Abstract
Avian migration, which involves billions of birds flying vast distances, is known to influence all aspects of avian life. Here we investigate how birds fit moult into an annual cycle determined by the need to migrate. Large variation exists in moulting patterns in relation to migration: for instance, moult can occur after breeding in the summer or after arrival in the wintering quarters. Here we use an optimal annual routine model to investigate why this variation exists. The modelled bird's decisions depend on the time of year, its energy reserves, breeding status, experience, flight feather quality and location. Our results suggest that the temporal and spatial variations in food are an important influence on a migratory bird's annual cycle. Summer moult occurs when food has a high peak on the breeding site in the summer, but it is less seasonal elsewhere. Winter moult occurs if there is a short period of high food availability in summer and a strong winter peak at different locations (i.e. the food is very seasonal but in opposite phase on these areas). This finding might explain why only long-distance migrants have a winter moult.
Keywords: optimal annual routines, moult, migration, birds, state-dependent dynamic models, dynamic programming
1. Introduction
Migration can be defined as a large-scale, seasonal and bidirectional movement of animals (Adriaensen & Dhondt 1990). Groups that are highly migratory include insects, fishes, whales, shorebirds and songbirds (Dingle 1996). Birds are especially preadapted for migratory life owing to their efficient mean of locomotion, powered flight (Alerstam 1991; Alexander 1998). Animals usually migrate to track resources that are unequally distributed in space and time (Aidley 1981). In other words, migration can be seen as an adaptation to deal with a high degree of environmental seasonality. In birds, this usually involves the movements between a breeding site and another location (or locations) where they spend the rest of the year (Greenberg & Marra 2005).
Avian migration is a fascinating event involving billions of birds flying vast distances, and considerable modelling efforts have been spent in recent years in an attempt to understand this. Early optimization models focused on isolated aspects of avian migration. Researchers first used the theory of bird flight (Pennycuick 1975, 1989) to understand the relationship between migratory flight and energetics (Alerstam 1990, 1991; Alerstam & Lindström 1990). For instance, with the theory of flight it is possible to predict the flight speeds which are optimal according to different criteria (minimizing the time, or the energy requirement of migration). This approach has also been used to predict the optimal fuel deposition rules for migratory birds at stopover sites and so the fat load of departing birds (Alerstam 1991). Despite their success in predicting speed and fat load (Alerstam 1991; Houston 1998), these models are, however, unable to tell us whether a bird should migrate or not, where it should fly to and when it should start. These models also do not allow us to investigate the effects of stochasticity, for instance, in foraging and flight conditions (e.g. wind speed and direction) on migratory behaviour.
If one considers only a single journey, most of the above problems can be handled by the now-standard technique of dynamic programming (Houston et al. 1988; Houston & McNamara 1999; Clark & Mangel 2000). For instance, in the case of spring migration, Weber et al. (1998) and Clark & Butler (1999) were able to investigate how stochasticity in wind and foraging conditions influences migratory fuelling. They also identified several circumstances when site skipping (passing over some of the stopover sites without landing) can occur along the migratory path. While these models are well suited to study the fine details of the behaviour of one individual over its journey, they are unable to address more general questions. Their limitations come from two sources: they consider (i) a single individual on (ii) a single journey.
Considering just a single individual is problematic if the focal individual's behaviour is influenced by others in the population. For instance, to determine the optimal arrival time on the breeding ground, one should consider not only the focal bird's state and the environmental conditions on the breeding ground but also the behaviour of other members of the population. This is because the density of birds already on the breeding area can influence the reproductive value of individuals on arrival; consider, for instance, the competition for limited territories (Kokko 1999). Therefore, a game theoretical approach is needed and the resulting optimal behaviour will in effect be an evolutionarily stable strategy (Maynard Smith 1982; Kokko 1999).
In avian migration research, we cannot avoid considering the behaviour of several individuals simultaneously in at least two other cases: (i) partial and (ii) differential migration. In partial migration, only one part of the population migrates from the breeding site while the other part remains resident there (Berthold 1984; Adriaensen & Dhondt 1990). If we, reasonably, assume that increasing density of birds on the breeding site during winter decreases the overwinter survival of residents, then the behaviour of migrants clearly influences the reproductive value of residents and vice versa. The question arises: who will stay and who will migrate? A possible answer is that a mixed evolutionarily stable strategy (Maynard Smith 1982; Kaitala et al. 1993) exists where both tactics have the same rewards and the behaviour is randomly decided. However, field studies show that residents usually do better in terms of fitness than migrants in the same population (Berthold 1984; Adriaensen & Dhondt 1990). This might indicate that migrants are making the best of a bad job (Adriaensen & Dhondt 1990), i.e. the individuals follow a unique but conditional strategy, and the decision about migration depends on the state of the individuals (e.g. energy reserves or immune condition). This indicates the necessity of a state-dependent game theoretical approach to model partial migration realistically.
Differential migration is similar to partial migration in that individuals of the same population follow different migratory strategies. An apparent difference, however, is that under differential migration, individuals of well-defined classes (e.g. sex or age) migrate differently (but this might only relate to the fact that currently we are only able to recognize differential migration in cases where the classes are different in some clearly observable respects). For instance, in their classic studies Ketterson & Nolan (1976, 1979) reported that in dark-eyed juncos, Junco hyemalis hyemalis, the females migrate further south than males do. To understand this phenomenon, one must consider the behaviour of males and females simultaneously at a range of locations.
The other problem of the single bird/single journey models stems from the fact that an individual's state and behaviour at a given time of year and location can significantly influence its behaviour at another time of year and location. For instance, Marra et al. (1998) found that the quality of winter habitats occupied by American redstarts, Setophaga ruticilla, determines their condition and spring departure dates, which, in turn, influence arrival schedules and condition in the breeding season, and so possibly affect the birds' reproductive success. In other words, wintering behaviour and state affect migration and breeding, which, in turn, can influence the behaviour and state in the next winter. Consequently, to fully understand migration, one needs to consider the whole annual cycle. These carry-over or ‘knock-on’ effects are the strongest under seasonal environments (Fretwell 1972), the environments to which migration seems to be an adaptation.
In seasonal environments, the timing of actions may considerably affect an organism's chances of survival and reproduction. It can thus be expected that the order and timing of behaviours will be shaped by natural selection, so that fitness is maximized. The proper timing of behaviours in the annual cycle is important for a number of reasons. First, the benefits from the different behaviours can vary notably over the year (Masman et al. 1988). Second, as we have seen above, performing a certain action may significantly influence the animal's future state, which, in turn, may affect the animal's ability to perform other behaviours in the future. Third, a number of behaviours are clearly exclusive. In birds, migration and reproduction cannot be performed at the same time. The optimal timing of behaviour will therefore be dependent not only on the best time for this action but also on whether there are other good times to perform the excluded activities. Houston & McNamara (1999) present a general technique for finding state-dependent optimal annual routines which allows the unified handling of game theoretical considerations and knock-on effects. Barta et al. (2006) apply the technique to find the optimal annual routine of reproduction and moult in non-migratory birds. Here we extend their analysis to include migration.
Holmgren & Hedenström (1995) have previously tackled the problem of the optimal timing of moult in migratory birds using dynamic programming. One main finding of their analysis was that birds should replace their feathers before the activity that benefits the most from fresh feathers. However, they had to make some restricting assumptions, such as a fixed time of breeding, and so fixed terminal reward, and a fixed rate of feather degradation, to make the problem tractable. The new technique of Houston & McNamara (1999) allows us to remove these restrictions and to investigate the evolution of optimal annual routines for migratory birds in a more general manner.
Feathers are constantly exposed to degrading agents, such as UV-B radiation (Bergman 1982), keratin-digesting bacteria (Burtt & Ichida 1999) and mechanical abrasion and wear (Bergman 1982; Bonser 1995; Merilä & Hemborg 2000; Butler & Johnson 2004). Unlike bones, feathers lack the capability of self-repair. Hence, moult, the regular replacement of feathers, has evolved into a major event in avian life histories. Moult is costly in terms of energy (Lustick 1970; Lindström et al. 1993b; Murphy 1996) and time (Jenni & Winkler 1994) and may be in conflict with other activities such as reproduction and migration (Nilsson & Svensson 1996; Hemborg et al. 1998). Long-distance avian migrants often make use of highly productive, but short, high-latitude summers for reproduction. However, during the short summers, time has also to be found for the preparations for migration, and moult has to be fitted into the routine as well.
A great deal of variation exists in moulting patterns in relation to migration (Stresemann & Stresemann 1966; Ginn & Melville 1983; Jenni & Winkler 1994; Leu & Thompson 2002; Rohwer et al. 2005): flight feather replacement may be undertaken in the summer immediately after breeding, after arrival in the wintering quarters or there may even be two complete moults every year, as in the willow warbler, Phylloscopus trochilus (Prys-Jones 1991; Underhill et al. 1992). This broad scheme of evolutionary solutions hides large amounts of finer temporal and spatial inter- and intraspecific variations in moult–migration patterns. Migrants on their tropical wintering quarters may, for instance, moult at the beginning or the end of their stay (Salewski et al. 2004). The great reed warbler, Acrocephalus arundinaceus, stops over in the savannah belt in West Africa for a rapid moult before migration is resumed for destinations further south (Hedenström et al. 1993). The river warbler, Locustella fluviatilis, is unusual in that it moults variable numbers of outer primaries twice a year; birds leave their breeding grounds with worn feathers, moult a few outer primaries in Sudan and then all primaries, including the recently renewed outer primaries, on their wintering grounds (Pearson & Backhurst 1983). Savi's warbler has a similarly complex routine (Neto et al. 2006). Leu & Thompson (2002) suggest that moult during migration in Neotropical migrants may be more common than previously assumed.
Among many waterfowl, such as geese and ducks, after breeding there is often a so-called moult–migration to areas where moult takes place before the onset of migration to the winter grounds (Piersma 1987, 1988). Typically, these moult–migrations are not on the way to the winter destinations, but may rather be in the opposite direction and so extend the annual migration distance significantly (Kjellén 1994).
Arctic-breeding waders show complex inter- and intraspecific relationships between the timing of breeding, moult and migration. As an example, in southern Alaskan populations of the dunlin, Calidris alpina, breeding, moult and migration are mutually exclusive, whereas in northern Alaska and eastern Siberia, dunlin overlap moult with egg incubation and chick attendance (Holmes 1971; Holmgren et al. 2001). European populations moult after migration on their wintering grounds. Dunlins migrating along the coast of the Baltic overlap moult and migration (Holmgren et al. 1993). Purple sandpipers, Calidris maritima, also show a complex pattern (Summers et al. 2004).
It is still unclear what factors may account for this still far from fully documented variation in moult–migration patterns (Leu & Thompson 2002). Observed patterns suggest migration distance and the seasonality of food supply as important causes. All short-distance migrants to temperate wintering grounds use the moult-after-breeding strategy. Among Palaearctic migratory songbirds, about half of the long-distance migrants moult directly after breeding while the other half moult on the wintering grounds. In some cases, the timing of moult is intermediate, such as in the barred warbler, Sylvia nisoria, where the flight feather moult is split between summer and winter grounds (Hasselquist et al. 1988; Lindström et al. 1993a). In addition, depending on the population, the degree of moult suspension may vary a great deal (Swann & Baillie 1979; Hedenström et al. 1995). There is hence some correlation between migration distance and the timing and location of moult (Svensson & Hedenström 1999). Whether Neotropical migrants moult on their breeding or wintering grounds apparently also depends on the relative food availability at these locations (Leu & Thompson 2002). Leu & Thompson (2002) show that a significantly larger proportion of Neotropical migrants moult on the breeding ground in eastern versus western North America. The western areas become dry and unproductive by the end of the breeding season and it may be impossible for the birds to find enough energy both to prepare for migration and to replace feathers. Furthermore, habitat preferences might affect feather wear and thus moult schedules (Rohwer et al. 2005). Svensson & Hedenström (1999) suggested that the willow warbler's preference for open habitats on its wintering grounds might accelerate feather degradation through UV-B exposure and therefore force the birds to moult a second time before spring migration.
Here we present a model which allows the systematic investigation of how various factors and their interaction affect optimal annual routines of avian migrants. In this paper, we are mainly concerned with the large-scale patterns, i.e. we investigate strategies of moult and migration but do not consider the fine details of these strategies. For example, although we are concerned with the location at which birds moult, we do not present results on the details of the timing of moult within a given area.
2. The model
We consider the behaviour of a female bird and all its female descendants over a period of many years. A year is divided into T=52 weeks, where week 0 is the middle of the winter in the Northern Hemisphere. At the start of each week (i.e. at times t=0, 1,…, T−1), the bird has four available classes of behavioural actions relating to (i) reproductive behaviour, (ii) foraging intensity, (iii) migration, and (iv) moult of the primaries. It simultaneously performs one action from each class. The performance of an action in one class does not constrain the action performed in another class a priori. The action taken by the bird can depend on the time of the year and its state which is represented by five state variables: quality of its feathers F; experience e; ‘family status’ a; energy reserves r; and location l. The action taken influences the bird's future state.
(a) Feathers and moult
We have chosen to model the moult of a bird's primary feathers rather than other feather groups for two reasons. First, primary moult extends over virtually the entire moult period and is usually taken as a reference for the process of moult in the other feather tracts (Jenni & Winkler 1994). Hence, the moult of primaries is well studied. Second, primaries are considered to be the feathers that have the strongest effect on flight ability (Jenni & Winkler 1994). As a consequence of these points, modelling the moult of primaries may provide the most valuable (and testable) predictions.
The feather quality variable F can vary (in steps of 1/10) between 0 and 1, where 0 means that feathers are in very poor condition while 1 indicates newly moulted feathers. We assume that the rate of abrasion of unmoulted feathers during a given week t is proportional to the amount of energy spent during that week. Decreasing feather quality decreases flight ability (see the electronic supplementary material, appendix A.1, equation (A.3)) and so increases predation risk and energy expenditure (for the functions that we use, see equations (2.10) and (A.13)).
The above quality measure applies to unmoulted feathers, but we also need to represent the state of feathers that are regrowing during the process of moult. To do this, we allow F to also take negative integer values (−mlength≤F≤−1) to code that moult is in progress. Here mlength represents the minimum duration of moult (see the electronic supplementary material, appendix A.1 for details). Starting moult results in an instantaneous change in the feathers' state at the beginning of the week to F=−mlength. The state of feathers then tends to increase stochastically (see the electronic supplementary material, appendix A.1, equation (A.2)) until Ft=−1 when the bird will deterministically have completely new feathers at the start of the next week, i.e. Ft+1=1. Since feathers are renewing gradually during moult, we assume that the flight ability of a moulting bird increases as moult progresses (see the electronic supplementary material, appendix A.1, equation (A.3)). The synthesis of new flight feathers during moult requires an amount of energy, Δm(F), per week (Lustick 1970; Lindström et al. 1993b; see the electronic supplementary material, appendix A.4 (A.12)).
(b) Locations
We consider that the birds can use several distinct locations labelled by l (l=0…lmax), where location 0 is considered as the most northern location while lmax is the southernmost one. Locations are assumed to be linearly arranged so that a bird can only migrate from location l to location l+1 or l−1. In the implementation of the model, all parameters can depend explicitly on the location but in the paper, to simplify notation, we mark this dependence only for those parameters that were set to different values at different locations.
The exact amount of the food available in week t at location l, g(t, l), depends on the environmental food supply on a given location l and the competition between birds for this food (see the electronic supplementary material, appendix A.2 for details). The environmental food supply, G(t, l), varies sinusoidally over the year in a given location l. Its yearly average is given by Afood(l) while its maximal deviation from the average is denoted by ϵ(l). The food supply in a given location is Afood(l)−ϵ(l) at time t=0 and is Afood(l)+ϵ(l) at time t=26. Locations can differ in both Afood and ϵ. If ϵ(l)>0, we consider location l as being north of the equator while ϵ(l)<0 indicates that location l is south of the equator. Note we assume that years are identical, i.e. Afood(l) and ϵ(l) are the same for all years in a given location, so that we do not consider randomly fluctuating environments here.
(c) Energy intake
The bird's energy intake in a given location depends on its foraging intensity u, the availability of food in that location g(l, t) and the bird's experience e. At the beginning of each week, the bird adopts a foraging intensity, u (0≤u≤1), and forages with this intensity throughout that week. Intensity 1 means that the bird gains the maximal possible gross energetic intake, while intensity 0 means that the bird does not feed at all.
Because it is reasonable to assume that newly fledged birds forage with lower efficiency than adults, we introduce the state variable experience, e. This variable takes integer values between 0 (inexperienced) and emax (fully experienced). The newly fledged birds are all inexperienced (e=0), and experience tends to increase after fledgling until full maturation (e=emax, see the electronic supplementary material, appendix A.2 for details). Foraging efficiency depends on experience as follows. Let γ be the gross energetic intake of a fully experienced individual. Then the gross energetic intake for a bird with experience e (adopting the same foraging behaviour as a fully experienced one) is , where θ<1.
Finally, we assume that the gross energy intake increases linearly with increasing foraging intensity. Then a bird which has experience e and forages with intensity u in week t at location l gains an amount of energy
| (2.1) |
(d) Reproduction
The reproductive actions available to a bird depend on its family status which is indicated by the state variable a. If the bird does not have a territory then a=0. A non-territorial bird can either search for a new territory (hereafter labelled as ‘search’) or alternatively ‘subsist’. If the bird subsists then a remains 0. If the bird searches for a new territory, it obtains one with probability pterr(Nterr, Ns). This probability depends on the number of free territories Nterr and the number of searching birds Ns: many free territories and a low number of searching birds increase the probability of territory occupation (see the electronic supplementary material, appendix A.3 for details). If a bird obtains a territory then a becomes 1, otherwise it remains 0. The bird pays an energetic cost Δterr for territory searching irrespective of whether it occupies one or not during the week. We also assume that the foraging benefits of holding a territory are balanced by the cost of territory defence, i.e. maintaining a territory is neutral in terms of energy. A territory is, however, needed for reproduction.
A territory owner (at=1) can either start a new brood (labelled as ‘start’) or maintain the territory (‘maintain’). If the bird starts a new brood, the bird's reserves are decreased by an amount of energy Δs and its family status will be at+1=2 at the beginning of the next week. The family status of a maintaining bird does not change (at+1=at=1).
If the bird has a brood younger than the maximum allowed brood age (2≤a<amax), it can either continue to care for it and so retain the brood (‘care’) or desert it (‘desert’). The brood dies after the desertion and the bird will have no brood during the next week. If the bird continues to care, the family status increases by one (at+1=at+1), given that the bird is alive and can provide enough food, γbrood, to prevent the starvation of brood members. For simplicity, we assume uniparental care. If the parent bird dies between t and t+1 or is unable to achieve a gross energetic intake γbrood during this period, then all brood members starve to death. In order to get gross intake γbrood, the bird must forage with intensity at least equal to ucrit(e,l,t), where γ(ucrite,l,t)=γbrood. Thus, if ucrit(e,l,t)>1, the bird is forced to desert the brood since brood members will starve even if the mother forages with maximum intensity, u=1. If ucrit(e,l,t)<1 and the parent bird forages with intensity u, where ucrit(e,l,t)≤u≤1, then the nestlings survive and the parent bird receives a gross energy intake of γ(u,e,l,t)−γbrood.
If the brood reaches the maximum brood age (a=amax) and the bird still cares during the week, then it abandons the brood (‘abandon’) at the end of the week. The nestlings become independent at this time. Their experience is then e=0. We assume that their reserves are r=0.5 and their feathers are in top quality, F=1. A bird who deserts or abandons its brood during week t retains its territory, but cannot start a new brood before week t+1, i.e. at+1=1. For simplicity, and because we are mainly interested in moult and migration, we do not optimize over brood size. Instead the number of female young at abandonment nbrood is a parameter of our model. Note, however, that the modelled birds can still control their reproductive effort per year by varying their number of breeding attempts.
(e) Energy reserves
The bird's energy reserves, r, vary (in steps of 1/12) between r=0 and the bird's maximal storage capacity r=1. If energy reserves reach zero, the bird dies of starvation. Energy reserves change as a consequence of foraging and the metabolic expenditure of the actions taken. First, we consider the cases where the bird does not have a territory, i.e. a=0. Suppose that the bird subsists and forages with intensity u during week t at location l and has feather quality F at t. Its reserves at the start of the next week t+1 are then given by the random variable rsubsist, where
| (2.2) |
Here R is a random variable with zero mean representing the stochasticity in metabolic expenditure. The distribution of this random variable is specified in the electronic supplementary material, appendix B.2. Csubsist is the energetic expenditure of a subsisting bird (see equation (A.11)). This expenditure depends on the bird's reserves, its foraging intensity and the quality of its feathers (see the electronic supplementary material, appendix A.4 for further details). If it searches for a territory, its reserves at the start of week t+1 are given by
| (2.3) |
If a bird has found a territory then a=1. We assume that maintaining a territory does not have any energetic consequence for the bird, i.e. rmaintain=rsubsist.
The dynamics of reserves for a bird starting a new brood (now with territory, a=1) are similar,
| (2.4) |
Now suppose that the bird has a brood (a≥2) and continues to care for it. Then it must forage with intensity u, ucrit(e,l,t)≤u≤1, in order to ensure the survival of its nestlings. Its reserves will then be
| (2.5) |
where act is either care or abandon because the brood is assumed to become independent only at the end of the week.
(f) Migration
Migration is instantaneous and happens at the beginning of the week. Birds make only one movement between sites. Let the bird start to migrate in state (F, e, a, r, l), and let its state after finishing migration be (Fmig, emig, amig, rmig, lmig). If the bird migrates north lmig=l−1 given that l>0, i.e. the bird is currently not on the northernmost location. Similarly, if the bird migrates south lmig=l+1, given that it is not on the southernmost location (l<lmax). If a bird migrates it loses its brood and territory, i.e. amig=0. The bird's experience does not change during the instantaneous migratory trip (emig=e). The bird's reserves after migration are given by
| (2.6) |
where
| (2.7) |
where E(F) is the effect of feather quality on flight efficiency and is given by equation (A.3). The bird's feather quality after migration will be
| (2.8) |
Here Cmig is the energetic cost of migration (see above) and ff is a parameter scaling the effect of Cmig.
The bird survives the migratory journey with probability
| (2.9) |
where Mm scales the migratory mortality. As can be seen from this equation, we assume that migratory mortality increases with increasing reserves and decreasing feather quality for the same reasons that overall mortality changes with these variables (see §2g).
(g) Sources of mortality
The bird can die owing to starvation or predation. We assume that predation risk is an accelerating function of foraging intensity (the higher the intensity, the less probable the bird detects an approaching predator), and body reserves (Hedenström 1992; Slagsvold & Dale 1996; Lind et al. 1999), and decreases as flight efficiency E(F) increases (equation (A.3)). The birds also suffer from background mortality, Mb, which is independent of behaviour. The probability of mortality per week which is unrelated to starvation is given by
| (2.10) |
where Mf is a parameter that scales the reserve-dependent predation hazard. Mortality acts during the week. Thus, if a bird dies between t and t+1, then any young that became independent at t are not affected, but any young that are still dependent at t (i.e. a≤amax at time t) die along with the parent bird.
(h) Determination of the optimal policy
The best policy to adopt depends on the food availability in the environment. Food availability over the annual cycle is specified by the function g(l, t). The best policy for a given g does not simply maximize the lifetime number of young produced by a bird. This is because young produced at some times of year are more likely to survive, and hence have greater reproductive value, than young produced at other times of year. Instead, fitness is maximized by maximizing the long-term rate of growth of descendants (Metz et al. 1992). Equivalently, fitness is maximized by maximizing the expected number of descendants left far into the future. Using this principle, the best policy for given g can be found by dynamic programming back over successive years (and generations) until convergence (McNamara 1991; Houston & McNamara 1999). Details are given in the electronic supplementary material, appendix B.
Conversely, because there is density dependence, the policy adopted by population members determines the food availability. To calculate g from the policy, we follow the population forward in time. In this procedure, we start with an initial specification of numbers of individuals in each state. Given such a population distribution at the start of week t, a measure of competition for food is determined (see the electronic supplementary material, appendix A.2). This then determines g(l, t) for that week (see the electronic supplementary material, appendix A.2) and the population distribution at the start of week t+1. This weekly update is repeated until the food availability over the annual cycle converges, so that it is the same at the same time of year in successive years.
We find the policy that is the best given the food supply that is generated by a population following this same policy. We refer to this self-consistent best policy as the optimal policy, but it is in fact an evolutionarily stable strategy. To find the policy and the corresponding food availability, we iterate over g; we calculate the best policy for a given g, and then update g to be the food availability generated when population members follow this policy (Houston et al. 1988). This iterative procedure usually converges.
3. Results
First, we present two baseline cases. In both of these cases, parameters are such that breeding occurs at site one (the most northerly location). We thus refer to this site as the breeding ground. In one case (the ‘summer moult scenario’), the birds moult on site 1 after breeding. In the other case (the ‘winter moult scenario’), birds moult on site 4. We chose these two cases as baselines because these are the two most common forms of moult–migration strategies reported (Ginn & Melville 1983; Jenni & Winkler 1994). Second, we investigate how moult–migration strategies change if we transform the food distribution of one baseline case to that of the other. Third, we investigate the stability of the two forms of moult–migration strategies by altering several parameters of the model (one at a time). Fourth, we investigate the effects of the parameters that characterize the stopover sites. It turns out that as a consequence of changing some parameters, birds breed at more than one site. Because this behaviour is rather uncommon among real birds (Jenni & Winkler 1994), we also constrain the birds to be able to breed only at site 1 by setting the number of available territories to zero on the other sites and repeat the above analyses.
In all cases, we consider birds to be able to migrate over four sites (site 1 is the northernmost, while site 4 is the southernmost).
(a) Baseline cases
The baseline parameters of the two scenarios differ only in the distribution of food on the sites (table 1, figure 1). Under the summer moult scenario, the food on all but site 2 is less seasonal than under the winter moult scenario. On the other hand, under the winter moult scenario, the food is very seasonal on both sites 1 and 4.
Table 1.
Model's parameters and their baseline values. (Where two values are given, the first one shows the value for the summer moult scenario while the second one those of winter moult scenario. All energetic values are given as the proportion of the bird's maximum fuel storage capacity.)
| parameter | symbol | value |
|---|---|---|
| the effect of energy expenditure on feather abrasion | ff | 0.03 |
| descriptor of feather quality effect | α | 0.6 |
| the flight ability for very worn feathers | mA | 0.2 |
| stochasticity in moult length | ν | 0.15 |
| feather abrasion during migration | fm | 0.05 |
| energetic cost of migration | Cmig | 0.3 |
| migratory mortality | Mm | 0.001 |
| energetic cost of foraging on foot | cu | 0.2 |
| energetic cost of foraging in flight | cf | 0.75 |
| relative importance of flight during foraging | pF | 0.5 |
| energetic cost of territory occupation | Δterr | 1 |
| energetic cost of starting a brood | Δs | 1.1 |
| food provided for brood | γbrood | 1.1 |
| energetic cost of moult | κ | 0.85 |
| reserve-dependent metabolic cost | cr | 0.04 |
| basic metabolic cost | cb | 0.3 |
| background mortality | Mb | 0.0005 |
| reserve-dependent predation hazard | Mf | 0.002 |
| foraging efficiency of inexperienced bird (e=0) | θ | 0.7 |
| reference amount of food available at site 1 | Afood(1) | 1.6, −0.3 |
| reference amount of food available at site 2 | Afood(2) | 1.25, 1.2 |
| reference amount of food available at site 3 | Afood(3) | 0.8, 1.25 |
| reference amount of food available at site 4 | Afood(4) | 1.4, −0.25 |
| extent of seasonality of food at site 1 | ϵ(1) | 1.25, 2.35 |
| extent of seasonality of food at site 2 | ϵ(2) | 0.7, 0.3 |
| extent of seasonality of food at site 3 | ϵ(3) | 0, −0.25 |
| extent of seasonality of food at site 4 | ϵ(4) | −0.3, −2.85 |
| environmental stochasticity in available food | ω | 0.01 |
| parameter of the distribution of environmental stochasticity | E | 3 |
| scaling factor of population size | N0 | 1000 |
| strength of the density dependence | D | 0.1 |
| probability of increasing experience | pe | 0.025 |
| total number of territories | Ntot | 106 |
| parameter of the probability of territory occupation equation | κ | 0.9 |
| parameter of the probability of territory occupation equation | β | 2 |
| minimal length of moult (in weeks) | mlength | 10 |
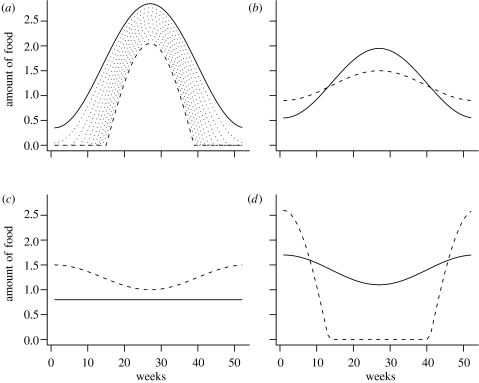
Figure 1.
Temporal distribution of food on the four sites: (a) site 1; (b) site 2; (c) site 3; and (d) site 4. Solid lines, summer moult scenario; dashed lines, winter moult scenario. The dotted lines on (a) illustrate how food is changed in the food manipulation runs.
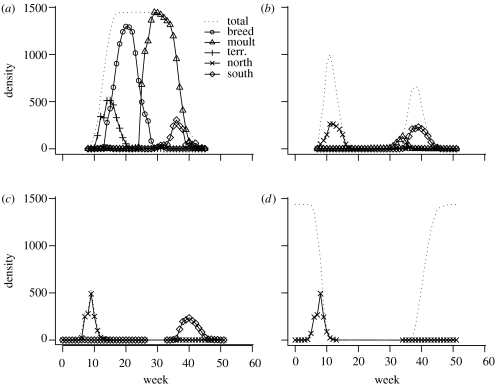
Under the summer moult scenario, the birds breed and moult on site 1 (figure 2). Some birds (10.2% of individuals) migrate from site 1 while still moulting and finish moult on site 2. Birds spend some time on site 2 during migration while most of them leave site 3 immediately after one week. The start date of the spring migration is less variable than that of the autumn migration (SDs of start dates: 1.36 versus 2.88 weeks), but the average time taken to complete the journey is slightly longer in spring than in autumn (4.7 versus 4.1 weeks).
Figure 2.
Modelled birds' behaviour during a year on the four sites: (a) site 1; (b) site 2; (c) site 3; and (d) site 4, under the summer moult scenario. The birds' breeding behaviour is not constrained (see text for details). ‘Total’, the total number of birds on the site; ‘breed’, the number of birds breeding; ‘moult’, the number of birds moulting; ‘terr.’, the number of birds searching for a territory; ‘north’, the number of birds leaving to migrate northward; ‘south’, the number of birds leaving to migrate southward.
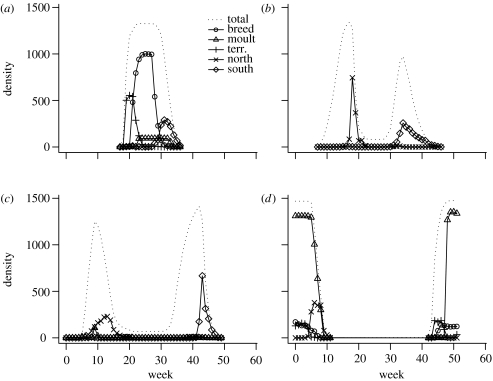
Under the winter moult scenario, most birds breed on site 1 and moult on site 4 (figure 3). Some of the birds (5.9%), however, moult on site 1 and many of them (22.1%) breed on site 4. Even after intensive search in the parameter space, we were unable to force the modelled birds to breed only on site 1 while moulting on site 4 just by changing the distribution of food. Birds spend several weeks on sites 2 and 3 during migration. The start of spring migration is also slightly less variable than the autumn one (1.23 versus 1.6 weeks), and again the duration of the spring journey is a little bit longer than that of the autumn journey (11 versus 10.5 weeks).
Figure 3.
Modelled birds' behaviour during a year on the four sites: (a) site 1; (b) site 2; (c) site 3; and (d) site 4, under the winter moult scenario. The birds' breeding behaviour is not constrained (see text for details). For detailed caption, see figure 2.
Intensive computations reveal that strong seasonality on both sites 1 and 4 is a prerequisite for winter moult to occur. As a consequence of strong seasonality, birds spend less time on the breeding (13.7 versus 24.1 weeks) as well as on the wintering ground (15.1 versus 19.2 weeks) under the winter moult scenario than under the summer one. The duration of the migratory journeys are also longer for the winter moult scenario than for the summer moult scenario (spring migration, 11 versus 4.7 weeks; autumn migration, 10.5 versus 4.1 weeks).
Apart from the fact that the birds do not breed at sites other than site 1, the birds' behaviour is remarkably similar in the runs where they were only allowed to breed on site 1 to those in the unconstrained runs both under the summer and winter moult scenarios. Even the timing of events shows great similarity (table 2).
Table 2.
A comparison of the timing of different events in the unconstrained and constrained baseline scenarios. (Column headings: ‘S-UC’ is summer moult, unconstrained; ‘S-C’ is summer moult, constrained; ‘W-UC’ is winter moult, unconstrained; ‘W-C’ is winter moult, constrained. All numbers are averages in weeks.)
| event | S-UC | S-C | W-UC | W-C |
|---|---|---|---|---|
| start of breeding | 16.6 | 16.5 | 21.9 | 21.8 |
| start of moult | 26 | 26.1 | 48.1 | 48.1 |
| start of spring migration | 7.6 | 7.4 | 6.7 | 6.5 |
| start of autumn migration | 36.3 | 36.4 | 32.1 | 32.1 |
| duration of summer stay | 24.1 | 24.2 | 13.7 | 14.1 |
| duration of winter stay | 19.2 | 18.9 | 15.1 | 14.8 |
| length of spring migration | 4.7 | 4.9 | 11 | 11.4 |
| length of autumn migration | 4.1 | 4.1 | 10.5 | 10.1 |
(b) Effects of food
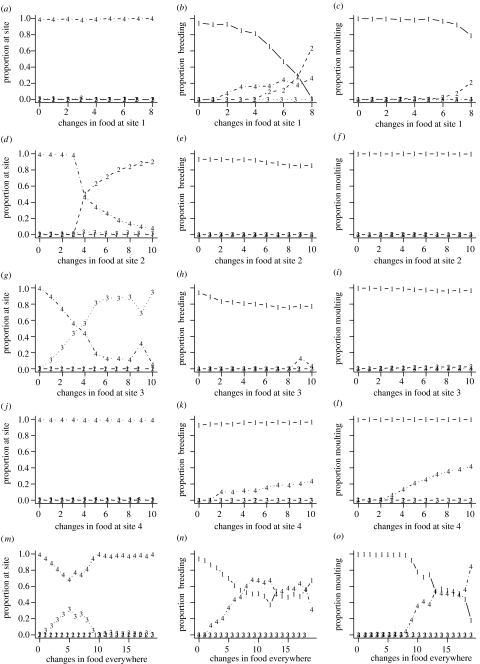
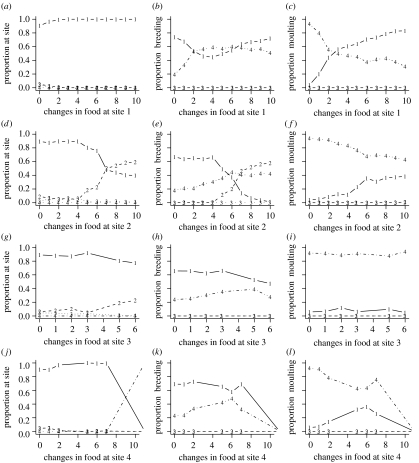
To investigate the effects of food, we have gradually transformed the food distribution at a site from its form under one scenario to that under the other scenario (see the dotted lines in figure 1a), first, at one site at a time, then at all sites simultaneously. We have only varied food from one scenario towards the other because computations show that changing food away from the other scenario quickly leads to the extinction of the birds.
At site 1 changing the food distribution from its summer moult form to its form under the winter moult scenario means more seasonal and less abundant food (figure 1a). As a consequence, birds increase their use of site 2 at the expense of site 1, for both breeding and moult (figure 4a–c). As food becomes more seasonal, the frequency of moult–migration also increases (table 3). Changing food at site 2 changed the migration patterns, birds only migrate to site 2 and spend the winter there (figure 4d–f). The resulting shortening of the migratory journey increases the survival prospect of birds, so they decrease the mean number of breeding attempts from 1.46 to 1.11. Similarly, modifying the food distribution at site 3 shortens the migratory journey (site 3 becomes the primary wintering site), and decreases the number of breeding attempts (figures 4g–i). In these two cases, the pattern of moult does not change. Increasing the seasonality of food at site 4 leads to more frequent biannual moult and breeding (figures 4j–l), using both sites 1 and 4. Note that variation in food has not caused a transition from summer moult to winter moult in any of these cases. Constraining the birds' breeding does not change their behaviour (not shown).
Figure 4.
Effects of transforming the distribution of food away from the summer moult scenario towards the winter moult scenario (for details see text); breeding is unconstrained. (a–l) The cases where food was transformed at sites 1, 2, 3 and 4, respectively. (m–o) Food was varied at all sites simultaneously. (a, d, g, j and m) The distribution of birds over the sites at week 0 (winter). (b, e, h, k and n) The proportion of birds breeding, and (c, f, i, l and o) the proportion of birds moulting over the year at the different sites. The proportion of birds is calculated as the proportion of all birds who survived the whole year. The ‘changes in food’ symbolizes the transition of food distribution from the summer moult scenario towards the winter moult scenario at a given site (as shown in figure 1a; the numbers are arbitrary labels). Number 0 is the baseline case shown in figure 2.
Table 3.
Effects of the manipulations of food on the birds' behaviour. ((a) Taking the unconstrained summer moult scenario, food is changed in the direction of the unconstrained winter moult scenario (as shown in figure 1a). (b) Food is changed from the unconstrained winter moult scenario in the direction of the unconstrained summer moult scenario. (c) Food is changed from the constrained winter moult scenario in the direction of the constrained summer moult scenario. The signs indicate how the given value changed with food (no sign means change less then 10%, one sign signals changes between 10 and 50% and two signs show changes more than 50%; n.a. means not applicable). ‘Sites’ marks where food was changed. ‘Pr. of moult’ shows the proportion of birds moulting at site 1 or 4. ‘Moult overlap’ gives the proportion of birds that actively moult during migration (‘migr.’) or breeding (‘breed.’). ‘No. of breed.’ shows the average number of breeding attempts per bird at site 1 or 4. ‘Distribution’ gives the proportion of birds at site 1 on week 26 (‘summ.’) and at site 4 on week 0 (‘wint.’).)
| sites | pr. of moult | moult overlap | no. of breed. | distribution | ||||
|---|---|---|---|---|---|---|---|---|
| site 1 | site 4 | migr. | breed. | site 1 | site 4 | summ. | wint. | |
| (a) unconstrained: from summer to winter moult scenario | ||||||||
| site 1 | − | ++ | − | −− | + | − | ||
| site 2 | − | −− | ||||||
| site 3 | − | −− | ||||||
| site 4 | + | + | + | |||||
| all | −− | ++ | − | ++ | + | |||
| (b) unconstrained: from winter to summer moult scenario | ||||||||
| site 1 | ++ | − | + | + | − | |||
| site 2 | + | − | + | −− | + | −− | ||
| site 3 | − | + | − | |||||
| site 4 | + | − | + | + | ||||
| (c) constrained: from winter to summer moult scenario | ||||||||
| site 1 | + | − | ++ | n.a. | − | |||
| site 2 | n.a. | |||||||
| site 3 | − | n.a. | − | |||||
| site 4 | n.a. | |||||||
The manipulation of the food from its winter moult scenario distribution to its summer moult one has a more uniform effect (table 3, figures 1 and 5). In almost all cases, under small deviations from the food distribution of the winter moult scenario, birds start to use both sites 1 and 4 for both breeding and moult (figure 5). A detailed analysis shows that in these cases the birds basically follow two separate life histories; one in which they moult on site 1 and breed on site 4, and one in which they moult on site 4 and breed on site 1. Note that, (i) in these cases the manipulation of food on one site cannot change the routine from winter moult to summer moult and (ii) the use of both endpoints of the migratory route for both breeding and moult occurs for small deviations from the baseline case.
Figure 5.
Effects of transforming the distribution of food away from winter moult scenario towards the summer moult scenario (for details, see text); breeding is unconstrained. (a, d, g and j) The distribution of birds over the sites at week 0 (winter). (b, e, h and k) The proportion of birds breeding and (c, f, i and l) the proportion of birds moulting over the year at the different sites. The proportion of birds is calculated as the proportion of all birds who survived the whole year. The ‘changes in food’ symbolizes the transition of food distribution from the winter moult scenario towards the summer moult scenario at a given site (as shown in figure 1a; the numbers are arbitrary labels). Number 0 is the baseline case shown in figure 2. (a–l) The cases where food was transformed on sites 1, 2, 3 and 4, respectively.
When birds are constrained in breeding, the effects of transforming food from the winter moult scenario are different from that under unconstrained breeding (table 3). Food manipulation at sites 2 and 4 has no effect, while at site 3 its effect can mainly be attributed to the high mortality. At site 1, increasing food raises the proportion of birds moulting at site 1, which, in turn, results in biannual moult in many birds.
Varying the food distribution at all sites simultaneously leads to a swap from summer to winter moult (figure 4m–o). Even in this case, however, the birds use both sites 1 and 4 more or less equally for moult as well as breeding under most intermediate food distributions. When food is varied at all sites at the same time and the birds are constrained, there is a smooth transition from summer to winter moult.
(c) The effects of parameters
To investigate how stable the modelled birds' behaviour is under the baseline cases, we systematically vary several parameters concerning general life history, cost of moult, feather quality and cost of migration (one at a time; table 4). Under the summer moult scenario, moult is only affected by the energetic cost of moult, κ (table 5); low cost increases the proportion of birds moulting at site 4 leading to biannual moult in many birds. Many parameters influence the overlap between moult and either migration or breeding. These effects can be traced back to the fact that increasing these parameters leads to decreasing survival, which, in turn, increases the number of breeding attempts in a year. This means less time for moult and so the moult–breeding overlap increases. Increasing the cost of migration (either the energetic cost, Cmig, or the mortality cost, Mm) decreases the number of sites used, resulting in fewer birds wintering at site 4. The reaction of constrained birds to varying parameters (not shown) is almost identical to that of unconstrained birds.
Table 4.
The manipulated parameters and the range of manipulation. (For the baseline values of the parameters, see table 1.)
| parameter | symbol | range |
|---|---|---|
| reserve-dependent metabolic cost | cr | 0 …0.06 |
| background mortality | Mb | 0 …0.01 |
| reserve-dependent predation hazard | Mf | 0 …0.01 |
| energetic cost of moult | κ | 0.5 …1.5 |
| descriptor of feather quality effect | α | 0.1 …1 |
| effect of energy expenditure on feather abrasion | ff | 0.02 …0.04 |
| the flight ability for very worn feathers | mA | 0.1 …0.5 |
| energetic cost of migration | Cmig | 0.1 …0.5 |
| feather abrasion during migration | fm | 0 …0.1 |
| migratory mortality | Mm | 0 …0.1 |
Table 5.
Effects of the parameter manipulations (see table 4 for details) on the birds' behaviour under the unconstrained summer moult scenario (see text for details). (For an explanation of headings and symbols, see table 3.)
| symbol | pr. of moult | moult overlap | no. of breed. | distribution | ||||
|---|---|---|---|---|---|---|---|---|
| site 1 | site 4 | migr. | breed. | site 1 | site 4 | summ. | wint. | |
| cr | + | + | ||||||
| mb | − | ++ | ++ | + | ||||
| mf | − | − | − | |||||
| κ | − | − | − | + | ||||
| α | − | + | ++ | |||||
| ff | ++ | ++ | ||||||
| mA | − | −− | ||||||
| Cmig | − | ++ | ++ | −− | ||||
| fm | + | ++ | ||||||
| Mm | − | ++ | ++ | − | ||||
Under the winter moult scenario, the changes in the parameters have more effects than under the summer moult scenario (table 6). Varying parameters results in decreased journey duration in more cases, but in contrast to the summer moult scenario birds use site 1 less frequently. This decrease in the proportion of birds spending the summer at site 1 naturally decreases the proportion of breeding birds there. Moult and moult–migration are affected only by the energetic cost of moult and parameters describing the effect of feather abrasion (α, ff, mA). When the cost of moult is low, birds use site 1 for moult and site 4 for breeding, which is just the opposite of that found under the baseline case. Low cost of moult again results in many birds migrating while moulting. When the effect of feather quality on flight ability is very nonlinear (small α), birds use both sites 1 and 4 for moult. As α increases, birds prefer site 4 for moult rather than site 1. When abrasion rate (ff) is low, many birds skip moult in some years. As a result, increasing abrasion rate leads to an increasing proportion of birds moulting. If mA is high (i.e. worn feathers do not decrease flight efficiency strongly), many birds skip moult in some years. Because moult and breeding usually occur at different sites separated in time by many weeks, the frequency of moult–breeding overlap is very low. More interestingly, the frequency of moult–migration is also very low (except in the case of very low moult cost).
Table 6.
The effects of the parameter manipulations (see table 4 for details) on the birds' behaviour under the winter moult scenario (see text for details). (For an explanation of headings and symbols, see table 3.)
| symbol | pr. of moult | moult overlap | no. of breed. | distribution | ||||
|---|---|---|---|---|---|---|---|---|
| site 1 | site 4 | migr. | breed. | site 1 | site 4 | summ. | wint. | |
| breeding unconstrained | ||||||||
| cr | ||||||||
| Mb | + | |||||||
| Mf | −− | |||||||
| κ | −− | ++ | −− | ++ | −− | − | ||
| α | − | ++ | −− | − | ||||
| ff | + | + | − | − | ||||
| mA | − | − | − | − | ||||
| Cmig | − | + | − | |||||
| fm | − | − | ||||||
| Mm | + | |||||||
| breeding constrained | ||||||||
| cr | n.a. | |||||||
| Mb | n.a. | |||||||
| Mf | n.a. | |||||||
| κ | − | + | − | + | n.a. | |||
| α | −− | n.a. | − | |||||
| ff | −− | n.a. | − | |||||
| mA | n.a. | |||||||
| Cmig | − | n.a. | − | |||||
| fm | − | n.a. | − | |||||
| Mm | n.a. | |||||||
When birds are constrained under the winter moult scenario, changing the parameters has less effect than under the unconstrained runs (table 6). Moult is affected only by the energetic cost of moult.
Under both the summer and winter moult scenarios, if high migration costs force the birds to shorten their migratory journey, they will eliminate the endpoint of the migratory route at which less food can be obtained; site 4 under the summer moult scenario and site 1 under the winter moult scenario (tables 5 and 6).
(d) Conditions on stopover sites
We have investigated the effects of stopover conditions on the birds' behaviour by varying parameters in table 4 on either site 2 or 3 under the two scenarios.
Under the summer moult scenario, the effects of changes on the two stopover sites are different in their consequences (table 7). Changes at site 2 influence moult, while changes at site 3 affect the number of sites used during the migratory journey. Both low energetic cost of moult, κ, and small effect of very worn feathers (mA close to 0.5) at site 2 result in many birds leaving site 1 (the breeding ground) immediately after breeding and moulting completely at site 2. The proportion of birds migrating while moulting is also increased. At site 3, low reserve-dependent metabolic rate, low feather abrasion, high energetic cost of migration and high migratory predation risk lead to a shortened migratory journey, i.e. many birds stop at site 3 and do not migrate further to site 4 (table 7). Other parameters changed at either site 2 or 3 do not influence moult–migration strategies. They do, however, significantly influence breeding; when conditions at either site 2 or 3 are worsened, birds breed more times at site 1 to compensate for the lowered survival prospect at the stopover sites. This also results in a high proportion of overlap between moult and breeding. The constrained and unconstrained calculations under the summer moult scenario were very similar to each other.
Table 7.
Effects of when parameters are manipulated (see table 4 for details) only at site 2 or 3 under the unconstrained summer moult scenario (see text for details). (For an explanation of headings and symbols, see table 3.)
| symbol | pr. of moult | moult overlap | no. of breed. | distribution | ||||
|---|---|---|---|---|---|---|---|---|
| site 1 | site 4 | migr. | breed. | site 1 | site 4 | summ. | wint. | |
| changes at site 2 | ||||||||
| cr | + | |||||||
| Mb | ++ | ++ | ||||||
| Mf | − | + | ++ | |||||
| κ | + | −− | + | |||||
| α | − | + | ||||||
| ff | + | |||||||
| mA | − | + | − | − | ||||
| Cmig | ++ | ++ | + | |||||
| fm | + | |||||||
| Mm | ++ | ++ | + | |||||
| changes at site 3 | ||||||||
| cr | + | + | ||||||
| Mb | ++ | ++ | + | |||||
| Mf | + | + | ||||||
| κ | ||||||||
| α | − | + | ++ | |||||
| ff | + | |||||||
| mA | − | −− | ||||||
| Cmig | ++ | ++ | −− | |||||
| fm | + | |||||||
| Mm | − | ++ | ++ | −− | ||||
When the parameters at site 2 are varied under the winter moult scenario only the energetic cost of moult and migratory mortality affect the moult strategy (table 8). When the cost of moult, κ, is low at site 2, many birds start to moult at site 1, instead of site 4, and finish moulting at site 2. Increasing κ leads to decreasing moult at sites 1 and 2 and increasing moult at site 4. Breeding changes with κ in the opposite way. Many parameter changes at site 3 influence moult strategy (table 8). Their effect is similar in that increasing the cost of moult (either energetic or flight efficiency cost) means that many birds do not moult at site 4 to avoid finishing moult on the costly site 3 owing to the unpredictable moult length.
Table 8.
Effects of when parameters are manipulated (see table 4 for details) only on site 2 or 3 under the unconstrained winter moult scenario (see text for details). (For an explanation of headings and symbols, see table 3.)
| symbol | pr. of moult | moult overlap | no. of breed. | distribution | ||||
|---|---|---|---|---|---|---|---|---|
| site 1 | site 4 | migr. | breed. | site 1 | site 4 | summ. | wint. | |
| changes at site 2 | ||||||||
| cr | ||||||||
| Mb | − | + | − | |||||
| Mf | + | |||||||
| κ | −− | ++ | −− | + | −− | |||
| α | ||||||||
| ff | + | |||||||
| mA | ||||||||
| Cmig | − | + | − | |||||
| fm | + | |||||||
| Mm | −− | ++ | −− | |||||
| changes at site 3 | ||||||||
| cr | ||||||||
| Mb | + | |||||||
| Mf | + | + | ||||||
| κ | + | − | ++ | |||||
| α | + | |||||||
| ff | + | + | + | |||||
| mA | − | − | − | − | ||||
| Cmig | + | − | + | |||||
| fm | ||||||||
| Mm | + | |||||||
The constrained and unconstrained runs differ most when parameters were varied only at one site under the winter moult scenario. At site 2, none of the parameters affected moult strategy (table 9). At site 3, only the energetic cost of moult, κ, has an effect (table 9). Low κ results in many birds leaving site 4 while still moulting. Another effect of the alteration of moult cost is that the birds moult earlier as the cost increases to avoid migration during moult to the area where the cost is high.
Table 9.
Effects of when parameters are manipulated (see table 4 for details) only at site 2 or 3 under the constrained winter moult scenario (see text for details). (For an explanation of headings and symbols, see table 3.)
| symbol | pr. of moult | moult overlap | no. of breed. | distribution | ||||
|---|---|---|---|---|---|---|---|---|
| site 1 | site 4 | migr. | breed. | site 1 | site 4 | summ. | wint. | |
| changes at site 2 | ||||||||
| cr | n.a. | |||||||
| Mb | −− | n.a. | −− | |||||
| Mf | n.a. | |||||||
| κ | n.a. | |||||||
| α | n.a. | |||||||
| ff | n.a. | |||||||
| mA | n.a. | |||||||
| Cmig | − | n.a. | − | |||||
| fm | n.a. | |||||||
| Mm | −− | n.a. | −− | |||||
| changes at site 3 | ||||||||
| cr | n.a. | |||||||
| Mb | n.a. | |||||||
| Mf | − | n.a. | ||||||
| κ | + | − | n.a. | |||||
| α | − | n.a. | ||||||
| ff | n.a. | + | ||||||
| mA | + | n.a. | ||||||
| Cmig | − | n.a. | − | |||||
| fm | n.a. | |||||||
| Mm | n.a. | |||||||
4. Discussion
(a) The moult–migration model
Our optimal annual routine model suggests that the temporal and spatial distributions of food have the most important role in determining the large-scale organization of migratory birds' annual cycle. This is supported by the following. First, only large differences in the distribution of food are able to produce very distinct moult–migration strategies. Summer moult (birds moult on the breeding ground immediately after breeding) occurs when food has a high peak in the summer, but it is less seasonal elsewhere. On the other hand, if there is a short period of high food availability in summer and a strong winter peak at different locations (i.e. the food is very seasonal but in opposite phase on these areas), the birds breed during the summer then migrate to the wintering area and moult there; they follow the winter moult scenario. Second, these two annual schedules are remarkably stable (especially if one considers the cases of constrained breeding). Neither changes in single parameter values nor those in the distribution of food in one location can force the modelled birds to switch from summer moult to winter moult or vice versa. Only changing the food simultaneously in all sites results in a transition from one scenario to the other.
Several empirical findings suggest that variations in the seasonality of the food supply on both breeding and wintering grounds can drive the timing of flight feather moult with respect to migration. First, winter moult occurs only among long-distance migrants (Jenni & Winkler 1994). It can be argued that only in this case can food on the breeding and wintering grounds be both strongly seasonal and out of phase with each other. According to our model, this is necessary to force the birds to moult on the wintering grounds. Second, the differences in moult–migration strategies between Nearctic and Palaearctic systems might also support our argument. In trans-Saharan migrants of the western Palaearctic, the moult of the flight feathers is very variable with respect to autumn migration. In contrast, Neotropical migrants typically moult their flight feathers in the autumn on the breeding grounds. Rohwer et al. (2005) suggest that the different characters of the wintering habitats may explain these differences in moult schedules between Nearctic and Palaearctic passerine migrants. New World migrants typically winter in tropical forests, a much buffered unseasonal environment (Tallman & Tallman 1997). Palaearctic migrants, on the other hand, mainly winter in scrub and acacia savannahs of sub-Saharan Africa, which are more exposed and so are probably more seasonal habitats than the winter habitats of Neotropical migrants (Moreau 1972; Jones 1995). This argument might be further supported by the observation that several long-distance Phylloscopus migrants that winter in Asian tropical forests also replace flight feathers in autumn before migration (Svensson & Hedenström 1999). Third, Neotropical migrants of the western US generally conform to a ‘push–pull’ scenario (Rohwer et al. 2005). Exceedingly dry and unproductive conditions in late summer ‘push’ the birds away and they are being ‘pulled’ towards the Mexican monsoon region or tropical Central and South America, where they then moult. This pattern conforms to our modelling results where a steep drop in resources in late summer selects for winter moult, and good conditions on an intermediate site allow the birds to moult there.
Our model predicts the occurrence of biannual flight feather moult as a consequence of cheap moult (owing to either richness of the food supply or the low energetic cost of moult), but not as a result of fast feather wear. This contradicts the current concept of biannual moult which states that birds moult twice a year owing to the fast abrasion of their feathers (Weber et al. 2005; Svensson & Hedenström 1999). One may argue, however, that even if fast abrasion would favour two moults in a year, the birds can perform them only if favourable conditions (e.g. rich food supply) allow. Therefore, fast abrasion of feathers might be a consequence, instead of the cause of the biannual moult. If favourable condition allows the birds to moult twice, then feathers have to last only half a year instead of a whole year. Therefore, the birds do not need to grow such durable feathers (thus decreasing the cost of moult further), which, in turn, leads to less resistance against wear.
An interesting aspect of our results is the occurrence of breeding on the wintering area. Note that this does not necessarily mean that individual birds breed (or moult) at both endpoints in the same year; rather, as our computations show, the population is split into two more or less separated subpopulations in which birds follow different schedules. According to our model, even a small alteration of food on the breeding or wintering areas under the winter moult scenario results in strategies under which it is optimal to breed (and moult) in both the summer and winter quarters. Consequently, one may expect this phenomenon to be very common in natural populations of birds which have their moult on their winter quarters. This behaviour of breeding on both the summer and winter grounds, however, occurs very rarely in reality. To our knowledge, this has been reported only in a Scandinavian population of dippers, Cinclus cinclus, where evidence shows that some females breed in the spring while still in their winter quarters and then once again later in the summer after a northward migration (Vuorinen 1999). Why is this so rare? One possibility is that birds are exhausted by breeding twice a year. But this explanation does not exclude the possibility of two subpopulations of birds breeding and moulting only once in a year. A more plausible explanation could be increased competition on the wintering areas (Cox 1968). The distribution of large landmasses on Earth is highly skewed towards the Northern Hemisphere. Owing to this uneven distribution of land, large numbers of migratory birds from large breeding areas gather into much smaller wintering areas in the Southern Hemisphere in each year (Mills 2006). This is then hypothesized to result in a high level of competition for food which prevents breeding at wintering sites. One may, however, argue that if competition for food prevents breeding, it might prevent moult too. On the other hand, birds might compete not just for food but for breeding sites too. Furthermore, resources on the wintering ground can vary widely in both space and time; consider, for example, the unpredictable onset of the rainy season. The well-specialized local residents, which are, for instance, quickly able to get into reproductive condition, can cope with this variability, and hence they can breed under these circumstances. The migrants, on the other hand, cannot. They can, however, still moult, because moulting does not immobilize them as breeding would do, so they are able to track these rich but locally ephemeral resources. We conclude this paragraph with two remarks. First, more empirical investigations are needed to clarify why migrants breed only at one of their journey's endpoints. Second, without our flexible optimal annual routine approach, this question cannot be theoretically addressed.
(b) Future directions
(i) Migration research
The annual routine model of migration that we have presented can be easily tailored to study the problems of migration research outlined in §1. Introducing sexes as state variables can allow us to investigate the factors leading to differential migration (Ketterson & Nolan 1976; Ketterson & Nolan 1979). Birds of different sexes can be allowed to follow different behaviours, e.g. males can obtain territories while females can obtain territory holding males. This would allow us to theoretically investigate the hypothesis that the need to obtain territories by males in spring drives males to winter closer to the breeding areas than females. In addition, males and females can differ in their energetics, foraging efficiency, etc., making it possible to study the effects of body size and tolerance of winter starvation on the evolution of partial migration. By choosing appropriate density functions for males and females, one can investigate the role of competition for food on the winter quarters too. By retaining feather quality and moult in this two-sex model, the differences between the sexes in breeding–moult overlap (Hemborg 1998, 1999a,b; Hemborg & Lundberg 1998) can also be studied. A crucial problem with the above approach is how to handle mate choice and parental care. As a first attempt, one might ignore parental care by assuming uniparental care by the female. On the other hand, mate choice must be handled in some explicit way, because male quality (or the quality of the territory obtained by the male) can depend directly on the male's migratory behaviour (e.g. on time of arrival at the breeding ground). Introducing territories of different qualities might be a way around this problem.
Partial migration can be studied in a model with only two sites. One can start with identical sites and then change the sites gradually and record when part of the population starts to migrate. Members of the population can be characterized by additional state variables, such as immune condition or foraging ability, to investigate which class of individuals is expected to start to migrate first. By changing the variables in which the sites differ, one can assess the role of different factors (e.g. food, predation, parasites) in the initiation of migration. This kind of modelling work can also shed light on the evolutionary origin of migratory behaviour.
It is widely hypothesized that competition and density-dependent effects play a significant role in the evolution of migratory behaviour (Cox 1968). Much less is known, however, about how the different forms of competition (e.g. for food or territories) influence migration. Information about how density dependence acting through different factors, such as food, predation or disease, can affect the evolution of migration is also limited. By applying different forms of density dependence, it is possible to separate these effects with our model.
A further possibility in studying the evolution of migration is that one can create a large array of sites which form a fine gradient of slightly different neighbouring environments in the model. This environmental gradient can then be used to investigate the factors leading to leap-frog or chain migration (Bell 2005).
(ii) General issues
One of the main advantages of the optimal annual routine approach is the ability to unify the study of several organizational levels under the umbrella of natural selection. Since Darwin's (1859) seminal work it is widely accepted that all forms of life on Earth are largely shaped by the process of natural selection, which mainly acts on the organizational level of individuals (Williams 1966). Consequently, the level of individuals should play a central role in the understanding of the biology of other levels of organization, most notably, the organizational level of physiology (e.g. the neuroendocrine system) and the level of populations. In the case of the within-individual level, one should note that all physiological mechanisms are functioning for the ‘benefits’ of the individual that contains them (Williams 1966). In the case of populations, all behaviours of a population (e.g. birth or death rate) emerge from its members' behaviour which is shaped by natural selection. Therefore, we argue that a new conceptual tool, which is based on the level of individuals but allows the easy connection between the different organizational levels, may help understand the properties and processes of both the organizational levels within an individual and the level of populations. We propose that the technique for finding optimal annual routines used here (McNamara et al. 1998; Houston & McNamara 1999; Barta et al. 2006) may serve as such an important conceptual tool. On one hand, this technique is based on the theory of state-dependent dynamic models, which is nowadays widely used to find optimal individual behaviour. Consequently, the technique describes the connection between within-individual entities (state variables) and optimized individual behaviour. On the other hand, the technique ‘automatically’ provides the lifetime reproductive success of the modelled individuals because finding the optimal behaviour involves maximizing the number of descendants left in the far future (Houston & McNamara 1999). Therefore, it might also be used to predict the behaviour of populations of optimally behaving individuals (especially if some form of density dependence is included).
This approach offers several novel ways to study within-individual and population processes. First, the technique can be used to study the possible attributes of a within-individual control system which is capable of producing optimal behaviour. The results of this kind of investigation might contribute to the development of a new evolutionary understanding of the neuroendocrine system. Second, a new class of ecological models can be developed, in which the population processes are analysed and predicted on the basis of individual behaviour shaped by natural selection. This might allow us to predict the effects of sudden environmental changes on population trends. Currently, we are using the annual routine model of moult–migration presented here to predict the effects of food shortage on sites along the migratory route. Third, investigations under the previous two points can be joined to study whether state variables respond more readily to environmental changes than population processes do, and so whether the monitoring of the state of individuals (e.g. body condition, health status, levels of hormones and parasite load) in populations might provide an effective non-invasive tool for predicting population trends and indicating impending disasters in conservation biology (Hill 1995; Piersma & Lindström 2004).
Acknowledgments
We are grateful to P. Bednekoff, D.M. Buehler and T. Piersma for their helpful comments on a previous version. ZB was supported by a Marie Curie Fellowship of the European Community programme “Improving Human Research Potential and the Socio-economic Knowledge Base”, contract number HPMF-CT-2001-01150. JMM acknowledges support of the Leverhulme Trust. TPW was supported by the Swedish Natural Research Council. The collaboration between Bristol and Lund was supported by a Royal Society Joint Project Grant. The study was supported by OTKA Grants (T046661, NF 61143) and a Marie Curie European Reintegration Grant (contract no. 005065).
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
Supplementary Material
Technical description of the optimal annual routine model
References
- Adriaensen F, Dhondt A.A. Population dynamics and partial migration of the European robin (Erithacus rubecula) in different habitats. J. Anim. Ecol. 1990;59:1077–1090. doi:10.2307/5033 [Google Scholar]
- Aidley D.J. Questions about migration. In: Aidley D.J, editor. Animal migration. Cambridge University Press; Cambridge, UK: 1981. pp. 1–8. [Google Scholar]
- Alerstam T. Cambridge University Press; Cambridge, UK: 1990. Bird migration. [Google Scholar]
- Alerstam T. Bird flight and optimal migration. Trends Ecol. Evol. 1991;6:210–215. doi: 10.1016/0169-5347(91)90024-R. doi:10.1016/0169-5347(91)90024-R [DOI] [PubMed] [Google Scholar]
- Alerstam T, Lindström A. Optimal bird migration: the relative importance of time, energy and safety. In: Gwinner E, editor. Bird migration: physiology and ecophysiology. Springer; Berlin, Germany: 1990. pp. 331–351. [Google Scholar]
- Alexander R.M. When is migration worthwhile for animals that walk, swim or fly? J. Avian Biol. 1998;29:387–394. doi:10.2307/3677157 [Google Scholar]
- Barta Z, Houston A.I, McNamara J.M, Welham R.K, Hedenström A, Weber T.P, Feró O. Annual routines of non-migratory birds: optimal moult strategies. Oikos. 2006;112:580–593. doi:10.1111/j.0030-1299.2006.14240.x [Google Scholar]
- Bell C.P. Inter- and intrapopulation migration patterns. In: Greenberg R, Marra P.P, editors. Birds of two worlds. The Johns Hopkins University Press; Baltimore, MD: 2005. pp. 41–52. [Google Scholar]
- Bergman G. Why are the wings of Larus f. fuscus so dark? Ornis Fenn. 1982;59:77–83. [Google Scholar]
- Berthold P. The control of partial migration in birds: a review. Ring. 1984;10:253–265. [Google Scholar]
- Bonser R.H.C. Melanin and the abrasion resistance of feathers. Condor. 1995;97:590–591. doi:10.2307/1369048 [Google Scholar]
- Burtt E.H, Jr, Ichida J.M. Occurence of feather-degrading bacilli in the plumage of birds. Auk. 1999;116:364–372. [Google Scholar]
- Butler M, Johnson A.S. Are melanized feather barbs stronger? J. Exp. Biol. 2004;207:285–293. doi: 10.1242/jeb.00746. doi:10.1242/jeb.00746 [DOI] [PubMed] [Google Scholar]
- Clark C.W, Butler R.W. Fitness components of avian migration: a dynamic model of western sandpiper migration. Evol. Ecol. Res. 1999;1:443–457. [Google Scholar]
- Clark C.W, Mangel M. Oxford University Press; New York, NY: 2000. Dynamic state variable models in ecology. [Google Scholar]
- Cox G.W. The role of competition in the evolution of migration. Evolution. 1968;22:180–192. doi: 10.1111/j.1558-5646.1968.tb03461.x. doi:10.2307/2406662 [DOI] [PubMed] [Google Scholar]
- Darwin C. John Murray; London, UK: 1859. On the origin of the species by means of natural selection or the preservation of favoured races in the struggle for life. [PMC free article] [PubMed] [Google Scholar]
- Dingle H. Oxford University Press; Oxford, UK: 1996. Migration: the biology of life on the move. [Google Scholar]
- Fretwell S.D. Number 5 in Monographs in population biology. Princeton University Press; Princeton, NJ: 1972. Populations in a seasonal environment. [PubMed] [Google Scholar]
- Ginn H.B, Melville D.S. Guide 19. British Trust for Ornithology; Tring, UK: 1983. Moult in birds. [Google Scholar]
- Greenberg R, Marra P.P, editors. Birds of two worlds. The Johns Hopkins University Press; Baltimore, MD: 2005. [Google Scholar]
- Hasselquist D, Hedenström A, Lindström A, Bensch S. The seasonally divided flight feather moult in the barred warbler Sylvia nisoria—a new moult pattern for European passerines. Ornis Scand. 1988;19:280–286. doi:10.2307/3676722 [Google Scholar]
- Hedenström A. Flight performance in relation to fuel load in birds. J. Theor. Biol. 1992;158:535–537. doi:10.1016/S0022-5193(05)80714-3 [Google Scholar]
- Hedenström A, Bensch S, Hasselquist D, Lockwood M, Ottosson U. Migration, stopover and moult of the great reed warbler Acrocephalus arundinaceus in Ghana, West Africa. Ibis. 1993;135:177–180. [Google Scholar]
- Hedenström A, Lindström A, Pettersson J. Interrupted moult of adult willow warblers Phylloscopus trochilus during autumn migration through Sweden. Ornis Svecica. 1995;5:69–74. [Google Scholar]
- Hemborg C. Sexual differences in the control of postnuptional moult in the pied flycatcher. Anim. Behav. 1998;56:1221–1227. doi: 10.1006/anbe.1998.0885. doi:10.1006/anbe.1998.0885 [DOI] [PubMed] [Google Scholar]
- Hemborg C. Annual variation in the timing of breeding and moulting in male and female pied flycatchers Ficedula hypoleuca. Ibis. 1999a;141:226–232. [Google Scholar]
- Hemborg C. Sexual differences in moult-breeding overlap and female reproductive costs in pied flycatchers, Ficedula hypoleuca. J. Anim. Ecol. 1999b;68:429–436. doi:10.1046/j.1365-2656.1999.00295.x [Google Scholar]
- Hemborg C, Lundberg A. Costs of overlapping reproduction and moult in passerine birds: an experiment with the pied flycatcher. Behav. Ecol. Sociobiol. 1998;43:19–23. doi:10.1007/s002650050462 [Google Scholar]
- Hemborg C, Lunberg A, Siikamäki P. Trade-off between reproduction and moult—a comparison of three Fennoscandian pied flycatcher populations. Oecologia. 1998;117:374–380. doi: 10.1007/s004420050670. doi:10.1007/s004420050670 [DOI] [PubMed] [Google Scholar]
- Hill G.E. Ornamental traits as indicators of environmental health. Bioscience. 1995;45:25–31. doi:10.2307/1312532 [Google Scholar]
- Holmes R.T. Latitudinal differences in the breeding and molt schedules of Alaskan red-backed sandpipers (Calidris alpina) Condor. 1971;73:93–99. doi:10.2307/1366128 [Google Scholar]
- Holmgren N, Hedenström A. The scheduling of molt in migratory birds. Evol. Ecol. 1995;9:354–368. doi:10.1007/BF01237759 [Google Scholar]
- Holmgren N, Ellegren H, Pettersson J. The adaptation of moult pattern in migratory dunlins Calidris alpina. Ornis Scand. 1993;24:21–27. doi:10.2307/3676405 [Google Scholar]
- Holmgren N, Jönsson P, Wennerberg L. Geographical variation in the timing of breeding and moult in dunlin Calidris alpina on the Palearctic tundra. Polar Biol. 2001;24:369–377. doi:10.1007/s003000000222 [Google Scholar]
- Houston A.I. Models of optimal migration: state, time and predation. J. Avian Biol. 1998;29:395–404. doi:10.2307/3677158 [Google Scholar]
- Houston A.I, McNamara J.M. Cambridge University Press; Cambridge, UK: 1999. Models of adaptive behaviour. [Google Scholar]
- Houston A.I, McNamara J.M, Clark C.W, Mangel M. Dynamic models in behavioural and evolutionary ecology. Nature. 1988;332:29–34. doi:10.1038/332029a0 [Google Scholar]
- Jenni L, Winkler R. Academic Press; London, UK: 1994. Moult and ageing of European passerines. [Google Scholar]
- Jones P.J. Migration strategies of Palearctic passerines in Africa. Israel J. Zool. 1995;41:393–406. [Google Scholar]
- Kaitala A, Kaitala V, Lundberg P. A theory of partial migration. Am. Nat. 1993;142:59–81. doi:10.1086/285529 [Google Scholar]
- Ketterson E.D, Nolan V., Jr Geographic variation and its climatic correlates in the sex ratio of eastern-wintering dark-eyed juncos (Junco hyemalis) Ecology. 1976;57:679–693. doi:10.2307/1936182 [Google Scholar]
- Ketterson E.D, Nolan V., Jr Seasonal, annual, and geographic variation in sex ratio of wintering populations of dark-eyed juncos (Junco hyemalis) Auk. 1979;96:532–536. [Google Scholar]
- Kjellén N. Moult in relation to migration—a review. Ornis Svecica. 1994;4:1–24. [Google Scholar]
- Kokko H. Competition for early arrival in migratory birds. J. Anim. Ecol. 1999;68:940–950. doi:10.1046/j.1365-2656.1999.00343.x [Google Scholar]
- Leu M, Thompson C.W. The potential of migratory stopover sites as flight feather molt staging areas: a review for neotropical migrants. Biol. Conserv. 2002;106:45–56. doi:10.1016/S0006-3207(01)00228-2 [Google Scholar]
- Lind J, Fransson T, Jakobsson S, Kullberg C. Reduced take-off ability in robins (Erithacus rubecula) due to migratory fuel load. Behav. Ecol. Sociobiol. 1999;46:65–70. doi:10.1007/s002650050593 [Google Scholar]
- Lindström A, Pearson D.J, Hasselquist D, Hedenström A, Bensch S, Åkesson S. The moult of barred warblers Sylvia nisoria in Kenya—evidence for a split wing-moult pattern initiated during the birds' first winter. Ibis. 1993a;135:403–409. [Google Scholar]
- Lindström Å, Visser G.H, Daan S. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 1993b;66:490–510. [Google Scholar]
- Lustick S. Energy requirement of molt in cowbirds. Auk. 1970;87:742–746. [Google Scholar]
- Marra P.P, Hobson K.A, Holmes R.T. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science. 1998;282:1884–1886. doi: 10.1126/science.282.5395.1884. doi:10.1126/science.282.5395.1884 [DOI] [PubMed] [Google Scholar]
- Masman D, Daan S, Beldhuis J.A. Ecological energetics of the kestrel: daily energy expenditure through the year based on on time–energy budget, food intake and doubly labeled water methods. Ardea. 1988;76:64–81. [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, MA: 1982. Evolution and the theory of games. [Google Scholar]
- McNamara J.M. Optimal life histories: a generalisation of the Perron–Frobenius theorem. Theor. Popul. Biol. 1991;40:230–245. doi:10.1016/0040-5809(91)90054-J [Google Scholar]
- McNamara J.M, Welham R.K, Houston A.I. The timing of migration within the context of an annual routine. J. Avian Biol. 1998;29:416–423. doi:10.2307/3677160 [Google Scholar]
- Merilä J, Hemborg C. Fitness and feather wear in the collared flycatcher Ficedula albicollis. J. Avian Biol. 2000;31:504–510. doi:10.1034/j.1600-048X.2000.310410.x [Google Scholar]
- Metz J.A.J, Nisbet R.M, Geritz S.A.H. How should we define ‘fitness’ for general ecological scenarios? Trends Ecol. Evol. 1992;7:198–202. doi: 10.1016/0169-5347(92)90073-K. doi:10.1016/0169-5347(92)90073-K [DOI] [PubMed] [Google Scholar]
- Mills A.M. Winter range compression of migrants in central america. J. Avian Biol. 2006;37:41–51. doi:10.1111/j.2005.0908-8857.03431.x [Google Scholar]
- Moreau R.E. Academic Press; London, UK: 1972. The Palearctic–African bird migration systems. [Google Scholar]
- Murphy M.E. Energetics and nutrition of moult. In: Carey C, editor. Avian energetics and nutritional ecology. Chapman and Hall; New York, NY: 1996. pp. 158–198. [Google Scholar]
- Neto J.M, Newton J, Gosler A.G, Perrins C.M. Using stable isotope analysis to determine the winter moult extent in migratory birds: the complex moult of Savi's warblers Locustella luscinioides. J. Avian Biol. 2006;37:117–124. doi:10.1111/j.2006.0908-8857.03563.x [Google Scholar]
- Nilsson J.-Å, Svensson E. The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc. R. Soc. B. 1996;263:711–714. doi:10.1098/rspb.1996.0106 [Google Scholar]
- Pearson D, Backhurst G. Moult in the river warbler Locustella fluviatilis. Ringing Migration. 1983;4:227–230. [Google Scholar]
- Pennycuick C.J. Mechanics of flight. In: Farner D.S, King J.R, editors. Avian Biol. vol. 5. Academic Press; London, UK and New York, NY: 1975. pp. 1–75. [Google Scholar]
- Pennycuick C.J. Oxford University Press; Oxford, UK: 1989. Bird flight performance. [Google Scholar]
- Piersma T. Population turnover in groups of wing-moulting waterbirds: the use of a natural marker in great crested grebes. Wildfowl. 1987;38:37–45. [Google Scholar]
- Piersma T. The annual moult cycle of great crested grebes. Ardea. 1988;76:82–95. [Google Scholar]
- Piersma T, Lindström A. Migrating shorebirds as integrative sentinels of global environmental change. Ibis. 2004;146(suppl. 1):61–69. doi:10.1111/j.1474-919X.2004.00329.x [Google Scholar]
- Prys-Jones R. The occurrence of biannual primary moult in passerines. Bull. Br. Ornithol. Club. 1991;111:150–152. [Google Scholar]
- Rohwer S, Butler L.K, Froehlich D.R. Ecology and demography of east–west differences in molt scheduling of Neotropical migrant passerines. In: Greenberg R, Marra P.P, editors. Birds of two worlds. The Johns Hopkins University Press; Baltimore, MD: 2005. pp. 87–105. [Google Scholar]
- Salewski V, Altwegg R, Erni B, Falk K.H, Bairlein F, Bernd L. Moult of three Palaearctic migrants in their West African winter quarters. J. Ornithol. 2004;145:109–116. doi:10.1007/s10336-004-0020-2 [Google Scholar]
- Slagsvold T, Dale S. Disappearance of female pied flycatchers in relation to breeding stage and induced moult. Ecology. 1996;77:461–471. doi:10.2307/2265622 [Google Scholar]
- Stresemann E, Stresemann V. Die Mauser der Vögel. J. Ornithol. 1966;107:1–448. [Google Scholar]
- Summers R, Underhill L, Nicoll M, Strann K.-B, Nilsen S. Timing and duration of moult in three populations of purple sandpipers Calidris maritima with different moult/migration patterns. Ibis. 2004;146:394–403. doi:10.1111/j.1474-919X.2004.00273.x [Google Scholar]
- Svensson E, Hedenström A. A phylogenetic analysis of the evolution of moult strategies in Western Palearctic warblers (Aves: Sylviidae) Biol. J. Linn. Soc. 1999;67:263–276. doi:10.1006/bijl.1998.0302 [Google Scholar]
- Swann R, Baillie S. Suspension of moult by trans-Saharan migrants in Crete. Bird Study. 1979;26:55–58. [Google Scholar]
- Tallman D.A, Tallman E.J. Timing of breeding by antbirds (Formicariidae) in an aseasonal environment in amazonian ecuador. Ornithol. Monogr. 1997;48:783–789. [Google Scholar]
- Underhill L.R, Prys-Jones R, Dowsett R, Herroelen P, Johnson D, Lawn M, Norman S, Pearson D, Tree A.J. The biannual primary moult of willow warblers Phylloscopus trochilus in Europe and Africa. Ibis. 1992;134:286–297. [Google Scholar]
- Vuorinen J. Successive spatial polyandry stated in dipper. Cinclus Scand. 1999;12:14–16. [Google Scholar]
- Weber T.P, Ens B.J, Houston A.I. Optimal avian migration: a dynamic model of fuel stores and site use. Evol. Ecol. 1998;12:377–401. doi:10.1023/A:1006560420310 [Google Scholar]
- Weber T.P, Borgudd J, Hedenström A, Persson K, Sandberg G. Resistance of flight feathers to mechanical fatigue covaries with moult strategy in two warbler species. Biol. Lett. 2005;1:27–30. doi: 10.1098/rsbl.2004.0244. doi:10.1098/rsbl.2004.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Technical description of the optimal annual routine model