Abstract
Polyploidy, hybridization and variation in mating systems are central issues for a deeper understanding of animal evolution. The Iberian species Squalius alburnoides represents an example combining all three phenomena. Previous studies showed that S. alburnoides populations are mainly composed of triploid and diploid hybrid forms (mainly females), and that the tetraploid forms are rare or absent. Both populations from the Douro drainage reveal a distinct scenario: tetraploid individuals represent 85.6–97.5% of the population, with no sex ratio bias observed. Based on the flow cytometry measurements of blood and spermatozoa cells, microsatellite loci and experimental crosses, we describe here, for the first time, two symmetric allotetraploid populations (CCAA) that resumed normal meiosis after undergoing intermediate processes of non-sexual reproduction to give rise to a new sexually reproducing polyploid species. Prezygotic (habitat selection and assortative mating) and postzygotic mechanisms (nonviable embryos) are responsible for the reproductive isolation from other forms of the S. alburnoides complex (e.g. CA, CAA). This example illustrates how hybrid polyploid complexes may lead to speciation.
Keywords: allopolyploid speciation, non-sexual reproduction, microsatellites, tetraploidization
1. Introduction
Several studies considered interspecific hybridization and polyploidization in animal species as key mechanisms for diversification and the origin of new evolutionary lineages (Schultz 1969; Dawley 1989; Arnold 1992; Dowling & Secor 1997; Otto & Whitton 2000; Allendorf et al. 2001; Barton 2001; Mallet 2007). Hybridization is an important event in many taxa, contributing to the adaptation in plants and animals and sometimes resulting in the formation of entirely new species (Baack & Rieseberg 2007; Chapman & Burke 2007). Combining the genomes of two genetically differentiated species may skew the sex ratio in hybrids towards females (Dawley 1989). Asexual complexes are frequently considered evolutionary dead ends, although some of them have existed for longer than initially expected (Hedges et al. 1992; Quattro et al. 1992; Spolsky et al. 1992; Schartl et al. 1995; Cunha et al. 2004). However these non-sexual taxa may constitute a critical intermediate step in the formation of even-ploid sexual lineages (Mable 2003, 2004). Among vertebrates, asexual forms seem to be restricted to the ‘lower’ vertebrates, for example, 80 asexual species complexes in fish (e.g. Poecilia formosa), amphibians (e.g. genus Ambystoma) and reptiles (e.g. genus Cnemidophorus). These taxa reproduce either sperm independently (parthenogenesis) or dependently (gynogenesis, hybridogenesis; Vrijenhoek et al. 1989; Alves et al. 2001 and references therein). Polyploidization events seem to play an important role in the evolution of some fish groups, for example, Cyprinidae (David et al. 2003), Cobitidae (Janko et al. 2003) and Salmonidae (Phillips & Ráb 2001). However, the relative contribution of sexual versus asexual reproduction in evolution is only starting to be clarified (Janko et al. 2005).
The evolutionary potential of an organism depends mainly on the levels and patterns of genetic diversity. The limited success of asexual species is the consequence of their inability to generate the necessary genetic variability to adapt to environmental changes or to compensate for deleterious mutations through heterozygosity (Vrijenhoek 1998). Nevertheless, the different reproductive modes exhibited by such complexes might represent the key attributes facilitating the formation of new sexually reproducing polyploid species (Schultz 1969; Mable 2003, 2004), a phenomenon that seems to be extremely rare in animals. To complete the speciation process, pre- or postzygotic isolation must occur (Baack & Rieseberg 2007). In consequence, the reproductively isolated hybrid lineages can escape close competition with their parental taxa (Chapman & Burke 2007). For instance, due to their hybrid origin, allopolyploids may exhibit a larger ecological differentiation from their parental species and might avoid intraspecific competition by the colonization of novel habitats (Baack 2005 and references therein). However, due to the lack of empirical examples, tetraploidization remains an evolutionary enigma.
Squalius alburnoides, a small endemic cyprinid fish inhabiting the rivers of the Iberian Peninsula, is among the most complex polyploid systems known among vertebrates. As such, it is an excellent model species for deciphering and understanding the evolutionary potential of tetraploidization. The complex arose as the result of interspecific hybridization between Squalius pyrenaicus females (P genome) and males of an unknown species closely related to Anaecypris hispanica (A genome; Alves et al. 1997; Carmona et al. 1997). Squalius alburnoides is not truly asexual, being involved in reproduction mechanisms where individuals of other species act as sperm donors (S. pyrenaicus in the southern river basins and Squalius carolitertii in the northern ones), reproducing without ‘normal’ meiosis, but frequently with recombination and meiotic reduction, although genomes may be clonally transmitted either by syngamy or karyogamy (Alves et al. 2001; Gromicho 2006). Previous studies have shown that S. alburnoides populations are mainly composed of triploid hybrids (mainly PAA and CAA) and diploid hybrids (PA or CA) and nuclear non-hybrid males (AA, absent in the northern river basins); tetraploid forms are scarce or absent (Collares-Pereira 1985; Carmona et al. 1997; Alves et al. 2001). The diploid hybrid females in southern populations transmit the intact genome to the egg, which develops by gynogenesis (less than 3% of the cases) or generates triploid progeny if fertilized (reviewed in Alves et al. 2001). In the Douro River, diploid and triploid hybrid females reproduce hybridogenetically, discarding the C genome (Carmona et al. 1997; figure 1a,b). Triploid hybrid females in southern Iberian Peninsula and Mondego populations produce haploid and, occasionally, diploid eggs by ‘meiotic hybridogenesis’ (Alves et al. 1998; Pala & Coelho 2005; figure 1b,c). Squalius alburnoides males are fertile and play an important role in the dynamics of the complex. Diploid S. alburnoides hybrid males produce unreduced fertile sperm, transmitting their intact hybrid genome to their offspring, whereas tetraploid S. alburnoides males produce reduced sperm by normal meiosis (Alves et al. 1999).
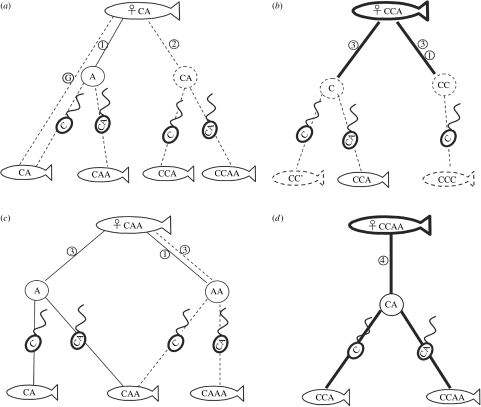
Figure 1.
Summary of the most probable modes of reproduction of S. alburnoides hybrid females found in northern regions of the Iberian Peninsula. (a) CA (diploid), (b) CCA (triploid), (c) CAA (triploid) and (d) CCAA (tetraploid). Fish forms and pathways (solid lines) previously described (Carmona et al. 1997; Pala & Coelho 2005) and hypothetical forms and pathways (dashed lines). Bold lines indicate crossing experiments. C is the genome of the parental host (S. carolitertii) and A is the genome of the ‘unknown’ ancestor. Dashed fish symbol with CC' indicates reconstituted S. carolitertii. Spermatozoid symbol with C indicates fertilization by C sperm; Spermatozoid symbol with CA indicates fertilization by CA sperm (produced by tetraploid males in our breeding experiments). G, gynogenesis; 1, hybridogenesis (observed exclusively in the Douro River); 2, unreduced eggs with high levels of syngamy; 3, meiotic hybridogenesis; and 4, normal meiosis.
Experimental crosses (Alves et al. 1999, 2004) have revealed two possible routes to tetraploidy in the S. alburnoides complex: one resulting from syngamy of diploid hybrid eggs with diploid clonal sperm from hybrid males and the other from syngamy of triploid eggs with reduced sperm.
To understand how even-ploid lineages in non-sexual complexes may be the stepping stones to biparental reproduction and speciation, we examined the genomic composition, genetic diversity and reproductive modes of two populations of S. alburnoides from the Douro basin, where the tetraploid form is predominant. We describe, for the first time, the existence of symmetric allotetraploid populations that resumed normal meiosis after intermediate processes of non-sexual reproduction to give rise to a new, sexually reproducing, polyploid species.
2. Material and methods
(a) Sample collection
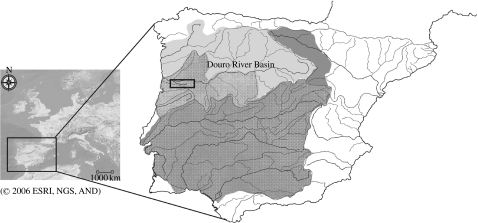
All samples were collected from two tributaries of the Douro basin (Lodeiro and Paiva Rivers) located on the northern distribution area of the S. alburnoides complex (figure 2). The tetraploids were found in this region during a population survey performed along the Douro River in the Iberian Peninsula from 2004 to 2005. Permission for sampling in this area was obtained from the Portuguese Ministry of Agriculture, Rural Development and Fisheries.
Figure 2.
Geographical distribution of the S. alburnoides complex. Map of the Iberian Peninsula indicating the collection area. The textured area represents the distributional range of the S. alburnoides complex; light grey indicates the distribution area of the northern S. carolitertii (sperm donor); dark grey indicates the distribution area of the southern S. pyrenaicus (sperm donor).
(b) Ploidy and sex determination
The ploidy of 230 individuals (193 S. alburnoides and 37 S. carolitertii) was determined through flow cytometry of blood cells as described previously (Dawley & Goddard 1988; Collares-Pereira & Moreira da Costa 1999). Blood samples were drawn from the caudal vein, stabilized in buffer (40 mM citric acid trisodium salt, 0.25 M saccharose and 5% DMSO) and immediately frozen at −80°C. Chicken erythrocytes were used as both an internal and external standard. Only peaks of the fluorescence histograms with a coefficient lower than 5% were scored. The DNA content of erythrocytes was estimated by calculating the ratio of mean fluorescence between the blood cells and the mean fluorescence of chicken cells, and multiplying by the standard DNA content value for chicken erythrocytes, 2.5 pg (Tiersch et al. 1989). All specimens were killed with an overdose of anaesthetic MS222. The sex of each fish was determined by examining the gonads using a dissection microscope. Deviations in the sex ratio were determined using the Χ2 test.
(c) Microsatellite typing
The total genomic DNA of 150 individuals (115 S. alburnoides and 35 S. carolitertii) was extracted from fins preserved in absolute ethanol or from frozen muscle tissue using standard methods (Sambrook et al. 1989). Out of eight tested PCR primers, four isolated from the Luxilus genus (Turner et al. 2004) and four from the Squalius genus (Mesquita et al. 2003; Pala & Coelho 2005), only six (n7j4, e1g6, lco1, lco3, lco4 and lco5) were used to amplify polymorphic microsatellite loci. PCRs were performed in 10 μl volumes, containing 25–50 ng of template DNA, 0.2 mM each dNTP, 2 mM MgCl2, 1 unit Taq DNA polymerase (Invitrogen) and 0.2 μM of each primer or using the Multiplex PCR Kit (Qiagen), using the reverse primer labelled with fluorescence and fitting the following profile: 94°C (2 min), 35 cycles at 94°C (30 s), 50–57°C (30 s), 72°C (1 min). The PCR products were electrophoresed in an automatic sequencer CEQ 2000XL (Beckman Coulter) and the molecular weights of alleles were determined with CEQ Fragment Analysis Software.
(d) Microsatellite data analysis
The alleles scored for S. carolitertii and the diploid hybrids enabled the identification of diagnostic alleles for both genomes and genotyping of the individuals. To examine possible scoring errors due to stuttering, large allele dropouts or the occurrence of null alleles, the program Micro-Checker v. 2 was used (Oosterhout et al. 2004). The genotypic diversity of the hybrid polyploids was estimated using SPAGeDi v. 1.1 (Hardy & Vekemans 2002). Because allotetraploids show disomic inheritance, the two genomes were analysed separately, since they contain the genetic information arising from the two diploid progenitor species. The allelic frequencies per locus for S. carolitertii and for the tetraploid S. alburnoides populations were determined using Genepop v. 3.4 (Raymond & Rousset 1995). This software was also used to test for linkage disequilibria and deviations from Hardy–Weinberg equilibrium (HWE). The ‘exact HW test’ was used to test the null hypothesis of a random union of gametes for each breed analysed. Significance of the obtained values was tested by 1000 permutations.
(e) Experimental crosses
To obtain further confirmation of the existing reproductive modes, parallel measurements of the DNA contents of erythrocytes and spermatozoa were made by flow cytometry in 42 male specimens (34 S. alburnoides and 8 S. carolitertii). Sperm was collected and flow cytometry measurements were conducted as previously described (see Alves et al. 1999). Experimental crosses were performed immediately after sample collection. Females were stripped and their eggs exposed to sperm on a Petri dish. Gametes were gently mixed with a brush and water was added. Fertilized eggs were laid on a mesh installed in an aquarium. The processes of egg fertilization and development of progeny were monitored using a dissection microscope. To reveal the particular features of reproduction and gametogenesis, eight crosses were performed: three crosses between tetraploid S. alburnoides females and S. carolitertii, or triploid or tetraploid S. alburnoides males; and five crosses between triploid S. alburnoides females and S. carolitertii or triploid or tetraploid S. alburnoides males (figure 1b,d). Crosses involving hybrid diploid forms were not performed due to their absence or scarcity in those populations.
3. Results
(a) Population composition
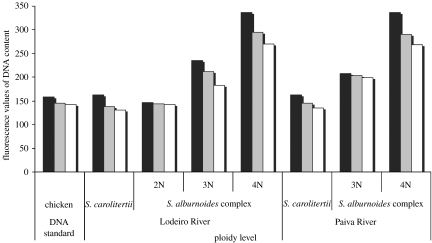
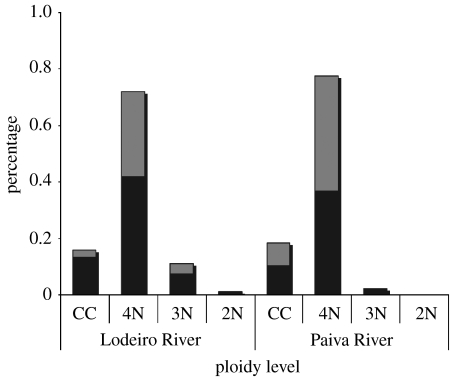
Squalius alburnoides individuals represent 83%, while S. carolitertii represents only 17% of all the animals studied. Flow cytometric measurements of DNA content in the erythrocytes (figure 3) revealed that 88.1% of all S. alburnoides sampled were tetraploids (mean DNA content 4.99±0.16 pg per cell), 10.8% were triploids (mean DNA content 3.61±0.11 pg per cell) and only 1% were diploids (mean DNA content 2.41±0.01 pg per cell). All specimens identified as S. carolitertii comprised only diploids (mean DNA content of 2.43±0.07 pg per cell). Also, according to flow cytometry data, the tetraploid individuals represent 85.6% of the total population in the Lodeiro River and 97.5% of the total population in the Paiva River (figure 4). Triploid and diploid forms were scarce or absent, only representing 13 and 1% of the population in the Lodeiro River and 2.5 and 0% in the Paiva River, respectively. Over a one-year monitoring period, the population composition remained the same. Both males and females are represented in almost all ploidy levels. Among tetraploid forms, in the Lodeiro River, 58% were males and 42% were females (figure 4). In the Paiva River, 53% of the tetraploids were females and 47% were males. Among the triploid forms in the Lodeiro River, 65% were males and 35% were females and in the Paiva River the triploid forms were all males (figure 4). In the diploid forms, only males were found. The Χ2 test indicated no significant differences between the proportions of each sex in the tetraploids from the Lodeiro (Χ0.05,12=3.41) and Paiva Rivers (Χ0.05,12=0.11; figure 4). For the triploids the Χ2 test indicated a significant difference between the proportions of each sex with a higher frequency towards males (Χ0.05,12=9.79).
Figure 3.
Histograms of fluorescence values of erythrocytes cells that distinguish ploidy levels. Chicken blood cells are used as a DNA standard. Black bars, maximum; grey bars, average; white bars, minimum.
Figure 4.
Population composition indicating the percentage of S. carolitertii (CC) and of the several ploidy forms of S. alburnoides (2N, 3N and 4N) determined by flow cytometry. Indicated also are the percentages of males (black bars) and females (grey bars) in the Lodeiro and Paiva Rivers.
(b) Genomic composition
Diagnostic alleles for each genome were determined through the analysis of alleles identified in S. carolitertii and in the diploid hybrids. In the C genome we identified 36 diagnostic alleles and in the A genome we only identified 16 diagnostic alleles across all loci (figure 5).
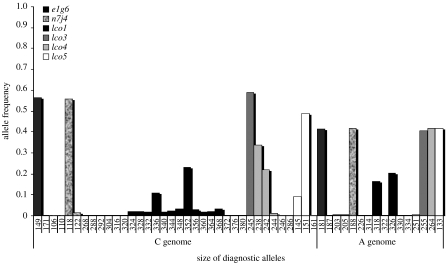
Figure 5.
Allele frequencies of the six polymorphic microsatellite loci used to genotype S. alburnoides. In the x-axis are represented all diagnostic alleles scored for each microsatellite in each genome (C and A) and in the y-axis are indicated the allelic frequencies for each locus across both genomes.
All loci were demonstrated to be polymorphic for both genomes (C and A), the lco1 was the most polymorphic locus (24 alleles scored) and n7j4 the least polymorphic one (two alleles scored). According to the results obtained with Micro-Checker v. 2.2 software, only the n7j4 locus might exhibit null alleles, as suggested by the general excess of homozygotes in both populations. Pala & Coelho (2005) reported two alleles for locus e2f8, a 106 bp fragment attributed to the C genome and a 138 bp fragment to the A genome. Here only the 106 bp allele was amplified, suggesting that for these populations this locus has suffered a mutation in the flanking primer region of the A genome. For the reasons mentioned above, these two loci were abandoned. In the six microsatellite loci examined, no null alleles were found, and all the specimens of S. alburnoides analysed were heterozygous for all loci. Out of the six microsatellite loci, four (lco1, lco3, lco4 and e1g6) were found to be useful for discriminating the genotypes, providing the indication of the absence of asymmetrical tetraploids. All triploids exhibited a CCA genome, whereas other forms such as those carrying a CAA genome or non-hybrid forms (AA) were not found.
(c) Genetic diversity
Average genotypic diversity across all loci was higher in the tetraploids (0.591–0.656) than in the triploids (0.344–0.404) of both populations. By juxtapositioning the C and A tetraploid genomes, it can be observed that the A genome exhibits a lower number of alleles (figure 5). The tetraploids show disomic inheritance, with their homologous pairs of chromosomes forming bivalents during meiosis, just like any diploid. Thus, the observed and expected heterozygosities were separately calculated for each genome within the Lodeiro (C genome Ho=0.241, He=0.242; A genome Ho=0.124, He=0.132) and Paiva Rivers (C genome Ho=0.175, He=0.174; A genome Ho=0.123, He=0.132). Hardy–Weinberg and linkage equilibria across the polymorphic loci were also calculated for each genome and no significant deviations were observed (p>0.05).
(d) Experimental crosses
The average of erythrocytes and spermatozoa DNA content ratio of hybrid males ranges from 1 : 0.99 (±0.00) to 1 : 0.48 (±0.04). Flow cytometry measurements of spermatozoa DNA content revealed that tetraploid hybrid males of the Douro River basin produce reduced sperm; 1 : 0.48 (±0.04). The diploid and triploid hybrid males produce unreduced sperm (1 : 0.99 (±0.00) and 1 : 0.96 (±0.02), respectively). The results of the crosses involving tetraploid S. alburnoides showed evidence of normal meiosis (table 1). The combined analysis of the six microsatellite loci (especially lco1) and the measurement of the DNA content of erythrocytes and spermatozoa indicated that the tetraploids carried a CCAA genome and that males produced diploid gametes (CA). We crossed a CCAA female with a CCAA male and obtained only tetraploid offspring (figure 1d, table 1). Moreover, the analysis of microsatellite variation indicates that females produce reduced eggs and transmit their diploid genome to the progeny, resulting in symmetric tetraploids (cross type I, table 1). If we cross a tetraploid female (CCAA) with a S. carolitertii (CC), all individuals in the progeny are triploid CCA. The female produces CA gametes and the S. carolitertii produces haploid C gametes (cross type II, table 1). In the six crosses performed involving triploids (CCA), fertilization occurred but no development was observed.
Table 1.
Summary of the results of two experimental crosses: alleles identified for each informative microsatellite locus. (P, ploidy level; G, ploidy of gametes; alleles in brackets were inferred and superscripts correspond to the diagnostic alleles for each genome of S. alburnoides (C or A).)
| crosses | genotypes | sex | P | G | lco1 | lco4 | e1g6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCAA | F | 4N | 2N | 326A | (326A) | 352C | (352C) | 238C | (238C) | 264A | (264A) | 149C | (149C) | 181A | 203A | |
| type I | CC | M | 2N | N | 348C | 356C | 238C | 242C | 149C | (149C) | ||||||
| I-1 | CCA | — | 3N | — | 326A | 348C | 352C | 238C | 242C | 264A | 149C | (149C) | 181A | |||
| I-2 | CCA | — | 3N | — | 326A | 352C | 356C | 238C | (238C) | 264A | 149C | (149C) | 203A | |||
| I-3 | CCA | — | 3N | — | 326A | 348C | 352C | 238C | 242C | 264A | 149C | (149C) | 203A | |||
| CCAA | F | 4N | 2N | 318A | 326A | 352C | 368C | |||||||||
| type II | CCAA | M | 4N | 2N | 326A | (326A) | 352C | (352C) | ||||||||
| II-1 | CCAA | — | 4N | — | 326A | (326A) | 352C | 368C | ||||||||
| II-2 | CCAA | — | 4N | — | 326A | (326A) | 352C | (352C) | ||||||||
| II-3 | CCAA | — | 4N | — | 326A | (326A) | 352C | 368C | ||||||||
| II-4 | CCAA | — | 4N | — | 318A | 326A | 352C | (352C) |
4. Discussion
Ancient polyploidy has been found at the root of many animal groups; however, present examples of polyploids are relatively rare among animals. Also, polyploid speciation is apparently very rare when compared with other speciation modes (Otto & Whitton 2000). Here, we describe an example that appears to lead to allopolyploid speciation after intermediate processes of non-sexual reproduction in the S. alburnoides complex (Cyprinidae).
Asexual reproduction is often associated with polyploidy (Bullini 1994; Otto & Whitton 2000) and even-ploidy is required to self-sustain polyploid strains in bisexual animals. Previous studies have shown that tetraploid forms are scarce or absent in S. alburnoides populations (Collares-Pereira 1985; Alves et al. 1997; Martins et al. 1998; Pala & Coelho 2005). According to previous studies (Collares-Pereira 1985; Carmona et al. 1997; C. Cunha et al. 2005, unpublished data) and similar to other basins (Pala & Coelho 2005), S. alburnoides populations from the Douro River basin are mostly composed of triploids (mainly CAA females). In the present study, however, flow cytometry data have indicated that the northern Douro basin exhibits a distinctly different population composition scenario compared with the other basins; in the two populations analysed here, tetraploid individuals represent 88% of the collected samples. How can we explain the differences observed in the composition of the different forms in S. alburnoides populations? Specific habitat preferences of this tetraploid lineage could be one explanation for its occurrence in nature. Shallow waters, gravel and cobble beds as well as fast stream flow characterize the habitat of these two tetraploid populations. This habitat type is not shared by other hybrid forms sampled in other locations (Martins et al. 1998), indicating divergent habitat preferences. Adaptation to this habitat that is also used as a mating site not only reduces competition with other forms, but also allows for assortative pairing, since it diminishes the possibility of cross-ploidy mating helping to form these large tetraploid populations and resulting in effective prezygotic isolation. However, it is legitimate to question whether the high frequency of S. alburnoides triploids observed in nature (mainly CAA females) could be a result of crosses where allotetraploids are involved. Under such a scenario, it would be expected that hybrid speciation would occur immediately by reproductive isolation through ploidy (reviewed by Mallet 2007). Triploids would, at least in an initial period, be a consequence of sympatry between tetraploids and their progenitors. However, according to previous studies, this hypothesis is unlikely because diploid females in the Douro River do not seem to produce clonal eggs. Thus, the only route to tetraploidy in this river would be the syngamy of unreduced triploid eggs and haploid sperm. Moreover, it seems that the origin of this hybrid complex is not recent (Cunha et al. 2004), which is in contrast to an apparently more recent origin of these two tetraploid populations. Microsatellite data from Douro River basin indicate that these two tetraploid populations have arisen from other neighbouring polyploid populations. The reduction in the number of alleles, the increased frequency of some of them, and the loss of variability observed in these two populations, when compared with the adjacent ones, could be a consequence of the founder effect (C. Cunha et al. 2005, unpublished data). Moreover, the contribution of tetraploids to the origin of the most common form of the complex, the CAA triploids, would be very difficult to explain not only due to the extinction of the paternal species, which might have resulted from genetic assimilation due to an extensive gene flow between hybrids and the paternal species, but also due to the scarcity or inexistence of allotetraploids in other river basins.
High abundance of tetraploids has previously been reported among asexual species (Bogart 1989; Vasil'ev et al. 1989). However, males are sterile, if present, and all females seem to propagate via asexual reproduction (Bogart 1989; Vasil'ev et al. 1989). Only the isogenic tetraploid forms (CCAA) allow stable meiosis, since intergenomic recombination compromises conservation of the two parental chromosomal complements (Comai 2005) and our data indicate the absence of asymmetrical tetraploids (figure 4).
Diploid females, which exhibit different reproductive modes (co-varying with geographical region), reproduce in the Douro River by hybridogenesis (Carmona et al. 1997) and are present at a very low frequency. Accordingly, the most probable route to tetraploidy here was through clonal CAA eggs fertilized by C-reduced sperm. In contrast to what has been frequently observed in other S. alburnoides populations, all the triploids analysed display a CCA composition. These triploids are the result of the fertilization of CA oocytes from symmetric tetraploid females (CCAA) with C sperm from S. carolitertii males, which was confirmed with breeding experiments (table 1). The low percentage of triploid forms and the rarity of diploid hybrids indicate that tetraploids do not return to triploidy. Therefore, for some generations, they must have subsisted through cross events among themselves by normal sexual reproduction. The crossing of triploid CCA females and males with tetraploids in breeding experiments resulted in the non-development of embryos. These results do not seem to be a constraint of our experimental crosses, since CCC, CCCA or CCCCA forms have not been observed in nature. This means that tetraploids are being reproductively isolated from the other forms through postzygotic isolation.
Flow cytometry measurements of erythrocyte and spermatozoa DNA contents indicate that tetraploid hybrid males from the Douro River basin produce reduced sperm with the ratio of erythrocytes and gamete DNA content being approximately 1 : 0.5 and microsatellite markers segregated according to Mendelian expectations. In addition, the other hybrid forms (2N and 3N) produce unreduced sperm showing ratios around 1 : 1. Crosses involving tetraploid S. alburnoides (cross type II, table 1) showed evidence of normal meiosis as noted in the southern populations (Alves et al. 2001). Microsatellite loci (especially lco1) and the DNA contents of erythrocytes and spermatozoa indicated that tetraploids exhibit the CCAA genome and produce diploid CA gametes. These results not only reveal that the tetraploid forms are symmetric and fertile, but also that by restoring even-ploidy, normal meiosis can take place in this complex, leading to biparental reproduction.
Asexual organisms normally show abnormal sex ratios (Dawley 1989), as has been shown for the S. alburnoides complex (imbalanced towards females; Alves et al. 2001). However, a Χ2 test indicated no significant differences between the proportions of each sex in tetraploids from the Lodeiro and Paiva Rivers (figure 4).
Our data on sex ratios, spermatozoa DNA contents, genome composition and Mendelian segregation at the microsatellite markers revealed through experimental crosses indicate that these tetraploid lineages are not clonal and represent convincing cases of bisexual reproduction.
The observed higher average genotypic diversity across all loci in the tetraploids when compared with the CCA triploids was expected, since bisexuality allows existing gene combinations to break up and recombine. The lower allelic genetic diversity found for the A genome may be explained by the founder effect during the formation of this complex, since the paternal species is extinct and nuclear non-hybrid males are absent from the northern rivers and there was no further introduction of this genome.
If these populations were partially sexual, departures from HWE would persist over time (Otto & Lenormand 2002). The absence of significant deviations from Hardy–Weinberg and linkage equilibria across the polymorphic loci reinforces the idea of random mating in the population, with genotype frequencies having remained unchanged in the past generations and random assortment of alleles resulting in equal proportions of each allele combination in the population.
5. Conclusions
Through recombination, sexual reproduction among the tetraploid forms of S. alburnoides has given rise to genetic variability and allowed the adaptation and long-term evolution of these fish. Our data suggest that we witness an ongoing process of speciation in tetraploids as a consequence of their evolution in isolation from the other forms of S. alburnoides complex. This process may occur via prezygotic (habitat selection and assortative mating) and possibly postzygotic mechanisms (non-viable embryos), and illustrates how these hybrid polyploid complexes can lead to speciation and highlights their important role in animal evolution.
Acknowledgments
We thank M. L. Arnold for suggestions at the initial preparation stage of the manuscript, C. Luís for help with the microsatellite analyses, and A. Perdices, J. Abrantes and I. Pala for their help in the field and breeding experiments. We thank M. Sparrow, R. Weld and I. Pala for their English revision. We thank Klaus Schwenk, Bruno Streit, Nora Brede and two anonymous reviewers for helpful comments. This study was financially supported by POCTI/BSE40868/2001 and CGL2004-00077/BOS and by a PhD grant, from FCT, attributed to C.C. (SFRH/BD/8637/2002).
Footnotes
One contribution of 16 to a Theme Issue ‘Hybridization in animals: extent, processes and evolutionary impact’.
References
- Allendorf F.M, Leary R.F, Spruell P, Wenburg J.K. The problems with hybrids: setting conservation guidelines. Trends Ecol. Evol. 2001;16:613–622. doi:10.1016/S0169-5347(01)02290-X [Google Scholar]
- Alves M.J, Coelho M.M, Collares-Pereira M.J, Dowling T.E. Maternal ancestry of the Rutilus alburnoides complex (Teleostei, Cyprinidae) as determined by analysis of cytochrome b sequences. Evolution. 1997;51:1584–1592. doi: 10.1111/j.1558-5646.1997.tb01481.x. doi:10.2307/2411210 [DOI] [PubMed] [Google Scholar]
- Alves M.J, Coelho M.M, Collares-Pereira M.J. Diversity in the reproductive modes of females of the Rutilus alburnoides complex (Telostei, Cyprinidae): a way to avoid the genetic constraints of uniparentalism. Mol. Biol. Evol. 1998;15:1233–1242. [Google Scholar]
- Alves M.J, Coelho M.M, Próspero M.I, Collares-Pereira M.J. Production of fertile unreduced sperm by hybrids males of the Rutilus alburnoides complex (Teleostei, Cyprinidae): an alternative route to genome tetraploidization in unisexuals. Genetics. 1999;151:277–283. doi: 10.1093/genetics/151.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves M.J, Coelho M.M, Collares-Pereira M.J. Evolution in action through hybridization and polyploidy in an Iberian fresh water fish: a genetic review. Genetica. 2001;111:375–385. doi: 10.1023/a:1013783029921. doi:10.1023/A:1013783029921 [DOI] [PubMed] [Google Scholar]
- Alves M.J, Gromicho M, Collares-Pereira M.J, Crespo-López E, Coelho M.M. Simultaneous production of triploid and haploid eggs by triploid Squalius alburnoides (Teleostei: Cyprinidae) J. Exp. Zool. 2004;301A:552–558. doi: 10.1002/jez.a.51. doi:10.1002/jez.a.51 [DOI] [PubMed] [Google Scholar]
- Arnold M.L. Natural hybridization as an evolutionary process. Annu. Rev. Ecol. Syst. 1992;23:237–261. doi:10.1146/annurev.es.23.110192.001321 [Google Scholar]
- Baack E.J. To succeed globally, disperse locally: effects of local pollen and seed dispersal on tetraploid establishment. Heredity. 2005;94:538–546. doi: 10.1038/sj.hdy.6800656. doi:10.1038/sj.hdy.6800656 [DOI] [PubMed] [Google Scholar]
- Baack E.J, Rieseberg L.H. A genomic view of introgression and hybrid speciation. Curr. Opin. Genet. Dev. 2007;17:1–6. doi: 10.1016/j.gde.2007.09.001. doi:10.1016/j.gde.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N.H. The role of hybridization in evolution. Mol. Ecol. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. doi:10.1046/j.1365-294x.2001.01216.x [DOI] [PubMed] [Google Scholar]
- Bogart J.P. A mechanism for interspecific gene exchange via all-female salamander hybrids. In: Dawley R.M, Bogart J.P, editors. Evolution and ecology of unisexual vertebrates. New York State Museum Bulletin; Albany, NY: 1989. pp. 170–179. [Google Scholar]
- Bullini L. Origin and evolution of animal hybrid species. Trends Ecol. Evol. 1994;9:422–426. doi: 10.1016/0169-5347(94)90124-4. doi:10.1016/0169-5347(94)90124-4 [DOI] [PubMed] [Google Scholar]
- Carmona J.A, Sanjur O.I, Doadrio I, Machordom A, Vrijenhoek R.C. Hybridogenetic reproduction and maternal ancestry of polyploid Iberian fish: the Tropidophoxinellus alburnoides complex. Genetics. 1997;146:983–993. doi: 10.1093/genetics/146.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman M.A, Burke J.M. Genetic divergence and hybrid speciation. Evolution. 2007;61:1773–1780. doi: 10.1111/j.1558-5646.2007.00134.x. doi:10.1111/j.1558-5646.2007.00134.x [DOI] [PubMed] [Google Scholar]
- Collares-Pereira M.J. The “Rutilus alburnoides (Steindachner, 1866) complex” (Pisces, Cyprinidae). II. First data on the karyology of a well-established diploid–triploid group. Arq. Mus. Boc. (Ser. A) 1985;3:69–89. [Google Scholar]
- Collares-Pereira M.J, Moreira da Costa L. Intraspecific and interspecific genome size variation in Iberian Cyprinidae and the problem of diploidy and poplyploidy, with review of genome sizes within the family. Folia Zool. 1999;48:61–76. [Google Scholar]
- Comai L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005;6:836–846. doi: 10.1038/nrg1711. doi:10.1038/nrg1711 [DOI] [PubMed] [Google Scholar]
- Cunha C, Coelho M.M, Carmona J.A, Doadrio I. Phylogeographical insights into the origins of the Squalius alburnoides complex via multiple hybridization events. Mol. Ecol. 2004;13:2807–2817. doi: 10.1111/j.1365-294X.2004.02283.x. doi:10.1111/j.1365-294X.2004.02283.x [DOI] [PubMed] [Google Scholar]
- David L, Blum S, Feldman M.W, Lavi U, Hillel J. Recent duplication of the common carp (Cyprinus carpio L.) genome as revealed by analyses of microsatellite loci. Mol. Biol. Evol. 2003;20:1425–1434. doi: 10.1093/molbev/msg173. doi:10.1093/molbev/msg173 [DOI] [PubMed] [Google Scholar]
- Dawley R.M. An introduction to unisexual vertebrates. In: Dawley R.M, Bogart J.P, editors. Evolution and ecology of unisexual vertebrates. New York State Museum Bulletin; Albany, NY: 1989. pp. 1–18. [Google Scholar]
- Dawley R.M, Goddard K.A. Diploid–triploid mosaics among unisexual hybrids of minnows Phoxinus neogaeus. Evolution. 1988;42:649–659. doi: 10.1111/j.1558-5646.1988.tb02484.x. doi:10.2307/2408857 [DOI] [PubMed] [Google Scholar]
- Dowling T.E, Secor C.L. The role of hybridization and introgression in the diversification of animals. Annu. Rev. Ecol. Syst. 1997;28:593–619. doi:10.1146/annurev.ecolsys.28.1.593 [Google Scholar]
- Gromicho, M. 2006 Genome polyploidisation in the Iberian fish complex Squalius alburnoides A case study of evolution-in-action through hybridization and non-sexuality: a cytogenetic approach. PhD thesis, University of Lisbon, Lisbon.
- Hardy O.J, Vekemans X. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes. 2002;2:618–620. doi:10.1046/j.1471-8278.2002.00305.x [Google Scholar]
- Hedges S.B, Bogart J.P, Maxson L.R. Ancestry of unisexual salamanders. Nature. 1992;356:708–710. doi: 10.1038/356708a0. doi:10.1038/356708a0 [DOI] [PubMed] [Google Scholar]
- Janko K, Kotlík P, Ráb P. Evolutionary history of asexual hybrid loaches (Cobitis: Teleostei) inferred from phylogenetic analysis of mitochondrial DNA variation. J. Evol Biol. 2003;16:1280–1287. doi: 10.1046/j.1420-9101.2003.00627.x. doi:10.1046/j.1420-9101.2003.00627.x [DOI] [PubMed] [Google Scholar]
- Janko K, Culling M.A, Ráb P, Kotlík P. Ice age cloning—comparison of the Quaternary evolutionary histories of sexual and clonal forms of spiny loaches (Cobitis; Teleostei) using the analysis of mitochondrial DNA variation. Mol. Ecol. 2005;14:2991–3004. doi: 10.1111/j.1365-294X.2005.02583.x. doi:10.1111/j.1365-294X.2005.02583.x [DOI] [PubMed] [Google Scholar]
- Mable B.K. Breaking down taxonomic barriers in polyploidy research. Trends Plant. Sci. 2003;8:582–590. doi: 10.1016/j.tplants.2003.10.006. doi:10.1016/j.tplants.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Mable B.K. ‘Why polyploid is rarer in animals than in plants’: myths and mechanisms. Biol. J. Linn. Soc. 2004;82:453–466. doi:10.1111/j.1095-8312.2004.00332.x [Google Scholar]
- Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. doi:10.1038/nature05706 [DOI] [PubMed] [Google Scholar]
- Martins M.J, Collares-Pereira M.J, Cowx I.G, Coelho M.M. Diploids v. triploids of Rutilus alburnoides: spatial segregation and morphological differences. J. Fish Biol. 1998;52:817–828. doi:10.1111/j.1095-8649.1998.tb00822.x [Google Scholar]
- Mesquita N, Cunha C, Hänfling B, Carvalho G.R, Zé-Zé L, Tenreiro R, Coelho M.M. Isolation and characterization of polymorphic microsatellite loci in the endemic Portuguese freshwater fish Squalius aradensis (Cyprinidae) Mol. Ecol. Notes. 2003;3:572–574. doi:10.1046/j.1471-8286.2003.00515.x [Google Scholar]
- Oosterhout C.V, Hutchinson W.F, Wills D.P.M, Shipley P. Micro-Checker: software for identifying and correcting genotype errors in microsatellite data. Mol. Ecol. Notes. 2004;4:535–538. doi:10.1111/j.1471-8286.2004.00684.x [Google Scholar]
- Otto S.P, Lenormand T. Resolving the paradox of sex and recombination. Nat. Rev. Genet. 2002;3:252–260. doi: 10.1038/nrg761. doi:10.1038/nrg761 [DOI] [PubMed] [Google Scholar]
- Otto S.P, Whitton J. Polyploid incidence and evolution. Annu. Rev. Genet. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. doi:10.1146/annurev.genet.34.1.401 [DOI] [PubMed] [Google Scholar]
- Pala I, Coelho M.M. Contrasting views over a hybrid complex: between speciation and evolutionary “dead-end”. Gene. 2005;347:283–294. doi: 10.1016/j.gene.2004.12.010. doi:10.1016/j.gene.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Phillips R, Ráb P. Chromosome evolution in the Salmonidae (Pisces): an update. Biol. Rev. 2001;76:1–25. doi: 10.1017/s1464793100005613. doi:10.1017/S1464793100005613 [DOI] [PubMed] [Google Scholar]
- Quattro J.M, Avise J.C, Vrijenhoek R.C. An ancient clonal lineage in the fish genus Poeciliopsis (Atheriniformes: Poeciliidae) Proc. Natl Acad. Sci. USA. 1992;89:348–352. doi: 10.1073/pnas.89.1.348. doi:10.1073/pnas.89.1.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Rousset F. Genepop (v. 1.2): a population genetics software for exact test and ecumenicism. J. Hered. 1995;86:248–249. [Google Scholar]
- Sambrook J, Fritsch E.F, Maniatis T. Cold Spring Harbor Laboratory Press; New York, NY: 1989. Molecular cloning: a laboratory manual. [Google Scholar]
- Schartl M, Nanda I, Schlupp I, Wilde B, Epplen J.T, Schmid M, Parzefall J. Incorporation of subgenomic amounts of DNA as compensation for mutational load in a gynogenetic fish. Nature. 1995;373:68–71. doi:10.1038/373068a0 [Google Scholar]
- Schultz R.J. Hybridization, unisexuality and polyploidy in the teleost Poeciliopsis (Poeciliidae) and other vertebrates. Am. Nat. 1969;103:605–619. doi:10.1086/282629 [Google Scholar]
- Spolsky C.M, Phillips C.A, Uzzell T. Antiquity of clonal salamander lineages revealed by mitochondrial DNA. Nature. 1992;356:706–708. doi: 10.1038/356706a0. doi:10.1038/356706a0 [DOI] [PubMed] [Google Scholar]
- Tiersch T.R, Chandler R.W, Wachtel S.S, Elias S. Reference standards for flow cytometry and application in comparative studies of nuclear DNA content. Cytometry. 1989;10:706–710. doi: 10.1002/cyto.990100606. doi:10.1002/cyto.990100606 [DOI] [PubMed] [Google Scholar]
- Turner T.F, Dowling T.E, Broughton R.E, Gold J.R. Variable microsatellite markers amplify across divergent lineages of cyprinid fishes (subfamily Leuciscinae) Conserv. Genet. 2004;5:279–281. doi:10.1023/B:COGE.0000029998.11426.ab [Google Scholar]
- Vasil'ev V.P, Vasil'ev K.D, Osinov A.G. Evolution of a diploid-tripoid-tetraploid complex in fish of the genus Cobittis (Pisces, Cobitidae) In: Dawley R.M, Bogart J.P, editors. Evolution and ecology of unisexual vertebrates. New York State Museum Bulletin; Albany, NY: 1989. pp. 153–169. [Google Scholar]
- Vrijenhoek R.C. Animal clones and diversity. BioScience. 1998;48:617–628. doi:10.2307/1313421 [Google Scholar]
- Vrijenhoek R.C, Dawley R.M, Cole C.J, Bogart J.P. A list of the known unisexual vertebrates. In: Dawley R.M, Bogart J.P, editors. Evolution and ecology of unisexual vertebrates. New York State Museum Bulletin; Albany, NY: 1989. pp. 19–23. [Google Scholar]