Abstract
Although it is axiomatic that males and females differ in relation to many aspects of reproduction related to physiology, morphology and behaviour, relatively little is known about possible sex differences in the response to cues from the environment that control the timing of seasonal breeding. This review concerns the environmental regulation of seasonal reproduction in birds and how this process might differ between males and females. From an evolutionary perspective, the sexes can be expected to differ in the cues they use to time reproduction. Female reproductive fitness typically varies more as a function of fecundity selection, while male reproductive fitness varies more as a function sexual selection. Consequently, variation in the precision of the timing of egg laying is likely to have more serious fitness consequences for females than for males, while variation in the timing of recrudescence of the male testes and accompanying territory establishment and courtship are likely to have more serious fitness consequences for males. From the proximate perspective, sex differences in the control of reproduction could be regulated via the response to photoperiod or in the relative importance and action of supplementary factors (such as temperature, food supply, nesting sites and behavioural interactions) that adjust the timing of reproduction so that it is in step with local conditions. For example, there is clear evidence in several temperate zone avian species that females require both supplementary factors and long photoperiods in order for follicles to develop, while males can attain full gonadal size based on photoperiodic stimulation alone. The neuroendocrine basis of these sex differences is not well understood, though there are many candidate mechanisms in the brain as well as throughout the entire hypothalamo–pituitary–gonadal axis that might be important.
Keywords: photoperiodism, circannual rhythms, sex differences, supplementary cues
1. Introduction
(a) Scope of the chapter
This paper concerns the question of whether there are sex differences in the nature and processing of environmental cues that regulate seasonal reproduction. The modern era for the study of the environmental regulation of reproduction was initiated based on the studies of Rowan (1929) on the photoperiodic regulation of migratory behaviour in dark-eyed juncos (Junco hyemalis). Since that time, a wealth of information has accumulated in vertebrate and invertebrate species about how cues such as variation in photoperiod as well as temperature, food availability, nest sites and social interactions can affect the timing of the onset and the end of reproduction (Goldman et al. 2004). The general approach has been to develop comprehensive theories about how such cues are processed by particular taxa (e.g. Goldman 2001; Goldman et al. 2004). Intraspecific variation in the response to such cues has not been a major research focus (but see Nelson 1987). One obvious aspect of intraspecific variation in reproduction relates to differences between the sexes. Males and females are defined based on the different strategies they adopt when reproducing (i.e. anisogamy), so the usual approach is to distinguish between them when considering most other aspects of reproductive physiology and behaviour (Andersson 1994). Despite this fact, sex differences in relation to the environmental control of reproduction are not a major research focus for the field overall. However, considering such differences will be necessary for a complete understanding of this problem. In this paper, we will review the relatively little that is known about such differences, and we discuss some reasons why a consideration of sex differences will be useful for framing future research questions.
Recognizing that it is not fashionable to organize a review on a particular taxon, we nevertheless focus our review on avian species. Many of the pioneering studies concerning the complex interrelations among hormones, brain, behaviour and environmental stimuli were conducted on birds (e.g. Berthold 1849; Rowan 1929; Lehrman 1959). Therefore, the history of avian studies in this field is as old as the field itself. Owing to the tireless efforts of many field ethologists and field ecologists, there also exists extensive knowledge of basic information on reproductive cycles and reproductive behaviour in nature among avian species (e.g. Wingfield & Farner 1993). Within birds, parallel studies involving both laboratory and field approaches are most common in the songbird order (Wingfield & Farner 1980, 1993; Ketterson et al. 2001), so most of our examples are from the songbird order. However, valuable studies often under captive conditions have been conducted on birds from other orders, including domesticated species, and they are discussed at times as well.
This knowledge base of birds is arguably more extensive than for any other vertebrate class. Therefore, a question such as whether the sexes differ from one another in the nature of the environmental cues they rely on to regulate reproduction in a natural context can arguably be considered more easily in birds than in other taxa. Birds also exhibit attributes that make them especially useful for this question such as well-defined neural substrates that mediate the activation of many behaviours, a high level of adult neuroplasticity that includes adult neurogenesis (Nottebohm 1989) and robust and well-characterized endocrine responses to environmental and social stimuli (Ball & Bentley 2000; Wingfield 2006). These attributes make avian species a valuable resource for the elucidation of basic cellular and molecular mechanisms over and above their ability to inform us about mechanisms regulating reproduction in a natural context.
The literature on the neuroendocrinology of reproduction in birds is smaller than that for mammalian species, and therefore a large number of topics can be considered at once while still being reasonably comprehensive. Studies of birds in the past have been a source of interesting insights into the mechanisms of organismal processes (Konishi et al. 1989); our goal in this chapter is to use bird studies to investigate a particular question in the field of seasonal reproduction which we think deserves more attention. Not only should an overview of sex differences in the response to environmental cues related to reproduction help to stimulate research on basic mechanisms of the control of seasonal breeding, but also should prove useful in the area of evolutionary and conservation biology. Without knowing how the sexes differ in the environmental regulation of their reproduction, it will not be possible to predict how populations might respond to environmental change—either in terms of phenotypically plastic responses to normal environmental variation or in their evolutionary response to climate change. If, for example, environmental changes alter cues that influence the readiness of males to reproduce without affecting cues that influence female readiness, coordination between the sexes could be affected. In this paper, we want to articulate why studying sex differences in the response to environmental cues might be interesting, and we will review some illustrative examples from the extant literature. However, many of the questions we raise have not been thoroughly addressed yet.
(b) How to consider mechanistic and functional approaches to the study of seasonal reproduction
It is common for non-tropical avian species to limit reproductive activity to the time of year when temperatures are relatively mild and the necessary food resources are present (Baker 1938; Perrins 1970; Wingfield 1983). In order to coordinate gonadal recrudescence and the associated increases in endocrine-mediated behaviour with conditions that are favourable for breeding, birds often use specific cues in the environment to time their reproduction. Environmental factors that influence timing of reproduction though their effects on fitness are typically referred to as ultimate causes (or factors) of variation in timing (Baker 1938; Wingfield & Kenagy 1991), and food is the common example. In contrast, environmental factors that act to initiate reproduction because they stimulate the neural substrates that lead to reproductive cascades are usually referred to as proximate causes (or cues), and here variation in day length is the common example (Baker 1938; Wingfield & Kenagy 1991).
This distinction between factors as ultimate or proximate based on whether they influence fitness or act to stimulate the neuroendocrine system has proved quite useful. The distinction has allowed investigators to ask not only why animals breed when they do—because they are more successful than they would have been at other times—but also how the animal ‘knows’ when it is best to breed, i.e. what cues it uses to initiate and maintain reproductive readiness. However, the distinction between proximate and ultimate factors can become muddied when applied in practice. Birds often rely on multiple cues to time their reproduction, and proximate cues are often subdivided into different categories not based on how they influence physiology, but rather based on why the animal has come to rely on them as it does. In other words, the discussion of proximate cues has addressed not only their impact on reproductive physiology but also their valence as predictors of good conditions for reproduction in the still distant or immediate future. For example, photoperiod is a powerful ‘initial predictive cue’ that initiates or terminates the period of reproduction in many non-tropical species (Wingfield 1980; Dawson et al. 2001). Changes in photoperiod set in motion aspects of reproduction that require time to prepare such as the development of the reproductive organs. Other cues provide ‘essential supplementary information’ and ‘synchronizing and integrating information’ (Wingfield 1980; Wingfield & Kenagy 1991). These latter cues include mild weather, the availability of nest sites and stimulatory social interactions (Wingfield & Kenagy 1991), i.e. cues that can trigger territorial behaviour, nest building and final stages of yolk accumulation. Questions related to ultimate factors have traditionally been referred to as ‘why questions’, and questions related to proximate factors as ‘how questions’. In practice, however, treatment of proximate cues has come to include a ‘why’ component as investigators have weighed cues in terms of their utility to the organism in predicting optimal conditions for reproduction. Additional room for confusion arises because some factors, e.g. food, can act as both ultimate and proximate depending on whether they are being invoked to explain why species of birds breed when they do or how individuals know when it is time to breed (e.g. O'Brien & Hau 2005).
Confusion can be relieved by the use of phylogenetic analyses (evolutionary comparative biology) to resolve which ultimate factors best explain variation in timing among species, microevolutionary techniques to compare the fitness of individuals that differ in timing (e.g. relative loss of nestlings to starvation in early and late breeders), statistical analyses to assess the degree of correlation between potentially predictive environmental cues and suitable conditions for breeding, and behavioural neuroendocrine approaches to explain variation in the response to environmental cues (e.g. Dawson et al. 2001).
In this paper, we will refer to both ultimate and proximate causes of variation in the timing of seasonal reproduction by males and females. Ultimate causes will be addressed by questions such as ‘why might it be adaptive for males and females to respond differently to the same cue or to respond to different cues altogether?’ Proximate causes will be addressed by questions such as ‘How do males and females differ in the environmental cues they respond to and in the nature of their response?’
It is also important to distinguish between the two sets of questions when considering proximate cues. One question relates to what cues a particular sex will be most likely respond to. For example, there is evidence that males and females both respond to photoperiod, but there may well be secondary cues that are salient in this regard in a sex-typical fashion. One could even envision cases in which a particular stimulus has opposite effects in males and females, being stimulatory in one but inhibitory in the other. A second related question concerns the degree of a response to the same cue. One could envision a situation in which the threshold for photoperiodic stimulation would be lower in males than in females (e.g. if males migrate sooner and defend territories in advance of females), as it is known to be for different populations of the same species breeding at different latitudes (e.g. Silverin et al. 1993). As we will discuss later, it is clear that the ability of photostimulation alone to induce full gonadal growth is different between males and females in many seasonally breeding species.
As alluded to previously, such sex differences could also have significance for our understanding of long-term adjustments of animal populations to climate change. It has been observed that certain avian populations, such as great tits (Parus major) nesting in the Netherlands, are shifting the timing of reproduction as the temperature increases (Visser et al. 1998, 2006). As is well known, the ultimate reason for the timing of laying in this species is to foster coincidence between the peak in caterpillar abundance and the hatching of the young (Perrins 1970; Visser et al. 1998). However, the caterpillar populations are responding to the change in temperature at a different rate from the bird population. Is it possible that males and females in the same species will adjust to climate change in different ways? If during a period of rapid environmental change males and females were to rely on different cues to time their reproduction, could the consequences be maladaptive? To answer this question, one needs to know which cues males and females respond to and whether their response to the same cues differs.
(c) Why is this question of sex differences interesting? Why should females be expected to respond differently than males?
(i) Aspects of avian life history that might bias males and females to respond differently to environmental cues
A general assumption often made by biologists is that something as fundamental as biological timing will not tend to differ between males and females. However, if one carefully considers a timed biological response such as seasonal reproduction, it is far from clear that this should be the case. In order to appreciate how and why males and females might differentially respond to environmental cues, it is useful to consider many aspects of the natural history and breeding biology of taxa of interest that can result in different selection pressures on males and females. For example, birds exhibit universal oviparity combined with endothermy, which together make a high degree of parental care essential (Oring 1982). Thus, there is no option of generating progeny and abandoning them to the elements as is the case in ectothermic taxa. The eggs and young of even brood parasitic species receive extensive parental care, they just receive it from the parasitized host species, not from their parental species and even mound builders such as species in the megapode order have males that tend the mounds. From this perspective, one can argue that if females are to commit to a substantial investment in an egg as well as the time and energy required for parental care, then the timing of the parental commitment should be optimized for successful reproduction. Mistakes in such timing can be catastrophic. In birds, food for the young at the time of hatching is particularly important for reproductive success because the successful survival of the progeny is closely tied to the types of food ingested during development (Baker 1938; Perrins & Birkhead 1983). In the case of altricial species, food is provided by the parents to the young, while in the case of precocial species, the young are guided to food sources where they feed themselves (Ricklefs 1983). In general, avian parents are unable to store energy as body fat and then provide it to their young at a later time. This is in contrast to certain mammalian species, in which the mother will overeat and store excess calories in the form of fat during a time of abundant food availability, then convert her fat to milk during lactation and feed her young on this milk at a later time when food availability may be low (Bronson 1989). This pattern is very common among Pinnepeds. There are of course exceptions among the over 9000 extant species of birds. Some species (especially in the corvid family) store food so that they can initiate breeding before appropriate quantities and types of food are available (e.g. grey jays, Perisoreus canadensis), and both males and females in columbiform species make a milk of sorts in their crop sac that is used to feed the altricial squab. An important concept to consider in this regard is that of capital versus income breeders (Doughty & Shine 1997). Income breeders rely on available resources while capital breeders may rely on energy stored previously, as is the case for geese or more dramatically penguins that lay eggs and incubate while relying on previously stored food. Thus, while birds lack a mechanism such as lactation that provides great flexibility as to when resources are provided by the mother to the young, they can adopt strategies to use resources collected previously.
(ii) Influence of parental care systems on sex differences in the cues regulating the timing of reproduction
An obvious prediction is that the greater the similarity in the reproductive behaviour of the sexes, the greater the similarity in the supplementary cues that males and females will use to time the onset, prolongation and termination of reproduction. Conversely, the more divergent the role of the sexes in reproduction, the less similar the cues should be. Thus, in seasonal migratory species in which the sexes arrive on the breeding grounds simultaneously and both sexes build the nest, share in incubation of the eggs and provide for young, we might expect the sexes to monitor similar aspects of the environment. Conversely, in lekking species in which males gather early in the breeding season to advertise and females visit only for copulation, we might expect males to monitor conditions on the lek, the behaviour or other males and the impact of resources on their ability to sustain advertisement early in the year, while females would assess the state of nesting sites located some distance from the lek and the impact of resources on their ability to sustain incubation and offspring care. The time course of these resources need not be the same, so selection should favour differential sensitivity to their enhancing or suppressive effects on readiness to reproduce.
Focusing on parental behaviour, a recent review by Cockburn (2006) recognized six distinct modes of parental care in 9456 species of birds belonging to 188 families and discussed their phylogeny and prevalence. Two modes, incubation of eggs by means of geothermal heat and brood parasitism, involve the absence of incubation or provisioning young and are quite rare (less than 1% of species). The other four, male-only care, female-only care, biparental care and cooperative breeding involve parenting by one to many individuals. Biparental care by pair-bonded males and females is the most common pattern (inferred as 81% of species), but is nevertheless less frequent than reported in earlier summaries. Instead, female-only care and cooperative breeding are more common than previously thought (e.g. Owens 2002), occurring with a prevalence of 8 and 9% of species, respectively.
Because we anticipate greater sex differences in the cues regulating reproduction in species in which the parental roles differ, future studies of cues should compare the sexes in brood parasites at one extreme and biparental species at the other. Of particular interest will be clades in which multiple transitions are believed to have occurred such as from male only to biparental care in the charadrii (Szekely & Reynolds 1995), allowing studies of cue sensitivity in closely related species that differ strikingly in their mode of parental care.
Importantly, however, even in species in which females care alone, males can be expected to be sensitive to cues that impact female fecundity. Many examples of female-only care involve species that are frugivores or nectivores. Females of such species are thought to rely on patchily fruiting trees as a food source for young, whereas males rely on the trees as a place to encounter females (Cockburn 2006).
To focus briefly on other obvious examples, exposure to nest predators might have a far greater suppressive effect on readiness to reproduce in the sex that makes the greater investment via incubation. Similarly, the availability of suitable, i.e. safe, nest sites would be predicted to have a greater stimulatory effect on the incubating than the non-incubating sex. In species in which males build the nest or display using nest materials, we might expect similar male and female sensitivity to the availability of nesting materials, although even in this case, nesting materials may function as male display, whereas for females they may function as a safe harbour, causing the sexes to respond to different aspects of the same class of cues.
Termination of breeding and onset of moult are also known to differ by sex, and thus we might expect the process to be mediated by different cues. Among shorebirds, relatively closely related species differ in whether males or females are first to desert offspring, leaving members of the other sex as sole parents. Szekely & Reynolds (1995) have explored adaptive and historical reasons for these sex differences, but less comparative attention has been paid to sex differences in parental responses to offspring cues as offspring age. In all species with parental behaviour, the sexes become decreasingly nurturing with time and as the cues presented by the young change during development. The ability of stimuli from younger offspring to reinduce high rates of parental care as well as to maintain physiological correlates of such rates of care has been experimentally demonstrated in avian taxa such as doves and pigeons (e.g. Hansen 1971, 1973). However, as with other questions raised in this review, sex differences in the time course of changing neuroendocrine sensitivity to offspring cues requires more study.
Another life-history trait that greatly influences patterns of breeding in many birds is related to their volant life style. The advantage conferred by the ability to fly favours birds of smaller size. This makes it especially important to reduce organ systems when they are unneeded. Extreme reduction in gonadal size to foster flight occurs seasonally in females and males. For example, there is remarkable seasonal variation in the size of the testis, with increases as large as 1000-fold in breeding compared with non-breeding males (Follett 1984). However, although most avian species exhibit some sort of sharing pattern of parental care (Silver et al. 1985; Clutton-Brock 1991), there are particular strains on females. Females lay the egg so that they make the final reproductive decision. A mistake in timing made by females is potentially far more costly than a mistake by males. Also, it may well take longer for a female to recover from a breeding mistake than a male due to the necessity of generating sufficient resources to produce another egg. The importance of this sex difference will interact with whether the species in question is a so-called ‘capital’ or ‘income’ breeder (Doughty & Shine 1997) as alluded to previously. Capital breeders, even among avian species, can rely on stored energy (e.g. if they store food) that may be impossible to replace easily when compared with income breeders which are relying on energy available in the environment that may still be present if a breeding mistake is made.
(d) Why has this question been ignored to some degree?
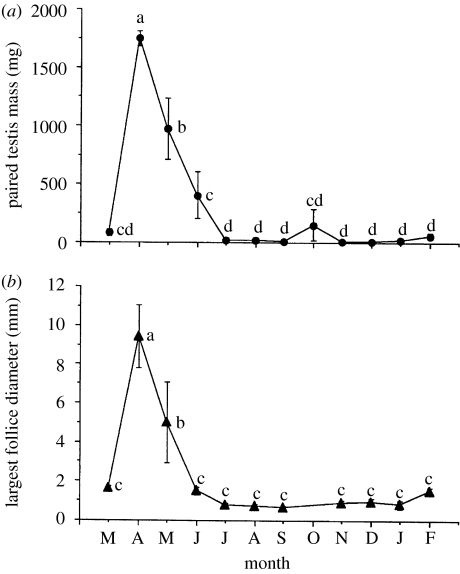
Given the possible significance of a sex difference in the response to environmental cues, why are there relatively few data that specifically address this issue? A big factor is merely experimental convenience. As mentioned previously, the study of avian photoperiodism has a long history (e.g. Rowan 1929), but very early on, key investigators, who studied basic mechanisms in wild species, decided to focus on males (Burger 1949). Field-caught males and females do exhibit similar relative seasonal changes in gonadal size (figure 1). However, in captivity males in most photoperiodic species exhibit robust gonadal responses to changes in day length (e.g. Farner & Wilson 1957), but females do not. Females generally require additional cues, often complex ones that are hard to replicate in the laboratory, and/or long experience with laboratory conditions, in order to exhibit robust ovarian growth and egg laying. This sex difference alone makes it clear that one needs to be cautious when generalizing about males and females. However, given that excellent progress was made on the study of the testicular response of males to photoperiod and other environmental cues, it is understandable that most work focused on them (Farner & Follett 1966, 1979; Farner 1986). Nonetheless, there are fundamental questions about sex differences in the responsiveness to cues that remain to be addressed.
Figure 1.
Seasonal changes in (a) paired testis mass (mg) and (b) largest ovarian follicle diameter (mm) in male and female European starlings (Sturnus vulgaris), respectively, collected each month from March 1994 to February 1995 in Baltimore, MD, USA. Points represent mean±s.e. of the mean. Points with different letters differ significantly, while points with the same letter do not. Note how similar the relative pattern of change is in these field-caught male and female starlings. Male starlings, like many other temperate zone songbird species, when held in captivity under artificial photoperiods exhibit changes of a similar magnitude to that observed in the field. In contrast, captive female starlings exhibit a change of a much smaller magnitude.
2. Basics of the environmental regulation of annual cycles in birds
(a) Types of cues: photoperiodic and extra-photoperiodic cues
Changes in day length (or photoperiod) provide a valuable cue in the environment to allow animals to assess the time of year. Extra-photoperiodic cues include a wide range of stimuli in the physical environment, such as temperature and nest site availability, as well as a range of social cues from conspecifics and perhaps even nearby heterospecific individuals. If an individual is able to measure the current photoperiod and ascertain whether the photoperiod is decreasing or increasing, it can in theory determine with precision any date of the year. A wide variety of avian species that live either in the tropics or in the temperate zone have evolved the ability to measure and respond to seasonal fluctuations in photoperiod (Murton & Westwood 1977; Nicholls et al. 1988; Wilson & Donham 1988). Most of our discussion focuses on temperate-zone species, but it should be noted that species in tropical climes can detect even the small changes in day length which occur in this habitat, and photoperiod may play some role in the regulation of reproduction even among birds from this region (Hau 2001). The photoperiodic response in birds can be characterized based on the physiological responses a given avian population exhibits as it experiences seasonal fluctuations in the photoperiod (refer figure 1 for an example of a seasonal gonadal cycle in wild-caught birds). These responses are mediated by a complex system that includes both a neural component and an endocrine component (Follett 1984; Sharp 2005). Variation in photoperiod is referred to as an initial predictive cue (Wingfield & Kenagy 1991), because photoperiod regulates the onset and offset of breeding independent of any year-to-year variation in weather, food or social conditions or local geographical variation in the quality of such factors that a bird might encounter. For successful breeding to occur, photoperiodic responses must be fine-tuned by other cues in the environment that allow egg laying to be timed optimally in response to variation in local conditions (Wingfield 1980, 1983). In our consideration of seasonal reproduction, we focus on the regulation of reproduction. However, it should be noted that many other traits change seasonally and are regulated by photoperiod (such as plumage, body mass, migratory restlessness or immune function), while other traits may be impervious to photoperiodic changes (see Nelson (1987) for a review). Some authors therefore argue that it is more accurate to refer to photoperiodic traits rather than to photoperiodic individuals or populations (e.g. Nelson 1987).
(b) Brief description of the photoperiodic response
Birds are traditionally viewed as being ‘long-day breeders’ (e.g. Murton & Westwood 1977). By this most authors mean that the hypothalamo–pituitary–gonadal (HPG) axis responds to increasing day lengths after the winter solstice with a marked increase in gonadotrophin secretion, gonadal growth and a wide range of steroid hormone-dependent processes, including changes in reproductive behaviours. This view of the photoperiodic regulation of reproduction leads logically to the simplistic hypothesis that photoperiods greater than 12 L:12 D should stimulate the reproductive axis, while day lengths shorter than 12 L:12 D should inhibit it. One would then predict a symmetrical function of gonadal activity centred on the summer solstice. It turns out that such breeding patterns are exceedingly rare. Perhaps the closest approximation of symmetrical cycles are the annual cycles of certain gallinaceous birds such as the California quail (Callipepla californica; Murton & Westwood 1977) or columbiforms such as the wood pigeon (Columba palumbus) in the UK (Lofts et al. 1966). It is more common that the photoperiodic response of birds exhibits physiological responses asymmetrical to seasonal changes in day length, in which the reproductive system will grow in the presence of short day lengths or regress in the presence of long day lengths. The effects on reproductive physiology of the lengthening periods of daylight, which are normally experienced by temperate-zone birds in the spring, are twofold (Dawson et al. 2001). One effect is to stimulate the HPG axis to prepare and maintain the bird for reproduction. Birds that are reproductively active due to the stimulating effects of long lengthening days are said to be photostimulated (Follett 1984; Nicholls et al. 1988; Dawson et al. 2001). A second effect of long lengthening days is to initiate an inhibitory process that later results in the regression of the gonadal portion of the HPG axis. This inhibitory process has been well documented in many temperate-zone songbird species such as the European starling (Sturnus vulgaris), tree sparrow (Passer montanus) and white-crowned sparrow (Zonotrichia lencophrys) (Nicholls et al. 1988; Wilson & Donham 1988). In these species, the reproductive system regresses before the summer solstice as a function of the number of long days the bird has experienced. It is impossible to photostimulate birds at this time. Even if one artificially lengthens the photoperiod so that the birds are on constant light, they will not exhibit an enhanced reproductive response (e.g. Hamner 1971). Birds with regressing or regressed reproductive systems that no longer exhibit reproductive responses to long day lengths are said to be photorefractory. A feature of photorefractoriness in birds is that even though it does not become apparent for several weeks after exposure to long days, it is initiated rapidly, and once initiated the reproductive system continues to proceed towards a future refractory state regardless of subsequent photoperiod (Dawson et al. 2001). The rate of onset of photorefractoriness is proportional to the length of the photoperiod (Hamner 1971; Dawson & Goldsmith 1983) or the length of the photoperiod beyond some critical day length (Moore et al. 2005).
In this context, a long day is one in which the duration of the light period exceeds a ‘critical day length’, defined as the length of day below which photorefractoriness is not induced. The critical day length to induce photorefractoriness is species typical or even population typical. It can also be influenced by the photoperiodic history the animal has experienced (Robinson & Follett 1982).
Photorefractoriness can only be dissipated or broken by the experience of short days that are normally encountered in the autumn when ‘photosensitivity’ is acquired. Photosensitivity in this sense means that the avian reproductive system will respond to long days with dramatic increases in various measures of reproductive physiology such as gonad size and hormone secretion. The effect of short days on the dissipation of photorefractoriness has been demonstrated experimentally based on the laboratory studies in many species (e.g. Dawson 1991). In wild populations of white-crowned sparrows in Washington State, USA, a long-day challenge was administered at various time points throughout the autumn, and it was discovered that photosensitivity naturally occurs in late October to early November (Farner & Mewaldt 1955). This reacquisition of photosensitivity in the autumn while on short days can result in an increase in reproductive physiology and general activity including mating behaviour in some species. This has been termed as ‘autumnal sexuality’ (Murton & Westwood 1977; Lincoln et al. 1980; Dawson 1983). This phenomenon is quite apparent in seasonally breeding ducks when they break photorefractoriness in the autumn. Some investigators have linked an autumn increase in plasma androgen concentrations with an increase in the male display rate (Balthazart & Hendrick 1976). It is important to note therefore that the experience of short days, which dissipates photorefractoriness, has the effect of resetting the system so that birds can respond once again to a stimulatory photoperiod and other cues. In some species, this dissipation of photorefractoriness and the resultant relatively small increase in reproductive endocrine activity can support breeding under short days (Sharp 1996), though in most species additional photostimulation is required (Sharp 1996).
The photoperiodic response in birds involves an endogenous component (Gwinner 1986). In many species, when maintained under conditions of constant photoperiod (such as 12 L : 12 D) and temperature, cycles of gonadal maturation and regression followed by feather moult can be observed for years (see Gwinner 1986, 1996 for reviews). This phenomenon seems to be especially prevalent in tropical species but has also been observed in temperate zone species (e.g. Gwinner & Scheuerlein 1999). For this reason, it has been hypothesized that one mechanism by which photoperiod regulates seasonal breeding is through the entrainment of an endogenous circannual rhythm (Gwinner 1986, 1996). The exact nature of these endogenous seasonal rhythms that regulate seasonal reproduction remains to be elucidated. In one useful test of the existence of a circannual rhythm, dark-eyed juncos were maintained under conditions of constant dim light (rather than a constant photoperiod of some sort) and a persistent rhythmicity of gonadal growth, regression and moult was observed (Holberton & Able 1992). However, the period of this rhythm varied widely from 6 to 21 months. Although endogenous rhythms do vary in period when free-running, this is much greater that one would normally expect for a single rhythmic endogenous process. Therefore, this endogenous regulation of reproduction in birds may not involve a true circannual rhythm as that seen in hibernating mammals and not be one that is analogous to circadian rhythms. Rather, the circannual rhythms in birds that persist under constant day lengths may involve an endogenous mechanism of a kind yet to be understood.
(c) Influence of non-photoperiodic cues on the timing of breeding
As discussed previously, in order to time breeding successfully, birds must integrate initial predictive cues such as photoperiod with other types of cues that provide information about local variation in the environment that results from year-to-year fluctuations in weather conditions or geographical variation in food availability and/or social interactions (Ball 1993). The extent to which a given species or population will integrate supplementary cues with an initial predictive cue such as photoperiod is related to the predictability of the environment in relation to breeding. If the timing of the suitability of the environment for a future event such as the onset of breeding is highly predictable, then only a select number of reliable cues will be required to time breeding while many other cues can be ignored (Cohen 1967). If the timing of the onset of suitable conditions for breeding season is less predictable, then many cues should be integrated to optimize the timing of breeding (Wingfield et al. 1992). Typically suitability is thought of as the coincidence of breeding with environmental factors that enhance reproductive success such as food and shelter. But in extreme environments such as the Arctic, the time available for breeding may be quite constrained, and in that case, birds may become relatively impervious or unresponsive to supplementary cues that would fine-tune breeding in more forgiving environments (Wingfield & Hunt 2002). Thus, Arctic environments, when compared with lower latitude environments, are predictable not in the suitability of the weather, but in the short duration of the season when breeding is possible and, interestingly, gonadal growth in arctic white-crowned sparrows is less responsive to variation in temperature than is gonadal growth in populations of white-crowned sparrows that breed in more temperate climates (Wingfield et al. 1996). This linking of the tendency to integrate non-photoperiodic cues into the decision of when to breed with the predictability or unpredictably of the environment has proved to be very useful for the interpretation of species variability in the importance of non-photoperiodic cues.
The integration of supplemental cues with initial predictive cues provided by photoperiod seems quite likely to be one way in which males and females will differ in their photoperiodic responses. In particular, one would expect supplementary cues to be relatively more important in regulating reproductive endocrine responses in females when compared with males. Although it is somewhat common to study population differences in the response to photoperiod (e.g. Silverin et al. 1993) and/or interactions between supplementary cues and photoperiodic cues (e.g. Silverin & Viebke 1994; Perfito et al. 2005), the same strategy has generally not been applied to the sexes (also see Wingfield et al. 1996). Progress in this area will require selection of comparable time points in the reproductive cycle of the sexes and their response to manipulation of combinations of potentially significant cues.
(d) The sensory and neuroendocrine control of seasonal reproduction in birds
(i) The encephalic photoreceptor and the photoperiodic response in birds
One interesting feature of the photoperiodic response in birds is that the sensory receptor mediating the response to photoperiod is not in the retina but rather appears to be located in the hypothalamic region of the brain. It was first suggested in the 1930s in ducks (Benoit 1935) that the photoinduction of gonadal growth in birds did not require the eyes. This was subsequently confirmed in several species including songbird species such as the white-crowned sparrow and the house sparrow (Passer domesticus; e.g. McMillan et al. 1975). Thorough studies by Wilson (1989, 1991) have led to the conclusive demonstration that at least in the Arctic breeding tree sparrow, neither the eyes nor the pineal gland are needed for the stimulation of gonadal growth by long days, the onset of photorefractoriness in response to long days or the breaking of refractoriness in response to short days. The eyes are not needed in either males or females in this species (Wilson 1991). It is thus clear that, at least in the photoperiodic species that have been studied, the sensory receptor mediating the response of initial predictive information seems quite independent of those mediating the response to supplementary cues. The exact location of this encephalic photoreceptor has still not been definitively identified. However, proteins related to photoreception have been found in the lateral septum as well as in the mediobasal hypothalamus (e.g. Silver et al. 1988; Saldanha et al. 1994). Also, recent studies of the photopigment melanopsin that is involved in non-image forming photoreception in mammals have identified it in the medial septum of birds (Chaurasia et al. 2005). Lesions to cells in the lateral septum that expresses this putative photoreceptor attenuate the effects of photostimulation on the maturation of the reproductive axis in male chicks (Rathinam & Kuenzel 2005). However, no studies have been conducted on possible sex differences in the localization or functioning of the avian extra-retinal photoreceptor. Given that both males and females use this photoreceptor to respond to photoperiodic variation one would not expect qualitative differences though as we learn more about male and female responses to photoperiod, it is worth considering that some aspects of the initial sensation of photoperiod may vary between the sexes.
(ii) The visual and the auditory systems: the response to supplementary cues
In contrast to the response to photoperiod, there is every indication that the endocrine response to supplementary information provided by the social context and to some degree the physical context is mediated by the sensory receptors in the visual system (with the use of the eyes) and in the auditory system. For example, a large amount of direct and indirect evidence has been collected in many avian species which indicates that the response to salient social cues such as a displaying conspecific requires stimulus cues derived primarily from visual and auditory stimulation (Lehrman 1959; Hinde & Steel 1978; Silver 1978; Cheng 1979; Wingfield 1980; Moore 1983; Crews & Silver 1985; Erickson 1985; Ball & Balthazart 2002). There is little evidence that other sensory systems such as the somatosensory and olfactory systems play a major role in mediating the effects of social stimuli on endocrine secretion as they clearly do in mammals (Wingfield 2006). However, this cannot be ruled out completely as in many species such as pigeons and doves, there is extensive courtship preening just prior to ovulation in females. The importance of these interactions has not been experimentally investigated, though it should be noted that visual and auditory cues in the absence of such interactions are quite effective in stimulating endocrine secretion (Silver 1978; Cheng 1979).
It should also be remembered that these supplementary cues may not always be stimulatory. For example, Yokoyama & Farner (1976) found that enucleation leads to an increase in gonadal activity in female white-crowned sparrows. This suggests the intriguing possibility that stimuli most commonly processed by the retinal pathway, i.e. social and non-photoperiodic physical information, may also exert an inhibitory influence. It could well be the case that this effect is particularly acute in females, but it has not been systematically tested.
An endocrine response to social supplementary cues may require rather complex neural processing to occur. For example, Friedman (1977), in an ingenious experiment, demonstrated that a female dove exposed to a courting male which is directing his behaviour towards her is more apt to show an enhanced endocrine response than a female that sees the same male but from a view which indicates that he is not directing his courtship towards that specific female. Also, Cheng (1986) has suggested, from her work with ring doves, that the female's endocrine response to male courtship is the result of the female behaviourally stimulating herself by nest cooing.
(iii) The importance of the gonadotrophin-releasing hormone neuronal system
Gonadotrophin-releasing hormone (GnRH) regulates the release of the pituitary gonadotrophins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and is therefore the neuropeptide that links the brain with the reproductive endocrine system. There are two forms in the avian brain named chicken GnRH-I (cGnRH-I) and chicken GnRH-II (cGnRH-II) due to the fact that they were first discovered in chicken (Gallus gallus), though similar peptides are generally present in avian species (see Ball & Hahn (1997) and Ball & Balthazart (2002) for reviews). Both these forms of GnRH have been found to be effective releasers of the gonadotrophins, LH and FSH, in vivo (Millar & King 1984; Sharp et al. 1990). However, based on its anatomical distribution, cGnRH-I appears to be the primary regulator of gonadotrophin secretion in birds (e.g. Millam et al. 1993; see Ball & Hahn (1997) for a review). This system exhibits remarkable seasonal plasticity in several avian species in that there is a marked decline in the expression of the peptide after the onset of photorefractoriness (see Ball & Hahn (1997), Dawson et al. (2001), MacDougall-Shackleton et al. (2005) for reviews) and even a seasonal decline in the release of gonadal steroids in response to GnRH stimulation of the pituitary–gonadal axis during the breeding season (Jawor et al. 2006). Although there has been an extensive discussion of species differences in this regulation, little is known about sex differences. One study in Japanese quail (Coturnix coturnix japonica) does indicate that there is a male-biased sex difference in the GnRH protein as measured by immunohistochemistry in short-day photosensitive birds but not in photostimulated birds (Foster et al. 1988). In bantam chicken (Gallus domesticus) chicks one induces rapid sexual maturity by taking chicks reared on short days and transferring them to long days (Sreekumar & Sharp 1998), this enhancement by photostimulation occurs earlier in males (by four weeks of age) than in females suggesting that the reproductive neuroendocrine system (that includes GnRH) develops sooner in males than in females (Sreekumar & Sharp 1998). These studies along with the well-recognized intraspecific plasticity of the GnRH system could make this a fruitful system to pursue in future studies of sex differences in the response to environmental cues regulating reproduction.
Cues from photoperiodic stimuli and supplementary stimuli converge on the GnRH system to implement the organized response in reproductive physiology that characterizes seasonal reproduction (Ball 1993). The neural processing of these classes of cues is somewhat different and these processing steps could in turn be different between the sexes. Photoperiod is perceived by the extra-retinal photoreceptor and interpreted by the circadian system since birds like other vertebrates measure day length based on the coincidence of light with the photoinducible phase of the circadian cycle (see Follett (1984) for discussion). Recent studies suggest that the clock for photoperiodic time measurement in birds is in the mediobasal hypothalamus (Yasuo et al. 2003; Sharp 2005). Thus, the neural circuit regulating photoperiod is fairly compact given that the extra-retinal photoreceptor, the clock for interpreting light and the GnRH neuronal system are all in the POA–hypothalamic area in the diencephalic region of the brain. Studies of the effects of supplementary cues such as those reviewed previously argue that at least some types of supplementary cues undergo complex neural processing involving telencephalic structures before they exert their modulatory effects on the GnRH system and through GnRH, on the endocrine system. The manner in which supplementary cues are perceived can be influenced by experience in ways that the perception of day length is not. For example, Baptista & Petrinovich (1986) describe how female white-crowned sparrows that were collected as nestlings in the wild and hand reared in the laboratory readily ovulated in captivity when housed in a situation under which ovulation has never been reported in wild-caught adult females. They suggest that young female white-crowned sparrows undergo an imprinting process during their first year to the stimuli supplementary to photoperiod, which are required for ovulation. When hand reared in captivity, they ‘imprinted’ on substitute supplementary stimuli and therefore ovulated under these conditions.
All these studies suggest that, in contrast to the apparently circumscribed locale of the neural pathway mediating the photoperiodic response, the neural pathways processing the endocrine response to supplementary cues will involve many more processing ‘steps’ and potentially could be connected to a wide variety of modulatory brain nuclei. Nonetheless, it seems essential that supplementary information exert whatever effect it may finally have on endocrine functioning via the GnRH neuron system. Thus, both types of information, photoperiodic and supplementary, appear to converge on the GnRH system. Therefore, the group of neurons in the preoptic–septal area, which project to the median eminence and are immunoreactive for GnRH, should be the starting point for the elucidation of the neural pathways processing both initial predictive and supplementary cues. Furthermore, based on the data just reviewed, tracking the pathway for the two different classes of cues should lead to very different places in the brain. It would be useful to investigate the manner in which these neural pathways process this information in males and females. At present, there is little information about functional links between the visual and auditory systems and the GnRH neuronal system and even associated hypothalamic areas. Connections have been described in female ring doves between the auditory thalamus and the regions in the preoptic area and hypothalamus, which contain GnRH neurons (Cheng & Zuo 1994). Cells in these GnRH-positive hypothalamic areas fire in response to the male and female nest coo calls (Cheng et al. 1998) perhaps based on this auditory input from the thalamus. Cheng et al. (1998) hypothesize that these cells are involved in mediating the effects of female nest coo on her own endocrine activity via the production of nest coos that increase when a male courts a female. Supporting this idea is the observation that although the neural units respond similarly to male and female calls, the playback of females coos are three times more effective in releasing the gonadotrophin LH than male coos (Cheng et al. 1998). The neural basis of this sex difference is unknown as only females were investigated in this study. Functional connections between the auditory midbrain and thalamus and brain areas containing GnRH cell groups have also been described in frogs (Wilczynski et al. 2005).
3. How are males and females different?
Up to this point, we have been discussing seasonal reproduction in birds in general terms while highlighting how different aspects of this process are relevant to a consideration of sex differences. In this final section, we will focus more on the relatively little that is known about sex differences in the response to environmental cues regulating seasonal reproduction. We will start with a consideration of ultimate causes and then focus on proximate causes.
(a) Comparing the sexes in relation to the environmental control of seasonal reproduction: a microevolutionary approach
Natural selection can be expected to have acted on the avian reproductive system to time reproduction so as to optimize both male and female reproductive success. One aspect of success will be reproductive synchrony such that males and females are ready to reproduce at the same time of year.
Males and females differ in their life histories, however, and thus the phenotypic characters that promote successful reproduction and consequently the environmental cues that promote breeding are likely to differ by sex. Male reproductive success typically varies more than female success, and reproductive competition can be expected to play a greater role in shaping male than female adaptations for breeding. In other words, male assessment of a suitable environment and time for breeding will reflect the greater role played by sexual selection in accounting for variation in male fitness; the female assessment will reflect the greater role played by fecundity selection. To illustrate this point from a male's perspective, being first to breed may allow more time for seeking additional mating partners with little attendant risk, but from a female's perspective, while being first may enhance her fecundity, it may also expose her to more stressful weather conditions.
Among temperate zone birds, given the common pattern of early establishment of territories by males and later laying of eggs by females, we are accustomed to thinking of males as initiating the sequence of behaviours that results in reproduction earlier than females, and we attribute that to the more intense competition just described. Once territories are established, coordination between the sexes is thought to require males to ‘wait for’ females to acquire sufficient energy reserves to lay eggs and initiate incubation. We typically explain this delay and thus part of the sex difference in timing to the greater pre-zygotic investment per reproductive event made by females as opposed to males. To protect that investment in eggs, females are thought to proceed more cautiously than males. In the language used earlier in this paper, males are more affected by initial predictive cues, and females more by supplementary cues.
Interestingly, a comparison with mammals suggests that intrasexual competition alone can force earlier reproductive readiness in males, regardless of the pre-zygotic investment made by females. Prendergast (2005) expressed this as follows: ‘Earlier vernal recrudescence [of gonads] in males may also facilitate intrasexual competition for mates in cases where the required behaviors are gonadal hormone-dependent, and may reflect a general sex difference in vertebrate seasonal timekeeping (cf Zucker & Boshes 1982; Crews 1983).’ Male hamsters, for example, break photorefractoriness as much as five weeks earlier than females, and this difference in timing is seen in part as an adaptation to male–male competition. Interestingly, however, female mammals invest more in their oocytes after they are fertilized than before, i.e. during pregnancy and lactation, and preparation for ovulation in females is seen as requiring less time than that needed by males to complete spermatogenesis (Beery et al. 2007). Consequently, earlier breaking of refractoriness in male hamsters is also seen as an adaptation to promote synchrony of reproductive competence between the sexes (see Prendergast (2005) for discussion). Clearly, proper interpretation of sex differences in timing requires full knowledge of the natural history of the species in question.
While sexual cooperation predicts that natural selection should act to coordinate reproductive readiness in males and females, regardless of which cues they rely upon, theory on reproductive conflict makes an additional prediction. Conflict theory suggests that selection should favour members of one sex that can successfully induce members of the other sex to invest more heavily in reproduction. In particular, given the advantages experienced by early hatched offspring (Perrins & Birkhead 1983), males might be expected to benefit if they could induce females to breed earlier than what might otherwise be in the females' best interest. Similarly, females able to elicit more investment from males in the form of guarding or provisioning should be favoured over females less able to do the same. Conflict theory thus leads us to consider not only how the behaviour of one sex coordinates itself with the behaviour of the other, but also how the behaviour of one sex influences and accelerates the reproductive behaviour of the other (e.g. Bentley et al. 2000).
Finally, when predicting how males and females respond to their environment and how avian populations might vary in their evolutionary response to environmental change like that predicted by climate change, it is critical to recall that the sexes share most of their genome. But for genes on sex chromosomes, loci present in males are also present in females. Thus, selection on males for earlier breeding and the loci that regulate breeding could give rise to a correlated response to selection in females such that daughters of early breeding males might breed earlier than the average female. If traits favoured by a changing environment are advantageous to one sex, but not to the other, we might see a rapid evolutionary response in males that proved disadvantageous to females. In that case, females might constrain further response in males and lead to a mismatch of optimal breeding times for males and females (Ketterson et al. 2005).
In sum, these considerations are useful when generalizing about how the sexes might differ or be the same in the timing of reproduction, but they also indicate a need for data. In the §3b, we consider empirical studies of responses to cues that time reproduction as well how the sexes might differ in their response to supplementary cues that can act to hasten or delay reproduction. For example, is breeding date heritable? There is evidence that this is the case in hamsters (Prendergast et al. 2004) and female birds (Brommer et al. 2005; Nussey et al. 2005). Is the threshold day length that stimulates gonadal recrudescence the same in males and females? Do males and females rely on the same supplementary cues to accelerate or delay breeding? Does food, for example, accelerate breeding equally in both sexes? Can male behaviour accelerate breeding in females? Do females vary in their susceptibility to stimulation by males?
(b) Sex differences in the response to environmental cues: mechanistic approaches
As a generalization, studies of the cues that time reproduction in birds have focused on males in the laboratory, particularly on testicular responses to change in day length, and females in the field, particularly on laying date as a function of food availability. Considering the size of the literature in this area, relatively few studies on captives have directly compared males and females using similar methods and dependent variables. Similarly, relatively few field studies have compared the sexes for the relationship between food and readiness to reproduce.
We shall consider briefly the kinds of studies that are currently being conducted and suggest that greater attention needs to be paid to obtaining measures that will allow us to compare the sexes.
Focusing first on studies of captives, only some of the dependent or response variables that can be measured in captive birds in relation to the onset of reproduction are the same in each sex. Temporal patterns in LH and gonadal development are common to males and females and have frequently been quantified. For example, captive birds of both sexes will show an increase in LH when photostimulated. Interestingly, female Zonotrichia leucophrys oriantha's LH response to increased photoperiod is considerably more robust than that of males (Wingfield et al. 2003), and stronger LH and FSH in response to photostimulation have been seen in female Zonotrichia leucophrys gambelli and to a lesser extent in female Zonotrichia leucophrys pugetensis (Follett et al. 1975).
As mentioned previously in this review, it has been noted by several authors that female birds seem to play a greater role in the ‘fine-tuning’ of the onset and termination of breeding than male birds do (Farner & Follett 1979; Wingfield 1980; Moore 1983). It is the female that makes the critical response of oviposition to local and yearly variation in resource availability. Therefore, it is not surprising that in many male birds, photoperiodic stimuli alone can induce full gonadal growth, while in females the appropriate complement of supplementary cues is required for egg laying (Wingfield 1980). The importance of the integration of initial predictive cues with supplementary cues and/or synchronizing cues from the physical and social environment is most apparent in females. Initial predictive cues such as photoperiod can stimulate ovarian development to a pre-breeding stage (Wingfield & Farner 1980). This stage is followed by an exponential growth stage that includes the synthesis and deposition of yolk, which will occur only if the local conditions are correct (e.g. Farner et al. 1966; Cheng 1974; Johnson 2000). Wild-caught females that are brought into captivity often do not develop beyond the pre-breeding stage of follicle development (Farner et al. 1966). It is generally assumed that captivity fails to provide some necessary stimulus to females or that females somehow perceive captive conditions as being unsuitable for breeding. There is some information suggesting that this latter hypothesis is correct. The fact that hand-reared captive female white-crowned sparrows readily ovulated in captivity when housed under conditions in which ovulation has never been reported in wild-caught adult females (Baptista & Petrinovich 1986) is consistent with this notion. Perhaps when hand reared in captivity, these females imprinted in some way on substitute supplementary stimuli and therefore would ovulate under these conditions. It is also clear that supplementary information can exert strong inhibitory effects on neuroendocrine physiology as well as stimulatory effects, especially in females. As mentioned previously, enucleation studies in white-crowned sparrows revealed that females without eyes actually had higher plasma concentration of LH than individuals with intact eyes (Yokoyama & Farner 1976). These data suggest that information from the eyes ordinarily conveyed to these sparrows under captive conditions was largely inhibitory of reproductive physiology.
The timing of male breeding can be influenced by manipulating the behaviour of the female, but the reverse does not seem to be the case. For example, Runfedlt & Wingfield (1985) implanted free-living female song sparrows with oestradiol pellets in the late summer. This treatment delayed the termination of reproductive behaviour in these females but also prolonged reproductive activity in the untreated male controls! Males in control pairs with untreated females went refractory and ceased reproduction several weeks before the treated pairs. These studies suggest the termination of breeding by males is influenced by what the female does. A similar situation may not apply to the initiation of breeding. Studies of two populations of Mediterranean blue tits (Parus caeruleus) in Corsica breeding in different habitats reveals a one-month difference in the initiation of egg laying that is related to timing of the availability of caterpillars at the two sites (Caro et al. 2006). Despite this difference in the timing of egg laying, males in the two populations initiate the seasonal recrudescence of the reproductive neuroendocrine system at approximately the same time in late winter (Caro et al. 2006). Thus, sensitivity to local environmental variation related to the fine-tuning of reproduction appears limited to females. Overall, these data suggest that the neural inputs to the GnRH system and the responsiveness of the GnRH system to photoperiodic and extra-photoperiodic cues may be quite different between the sexes. These data also suggest that males and females may exhibit different patterns of flexibility in their response to supplementary cues that relate to the initiation and termination of breeding. Based on the song sparrow data, it appears that for male reproductive competence, once reproduction has been initiated it can be maintained by the presence of a female, while the reverse is not necessarily the case. This scenario is reminiscent of studies of ring doves concerning the social and hormonal mechanism that regulates the synchronization of incubation behaviour. These studies concluded that females played the more important role in mediating the transition from courtship to incubation behaviour as well as in controlling the pattern of incubation sharing, and that male behaviour was cued to the behaviour of the female (Silver 1978; Ball & Silver 1983).
Because specific empirical comparisons of how the sexes fine-tune the timing of their reproduction are not common, we will describe current trends in seasonality research and consider how they might be applied to this issue of sex differences. In a sentence, the current emphasis is on comparisons involving situations in which timing of breeding varies even though day length does not. The goal is to determine which aspects of the environment are driving the variation. The application to the goal of this paper is to suggest that the questions and the methods that are currently being employed should include the question of coordination and potential conflict between the sexes.
The field of biological timing has a long history of comparing populations of the same species that reside at different latitudes and consequently experience different photoperiodic regimes (e.g. Silverin et al. 1993). The more recent application has been to compare populations that share a photoperiodic regime but occupy otherwise different environments, e.g. coastal and montane populations living at the same latitude. Such studies report considerable population-based differences in breeding schedules (Wingfield et al. 2003; Hau et al. 2004; Perfito et al. 2005), some of which appear to have a genetic basis (Moore et al. 2005)
A second approach that has a long history is to employ long-term studies to compare the same population over time, i.e. to relate annual variation in the onset of reproduction to annual variation in the environment. The recent emphasis on temperature as a timing cue has led people to add an experimental component to this work (Wingfield et al. 2003). Thus, in the field, one would seek correlations between environmental variables and breeding dates, and in the laboratory, one would observe the response of captive populations to manipulations of supplementary cues.
Together, the combination of these classic and newer approaches has clearly revealed a highly significant role for supplementary cues in the timing of breeding, even suggesting that in some situations they may be just as important to the induction of reproductive readiness as photic cues and may interact with photic cues on what might be considered ‘equal footing’ (Hau 2001).
Despite advances in our empirical understanding, it is still true that few studies of captive birds compare the sexes directly regarding their response with cues that time reproduction. Consequently, we briefly summarize some of what has been learned from comparative studies of populations and use this knowledge to speculate about how males and females might differ in their responses. To the extent that the sexes are analogous to separate populations, such speculation may prove accurate or at least serve as a basis for future studies comparing the sexes.
(c) Measuring the impact of supplemental cues on timing of reproduction: impact of temperature
Wingfield et al. (2003) reported yearly variation in laying dates in a montane population of white-crowned sparrows, Z. l. oriantha. The level of variation reported revealed that photoperiod is key to reproductive timing but environmental variables such as temperature were clearly playing a role. They brought individual male and female sparrows to the laboratory and manipulated day length and temperature. The birds were measured for a suite of characters, some of which are shared by both sexes, e.g. LH, gonadal growth and regression, thyroid hormone, body mass, fat and moult, and some of which were sex specific, e.g. brood patch development in females. No sex differences were reported. Both sexes responded to both photoperiod and temperature in similar ways, e.g. gonadal growth was accelerated at warmer temperatures, and both sexes regressed their gonads sooner at warmer temperatures. In a similar study conducted on the high latitude breeding Gambell's subspecies of the white-crowned sparrow, no sex difference was reported for the effect of temperature on gonadal growth (Wingfield et al. 1996). However, low temperatures (5°C) inhibited the premigratory increase in fattening and body mass in females but not in males (Wingfield et al. 1996). This difference may reflect sex differences in selection for males to arrive on breeding ground early to set up territories to attract mates, regardless of local conditions, while females may be selected to wait until conditions are optimal to lay on fat and migrate (Wingfield et al. 1996). Dawson (2005) reports that although female starlings have advanced laying date in the field over recent decades, captive males do not accelerate gonadal development in response to temperature manipulations in the laboratory. A study comparing males belonging to a coastal and montane population of song sparrows known to differ in reproductive timing also failed to attribute the differences to temperature, because temperature manipulations had little effect on testis development in captives (Perfito et al. 2005). Hau et al. (2004), based on correlative comparisons across free-living populations of a tropical species, concluded that temperature could not explain the variation; rather ‘factors such as humidity, barometric pressure or rainfall itself may stimulate reproduction.’ In sum, studies comparing the impact of temperature on males and females are needed, but there is reason to think that other variables will need to be studied as well.
(d) Impact of food
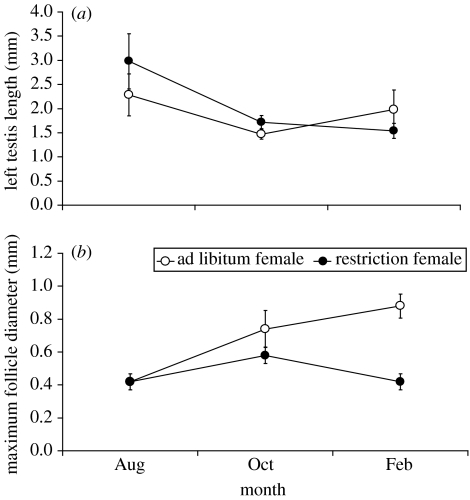
It is well known based both on correlational studies and on experimental investigations that food availability is a supplemental factor which can influence the timing of egg laying in birds (Perrins 1970; Martin 1987; Boutin 1990; Svensson 1995). Thus, in this section, we focus on some experimental studies that have specifically considered food as an environmental cue which can affect the timing of egg laying. Hahn and co-investigators have studied the impact of food availability and day length on the timing of reproduction in opportunistically breeding species. Such species have often been thought to be heavily or entirely reliant on food to time their breeding, but work by this group has shown that day length and food interact in their effect on readiness to reproduce. Most studies have focused on males, but one study that compared the sexes directly and found that moving red crossbills (Loxia curvirostra) from mild food restriction to ad libitum food in winter enhanced ovarian development in females, while having no significant effect on males, suggesting that females may be more affected by food than males (Hahn et al. 2005; figure 2).
Figure 2.
Comparison of effect of ad libitum food versus mild food restriction on seasonal variation in gonad size in (a) male and (b) female red crossbills (adapted from Hahn et al. 2005).
Timing of reproduction can be affected by food quality as well as quantity. Female crossbills increase LH when food is supplemented, and the increase in LH was greater when the females were given conifer cones along with the supplemental food (reviewed in Hahn et al. 2005). Schoech et al. (2004) have shown that the supplemental food significantly advanced laying date of female Florida scrub jays (Aphelocoma coerulescens) in the field. When they offered free-living scrub jays experimental diets that were the same in caloric value but differed in the ratio of fat to protein, they found that diet quality did not influence laying date, although it did affect egg size in relation to laying order (Reynolds et al. 2003).
Returning to sex differences in response to food, it is of interest to know whether food influences the reproductive axis through an internal perception of energy balance or nutrient availability or whether food itself provides some stimulatory sensation. Early in spring in Alaska, alfalfa sprouts increase testis growth in male common redpolls (Carduelis flammea) but do not increase ovarian development in females (Hahn et al. 2005). Hau et al. (2000) conducted an elegant study on the visual impact of crickets on male song and gonadal development in spotted antbirds (Hylophylax naevioides). They found that both song and testis size were enhanced simply by seeing and handling the crickets, controls that received dead crickets of similar nutritional quality exhibited no such enhancing effects (Hau et al. 2000). It would be interesting to see whether females would respond in the same way.
4. Conclusion
Many interesting questions remain about whether males and females respond to the environment and thus will respond to climate change in similar fashion. There is also very little literature exploring the effects of global climate change, and/or differential responses of the sexes to environmental cues in breeding birds. It is clear that coordinated timing of events that directly affect the ability to reproduce should be selected for, but much remains to be learned, and variation between species in the ability to coordinate responses could provide clues about which species are in most need of observation over the coming years.
Acknowledgments
Our research relevant to these topics has been approved by the appropriate animal care and use committee at either John Hopkins University or Indiana University.
We thank Steve Schoech, Eric Snajdr, Tom Hahn, Alistair Dawson, Scott McDougall-Shackleton, Sara Schrock and Tim Greives for their useful discussions. We thank Daniel Bernard for providing the data on gonadal changes in wild caught male and female starlings. We also thank the NSF and NIH (R01 NS 35467) for the support of our research programmes. Figure 2 reproduced from Functional Avian Ecology, copyright 2005, Narosa Publishing House Pvt. Ltd., New Delhi.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Baker J. The evolution of breeding seasons. In: DeBeer G.B, editor. Evolution: essays on aspects of evolutionary biology. Clarendon Press; Oxford, UK: 1938. pp. 161–177. [Google Scholar]
- Ball G.F. The neural integration of environmental information by seasonally breeding birds. Am. Zool. 1993;33:185–199. [Google Scholar]
- Ball G.F, Balthazart J. Neuroendocrine mechanisms regulating reproductive cycles and reproductive behavior in birds. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. vol. 2. Academic Press; San Diego, CA: 2002. pp. 649–798. [Google Scholar]
- Ball G.F, Bentley G.E. Neuroendocrine mechanisms mediating the photoperiodic and social regulation of seasonal reproduction in birds. In: Wallen K, Schneider J.E, editors. Reproduction in context: social and environmental influences on reproductive physiology and behavior. MIT Press; Cambridge, MA: 2000. pp. 129–158. [Google Scholar]
- Ball G.F, Hahn T.P. GnRH neuronal systems in birds and their relation to the control of seasonal reproduction. In: Parhar I.S, Sakuma Y, editors. GnRH neurons: gene to behavior. Brain Shuppan; Tokyo, Japan: 1997. pp. 325–342. [Google Scholar]
- Ball G.F, Silver R. Timing of incubation bouts by ring doves (Streptopelia risoria) J. Comp. Psych. 1983;97:213–225. doi:10.1037/0735-7036.97.3.213 [PubMed] [Google Scholar]
- Balthazart J, Hendrick J. Annual variation in reproductive behavior, testosterone, and plasma FSH levels in the Rouen duck, Anas platyrhynchos. Gen. Comp. Endocrinol. 1976;28:171–183. doi: 10.1016/0016-6480(76)90169-6. doi:10.1016/0016-6480(76)90169-6 [DOI] [PubMed] [Google Scholar]
- Baptista L.F, Petrinovich L. Egg production in hand-reared white-crowned sparrows. Condor. 1986;88:379–380. doi:10.2307/1368888 [Google Scholar]
- Beery A.K, Trumbull J.J, Tsao J.M, Costatini R.M, Zucker I. Sex differences in the onset of seasonal reproductive quiescence in hamsters. Proc. R. Soc. B. 2007;274:281–286. doi: 10.1098/rspb.2006.3726. doi:10.1098/rspb.2006.3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit J. Stimulation par la lumiere artificiell du developpement testiculaire chez des canards aveugles par enucleation des globes oculaires. C.R. Soc. Biol. (Paris) 1935;120:136–139. [Google Scholar]
- Bentley G.E, Wingfield J.C, Morton M.L, Ball G.F. Stimulatory effects on the reproductive axis in female songbirds by conspecific and heterospecific male song. Horm. Behav. 2000;37:179–189. doi: 10.1006/hbeh.2000.1573. doi:10.1006/hbeh.2000.1573 [DOI] [PubMed] [Google Scholar]
- Berthold A.A. Transplantation der Hoden. Arch. F. Anat. U. Physiol. 1849;16:42–46. [Google Scholar]
- Boutin S. Food supplementation experiments with terrestrial vertebrates—patterns, problems, and the future. Can. J. Zool. 1990;68:203–220. [Google Scholar]
- Brommer J.E, Merila J, Sheldon B.C, Gustafsson L. Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution. 2005;59:1362–1371. [PubMed] [Google Scholar]
- Bronson F. University of Chicago Press; Chicago, IL: 1989. Mammalian reproductive biology. [Google Scholar]
- Burger J.W. A review of experimental investigations of seasonal reproduction in birds. Wilson Bull. 1949;61:211–230. [Google Scholar]
- Caro S.P, Lambrechts M.M, Chastel O, Sharp P.J, Thomas D.W, Balthazart J. Simultaneous pituitary–gonadal recrudescence in two Corsican populations of male blue tits with asynchronous breeding dates. Horm. Behav. 2006;50:347–360. doi: 10.1016/j.yhbeh.2006.03.001. doi:10.1016/j.yhbeh.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Chaurasia S.S, et al. Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation and expression in pineal and retinal cell types. J. Neurochem. 2005;92:158–170. doi: 10.1111/j.1471-4159.2004.02874.x. doi:10.1111/j.1471-4159.2004.02874.x [DOI] [PubMed] [Google Scholar]
- Cheng M.F. Ovarian development in the female ring dove in response to stimulation by intact and castrated male ring doves. J. Endocrinol. 1974;63:43–53. doi: 10.1677/joe.0.0630043. [DOI] [PubMed] [Google Scholar]
- Cheng M.F. Progress and prospects in ring dove research: a personal view. Adv. Study Anim. Behav. 1979;9:97–129. [Google Scholar]
- Cheng M.F. Female cooing promotes ovarian development in ring doves. Physiol. Behav. 1986;37:371–374. doi: 10.1016/0031-9384(86)90248-9. doi:10.1016/0031-9384(86)90248-9 [DOI] [PubMed] [Google Scholar]
- Cheng M.F, Zuo M. Proposed pathways for vocal self-stimulation: met-enkephalinergic projections linking the midbrain vocal nucleus, auditory responsive thalamic regions and neuroscretory hypothalamus. J. Neurobiol. 1994;25:361–379. doi: 10.1002/neu.480250403. doi:10.1002/neu.480250403 [DOI] [PubMed] [Google Scholar]
- Chen M.F, Peng J.P, Johnson P. Hypothalamic neurons preferentially respond to female nest coo stimulation: demonstration of direct acoustic stimulation of luteinizing hormone release. J. Neurosci. 1998;18:5477–5489. doi: 10.1523/JNEUROSCI.18-14-05477.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Princeton University Press; Princeton, NJ: 1991. The evolution of parental care. [Google Scholar]
- Cockburn A. Prevalence of different modes of parental care in birds. Proc R. Soc. B. 2006;273:1375–1383. doi: 10.1098/rspb.2005.3458. doi:10.1098/rspb.2005.3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. Optimizing reproduction in a varying environment. J. Theor. Biol. 1967;16:1–14. doi: 10.1016/0022-5193(67)90050-1. doi:10.1016/0022-5193(67)90050-1 [DOI] [PubMed] [Google Scholar]
- Crews D. Control of male sexual behavior in Canadian red-sided garter snake. In: Balthazart J, Pröve E, Gilles R, editors. Hormones and behavior in higher vertebrates. Plenum; London, UK: 1983. pp. 398–406. [Google Scholar]
- Crews D, Silver R. Reproductive physiology and behavior interactions in nonmammalian vertebrates. In: Adler N, Pfaff D, Goy R.W, editors. Handbook of behavioral neurobiology. vol. 7. Plenum Publishing Co.; New York, NY: 1985. pp. 101–182. [Google Scholar]
- Dawson A. Plasma gonadal steroid levels in wild starlings (Sturnus vulgaris) during the annual cycle and in relation to the stages of breeding. Gen. Comp. Endocrinol. 1983;49:286–294. doi: 10.1016/0016-6480(83)90146-6. doi:10.1016/0016-6480(83)90146-6 [DOI] [PubMed] [Google Scholar]
- Dawson A. Effect of daylength on the rate of recovery of photosensitivity in male starlings (Sturnus vulgaris) J. Reprod. Fert. 1991;93:521–524. doi: 10.1530/jrf.0.0930521. [DOI] [PubMed] [Google Scholar]
- Dawson A. The effect of temperature on photoperiodically regulated gonadal maturation, regression and moult in starlings—potential consequences of climate change. Funct. Ecol. 2005;19:995–1000. doi:10.1111/j.1365-2435.2005.01061.x [Google Scholar]
- Dawson A, Goldsmith A.R. Plasma prolactin and gonadotrophins during gonadal development and the onset of photorefractoriness in male and female starlings (Sturnus vulgaris) on artificial photoperiods. J. Endocr. 1983;97:253–260. doi: 10.1677/joe.0.0970253. [DOI] [PubMed] [Google Scholar]
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. doi:10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Doughty P, Shine R. Detecting life history trade-offs: measuring energy stores in “capital” breeders reveals costs of reproduction. Oecologia. 1997;110:508–513. doi: 10.1007/s004420050187. doi:10.1007/s004420050187 [DOI] [PubMed] [Google Scholar]
- Erickson C.J. Mrs. Harvey's parrot and some problems of socioendocrine response. In: Bateson P.P.G, Klopfer P, editors. Perspectives in ethology 6: mechanisms. Plenum Press; New York, NY: 1985. pp. 261–285. [Google Scholar]
- Farner D. Generation and regulation of annual cycles in migratory passerine birds. Am. Zool. 1986;26:493–501. [Google Scholar]
- Farner D.S, Follett B.K. Light and other environmental factors affecting avian reproduction. J. Anim. Sci. 1966;26:90–118. doi: 10.2527/jas1966.25supplement90x. [DOI] [PubMed] [Google Scholar]
- Farner D.S, Follett B.K. Reproductive periodicity in birds. In: Barrington E.J.W, editor. Hormones and evolution. Academic Press; London, UK; New York, NY: 1979. pp. 829–872. [Google Scholar]
- Farner D.S, Mewaldt L.R. The natural termination of the refractory period in the white-crowned sparrow. Condor. 1955;57:112–116. doi:10.2307/1364552 [Google Scholar]
- Farner D.S, Wilson A.C. A quantitative examination of testicular growth in the white-crowned sparrow. Biol. Bull. 1957;113:254–267. doi: 10.2307/1539953. doi:10.2307/1539083 [DOI] [PubMed] [Google Scholar]
- Farner D.S, Follett B.K, King J.R, Morton M.L. A quantitative examination of ovarian growth in white-crowned sparrow. Biol. Bull. 1966;130:67–75. doi: 10.2307/1539953. doi:10.2307/1539953 [DOI] [PubMed] [Google Scholar]
- Follett B.K. Birds. In: Lamming G.E, editor. Marshall's physiology of reproduction. Longman Greene; Edinburgh, UK: 1984. pp. 283–350. [Google Scholar]
- Follett B.K, Farner D.S, Mattocks P.W., Jr Luteinizing hormone in the plasma of white-crowned sparrows, Zonotrichia leucophrys gambelii, during artificial photostimulation. Gen. Comp. Endocrinol. 1975;26:126–134. doi: 10.1016/0016-6480(75)90223-3. doi:10.1016/0016-6480(75)90223-3 [DOI] [PubMed] [Google Scholar]
- Foster R.G, Panzica G.C, Parry D.M, Viglietti-Panzica C. Immunocytochemical studies on the LHRH system of the Japanese quail—influence by photoperiod and aspects of sexual-differentiation. Cell Tissue Res. 1988;253:327–335. doi: 10.1007/BF00222289. doi:10.1007/BF00222289 [DOI] [PubMed] [Google Scholar]
- Friedman M.B. Interactions between visual and vocal courtship stimuli in the neuroendocrine response of female doves. J. Comp. Physiol. Psychol. 1977;91:1408–1416. doi:10.1037/h0077407 [Google Scholar]
- Goldman B.D. Mammalian phooperiodic system: formal properties and neuroendorince mechanisms of photoperiodic time measurement. J. Biol. Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. doi:10.1177/074873001129001980 [DOI] [PubMed] [Google Scholar]
- Goldman B.D, Gwinner E, Karsch F.J, Saunders D, Zucker I, Ball G.F. Circannual rhythms and photoperiodism. In: Dunlap J.C, Loros J.J, DeCoursey P.J, editors. Chronobiology: biological timekeeping. Sinauer Associates, Inc.; Sunderland, MA: 2004. pp. 107–142. [Google Scholar]
- Gwinner E. Springer; Berlin, Germany: 1986. Circannual rhythms. [Google Scholar]
- Gwinner E. Circadian and circannual programmes in avian migration. J. Exp. Biol. 1996;199:39–48. doi: 10.1242/jeb.199.1.39. [DOI] [PubMed] [Google Scholar]
- Gwinner E, Scheuerlein A. Photoperiodic responsiveness of equatorial and temperate-zone stonechats. Condor. 1999;101:347–359. doi:10.2307/1369998 [Google Scholar]
- Hahn T.P, Pereyra M.E, Katti M, Ward G.M, MacDougall-Shackleton S.A. Effects of food availability on the reproductive system. In: Dawson A, Sharp P.J, editors. Functional avian endocrinology. Narosa Publishing House; New Delhi, India: 2005. pp. 167–180. [Google Scholar]
- Hamner W.M. On seeking an alternative to the endogenous reproductive rhythm hypothesis in birds. In: Menaker M, editor. Biochronometry. National Academy of Sciences; Washington, DC: 1971. [Google Scholar]
- Hansen E.W. Squab-induced crop growth in experienced and inexperienced ring dove (Streptopelia risoria) foster parents. J. Comp. Physiol. Psychol. 1971;77:375–382. doi: 10.1037/h0031897. doi:10.1037/h0031897 [DOI] [PubMed] [Google Scholar]
- Hansen E.W. Further analysis of responsiveness of experienced and inexperienced ring dove (Streptopelia risoria) foster parents to squabs. Dev. Psychobiol. 1973;6:557–565. doi: 10.1002/dev.420060612. doi:10.1002/dev.420060612 [DOI] [PubMed] [Google Scholar]
- Hau M. Timing of breeding in variable environments: tropical birds as model systems. Horm. Behav. 2001;40:281–290. doi: 10.1006/hbeh.2001.1673. doi:10.1006/hbeh.2001.1673 [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. Visual and nutritional food cues fine-tune timing of reproduction in a neotropical rainforest bird. J. Exp. Zool. 2000;286:494–504. doi:10.1002/(SICI)1097-010X(20000401)286:5<494::AID-JEZ7>3.0.CO;2-3 [PubMed] [Google Scholar]
- Hau M, Wikelski M, Gwinner H, Gwinner E. Timing of reproduction in a Darwin's finch: temporal opportunism under spatial constraints. Oikos. 2004;106:489–500. doi:10.1111/j.0030-1299.2004.13206.x [Google Scholar]
- Hinde R.A, Steel E. The influence of daylength and male vocalizations on the estrogen-dependent behavior of female canaries and budgerigars, with discussion of data from other species. Adv. Study Anim. Behav. 1978;8:39–73. [Google Scholar]
- Holberton R, Able K. Persistence of circannual cycles in a migratory bird held in constant dim light. J. Comp. Physiol. A. 1992;171:477–481. doi:10.1007/BF00194580 [Google Scholar]
- Jawor J.M, McGlothlin J.W, Casto J.M, Greives T.J, Snajdr E.A, Bentley G.E, Ketterson E.D. Seasonal and individual variation in response to GnRH challenge in male dark-eyed juncos (Junco hyemalis) Gen. Comp. Endocrinol. 2006;149:182–189. doi: 10.1016/j.ygcen.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Johnson A.L. Reproduction in the female. In: Whittow G.C, editor. Sturkie's avian physiology. Academic Press; San Diego, CA: 2000. pp. 569–596. [Google Scholar]
- Ketterson E.D, et al. Testosterone, phenotype, and fitness: a research program in evolutionary behavioral endocrinology. In: Dawson A, Chaturvedi C.M, editors. Avian endocrinology. Narosa Publishing House; New Delhi, India: 2001. pp. 19–40. [Google Scholar]
- Ketterson E.D, Nolan V, Jr, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am. Nat. 2005;166:S85–S98. doi: 10.1086/444602. doi:10.1086/444602 [DOI] [PubMed] [Google Scholar]
- Konishi M, Emlen S.T, Ricklefs R.E, Wingfield J.C. Contributions of bird studies to biology. Science. 1989;246:465–472. doi: 10.1126/science.2683069. doi:10.1126/science.2683069 [DOI] [PubMed] [Google Scholar]
- Lehrman D.S. Hormonal responses to external stimuli in birds. Ibis. 1959;101:478–495. [Google Scholar]
- Lincoln G.A, Racy P.A, Sharp P.J, Kandorf H. Endocrine changes associated with spring and autumn sexuality in the rook Corvus frugilegus. J. Zool. 1980;190:137–153. [Google Scholar]
- Lofts B, Murton R.K, Westwood N.J. Gonad cycles and the evolution of breeding seasons in British Columbidae. J. Zool. 1966;150:249–272. [Google Scholar]
- MacDougall-Shackleton S.A, Pereyra M.E, Hahn T.P. GnRH, photorefractoriness and breeding schedules of cardueline finches. In: Dawson A, Sharp P.J, editors. Functional avian endocrinology. Narosa Publishing House; New Delhi, India: 2005. pp. 97–110. [Google Scholar]
- Martin T.E. Food as a limit on breeding birds—a life-history perspective. Annu. Rev. Ecol. Syst. 1987;18:453–487. doi:10.1146/annurev.es.18.110187.002321 [Google Scholar]
- McMillan J, Underwood H, Elliot J, Stetson M, Menaker M. Extra-retinal light perception. 4. Further evidence that eyes do not participate in photperiodic photoreception. J. Comp. Physiol. A. 1975;97:205–214. doi:10.1007/BF00617543 [Google Scholar]
- Millam J.R, Faris P.L, Youngren O.M, El Halawani M.E, Hartman B.K. Immunohistochemical localization of chicken gonadotropin-releasing hormones I and II (cGnRH I and II) in turkey hen brain. J. Comp. Neurol. 1993;333:68–82. doi: 10.1002/cne.903330106. doi:10.1002/cne.903330106 [DOI] [PubMed] [Google Scholar]
- Millar R, King J. Structure–activity relations of LHRH in birds. J. Exp. Zool. 1984;232:425–430. doi: 10.1002/jez.1402320307. doi:10.1002/jez.1402320307 [DOI] [PubMed] [Google Scholar]
- Moore M.C. Effect of female sexual displays on the endocrine physiology and behaviour of male white-crowned sparrows. Zonotrichia leucophrys. J. Zool., Lond. 1983;199:137–148. [Google Scholar]
- Moore I.T, Bonier F, Wingfield J.C. Reproductive asynchrony and population divergence between two tropical bird populations. Behav. Ecol. 2005;16:755–762. doi:10.1093/beheco/ari049 [Google Scholar]
- Murton R, Westwood N. Clarendon Press; Oxford, UK: 1977. Avian breeding cycles. [Google Scholar]
- Nelson R.J. Photoperiod-nonresponsive morphs: a possible variable in microtine population-density fluctuations. Am. Nat. 1987;130:350–369. doi:10.1086/284715 [Google Scholar]
- Nicholls T, Goldsmith A, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiol. Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. From bird song to neurogenesis. Sci. Am. 1989;260:74–79. doi: 10.1038/scientificamerican0289-74. [DOI] [PubMed] [Google Scholar]
- Nussey D.H, Postma E, Gienapp P, Visser M.E. Selection on heritable phenotypic plasticity in a wild bird population. Science. 2005;310:304–306. doi: 10.1126/science.1117004. doi:10.1126/science.1117004 [DOI] [PubMed] [Google Scholar]
- O'Brien S, Hau M. Food cues and gonadal development in neotropical spotted antbirds (Hylophylax naevioides) J. Ornithol. 2005;146:332–337. doi:10.1007/s10336-005-0013-9 [Google Scholar]
- Oring L.W. Avian mating systems. In: Farner D.S, King J.R, Parkes K.C, editors. Avian biology. vol. VI. Academic Press; New York, NY: 1982. pp. 1–92. [Google Scholar]
- Owens I.P.F. Male-only care and classical polyandry in birds: phylogeny, ecology and sex differences in remating opportunities. Proc. R. Soc. B. 2002;357:283–293. doi: 10.1098/rstb.2001.0929. doi:10.1098/rstb.2001.0929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfito N, Meddle S.L, Tramontin A.D, Sharp P.J, Wingfield J.C. Seasonal gonadal recrudescence in song sparrows: response to temperature cues. Gen. Comp. Endocrinol. 2005;143:121–128. doi: 10.1016/j.ygcen.2005.03.004. doi:10.1016/j.ygcen.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Perrins C. The timing of birds breeding seasons. Ibis. 1970;112:242–255. [Google Scholar]
- Perrins C, Birkhead T. Blackie; Glasgow, UK; London, UK: 1983. Avian ecology. [Google Scholar]
- Prendergast B.J. Internalization of seasonal time. Horm. Behav. 2005;48:503–511. doi: 10.1016/j.yhbeh.2005.05.013. doi:10.1016/j.yhbeh.2005.05.013 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Renstrom R.A, Nelson R.J. Genetic analyses of a seasonal interval timer. J. Biol. Rhythms. 2004;19:298–311. doi: 10.1177/0748730404266626. doi:10.1177/0748730404266626 [DOI] [PubMed] [Google Scholar]
- Rathinam T, Kuenzel W.J. Attenuation of gonadal response to photostimulation following ablation of neurons in the lateral septal organ of chicks. Brain Res. Bull. 2005;64:455–461. doi: 10.1016/j.brainresbull.2004.10.003. doi:10.1016/j.brainresbull.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Reynolds S.J, Schoech S.J, Bowman R. Diet quality during pre-laying and nestling periods influences growth and survival of Florida scrub-jay (Aphelocoma coerulescens) chicks. J. Zool. 2003;261:217–226. doi:10.1017/S0952836903004023 [Google Scholar]
- Ricklefs R.E. Avian postnatal development. In: Farner D.S, King J.R, Parkes K.C, editors. Avian biology. vol. VII. Academic Press; New York, NY: 1983. pp. 1–83. [Google Scholar]
- Robinson J, Follett B.K. Photoperiodism in Japanese quail: termination of season breeding by photorefractoriness. Proc. R. Soc. B. 1982;215:96–116. doi: 10.1098/rspb.1982.0030. doi:10.1098/rspb.1982.0030 [DOI] [PubMed] [Google Scholar]
- Rowan W. Experiments in bird migration I. Manipulation of the reproductive cycle: seasonal histological changes in the gonads. Proc. Boston Soc. Nat. Hist. 1929;39:151–208. [Google Scholar]
- Runfedlt S, Wingfield J.C. Experimentally prolonged sexual activity in female sparrows delays termination of reproductive activity in their untreated mates. Anim. Behav. 1985;33:403–410. doi:10.1016/S0003-3472(85)80064-6 [Google Scholar]
- Saldanha C.J, Leak R.K, Silver R. Detection and transduction of daylength in birds. Psychoneuroendocrinology. 1994;19:641–656. doi: 10.1016/0306-4530(94)90047-7. doi:10.1016/0306-4530(94)90047-7 [DOI] [PubMed] [Google Scholar]
- Schoech S.J, Bowman R, Reynolds S.J. Food supplementation and possible mechanisms underlying early breeding in the Florida scrub-jay (Aphelocoma coerulescens) Horm. Behav. 2004;46:565–573. doi: 10.1016/j.yhbeh.2004.06.005. doi:10.1016/j.yhbeh.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Sharp P.J. Strategies in avian breeding cycles. Anim. Reprod. Sci. 1996;42:505–513. doi:10.1016/0378-4320(96)01556-4 [Google Scholar]
- Sharp P.J. Photoperiodic regulation of seasonal breeding in birds. Ann. NY Acad. Sci. 2005;1040:189–199. doi: 10.1196/annals.1327.024. doi:10.1196/annals.1327.024 [DOI] [PubMed] [Google Scholar]
- Sharp P, Dunn I, Main G, Sterling R, Talbot R. Gonadotropin releasing hormones: distribution and function. In: Wada M, Ishii S, Scanes C, editors. Endocrinology of birds. Springer; Berlin, Germany: 1990. pp. 31–42. [Google Scholar]
- Silver R. The parental behavior of ring doves. Am. Sci. 1978;66:209–215. [Google Scholar]
- Silver R, Andrews H, Ball G.F. Parental care in an ecological perspective: a quantitative analysis of avian subfamilies. Am. Zool. 1985;25:823–840. [Google Scholar]
- Silver R, Witkovsky P, Horvath P, Alones V, Barnstable C.J, Lehman M.N. Coexpression of opsin- and VIP-like immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. doi:10.1007/BF00221754 [DOI] [PubMed] [Google Scholar]
- Silverin B, Viebke P.A. Low temperatures affect the photoperiodically induced LH and testicular cycles differently in closely related species of tits (Parus spp.) Horm. Behav. 1994;28:199–206. doi: 10.1006/hbeh.1994.1017. doi:10.1006/hbeh.1994.1017 [DOI] [PubMed] [Google Scholar]
- Silverin B, Massa R, Stokkan K. Photoperiodic adaptation to breeding at different latitudes in great tits. Gen. Comp. Endocrinol. 1993;90:14–22. doi: 10.1006/gcen.1993.1055. doi:10.1006/gcen.1993.1055 [DOI] [PubMed] [Google Scholar]
- Sreekumar K.P, Sharp P.J. Ontogeny of the photoperiodic control of prolactin and luteinizing hormone secretion in male and female bantams (Gallus domesticus) Gen. Comp. Endocrinol. 1998;109:69–74. doi: 10.1006/gcen.1997.7009. doi:10.1006/gcen.1997.7009 [DOI] [PubMed] [Google Scholar]
- Svensson E. Avian reproductive timing—when should parents be prudent. Anim. Behav. 1995;49:1569–1575. doi:10.1016/0003-3472(95)90078-0 [Google Scholar]
- Szekely T, Reynolds J.D. Evolutionary transitions in parental care in shorebirds. Proc. R. Soc. B. 1995;262:57–64. doi:10.1098/rspb.1995.0176 [Google Scholar]
- Visser M.E, van Noordwijk A.J, Tinbergen J.M, Lessells C.M. Warmer springs lead to mistimed reproduction in great tits (Parus major) Proc. R. Soc. B. 1998;265:1867–1870. doi:10.1098/rspb.1998.0514 [Google Scholar]
- Visser M.E, Holleman L.J.M, Gienapp P. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia. 2006;147:164–172. doi: 10.1007/s00442-005-0299-6. doi:10.1007/s00442-005-0299-6 [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Lynch K.S, O'Bryant E.L. Current research in amphibians: studies integrating endocrinology, behavior, and neurobiology. Horm. Behav. 2005;48:440–450. doi: 10.1016/j.yhbeh.2005.06.001. doi:10.1016/j.yhbeh.2005.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson F, Donham R. Daylength and control of seasonal reproduction in male birds. In: Stetson M.H, editor. Processing of environmental information in vertebrates. Springer; Berlin, Germany: 1988. pp. 101–120. [Google Scholar]
- Wilson F.E. Extraocular control of photorefractoriness in American tree sparrows (Spizella arobrea) Biol. Reprod. 1989;41:111–116. doi: 10.1095/biolreprod41.1.111. doi:10.1095/biolreprod41.1.111 [DOI] [PubMed] [Google Scholar]
- Wilson F.E. Neither retinal nor pineal photoreceptors mediate photoperiodic control of seasonal reproduction in American tree sparrows (Spizella arborea) J. Exp. Zool. 1991;259:117–127. doi:10.1002/jez.1402590114 [Google Scholar]
- Wingfield J.C. Fine temporal adjustment of reproductive functions. In: Epple A, Stetson M.H, editors. Avian endocrinology. Academic Press; New York, NY: 1980. pp. 367–389. [Google Scholar]
- Wingfield J.C. Environmental and endocrine control of avian reproduction: an ecological approach. In: Mikami S.I, Homma K, Wada M, editors. Avian endocrinology. Springer; Japan Science Society Press; Berlin, Germany; Tokyo, Japan: 1983. pp. 265–288. [Google Scholar]
- Wingfield J.C. Communicative behaviors, hormones–behavior interactions, and reproduction in vertebrates. In: Neil J.D, editor. Knobil and Neill's physiology of reproduction. vol. 2. Academic Press; San Diego, CA: 2006. pp. 1995–2040. [Google Scholar]
- Wingfield J.C, Farner D.S. Control of seasonal reproduction in temperate-zone birds. Prog. Reprod. Biol. 1980;5:62–101. [Google Scholar]
- Wingfield J.C, Farner D.S. Endocrinology of reproduction in wild species. In: Farner D.S, King J, Parkes K.C, editors. Avian biology. vol. 9. Academic Press; New York, NY: 1993. pp. 163–327. [Google Scholar]
- Wingfield J.C, Hunt K.E. Arctic spring: hormone–behavior interactions in a severe environment. Comp. Biochem. Physiol. B. 2002;132:275–286. doi: 10.1016/s1096-4959(01)00540-1. doi:10.1016/S1096-4959(01)00540-1 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Kenagy G.J. Natural regulation of reproductive cycles. In: Pang P.K.T, Schreibman M.P, editors. Vertebrate endocrinology: fundamentals and biomedical implications. Academic Press; San Diego, CA: 1991. pp. 181–241. [Google Scholar]
- Wingfield J.C, Hahn T.P, Wada M, Schoech S.J. Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 1992;261:214–231. doi:10.1002/jez.1402610212 [Google Scholar]
- Wingfield J.C, Hahn T.P, Wada M, Astheimer L.B, Schoech S. Interrelationship of day length and temperature on the control of gonadal development, body mass and fat score in white-crowned sparrows, Zonotrichia leucophrys gambelii. Gen. Comp. Endocrinol. 1996;101:242–255. doi: 10.1006/gcen.1996.0027. doi:10.1006/gcen.1996.0027 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hahn T.P, Maney D.L, Schoech S.J, Wada M, Morton M.L. Effects of temperature on photoperiodically induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white-crowned sparrows, Zonotrichia leucophrys oriantha. Gen. Comp. Endocrinol. 2003;131:143–158. doi: 10.1016/s0016-6480(02)00648-2. doi:10.1016/S0016-6480(02)00648-2 [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Okabayashi N, Ebihara S, Yoshimura T. Circadian clock genes and photoperiodism: comprehensive analysis of clock genes expression in the mediobasal hypothalamus, the suprachiastmatic nucleus and the pineal gland of Japanese quail under various light schedules. Endocrinology. 2003;144:3742–3748. doi: 10.1210/en.2003-0435. doi:10.1210/en.2003-0435 [DOI] [PubMed] [Google Scholar]
- Yokoyama K, Farner D. Photoperiodic responses in bilaterally enucleated female white-crowned sparrows. Zonotrichia leucophrys gambelii. Gen. Comp. Endocrinol. 1976;30:528–533. doi: 10.1016/0016-6480(76)90124-6. doi:10.1016/0016-6480(76)90124-6 [DOI] [PubMed] [Google Scholar]
- Zucker I, Boshes M. Circannual body weight rhythms of ground squirrels: role of gonadal hormones. Am. J. Physiol. 1982;243:R546–R551. doi: 10.1152/ajpregu.1982.243.5.R546. [DOI] [PubMed] [Google Scholar]