Abstract
The annual life cycle of many birds includes breeding, moult and migration. All these processes are time and energy consuming and the extent of investment in any one may compromise the others. The output from breeding is of course the ultimate goal for all birds, while the investment in moult and migration should be selected so that lifetime fitness is maximized. In particular, long-distance migrants breeding at high latitudes face severe time pressures, which is a probable reason why natural selection has evolved efficient behaviours, physiological and morphological adaptations allowing the maximum possible migration speed. Optimal migration theory commonly assumes time minimization as an overall strategy, but the minimization of energy cost and predation risk may also be involved. Based on these assumptions, it is possible to derive adaptive behaviours such as when and at which fuel load a stopover site should be abandoned. I review some core components of optimal migration theory together with some key predictions. A review of accumulated empirical tests of the departure rule indicates that time minimization is an important component of the overall migration strategy, and hence gives support to the assumption about time-selected migration. I also briefly discuss how the optimal policy may be implemented by the bird by applying a set of simple rules. The time constraints on migrants increase with increasing body size. Some consequences of this are discussed.
Keywords: bird migration, time minimization, morphology, physiological flexibility, moult, scaling
1. Introduction
Migration is widespread in birds living in seasonal environments with fluctuating food sources and its adaptive value is well understood (Lack 1968a; Alerstam et al. 2003). Migration distance and strategies do, however, vary a great deal among species and populations, probably depending on a multitude of ecological factors. Since migration serves to take the individual from one breeding season to the next, we may expect adaptations to enhance this function. But what are these adaptations? The suite of modified characters that makes migrants better adapted for migration than resident species is often refereed to as the migration syndrome (e.g. Dingle 1996; Piersma et al. 2005). The migration syndrome includes evolutionary modifications of morphology, the acquisition of orientation and navigation skills and behavioural adjustments in relation to ecological and external factors. We may look at the migration syndrome from an engineering reductionist's viewpoint (Piersma et al. 2005), while the bird solves all its problems at once and with apparent ease. Some of the feats we consider as ‘adaptations’ may not be that, but rather exaptations if they originally evolved for another function than their current one (Gould & Vrba 1982). For instance, fat deposition occurs not only in migratory birds, but also in temperate resident species during winter to be used as energy source for long nights or insurance against periods of unfavourable weather. Be that as it may, it is clear that long-distance migration in birds requires special capacities that are of special interest to ecologists, physiologists and bioengineers.
Over the last two decades, a set of optimality analyses of bird migration have been developed (e.g. Alerstam & Hedenström 1998; Houston 1998), with roots in foraging theory (Stephens & Krebs 1986). This set of optimality analyses together with predictions about flight behaviour derived from flight mechanics will be referred to as ‘migration theory’ in this paper. This theory has reached a stage of maturity that now allows an evaluation of some of its assumptions and experimental tests of predictions derived from it. A cornerstone in migration theory is the assumption of an appropriate surrogate currency. In optimal migration models, it is often assumed that the minimization of time is the most relevant currency and that natural selection favours those traits that maximize the overall migration speed (Alerstam & Lindström 1990). Alternative currencies are energy and mortality risk or some combination of different currencies (Houston 1998). I will evaluate the evidence that has accumulated for or against the notion of time-minimized migration. In the context of optimality models, it is also important to consider relevant constraints that may affect the solution for an ‘optimal’ behaviour (Stephens & Krebs 1986). Body size is a potential constraint regarding many biological functions, and so we should expect effects of size on certain aspects of migratory performance. Similarly, over evolutionary time, we may expect selection of migration performance to affect the body size of migrants. Finally, optimality solutions tell us what is optimal and what an animal should do, but it does not mean that animals arrive at the optimal solutions in the same way as we do. To understand how migration is implemented, we may therefore also look for simple mechanisms, or so-called ‘rules of thumb’ that implement an acceptably good approximation to an optimal solution (e.g. McNamara & Houston 1980; Stephens & Krebs 1986; Wehner 1998). In this paper, I will present some key components of simple optimality models of bird migration and briefly discuss what the relevant optimization currency applicable to migration should be and how birds actually implement optimal behaviours. This paper deals with simple optimization models about migration; migration strategies and annual routine models of migration and moult have also been developed using state-dependent optimization. For those interested in state-dependent models of migration, please refer to Barta et al. (2008) and McNamara & Houston (2008).
2. Some basic equations
When a migrant bird is depositing fuel, this can conveniently be viewed as the accumulation of potential flight range. Let f be the relative fuel load, relating the lean body mass m0 to body mass including fuel as m=(1+f) m0. The potential flight range is a diminishing return function (derived from the Breguet (1922) equation) of added fuel mass owing to increased flight costs due to the added mass and drag, and if the added fuel increases the body drag through an increased body frontal area in direct proportion to the fuel load, the range is
| (2.1a) |
where the constant c includes factors related to fuel composition, muscle work efficiency, bird shape and wind condition (Alerstam & Lindström 1990; Alerstam & Hedenström 1998). In cases where added fuel does not affect the drag, or when the range is based on the assumption that fuel consumption is a constant proportion of the mass, an alternative range equation is
| (2.1b) |
where k is the rate of mass loss (dm/dt=km) and U is the flight speed (Alerstam 1981).
Another useful relationship is that between power required to fly and forward speed through the air. In a simple form, it can be expressed as the sum of three power components: induced, parasite and profile power, representing the cost of generating lift, overcoming the drag of the body and wings (Pennycuick 1989; Hedenström 2002). If U is the airspeed, then the power equation is
| (2.2) |
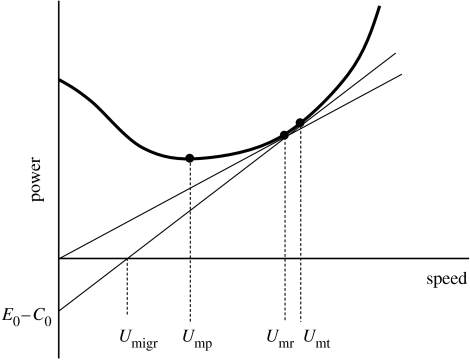
where α, β and γ include different physical and morphological properties of air and the animal. This function has a characteristic U-shape (figure 1). From this equation, different characteristic speeds can be derived that are applicable in different ecological situations such as display, foraging and migration flight (Hedenström & Alerstam 1995).
Figure 1.
Power required for a bird to fly by horizontal flapping flight in relation to speed through the air. Characteristic speeds are minimum power speed (Ump), maximum range speed (Umr) and speed of time-minimization migration (Umt). The negative ordinate shows the rate of fuel deposition (E0−C0) increasing downwards. The tangents show how Umr and Umt can be derived graphically. The point where the tangent associated with Umt intersects the speed axis is the overall migration speed (Umigr), which increases with increasing rate of fuel deposition.
3. Currencies of migration
It is commonly assumed that lifetime reproductive success is a measure close to true fitness. However, this measure is impractical in most situations when evaluating behavioural strategies in migrating birds, and therefore some more immediate currencies are often used as a surrogate for fitness. A prerequisite for this approach to be successful is that true fitness is a function of the surrogate currency, which a priori is never guaranteed. The maximization of survival from one breeding season to the next is probably a major reason for migration to occur in the first place (Lack 1968a), and hence survival per unit migration distance is one possible currency (Alerstam & Lindström 1990). Even if the mortality rate may be high during migration, it may still be a better strategy to migrate than to over-winter at a temperate or Arctic breeding site, especially in birds whose food source dwindles in the winter. Given that the majority of annual mortality occurs during migration (Sillett & Holmes 2002), survival could be maximized by minimizing the overall time of migration and hence the exposure to danger. Alternatively, an economical use of energy could lead to reduced exposure to predation during foraging, or reducing the exposure to food-related parasites, and hence an energy minimization strategy may be optimal. In the following paragraphs, I will present some simple currencies often used when evaluating migration strategies.
(a) Migration speed
Long-distance migration typically involves several cycles of fuelling and transportation, and so the overall migration speed is the migration distance divided by the time for fuelling and flight. The stopover time to build up fuel, Tfuel, that covers a migration distance D is Tfuel=PflightDU−1(E0−C0)−1, where Pflight is the rate of energy consumption during flight (equation (2.2)), U is flight speed, E0 is the rate of energy accumulation at stopovers, C0 is the rate of energy consumption when fuelling (Hedenström & Alerstam 1995). The flight time is Tflight=D/U. Dividing the migration distance by the migration time Tmigr (=Tfuel+Tflight) yields the migration speed
| (3.1) |
Migration speed according to equation (3.1) must not be confused with flight speed U, which is a component of the overall migration speed. Migration speed is the flight speed multiplied by a factor that represents the fraction of the total time spent in flight (Alerstam 2003). Maximizing the speed of migration is synonymous with minimizing the time of migration, hence the term time-selected migration (sensu Alerstam & Lindström 1990). A high migration speed is achieved by a high flight speed, but the rate of energy consumption in flight (henceforth power) increases steeply with increasing speed (equation (2.2); Pennycuick 1975; Hedenström 2002; Tobalske et al. 2003). The optimum flight speed, Umt (mt for minimum time), associated with time-selected migration is illustrated in figure 1. The overall migration speed is where the tangent from an extended ordinate, representing the net fuelling rate, intersects the speed axis. Migration speed is also increased by an increased net energy accumulation rate. Migratory birds are likely to be constrained by their metabolic capacity to process ingested food, such as the maximum rate for assimilating energy (Kirkwood 1983; Hammond & Diamond 1997; Kvist & Lindström 2003). The deposition rate of fuel energy (Edep) is the surplus from the gross energy intake rate (E) minus the energy expenditure when foraging (C) and the non-foraging energy expenditure (A), and is written as
| (3.2) |
The metabolic ceiling (K) constrains the foraging time to t=K/E, which when inserted into equation (3.2) yields
| (3.3) |
which is maximized if the ratio E(C−A)−1, a currency termed the foraging gain ratio by Hedenström & Alerstam (1995), is maximized. The same currency is also expected to yield the best result in other foraging situations when an animal is restricted by a metabolic ceiling (Ydenberg et al. 1994; Houston 1995) or when meeting an energy requirement below the metabolic ceiling (Hedenström & Alerstam 1995; Nolet 2002).
(b) Energy cost of migration
The overall energy cost of migration (Emigr) is the gross energy intake rate (E) multiplied by the total stopover duration (Tfuel, see above), which includes maintenance energy costs during stopover and the surplus energy accumulated as flight fuel, hence
| (3.4) |
A minimum total energy cost of migration is achieved if the ratio E0/C0 is maximized, which is the overall stopover gain ratio (Hedenström & Alerstam 1995). If we assume that the cost during stopovers is divided into foraging activity at a rate C and non-foraging metabolic rate A, then the total stopover energy cost is
| (3.5) |
and with we can write the stopover gain ratio for a bird that is not metabolically constrained as
| (3.6) |
Most birds are probably restricted to foraging during approximately half of the stopover owing to the light/dark periods of the day, and so with t=0.5 the foraging gain ratio defined as E(C+A)−1 will maximize the stopover efficiency. Obviously, this is accomplished by low foraging and non-foraging costs. In situations where the foraging time approaches 1 or 0, the relevant gain ratio and the gross energy intake rate are EC−1 and E, respectively (Hedenström & Alerstam 1995). Concerning the flight behaviour, equation (3.4) implies that the ratio P/U should be minimized, which is achieved at the maximum range speed Umr (figure 1).
If the migrant operates at a metabolic ceiling, then E0=K=Et, which combined with equation (3.5) results in stopover efficiency expressed as
| (3.7) |
Again, stopover efficiency is maximized when the foraging gain ratio E(C−A)−1 is maximized.
4. Stopover behaviour
The optimal stopover duration and the associated fuel load can be derived using equation (2.1a) and (2.1b). The maximum speed of migration is achieved when
| (4.1) |
where Y is the flight range according to equation (2.1a) and (2.1b), f0 is the search/settling energy cost, t0 is the search/settling time cost and t is the duration of fuel deposition at a stopover (Alerstam & Hedenström 1998). Search/settling costs are assumed to arise upon arrival at a new stopover site because the bird is initially unable to locate food sources at new (unfamiliar) sites, or because there is a queue to get access to temporary stopover territories required for efficient fuelling. Let f=kt and thus df/dt=k, where k is the daily fuel deposition rate (FDR) expressed as a proportion of m0, which substituted into equation (4.1) yields
| (4.2) |
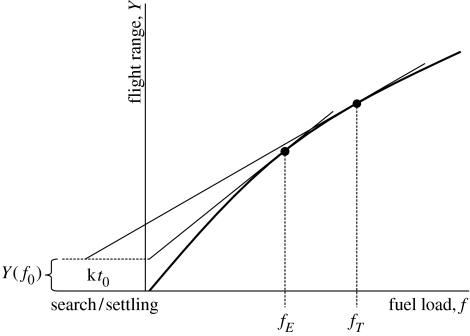
This criterion can be graphically illustrated as shown in figure 2, where it is evident that the optimum departure load depends on the three parameters f0, t0 and k.
Figure 2.
Gain in flight range with increasing relative fuel load. For a certain relative FDR (k), search/settling time (t0), and initial fuel loss (f0), the optimal departure fuel load of time-minimization migration (global variation) is given by constructing a tangent from the point (kt0, Y(f0)) to the gain curve to yield departure load fT. If t0=0, the optimal solution is identical to the minimization of energy cost of transport and the associated departure fuel load is fE. Adapted from Alerstam & Hedenström (1998).
The optimal fuel load associated with minimum energy cost of transport can be obtained from equation (4.2) by setting k=0, or graphically as shown in figure 2. The optimum fuel load associated with time-selected migration is always higher than for energy-selected migration (figure 2). The minimization of the total energy cost of migration involves an element of time, because extended stopover times increase the overall energy consumption during migration (Hedenström & Alerstam 1997); as a result, fuel loads are intermediate between time minimization and minimum energy cost of transport.
The first model presented by Alerstam & Lindström (1990) assumed that birds would react to food availability and concomitant increased FDR as if this new rate would apply for the current site only (local variation), while the expected subsequent FDR would remain unaffected by local variations like experimentally supplemented food (the field test paradigm). This assumption leads to rather steep slopes of the predicted relationship between departure fuel load and FDR (Lindström & Alerstam 1992). Instead, if the bird updates its future expectation according to the experienced FDR at the current site (global variation), then the slope between predicted fdep and k will be lower than with local variation (Houston 1998; Weber et al. 1999). Note that the optimal departure loads derived from the graphical construction in figure 2 refer to global variation.
Another way of viewing the departure decision is by deriving an expression for the instantaneous migration speed. By noting that f=kt and considering the range equation as the accumulation in flight distance as fuel is loaded at a stopover, the instantaneous speed of migration is
| (4.3) |
where symbols are as defined before (e.g. Alerstam & Hedenström 1998). This function is declining over time and the bird should depart when S has dropped to the overall expected speed of migration along the route (Lindström & Alerstam 1992).
5. Scaling of migration speed and distance
Depending on flight mode, equation (2.2) evaluated in relation to body size gives differing trends of migration speed (Hedenström & Alerstam 1998; Hedenström 2003a). For flapping flight, the migration speed scales as
in allometrically scaled (real) birds, while soaring flight yields the scaling
Hence, there is a certain body size where the migration speed is equal for the two flight strategies, given the fuelling rate and rate of climb in thermal soaring. These scaling proportionalities also imply that given one of the alternative flight strategies has been adopted, there should be selection for reduced and increased body size for flapping flight and soaring flight migration, respectively.
Given a migratory species breeding annually in a seasonal environment, the main time-consuming processes will sum up to 1 year as follows:
| (5.1) |
The first two terms on the right-hand side of equation (5.1) scale positively with body size and so does Tmigr (which equals D/Umigr) in flapping flight migration. Whether there are any other activities that require time during the annual cycle is unclear, but they should also scale positively with body size. Imposing the requirement T≤365 days, the available time for migration will decrease with increasing size, and hence for flapping flight the potential migration distance will decrease with increasing size. Given a migration distance D, it is possible to calculate the critical body size for which the first three terms on the right-hand side of equation (5.1) equals 1 year. In the three swan species in Europe, the migration distances are in reverse order of the body size, perhaps an effect of limitations due to migration speed (Hedenström 2006).
In soaring flight, body size does not impose the same strong restriction, although time for breeding and moult may last for such long times that annual breeding is not possible (Lack 1968b; Jouventin & Dobson 2002).
In isometrically scaled birds, the flight range is directly proportional to the fuel load (Pennycuick 1975), while the maximum relative fuel-load (expressed as proportion of lean body mass) capacity declines with increasing body size (Hedenström & Alerstam 1992). Real birds compensate for this to some degree by having relatively longer wings than isometrically scaled birds (Rayner 1988), but this is far from compensating for the reduced fuel-loading capacity. This means that the potential non-stop flight range is progressively circumscribed with increasing body size.
6. Realized migration speed
Migration speeds as recorded by ringing recoveries and satellite telemetry in a selected set of species using different flight strategies are shown in table 1. Among powered flyers, maximum speeds are achieved by the medium–small birds of body mass approximately 0.1 kg with overall travel rates approximately 200 km d−1. In smaller long-distance migrating songbirds, the travel rate is only half or even less than that, and in short-range or partial migrants it is still lower. In the 6.5 kg Bewick's swan (Cygnus columbianus), the migration speed is similarly very low (26 km d−1), as expected from the scaling relationship (§5). For flapping flight, it thus seems as if the fastest migrants are relatively small (0.1 kg) birds with relatively high aspect ratio (AR=b2/S, where b is wing span and S is wing area; a high value of AR means a long and slender wing) wings resulting in low cost of flight. Other factors that result in different migration speeds are nocturnal versus day migration (Hildén & Saurola 1982), whether birds are short-/long-distance migrants (Alerstam 2003), or the seasonal timing of migration (Alerstam & Lindström 1990). Some of the differences between species may arise due to common factors rather than specific ecological circumstances, such as shortened day length with the progress of the autumn and the fact that migrants bound for the tropics migrate early in the season resulting in relatively fast migration speed, while short-distance migrants move late in the season resulting in a relatively slow migration speed. In the case of nocturnal migrants, flights are performed in ‘free time’ that could usually not be used for feeding, while day migrants must use potential foraging time for migratory flights.
Table 1.
Migration speed for a selected sample of bird species.
| species | scientific name | season | age | body mass (kg) | flight modea | speedb (km d−1) |
|---|---|---|---|---|---|---|
| Bewick's swan | Cygnus columbianus | spring | 6.0 | f | 26 | |
| red knot | Calidris canutus | autumn | 0.134 | f | 175 | |
| Arctic tern | Sterna paradisaea | autumn | 0.11 | f | 200 | |
| swift | Apus apus | autumn | 0.040 | f | 150 | |
| wheatear | Oenanthe oenanthe | autumn | 0.023 | f | 110 | |
| willow warbler | Phylloscopus trochilus | autumn | 0.009 | f | 84 | |
| goldcrest | Regulus regulus | autumn | 0.005 | f | 57 | |
| blue tit | Parus caeruleus | autumn | 0.013 | f | 17 | |
| lesser-spotted eagle | Aquila pomarina | autumn | 1.5 | ts | 133 | |
| honey buzzard | Pernis apivorus | autumn | ad | 0.8 | ts | 163 |
| juv | ts | 104 | ||||
| Swainson's hawk | Buteo swainsoni | autumn spring | 1.0 | ts | 188 | |
| ts | 150 | |||||
| peregrine | Falco peregrinus | autumn | 0.7 | ts/f | 172 | |
| spring | ts/f | 198 | ||||
| grey-headed albatross | Thalassarche chrystostoma | 3.5 | ds | 880 |
f, flapping flight; ts, thermal soaring; ds, dynamic soaring.
When external energy provided by thermals for cross-country soaring or wind gradients for dynamic soaring is used, migration speed approaches 200 km d−1 even for quite large birds using thermal soaring and surpasses this by far in albatrosses (table 1).
7. What is the evidence for time-selected migration?
Ever since optimality reasoning was introduced more explicitly to the analysis of migration strategies (Alerstam & Lindström 1990), some effort has been made to test predictions based on time minimization. Modifications of the baseline predictions have successively been added to the theory in order to improve the agreement between theory and data (Alerstam & Hedenström 1998; Houston 1998). The predictions mainly fall into three categories: (i) flight behaviour, (ii) stopover behaviour and flight distances, and (iii) physiological adaptations.
(a) Flight behaviour
In time-selected migration, the optimal flight speed should be greater than the maximum range speed associated with energy-selected migration, Umt>Umr, where the magnitude of this difference depends on the rate of energy deposition (figure 1). It has not yet been possible to distinguish between Umt and Umr by direct flight speed measurements, mainly because speeds vary quite a lot and depend also on other factors such as body mass (usually unknown) and winds. Another reason could also be that the selection of accurate choice of speed in the neighbourhood of Umr/Umt is not as strong as near Ump (Houston 2000). Skylarks Alauda arvensis clearly select a speed above Ump during migration and the mean speed (14 m s−1) was larger than calculated Umr (10 m s−1), indicating the possibility of a time-selected flight strategy (Hedenström & Alerstam 1996). Testing predictions about flight behaviour is fraught with uncertainties due to unknown parameters such as rate of energy accumulation and uncertainties in flight mechanical theory itself. A related problem concerns the predicted flight speed (above Umr) in birds transporting energy to a central place (Norberg 1981), which also lacks empirical support in spite of a more tractable experimental situation. We may have to accept that selection of accurate choice of speed is not strong enough in the neighbourhood of Umr to result in fine-tuned behavioural differences in response to small variations in energy deposition rate (Pennycuick 1997; Hedenström & Alerstam 1998; Houston 2000).
Cross-country soaring is limited to the daytime hours when thermal activity provides raising air, which is used by soaring birds. Thermals are typically available for about 8 hours in temperate regions (Konrad 1970; Rowland 1973) and not much longer in the tropics. In situations where soaring migrants experience a positive energy budget and yet are able to soar for the entire thermal period, a time minimizer is predicted to continue by flapping flight outside the thermal period until the surplus energy is consumed (Hedenström 1993). Observations of nocturnal migratory flight in otherwise typical soaring migrants therefore provide support for a time-selected strategy (Stark & Liechti 1993).
(b) Stopover behaviour
A central prediction for time-selected migration is that departure fuel load (fdep) depends on the rate of fuel deposition (k, %LBM d−1). This prediction has been tested on nine occasions by providing additional food at stopover sites, followed by estimating k and the stopover duration for individual birds and in one case by monitoring natural variation in k (table 1). In seven studies (out of nine), there was a significant positive relationship between fdep and k, which was also the case for the whitethroat (Sylvia communis) in an augmented dataset (Weber et al. 1999). In one study of spring migrating wheatears Oenanthe oenanthe, it was only the males that showed a significantly positive relationship while females departed with the same fdep irrespective of k (Dierschke et al. 2005), suggesting a time-selected strategy in males and an energy minimization strategy in females. In the robin (Erithacus rubecula) study, the experimentally fed birds departed with larger fuel reserves than non-fed birds, suggesting a time-minimization strategy (Dänhardt & Lindström 2001). The slope of the relationship between fdep and k is invariably lower than that predicted under local variation (see above). Instead, if birds update their expected migration speed according to their current experience (global variation), then the slopes observed are close to the prediction (see references in table 2). In energy-selected migration, there is no predicted relationship between fdep and k (Hedenström & Alerstam 1997), which was generally not the case in the tests (table 1). If birds are minimizing the total energy cost of migration, the expected slope between fdep and k is lower than under time-selected migration and global variation. This currency however includes an element of time, and may be difficult to distinguish from purely time-selected migration. In conclusion, there is strong support for a time-selected migration from experimental field tests of departure fuel loads in migratory song birds.
Table 2.
Summary of studies testing the effect of FDR (k, %LBM d−1) on departure fuel load (f) in small migratory birds. (m0 is lean body mass. A ‘+’ means that a significant relationship was found as predicted from a time-minimization migration policy, while a ‘0’ indicates no significant relationship. nat, natural; exp, experimental.)
| species | m0 (kg) | nat/exp variation in k | f (k) | source |
|---|---|---|---|---|
| Selasphorus rufus | 0.003 | nat | + | Carpenter et al. (1983) |
| Luscinia svecica | 0.016 | exp | + | Lindström & Alerstam (1992) |
| Erithacus rubecula | 0.014 | exp | 0 | Dänhardt & Lindström (2001) |
| Sylvia communis | 0.014 | exp | 0 (+) | Fransson (1998), Weber et al. (1999) |
| Acrocephalus scirpaceus | 0.0095 | exp | + | Bayly (2006) |
| Acrocephalus schoenobaenus | 0.010 | exp | + | Bayly (2007) |
| Oenanthe oenanthe, males | 0.023 | exp | + | Dierschke et al. (2005) |
| Oenanthe oenanthe, females | 0.023 | exp | 0 | Dierschke et al. (2005) |
| Oenanthe oenanthe | 0.021 | exp | + | Schmaljohann & Dierschke (2005) |
| Oenanthe oenanthe | 0.023 | exp | + | Delingat et al. (2006) |
In many birds, stopover sites occur as discrete patches in an otherwise non-useful habitat. For such birds, the migration is divided into a few long flights between a subset of the available stopover sites. Which of these should be used can be analysed by using the instantaneous speed of migration (equation (4.3)), together with information about the FDRs (ki) at the sites. A bird should leave for the next site if S(k1, Y=D)≤S(k2, Y=0), where k1 and k2 are fuelling rates at two consecutive sites, Y is the current potential flight distance using accumulated fuel and D is the distance between the sites (Alerstam & Hedenström 1998). Depending on the relative values of the ki:s among the potential route sites, it may be advantageous to accumulate more fat (overload) than necessary for the flight to the next site, a situation that may occur during spring migration when southern sites provide more food than phenologically earlier sites further to the north. Overloading should occur when S(k1, Y)=S(k2, Y−D) and Y>D, where the expected overload can be solved by inserting f(Y) into this equation (Alerstam & Hedenström 1998). Implicit in this scenario is also the bypassing of potential stopovers providing FDRs that are too low. An interesting theoretical observation is that overloading will not occur if range equation (2.1b) is the correct one, while with equation (2.1a) it is predicted to occur (Weber & Houston 1997). Overall, overloading is expected only under a rather restricted range of conditions (Weber et al. 1994). Even if many birds, e.g. shorebirds, do arrive at stopovers with some surplus energy, it remains to be demonstrated that overloading according to time-selected migration is the actual cause. Alternative reasons for accumulating more energy than actually required to fly between consecutive stopovers could include the need for insurance against unpredictable head/cross wind conditions during the flight or unpredictable feeding conditions at the destination.
A related phenomenon may be seen in birds approaching their breeding site. Early arrival and onset of breeding is usually considered advantageous (Kokko 1999). Pre-laying activities such as display and song require energy, and nest building and the formation of eggs also similarly require energy. Hence, it may be advantageous for birds to accumulate this energy (or part of it) at the final stopover site, and thus arrive with an energy capital (hence the term ‘capital breeders’; Drent & Daan 1980). If a fuel load f0 is required at arrival for breeding activities, f1 is the fuel required for the flight only, then it will be optimal to depart with a fuel load f2 if
where k1 and k2 are the FDRs at the final stopover site and the breeding site, respectively (Gudmundsson et al. 1991; Hedenström 2006). This phenomenon occurs in certain arctic-breeding geese and eiders (Meijer & Drent 1999) but to much less degree in shorebirds (Klaassen et al. 2001), although there may be variation between years (Morrison & Hobson 2004). If brent geese Branta bernicla encounter headwinds during spring migration, they will consume more fuel during the migration than in neutral or following winds, with measurable consequences for their breeding success (Ebbinge 1989).
(c) Physiological flexibility
It has become clear that migratory birds undergo considerable physiological changes during fuelling and flight. Not only do fat loads change, but also nutritional organs and flight muscles vary in mass during the fuelling–flight cycle (e.g. Piersma 1998; Buehler & Piersma 2008). Increased gizzard, stomach, intestine and liver may serve to increase the metabolic capacity and energy assimilation rate and thus the rate of fuelling. Shortly before departure on (long) migratory flights, parts of the nutritional organs may be discarded to reduce the payload, and hence flight cost, and thereby increase flight range. The flight muscles change in parallel with overall body mass (e.g. Lindström et al. 2000), but the increase does not match the increase in power requirements in the red knot Calidris canutus (Dietz et al. 2007). When birds are very fat, the power margin is reduced compared with when they are lighter, suggesting that they are trading escape flight capacity (predation risk) against migratory flight economy. Flight muscle mass declines during long flights (e.g. Battley et al. 2000), and it has been suggested that some protein breakdown may be necessary to serve as intermediates of the citric acid cycle and for maintenance and repair of organs (Jenni & Jenni-Eiermann 1998). Sustained flight causes damage to the flight muscles and protein could serve as repair substrate (Guglielmo et al. 2001). In an optimization model, Weber & Hedenström (2001) showed that organ dynamics of the kind presented here is mainly reconcilable with time-minimizing migration or minimization of total energy cost of migration, which is also a function of time. Nutritional organ flexibility is expected mainly in association with long migratory flights. The reduction of nutritional organs before long flights may be seen as a way of increasing the flight range and thereby the probability of successful migration, especially during long transoceanic flights. This may be crucial in species such as the bar-tailed godwit (Limosa lapponica) where the flight distance is close to the limit. However, the build-up of these organs before episodes of fuelling must be interpreted as support for a time-selected strategy.
8. Morphology
Since flight is costly, there should be adaptations in the flight apparatus, i.e. wings and musculoskeletal systems, that reduce these costs. Aerodynamic theory provides predictions regarding wing and tail morphology (Rayner 1988; Thomas 1993). Migrants generally have wings of higher aspect ratio and more pointed wing tips than residents (Mönkkönen 1995; Lockwood et al. 1998; Voelker 2001), and the tails tend to be short and square rather than long and graduated (Leisler & Winkler 2003). In addition, more subtle differences occur, for example the notch of the inner web of primaries is shorter in migrants than residents (Winkler & Leisler 2005). Comparison also showed that migrants have smaller and flatter skulls than residents (Winkler & Leisler 2005), which was paralleled in measures of brain volume (Sol et al. 2005). This has been interpreted as an adaptation in residents for increased innovative capacity needed to cope with a seasonally changing environment (Sol et al. 2005), while migrants would rely on more stereotyped behaviours and migratory programmes. Why migrants would be disfavoured by the same innovative capacity when constantly visiting new habitats is perhaps not so obvious. Many species revisit the same route and stopover sites in successive migrations (e.g. Alerstam et al. 2006), which would require some spatial memory capacity and navigation skills. In line with this, it has been hypothesized that migrants should have an enlarged hippocampal region, a region known to be involved in processing spatial information, compared with residents. There was no apparent difference in hippocampus volume between migrants and residents (Healy et al. 1991), although it increased with age and experience in a migratory passerine but not in a resident relative (Healy et al. 1996).
A more general prediction is that migrants using flapping flight should be favoured by small overall body size (Hedenström & Alerstam 1998), which has gained some empirical support (Sol et al. 2005). Apparently, migrants exhibit a suite of morphological modifications compared with residents. Body size and wing morphology are easily interpreted in terms of aerodynamic efficiency and migration speed, but there may be similar adaptations in relation to habitat that could confound the adaptive explanation (Leisler & Winkler 2003). The explanation for the smaller forebrain in migrants, or rather enlarged brain in residents for improved innovative capacity, remain a speculation.
9. Moult
Feathers get worn by use and exposure to light, which affects their aerodynamic and insulatory function (Hedenström 2003b; Williams & Swaddle 2003). Therefore, the plumage is replaced periodically through a process known as moult. Moult resets the function of the feathers, but has direct energy costs from synthesizing new feathers (e.g. Lindström 1993) and indirect costs caused by the gaps due to missing flight feathers (Rayner & Swaddle 2000). Moult also takes time and has to be fitted into the annual cycle with respect to breeding and migration, with which it normally does not overlap. In principle, moult could overlap with migration if the reduced migration speed due to elevated costs of moult is balanced by the time saved by not having to remain stationary for moulting. Some birds may moult during migration, but moult is then slow and involves few feathers and relatively small wing gaps (Holmgren et al. 1993). The main reason for avoiding a moult/migration overlap is probably the increased predation risk associated with moult during a period when mortality rate is high anyway (Sillett & Holmes 2002).
The two main moult patterns are either post-breeding moult while still in the breeding area or a post-migration moult in the wintering area (Jenni & Winkler 1994). There is a general trend for moult to be more likely to occur after autumn migration in the winter quarters as migration distance increases (Svensson & Hedenström 1999; Hall & Tullberg 2004). Populations breeding at northerly latitudes, with increased time stress, tend to interrupt post-breeding moult more often than southern conspecifics (Swann & Baillie 1979; Hedenström et al. 1995). Similarly, late breeders among American redstarts Setophaga ruticilla initiate southbound migration but stop for moult during migration (Norris et al. 2004). This has been taken further in the great reed warbler Acrocephalus arundinaceus, which stops for moult immediately after reaching the savannah belt south of the Sahara and then commences migration further to the south (Hedenström et al. 1993). Variations to the most common moult patterns involve biannual moult, i.e. two complete moults annually (Underhill et al. 1992), split moult between seasons (Lindström et al. 1993), or the moult of a few primaries at intermediate sites during migration (Pearson & Backhurst 1983). The variation in moult–migration patterns is quite large and the adaptive values are far from understood.
There are differences in the mechanical properties of feathers depending on whether they have grown during summer or winter (Weber et al. 2005), which are likely to affect the selection of moult strategy. Moult during winter usually takes longer than post-breeding moult in the summer (Underhill et al. 1992), which could be due to constraints in obtaining resources or, alternatively, be due to relaxed time constraints in completing the moult. Prolonged moult may result in the production of higher-quality and more durable feathers that resist wear better than feathers that have grown faster (Serra 2001). In addition, experimentally time-stressed lesser whitethroats Sylvia curruca speeded up moult and grew shorter primary feathers (Hall & Fransson 2000), which is likely to be penalized in terms of a reduced subsequent migration speed. The scheduling of moult in a migratory species is probably the result of many factors, which makes it tractable for state-dependent optimization approaches (Holmgren & Hedenström 1995; Barta et al. 2006a,b).
10. Discussion
Development of optimal migration theory has provided a framework for understanding and testing how different factors influence migration performance. This theory allows the derivation of a number of testable predictions regarding the migration process. As with evolutionary optimization theory in general (Parker & Maynard Smith 1990), the consistency and validity of the theory can be tested by evaluating the applicability of key assumptions, or as tests of more specific predictions derived from the theory. In the case of migration, the theory provides differing predictions if migration is selected to minimize time, energy or predation risk (Alerstam & Lindström 1990). It could be argued that migration serves the function of maximizing individual survival between consecutive breeding seasons, implying that the minimization of predation risk is the appropriate currency. This notion is supported by the fact that migration is responsible for more than 85% of the annual mortality in a passerine long-distance migrant (Sillett & Holmes 2002). However, all mortality during migration is unlikely to be due to direct predation, but due to other failures as well. The review of empirical tests about stopover behaviour (departure fuel load; table 2) showed that time minimization is at least a significant component of the migration strategy. The true currency could be a composite function of several variables, for example time and predation risk minimization, which can be analysed as illustrated by Houston (1998). However, recent data from wheatears showed that they experienced reduced fuelling rates in relation to the density of predators, but this did not affect their departure decision away from the optimum under a time-minimizing policy (Schmaljohann & Dierschke 2005). The conclusion is therefore that time minimization (equivalent to speed maximization) or the minimization of total energy is a reasonable first-order single currency in bird migration.
A related problem concerns the appropriate currency for foraging behaviour during stopovers. Time minimizers should maximize their FDR, which is achieved by a high rate of food intake. One way of achieving this is by operating food processing at the metabolic ceiling and simultaneously minimizing the foraging energy costs. This, in turn, leads to the maximization of the foraging gain ratio as the best policy (equation (3.3); Hedenström & Alerstam 1995; Houston 1995). That birds actually comply with this rule has been shown for red knots C. canutus (Gils et al. 2003). Hence, the fact that migratory birds seem to ingest and process food at their maximum capacity is itself an indirect indication that they are time minimizers. However, in many cases, a high food intake rate at a stopover may also be associated with a high predation risk. Migratory birds fuelling under a predation risk should forage in a risk-prone way (Bednekoff & Houston 1994). Risk proneness has been observed during migratory fuelling in the yellow rumped-warbler Dendroica coronata, where the birds preferred the high-variation food source (Moore & Simm 1986).
The energy budget of migration can be subdivided into the actual transportation costs and the maintenance and activity costs during stopovers. By rearranging equation (3.4), the ratio between migratory flight costs and stopover energy expenditure can be written as 1/(C0/(E0−C0)), which evaluates to approximately 1 : 2 for a small passerine (Hedenström & Alerstam 1997). Wikelski et al. (2003) measured energy costs in free-flying Catharus thrushes using the doubly labelled water technique and obtained the ratio 1 : 2.4 between flight and stopover energy cost, which is in broad agreement with the prediction. Similarly, the time divided between flight and stopover was predicted as 1 : 7 for a small passerine (Hedenström & Alerstam 1997), a prediction that also enjoys empirical support (Fransson 1995).
During migratory flight, birds should select a cruising speed associated with minimum energy cost of transport (Umr) or maximum speed of migration (Umt). There is observational support for the selection of either of these two speeds, rather than the lower minimum power speed (Ump), but distinguishing between Umr and Umt is difficult considering the many factors that also influence the optimal speed (e.g. winds, body mass, flocking). Birds generally adjust their airspeed in relation to head/tail winds (Hedenström et al. 2002), which should occur only when flying at Umr or Umt but not at Ump. Hence, there is agreement between theory and data at different levels of what we can loosely call migration theory, suggesting that it is a consistent scientific platform that is useful for studying many aspects of bird migration.
Since migrating birds behave in many respects as if informed by optimal migration theory, a relevant question is how they compute the solution to different strategic problems. They hardly use calculus as we do and they definitely do not use Matlab. We may therefore look for more simple rules of thumb that provide sufficiently good approximations to the actual optimal policy. Erni et al. (2002) proposed that birds should stay at a stopover site (i) until a certain fuel load is reached or (ii) for a constant duration. Simulations of migratory journeys following these rules showed that rule (2.2) resulted in only slightly longer migration than under the optimal time-minimization strategy. Reed warblers Acrocephalus scirpaceus at a stopover showed quite constant stopover durations, although supplemented food resulted in shorter stopover time as the FDR increased (Bayly 2006). On the basis of tracking of individual Catharus thrushes, Cochran & Wikelski (2005) found that migratory departures were triggered by certain circumstances, including a threshold fat load, a daily maximum temperature of 21°C or more and weak surface winds. Other studies of passerine departures have found that departure is more likely on occasions with tail winds (Åkesson & Hedenström 2000) and when weather conditions allow the observation of celestial cues for accurate orientation (Åkesson et al. 2001). Hence, it seems probable that departure is triggered by some minimum fuel load and further conditioned by local weather and geographical factors. Note that the current winds change the exchange rate of fuel into distance, which can be accommodated within a time-minimization strategy (Weber et al. 1998a). In birds having a relatively low energy cost of transport, such as large raptors using thermal soaring, and where refuelling opportunities are limited along the flight route, the best strategy may be not to be wind sensitive and migrate irrespective of wind direction, at least with moderate wind strengths (Thorup et al. 2006). Potential sets of rules implementing an overall migration strategy can be evaluated by state-dependent optimization (Weber et al. 1998b). Further studies involving the tracking of individual birds are likely to give insights into how rigid/flexible behaviours are during migration and which set of rules are likely to be used in order to achieve an efficient migration strategy.
Migratory birds are guided through the annual cycle by genetic programmes, responsive to external inputs (‘zeitgebers’) for calibration and fine-tuning of events (e.g. Gwinner 1996). Such inherited instructions are often claimed to be part of the migration syndrome, even though resident populations may also show migratory restlessness (Helm & Gwinner 2006). Populations of differing migratory habits appear to respond differently to external cues (e.g. shortened day length), with long-distance migrants breeding at high latitudes showing less flexibility than non-migrants at equatorial latitudes (Helm et al. 2005). This could be an insurance adaptation in migrants to get away from deteriorating ecological conditions in time, but it also shows that residents are ‘pre-adapted’ to develop migration or, alternatively, that they have evolved from migrants and retain certain characteristics from their migratory past. Be that as it may, the presence of migratory feats also in residents casts doubts on whether there are migration syndromes unique to migrants (Piersma et al. 2005). Perhaps migration is the original characteristic of all birds and the differences we see between migrants and residents are only the loss of some adaptations among residents when not needed any longer. The reason for such changes when a migrant becomes resident could be that the overall selection regime from demands on efficient migration and exploitation of the habitat during non-migratory seasons changes. The resident experiences relaxed selection for migration performance to an emphasis on purely selection in relation to its habitat. In this sense, the migrant could be seen as an evolutionary compromise, while residents are better adapted to their habitats.
The annual cycle and the scheduling of different activities differ a lot between populations and species of different migratory habits. The time divided between migration, moult and breeding can be illustrated by a real example. The willow warbler Phylloscopus phylloscopus has two annual moults, which take approximately 40 and 60 days, respectively (Underhill et al. 1992). Populations that breed in Finland migrate 9500 km to their African winter quarters (Hedenström & Pettersson 1987), which takes 113 days one way at an average speed of 84 km d−1 (table 1). The breeding season is approximately 70 days (Cramp & Brooks 1992). Added together, these times sum up to 396 days, which clearly implies some time stress on the annual budget, and is the reason for why northerly populations interrupt summer moult and depart on migration (Hedenström et al. 1995). Now, this species is extreme in having two annual complete moults, while most species have one annual moult. In addition, breeding and moult take longer with increasing body size and migration speed declines with increasing size for flapping flight migrants. Hence, increasing body size increases the pressure on the annual time budget to the degree that some birds skip breeding in some years, and that large birds cannot migrate as far as smaller species. It seems uncontroversial to say that the understanding of annual cycle adaptations in relation to migration, moult, breeding and overall body size is incomplete.
11. Future directions
What have we learnt about bird migration since the emergence of optimal migration theory and what gaps remain to be filled by new research? All models are by definition wrong and will ultimately be replaced by an alternative. The main purpose of a model is, however, to focus our attention on a problem and generate testable predictions. In the case of migration ecology, theoretical and empirical exploration lives in a very fruitful symbiosis and optimality thinking has generated a number of experimental tests of, for example, the dependence of departure fuel load on fuelling rate. Interestingly, all experimental studies on departure fuel load since 1990 have been conducted in Europe and none in America (table 2). This probably reflects the differences in research tradition where Europeans are more inclined towards ecology (David Lack legacy), while Americans tend to favour a more physiological approach (Donald Farner and James King legacy). While compiling this review, it has become clear where new research would be welcomed regarding bird migration strategies. One omission in the current theory is the potential effect on migration performance of infections and the associated mounting of an immune defence. Some variation among individuals in migration performance may well be due to differences in coping with disease (see Buehler & Piersma 2008). Migration activity could, for example, reactivate latent Borrelia infection (Gylfe et al. 2000), perhaps owing to suppression of the immune system during migration. Even though the energy cost of mounting an immune response in birds is relatively low (Svensson et al. 1998), there may be costs other than direct energy costs of activated immune function. For example, it has been shown that when birds are working hard during nest feeding, a diphtheria–tetanus vaccination (to provoke an immune reaction) causes a reduced feeding rate (Råberg et al. 2000). Hence, migrating birds may be similarly affected with a reduced performance (e.g. migration speed or survival) due to immune system processes.
Another emerging topic of interest concerns the interaction between migration, breeding and moult—a problem which is perhaps best addressed by the annual routine approach (e.g. Barta et al. 2008). A complication must be considered in that the timing of moult and migration may be genetically correlated and may therefore not evolve independent of each other (Pulido & Coppack 2004). A number of recent studies have found an advancement in spring migration among many bird species (e.g. Hüppop & Hüppop 2003; Stervander et al. 2005), which sometimes is also followed by an advancement in the timing of autumn migration (Cotton 2003; Jenni & Kéry 2003). The reason for these changes are believed to be associated to global climatic changes that shift the phenology of food sources and the optimal timing of breeding (e.g. Both et al. 2005). Problems to solve concern how birds will react to climatic changes, such as whether they depart sooner from wintering sites or whether migration speed is changed due to changes in stopover conditions along the route. Birds may not be able to increase migration speed beyond the maximum rate set by physiological constraints of fuel accumulation and therefore may not be able to track rapid climatic changes. New and challenging research problems present themselves continuously and, not surprisingly, I predict that the study of bird migration will continue to be a lively research topic.
Acknowledgments
This research was supported by the Swedish Research Council. A.H. is a Royal Swedish Academy of Sciences Research Fellow supported by a grant from the Knut and Alice Wallenberg Foundation. The author is very grateful to A. I. Houston, J. McNamara and two anonymous reviewers for their constructive comments on the manuscript.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Åkesson S, Hedenström A. Wind selectivity of migratory flight departures in birds. Behav. Ecol. Sociobiol. 2000;47:140–144. doi:10.1007/s002650050004 [Google Scholar]
- Åkesson S, Walinder G, Karlsson L, Ehnbom S. Reed warbler orientation: initiation of nocturnal migratory flights in relation to visibility of celestial cues at dusk. Anim. Behav. 2001;61:181–189. doi: 10.1006/anbe.2000.1562. doi:10.1006/anbe.2000.1562 [DOI] [PubMed] [Google Scholar]
- Alerstam T. The course and timing of bird migration. In: Aidley D.J, editor. Animal migration. Society for experimental biology seminar series. vol. 13. Cambridge University Press; Cambridge, UK: 1981. pp. 9–54. [Google Scholar]
- Alerstam T. Determinants of migration speed. In: Berthold P, Gwinner E, Sonneschein E, editors. Avian migration. Springer; Berlin, Germany: 2003. pp. 253–267. [Google Scholar]
- Alerstam T, Hedenström A. The development of bird migration theory. J. Avian Biol. 1998;29:343–369. doi:10.2307/3677155 [Google Scholar]
- Alerstam T, Lindström Å. Optimal bird migration: the relative importance of time, energy and safety. In: Gwinner E, editor. Bird migration: physiology and ecophysiology. Springer; Berlin, Germany: 1990. pp. 331–351. [Google Scholar]
- Alerstam T, Hedenström A, Åkesson S. Long-distance migration: evolution and determinants. Oikos. 2003;103:247–260. doi:10.1034/j.1600-0706.2003.12559.x [Google Scholar]
- Alerstam T, Hake M, Kjellén N. Temporal and spatial patterns of repeated migratory journeys by ospreys: implications for strategies and navigation in bird migration. Anim. Behav. 2006;71:555–556. doi:10.1016/j.anbehav.2005.05.016 [Google Scholar]
- Barta Z, Houston A.I, McNamara J.M, Welham R.K, Hedenström A, Weber T.P, Feró O. Annual routines of non-migratory birds: optimal moult strategies. Oikos. 2006;112:580–593. doi:10.1111/j.0030-1299.2006.14240.x [Google Scholar]
- Barta Z, McNamara J.M, Houston A.I, Weber T.P, Hedenstro¨m A, Fero´ O. Optimal moult strategies in migratory birds. Phil. Trans. R. Soc. B. 2008;363:211–229. doi: 10.1098/rstb.2007.2136. doi:10.1098/rstb.2007.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battley P.F, Piersma T, Dietz M.W, Tang S, Dekinga A, Hulsman K. Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc. R. Soc. B. 2000;267:191–195. doi: 10.1098/rspb.2000.0986. doi:10.1098/rspb.2000.0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayly N.J. Optimality in avian migratory fuelling behaviour: a study of a trans-Saharan migrant. Anim. Behav. 2006;71:173–182. doi:10.1016/j.anbehav.2005.04.008 [Google Scholar]
- Bayly N.J. Extreme fattening by sedge warblers Acrocephalus schoenobaenus is not triggered by food availability alone. Anim. Behav. 2007;74:471–479. doi:10.1016/j.anbehav.2006.11.030 [Google Scholar]
- Bednekoff P.A, Houston A.I. Dynamic models of mass-dependent predation, risk-sensitive foraging, and premigratory fattening in birds. Ecology. 1994;75:1131–1140. doi:10.2307/1939436 [Google Scholar]
- Both C, Bijlsma R.G, Visser M.E. Climatic effects on timing of spring migration and breeding in a long-distance migrant, the pied flycatcher Ficedula hypoleuca. J. Avian Biol. 2005;36:368–373. doi:10.1111/j.0908-8857.2005.03484.x [Google Scholar]
- Breguet L. Aerodynamic efficiency and the reduction of air transport costs. Aeronaut. J. 1922;26:307–313. [Google Scholar]
- Buehler D.M, Piersma T. Travelling on a budget: predictions and ecological evidence for bottlenecks in the annual cycle of long-distance migrants. Phil. Trans. R. Soc. B. 2008;363:247–266. doi: 10.1098/rstb.2007.2138. doi:10.1098/rstb.2007.2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter F.L, Paton D.C, Hixon M.A. Weight gain and adjustment of feeding territory size in migrant hummingbirds. Proc. Natl Acad. Sci. USA. 1983;80:7259–7263. doi: 10.1073/pnas.80.23.7259. doi:10.1073/pnas.80.23.7259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran W.W, Wikelski M. Individual migratory tactics of New World Catharus thrushes: current knowledge and future tracking options from space. In: Greenberg R, Marra P, editors. Birds of two worlds. Johns Hopkins University Press; Baltimore, MD: 2005. pp. 274–289. [Google Scholar]
- Cotton P.A. Avian migration phenology and global climate change. Proc. Natl Acad. Sci. USA. 2003;100:12 219–12 222. doi: 10.1073/pnas.1930548100. doi:10.1073/pnas.1930548100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramp S, Brooks D.J, editors. The birds of the Western Palearctic. vol. VI. Oxford University Press; Oxford, UK: 1992. [Google Scholar]
- Croxall J.P, Silk J.R.D, Phillips R.A, Afanasyev V, Briggs D.R. Global circumnavigations: tracking year-round ranges of nonbreeding albatrosses. Science. 2005;307:249–250. doi: 10.1126/science.1106042. doi:10.1126/science.1106042 [DOI] [PubMed] [Google Scholar]
- Delingat J, Dierschke V, Scmaljohann H, Mendel B, Barlein F. Daily stopovers as optimal migration strategy in a long-distance migrating passerine: the northern wheatear (Oenanthe oenanthe) Ardea. 2006;94:593–605. [Google Scholar]
- Dierschke V, Mendel B, Schmaljohann H. Differential timing of spring migration in northern wheatears Oenanthe oenanthe: hurried males or weak females. Behav. Ecol. Sociobiol. 2005;57:470–480. doi:10.1007/s00265-004-0872-8 [Google Scholar]
- Dietz M.W, Piersma T, Hedenström A, Brugge M. Intraspecific variation in avian pectoral muscle mass: constraints on maintaining manoeuvrability with increasing body mass. Funct. Ecol. 2007;21:317–326. doi:10.1111/j.1365-2435.2006.01234.x [Google Scholar]
- Dingle H. Oxford University Press; Oxford, UK: 1996. Migration: the biology of life on the move. [Google Scholar]
- Drent R, Daan S. The prudent parent: energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Dänhardt J, Lindström Å. Optimal departure decisions of songbirds from an experimental stopover site and the significance of weather. Anim. Behav. 2001;62:235–243. doi:10.1006/anbe.2001.1749 [Google Scholar]
- Ebbinge B.S. A multifactorial explanation for variation in breeding performance of brent geese Branta bernicla. Ibis. 1989;131:196–204. [Google Scholar]
- Ellegren H. Speed of migration and migratory flight lengths of passerine birds ringed during autumn migration in Sweden. Ornis Scand. 1993;24:220–228. doi:10.2307/3676737 [Google Scholar]
- Erni B, Liechti F, Bruderer B. Stopover strategies in passerine bird migration: a simulation study. J. Theor. Biol. 2002;219:479–493. doi: 10.1006/jtbi.2002.3138. doi:10.1006/jtbi.2002.3138 [DOI] [PubMed] [Google Scholar]
- Fransson T. Timing and speed of migration in North and West European populations of Sylvia warblers. J. Avian Biol. 1995;26:39–48. doi:10.2307/3677211 [Google Scholar]
- Fransson T. A feeding experiment on migratory fuelling in whitethroats, Sylvia communis. Anim. Behav. 1998;55:153–162. doi: 10.1006/anbe.1997.0573. doi:10.1006/anbe.1997.0573 [DOI] [PubMed] [Google Scholar]
- Fuller M.R, Seegar W.S, Schueck L.S. Routes and travel rates of migrating peregrine falcons Falco peregrinus and Swainson's hawks Buteo swainsoni in the Western Hemisphere. J. Avian Biol. 1998;29:433–440. doi:10.2307/3677162 [Google Scholar]
- Gils J.A, van Schenk I.W, Bos O, Piersma T. Incompletely informed shorebirds that face a digestive constraint maximize net energy gain when exploiting patches. Am. Nat. 2003;161:777–793. doi: 10.1086/374205. doi:10.1086/374205 [DOI] [PubMed] [Google Scholar]
- Gould S.J, Vrba E. Exaptation—a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- Gudmundsson G.A, Lindström Å, Alerstam T. Optimal fat loads and long-distance flights by migrating knots Calidris canutus, sanderlings C. alba and turnstones Arenaria interpres. Ibis. 1991;133:140–152. [Google Scholar]
- Guglielmo C.G, Piersma T, Williams T.D. A sport-physiological perspective on bird migration: evidence for flight-induced muscle damage. J. Exp. Biol. 2001;204:2683–2690. doi: 10.1242/jeb.204.15.2683. [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circadian and circannual programmes in avian migration. J. Exp. Biol. 1996;199:39–48. doi: 10.1242/jeb.199.1.39. [DOI] [PubMed] [Google Scholar]
- Gylfe Å, Bergström S, Lundström J, Olsen B. Reactivation of Borrelia infection in birds. Nature. 2000;403:724–725. doi: 10.1038/35001663. doi:10.1038/35001663 [DOI] [PubMed] [Google Scholar]
- Hake M, Kjellén N, Alerstam T. Age-dependent migration strategy in honey buzzards Pernis apivorus tracked by satellite. Oikos. 2003;103:385–396. doi:10.1034/j.1600-0706.2003.12145.x [Google Scholar]
- Hall K.S.S, Fransson T. Lesser whitethroats under time-contraint moult more rapidly and grow shorter wing feathers. J. Avian Biol. 2000;31:583–587. doi:10.1034/j.1600-048X.2000.310419.x [Google Scholar]
- Hall K.S, Tullberg B.S. Phylogenetic analyses of the diversity of moult strategies in Sylviidae in relation to migration. Evol. Ecol. 2004;18:85–105. doi:10.1023/B:EVEC.0000017848.20735.8b [Google Scholar]
- Hammond K.A, Diamond J. Maximum sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. doi:10.1038/386457a0 [DOI] [PubMed] [Google Scholar]
- Healy S.D, Krebs J.R, Gwinner E. Hippocampal volume and migration in passerine birds. Naturwissenschaften. 1991;78:424–426. doi:10.1007/BF01133419 [Google Scholar]
- Healy S.D, Gwinner E, Krebs J.R. Hippocampal volume in migratory and non-migratory warblers: effects of age and experience. Behav. Brain Res. 1996;81:61–68. doi: 10.1016/s0166-4328(96)00044-7. doi:10.1016/S0166-4328(96)00044-7 [DOI] [PubMed] [Google Scholar]
- Hedenström A. Migration by soaring or flapping flight in birds: the relative importance of energy cost and speed. Phil. Trans. R. Soc. B. 1993;342:353–361. doi:10.1098/rstb.1993.0164 [Google Scholar]
- Hedenström A. Aerodynamics, evolution and ecology of bird flight. Trends Ecol. Evol. 2002;17:415–422. doi:10.1016/S0169-5347(02)02568-5 [Google Scholar]
- Hedenström A. Scaling migration speed in animals that run, swim and fly. J. Zool. 2003a;259:155–160. doi:10.1017/S0952836902003096 [Google Scholar]
- Hedenström A. Flying with holey wings. J. Avian Biol. 2003b;34:324–327. doi:10.1111/j.0908-8857.2003.03324.x [Google Scholar]
- Hedenström A. Scaling of migration and the annual cycle of birds. Ardea. 2006;94:399–408. [Google Scholar]
- Hedenström A, Alerstam T. Climbing performance of migrating birds as a basis for estimating limits for fuel-carrying capacity and muscle work. J. Exp. biol. 1992;164:19–38. [Google Scholar]
- Hedenström A, Alerstam T. Optimal flight speed of birds. Phil. Trans. R. Soc. B. 1995;348:471–487. doi:10.1098/rstb.1995.0082 [Google Scholar]
- Hedenström A, Alerstam T. Skylark optimal flight speeds for flying nowhere and somewhere. Behav. Ecol. 1996;7:121–126. doi:10.1093/beheco/7.2.121 [Google Scholar]
- Hedenström A, Alerstam T. Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J. Theor. Biol. 1997;189:227–234. doi: 10.1006/jtbi.1997.0505. doi:10.1006/jtbi.1997.0505 [DOI] [PubMed] [Google Scholar]
- Hedenström A, Alerstam T. How fast can birds migrate? J. Avian Biol. 1998;29:424–432. doi:10.2307/3677161 [Google Scholar]
- Hedenström A, Pettersson J. Migration routes and wintering areas of willow warblers Phylloscopus trochilus (L.) ringed in Fennoscandia. Ornis Fenn. 1987;64:137–143. [Google Scholar]
- Hedenström A, Bensch S, Hasselquist D, Lockwood M, Ottosson U. Migration, stopover and moult of the great reed warbler Acrocephalus arundinaceus in Ghana, West Africa. Ibis. 1993;135:177–180. [Google Scholar]
- Hedenström A, Lindström Å, Pettersson J. Interrupted moult of adult willow warblers Phylloscopus trochilus during autumn migration through Sweden. Ornis Svecica. 1995;5:69–74. [Google Scholar]
- Hedenström A, Alerstam T, Green M, Gudmundsson G.A. Adaptive variation in airspeed: tracking radar observations of Arctic birds from the Northwest Passage. Behav. Ecol. Sociobiol. 2002;52:308–317. doi:10.1007/s00265-002-0504-0 [Google Scholar]
- Helm B, Gwinner E. Migratory restlessness in an equatorial non-migratory bird. PLoS Biology. 2006;4:611–614. doi: 10.1371/journal.pbio.0040110. doi:10.1371/journal.pbio.0040110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm B, Gwinner E, Trost L. Flexible seasonal timing and migratory behaviour: results from stonechat breeding programs. Ann. NY Acad. Sci. 2005;1046:1–12. doi: 10.1196/annals.1343.019. doi:10.1196/annals.1343.019 [DOI] [PubMed] [Google Scholar]
- Hildén O, Saurola P. Speed and autumn migration of birds ringed in Finland. Ornis Fenn. 1982;59:140–143. [Google Scholar]
- Holmgren N, Ellegren H, Pettersson J. The adaptation of moult pattern in migratory dunlins Calidris alpina. Ornis Scand. 1993;24:21–27. doi:10.2307/3676405 [Google Scholar]
- Holmgren N, Hedenström A. The scheduling of moult in migratory birds. Evol. Ecol. 1995;9:354–368. doi:10.1007/BF01237759 [Google Scholar]
- Houston A.I. Energetic constraints and foraging efficiency. Behav. Ecol. 1995;6:393–396. doi:10.1093/beheco/6.4.393 [Google Scholar]
- Houston A.I. Models of optimal avian migration: state, time and predation. J Avian Biol. 1998;29:395–404. doi:10.2307/3677158 [Google Scholar]
- Houston A.I. The strength of selection in the context of migration speed. Proc. R. Soc. B. 2000;267:2393–2395. doi: 10.1098/rspb.2000.1296. doi:10.1098/rspb.2000.1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüppop O, Hüppop K. North Atlantic oscillation and timing of spring migration in birds. Proc. R. Soc. B. 2003;270:233–240. doi: 10.1098/rspb.2002.2236. doi:10.1098/rspb.2002.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni L, Jenni-Eiermann S. Fuel supply and metabolic constraints in migrating birds. J. Avian Biol. 1998;29:521–528. doi:10.2307/3677171 [Google Scholar]
- Jenni L, Kéry M. Timing of autumn bird migration under climate change: advances in long-distance migrants, delays in short-distance migrants. Proc. R. Soc. B. 2003;270:1467–1471. doi: 10.1098/rspb.2003.2394. doi:10.1098/rspb.2003.2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni L, Winkler R. Academic Press; London, UK: 1994. Moult and ageing in European passerines. [Google Scholar]
- Jouventin P, Dobson F.S. Why breed every other year? The case of albatrosses. Proc. R. Soc. B. 2002;269:1955–1961. doi: 10.1098/rspb.2002.2080. doi:10.1098/rspb.2002.2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood J.K. A limit to metabolisable energy intake in mammals and birds. Comp. Biochem. Physiol. A. 1983;75:1–3. doi: 10.1016/0300-9629(83)90033-6. doi:10.1016/0300-9629(83)90033-6 [DOI] [PubMed] [Google Scholar]
- Klaassen M, Lindström Å, Meltofte H, Piersma T. Arctic waders are not capital breeders. Nature. 2001;413:795. doi: 10.1038/35101654. doi:10.1038/35101654 [DOI] [PubMed] [Google Scholar]
- Kokko H. Competition for early arrival in migratory birds. J. Anim. Ecol. 1999;68:940–950. doi:10.1046/j.1365-2656.1999.00343.x [Google Scholar]
- Konrad T.G. The dynamics of the convective process in clear air as seen by radar. J. Atmos. Sci. 1970;27:1138–1147. doi:10.1175/1520-0469(1970)027<1138:TDOTCP>2.0.CO;2 [Google Scholar]
- Kvist A, Lindström Å. Gluttony in migratory waders—unprecedented energy assimilation rates in vertebrates. Oikos. 2003;103:397–402. doi:10.1034/j.1600-0706.2003.12259.x [Google Scholar]
- Lack D. Bird migration and natural selection. Oikos. 1968a;19:1–9. doi:10.2307/3564725 [Google Scholar]
- Lack D. Methuen; London, UK: 1968b. Ecological adaptations for breeding in birds. [Google Scholar]
- Leisler B, Winkler H. Morphological consequences of migration in passerines. In: Berthold P, Gwinner E, Sonnenschein E, editors. Bird migration. Springer; Berlin, Germany: 2003. pp. 175–176. [Google Scholar]
- Lindström Å. The energetic cost of feather synthesis is proportional to basal metabolic rate. Physiol. Zool. 1993;66:490–510. [Google Scholar]
- Lindström Å, Alerstam T. Optimal fat loads in migrating birds: a test of the time-minimization hypothesis. Am. Nat. 1992;140:477–491. doi: 10.1086/285422. doi:10.1086/285422 [DOI] [PubMed] [Google Scholar]
- Lindström Å, Pearson D.J, Hasselquist D, Hedenström A, Bensch S, Åkesson S. The moult of barred warblers Sylvia nisoria in Kenya-evidence for a split wing-moult pattern initiated during the birds' first winter. Ibis. 1993;135:403–409. [Google Scholar]
- Lindström Å, Kvist A, Piersma T, Dekinga A, Dietz M.W. Avian pectoral muscle size rapidly tracks body mass changes during flight, fasting and fuelling. J. Exp. Biol. 2000;203:913–919. doi: 10.1242/jeb.203.5.913. [DOI] [PubMed] [Google Scholar]
- Lockwood R, Swaddle J.P, Rayner J.M.V. Avian wingtip shape reconsidered: wingtip shape indices and morphological adaptations to migration. J. Avian. Biol. 1998;29:273–292. doi:10.2307/3677110 [Google Scholar]
- McNamara J.M, Houston A.I. The application of statistical decision theory to animal behaviour. J. Theor. Biol. 1980;85:673–690. doi: 10.1016/0022-5193(80)90265-9. doi:10.1016/0022-5193(80)90265-9 [DOI] [PubMed] [Google Scholar]
- McNamara J.M, Houston A.I. Optimal annual routines: behaviour in the context of physiology and ecology. Phil. Trans. R. Soc. B. 2008;363:301–319. doi: 10.1098/rstb.2007.2141. doi:10.1098/rstb.2007.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer T, Drent R. Re-examination of the capital and income dichotomy in breeding birds. Ibis. 1999;141:399–414. [Google Scholar]
- Moore F.R, Simm P.A. Risk-sensitive foraging by a migratory bird (Dendroica coronata) Experientia. 1986;42:1054–1056. doi:10.1007/BF01940730 [Google Scholar]
- Morrison R.I.G, Hobson K.A. Use of body stores in shorebirds after arrival on high-Arctic breeding grounds. Auk. 2004;121:333–344. doi:10.1642/0004-8038(2004)121[0333:UOBSIS]2.0.CO;2 [Google Scholar]
- Mönkkönen M. Do migrant birds have more pointed wings? A comparative study. Evol. Ecol. 1995;9:520–528. doi:10.1007/BF01237833 [Google Scholar]
- Nolet B.A. Efficiency as a foraging currency in animals attaining a gain below the energetic ceiling. Behav. Ecol. 2002;13:571–574. doi:10.1093/beheco/13.4.571 [Google Scholar]
- Norberg R.Å. Optimal flight speed in birds when feeding young. J. Anim. Ecol. 1981;50:473–477. doi:10.2307/4068 [Google Scholar]
- Norris D.R, Marra P.P, Montgomerie R, Kyser T.K, Ratcliffe L.M. Reproductive effort, molting latitude, and feather color in a migratory songbird. Science. 2004;306:2249–2250. doi: 10.1126/science.1103542. doi:10.1126/science.1103542 [DOI] [PubMed] [Google Scholar]
- Parker G.A, Maynard Smith J. Optimality theory in evolutionary biology. Nature. 1990;348:27–33. doi:10.1038/348027a0 [Google Scholar]
- Pearson D.J, Backhurst G. Moult in the river warbler Locustella fluviatilis. Ring. Migr. 1983;4:227–230. [Google Scholar]
- Pennycuick C.J. Mechanics of flight. In: Farner D.S, King J.R, Parkes K.C, editors. Avian biology, vol. 5. vol. 5. Academic Press; New York, NY: 1975. pp. 1–75. [Google Scholar]
- Pennycuick C.J. Oxford University Press; Oxford, UK: 1989. Bird flight performance: a practical calculation manual. [Google Scholar]
- Pennycuick C.J. Actual and ‘optimum’ flight speeds: field data reassessed. J. Exp. Biol. 1997;200:2355–2361. doi: 10.1242/jeb.200.17.2355. [DOI] [PubMed] [Google Scholar]
- Piersma T. Phenotypic flexibility during migration: optimization of organ size contingent on the risks and rewards of fueling and flight? J. Avian Biol. 1998;29:511–520. doi:10.2307/3677170 [Google Scholar]
- Piersma T, Pérez-Tris J, Mouritsen H, Bauchinger U, Bairlein F. Is there a ‘migratory syndrome’ common to all migrant birds? Ann. NY Acad. Sci. 2005;1046:282–293. doi: 10.1196/annals.1343.026. doi:10.1196/annals.1343.026 [DOI] [PubMed] [Google Scholar]
- Pulido F, Coppack T. Correlation between timing of juvenile moult and onset of migration in the blackcap, Sylvia atricapilla. Anim. Behav. 2004;68:167–173. doi:10.1016/j.anbehav.2003.11.006 [Google Scholar]
- Råberg L, Nilsson J.-Å, Ilmonen P, Stjernman M, Hasselquist D. The cost of an immune response: vaccination reduces parental effort. Ecol. Lett. 2000;3:382–386. doi:10.1046/j.1461-0248.2000.00154.x [Google Scholar]
- Rayner J.M.V. Form and function in avian flight. Curr. Ornithol. 1988;5:1–66. [Google Scholar]
- Rayner J.M.V, Swaddle J.P. Aerodynamics and behaviour of moult and take-off in birds. In: Domenici P, Blake R.W, editors. Biomechanics in animal behaviour. BIOS Scientific Publishers Ltd; Oxford, UK: 2000. pp. 125–157. [Google Scholar]
- Rowland J.R. Intensive probing of the clear convective field by radar and instrumented drone aircraft. J. Appl. Meteorol. 1973;12:149–155. doi:10.1175/1520-0450(1973)012<0149:IPOACA>2.0.CO;2 [Google Scholar]
- Schmaljohann H, Dierschke V. Optimal bird migration and predation risk: a field experiment with northern wheatears Oenanthe oenanthe. J. Anim. Ecol. 2005;74:131–138. doi:10.1111/j.1365-2656.2004.00905.x [Google Scholar]
- Serra L. Duration of primary moult affects primary quality in grey plovers Pluvialis squatarola. J. Avian Biol. 2001;32:377–380. doi:10.1111/j.0908-8857.2001.320415.x [Google Scholar]
- Sillett T.S, Holmes R.T. Variation in survivorship of a migratory songbird throughout its annual cycle. J. Anim. Ecol. 2002;71:296–308. doi:10.1046/j.1365-2656.2002.00599.x [Google Scholar]
- Sol D, Lefebvre L, Rodriguez-Teijeiro J.D. Brain size, innovative propensity and migratory behaviour in temperate Palaearctic birds. Proc. R. Soc. B. 2005;272:1433–1441. doi: 10.1098/rspb.2005.3099. doi:10.1098/rspb.2005.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H, Liechti F. Do Levant sparrowhawks Accipiter brevipes also migrate at night? Ibis. 1993;135:233–236. [Google Scholar]
- Stephens D.W, Krebs J.R. Princeton University Press; Princeton, NJ: 1986. Foraging theory. [Google Scholar]
- Stervander M, Lindström Å, Jonzén N, Andersson A. Timing of spring migration in birds: long-term trends, North Atlantic Oscillation and the significance of different migration routes. J. Avian. Biol. 2005;36:210–221. doi:10.1111/j.0908-8857.2005.03360.x [Google Scholar]
- Svensson E, Hedenström A. A phylogenetic analysis of the evolution of moult strategies in Western Palearctic warblers (Aves: Sylviidae) Biol. J. Linn. Soc. 1999;67:263–276. doi:10.1006/bijl.1998.0302 [Google Scholar]
- Svensson E, Råberg L, Koch C, Hasselquist D. Energetic stress, immunosupression and the costs of an antibody response. Funct. Ecol. 1998;12:912–919. doi:10.1046/j.1365-2435.1998.00271.x [Google Scholar]
- Swann R.L, Baillie S.R. The suspension of moult by trans-Saharan migrants in Crete. Bird Study. 1979;26:55–58. [Google Scholar]
- Thomas A.L.R. On the aerodynamics of birds' tails. Phil. Trans. R. Soc. B. 1993;340:361–380. doi:10.1098/rstb.1993.0079 [Google Scholar]
- Thorup K, Alerstam T, Hake M, Kjellén N. Traveling or stopping of migrating birds in relation to wind: an illustration for the osprey. Behav. Ecol. 2006;17:497–502. doi:10.1093/beheco/arj054 [Google Scholar]
- Tobalske B.W, Hedrick T.L, Dial K.P, Biewener A.A. Comparative power curves in bird flight. Nature. 2003;421:363–366. doi: 10.1038/nature01284. doi:10.1038/nature01284 [DOI] [PubMed] [Google Scholar]
- Underhill L.G, Prŷs-Jones R.P, Dowsett R.J, Herroelen P, Johnson D.N, Lawn M.R, Norman S.C, Pearson D.J, Tree A.J. The biannual primary moult of willow warblers Phylloscopus trochilus in Europe and Africa. Ibis. 1992;134:286–297. [Google Scholar]
- Voelker G. Morphological correlates of migratory distance and flight display in the avian genus Anthus. Biol. J. Linn. Soc. 2001;73:425–435. doi:10.1006/bijl.2001.0533 [Google Scholar]
- Weber T.P, Hedenström A. Long-distance migrants as a model system of structural and physiological plasticity. Evol. Ecol. Res. 2001;3:255–271. [Google Scholar]
- Weber T.P, Houston A.I. Flight costs, flight range and the stopover ecology of migrating birds. J. Anim. Ecol. 1997;66:297–306. doi:10.2307/5976 [Google Scholar]
- Weber T.P, Houston A.I, Ens B.J. Optimal departure fat loads and site use in avian migration: an analytical model. Proc. R. Soc. B. 1994;258:29–34. doi:10.1098/rspb.1994.0137 [Google Scholar]
- Weber T.P, Alerstam T, Hedenström A. Stopover decisions under wind influence? J. Avian Biol. 1998a;29:552–560. doi:10.2307/3677175 [Google Scholar]
- Weber T.P, Ens B.J, Houston A.I. Optimal avian migration: A dynamic model of fuel stores and site use. Evol. Ecol. 1998b;12:377–401. doi:10.1023/A:1006560420310 [Google Scholar]
- Weber T.P, Fransson T, Houston A.I. Should I stay or should I go? Testing optimality models of stopover decisions in migrating birds. Behav. Ecol. Sociobiol. 1999;46:280–286. doi:10.1007/s002650050621 [Google Scholar]
- Weber T.P, Borgudd J, Hedenström A, Persson K, Sandberg G. Resistance of flight feathers to mechanical fatigue covaries with moult strategy in two warbler species. Biol. Lett. 2005;1:27–30. doi: 10.1098/rsbl.2004.0244. doi:10.1098/rsbl.2004.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner R. Navigation in context: grand theories and basic mechanisms. J. Avian. Biol. 1998;29:370–386. doi:10.2307/3677156 [Google Scholar]
- Wikelski M, Tarlow E.M, Raim A, Diehl R.H, Larkin R.P, Visser G.H. Costs of migration in free-flying songbirds. Nature. 2003;423:704. doi: 10.1038/423704a. doi:10.1038/423704a [DOI] [PubMed] [Google Scholar]
- Williams E.V, Swaddle J.P. Moult, flight performance and wingbeat kinematics during take-off in European starlings Sturnus vulgaris. J. Avian. Biol. 2003;34:371–378. doi:10.1111/j.0908-8857.2003.02964.x [Google Scholar]
- Winkler H, Leisler B. To be a migrant: ecomorphological burdens and chances. In: Greenberg R, Marra P, editors. Birds of two worlds. Johns Hopkins University Press; Baltimore, MD: 2005. pp. 79–86. [Google Scholar]
- Ydenberg R.C, Welham C.V.J, Schmid-Hempel R, Schmid-Hempel P, Beauchamp G. Time and energy constraints and the relationship between currencies and foraging theory. Behav. Ecol. 1994;5:28–34. doi:10.1093/beheco/5.1.28 [Google Scholar]