold M.L. Oxford University Press; Oxford, UK: 1997. Natural hybridization and evolution. [Google Scholar]
- Avise J.C, Trexler J.C, Travis J, Nelson W.S. Poecilia mexicana is the recent female parent of the unisexual fish P. formosa. Evolution. 1991;46:1530–1533. doi: 10.1111/j.1558-5646.1991.tb02657.x. doi:10.2307/2409901 [DOI] [PubMed] [Google Scholar]
- Balsano J.S, Darnell R.M, Abramoff P. Electrophoretic evidence of triploidy associated with populations of the gynogenetic teleost Poecilia formosa. Copeia. 1972;1972:292–297. doi:10.2307/1442490 [Google Scholar]
- Bell G. University of California Press; Berkeley, CA: 1982. The masterpiece of nature: the evolution and genetics of sexuality. [Google Scholar]
- Berger L. Systematics and hybridization in European green frogs of Rana esculenta complex. J. Herpetol. 1973;7:1–10. doi:10.2307/1562822 [Google Scholar]
- Bougourd S.M, Jones R.N. B chromosomes: a physiological enigma. New Phytol. 1997;137:43–54. doi:10.1046/j.1469-8137.1997.00823.x [Google Scholar]
- Burke J.M, Arnold M.L. Genetics and the fitness of hybrids. Annu. Rev. Genet. 2001;35:31–52. doi: 10.1146/annurev.genet.35.102401.085719. doi:10.1146/annurev.genet.35.102401.085719 [DOI] [PubMed] [Google Scholar]
- Camacho J.P.M, Sharbel T.F, Beukeboom L.W. B-chromosome evolution. Phil. Trans. R. Soc. B. 2000;355:163–178. doi: 10.1098/rstb.2000.0556. doi:10.1098/rstb.2000.0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman M.A, Burke J.M. Genetic divergence and hybrid speciation. Evolution. 2007;61:1773–1780. doi: 10.1111/j.1558-5646.2007.00134.x. doi:10.1111/j.1558-5646.2007.00134.x [DOI] [PubMed] [Google Scholar]
- Comai L. The advantages and disadvanatages of being polyploid. Nat. Rev. Genet. 2005;6:836–846. doi: 10.1038/nrg1711. doi:10.1038/nrg1711 [DOI] [PubMed] [Google Scholar]
- Comings D.E, MacMurray J.P. Molecular heterosis: a review. Mol. Genet. Metabol. 2000;71:19–31. doi: 10.1006/mgme.2000.3015. doi:10.1006/mgme.2000.3015 [DOI] [PubMed] [Google Scholar]
- Coyne J.A. Genetics and speciation. Nature. 1992;355:511–515. doi: 10.1038/355511a0. doi:10.1038/355511a0 [DOI] [PubMed] [Google Scholar]
- Darnell R.M, Abramoff P. Distribution of the gynogenetic fish, Poecilia formosa, with remarks on the evolution of the species. Copeia. 1968;1968:354–361. doi:10.2307/1441764 [Google Scholar]
- Dawley R.M. An introduction to unisexual vertebrates. In: Dawley R.M, Bogart J.B, editors. Evolution and ecology of unisexual vertebrates. New York State Museum; Albany, NY: 1989. pp. 1–18. [Google Scholar]
- Dawley R.M, Bogart J.B. New York State Museum; Albany, NY: 1989. Evolution and ecology of unisexual vertebrates. [Google Scholar]
- Dowling T.E, DeMarais B.D. Evolutionary significance of introgressive hybridization in cyprinid fishes. Nature. 1993;362:444–446. doi:10.1038/362444a0 [Google Scholar]
- Dowling T.E, Secor C.L. The role of hybridization and introgression in the diversification of animals. Annu. Rev. Ecol. Syst. 1997;28:593–619. doi:10.1146/annurev.ecolsys.28.1.593 [Google Scholar]
- FitzPatrick D.R, Ramsay J, McGill N.I, Shade M, Carothers A.D, Hastie N.D. Transcriptome analysis of human autosomal trisomy. Hum. Mol. Genet. 2002;11:3249–3256. doi: 10.1093/hmg/11.26.3249. doi:10.1093/hmg/11.26.3249 [DOI] [PubMed] [Google Scholar]
- Futami K, Zhang H, Okamato N. Functional divergence of duplicated c-myc genes in a tetraploid fish, the common carp (Cyprinus carpio) Gene. 2005;363:61–66. doi: 10.1016/j.gene.2005.06.041. doi:10.1016/j.gene.2005.06.041 [DOI] [PubMed] [Google Scholar]
- Goddard K.A, Dawley R.M, Dowling T.H. Origin and genetic relationships of diploid, triploid and diploid–triploid mosaic biotypes in the Phoxinus eos-neogaeus unisexual complex. In: Dawley R.M, Bogart J.B, editors. Evolution and ecology of unisexual vertebrates. New York State Museum; Albany, NY: 1989. pp. 268–280. [Google Scholar]
- Grant P.R, Grant B.R. Hybridization of bird species. Science. 1992;256:193–197. doi: 10.1126/science.256.5054.193. doi:10.1126/science.256.5054.193 [DOI] [PubMed] [Google Scholar]
- Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002;11:2467–2474. doi: 10.1046/j.1365-294x.2002.01644.x. doi:10.1046/j.1365-294X.2002.01644.x [DOI] [PubMed] [Google Scholar]
- Harrison R.G. Hybrids and hybrid zones: historical perspective. In: Harrison R.G, editor. Hybrid zones and the evolutionary process. Oxford University Press; Oxford, UK: 1993. pp. 3–12. [Google Scholar]
- Hotz H, Semlitsch R.D, Gutmann E, Guex G.-D, Beerli P. Spontaneous heterosis in larval life-history traits of hemiclonal frog hybrids. Proc. Natl Acad. Sci. USA. 1999;96:2171–2176. doi: 10.1073/pnas.96.5.2171. doi:10.1073/pnas.96.5.2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D.J, Berlocher S.H. Oxford University Press; New York, NY: 1998. Endless forms: species and speciation. [Google Scholar]
- Hubbs C.L, Hubbs L.C. Apparent parthenogenesis in nature, in a form of fish of hybrid origin. Science. 1932;76:628–630. doi: 10.1126/science.76.1983.628. doi:10.1126/science.76.1983.628 [DOI] [PubMed] [Google Scholar]
- Janko K, Kotlik P, Rab P. Evolutionary history of asexual hybrid loaches (Cobitis: Teleostei) inferred from phylogenetic analysis of mitochondrial DNA variation. J. Evol. Biol. 2003;16:1280–1287. doi: 10.1046/j.1420-9101.2003.00627.x. doi:10.1046/j.1420-9101.2003.00627.x [DOI] [PubMed] [Google Scholar]
- Jones R, Rees H. Academic Press; New York, NY: 1982. B-chromosomes. [Google Scholar]
- Kearney M, Blacket M.J, Strasburg J.L, Moritz C. Waves of parthenogenesis in the desert: evidence of the parallel loss of sex in a grasshopper and a gecko from Australia. Mol. Ecol. 2006;15:1743–1748. doi: 10.1111/j.1365-294X.2006.02898.x. doi:10.1111/j.1365-294X.2006.02898.x [DOI] [PubMed] [Google Scholar]
- Kondrashov A.S. Deleterious mutations and the evolution of sexual reproduction. Nature. 1988;336:435–440. doi: 10.1038/336435a0. doi:10.1038/336435a0 [DOI] [PubMed] [Google Scholar]
- Krämer A, Ho A.D. Centrosome aberrations and cancer. Onkologie. 2001;24:538–544. doi: 10.1159/000055141. doi:10.1159/000055141 [DOI] [PubMed] [Google Scholar]
- Lamatsch, D. K. 2001 Molekulargenetische und zytologische Untersuchungen zur paternalen Introgression beim gynogenetischen Amazonenkärpfling, Poecilia formosa PhD thesis, University of Wuerzburg.
- Lamatsch D.K, Nanda I, Schlupp I, Epplen J.T, Schmid M, Schartl M. Distribution and stability of supernumerary microchromosomes on natural populations of the Amazon molly, Poecilia formosa. Cytogenet. Genome Res. 2004;106:189–194. doi: 10.1159/000079286. doi:10.1159/000079286 [DOI] [PubMed] [Google Scholar]
- Lamatsch, D. K., Lampert, K. P., Fischer, P., Geiger, M., Schlupp, I. & Schartl, M. Submitted. Diploid Amazon mollies outcompete triploids in reproduction.
- Lampert K.P, Lamatsch D.K, Epplen J.T, Schartl M. Evidence for a monophyletic origin of triploid clones of the Amazon molly, Poecilia formosa. Evolution. 2005;59:881–889. doi:10.1111/j.0014-3820.2005.tb01761.x [PubMed] [Google Scholar]
- Lampert K.P, Lamatsch D.K, Schories S, Hopf A, Garcia de León F.J, Schartl M. Microsatellites for the gynogenetic Amazon molly, Poecilia formosa: useful tools for detection of mutation rate, ploidy determination and overall genetic diversity. J. Genet. 2006;1:67–71. doi: 10.1007/BF02728973. doi:10.1007/BF02728973 [DOI] [PubMed] [Google Scholar]
- Lampert K.P, Lamatsch D.K, Fischer P, Epplen J.T, Nanda I, Schmid M, Schartl M. Automictic reproduction in interspecific hybrids of poeciliid fish. Curr. Biol. 2007;17:1948–1953. doi: 10.1016/j.cub.2007.09.064. doi:10.1016/j.cub.2007.09.064 [DOI] [PubMed] [Google Scholar]
- Lampert K.P, Lamatsch D.K, Fischer P, Schartl M. A tetraploid Amazon molly, Poecilia formosa. J. Hered. 2008;99:223–226. doi: 10.1093/jhered/esm102. doi:10.1093/jhered/esm102 [DOI] [PubMed] [Google Scholar]
- Leberg P, Vrijenhoek R.C. Variation among desert topminnows in their susceptibility to attack by exotic parasites. Conserv. Biol. 1994;8:419–424. doi:10.1046/j.1523-1739.1994.08020419.x [Google Scholar]
- Le Comber S.C, Smith C. Polyploidy in fishes: patterns and processes. Biol. J. Linn. Soc. 2004;82:431–442. doi:10.1111/j.1095-8312.2004.00330.x [Google Scholar]
- Leggatt R.A, Iwama G.K. Occurrence of polyploid in the fishes. Rev. Fish Biol. Fish. 2003;13:237–246. doi:10.1023/B:RFBF.0000033049.00668.fe [Google Scholar]
- Leslie J.F, Vrijenhoek R.C. Genetic dissection of clonally inherited genomes of Poeciliopsis I. Linkage analysis and preliminary assessment of deleterious gene loads. Genetics. 1978;90:801–811. doi: 10.1093/genetics/90.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively C.M, Craddock C, Vrijenhoek R.C. The Red Queen hypothesis supported by parasitism in sexual and clonal fish. Nature. 1990;344:864–866. doi:10.1038/344864a0 [Google Scholar]
- Losey J.E, Ives A.R, Harmon J, Ballantyne F, Brown C. A polymorphism maintained by opposite patterns of parasitism and predation. Nature. 1997;388:269–272. doi:10.1038/40849 [Google Scholar]
- Lynch M. Destabilizing hybridization, general purpose genotypes and geographic parthenogenesis. Q. Rev. Biol. 1984;59:257–290. doi:10.1086/413902 [Google Scholar]
- Lynch M, Gabriel W. Mutation load and the survival of small populations. Evolution. 1990;44:1725–1737. doi: 10.1111/j.1558-5646.1990.tb05244.x. doi:10.2307/2409502 [DOI] [PubMed] [Google Scholar]
- Mable B.K. Why polyploidy is rarer in animals than in plants: myths and mechanisms. Biol. J. Linn. Soc. 2004;82:453–466. doi:10.1111/j.1095-8312.2004.00332.x [Google Scholar]
- Mabry M, Verrell P. Stifled sex in sympatry: patterns of sexual incompatibility among desmognathine salamanders. Biol. J. Linn. Soc. 2004;82:367–375. doi:10.1111/j.1095-8312.2004.00364.x [Google Scholar]
- Mallet J. Mimicry and warning colors at the boundary between races and species. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; Oxford, UK: 1998. pp. 390–403. [Google Scholar]
- Mallet J. Hybridization as an invasion of the genome. Trends Ecol. Evol. 2005;20:229–237. doi: 10.1016/j.tree.2005.02.010. doi:10.1016/j.tree.2005.02.010 [DOI] [PubMed] [Google Scholar]
- Mateos M, Vrijenhoek R.C. Independent origins of allotriploidy in the fish genus Poeciliopsis. J. Hered. 2005;96:32–39. doi: 10.1093/jhered/esi010. doi:10.1093/jhered/esi010 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, UK: 1978. The evolution of sex. [Google Scholar]
- Maynard Smith J. Contemplating life without sex. Nature. 1986;324:300–301. doi: 10.1038/324300a0. doi:10.1038/324300a0 [DOI] [PubMed] [Google Scholar]
- Mayr E. Harvard University Press; Cambridge, UK: 1963. Animal species and evolution. [Google Scholar]
- Meyer M.M, Salzburger W, Schartl M. Hybrid origin of a swordtail species (Teleostei: Xiphophorus clemenciae) driven by sexual selection. Mol. Ecol. 2006;15:721–730. doi: 10.1111/j.1365-294X.2006.02810.x. doi:10.1111/j.1365-294X.2006.02810.x [DOI] [PubMed] [Google Scholar]
- Moghadam H.K, Ferguson M.M, Danzmann R.G. Evidence for a Hox gene duplication in rainbow trout (Oncorhynchus mykiss): a tetraploid model species. J. Mol. Evol. 2005;61:804–818. doi: 10.1007/s00239-004-0230-5. doi:10.1007/s00239-004-0230-5 [DOI] [PubMed] [Google Scholar]
- Mogie M. On the relationship between asexual reproduction and polyploidy. J. Theor. Biol. 1986;122:493–498. doi:10.1016/S0022-5193(86)80189-8 [Google Scholar]
- Moore W.S. Stability of unisexual–bisexual populations of Poeciliopsis (Pisces: Poeciliidae) Ecology. 1975;56:791–808. doi:10.2307/1936292 [Google Scholar]
- Moore W.S, McKay F.E. Coexistence in unisexual–bisexual species complexes of Poeciliopsis (Pisces: Poeciliidae) Ecology. 1971;52:791–799. doi:10.2307/1936026 [Google Scholar]
- Moritz C, Heideman A. The origin and evolution of parthenogenesis in Heteronotia binoei (Gekkonidae): reciprocal origins and diverse mitochondrial DNA in western populations. Syst. Biol. 1993;42:293–306. doi:10.2307/2992465 [Google Scholar]
- Moritz C, McCallum H, Donnellan S, Roberts J.D. Parasite load in parthenogenetic and sexual lizards (Heteronotia binoei): support for the Red Queen hypothesis. Proc. R. Soc. B. 1991;224:145–149. doi:10.1098/rspb.1991.0063 [Google Scholar]
- Muller H.J. Some genetic aspects of sex. Am. Nat. 1932;66:118–138. doi:10.1086/280418 [Google Scholar]
- Nanda I, Schartl M, Feichtinger W, Schlupp I, Parzefall J, Schmid M. Chromosomal evidence for laboratory synthesis of a triploid hybrid between the gynogenetic teleost Poecilia formosa and its host species. J. Fish Biol. 1995;47:619–623. [Google Scholar]
- Nanda I, Schlupp I, Lamatsch D.K, Lampert K.P, Schmid M, Schartl M. Stable inheritance of host species-derived microchromosomes in the gynogenetic fish, Poecilia formosa. Genetics. 2007;177:917–926. doi: 10.1534/genetics.107.076893. doi:10.1534/genetics.107.076893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Verlag; New York, NY: 1970. Evolution by gene duplication. [Google Scholar]
- Pandian T.J, Koteeswaran R. Ploidy induction and sex control in fish. Hydrobiologia. 1998;384:167–243. doi:10.1023/A:1003332526659 [Google Scholar]
- Quattro J.M, Avise J.C, Vrijenhoek R.C. An ancient clonal lineage in the fish genus Poeciliopsis (Atheriniformes: Poeciliidae) Proc. Natl Acad. Sci. USA. 1992;89:348–352. doi: 10.1073/pnas.89.1.348. doi:10.1073/pnas.89.1.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch E.M, Balsano J.S. Biochemical and cytogenetic studies of Poecilia from eastern Mexico. II. Frequency, perpetuation and probable origin of triploid genomes in females associated with Poecilia formosa. Rev. Biol. Trop. 1974;21:351–381. [Google Scholar]
- Rasch E.M, Darnell R.M, Kallman K.D, Abramoff P. Cytophotometric evidence fo triploidy in hybrids of the gynogenetic fish, Poecilia formosa. J. Exp. Zool. 1965;160:155–169. doi: 10.1002/jez.1401600203. doi:10.1002/jez.1401600203 [DOI] [PubMed] [Google Scholar]
- Rezneat M.D, Abramoff P. Distribution of the gynogentic fish, Poecilia formasa, with remarks on the evolution of the species. Copeia. 1968;1968:354–361. doi:10.2307/1441764 [Google Scholar]
- Salzburger W, Baric S, Sturmbauer C. Speciation via introgressive hybridization in East African cichlids? Mol. Ecol. 2002;11:619–625. doi: 10.1046/j.0962-1083.2001.01438.x. doi:10.1046/j.0962-1083.2001.01438.x [DOI] [PubMed] [Google Scholar]
- Schartl M, Nanda I, Schlupp I, Schmid M, Epplen J.T. Genetic variation in the clonal vertebrate Poecilia formosa is limited to few truly hypervariable loci. Fingerprint News. 1990;2:22–24. [Google Scholar]
- Schartl M, Nanda I, Schlupp I, Wilde B, Epplen J.T, Schmid M, Parzefall J. Incorporation of subgenomic amounts of DNA as compensation for mutational load in a gynogenetic fish. Nature. 1995a;373:68–71. doi:10.1038/373068a0 [Google Scholar]
- Schartl M, Wilde B, Schlupp I, Parzefall J. Evolutionary origin of a parthenoform, the Amazon molly Poecilia formosa, on the basis of a molecular genealogy. Evolution. 1995b;49:827–835. doi: 10.1111/j.1558-5646.1995.tb02319.x. doi:10.2307/2410406 [DOI] [PubMed] [Google Scholar]
- Schenck R.A, Vrijenhoek R.C. Coexistence among sexual and asexual Poeciliopsis: foraging behavior and microhabitat selection. In: Dowley R.M, Bogart J.B, editors. Evolution and ecology of unisexual vertebrates. New York State Museum; Albany, NY: 1989. pp. 39–48. [Google Scholar]
- Schlupp I. The evolutionary ecology of gynogenesis. Annu. Rev. Ecol. Evol. Syst. 2005;36:399–417. doi:10.1146/annurev.ecolsys.36.102003.152629 [Google Scholar]
- Schlupp I, Ryan M.J. Mixed-species shoals and the maintenance of a sexual–asexual mating system in mollies. Anim. Behav. 1996;52:885–890. doi:10.1006/anbe.1996.0236 [Google Scholar]
- Schlupp I, Parzefall J, Schartl M. Male mate choice in mixed bisexual/unisexual breeding complexes of Poecilia (Teleostei: Poeciliidae) Ethology. 1991;88:215–222. [Google Scholar]
- Schlupp I, Marler C, Ryan M.J. Benefit to male sailfin mollies of mating with heterospecific females. Science. 1994;263:373–374. doi: 10.1126/science.8278809. doi:10.1126/science.8278809 [DOI] [PubMed] [Google Scholar]
- Schlupp I, Parzefall J, Schartl M. Biogeography of the Amazon molly, Poecilia formosa. J. Biogeogr. 2002;29:1–6. doi:10.1046/j.1365-2699.2002.00651.x [Google Scholar]
- Schories S, Lampert K.P, Lamatsch D.K, Garcia de León F.J, Schartl M. Evidence for an independent origin of the triploid P. formosa outside the Río Purificación river system. Front. Zool. 2007;4:13. doi: 10.1186/1742-9994-4-13. doi:10.1186/1742-9994-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R.J. Hybridization, unisexuality, and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am. Nat. 1969;103:606–619. doi:10.1086/282629 [Google Scholar]
- Schultz R.J. The role of polyploidy in the evolution of fishes. In: Lewis H.W, editor. Polyploidy: biological relevance. Plenum Publishing; New York, NY: 1980. pp. 313–339. [Google Scholar]
- Schultz R.J, Kallman K.D. Triploid hybrids between the all-female teleost Poecilia formosa and Poecilia sphenops. Nature. 1968;219:280–284. doi: 10.1038/219280a0. doi:10.1038/219280a0 [DOI] [PubMed] [Google Scholar]
- Seehausen O. Hybridization and adaptive radiation. Trends Ecol. Evol. 2004;19:198–207. doi: 10.1016/j.tree.2004.01.003. doi:10.1016/j.tree.2004.01.003 [DOI] [PubMed] [Google Scholar]
- Shaw M.W, Hewitt G.M. B chromosomes, selfish DNA and theoretical models: where next? Oxf. Surv. Evol. Biol. 1990;7:197–223. [Google Scholar]
- Smith D.A.S, Owen D.F, Gordon I.J, Owiny G.M. Polymorphism and evolution in the butterfly Danaus chrysippus (L.) (Lepidoptera: Danainae) Heredity. 1993;71:242–251. doi:10.1038/hdy.1993.132 [Google Scholar]
- Spinella D.G, Vrijenhoek R.C. Genetic dissection of clonally inherited genomes of Poeciliopsis: II. Investigation of a silent carboxylesterase allele. Genetics. 1982;100:279–285. doi: 10.1093/genetics/100.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer N.M, Stupar R.M. Allelic variation and hetrosis in maize: how do two halves make more than a whole? Genome Res. 2007;17:264–275. doi: 10.1101/gr.5347007. doi:10.1101/gr.5347007 [DOI] [PubMed] [Google Scholar]
- Stace C.A. Hybridization and the plant species. In: Urbanska K.M, editor. Differentiation patterns in higher plants. Academic Press; London, UK: 1987. pp. 115–127. [Google Scholar]
- Taylor J.S, Raes J. Duplication and divergence: the evolution of new genes and old ideas. Annu. Rev. Genet. 2004;38:615–643. doi: 10.1146/annurev.genet.38.072902.092831. doi:10.1146/annurev.genet.38.072902.092831 [DOI] [PubMed] [Google Scholar]
- Templeton A.R. Mechanisms of speciation—a population genetic approach. Annu. Rev. Ecol. Syst. 1981;12:23–48. doi:10.1146/annurev.es.12.110181.000323 [Google Scholar]
- Tiedemann R, Moll K, Paulus K.B, Schlupp I. New microsatellite loci confirm hybrid origin, parthenogenetic inheritance, and mitotic gene conversion in the gynogenetic Amazon molly (Poecilia formosa) Mol. Ecol. Notes. 2005;5:586–589. doi:10.1111/j.1471-8286.2005.00993.x [Google Scholar]
- Tobler M, Schlupp I. Parasites in sexual and asexual molly species of the genus Poecilia (Poeciliidae teleostei): a case for the Red Queen? Biol. Lett. 2005;1:166–168. doi: 10.1098/rsbl.2005.0305. doi:10.1098/rsbl.2005.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R, Burt A, Palestis B.G. B chromosomes and genome size in flowering plants. Genome. 2004;47:1–8. doi: 10.1139/g03-088. doi:10.1139/g03-088 [DOI] [PubMed] [Google Scholar]
- Turner B.J. The evolutionary genetics of a unisexual fish Poecilia formosa. In: Berigozzi C, editor. Mechanisms of Speciation. Alan R. Liss; New York, NY: 1982. pp. 265–305. [PubMed] [Google Scholar]
- Turner B.J, Brett B.-L.H, Miller R.R. Interspecific hybridization and the evolutionary origin of a gynogenetic fish, Poecilia formosa. Evolution. 1980a;34:917–922. doi: 10.1111/j.1558-5646.1980.tb04029.x. doi:10.2307/2407997 [DOI] [PubMed] [Google Scholar]
- Turner B.J, Brett B.-L.H, Rasch E.M, Balsano J.S. Evolutionary genetics of a gynogenetic fish, Poecilia formosa, The Amazon molly. Evolution. 1980b;34:246–258. doi: 10.1111/j.1558-5646.1980.tb04813.x. doi:10.2307/2407389 [DOI] [PubMed] [Google Scholar]
- Turner B.J, Balsano J.S, Monaco P.J, Rasch E.M. Conal diversity and evolutionary dynamics in a diploid–triploid breeding complex of unisexual fishes (Poecilia) Evolution. 1983;37:798–809. doi: 10.1111/j.1558-5646.1983.tb05601.x. doi:10.2307/2407920 [DOI] [PubMed] [Google Scholar]
- Turner B.J, Elder J.F, Laughlin T.F, Davis W.P. Genetic variation in clonal vertebrates detected by simple-sequence DNA fingerprinting. Proc. Natl Acad. Sci. USA. 1990;87:5653–5657. doi: 10.1073/pnas.87.15.5653. doi:10.1073/pnas.87.15.5653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzzell T, Darevsky I.S. Biochemical evidence for the hybrid origin of the parthenogenetic species of the Lacerta saxicola complex (Sauria lacertidae), with a discussion of some evolutionary and ecological implications. Copeia. 1975;1975:204–222. doi:10.2307/1442879 [Google Scholar]
- Van Valen L. A new evolutionary law. Evol. Theor. 1973;1:1–30. [Google Scholar]
- Vasil'ev V.P, Vasil'eva E.D, Osinov A.G. Evolution of a diploid–triploid–tetraploid complex in fishes of the genus Cobitis (Pisces, Cobitidae) In: Dawley R.M, Bogart J.B, editors. Evolution and ecology of unisexual vertebrates. New York State Museum; Albany, NY: 1989. [Google Scholar]
- Volff J.-N. Genome evolution and biodiversity in teleost fish. Heredity. 2005;94:280–294. doi: 10.1038/sj.hdy.6800635. doi:10.1038/sj.hdy.6800635 [DOI] [PubMed] [Google Scholar]
- Vrijenhoek R.C. Genetic and ecological constraints in the origins and establishment of unisexual vertebrates. In: Dawley R.M, Bogart J.B, editors. Evolution and ecology of unisexual vertebrates. New York State Museum; Albany, NY: 1989. pp. 24–31. [Google Scholar]
- Vrijenhoek R.C. Unisexual fish: model systems for studying ecology and evolution. Annu. Rev. Ecol. Syst. 1994;25:71–96. doi:10.1146/annurev.es.25.110194.000443 [Google Scholar]
- Vrijenhoek R.C, Angus R.A, Schultz R.J. Variation and heterozygosity in sexually vs. clonally reproducing populations of Poeciliopsis. Evolution. 1977;31:767–781. doi: 10.1111/j.1558-5646.1977.tb01069.x. doi:10.2307/2407438 [DOI] [PubMed] [Google Scholar]
- Vrijenhoek R.C, Dawley R.M, Cole C.J, Bogart J.B. A list of the known unisexual vertebrates. In: Dawley R.M, Bogart J.B, editors. Evolution and ecology of unisexual vertebrates. New York State Museum; Albany, NY: 1989. pp. 19–23. [Google Scholar]
- Wirtz P. Mother species–father species: unidirectional hybridization in animals with female choice. Anim. Behav. 1999;58:1–12. doi: 10.1006/anbe.1999.1144. doi:10.1006/anbe.1999.1144 [DOI] [PubMed] [Google Scholar]