Abstract
Long-distance migration, and the study of the migrants who undertake these journeys, has fascinated generations of biologists. However, many aspects of the annual cycles of these migrants remain a mystery as do many of the driving forces behind the evolution and maintenance of the migrations themselves. In this article we discuss nutritional, energetic, temporal and disease-risk bottlenecks in the annual cycle of long-distance migrants, taking a sandpiper, the red knot Calidris canutus, as a focal species. Red knots have six recognized subspecies each with different migratory routes, well-known patterns of connectivity and contrasting annual cycles. The diversity of red knot annual cycles allows us to discuss the existence and the effects of bottlenecks in a comparative framework. We examine the evidence for bottlenecks focusing on the quality of breeding plumage and the timing of moult as indicators in the six subspecies. In terms of breeding plumage coloration, quality and timing of prealternate body moult (from non-breeding into breeding plumage), the longest migrating knot subspecies, Calidris canutus rogersi and Calidris canutus rufa, show the greatest impact of bottlenecking. The same is true in terms of prebasic body moult (from breeding into non-breeding plumage) which in case of both C. c. rogersi and C. c. rufa overlaps with southward migration and may even commence in the breeding grounds. To close our discussion of bottlenecks in long-distance migrants, we make predictions about how migrants might be impacted via physiological ‘trade-offs’ throughout the annual cycle, using investment in immune function as an example. We also predict how bottlenecks may affect the distribution of mortality throughout the annual cycle. We hope that this framework will be applicable to other species and types of migrants, thus expanding the comparative database for the future evaluation of seasonal selection pressures and the evolution of annual cycles in long-distance migrants. Furthermore, we hope that this synthesis of recent advancements in the knowledge of red knot annual cycles will prove useful in the ongoing attempts to model annual cycles in migratory birds.
Keywords: red knot, Calidris canutus, migration, annual cycle, plumage, moult
1. Introduction: annual cycles and life-history trade-offs in avian migrants
The Earth provides an ever-fluctuating environment. Yearly, the Earth revolves around the Sun and its 23.5° tilt gives seasons. These seasons bring about fluctuations in resource availability and temperature that can be potent selective forces in the evolution of life (Alerstam 1990). Non-tropical animals display a variety of behavioural adaptations to cope with the challenge of seasonal survival. These adaptations influence the seasonal pattern of behaviour in the animal's life and influence the timing of major events throughout the year. In birds, these patterns of behaviour include breeding, moult, winter survival and migration, and are thought of as the bird's annual cycle.
This article focuses on the annual cycles in migrant birds and considers migration as an adaptation for exploiting seasonal peaks of resource abundance while avoiding seasonal resource depression (Alerstam et al. 2003). Long-distance migration in particular allows migrants to exploit widely spaced resources during periods of high productivity. For example, shorebirds and gulls exploit an abundance of horseshoe crab Limulus polyphemus eggs on the beaches of Delaware Bay, USA, using a short window of opportunity during which their spring migration coincides with horseshoe crab spawning (Schuster et al. 2003). After Delaware Bay, they continue to Arctic tundra areas, where they are able to reproduce in remote and extreme environments suitable for only two to three months each year. During the short summer, these habitats offer the advantages of long days, sufficient food resources and fewer pathogens and parasites than temperate breeding areas further south (Greiner et al. 1975; Piersma 1997; Mendes et al. 2005). However, to gain these benefits, the migrants must perform demanding migrations often covering thousands of kilometres and require a complete change in physiology as they alternate between phases of the annual cycle (Piersma & Lindström 1997; Piersma et al. 1999b; Wingfield 2005).
Both migrants and residents must cope with different environments; however, the annual cycles of residents and migrants differ markedly. Residents must survive and find food in a changing seasonal environment requiring a high capacity for behavioural flexibility (Sol et al. 2005). Residents, however, do not have to carry out long and demanding travels. In contrast, migrants travel to a great diversity of habitats throughout their annual cycle. These travels reduce the amplitude of fluctuation in seasonal resource levels, but while travelling migrants must adjust to unfamiliar surroundings, balance conflicting demands between predator avoidance and fast fuel acquisition, cope with unfavourable weather and determine the correct direction for the next leg of their journey (Piersma 1987; Piersma et al. 1990). Furthermore, they must satisfy nutritional demands not only for survival but also to fuel the energy cost of transport. Finally, all of this must be precisely timed to best exploit food resources along their migration route.
One way to look at the differences in the annual cycles of residents and migrants and to examine annual cycles in general is through the framework of finite state machine theory (Jacobs & Wingfield 2000; Wingfield 2008). This theory describes an organism's life cycle as a series of life-history stages, such as breeding, moult, winter survival and migration. These stages are distinct, independent of one another and occur in a set sequence that cannot be reversed. The finite nature of these life-history stages, and the states which occur within the stages, provides the analogy of the annual cycle as a finite state machine (Jacobs & Wingfield 2000). Since migrants must pass through both northward and southward migrations in addition to breeding, winter survival and moult, they have more life-history stages than residents and this may mean less flexibility in timing throughout the annual cycle (Wingfield 2005).
This decrease in flexibility can be examined by looking at the overlap between potentially costly life-history stages such as migration or moult. Indeed, moult rarely overlaps with breeding or with the cruellest months of the winter (Payne 1972; Masman et al. 1988; Dietz et al. 1992). Furthermore, migration and moult rarely overlap (Payne 1972) and in some taxa, such as waterfowl and grebes, moult completely constrains migration as it prevents the birds from flying (see Jehl (1990) for a review). However, in other taxa flying remains possible but moult imparts an energetic cost, limiting overlap with migration. For example, in a split-brood experiment, blackcaps Sylvia atricapilla were kept in either a natural photoperiod or an experimental photoperiod to advance and prolong moult (Pulido & Coppack 2004). In both groups the onset of migratory activity was significantly correlated with the termination but not with the onset of moult, and moult intensity at the onset of migration was low. If moult and migration do overlap then active moult is suspended for the duration of active migration. This phenomenon is found in several shorebirds breeding at temperate and boreal latitudes in Eurasia and wintering in inland Africa, notably ruffs Philomachus pugnax (Koopman 1986) and black-tailed godwits Limosa limosa limosa (van Dijk 1980).
Another potential indicator of decreased flexibility in the annual cycle of migrants is decreased investment or a shift in immune function during potentially costly life-history stages. This idea is based on the assumption that trade-offs exist between immune defence and other functions that share common resources and contribute to fitness (Sheldon & Verhulst 1996). Immunity can be divided into innate (non-specific) and acquired (specific) responses, and further divided into constitutive (always present) and induced aspects. Owing to the complexity of the immune system, it is difficult to define a single ecological or evolutionary currency with which to measure the cost of immune function (Martin et al. 2008). From a physiological standpoint, the costs of immunity can be subdivided into three components: development; maintenance; and use, each of which differs among different types of immunity (Klasing 2004). Inflammatory responses such as induced innate and cell-mediated acquired immunity are considered the most costly in terms of use owing to the metabolic requirements of immune cells and due to indirect consequences, such as tissue degradation or anorexia (Lochmiller & Deerenberg 2000; Klasing 2004). In contrast, mounting a specific antibody-mediated acquired response is thought to be less costly (Klasing 2004). Maintenance costs remain extremely difficult to measure, but it is thought that maintenance costs for constitutive immunity, both innate and acquired, are quite low (Klasing 2004; Lee 2006). Taking the complexities of the immune system into account, Lee (2006) provides a framework predicting a switch from reliance on inflammatory (cell-mediated acquired and constitutive innate) to specific (antibody-mediated acquired) immunity during high-intensity effort or more demanding seasons.
Seasonal patterns in immune function have been well studied in temperate resident mammals (Nelson & Demas 1996) and research is accumulating in resident birds (Martin et al. 2008). But how does immune function change throughout the year in migrants? To date, very little is known. Immune function has been studied in long-distance migrating ruffs and has shown that captive birds show decreased cell-mediated immune responses during the breeding versus the non-breeding season (Lozano & Lank 2003). However, to our knowledge, nothing is known about seasonal variability in immune function in long-distance migrants throughout the entire annual cycle, and it is beyond the scope of this paper to address this issue with empirical evidence. We do, however, make predictions about immune function in the context of trade-offs and bottlenecks in the annual cycle of red knots.
Closely tied to the concept of the annual cycle are annual routine models of optimal behaviour. Houston & McNamara (1999) introduced a modelling framework that takes into account the fact that optimal behaviour relies not only on isolated ‘decisions’ but also on circumstances during the annual cycle as a whole (see also McNamara & Houston 2008). This approach has been used to model optimal migration timing and reproductive effort under varying circumstances (McNamara et al. 1998, 2004). When first using this approach to model migration timing, McNamara et al. (1998) pointed out that the problem is that a lot of information about the organism and their annual cycles is required. They then suggested red knots Calidris canutus as a realistic species to model given that much information is known about these birds and their migrations. Well-studied species are important in the development of realistic models because the details of their annual cycles are necessary as a framework from which to hang state variables used to examine interactions between factors affecting behaviour. Once developed, these models may be generalized and can be useful for species where such detailed knowledge is not available. Red knots remain an excellent species to model and nearly a decade later even more is known about their annual cycles. We hope that this review pushes forward theoretical as well as empirical studies on migrants and their annual cycles by providing a framework of bottlenecks through which selection pressures may act and by synthesizing what is known about these bottlenecks in the annual cycle of a representative migrant.
The goal of this article is to examine ecological evidence for bottlenecks in the annual cycle of long-distance migrants and to make predictions using this framework. To establish the comparative setting, we first introduce red knots as a focal species and describe their subspecies and annual cycles. We then define possible nutritional, energetic, temporal and disease-risk bottlenecks in their annual cycle and discuss possible evidence for these bottlenecks, looking in depth at the quality of breeding plumage and the timing of moult in the six subspecies as indicators. Finally, we make predictions about how these bottlenecks might impact long-distance migrants via physiological trade-offs such as investment in immune function, and how these bottlenecks may affect the distribution of mortality during the annual cycle. Throughout this paper, we limit ourselves to the migration and annual cycle of adults. The selection pressures affecting juvenile birds may be very different from those affecting adults and are excluded from this discussion to limit the article to a manageable length.
2. Red knots as a model system
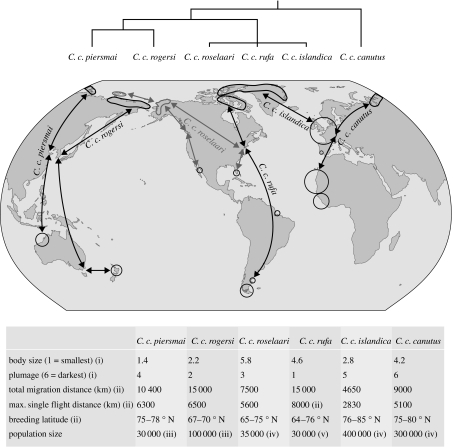
Red knots (hereafter referred to simply as knots) provide a beautiful study system for the investigation of bottlenecks throughout the annual cycle of a long-distance migrant. Knots are medium-sized shorebirds with six subspecies and a web of migratory routes that spans the globe. Knot flyways cover a diversity of environments from the High Arctic to the American and European North Temperate Zone, to the desert coasts of Africa and Australia and to the sub-Antarctic flats of Tierra del Fuego (figure 1). It is this wide diversity of migratory routes, occurring within a single species, that make knots an ideal focal organism. Another benefit of knots as a focal species is that among the six subspecies, patterns of connectivity and population genetics are relatively well known (figure 1).
Figure 1.
The global distribution of knots (updated from Piersma & Davidson 1992) highlighting morphological and behavioural congruence as well as genetic structuring in knot flyways. Migratory routes are colour coded to subspecies and grey lines represent routes requiring further study. The South African wintering area present in Piersma & Davidson (1992) is not shown as knots no longer seem to winter there (L. Underhill 2004, personal communication). Shaded areas in the Arctic indicate breeding areas and circles indicate wintering areas. The size of the circle indicates the relative number of birds using the area. Projected above the contemporary distribution of knots is a phenogram summarizing knot population structure (Buehler & Baker 2005) and below a table outlining morphological, migration and population size details: (i) Bill, tarsus and wing length measurements were ranked and averaged for size score, and overall extent of redness and depth of colour were taken into account for rank plumage score (Tomkovich 1992, 2001); (ii) Piersma et al. (2005); (iii) P. F. Battley 2005, personal observation; (iv) T. Piersma & B. Spaans 2005, unpublished data; (v) Baker et al. (2004, 2005a).
As a species, knots share several common traits. All subspecies breed in harsh High Arctic habitats, all winter in coastal areas, and like most shorebird species, they lay a four-egg clutch. On the breeding grounds incubation is shared, but females depart right after the eggs hatch leaving the males to care for the chicks until they fledge (Whitfield & Brade 1991; Tomkovich & Soloviev 1996). After fledging the males depart and the chicks undertake their first migration south independently. On the breeding grounds, knots eat mostly spiders and arthropods obtained by surface pecking (Tulp et al. 1998) and on the wintering grounds, they eat a variety of hard-shelled prey such as bivalves, gastropods and small crabs obtained by high-frequency probing and the use of a specialized bill tip organ used to find hard objects in soft sediments (Piersma et al. 1993a, 1998). Prey are ingested whole and crushed by a muscular stomach (Piersma et al. 1993b, 1999a). Yet, beyond this brief description, the uniformity stops.
Knots comprise six distinct breeding populations, all of which are currently recognized as subspecies based on morphological characteristics and distinct migratory routes: Calidris canutus canutus; Calidris canutus piersmai; Calidris canutus rogersi; Calidris canutus roselaari; Calidris canutus rufa and Calidris canutus islandica. Morphologically, knots have been subdivided by tarsus length, wing length and bill length as well as various fine points pertaining to plumage (Tomkovich 1992, 2001). Overall, C. c. piersmai is the smallest in size, followed by C. c. rogersi, then C. c. islandica and C. c. canutus, then C. c. rufa and finally C. c. roselaari, the largest subspecies. With respect to plumage characteristics, C. c. canutus, C. c. islandica and C. c. piersmai are the ‘darker’ subspecies. C. c. rogersi have lighter bellies than both C. c. roselaari and C. c. piersmai, and C. c. rufa is the lightest in overall plumage (figure 1).
Remarkably, in knots there is complete congruence between morphological typing and behavioural differences in migratory routes, leading to relatively well-known patterns of connectivity. Evidence from long-term ringing programmes indicates that distinct flyways exist and correspond to separate breeding areas in the Arctic (Piersma & Davidson 1992). During the course of migration, some subspecies share certain staging areas; for example, C. c. rufa and C. c. roselaari in Delaware Bay and the southeastern USA (Atkinson et al. 2005), and C. c. islandica and C. c. canutus in the Wadden Sea in Europe (Nebel et al. 2000). However, the subspecies can usually be distinguished using the timing of passage and their primary moult status. For example, during southward migration in South Carolina and Georgia, C. c. roselaari moult their primary feathers, whereas C. c. rufa do not because they still need to cross the Caribbean Sea to South America (B. A. Harrington 2005, personal communication). Wintering areas are geographically distant from one another and banding studies to date have shown that subspecies are not mixing on the wintering grounds (Piersma & Davidson 1992).
Close congruence has also been found between migratory routes and genetic differences between populations in knots. Genetic differentiation has been found between four groups: C. c. canutus; C. c. piersmai; C. c. rogersi; and a North American breeding lineage that comprises C. c. roselaari, C. c. rufa and C. c. islandica (figure 1; Buehler & Baker 2005; Buehler et al. 2006). The single discordance arises in the deep separation between C. c. canutus and C. c. islandica which are very close in terms of morphology and the timing of annual cycle events (Piersma & Davidson 1992). This incongruence may be explained by that fact that many genes code for size, plumage characteristics and annual cycling, and the fact that these genes are under strong selection. For example, the Arctic tundra areas used by C. c. canutus and C. c. islandica may be more similar than those used by C. c. rufa, possibly leading to selection for similar breeding plumage and size characteristics. Furthermore, as discussed later, bottlenecks on the timing of moult are more similar in C. c. canutus and C. c. islandica than in the more closely related C. c. islandica and C. c. rufa. Since characteristics such as size and plumage are polygenetic (coded for by many genes) and are under selection, they can evolve much faster than the single, and by definition selectively neutral genes, used in genetic typing.
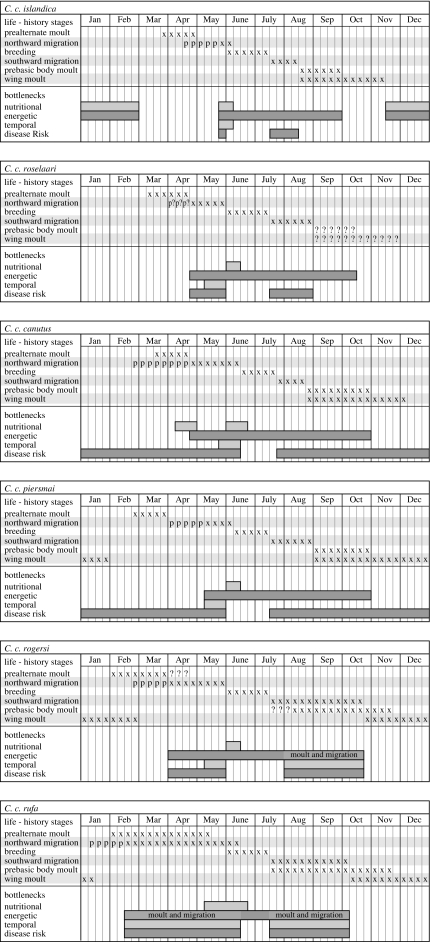
The next few sections (§§3–5) are dedicated to describing the six recognized subspecies of knot and details of their migrations; phenology and moult are summarized in table 1 and figure 2.
Table 1.
A summary of knot migrations, phenology and moult locations. (i) Piersma & Davidson (1992), (ii) Higgins & Davies (1996) and (iii) Cramp & Simmons (1985).
| subspecies | non-breeding mass peak (i) | phenology wing moult (i), (ii), (iii) | phenology prebasic moult (breeding to winter) (i), (ii), (iii) | location prebasic and wing moult (i) | phenology prealternate moult (winter to breeding) (i), (ii), (iii) | location prealternate moult (i) |
|---|---|---|---|---|---|---|
| C. c. islandica | yes | mid-August to mid-November | mid-August to end September | Wadden Sea and United Kingdom Estuaries | late March to end April | Wadden Sea |
| C. c. roselaari | yes—predicted | early September likely to end November | early September to end October | southeast USA | mid-March to mid-April | southeast USA |
| C. c. canutus | no | late August to early December | late August to end October | northwest Africa | mid-March to end April | northwest Africa |
| C. c. piersmai | no | early September to early February | early September to end October | northwest Australia | Late February to end March | northwest Australia |
| C. c. rogersi | no | late October to early March | possibly as early as August to end November | New Zealand (some prebasic body moult on southward migration and even breeding grounds) | Early February to end March (possibly into northward migration) | New Zealand |
| C. c. rufa | no | mid-October to mid-January | begins mid-July ends end November | Tierra del Fuego (some prebasic body moult on southward migration and breeding grounds) | begins mid-February ends mid-May | Tierra del Fuego (some prealternate body moult on northward migration) |
Figure 2.
A graphical representation of the annual cycles and life-history stages of the knot subspecies. For the life-history stage ‘northward migration’, ‘x’ represents flight and staging, whereas ‘p’ represents pre-migratory mass gain (extrapolated from table 21.1 in Piersma et al. 2005). In all life-history stages, ‘?’ indicates parts of the annual cycle that are not known with certainty. Periods of bottleneck are shown below the life-history stages. In this figure simultaneous prebasic and wing moult is highlighted as an energetic bottleneck, and periods where both moult and migration overlap are represented by lighter grey shading and labelled ‘moult and migration’ in the C. c. rogersi and C. c. rufa subspecies. Periods of the annual cycle in which individuals experience three or more bottlenecks at a time are considered severely bottlenecked. For example, all subspecies are severely bottlenecked during the final stages of northward migration and arrival on the breeding grounds due to the overlap of energetic, temporal and disease-risk bottlenecks.
3. North temperate winterers
(a) Calidris canutus islandica
Calidris canutus islandica is one of the best-studied subspecies. Its breeding grounds extend further north of any other subspecies, spanning from 75° N to 85° N and covering northern Greenland and the Queen Elisabeth Islands west to Prince Patrick Island. Adults leave the breeding grounds between mid-July and early August and fly non-stop (R. I. G. Morrison 2005, personal communication) to staging grounds in western Iceland where they refuel from late July to early August (Davidson & Wilson 1992). From Iceland the birds fly to the Wadden Sea where prebasic body moult (from breeding into non-breeding plumage) occurs in August and September and wing moult extends from August to mid-October for adults. From October to December the birds disperse to wintering grounds, moving west from the Wadden Sea to Britain, and moving northwest within Britain (Davidson 2002), where they remain until March. In late March, some of the birds return eastwards to the Wadden Sea, and some remain the UK estuaries where they undergo prealternate moult (from non-breeding into breeding plumage) and fuel for the journey north. From there they travel north to their final staging areas in Iceland and northern Norway (Davidson et al. 1986; Wilson & Strann 2005). In the last week of May, there are synchronous departures to the breeding grounds where birds arrive in early June.
(b) C. c. roselaari
In contrast to C. c. islandica, the C. c. roselaari subspecies is the least studied of the six and as such details about its migration route and breeding areas are not well known. Calidris canutus roselaari are thought to breed in northwest Alaska and Wrangel Island (67° N–73° N) probably from early June to mid-July (Tomkovich 1992). From the breeding grounds they may migrate across the North American continent perhaps using the large probably brackish lakes along this flyway as staging areas. It is also possible that C. c. roselaari birds fly non-stop over the continent and then intermingle with birds of the C. c. rufa subspecies on the Atlantic coast of North America. Calidris canutus roselaari also uses the Pacific coast flyway and at least some individuals winter in California, USA and Baja California, Mexico (Tomkovich 1992; Page et al. 1997, 1999). More investigation on this subspecies is needed to clarify the details of their migration routes. Calidris canutus roselaari individuals that fly to Florida and the Gulf of Mexico reach their wintering grounds in September. These birds probably spend September to April on the wintering grounds and begin prebasic body moult and wing moult as soon as they arrive. The duration of wing moult is not definitively known for this subspecies, but it is probable, given that wing moult does not render knots flightless at any stage, that wing moult continues into the end of November. Prealternate moult probably takes place from mid-March to mid-April and migration northwards can be traced around the west coast of the Gulf of Mexico by late April to early May and across the prairie provinces of Canada by late May (Morrison & Harrington 1992). In addition, stable isotope analysis has shown that some birds of the Florida wintering C. c. roselaari subspecies most probably join members of the C. c. rufa subspecies on the beaches of Delaware Bay in May where they take advantage of abundant horseshoe crab eggs (Atkinson et al. 2005).
4. Tropical winterers
(a) C. c. canutus
Birds belonging to the C. c. canutus subspecies breed in the coastal tundra of the Taymyr Peninsula (75° N–80° N). From there they migrate to staging areas in the Wadden Sea where they pass from late July to early August (Nebel et al. 2000). The birds do not moult in the Wadden Sea but continue on to wintering areas in the equatorial coasts of Mauritania and Guinea-Bissau, Africa where prebasic body moult occurs from late August until the end of October and wing moult takes place from late August to early December (Piersma et al. 1992, B. Spaans et al. 2005, unpublished data). Birds remain in the wintering areas from September through April, and prealternate moult occurs there from mid-March through the end of April. Departure northwards begins in late April and the birds arrive in staging areas in the Wadden Sea in mid-May for refuelling (Prokosch 1988). Staging areas in Portugal and France are used only briefly often as ‘emergency sites’ when birds encounter headwinds and cannot make it directly to the Wadden Sea (Piersma 1987; Smit & Piersma 1989). Calidris canutus canutus leave the Wadden Sea in early June passing southern Sweden and reaching the breeding grounds in mid-June (Gudmundsson 1994).
(b) C. c. piersmai
Calidris canutus piersmai is the most recently defined of the six subspecies (Tomkovich 2001) and breeds in the New Siberian Islands probably from early June to mid-July. From there they most probably migrate to staging areas along the coast of eastern Asia but the details of this flight are not fully known. Their migrations then continue to wintering grounds mainly in tropical northwest Australia where they arrive by the end of August to early September (D. I. Rogers 2005, personal communication) and remain until the end of April. Wing moult takes place in the wintering areas from late August to early September and is completed between mid-January and early February (D. I. Rogers 2005, personal communication). Prebasic body moult is faster beginning upon arrival in the wintering grounds in late August and early September and is completed by October (Higgins & Davies 1996). Some birds arrive on the wintering areas already showing non-breeding body plumage indicating that some prebasic body moult might take place on staging areas (D. I. Rogers 2005, personal communication). Prealternate moult takes place from late February until the end of March. Calidris canutus piersmai differs from other subspecies in that there is a long time lag between the completion of prealternate moult and departure on northward migration (Battley et al. 2005). Furthermore, departure to north from their wintering grounds takes place very late; for instance, on 5 May 2000 many birds were still on the wintering grounds (Battley et al. 2005). This late departure indicates that staging on the shores of the Yellow Sea on northward migration is very rapid, and only feasible if high-quality prey are available (Battley et al. 2005). Knots in the Americas use horseshoe crab eggs as a high-quality food source during northward migration and it may be possible that spawning horseshoe crabs in China and Southeast Asia (Tachypleus tridentatus, Tachypleus gigas and Carcinoscorpius rotundicauda) provide a food resource for C. c. piersmai. However, to date knots have not been observed feeding on horseshoe crab eggs in Asia and this possibility remains to be investigated further.
5. Transequatorial south temperate winterers
(a) C. c. rogersi
The C. c. rogersi subspecies breeds in the northern Chukotski Peninsula probably from early June to mid-July. From there these knots most probably migrate to staging areas in the Sea of Okhotsk and the northern Yellow Sea in East Asia, but the details of this part of their migration require further study. Birds begin to arrive on the wintering grounds in southeast Australia and New Zealand in late September (Barter 1992). Prebasic body moult begins in unknown staging areas and ends on the wintering areas in southeast Australia and New Zealand from mid-October to the end of November (Higgins & Davies 1996). The combination of moult and migration is unique to this subspecies and to C. c. rufa described below. The birds spend October to the end of March on the wintering grounds and begin wing moult from mid-October to the end of November. This wing moult is completed between the middle of January and early March (Higgins & Davies 1996; P. F. Battley 2005, personal communication). Prealternate moult begins from the middle of January to end of March (Battley 1997; Battley & Piersma 1997), but it may not be completed before departure and it is possible that this body moult is continued on staging areas during northward migration as seen in C. c. rufa. Departure northward takes place from mid-March to the beginning of April (Battley 1997; P. F. Battley, personal communication) and the birds fly to staging areas in the northern Yellow Sea where they have been seen in early May, with New Zealand birds probably making an intermediate stopover en route.
(b) C. c. rufa
The C. c. rufa subspecies is one of the best-studied groups and is known to breed in the central Canadian Arctic from Victoria Island southeast to South Hampton Island (65° N–75° N) from early June to mid-July (Morrison & Harrington 1992; Harrington 2001). Prebasic body moult begins on the breeding grounds but is suspended after departure. This subspecies is most probably unique in beginning moult in the breeding grounds, although C. c. rogersi may also begin prebasic body moult on the breeding areas. In addition, C. c. rufa is one of the only subspecies, along with C. c. rogersi, that suspends moult for migration.
Adults leave the breeding grounds towards mid-July and peak in late July and early August at staging areas in James Bay and the Bay of Fundy. Adults also appear around the same time in the staging areas along the northeast coast of the United States seaboard and most have departed by late August to early September towards staging areas further south. From the second half of August to the first half of September, C. c. rufa adults fly across the Atlantic to staging areas in Maranhão, northern Brazil and then on to staging areas in Rio Grande do Sul, southern Brazil. From there they depart to their wintering grounds in Tierra del Fuego where they arrive in late September through October already moulting into winter plumage (Baker et al. 2005a). Wing moult also takes place in the wintering grounds from arrival in late September and October until mid-January (Baker et al. 2005a). Calidris canutus rufa birds spend only three months in winter plumage, the shortest period among the subspecies, and they depart northward in February already moulting into summer plumage (Baker et al. 2005a). After departure from the wintering grounds, C. c. rufa birds migrate northward along the eastern Argentine coast with many individuals staging in Golfo San Matias (González et al. 1996) and often continuing onto Rio Grande do Sul, Brazil (Baker et al. 2001). From southern Brazil, C. c. rufa are faced with a transamazon crossing immediately followed by a transatlantic crossing. The birds probably make a short stop in northern Brazil (Wilson et al. 1998; Rodrigues 2000), but major refuelling in such a short period and on tropical mudflats is unlikely (Piersma et al. 2005). Furthermore, it is possible that some birds make a spectacular 8000 km flight directly to the southeastern United States. Whichever route they choose, the birds make landfall in early May on the Atlantic coast of the United States and in staging areas in Delaware Bay, they feed almost exclusively on the superabundant eggs of spawning horseshoe crabs (Tsipoura & Burger 1999). The use of horseshoe crab eggs as opposed to hard-shelled mollusc prey is unique to C. c rufa and C. c. roselaari and has resulted in adaptations such as ingesting small stones to grind the leathery outer shell of the eggs (Piersma et al. 1993b). The birds depart Delaware Bay en masse around 28–30 May (Baker et al. 2001) and make a direct flight to the breeding grounds where they begin to arrive in the first week of June.
A portion of the birds using the C. c. rufa flyway stop migration in Maranhão, Brazil where they spend the winter (Baker et al. 2005b). This makes them, in fact, tropical winterers rather than transequatorial south temperate winterers. These birds were long thought to be C. c. rufa and are thus included in this section. However, stable isotope analysis is currently underway to examine the possibility that these birds might belong to the C. c. roselaari subspecies. Thus, most of the discussion about C. c. rufa in this paper focuses on the Tierra del Fuego wintering population.
6. Bottlenecks in the annual cycle of knots
We have now presented information on the annual cycles of long-distance migrants by example of the six subspecies of our focal migrant, the knot. We will now define possible bottlenecks in these annual cycles and make predictions regarding possible trade-offs as a result of these bottlenecks. For the purpose of this paper we focus on nutritional, energetic, temporal and disease-risk bottlenecks. Figure 2 summarizes these bottlenecks for each of the subspecies throughout their life-history stages and table 2 shows the predicted impact of these bottlenecks for long-distance migrant shorebirds, in general, throughout the annual cycle.
Table 2.
Predicted impact of four types of bottleneck, as well as predicted immune function and mortality throughout the annual cycle.
| nutritional bottleneck | energetic bottleneck | temporal bottleneck | disease risk bottleneck | predicted immune function | predicted mortality | |
|---|---|---|---|---|---|---|
| northward migration fuelling | weak | weak | high | high risk | less inflammatory more antibody | low |
| northward migration flight | moderate | high | high | low risk | less inflammatory more antibody | high? |
| northward migration arrival | strong | strong | strong | low risk | low | high |
| reproduction | weak (high upon arrival) | strong | strong | low risk | low | high to moderate |
| southward migration fuelling | weak | weak | moderate | moderate risk | less inflammatory more antibody | low |
| southward migration flight | moderate | strong | strong | low risk | less inflammatory more antibody | high? |
| southward migration arrival | strong | strong | weak | moderate risk | low | moderate |
| wintering temperate | moderate | moderate to strong | weak | low to moderate risk | high (moderate during moult) | low to moderate |
| wintering tropical | moderate | weak | weak | high risk | high (moderate during moult) | low |
(a) Nutritional bottlenecks
A nutritional bottleneck is defined as a time when food resources are unpredictable, of low quality or found in low density. For knots, the nutritional bottlenecks in the wintering areas may be quantified by measuring the presence of a midwinter mass peak as an indication of food unpredictability (Piersma 1994). Furthermore, in view of the fact that knots ingest their shellfish prey whole, crushing the shells in their muscular gizzards and evacuating the crushed shell remains through the intestine (Piersma et al. 1993b; Battley & Piersma 2005), prey qualities at both wintering and stopover sites can be measured as the ratio of bivalve flesh and shell mass (van Gils et al. 2005b).
For long-distance migrants that breed in the High Arctic, and this includes all six subspecies of knots, the most severe nutritional bottleneck might be predicted upon early arrival on the Arctic breeding grounds (figure 2). Although food is of high quality and abundant during reproduction, birds arrive in the Arctic before the peak in insect availability (I. Tulp & H. Schekkerman 2005, personal communication) and must endure starvation conditions after days of flight. Furthermore, the timing of snowmelt in the High Arctic is unpredictable and in some years the birds may arrive before the snow has gone. The existence of this bottleneck is evidenced by that fact that knots often carry a much greater fuel load on the last leg of migration than is necessary for the flight alone. This extra fuel is most probably an ‘insurance policy’ against harsh conditions upon Arctic arrival (Morrison et al. 2005). After snowmelt, nutritional stress in the Arctic breeding grounds is expected to be lower despite the extra energetic demands of reproduction, because food sources in the Arctic are abundant and long photoperiod offers more hours to feed (e.g. Schekkerman et al. 2003).
During both northward and southward migrations, departures and arrivals are timed to exploit high quality and abundant food resources and, in general, prey quality at stopover sites is higher than that at wintering sites (van Gils et al. 2005a). This is important since low-quality prey necessitates an increase in gizzard size in knots owing to the need to process more shell material. During migration, however, there is a trade-off between increased shell-processing capacity and the costs of having to carry a heavy gizzard. Thus the combination of high prey quality and the knots' ability to adjust their gizzard size enables timely migrations (Battley & Piersma 2005; Battley et al. 2005) and due to generally abundant and high-quality food, nutritional bottlenecks during migration should be low. The one exception to this rule may be a human-related nutritional bottleneck during the final stopover in the C. c. rufa and C. c. roselaari subspecies flyways in Delaware Bay, USA (figure 2). These subspecies feed on horseshoe crab eggs and recent overharvesting of horseshoe crabs may be depleting food stocks for these birds with possible carry over effects throughout the annual cycle (Baker et al. 2004).
During the Northern Hemisphere winter knots use both temperate and tropical wintering areas. Nutritional bottlenecks in these areas are defined by both prey quality and prey predictability. For example, temperate wintering areas such as the Wadden Sea in northwest Europe, and coastal areas near Rio Grande, Tierra del Fuego, have high prey quality relative to tropical wintering areas such as Banc d'Arguin, Mauritania and Roebuck Bay, Northwest Australia (van Gils et al. 2005a). This higher prey quality in south temperate regions may offset the longer migrations and the higher thermoregulation costs required to migrate and survive there. Tropical wintering subspecies need not fly as far but may experience a moderate nutritional bottleneck as a result of predictable but low-quality food resources. In the C. c. canutus subspecies for example, there is evidence that the birds may have trouble feeding at the rate-maximizing levels necessary to gain fuel stores quickly before departure on northward migration (figure 2; Piersma et al. 2005). Northern Hemisphere winterers such as C. c. islandica appear to escape both transequatorial migrations and low prey quality found in tropical climes. However, thermoregulation is costly during the Northern Hemisphere winter (Wiersma & Piersma 1994) and cold weather can make food unpredictable if the mudflats freeze (figure 2; Johnson 1985; Zwarts et al. 1996). Evidence of this north temperate nutritional bottleneck in C. c. islandica is indicated by the presence of a midwinter mass peak.
(b) Energetic bottlenecks
Energetic bottlenecks are defined as periods when field metabolic rate (FMR) approaches or even exceeds the maximum sustained metabolic rate of approximately five times basal metabolic rate (BMR; Drent & Daan 1980; Hammond & Diamond 1997). Energetic bottlenecks can further be defined as periods of high-energy turnover as quantified by fuelling rates on staging and wintering grounds (Piersma et al. 2005).
For migratory shorebird species such as knots, energetic bottlenecks are expected during the flight phase of migration, when expenditure levels reach seven to eight times BMR (Wiersma & Piersma 1994; Kvist & Lindström 2001). In addition to the migratory period, knots are energetically bottlenecked during the reproductive period when energy is invested in breeding and where thermoregulation costs are high even during the Arctic summer (figure 2; Piersma et al. 2003). Indeed, for tropical winterers such as C. c. canutus, the highest thermoregulation costs of the year occur during breeding (Wiersma & Piersma 1994).
Only in the middle of the non-breeding season is energy expenditure relatively low because energy is not needed for either migration or reproduction. This may be why most knot subspecies postpone prebasic body moult and all subspecies postpone wing moult until arrival in the wintering areas. For C. c. islandica knots wintering in temperate areas such as the Wadden Sea, energy requirements for thermoregulation are high even during winter, with expenditure levels of four to five times BMR (figure 2; Wiersma & Piersma 1994). Perhaps this is why C. c. islandica performs prebasic body moult quite early in the season before the harsh winter weather begins.
In C. c. rufa and C. c. rogersi, moult and migration overlap and a strong energetic bottleneck is predicted during these periods of overlap (figure 2). In fact, C. c. rufa individuals are particularly bottlenecked. They face relatively harsh conditions during both the Arctic summer and the austral summer in Tierra del Fuego, as well as an extremely long migration period, and extremely long periods of moult that stretch from the breeding areas through migration and into the wintering grounds.
(c) Temporal bottlenecks
Migrants must pass through more life-history stages than resident birds within the annual cycle because they must perform northward and southward migrations in addition to breeding, winter survival and moult. Furthermore, the migrations themselves require precise timing in order to fully exploit peaks in prey species abundance. Thus, timing is very important in the annual cycle of a migrant and time can be important in assessing the cost of migration (Hedenström & Alerstam 1997). A temporal bottleneck can be defined as a period in which migrants are ‘pressed for time’, and can be measured using the synchrony of departure during migration and the amount of overlap between high energy-demanding life-history stages.
Temporal bottlenecks are most severe during the last leg of northward migration and during the breeding season (figure 2). During this period, knots of all subspecies are in a race against time because arrival on their Arctic breeding grounds must be precise in order to exploit the insect bloom for breeding, and to have time during the short Arctic summer to raise their young. This temporal bottleneck, based on the idea that the timing of migration becomes more constrained closer to the breeding grounds, is evidenced by increasingly synchronous departures as the breeding areas are approached in a number of waders including great knots Calidris tenuirostris and all subspecies of red knot (Battley et al. 2004).
During southward migration temporal bottlenecks may also come into play as the birds must arrive at staging areas in time to exploit food resources (Schneider & Harrington 1981; Zwarts et al. 1992) and must leave the Arctic and northern staging areas before the winter storms begin. In the Wadden Sea for example, crustacean density declines from July to September highlighting the importance of a timely arrival for C. c. canutus if they are to use this stopover before migration further south (van Gils et al. 2005c). However, the timing of northward migration may be more constrained than southward migration and models have indicated that northward migration is more compressed (e.g. fig. 1 in McNamara et al. 1998). Finally in the wintering grounds, time pressure is low until fattening for northward migration commences and the race begins anew.
Migration distance can also increase temporal bottlenecks in the annual cycle; the longer the distance to be covered, the longer the migration life-history stage lasts. In the longest-distance migrating C. c. rogersi and C. c. rufa, temporal bottlenecking is severe enough to necessitate an overlap between body moult and migration. In C. c. rogersi this overlap occurs only during southward migration, but in C. c. rufa overlap occurs during both northward and southward migrations. A further indication of temporal bottlenecking in C. c. rogersi and C. c. rufa is a complete lack of ‘down time’ between the end of primary wing moult and preparation (via pre-migratory fuelling and prealternate moult) for migration and breeding (figure 2).
(d) Habitat- and behaviour-related disease-risk bottlenecks
In general, migrants encounter a wider diversity of environments, and possibly a wider diversity of pathogens and parasites throughout their annual cycle than do resident birds. This has lead to the prediction that migrant birds might have more robust immune defences than resident birds (Møller & Erritzøe 1998).
Among migrating species, it has been suggested that marathon migrants, such as knots, may have poorer immune resistance than shorter distance migrants (Piersma 1997, 2003). The hypothesis is based on the idea that in marathon migrants demanding migrations and a history of genetic bottlenecking on an evolutionary time scale may have led to poor immune resistance that further restricts them to low pathogen habitats with less disease risk (Piersma 1997, 2003). Furthermore, on an immediate time scale, the demanding migrations themselves may cause immunosuppression (Råberg et al. 1998). Chronic muscle damage has been shown to cause a state of mild inflammation followed by immunosuppression in overtraining athletes (Shephard & Shek 1998), and muscle damage (although slight) has been detected during migration in long-distance migrating western sandpipers Calidris mauri and bar-tailed godwits Limosa lapponica (Guglielmo et al. 2001).
Even within long-distance migrants restricted to low pathogen environments, like knots, some variability in disease risk and immune function can be predicted throughout the annual cycle and between subspecies making use of different environments. In knots disease-risk bottlenecks have two components: (i) habitat-related disease risk pertaining to the variety and prevalence of pathogens along the flyway, (ii) seasonal variation in flocking behaviour (aggregated or not aggregated). These two components are closely tied to individual seasonal investment in the immune system, which is discussed later in this article.
The highest levels of disease risk in knots are predicted during migration and tropical wintering (figure 2). During migration, birds are aggregated into very dense and synchronously moving flocks and are passing through a variety of environments with novel pathogens. During tropical wintering the prevalence of disease is generally higher and pathogens are more varied, whereas disease risk is expected to be lower in temperate wintering areas where conditions are colder and harsher. The lowest disease risk is predicted in the Arctic where conditions are generally cold and breeding birds are widely dispersed.
These predictions are based on two assumptions that: (i) disease risk is higher in the tropics and (ii) disease risk is elevated when birds are densely aggregated during migration. Very little testing has been applied to these assumptions within long-distance migrants. However, in general, theory predicts that the spread, abundance and diversity of parasites, and thus disease risk, should be higher in hosts living at high density or with frequent intraspecific contacts (Anderson & May 1992; Arneberg 2002; Roberts et al. 2002). Furthermore, research on blood parasites has long shown that bird species inhabiting high Arctic and marine habitats have lower parasites levels than species inhabiting lower latitude and aquatic environments (Greiner et al. 1975; Bennet et al. 1992; Figuerola et al. 1996, Figuerola 1999). This conclusion has recently been confirmed specifically within shorebird species. The prevalence of avian malaria was compared in shorebird species sampled in the Arctic, in temperate Europe and in tropical West Africa and infected individuals were found mainly in tropical freshwater habitats (Mendes et al. 2005). In addition, a small population of knots that winter in tropical Brazil seem to suffer from high loads of feather lice and mites (Baker et al. 2005b).
7. Ecological evidence
We need ecological evidence to evaluate the existence and impact of these proposed bottlenecks. A particularly promising way to do this is to look at various aspects of the expression of nuptial plumage and moult itself throughout the annual cycle (Hill 1995; von Schantz et al. 1999).
(a) Breeding plumage: where and when to moult, and why fly dressed to impress?
Breeding plumage can be an important signal for sexual selection (Darwin 1871; see Baker & Parker (1979) for a review of theory). During breeding it is important for individuals to show their quality in order to ensure a high-quality partner and in many shorebird species, including knots, both partners are involved in mate selection (Piersma et al. 2001). Breeding plumage, its completeness and its colour, has been shown to be an honest quality signal. For example, in bar-tailed godwits the completeness of nuptial plumage during spring stopover in the Wadden Sea correlates positively with body mass (Piersma & Jukema 1993) and local survival (Drent et al. 2003), and negatively with the amount of intestinal cestode parasites (Piersma et al. 2001), although there may be an age component to these relationships (Battley 2007).
As their name suggests, breeding plumage in knots includes a rusty red colour acquired on the breast, belly, neck and face. Like many other sexually selected ornaments, this rusty colour is associated with pigments in the diet (namely melanin) and for the birds, the darker the rusty colour the better. We know that melanin occurs in two forms: eumelanins confer black and grey colours, and phaeomelanins are responsible for the chestnut and buff colours like the rusty red seen in knots. However, the physiological mechanisms that relate overall condition and melanin pigmentation are uncertain (McGraw 2005). For carotenoids, which stand as a model system to study honest signalling in pigments (von Schantz et al. 1999), one direct connection with condition is that individuals with more carotenoid pigmentation are better foragers and therefore more viable. Melanin pigments are large polymers synthesized endogenously from amino acids (Prota 1992), making the connection between the ingested building blocks of the pigment and coloration less direct. However, if not an indicator of foraging ability, melanin may well serve as an indicator of oxidative stress and immune function (McGraw 2005). Melanins, like carotenoids, are potent antioxidants containing both oxidizing and reducing functional groups which give them the capacity to quench reactive oxygen and nitrogen free radicals via electron donation or capture (Borovansky 1996). Melanin is also an immunostimulant via several mechanisms including phagocytosis, lysosomal enzyme activity, cytokine regulation and nitric oxide production in vertebrates (reviewed in Mackintosh 2001). Since they are synthesized internally, manipulation experiments to examine the direct antioxidative potential of melanin have been challenging (McGraw 2005). Nevertheless, increased pigmentation is correlated with improved health. For example, male house sparrows Passer domesticus that develop large patches of melanized throat feathers are in better body condition and show lower levels of parasitic infection than sparrows with smaller throat patches (Møller et al. 1996).
Both the immune system (especially the inflammatory response) and strenuous exercise such as migratory flight generate reactive metabolites and free radicals which contribute to oxidative stress (von Schantz et al. 1999). In migratory birds, pigmentation could then signal quality because pigments used by the immune response or for antioxidant purposes cannot be invested in breeding plumage. Thus, the ‘redness’ of the plumage gives an indication of the condition of the individual at the time of moult. Unlike carotenoids present in wattles, combs and skin of birds, the investment of melanin into plumage is non-reversible. Once these nutrients are imbedded into the plumage they are no longer available and are lost at the next moulting (Lozano 1994). Though in some species plumage colour is important for camouflage, it is not a life-saving body structure in knots; thus, if nutrients are directed away from investment in this ‘non-essential’ ornament when energy is limited, then plumage coloration should be a particularity sensitive indicator of conditions faced by the individual at the time of moult (Hill 1995).
Thus, one would expect birds to moult into breeding plumage in areas with sufficient food, benign thermal conditions and the necessary amino acids for pigments–-areas where they could best afford to invest energy into plumage coloration. Further, one would expect the birds to moult into breeding plumage as close to the breeding grounds as possible to avoid ‘wear and tear’.
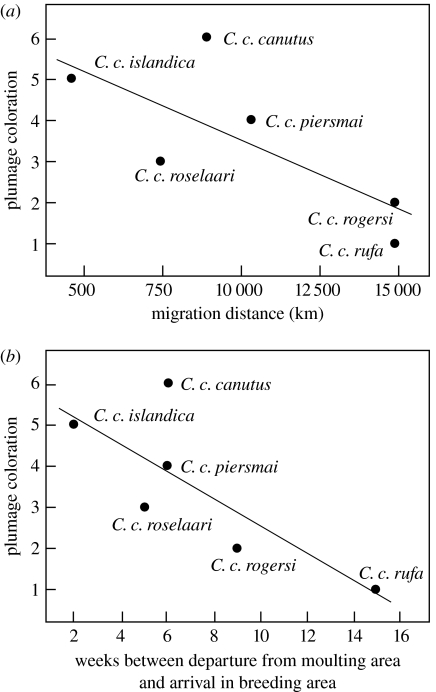
Is there ecological evidence supporting these predictions in knots and is there evidence of bottlenecking in certain subspecies? With respect to plumage characteristics, C. c. canutus, C. c. islandica and C. c. piersmai are the darker subspecies. Calidris canutus rogersi have lighter bellies than both C. c. roselaari and C. c. piersmai, and C. c. rufa is the palest in overall plumage (figure 1). Might pale plumage indicate a bottleneck? Calidris canutus rufa moult into breeding plumage while simultaneously fuelling for northward migration, thus energy and nutrients must be shared between fat stores and deeper red plumage. Furthermore, C. c. rufa is one of the longest distance migrants of the six subspecies, together with C. c. rogersi, which similarly has pale breeding plumage. Finally, both of these subspecies fly many kilometres, from wintering grounds in the Southern Hemisphere, ‘dressed up’ in their nuptial plumage. This may indicate a lack of sufficient food resources (nutritional bottleneck) or time (temporal bottleneck) to moult into breeding plumage closer to the breeding grounds to avoid fading and wear and tear on their plumage. The darker subspecies (C. c. canutus, C. c. islandica and C. c. piersmai) also moult into breeding plumage on the wintering grounds, but they do so closer to the breeding grounds, in terms of both distance and time. Indeed, darker breeding plumage is associated with shorter overall migration distance, as well as with a shorter period between departure from moulting area and arrival in the breeding area (figure 3).
Figure 3.
Trends between breeding plumage and aspects of migration. (a) Breeding plumage coloration, where 6 signifies the darkest colouration (taking into account both overall extent of redness and depth of colour from Tomkovich (1992, 2001), and overall migration distance for a one-way journey (Pearson correlation, 0.751; p=0.043, one tailed). (b) Breeding plumage coloration (as in (a)) and the number of weeks between departure from the moulting area and arrival on the breeding area (Pearson correlation, 0.781; p=0.033, one tailed).
(b) Wing moult and prebasic body moult: what is the cost and where and when to moult?
Unlike prealternate moult which is thought to produce an honest signal for sexual selection, prebasic body moult and wing moult are necessary for survival in knots. The yearly renewal of body and flight feathers is essential for both thermoregulation and flight and this moulting period is a potentially costly portion of the annual cycle. A general rise in energy metabolism during moult has been reported for a wide range of species (reviewed in King (1981) and Payne (1972)) and factors that contribute to this increase in energy consumption include the synthesis of the feathers themselves, as well as the indirect cost of increased thermoregulation due to decreased insulation of the plumage during moult and increased exposure of blood engorged quills. Studies have been done on the cost of feather synthesis (e.g. Dietz et al. 1992; Lindström et al. 1993; Schieltz & Murphy 1997), and all indicate that feather production alone cannot entirely explain the increase in energy metabolism seen during moult. The study of Schieltz & Murphy (1997) was particularly convincing as they experimentally plucked up to 36% of plumage in white-crowned sparrows Zonotrichia leucophrys gambelii and measured metabolic rate during regrowth. They found that under thermoneutral conditions, feather regrowth of even 36% of plumage did not increase oxygen consumption. Furthermore, a comparison between naturally moulting birds and birds that were plucked outside the natural moulting period revealed that naturally moulting birds showed a 25–54% higher metabolic rate than plucked birds. This result strongly suggests that the energy cost of moult is caused by other metabolic changes (still undiscovered) associated with moult and not the process of feather growth itself. Whatever the cause, moult appears to be metabolically costly.
In addition to the physiological costs of moult, there are indirect costs associated with wing moult including an increased cost of flight due to a reduction of wing area and an increase in foraging effort and predation risk due to decreased manoeuvrability (review by Hedenström 2003). These indirect costs of wing moult have been uncoupled from the physiological costs of feather synthesis in an elegant experiment by Swaddle & Witter (1997). They simulated wing moult by using scissors to reduce primary length in non-moulting birds and showed that this simulated moult resulted in reduced flight performance and body mass.
Given evidence for both direct and indirect costs of prebasic body moult and wing moult, one would predict that moult would be timed to avoid overlap with other costly life-history stages. Furthermore, because prebasic body moult and wing moult differ, we can make more specific predictions. For example, for temperate wintering subspecies, we would predict the timing of prebasic body moult to minimize overlap with short daylight and cold temperatures (Schieltz & Murphy 1997). In addition, given evidence for a reduction in flight performance, which would affect both predator avoidance and foraging, we would predict that wing moult would occur in an environment with sufficient food and low predation. Here again we can ask, is there ecological evidence supporting these predictions in knots and is there evidence of bottlenecking in certain subspecies?
In terms of overlap between prebasic body moult and other costly life-history stages, in general, knots delay body moult until arrival in the wintering areas. Only C. c. rufa is known to begin prebasic body moult on the breeding grounds. Moult is then suspended and is ultimately finished in the wintering areas 15 000 km away from Tierra del Fuego (Baker et al. 2005a). The reason why C. c. rufa commences prebasic body moult on the breeding grounds is unknown, but this phenomenon may be the evidence of temporal bottlenecking throughout the flyway, especially a lack of adequate time or possibly nutritional resources for complete moult in the wintering areas. Another possibility may be a carry-over effect from the need to moult into prealternate plumage very early in the season in this subspecies. By the end of breeding, C. c. rufa birds will have carried their breeding plumage for nearly six months and a distance of 15 000 km! Perhaps a small ‘touch-up’ moult is necessary for migration, assuming that worn plumage offers poor thermal capacity or decreases flight performance. Birds of the C. c. rogersi subspecies also show overlap between prebasic body moult and migration (figure 2), and moult on the breeding grounds is a possibility. As in C. c. rufa, the timing of moult and overlap with migration may indicate a temporal bottleneck throughout the flyway, possibly caused by the marathon distances covered by these two subspecies. Alternatively, moult and migration in C. c. rufa and C. c. roselaari might be a consequence of a shift in the temporal bottleneck relative to the other subspecies. Calidris canutus rufa and C. c. roselaari breed in the warmest spring conditions of the six subspecies (Battley et al. 2005). It is possible that these subspecies might commence breeding slightly earlier, finish it slightly earlier and have the opportunity to start prebasic moult on the breeding areas where the other subspecies do not.
In terms of the timing of prebasic body moult in relation to climatic conditions in the wintering areas, temperate winterers C. c. roselaari and C. c. islandica should moult as early as possible to avoid overlap with the cold dark winter months (see Summers et al. 2004). Calidris canutus islandica birds do in fact perform prebasic body moult quite early, perhaps to minimize heat loss during body moult (although there is no evidence that thermogenic capacity of the feathers is reduced during moult under captive conditions, A. Gustowska & F. Vézina 2006, personal communication). The moult schedule for C. c. roselaari is not precisely known, but it seems probable that they too complete prebasic body moult quickly before the onset of the winter months. Transequatorial migrants such as C. c. rogersi and C. c. rufa would have the longest daylight hours for feeding and the highest temperatures during the height of the austral summer. Thus, they might be predicted to moult later in the non-breeding season than do tropical or north temperate winters. In fact, instead of postponing moult, C. c. rogersi and C. c. rufa begin moulting even before reaching the wintering areas, possibly because time constraints do not allow optimization in terms of when and where to moult. However, as discussed above, many factors may be involved in the moult and migration overlap displayed in these subspecies.
In terms of overlap between wing moult and other costly life-history stages, all knot subspecies delay wing moult until arrival in the wintering areas, or very close to them in the case of C. c. islandica individuals, indicating that other times of the annual cycle may already be too nutritionally, energetically or temporally bottlenecked for overlap with costly wing moult.
The duration of wing moult may also be instructive in terms of ecological evidence for bottlenecking in knots. Stretching wing moult over as long a period as possible would have the advantage of diluting the physiological costs of feather synthesis as well as reducing the indirect costs on flight performance by reducing the size of the gap in the wing. Furthermore, there is evidence that a slower moult may also improve the quality of the feathers grown (Serra 2001). Even in the absence of statistically formal analyses, both C. c. rufa and C. c. islandica seem to have relatively short primary moult durations. Calidris canutus rufa is temporally bottlenecked and spends only three to four months on the wintering grounds, undergoing wing moult from arrival until fattening for northward migration. Calidris canutus islandica probably moult their primary feathers quickly to avoid elevated flight and foraging costs during the darker and colder months of winter when food is unpredictable and thermoregulatory costs are high (nutritional/energetic bottleneck).
8. Future directions
In summary, as a species, knots appear to experience nutritional, energetic, temporal and disease-risk bottlenecks throughout the annual cycle, and there appear to be critical periods in the course of this generally busy lifestyle. For example, in all subspecies timing seems to be paramount during departures from the final staging area before the breeding grounds, and all subspecies show synchronous departure during this period indicating little room for flexibility. Furthermore, the extent, type and timing of ecological bottlenecks vary between the subspecies, depending on the environments that they encounter throughout their annual cycle.
We have developed a framework to look at potential bottlenecks within the annual cycle of a long-distance migrant. We now use our framework to make predictions about investment in immunity in migrants, highlighting the idea that disease risk and immune function are not constant throughout the annual cycle. We also discuss the occurrence of mortality in the annual cycle with reference to nutritional, energetic, temporal and disease-risk bottlenecks.
(a) Predictions for seasonal variation in immune function
We have discussed how disease risk may play an important role in the annual cycle of long-distance migrants. We would now like to make a few predictions about defence against disease, in relation to both the predicted risk of pathogens themselves, and the nutrition and energy requirements needed to maintain and use immune system defences. As discussed before, it is difficult to define a single currency with which to measure the cost of immune function. The discipline of ecological immunology is still in an exploratory phase (Klasing 2004) and we are only beginning to understand this enormously interactive branch of biology (Martin et al. 2008), thus it is difficult to come up with simple predictions. Though it is clear that mounting an inflammatory immune response is costly (Lochmiller & Deerenberg 2000), knots and other migrants have to deal with several constraints acting together and it is not clear how they will respond to these constraints in the wild in terms of integrated trade-offs. Thus, until controlled laboratory experiments have been conducted, we will consider various different predictions to try to encompass the complexity of the immune system and the different conditions encountered during the annual cycle. It is important to keep in mind that this is a ‘cost–benefit’ framework over the annual cycle in which individuals are aiming to maximize fitness. During high-cost periods of the annual cycle such as breeding and migration, the cost of eliminating or preventing infection in terms of high-cost inflammatory defences might outweigh the cost of living with the infection (Viney et al. 2005). Thus, when we refer to reduced investment or a shift in the type of immune function, we are not implying that this is non-adaptive, only that a trade-off is possible (Lee 2006).
Migration is a time of considerable energetic and temporal bottlenecking for knots and all subspecies endure several bottlenecks simultaneously during the last leg of northward migration and arrival on the breeding grounds (figure 2). Furthermore, the risk of autoimmunity is increased during strenuous activity such as prolonged flight (Råberg et al. 1998). As such, a trade-off between immune function, migration and preparation for reproduction can be hypothesized in terms of resource limitation and the risk of autoimmunity. This hypothesis would predict relatively low immune function during the flight phase of migration during both northward and southward journeys and at arrival at stopover sites and the breeding grounds (table 2). However, disease risk differs between the flight and fuelling stages of migration with disease risk relatively higher during fuelling because birds are landing in diverse habitats and foraging in dense aggregations. Examined from this angle, immune investment should be relatively high during the fuelling phase of migration. It is not yet known whether immune function is plastic between flight and fuelling; however, this apparent paradox may be resolved by downregulation of costly inflammatory responses (such as the acute phase sickness response) and reliance on antibody-mediated acquired immunity during both the flight and the fuelling phases of migration (table 2; Klasing 2004; Lee 2006). Indeed, a recent study shows no evidence for reduced antibody or phytohaemagglutinin (PHA)-swelling response in knots flown in a wind tunnel (Hasselquist et al. 2007). However, further controlled experiments measuring many immune parameters will be needed to test this hypothesis (reviewed in Martin et al. 2008).
Breeding is also a period of energetic bottlenecking in knots. Furthermore, disease risk during reproduction should be low because knots breed in the relatively low risk Arctic and are widely dispersed in the breeding areas (Piersma 1997). As such, the need for immune function in terms of disease risk should be reduced. Thus, we predict low immune function during breeding for all subspecies (table 2).
We predict immune investment to be highest during wintering when knots are no longer investing resources, energy or time in migration or reproduction (table 2). During wintering, knots engage in only one potentially costly activity, i.e. moult. Studies on domestic fowl have demonstrated trade-offs between moult and immune activity (Kuenzel 2003). However, studies examining immune function and moult in wild birds are rare and often offer conflicting results (i.e. Silverin et al. 1999; Martin 2005) perhaps because they measured different types of immune function and may not be directly comparable. In general, during the wintering period we predict that knots should invest less in immune function during moult, though still more than during migration or reproduction.
A final consideration when making predictions about immune function is that it is probably a plastic response to the environment. As such predictions among subspecies can be made because different subspecies winter in different environments. Tropical winterers such as C. c. canutus and C. c. piersmai have low energetic demands, predictable food and relatively high disease risk in winter and are predicted to invest the most in immune function relative to the other subspecies on the wintering grounds. Calidris canutus islandica winter in more energetically demanding conditions with unpredictable food and lower disease risk, thus are predicted to invest comparably less in immune function. Finally, C. c. rogersi and C. c. rufa have long migrations resulting in relatively short stays on wintering grounds coupled with simultaneous moult and fuelling, thus these subspecies are predicted to invest the least in immune function during winter in comparison with the other subspecies.
(b) Predictions about the occurrence of mortality during the annual cycle of long-distance migrants
All the events within the annual cycle boil down to survival and reproduction. Owing to the difficulty involved in measuring the reproductive success of knots in the High Arctic (Meltofte 2001; Piersma et al. 2006), we only briefly discuss indirect measures of reproductive success in relation to a larger discussion on mortality (the inverse of survival) over the annual cycle. To simplify this discussion, we consider mortality from three broad sources: starvation; predation; and disease (McNamara et al. 1998). These sources of mortality can be placed into our framework of bottlenecks without difficulty. Mortality from starvation is tied to both nutritional and energetic bottlenecks since a bird starves as a result of expending more energy than it takes in. Predation pressure on adult knots is closely linked with factors involved in our temporal bottleneck in which birds must time their migrations in order to maximize survival and reproduction, balancing the need for energy with increased predation risk. For example, predation may be higher during migratory fattening when a bird's chances of being captured by a predator are thought to increase as their fat stores increase due to wing loading and a lack of compensatory pectoral muscle increase (Lank & Ydenberg 2003; Dietz et al. 2007) and as their foraging intensity increases due to lack of vigilance (Dierschke 2003). Finally, death from disease is linked with both disease risk, which can vary over the annual cycle, and the amount of resources available to invest into immune defences.
Our framework considers four bottlenecks that overlap during different times in the year, and for different proportions of the year, for different subspecies. We define a period in which individuals experience three or more bottlenecks simultaneously as severely bottlenecked (figure 2). Using this definition, all subspecies are severely bottlenecked during the last leg of northward migration and arrival on the breeding grounds for a period of about a month when energetic, temporal, disease risk and sometimes nutritional bottlenecks occur together. In addition, C. c. rogersi and C. c rufa are severely bottlenecked (energetic, temporal and disease risk) during migration. In C. c. rogersi this amounts to nearly four months and in C. c rufa nearly seven months of severe bottlenecking. Keeping this in mind, we predict high mortality in knots during the last leg of northward migration and arrival on the breeding grounds for all subspecies and during migration for C. c. rogersi and C. c rufa. Furthermore, we might predict lower overall adult survival in C. c. rogersi and C. c rufa since they spend a greater proportion of the annual cycle severely bottlenecked.
Unlike resident birds and mammals that tend to suffer high mortality during severe winter weather (Nelson et al. 2002), migratory birds tend to have higher mortality during migration and reproduction as predicted by our framework. For example, a study of black-throated blue warblers Dendroica caerulescens found that mortality during migration occurs at a rate at least 15 times higher than stationary periods (Sillett & Holmes 2002), with more than 85% of annual mortality occurring during migration. Beyond this study, however, few data exist on periods of high mortality in the annual cycle of migrating birds, even in a well-studied species such as the knot. Thus, at present we cannot test the hypothesis that mortality should be higher during the last leg of northward migration and arrival on the breeding grounds. Predictions can, however, be examined in more detail in C. c. islandica and C. c. rufa for which long-term demographic studies have been performed.
In C. c. islandica, demographic studies go back as far as 1969 and birds show episodes of high mortality, closely tied to climate and starvation, during both wintering and arrival for reproduction (Boyd & Piersma 2001). For example, knot mortality was high during the very cold winter of 1962–1963. Within our framework, C. c. islandica is the only subspecies predicted to have high mortality during winter due to nutritional and energetic bottlenecks. Cold summers in 1972 and 1974 also had a great impact and resulted in high adult mortality and low juvenile recruitment. In addition, the summer of 1979 was one of the coldest on record in the Canadian breeding grounds of C. c. islandica, and between 1979 and 1980 there was a 29% drop in the numbers of C. c. islandica birds wintering in Britain and a high proportion of juveniles in the wintering flocks (Boyd 1992). This pattern indicates high adult mortality during northward migration and upon arrival, as predicted by our framework, but high fecundity in the few adults that survived to breed in the summer of 1979.
While demographic studies on C. c. islandica point to high mortality due to climate-related nutritional and energetic bottlenecks during reproduction and wintering, a study of C. c. rufa from 1997 to 2002 indicates a nutritional and energetic bottleneck tied closely with human interference during migration. Our framework predicts severe bottlenecking in C. c. rufa in over 50% of the year and all four bottlenecks overlap during May and early June. The C. c. rufa subspecies exploits horseshoe crab eggs on the beaches of Delaware Bay, USA, during northward migration. However, increased harvesting of horseshoe crabs may have decreased the abundance of food enough to exacerbate and prolong the already existing nutritional bottleneck in this subspecies to overwhelming levels. In fact, adult survival in C. c. rufa has dropped significantly from 84.6% from 1994 to 1997 to only 56.4% from 1998 to 2001 (Baker et al. 2004). Baker et al. (2004) also show that birds are failing to meet minimum fuelling requirements and the failure to fuel adequately may result in high adult mortality on the breeding grounds due to insufficient ‘emergency stores’ upon arrival (cf. Morrison et al. 2005).
High winter mortality due to disease could be predicted during migration in tropical stopover sites in Brazil for C. c. rufa or C. c. roselaari when stores are low and disease risk is high. Indeed, the small population of birds that winter in Maranhão have high ectoparasite loads, indicating that disease risk in the area is probably high (Baker et al. 2005b). Furthermore, a large die off of C. c. rufa birds, possibly as a result of a viral infection combined with an infestation of acanthocephalan worms, has been witnessed at the southern Brazil stopover of Lagoa do Peixe in April (Baker et al. 1999). High winter mortality due to disease could also be predicted during wintering for C. c. canutus and C. c. piersmai, but it is possible that low energetic costs and predictable food allow these subspecies to invest enough in immune defence to survive this risk.
Large-scale demographic studies examining mortality in knots throughout the annual cycle are now needed to integrate data from C. c. islandica during reproduction and wintering and C. c. rufa during northward migration. Long-term survival studies are currently underway for five of the six subspecies (no programme of focused studies on C. c. roselaari is taking place) and hopefully these studies can begin to address the questions about mortality within the annual cycle. Furthermore, technological advances in radio transmitting are close to providing transmitters small enough to track knots, and other migrants, throughout their annual cycle (A. Purgue, D.W. Winkler & K. Fristrup 2006, personal communication). This technology should allow us to discover when and where these birds die and may also help us to discover whether mortality differs between habitats of differing quality or whether condition differences in one part of the annual cycle carry over into the next affecting survival and reproduction. This information can then be examined within the framework of ecological bottlenecks and other frameworks that theoretically model the annual cycle (e.g. Houston & McNamara 1999; McNamara & Houston 2008), helping to unravel the remaining mysteries of the annual cycle of long-distance migrants.
9. Concluding remarks
As a heuristic tool for the examination of selection pressures during the annual cycle of long-distance migrant birds, we have reviewed nutritional, energetic, temporal and disease bottlenecks. We have applied this framework in a comparative way to the six subspecies knots, a globally distributed shorebird, and have come up with predictions on immune function and seasonal mortality patterns that we hope will prove testable in the near future. We hope this framework will be applicable to other species and types of migrants, thus expanding the comparative database for future evaluation of seasonal selection pressures and the evolution of annual cycles in long-distance migrants. This description of the annual cycle of a representative migrant offers details not previously considered in models. We hope that these details can aid in the development of more realistic annual routine models for migrants and that these models can be generalized to examine factors that affect behaviour during the annual cycle, not only in well-studied species but also in species where such detailed knowledge is not available.
Acknowledgments
This research took place in compliance with ethical guidelines from the Animal Experiment Committee (DEC) of the Royal Netherlands Academy for Arts and Sciences (KNAW).
We would like to thank John McNamara and Alasdair Houston for inspiring this contribution and for their helpful editorial comments. Danny Rogers, Luisa Mendes, Patricia González, Phil Battley and two anonymous reviewers gave valuable comments and feedback on this manuscript and Dick Visser finalized the figures. This work was supported by a Natural Science and Engineering Research Council of Canada (NSERC) Post Graduate Scholarship (PGSB-267701-2003) and a University of Groningen Ubbo Emmius Scholarship to D.M.B. and operational grants by the Netherlands Organization for Scientific Research (NWO), NIOZ and University of Groningen to T.P.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Alerstam T. Cambridge University Press; Cambridge, UK: 1990. Bird migration. [Google Scholar]
- Alerstam T, Hedenström A, Åkesson S. Long-distance migration: evolution and determinants. Oikos. 2003;103:247–260. doi:10.1034/j.1600-0706.2003.12559.x [Google Scholar]
- Anderson R.M, May R.M. Oxford University Press; New York, NY: 1992. Infectious diseases of humans: dynamics and control. [Google Scholar]
- Arneberg P. Host population density and body mass as determinants of species richness in parasite communities: comparative analysis of directly transmitted nematodes of mammals. Ecography. 2002;25:88–94. doi:10.1034/j.1600-0587.2002.250110.x [Google Scholar]
- Atkinson P.W, Baker A.J, Bevan R.M, Clark N.A, Cole K.B, Gonzalez P.M, Newton J, Niles L.J, Robinson R.A. Unravelling the migration and moult strategies of a long-distance migrant using stable isotopes: red knot Calidris canutus movements in the Americas. Ibis. 2005;147:738–749. doi:10.1111/j.1474-919x.2005.00455.x [Google Scholar]
- Baker R.R, Parker G.A. The evolution of bird coloration. Phil. Trans. R. Soc. B. 1979;287:63–130. doi:10.1098/rstb.1979.0053 [Google Scholar]
- Baker A, et al. Northbound migration of red knots Calidris canutus rufa in Argentina and Brazil: report on results obtained by the international expedition in March–April 1997. Wader Study Group Bull. 1999;88:64–75. [Google Scholar]
- Baker, A. J., González, P. M., Minton, C. D. T., Carter, D. B., Niles, L. J., do Nascimento, I. L. S. & Piersma, T. 2001 Hemispheric problems in the conservation of red knots (Calidris canutus rufa). In Proc. VI Neotropical Ornithological Cong., Int. shorebird Symp. Monterrey, Mexico, pp. 21–28. Manomet, MA: Western Hemisphere Shorebird Reserve Network.
- Baker A.J, et al. Rapid population decline in red knots: fitness consequences of decreased refuelling rates and late arrival in Delaware Bay. Proc. R. Soc. B. 2004;271:875–882. doi: 10.1098/rspb.2003.2663. doi:10.1098/rspb.2003.2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A.J, González P.M, Benegas L, Rice S, D'Ámico V.L, Abril M, Farmer A, Peck M. Annual international shorebird expeditions to study the red knot population in Rio Grande, Tierra del Fuego, 2000–2004. Wader Study Group Bull. 2005a;107:19–23. [Google Scholar]
- Baker A.J, González P.M, Serrano I.L, Júnior W.R.T, Efe M.A, Rice S, D'Ámico V.L, Rocha M.C, Echave M.E. Assessment of the wintering area of red knots in Maranhão, northern Brazil, in February 2005. Wader Study Group Bull. 2005b;107:10–18. [Google Scholar]
- Barter M. Distribution, abundance, migration and moult of the red knot Calidris canutus rogersi. Wader Study Group Bull. 1992;64:64–70. [Google Scholar]
- Battley P.F. The northward migration of Arctic waders in New Zealand: departure behaviour, timing and possible migration routes of red knots and bar-tailed godwits from Farewell Spit, North-West Nelson. Emu. 1997;97:108–120. doi:10.1071/MU97014 [Google Scholar]
- Battley P.F. Plumage and timing of migration in bar-tailed godwits: a comment on Drent et al. (2003) Oikos. 2007;116:349–352. doi:10.1111/j.2006.0030-1299.15474.x [Google Scholar]
- Battley P.F, Piersma T. The body composition of lesser knots Calidris canutus rogersi preparing to take off on migration from northern New Zealand. Notornis. 1997;44:137–150. [Google Scholar]
- Battley P.F, Piersma T. Adaptive interplay between feeding ecology and features of the digestive tract in birds. In: Starck J.M, Wang T, editors. Physiological and ecological adaptations to feeding in vertebrates. Science Publishers; Enfield, NH: 2005. pp. 201–228. [Google Scholar]
- Battley P.F, Piersma T, Rogers D.I, Dekinga A. Do body condition and plumage during fuelling predict northwards departure dates of great knots Calidris tenuirostris from north-west Australia? Ibis. 2004;146:46–60. doi:10.1111/j.1474-919X.2004.00210.x [Google Scholar]
- Battley P.F, Rogers D.I, van Gils J.A, Piersma T, Hassell C.J, Boyle A, Hong-Yan Y. How do red knots leave Northwest Australia in May and reach the breeding grounds in June? Predictions of stopover times, fuelling rates and prey quality in the Yellow Sea. J. Avian Biol. 2005;36:494–500. doi:10.1111/j.0908-8857.2005.03730.x [Google Scholar]
- Bennet G.F, Montgomerie R, Seutin G. Scarcity of Haematozoa in birds breeding on the arctic tundra of North America. Condor. 1992;94:289–292. doi:10.2307/1368821 [Google Scholar]
- Borovansky J. Free radical activity of melanins and related substances: biochemical and pathobiochemical aspects. Sbornik Lekarsky. 1996;97:49–70. [PubMed] [Google Scholar]
- Boyd H. Arctic summer conditions and British knot numbers: an exploratory analysis. Wader Study Group Bull. 1992;64:144–152. [Google Scholar]
- Boyd H, Piersma T. Changing balance between survival and recruitment explains population trends in red knots Calidris canutus islandica wintering in Britain, 1969–1995. Ardea. 2001;89:301–317. [Google Scholar]
- Buehler D.M, Baker A.J. Population divergence times and historical demography in red knots and dunlins. Condor. 2005;107:497–513. doi:10.1650/0010-5422(2005)107[0497:PDTAHD]2.0.CO;2 [Google Scholar]
- Buehler D.M, Baker A.J, Piersma T. Reconstructing palaeoflyways of the late Pleistocene and early Holocene red knot (Calidris canutus) Ardea. 2006;94:485–498. [Google Scholar]
- Cramp S, Simmons K.E.L, editors. The birds of the Western Palearctic. Waders to gulls. vol. III. Oxford University Press; Oxford, UK: 1985. [Google Scholar]
- Darwin C. John Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Davidson N.C. Red knot (knot) Calidris canutus. In: Wernham C, Torris M, Marchant J, Clark J, Siriwardena G, Baille S, editors. The migration atlas: movements of the birds of Britain and Ireland. T. & A. D. Poyser; London, UK: 2002. pp. 293–296. [Google Scholar]
- Davidson N.C, Wilson J.R. The migration system of European-wintering knots Calidris canutus islandica. Wader Study Group Bull. 1992;64:39–51. [Google Scholar]
- Davidson N.C, et al. The origins of knots Calidris canutus in arctic Norway in spring. Ornis Scand. 1986;17:175–179. doi:10.2307/3676866 [Google Scholar]
- Dierschke V. Predation hazard during migratory stopover: are light or heavy birds under risk? J. Avian Biol. 2003;34:24–29. doi:10.1034/j.1600-048X.2003.03049.x [Google Scholar]
- Dietz M.W, Daan S, Masman D. Energy requirements for molt in the kestrel Falco tinnunculus. Physiol. Zool. 1992;65:1217–1235. [Google Scholar]
- Dietz M.W, Piersma T, Hedenström A, Brugge M. Intraspecific variation in avian pectoral muscle mass: constraints on maintaining maneuverability with increasing body mass. Funct. Ecol. 2007;21:317–326. doi:10.1111/j.1365-2435.2006.01234.x [Google Scholar]
- Drent R.H, Daan S. The prudent parent—energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Drent R, Both C, Green M, Madsen J, Piersma T. Pay-offs and penalties of competing migratory schedules. Oikos. 2003;103:274–292. doi:10.1034/j.1600-0706.2003.12274.x [Google Scholar]
- Figuerola J. Effects of salinity on rates of infestation of waterbirds by Haematozoa. Ecography. 1999;22:681–685. doi:10.1111/j.1600-0587.1999.tb00517.x [Google Scholar]
- Figuerola J, Velarde R, Bertolero A, Cerdá F. Absence of Haematozoa in a breeding population of Kentish plover Charadrius alexandrinus in northwest Spain. J. für Ornithologie. 1996;137:523–525. doi:10.1007/BF01661106 [Google Scholar]
- González P.M, Piersma T, Verkuil Y. Food, feeding and refuelling of red knots during northward migration at San Antonio Oeste, Rio Negro, Argentina. J. Field Ornithol. 1996;67:575–591. [Google Scholar]
- Greiner E.C, Bennet G.F, White E.M, Coombs R.F. Distribution of the avian hematoza of North America. Can. J. Zool. 1975;53:1762–1787. doi: 10.1139/z75-211. [DOI] [PubMed] [Google Scholar]
- Gudmundsson G.A. Spring migration of the knot Calidris c. canutus over southern Scandinavia as recorded by radar. J. Avian Biol. 1994;25:15–26. doi:10.2307/3677290 [Google Scholar]
- Guglielmo C.G, Piersma T, Williams T.D. A sport-physiological perspective on bird migration: evidence for flight-induced muscle damage. J. Exp. Biol. 2001;204:2683–2690. doi: 10.1242/jeb.204.15.2683. [DOI] [PubMed] [Google Scholar]
- Hammond K.A, Diamond J. Maximal sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. doi:10.1038/386457a0 [DOI] [PubMed] [Google Scholar]
- Harrington B.A. Red knot (Calidris canutus) In: Poole A, Gill F, editors. The birds of North America. vol. 563. The Birds of North America, Inc; Philadelphia, PA: 2001. pp. 1–32. [Google Scholar]
- Hasselquist D, Lindström Å, Jenni-Eiermann S, Koolhaas A, Piersma T. Long flights do not influence immune responses of a long-distance migrant bird: a wind-tunnel experiment. J. Exp. Biol. 2007;210:1123–1131. doi: 10.1242/jeb.02712. doi:10.1242/jeb.02712 [DOI] [PubMed] [Google Scholar]
- Hedenström A. Flying with holey wings. J. Avian Biol. 2003;34:324–327. doi:10.1111/j.0908-8857.2003.03324.x [Google Scholar]
- Hedenström A, Alerstam T. Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J. Theor. Biol. 1997;189:227–234. doi: 10.1006/jtbi.1997.0505. doi:10.1006/jtbi.1997.0505 [DOI] [PubMed] [Google Scholar]
- Higgins P.J, Davies S.J.J.F, editors. Handbook of Australian, New Zealand and Antarctic birds. Snipes to pigeons. vol. 3. Oxford University Press; Melbourne, Australia: 1996. [Google Scholar]
- Hill G.E. Ornamental traits as indicators of environmental health. Bioscience. 1995;45:25–31. doi:10.2307/1312532 [Google Scholar]
- Houston A.I, McNamara J.M. Cambridge University Press; Cambridge, UK: 1999. Models of adaptive behaviour. [Google Scholar]
- Jacobs J.D, Wingfield J.C. Endocrine control of life stages: a constraint on response to the environment? Condor. 2000;102:35–51. doi:10.1650/0010-5422(2000)102[0035:ECOLCS]2.0.CO;2 [Google Scholar]
- Jehl J.R., Jr . Aspects of the molt migration. In: Gwinner E, editor. Bird migration: physiology and ecophysiology. Springer; Berlin, Germany: 1990. pp. 102–113. [Google Scholar]
- Johnson C. Patterns of seasonal weight variation in waders on the Wash. Ring. Migr. 1985;6:19–32. [Google Scholar]
- King J.R. Energetics of avian moult. In: Nöhring R, editor. Acta XVII Congressus Internationalis Ornithologici. Verlag der Deutschen Ornithologen Gesellschaft; Berlin, Germany: 1981. pp. 312–317. [Google Scholar]
- Klasing K.C. The costs of immunity. Acta Zoologica Sinica. 2004;50:961–969. [Google Scholar]
- Koopman K. Primary moult and weight changes of ruffs in The Netherlands in relation to migration. Ardea. 1986;74:69–77. [Google Scholar]
- Kuenzel W.J. Neurobiology of molt in avian species. Poult. Sci. 2003;82:981–991. doi: 10.1093/ps/82.6.981. [DOI] [PubMed] [Google Scholar]
- Kvist A, Lindström A. Basal metabolic rate in migratory waders: intra-individual, intraspecific, interspecific and seasonal variation. Funct. Ecol. 2001;15:465–473. doi:10.1046/j.0269-8463.2001.00549.x [Google Scholar]
- Lank D.B, Ydenberg R.C. Death and danger at migratory stopovers: problems with ‘predation risk’. J. Avian Biol. 2003;34:225–228. doi:10.1034/j.1600-048X.2003.03250.x [Google Scholar]
- Lee K.L. Linking immune defenses and life history at the levels of the individual and the species. Integr. Comp. Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. doi:10.1093/icb/icl049 [DOI] [PubMed] [Google Scholar]
- Lindström Å, Visser G.H, Daan S. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 1993;6:490–510. [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi:10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Lozano G.A. Carotenoids, parasites, and sexual selection. Oikos. 1994;70:309–311. doi:10.2307/3545643 [Google Scholar]
- Lozano G.A, Lank D.B. Seasonal trade-offs in cell-mediated immunosenescence in ruffs (Philomachus pugnax) Proc. R. Soc. B. 2003;270:1203–1208. doi: 10.1098/rspb.2002.2309. doi:10.1098/rspb.2002.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh J.A. The antimicrobial properties of melanocytes, melanosomes and melanin and the evolution of black skin. J. Theor. Biol. 2001;212:101–113. doi: 10.1006/jtbi.2001.2331. doi:10.1006/jtbi.2001.2331 [DOI] [PubMed] [Google Scholar]
- Martin L.B. Trade-offs between molt and immune activity in two populations of house sparrows (Passer domesticus) Can. J. Zool. 2005;83:780–787. doi:10.1139/z05-114 [Google Scholar]
- Martin L.B, Weil Z.M, Nelson R.J. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil. Trans. R. Soc. B. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. doi:10.1098/rstb.2007.2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masman D, Daan S, Beldhuis J.A. Ecological energetics of the kestrel: daily energy expenditure throughout the year based on time-energy budget, food intake and doubly labeled water methods. Ardea. 1988;76:64–81. [Google Scholar]
- McGraw K.J. The antioxidant function of many animal pigments: are there consistent health benefits of sexually selected colourants? Anim. Behav. 2005;69:757–764. doi:10.1016/j.anbehav.2004.06.022 [Google Scholar]
- McNamara J.M, Houston A.I. Optimal annual routines: behaviour in the context of physiology and ecology. Phil. Trans. R. Soc. B. 2008;363:301–319. doi: 10.1098/rstb.2007.2141. doi:10.1098/rstb.2007.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J.M, Welham R.K, Houston A.I. The timing of migration within the context of an annual routine. J. Avian Biol. 1998;29:416–423. doi:10.2307/3677160 [Google Scholar]
- McNamara J.M, Welham R.K, Houston A.I, Daan S, Tinbergen J.M. The effects of background mortality on optimal reproduction in a seasonal environment. Theor. Popul. Biol. 2004;65:361–372. doi: 10.1016/j.tpb.2003.10.006. doi:10.1016/j.tpb.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Meltofte H. Wader population censuses in the Arctic: getting the timing right. Arctic. 2001;54:367–376. [Google Scholar]
- Mendes L, Piersma T, Lecoq M, Spaans B, Ricklefs R.E. Disease-limited distributions? Contrasts in the prevalence of avian malaria in shorebird species using marine and freshwater habitats. Oikos. 2005;109:396–404. doi:10.1111/j.0030-1299.2005.13509.x [Google Scholar]
- Møller A.P, Erritzøe J. Host immune defence and migration in birds. Evol. Ecol. 1998;12:945–953. doi:10.1023/A:1006516222343 [Google Scholar]
- Møller A.P, Kimball R.T, Erritzøe J. Sexual ornamentation, condition, and immune defence in house sparrow Passer domesticus. Behav. Ecol. Sociobiol. 1996;39:317–322. doi:10.1007/s002650050295 [Google Scholar]
- Morrison R.I.G, Harrington B.A. The migration system of the red knot Calidris canutus rufa in the new world. Wader Study Group Bull. 1992;64:71–84. [Google Scholar]
- Morrison R.I.G, Davidson N.C, Piersma T. Transformations at high latitudes: why do red knots bring body stores to the breeding grounds? Condor. 2005;107:449–457. doi:10.1650/7614 [Google Scholar]
- Nebel S, Piersma T, van Gils J.A, Dekinga A, Spaans B. Length of stopover, fuel storage and a sex-bias in the occurrence of red knots Calidris canutus canutus and C. c. islandica in the Wadden Sea during southward migration. Ardea. 2000;88:165–176. [Google Scholar]
- Nelson R.J, Demas G.E. Seasonal changes in immune function. Q. Rev. Biol. 1996;71:511–548. doi: 10.1086/419555. doi:10.1086/419555 [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Demas G.E, Klien S.L, Kriegsfeld L.J. Cambridge University Press; Cambridge, UK: 2002. Seasonal patterns of stress, immune function and disease. [Google Scholar]
- Page G.W, Palacios E, Alfaro L, Gonzalez S, Stenzel L.E, Jungers M. Numbers of wintering shorebirds in coastal wetlands of Baja California, Mexico. J. Field Ornithol. 1997;68:562–574. [Google Scholar]
- Page G.W, Stenzel L.E, Kjelmyr J.E. Overview of shorebird abundance and distribution in wetlands of the Pacific Coast of the Contiguous United States. Condor. 1999;101:461–471. doi:10.2307/1370176 [Google Scholar]
- Payne R.B. Mechanisms and control of molt. In: Farner D.S, King J.R, editors. Avian biology. vol. 2. Academic Press; New York, NY: 1972. pp. 103–155. [Google Scholar]
- Piersma T. Hop, skip or jump? Contraints on migration of arctic waders by feeding, fattening and flight speed. Limosa. 1987;60:185–194. [Google Scholar]
- Piersma T. Uitgeverij Het Open Boek; Den Burg, Texel, The Netherlands: 1994. Close to the edge: energetic bottlenecks and the evolution of migratory pathways in knots. [Google Scholar]
- Piersma T. Do global patterns of habitat use and migration strategies co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos. 1997;80:623–631. doi:10.2307/3546640 [Google Scholar]
- Piersma T. ‘Coastal’ versus ‘inland’ shorebird species: interlinked fundamental dichotomies between their life- and demographic histories? Wader Study Group Bull. 2003;100:5–9. [Google Scholar]
- Piersma T, Davidson N.C. The migrations and annual cycles of five subspecies of knots in perspective. Wader Study Group Bull. 1992;64:187–197. [Google Scholar]
- Piersma T, Jukema J. Red breasts as honest signals of migratory quality in a long distance migrant, the bar-tailed godwit. Condor. 1993;95:163–177. doi:10.2307/1369398 [Google Scholar]
- Piersma T, Lindström Å. Rapid reversible changes in organ size as a component of adaptive behavior. Trends Ecol. Evol. 1997;12:134–138. doi: 10.1016/s0169-5347(97)01003-3. doi:10.1016/S0169-5347(97)01003-3 [DOI] [PubMed] [Google Scholar]
- Piersma T, Zwarts L, Bruggemann J.H. Behavioral aspects of the departure of waders before long-distance flights: flocking, vocalizations, flight paths and diurnal timing. Ardea. 1990;78:157–184. [Google Scholar]
- Piersma T, Prokosch P, Bredin D. The migration system of Afro-Siberian knots Calidris canutus canutus. Wader Study Group Bull. 1992;64:52–63. [Google Scholar]
- Piersma T, Hoekstra R, Dekinga A, Koolhaas A, Wolf P, Battley P.F, Wiersma P. Scale and intensity of intertidal habitat use by knots Calidris canutus in the western Wadden Sea in relation to food, friends and foes. Neth. J. Sea Res. 1993a;31:331–357. doi:10.1016/0077-7579(93)90052-T [Google Scholar]
- Piersma T, Koolhaas A, Dekinga A. Interactions between stomach structure and diet choice in shorebirds. Auk. 1993b;110:552–564. [Google Scholar]
- Piersma T, van Aelst R, Kurk K, Berkhoudt H, Maas L.R.M. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proc. R. Soc. B. 1998;265:1377–1383. doi:10.1098/rspb.1998.0445 [Google Scholar]
- Piersma T, Dietz M.W, Dekinga A, Nebel S, van Gils J.A, Battley P.F, Spaans B. Reversible size-changes in stomachs of shorebirds: when, to what extent, and why? Acta Ornithol. 1999a;34:175–181. [Google Scholar]
- Piersma T, Gudmundsson G.A, Lilliendahl K. Rapid changes in the size of different functional organ and muscle groups during refueling in a long-distance migrating shorebird. Physiol. Biochem. Zool. 1999b;72:405–415. doi: 10.1086/316680. doi:10.1086/316680 [DOI] [PubMed] [Google Scholar]
- Piersma T, Mendes L, Hennekens J, Ratiarison S, Groenewold S, Jukema J. Breeding plumage honestly signals likelihood of tapeworm infestation in females of a long-distance migrating shorebird, the bar-tailed godwit. Zoology. 2001;104:41–48. doi: 10.1078/0944-2006-00003. doi:10.1078/0944-2006-00003 [DOI] [PubMed] [Google Scholar]
- Piersma T, Lindström Å, Drent R.H, Tulp I, Jukema J, Morrison R.I.G, Reneerkens J, Schekkerman H, Visser G.H. High daily energy expenditure of incubating shorebirds on High Arctic tundra: a circumpolar study. Funct. Ecol. 2003;17:356–362. doi:10.1046/j.1365-2435.2003.00741.x [Google Scholar]
- Piersma T, Rogers D.I, González P.M, Zwarts L, Niles L.J, de Lima Serrano do Nascimento I, Minton C.D.T, Baker A.J. Fuel storage rates before northward flights in red knots worldwide: facing the severest constraint in tropical intertidal environments? In: Greenberg R, Marra P.P, editors. Birds of two worlds: the ecology and evolution of migration. Johns Hopkins University Press; Baltimore, MD: 2005. pp. 262–273. [Google Scholar]
- Piersma T, Meltofte H, Jukema J, Reneerkens J, de Goeij P, Ekster W. A single-year comparison of two methods of censusing breeding red knot and sanderling in High Arctic Greenland. Wader Study Group Bull. 2006;109:83–87. [Google Scholar]
- Prokosch P. Das Schleswig-Holsteinische Wattenmeer als Frühjahrs-Aufenthaltsgebiet arktischer Watvogelpopulationen am Beispiel von Kiebitzregenpfeifer (Pluvialis squatarola, L. 1758), Knutt (Calidris canutus, L. 1758) und Pfuhlschnepfe (Limosa lapponica, L. 1758) Corax. 1988;12:273–442. [Google Scholar]
- Prota G. Academic Press; San Diego, CA: 1992. Melanins and melanogenesis. [Google Scholar]
- Pulido F, Coppack T. Correlation between timing of juvenile moult and onset of migration in the blackcap, Sylvia atricapilla. Anim. Behav. 2004;68:167–173. doi:10.1016/j.anbehav.2003.11.006 [Google Scholar]
- Råberg L, Grahn M, Hasselquist D, Svensson E. On the adaptive significance of stress-induced immunosuppression. Proc. R. Soc. B. 1998;265:1637–1641. doi: 10.1098/rspb.1998.0482. doi:10.1098/rspb.1998.0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.G, Dobson A.P, Arneberg P, de Leo G.A, Krecek R.C. Parasite community ecology and biodiversity. In: Hudson P.J, Rizzoli A, Grenfell B.T, Heesterbeek H, Dobson A.P, editors. The ecology of wildlife diseases. Oxford University Press; New York, NY: 2002. pp. 63–82. [Google Scholar]
- Rodrigues A.A.F. Seasonal abundance of Nearctic shorebirds in the Gulf of Maranhão, Brazil. J. Field Ornithol. 2000;71:665–675. doi:10.1648/0273-8570(2000)071[0665:SAONSI]2.0.CO;2 [Google Scholar]
- Schekkerman H, Tulp I, Piersma T, Visser G.H. Mechanisms promoting higher growth rates in Arctic than in temperate shorebirds. Oecologia. 2003;134:332–342. doi: 10.1007/s00442-002-1124-0. doi:10.1007/s00442-002-1124-0 [DOI] [PubMed] [Google Scholar]
- Schieltz P.C, Murphy M.E. The contribution of insulation changes to the energy cost of molt. Can. J. Zool. 1997;75:396–400. [Google Scholar]
- Schneider D.C, Harrington B.A. Timing of shorebird migration in relation to prey depletion. Auk. 1981;98:801–811. [Google Scholar]
- Schuster C.N.J, Barlow R.B, Brockman H.J. Harvard University Press; Cambridge, MA: 2003. The American horseshoe crab. [Google Scholar]
- Serra L. Duration of primary moult affects primary quality in grey plovers Pluvialis squatarola. J. Avian Biol. 2001;32:377–380. doi:10.1111/j.0908-8857.2001.320415.x [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. doi:10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Shephard R.J, Shek P.N. Acute and chronic over-exertion: do depressed immune responses provide useful markers? Int. J. Sports Med. 1998;19:159–171. doi: 10.1055/s-2007-971898. doi:10.1055/s-2007-971898 [DOI] [PubMed] [Google Scholar]
- Sillett T.S, Holmes R.T. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 2002;71:296–308. doi:10.1046/j.1365-2656.2002.00599.x [Google Scholar]
- Silverin B, Fänge R, Viebke P.-A, Westin J. Seasonal changes in mass and histology of the spleen in willow tits Parus montanus. J. Avian Biol. 1999;30:255–262. doi:10.2307/3677351 [Google Scholar]
- Smit C.J, Piersma T. Numbers, midwinter distribution, and migration of wader populations using the East Atlantic flyway. In: Boyd H, Pirot J.-Y, editors. Flyways and reserve networks for water birds. International Waterfowl Research Bureau; Slimbridge, UK: 1989. pp. 24–63. [Google Scholar]
- Sol D, Lefebvre L, Rodríguez-Teijeiro J.D. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B. 2005;272:1433–1441. doi: 10.1098/rspb.2005.3099. doi:10.1098/rspb.2005.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers R.W, Underhill L.G, Nicoll M, Strann K.-B, Nilsen S.Ø. Timing and duration of moult in three populations of purple sandpipers Calidris maritima with different moult/migration patterns. Ibis. 2004;146:394–403. doi:10.1111/j.1474-919X.2004.00273.x [Google Scholar]
- Swaddle J.F, Witter M.S. The effects of molt on the flight performance, body mass, and behavior of European starlings (Sturnus vulgaris): an experimental approach. Can. J. Zool. 1997;75:1135–1146. [Google Scholar]
- Tomkovich P.S. An analysis of the geographic variability in knots Calidris canutus based on museum skins. Wader Study Group Bull. 1992;64:17–23. [Google Scholar]
- Tomkovich P.S. A new subspecies of red knot Calidris canutus from the New Siberian Islands. Bull. Br. Ornithol. Club. 2001;121:257–263. [Google Scholar]
- Tomkovich P.S, Soloviev M.Y. Distribution, migrations and biometrics of knots Calidris canutus canutus on Taimyr, Siberia. Ardea. 1996;84:85–98. [Google Scholar]
- Tsipoura N, Burger J. Shorebird diet during spring migration stopover on Delaware Bay. Condor. 1999;101:635–644. doi:10.2307/1370193 [Google Scholar]
- Tulp I, Schekkerman H, Piersma T, Jukema J, de Goeij P, van de Kam J. Report 61. WIWO; Zeist, The Netherlands: 1998. Breeding waders at Cape Sterlegova, northern Taimyr, in 1994. [Google Scholar]
- van Dijk A.J. Observations on the moult of the black-tailed godwit Limosa limosa. Limosa. 1980;53:49–57. [Google Scholar]
- van Gils J.A, Battley P.F, Piersma T, Drent R. Reinterpretation of gizzard sizes of red knots world-wide, emphasizes overriding importance of prey quality at migratory stopover sites. Proc. R. Soc. B. 2005a;272:2609–2618. doi: 10.1098/rspb.2005.3245. doi:10.1098/rspb.2005.3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gils J.A, de Rooij S.R, van Belle J, van der Meer J, Dekinga A, Piersma T, Drent R. Digestive bottleneck affects foraging decisions in red knots Calidris canutus. I. Prey choice. J. Anim. Ecol. 2005b;74:105–119. doi:10.1111/j.1365-2656.2004.00903.x [Google Scholar]
- van Gils J.A, Dekinga A, Spaans B, Vahl W.K, Piersma T. Digestive bottleneck affects foraging decisions in red knots Calidris canutus. II. Patch choice and length of working day. J. Anim. Ecol. 2005c;74:120–130. doi:10.1111/j.1365-2656.2004.00904.x [Google Scholar]
- Viney M.E, Riley E.M, Buchanan K.L. Optimal immune responses: immunocompetence revisited. Trends Ecol. Evol. 2005;20:665–669. doi: 10.1016/j.tree.2005.10.003. doi:10.1016/j.tree.2005.10.003 [DOI] [PubMed] [Google Scholar]
- von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. Good genes, oxidative stress and condition-dependent sexual signals. Proc. R. Soc. B. 1999;266:1–12. doi: 10.1098/rspb.1999.0597. doi:10.1098/rspb.1999.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield D.P, Brade J.J. The breeding behaviour of the knot Calidris canutus. Ibis. 1991;133:246–255. [Google Scholar]
- Wiersma P, Piersma T. Effects of microhabitat, flocking, climate and migratory goal on energy expenditure in the annual cycle of knots. Condor. 1994;96:257–279. doi:10.2307/1369313 [Google Scholar]
- Wilson J.R, Strann K.-B. The migration of red knots through Porsangerfjord in spring 2005: a progress report on the Norwegian knot project. Wader Study Group Bull. 2005;108:66–69. [Google Scholar]
- Wilson J, Rodrigues A.A.F, Graham D. Red knots Calidris canutus rufa and other shorebirds on the north-central coast of Brazil in April and May 1997. Wader Study Group Bull. 1998;85:41–45. [Google Scholar]
- Wingfield J.C. Flexibility in annual cycles of birds: implications for endocrine control mechanisms. J. Ornithol. 2005;146:291–304. doi:10.1007/s10336-005-0002-z [Google Scholar]
- Wingfield J.C. Organization of vertebrate annual cycles: implications for control mechanisms. Phil. Trans. R. Soc. B. 2008;363:425–441. doi: 10.1098/rstb.2007.2149. doi:10.1098/rstb.2007.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarts L, Blomert A.-M, Wanink J.H. Annual and seasonal variation in the food supply harvestable by knot Calidris canutus staging in the Wadden Sea in late summer. Mar. Ecol. Prog. Ser. 1992;83:129–139. [Google Scholar]
- Zwarts L, Hulscher J.B, Koopman K, Piersma T, Zegers P.M. Seasonal and annual variation in body weight, nutrient stores and mortality of oystercatchers Haematopus ostralegus. Ardea. 1996;84A:327–356. [Google Scholar]