Abstract
Animals living in temporally dynamic environments experience variation in resource availability, climate and threat of infection over the course of the year. Thus, to survive and reproduce successfully, these organisms must allocate resources among competing physiological systems in such a way as to maximize fitness in changing environments. Here, we review evidence supporting the hypothesis that physiological trade-offs, particularly those between the reproductive and immune systems, mediate part of the seasonal changes detected in the immune defences of many vertebrates. Abundant recent work has detected significant energetic and nutritional costs of immune defence. Sometimes these physiological costs are sufficiently large to affect fitness (e.g. reproductive output, growth or survival), indicating that selection for appropriate allocation strategies probably occurred in the past. Because hormones often orchestrate allocations among physiological systems, the endocrine mediators of seasonal changes in immune activity are discussed. Many hormones, including melatonin, glucocorticoids and androgens have extensive and consistent effects on the immune system, and they change in systematic fashions over the year. Finally, a modified framework within which to conduct future studies in ecological immunology is proposed, viz. a heightened appreciation of the complex but intelligible nature of the vertebrate immune system. Although other factors besides trade-offs undoubtedly influence seasonal variation in immune defence in animals, a growing literature supports a role for physiological trade-offs and the fitness consequences they sometimes produce.
Keywords: immune, life history, passerine, rodent, seasonality, trade-offs
1. Introduction
Parasitism is one of the most common modes of life used to obtain resources. Consequently, one might expect that organisms would possess strong anti-parasite defences at all times of their lives to prevent or at least hinder potential infections. Counter-intuitively, a recent zeitgeist among ecology, physiology and life-history research has led to the observation that variability in these defences, particularly immune defences, is more often the rule than the exception (Nelson & Demas 1996; Ricklefs & Wikelski 2002). Variation in immune defence over the year may occur for several reasons including (i) changes in disease threat over time, (ii) dynamism in the relative benefits of immune defence at certain times of the year versus others, or (iii) changes in environmental signals which portend impending disease threats (Nelson et al. 2002). Another viable explanation that has only recently garnered attention involves the costs of immune defence and the subsequent trade-offs animals face between investing in immune defence versus other expensive processes (Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000; Norris & Evans 2000). For years, it has been known that current reproductive investments often impinge upon future reproductive success (Stearns 1992). Until recently however, few mechanisms that could lead to these outcomes had been proposed (Nilsson & Svensson 1996; Wiersma et al. 2004; Monaghan & Haussmann 2006). Recent studies have suggested that direct antagonism between reproduction and immune defence could be responsible (Norris & Evans 2000; Ardia et al. 2003; Hanssen et al. 2005; Martin et al. 2006a). Apparently, the costs of immune activity themselves can be large (Lochmiller & Deerenberg 2000) and thus sometimes negatively affect reproduction (Ilmonen et al. 2000), growth (Prendergast et al. 2004) or other aspects of fitness.
In this paper, we propose an extension of this hypothesis to explain seasonal changes in immune defence in small mammals and birds (Sheldon & Verhulst 1996), emphasizing existing evidence identifying trade-offs between reproduction and immune function. Trade-offs are defined here as direct or indirect antagonistic interactions between two physiological processes, which can (but do not necessarily) have long-term fitness consequences for organisms. This paper focuses predominantly on work in small mammals and birds because these species provide some of the best examples that immune activity and reproduction are often in conflict (Sheldon & Verhulst 1996; Zuk & Stoehr 2002; Demas 2004). Further, the income-breeding strategy of these species (Drent & Daan 1980) makes them appropriate models for investigating counterbalances between current and future reproduction. Income breeders by definition do not possess the resource reserve capacity (i.e. capital) to compensate for increased reproductive or immunological demands (Martin et al. 2006b), so in these species physiological trade-offs probably more often have consequences for fitness than would be the case in larger taxa.
In addition to reviewing the role of trade-offs in seasonal variation in the immune system, the hormonal mediators of these changes are discussed. Androgens, glucocorticoids, melatonin and other hormones mediate most changes in immune activity and are particularly important in seasonal contexts (Nelson et al. 2002). Finally, in an effort to refine future studies in ecological immunology, a recent hypothesis proposing a greater appreciation of the intricacies of the immune system is presented in the context of our review. Overall, our goal for this paper is to highlight how trade-offs might be important mediators of seasonal changes in immune defence; obviously, many other factors can also drive changes in immune activity across the year, but it is becoming apparent that trade-offs are indeed important.
2. The vertebrate immune system
The vertebrate immune system is diffusely distributed throughout the body and consists of many interacting cells, tissues and soluble proteins (Janeway et al. 2004). Collectively, these substances protect the body from infection, as well as rid or control infections once they have taken hold. Broadly, the immune system comprises two arms that differ in function and evolutionary history: the adaptive arm and the innate arm. The adaptive immune system is unique to the jawed vertebrates and consists of two branches termed humoral (B-cell) and cell mediated (T-cell). Humoral immunity is predominantly responsible for extracellular pathogen control through generation of soluble proteins (antibodies) specific to particular components (antigens) of invading cells or organisms. Cell-mediated immunity is generally responsible for intracellular pathogen control (cytotoxic T-cells) and/or managing B-cell and other immune responses (T-helper cells). Unlike other immune cells, B- and T-cells are capable of targeted defence against non-self substances via a diverse set of membrane receptors generated early in ontogeny (see below). Although these defences can provide immunological memory of prior infections, they are developmentally expensive and slower to reach effectiveness in terms of pathogen control relative to the other main immune defence system, innate immunity.
The innate immune system consists of a diverse array of cell types, such as macrophages, granulocytes and natural killer cells, and a variety of substances secreted by these cells including antimicrobial peptides, destructive enzymes and complement, a protein complex responsible for rapid control of extracellular pathogens (Janeway et al. 2004). Innate immune defences are effective at controlling multiple parasite types, and they are much more quickly engaged than adaptive defences. However, they are often more expensive (especially if an acute phase response is induced; see below), and they can be more self-damaging than adaptive defences (Klasing 2004). Also, it is often innate defences that determine the nature and intensity of the adaptive immune response (e.g. a T-helper cell type 1 (Th1) versus T-helper cell type 2 (Th2) bias; Janeway et al. 2004). To date, many techniques have been used to measure both innate and adaptive immune responses in seasonally breeding birds and mammals. Table 1 summarizes the salient details of some of the most popular tests including the conventional interpretations of strong responses (the putative important parameter) for each test.
Table 1.
Most common assays used to measure immune activity in small mammals and birds.
| arm measured | treatment | abbreviation | strong response | indicative of | reference |
|---|---|---|---|---|---|
| in vivo | |||||
| cell mediated | phytohaemagglutinina | PHA | delayed-type hypersensitivity (DTH) | resistance to intracellular infection (e.g. viruses) | Lochmiller et al. (1993), Martin et al. (2006c) |
| dinitrofluorobenzene | DNFB | Dhabhar & McEwen (1997) | |||
| keyhole limpet haemocyanin | KLH | Martin et al. (2006b) | |||
| humoral | sheep red blood cells | SRBC | generation of antibodies against novel protein(s) | resistance to extracellular infection (e.g. bacteria) | Deerenberg et al. (1997) |
| keyhole limpet haemocyanin | KLH | Demas & Nelson (1998) | |||
| diphtheria–tetanus virus | DPT | Ilmonen et al. (2000) | |||
| innate | lipopolysaccharide | LPS | variable | resistance to multiple novel pathogens | Aubert et al. (1997), Owen-Ashley et al. (2004) |
| in vitro | |||||
| cell mediated | concanavalin A | Con A | extensive proliferation of responsive T-cells | cytokine production, T-cell activity | Fitzgerald et al. (1992) |
Unlike KLH and DNFB, PHA is a mitogen that activates a variety of T-cell types, which may lead to different degrees of swelling compared with other antigens (see Martin et al. 2006a,c)
3. Seasonal variation in immune activity
(a) Intra-annual
Environmental conditions are in temporal flux over much of the planet. At temperate and boreal latitudes, summer and spring represent conditions in which individuals of most species of small birds and mammals can thrive. Winter and its associated low temperatures and food availability, however, make breeding and sometimes even survival difficult (Nelson et al. 2002). For these reasons, parturition usually coincides with summer and spring in small mammals, whereas reductions in gonad size occur in response to shortening day lengths (Bronson 1988). In seasonally breeding passerine birds, most reproductive activities take place in spring, then males and females become refractory to long day lengths, spontaneously regressing their reproductive organs before winter begins (Gwinner 2003). These reductions in gonad size in males are typically accompanied by decreases in androgen secretion and sperm production (Korytko et al. 1997; Kuwahara et al. 2000).
In contrast to these decreases in reproductive activity, most small mammals and birds enhance thermogenic activity, cellular maintenance and other processes that promote survival in winter (Nelson et al. 2002). In particular, immune activity tends to be elevated at this time of year (Nelson & Demas 1996; Nelson 2004), presumably to improve the likelihood of survival to the next breeding season (Goldman 2001). Extensive work in birds, mammals and particularly reptiles has demonstrated that immunological tissues including thymus, spleen, bursa of Fabricius and gut-associated lymphoid tissues (GALT) change in size over the year (Nelson et al. 2002). Similarly, changes in the abundance and distribution of immune cells on a seasonal basis are common (Nelson et al. 2002), with circulating cell densities tending to be higher in the winter months. Although intriguing, such changes in immune organ size and numbers of immune cells in circulation are difficult to interpret in a functional sense; increased cellularity, for example, may represent greater infection or enhanced capacity for disease resistance (Norris & Evans 2000). Direct measurements of immune responses are therefore thought to better reflect an individual's capacity for immune defence.
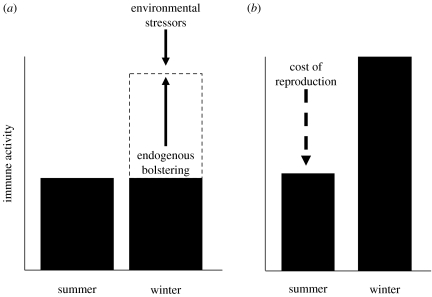
Table 2 reports the extensive seasonal variation in immune responses found in both captive and wild vertebrates. To date, two conceptual models have proposed that trade-offs must be important for explaining seasonal variation in immune activity. One hypothesis posits that winter immunoenhancement reflects active upregulation of immune activity to counteract the immunosuppressive effects of stressors that occur in winter including low ambient temperatures and reduced food availability (Nelson & Demas 1996; Sinclair & Lochmiller 2000; Nelson 2004). Such stressors often increase circulating glucocorticoids in laboratory conditions. Thus, because elevated corticosteroids (over long periods) tend to decrease immune activity (McEwen et al. 1997), animals must buffer environmentally induced immunosuppression by increasing immune activity to higher levels than are maintained in less-demanding environmental conditions (e.g. summer; figure 1a). An alternative hypothesis proposes that the costs of immune activity underlie seasonal fluctuations (Martin et al. 2004, 2006b; Greenman et al. 2005). That is, immune activity is traded off during specific phases of animals' lives, such as breeding, owing to the incompatible costs of simultaneous demanding physiological activities (figure 1b).
Table 2.
Seasonal variation in in vitro and in vivo measures of immune activity in small mammals and birds.
| species | measure | arm | in SD (winter) | reference |
|---|---|---|---|---|
| mammals | ||||
| Spermophilus beldingi | SRBC antibodies | humoral | decrease | Sidky et al. (1972) |
| Clethrionomys glareolus | SRBC antibodies | humoral | increase | Saino et al. (2000) |
| Sigmodon hispidus | spleen PFC to SRBC | cell mediated | increase | Lochmiller et al. (1994) |
| P. maniculatus | splenocyte proliferation | cell mediated | increase | Demas & Nelson (1998) |
| Mesocricetus auratus | splenocyte proliferation | cell mediated | increase | Brainard et al. (1987) |
| Phodopus sungorus | lymphocyte proliferation | cell mediated | decrease | Yellon et al. (1999) |
| DTH to DNFB | cell mediated | increase | Bilbo et al. (2002) | |
| NK cell cytolytic activity | innate | increase | Yellon et al. (1999) | |
| phagocytosis | innate | decrease | Yellon et al. (1999) | |
| fever | innate | decrease | Bilbo & Nelson (2002) | |
| wound healing | integrative | decrease | Kinsey et al. (2003) | |
| birds | ||||
| Sturnus vulgaris | splenocyte proliferation | cell mediated | increase | Bentley et al. (1998) |
| Philomachus pugnax | DTH to PHA | cell mediated | increase | Lozano & Lank (2003) |
| Passer domesticus | DTH to PHA | cell mediated | increase | Martin et al. (2004, 2005, 2006a) |
| increase | Greenman et al. (2005) | |||
| no change | Gonzalez et al. (1999) | |||
| Zonotrichia leucophrys | fever | innate | decrease | Owen-Ashley & Wingfield (2006) |
Figure 1.
Comparisons of the (a) winter immunoenhancement versus the (b) trade-off models to explain seasonal variation in immune activity. According to the winter immunoenhancement model (a), immune activity is suppressed during winter (short days for most non-tropical seasonal breeders) due to the stressors of winter conditions (downward-pointing arrow), such as low ambient temperatures and food availability. This stress-induced immunosuppression is buffered by animals (upward-pointing arrow) in winter (short-day conditions in the laboratory) by increasing immune responses to levels greater than those that occur in long days. According to the trade-off model (b), immune activity is depressed when other costly physiological activities are concurrent, such as breeding (downward-pointing arrow). The schematic here depicts resource-driven immunosuppression during breeding, but this result would presumably hold for any other costly physiological process, such as tissue regeneration and growth, or perhaps even expensive behaviours (e.g. territory defence).
Both hypotheses are supported by studies in multiple taxa and together they explain a significant proportion of the immunological variation seen in animals across the year. However, both hypotheses require refinement. For instance, it is unclear how animals could buffer themselves against winter stressors in the wild if (i) immune activity is costly, (ii) thermogenic requirements are high and also expensive at this time of year, and (iii) resource availability is low. If additional resources cannot be obtained then compensation must be made within some physiological system. Thus, if the immune system is to be bolstered and physiological trade-offs are unavoidable, other physiological systems must be weakened; just which system(s) are adjusted (other than the reproductive system) remain unspecified. Further, it is not apparent that persistent and predictable environmental conditions (e.g. low food availability and temperature) would necessarily induce stress responses each time they were encountered by wild animals. Arctic passerine birds only show elevated corticosteroid responses to large and prolonged storms, and baseline glucocorticoids are actually lower in most birds during winter when compared with summer (Romero 2002).
A shortcoming of the second (direct trade-off) hypothesis is that it cannot account for the known effects of environmental factors, particularly photoperiod, on immune activity in captive and wild non-tropical species (Nelson et al. 2002). That is, by using changing day length alone, many temperate and boreal species begin adjusting their phenotypes before changes in environmental conditions actually occur, and these changes occur even in laboratory conditions when resources are generally available ad libitum. Decreasing day lengths tend to enhance immune responses and induce regression of the reproductive system (Nelson & Demas 1996). Thus, seasonal changes in immune activity under laboratory conditions may represent manifestations of prior selection for phenotypes that use photoperiod to allocate resources appropriately among physiological systems (Ricklefs & Wikelski 2002).
Of course, these two hypotheses are not the only explanations for why immune defences may vary over the year. Another possibility that has been relatively unexplored involves changes in the abundance and distribution of pathogens over time. In temperate regions of the world, disease threats fluctuate depending on the time of year. In spring, vector-transmitted diseases, such as malaria, tend to peak; in autumn and winter, however, contact-transmitted diseases are more common (Nelson 2004). Thus, temporal changes in animals' immune defences may represent an effort to resist infection at those times of the year when certain diseases are most prevalent. Even though this hypothesis (and others) may be valid, it is apparent, based on a growing literature, that trade-offs must in part be responsible for seasonal variation in immune defence, the essence of the two hypotheses discussed earlier.
To establish the validity of these hypotheses, however, refinement and reinforcement are critical. Indeed, a historical emphasis on captive, temperate-dwelling seasonal breeders may have led to misconceptions regarding whether trade-offs alone can explain fluctuations in immune function in natural systems (Bronson 1988). For example, previous work by Lochmiller and colleagues on wild rodents highlights inconsistencies between patterns detected in captivity versus those found in the field. In one study, cotton rats (Sigmodon hispidus) from Oklahoma exhibited extensive intra-annual variation in both cell-mediated and humoral immune activity but not in any systematic (seasonal) fashion (Lochmiller et al. 1994). The authors suggested that the pattern they found reflected changes in the genotypic composition of the population more than phenotypic changes within individuals. In a second study, capacity of resistance to Listeria monocytogenes was compared over the year in both cotton rats and prairie voles (Microtus ochrogaster; Sinclair & Lochmiller 1999). Neither species showed seasonal differences in mortality due to a large inoculation of bacteria (LD50) despite the extensive seasonal variation in multiple aspects of immune activity detected in both species. Such results could indicate that the seasonal decrements in immune function that ecologists have commonly detected might not be functionally important (Adamo 2004). Alternatively, studies in which potentially lethal doses of bacteria are directly administered to study subjects may be inappropriate for determining the relevance of changes in immune measures in natural contexts. Indeed, this method circumvents potentially important upstream immune defences. Moreover, longitudinal studies cannot always provide the resolution necessary to determine if trade-offs are important mediators of seasonal changes in immune activity. Variability in the physiological condition of individuals at different time points could obscure important relationships.
Altogether, the work of Lochmiller and colleagues indicates that an argument justifying trade-offs as mediators of seasonal variation in vertebrates immune defence has not been made. Although studies have indicated that immune activity is expensive in terms of resources/energy (Lochmiller & Deerenberg 2000; Demas 2004) or self-damage (Råberg et al. 1998), evidence has yet to be presented en masse to indicate that the costs of immune defence are important in explaining seasonal changes in immune activity. A first step towards testing this hypothesis would include examining evidence indicating that immune activity fluctuates on shorter time scales than seasons, particularly if immune activity was compromised when other expensive processes were ongoing. Such evidence is plentiful.
(b) Intra-seasonal
Pregnancy and lactation represent the most energetically demanding periods of the life cycle for small female mammals (Thompson & Nicoll 1986; Speakman 2000). Subsequently, multiple types of immune activity are compromised during these stages in rodents. For instance, lactating and pregnant Siberian hamsters (Phodopus sungorus) had lower antibody responses to a novel protein when compared with nulliparous animals (Drazen et al. 2003). Similarly, the degree of lipopolysaccharide-induced fevers of pregnant and lactating rats (Rattus rattus) was reduced when compared with virgin animals (Martin et al. 1995); lipopolysaccharide (LPS) is a component of Gram-negative bacterial cell walls that is commonly used to induce fever in vertebrates but is not a replicating pathogen. Moreover, in the latter study, if fever was induced within 24 h of parturition then no fever was detected, but almost all animals died 3–15 h after giving birth. Similar dosages of LPS did not cause mortality in either virgin or pregnant rats when given outside of this temporal window (Martin et al. 1995).
Changes in immune function during breeding are not limited to rodents. Insectivorous passerines exhibited weak cell-mediated immune activity when ambient temperature (and hence food availability) was low (Lifjeld et al. 2002). In nestling great tits (Parus major), cell-mediated responses decreased as the breeding season progressed, presumably in response to deteriorating environmental conditions (Dubiec & Cichon 2005). As with small mammals, increased parental responsibilities decreased both cell-mediated and humoral immune activity in avian species (Deerenberg et al. 1997; Nordling et al. 1998; Moreno et al. 1999). Even increased incubation efforts negatively impinged on antibody production in collared (Ficedula albicollis) and pied (Ficedula hypoleuca) flycatchers (Cichon 2000; Ilmonen et al. 2002).
Short-term trade-offs between immune activity and reproduction can be complex. For instance, pregnant greater mouse-eared bats (Myotis myotis) had weaker cell-mediated responses and harboured more ectoparasites than non-reproductive females from the same roost (Christe et al. 2000). Unexpectedly, T-cell immune activity progressively increased over the course of the pregnancy, reaching a maximum during lactation. This phenomenon may have represented a sampling bias whereby early breeding bats (lactating by the time the authors measured immune activity) had stronger T-cell responses than late-breeding bats (early pregnancy; sensu Lochmiller et al. 1994). Alternatively, this pattern could represent an effort by females to ensure that they reared their litters to independence in spite of the long-term consequences of concurrent, large reproductive and immune investments (Christe et al. 2000).
Similar shifts in allocation priority to current versus future reproduction (once a large reproductive investment has already been made) have been reported in passerines. Female house sparrows (Passer domesticus) injected with LPS were less likely to abandon their nests when their clutches were enlarged versus reduced (Bonneaud et al. 2003), which the authors suggested as evidence for terminal investment. Recently, we found evidence for terminal investment in rodents. Typically, seasonally breeding small mammals regress gonads in short days, presumably as an energy-saving mechanism to promote overwinter survival (Bronson 1988). Siberian hamsters injected with LPS, however, slowed gonad regression, indicating that when infected, hamsters may remain reproductively active late into the year to maximize current reproduction as future reproductive opportunities may never occur (Weil et al. 2006c).
Short-term changes in immune activity also occur during hibernation. Many small mammals and birds use hibernation or torpor to survive the winter when food resources are scarce. Immune function seems to operate in a unique manner during these energy-saving bouts. For instance, many species exhibit short, periodic bouts of normothermia during the hibernation period. Although it is unlikely that these arousals are solely for immune defence, a recent study in ground squirrels suggests that one benefit of these arousals may include reactivation of the immune system. Hibernating animals injected with LPS showed no fever until arousal, and LPS expedited arousals via increased prostaglandin secretion in the brain (Prendergast et al. 2002a).
4. Are trade-offs responsible for seasonal variation in immune defence?
(a) The costs of immune defence
Presumably, all animals would benefit from strong immune defences at all times of their lives (Nelson et al. 2002; Nelson 2004). Some have suggested that variability in defence is a consequence of selection against immunopathology, influencing organisms to decrease immune responses to avoid self-damage during sensitive periods of their lives (Råberg et al. 1998). Thus far, data to support this possibility are lacking. A more substantiated hypothesis proposes that immune activity varies because it is too expensive to use when other physiological activities are operating at a maximum (Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000). It is well documented that reproduction is expensive (Speakman 2008). In female rodents, food intake can double over the course of the gestational period and triple with the onset of lactation (Bronson 1988). In passerines, the costs of provisioning nestlings and even incubating, brooding and laying eggs can be substantial (Monaghan & Nager 1997). Recent work in ecological immunology shows that the costs of immune activity are often sufficiently large to influence fitness (Lochmiller & Deerenberg 2000; Martin et al. 2003).
Although physiological costs may not always directly lead to fitness costs, fitness can be compromised by the activation of immune activity, especially if physiological costs are proportional to other fitness-related processes and compensation for increased resource use is not possible (Martin et al. 2003). Some of the first work demonstrating substantive costs of immune activity in wild vertebrates comes from work on the collared flycatcher (Gustafsson et al. 1994). Over the past several years, these results have been expanded to other species of birds and mammals. For instance, experimental increases of clutch size or brood size dampened immune activity (Nordling et al. 1998; Ardia et al. 2003). In some cases, these reductions in immune activity could be directly linked to compromised future reproductive success (Hanssen et al. 2004), but in others, individuals favouring strong immune responses over large reproductive efforts experienced greater future reproductive success by increasing lifespan (Ardia et al. 2003).
Although the above results indicate costs of immune activity, they do not demonstrate direct negative effects of induced immune activity on reproduction or other components of fitness (Sheldon & Verhulst 1996). Many other studies, however, have provided this evidence. Female house sparrows (P. domesticus) injected with LPS provisioned offspring at a lower rate than saline-injected birds, and hence had reduced reproductive success (Bonneaud et al. 2003). Studies in pied flycatchers (F. hypoleuca; Ilmonen et al. 2000) and blue tits (Parus caeruleus; Råberg et al. 2000) have detected similar outcomes. Fitness costs of immune activation are not limited to the effects on reproduction. Activation of the immune system reduced feather regrowth (moult) in house sparrows (Martin 2005). Similar suppressive effects of induced immune activity on the development of sexual ornaments in passerines have been reported (Kilpimaa et al. 2004). In white-footed mice (Peromyscus leucopus), immune challenge reduced testes and small intestine mass of adults (Derting & Compton 2003); likewise, immune challenge retarded both somatic and reproductive tissue growth in peripubertal Siberian hamsters (Prendergast et al. 2004) and Eastern bluebirds (Sialia sialis; Fair & Myers 2002) and attenuated reproductive behaviour (Aubert et al. 1997; Weil et al. 2006a). Even induction of one immune response can compromise another; female white-footed mice showed less inflammation at the site of wounds when T-cell-mediated activity was induced 1 day prior to wounding. Similarly, T-cell-mediated inflammation was compromised in mice wounded 1 day prior to T-cell challenge (Martin et al. 2006c). Some of the best evidence for the high costs of immune activity comes from work on domestic fowl. Turkeys (Meleagris gallopavo) selected for larger adult body size exhibited weaker cell-mediated responses, fewer lymphocytes in circulation and smaller spleens relative to birds selected for small body size (Bayyari et al. 1997). Conversely, chickens (Gallus gallus) selected for strong antibody responses to sheep red blood cells are smaller as adults than lines selected for weaker antibody responses (Parmentier et al. 1996).
To mount immune responses, animals require sufficient energy and nutrient resources. Oftentimes immune responses are condition dependent with animals in better health or possessing greater endogenous energy/resource reserves typically mounting larger immune responses than those in poor condition (Lifjeld et al. 2002). Adequate protein is critical to mounting many immune responses (Lochmiller et al. 1993; Klasing 1998). Likewise, adequate energy stores determine the strength and character of immune responses (Lochmiller & Deerenberg 2000; Demas 2004). Lipectomy in both prairie voles (M. ochrogaster) and Siberian hamsters reduced antibody production to a novel antigen (Demas et al. 2003). Similarly, immune challenge directly increases energy turnover in some, but not all, mammals and birds (table 3).
Table 3.
Costs of immune responses in mammals and birds as a proportion of resting metabolic rate.
| species | arm | percentage of increase | reference |
|---|---|---|---|
| mammals | |||
| Cavia porcellus | humoral | — | Pilorz et al. (2005) |
| Mus musculus | humoral | 27 | Demas et al. (1997) |
| Peromyscus leucopus | humoral and cell mediated | — | Derting & Compton (2003) |
| birds | |||
| Parus caeruleus | humoral | — | Svensson et al. (1998) |
| Parus major | humoral | 8 | Ots et al. (2001) |
| Passer domesticus | cell mediated | 29 | Martin et al. (2003) |
| 32a | Martin et al. (2006a) | ||
| Streptopelia risoria | humoral | 9 | Eraud et al. (2005) |
| Taeniopygia guttata | humoral | —b | Verhulst et al. (2005) |
Data from an equatorial population of house sparrows.
Decrease in metabolic rate detected.
(b) Parsing the costs of immune defence
The lack of consistent increases in energy expenditure in response to immune challenges in the studies listed in table 3 and the lack of both physiological and fitness costs of immune activity in other studies (Williams et al. 1999; Lozano & Ydenberg 2002; Horak et al. 2003; Martinez et al. 2004; Verhulst et al. 2005) indicate that immune responses may not always be expensive. Indeed due to a publication bias against negative results, evidence supporting a lack of detectable immunity costs may be greater than that is apparent from the above studies. It is impossible to know, however, how large this bias may be, and more importantly the absence of costs in some studies cannot explain the presence of costs in others. We expect that some of the lack of detection of energetic costs may reflect the imprecise nature of using whole body energy turnover as an endpoint (Ksiazek et al. 2003; Klasing 2004). That is, energetic costs of using the immune system may be hard to identify owing to compensation among other physiological systems (Derting & Compton 2003). Furthermore, anorexia and inactivity are hallmarks of fever responses in many vertebrates (Hart 1988), so decreased energy turnover in some cases may be part of the defence strategy itself (Klasing 2004). For these reasons, most accounting of the costs of immune activity in domestic fowl has relied on critical amino acids as currencies. This work has provided strong evidence that the immune system is expensive but also highlights that the (i) development, (ii) maintenance, and (iii) use of the immune system impart distinct costs (table 4).
Table 4.
Parsing the costs of the vertebrate immune system. (Adapted from Klasing (2004).)
| development | cost of maintenance | use | pathological damage | effectiveness | ||
|---|---|---|---|---|---|---|
| novel pathogen | repeated exposure | |||||
| arm | ||||||
| innate | low | intermediate | high | high | good | good |
| adaptive | high | low | low | variable | poor | excellent |
Development of the innate immune system is not especially costly when compared with that of other physiological systems. Development of the adaptive immune system, however, is potentially the largest immunological investment vertebrates make. This high cost stems from the semi-random nature by which the T- and B-cell repertoires are generated. Early in ontogeny, T- and B-cells are produced that are either sensitive to self-antigens or not sensitive to self-antigens; self-sensitive cells are common whereas self-insensitive cells are rare (Cohn & Langman 1990). Those that are sensitive are immediately destroyed so as not to initiate autoimmune responses, whereas those that are not sensitive to self-antigens reach the circulation and are maintained in a semi-quiescent state until they encounter a pathogen that binds to the receptors they possess (Janeway et al. 2004). In chickens (G. gallus), approximately 90% of B-cells and 95% of T-cells produced are eliminated before they reach circulation (Reynaud & Weill 1996). At four weeks post-hatch, chicks export 5 × 108 B-cells per day from their bursae of Fabricius, constituting 0.04% of the weight of the growing bird (Paramithiotis & Ratcliffe 1994).
It has been suggested that the high costs of the adaptive immune system development are related to the extensive variation in the length of incubation/gestation periods among different vertebrate species. Duration of the developmental period varies from 18 to 660 days among placental mammals (Promislow & Harvey 1990); similar variation can be seen among avian species (Ricklefs 1992). Indeed, recent work has indicated that prolongation of the developmental period is correlated with greater cell-mediated immune responses in some cases (Tella et al. 2002), although these results require further validation (Palacios & Martin 2006a).
Maintenance of the immune system appears cheap relative to development. Most inactive immune cells possess little cytoplasm and thus have modest metabolic demands. Furthermore in chickens, the cost in terms of the amino acid lysine for maintaining the entire immune system has been estimated to be only 3% of the total body requirements (Klasing 2004). In small mammals, immunological maintenance appears comparably cheap. Administration of cyclophosphamide (a protein synthesis inhibitor with immunosuppressive effects) greatly decreased circulating lymphocytes in P. leucopus, but it had no effect on energy turnover (Derting & Compton 2003). Also, basal metabolic rate was higher in lymphocyte-deficient mice relative to normal mice (Råberg et al. 2002), which the authors suggested may have been related to increased reliance on innate defence in the lymphocyte-deficient groups. Further study of the maintenance costs of immune activity is critical, however, as maintenance costs may be important mediators of physiological trade-offs (Schmid-Hempel 2003; Verhulst et al. 2005; Martin et al. 2007)
In terms of use, the cost of the adaptive immune system is modest. Generating antibodies in domestic chickens (G. gallus) requires only 28 mg of lysine kg−1 d−1 (Klasing 2004), a small amount relative to other physiological processes. Further, although activation of the adaptive immune system may elevate energy expenditure in some species (table 3), measurements in chickens indicate that the innate immune system is much more costly to mobilize, particularly processes associated with the acute phase response. Indeed, production of acute phase proteins during fever is more expensive (in terms of lysine) than any other metabolic process except somatic growth (Lochmiller & Deerenberg 2000; Klasing 2004). It has been suggested that usage costs of innate immune activity mediate many of the fitness consequences detected in ecological immunology studies. In pied flycatchers, for example, decreased reproductive success of females was originally attributed to the activation costs of the humoral immune system (Ilmonen et al. 2000). However, because the virus particles were emulsified in an adjuvant (aluminium phosphate) prior to injection, a technique specifically meant to induce a robust acute phase response and hence boost the antibody response (Janeway et al. 2004), it is probable that fitness costs in this study were due to induction of an acute phase response, not the generation of antibodies themselves (Klasing 2004). This issue does not invalidate the results of this and other studies; it simply highlights the need to appreciate the immunological details of the assays that are chosen. Indeed, even SRBC and PHA can induce mild acute phase responses in birds (Klasing & Peng 1987), which may explain why these challenges sometimes influence fitness (Garamszegi et al. 2004) or elevate energy expenditure (table 3).
5. Geographical variation in immune defence
Although immune function varies temporally within species, little work has addressed whether investments in immune defence versus other physiological processes change depending on where animals live. If seasonal changes in immune activity are partly driven by resource availability over the year, then changes in environment across habitats (and especially latitudes and altitudes) should influence how resources are allocated among competing physiological systems. For instance, animals living in the humid tropics would presumably be exposed to greater numbers of and more diverse parasites than animals living in inland, boreal sites where most parasites (and their vectors) are active briefly each year (Piersma 1997). Some evidence from birds indicates that species living near the equator (Ricklefs 1992) or with broad geographical ranges show the highest prevalence of haematozoan infections (Tella et al. 1999). Variation in parasite threats across habitats can influence investments in immune defences in some cases. For example, small ground finches (Geospiza fulginosa) on large islands in the Galápagos carried more ectoparasites than conspecifics on small islands; investment in humoral (but not cell mediated) immune defence paralleled these patterns (Lindström et al. 2004).
Another apparently consistent geographical influence on immune activity is latitude. Temperate-dwelling house sparrows (40° N latitude), which are exposed to a more dramatic climatic variability, decreased immune investments during the breeding season but increased them later when reproductive activity ended; a near-equatorial population (9° N) exhibited static immune investments year-round (Martin et al. 2004). A more recent study on the same populations found that the immune systems of each population were distinct, and that the tropical birds generally invested more in defence than did the temperate ones (Martin et al. 2006a). Further, endocrine mediation of immune activities in these populations was distinct; corticosterone (see below) suppressed immune activity in the temperate but not the tropical population.
Such patterns are not limited to house sparrows. Tree swallows showed a similar latitudinal pattern in immune versus reproductive investments; more southerly populations (35° N) maintained immune responses irrespective of brood-size manipulations whereas northerly populations (65° N) decreased immune responses when brood sizes were enlarged (Ardia 2005). Some rodents also allocate to immune defence differently depending on latitude. Aztec mice (Peromyscus aztecus), which reside in southern Mexico, show no immunological responsiveness to the changes in day length (Demas & Nelson 2003), whereas more northerly distributed species (northern USA) show enhanced responses in short days coupled with reduced responses in long days when breeding typically occurs (Pyter et al. 2005a). Recent work in meadow voles (Microtus pennsylvanicus) indicates that the effects of latitude on immune activity may be complicated in some cases. Voles from the Northwest Territory of Canada enhanced cell-mediated responses in long days but reduced them in short days; voles from central Ohio (USA) showed the opposite pattern (Pyter et al. 2005b).
The character of trade-offs themselves may change with latitude. In high-latitude birds for example, breeding and postnuptial moult often overlap owing to the short window of time available to animals to breed and regrow feathers (Hemborg & Merila 1999). In the tropics, a larger temporal window allows birds to minimize or even eliminate this overlap. Subsequently, trade-offs between moult and immune function should be dramatic at temperate or boreal latitudes, but minimal near the equator. To date, only one study has addressed this possibility in passerines, but did not find an effect of latitude on trade-offs. Moult and immune activity did interact antagonistically in that study (Martin 2005), implying a trade-off between the two processes however. One possible explanation for this outcome may involve the relatively recent arrival of the study species (P. domesticus) to the tropics. Invasion status probably impinges on how the immune systems of birds and other vertebrates are organized (Lee & Klasing 2004; Lee et al. 2005), perhaps because parasites often perform better on local versus foreign hosts (Ebert 1994).
Conditions during birth can also influence both the immune and the reproductive systems. White-footed mice (P. leucopus) born at high latitudes are reproductively more sensitive to decreasing day lengths than low-latitude populations (Lynch et al. 1981). Similarly, high-latitude populations are slower to mature than low-latitude populations when exposed to decreasing day lengths (Dark et al. 1983), perhaps in an effort to delay the onset of puberty until environmental conditions permit rapid maturation. These reproductive strategies may ensure that immune defences are optimized to promote overwinter survival. Both male and female Siberian hamsters born in short days and reared in short days (e.g. an autumn birth) exhibited stronger cell-mediated immune responses than hamsters short-day born, long-day reared (e.g. a spring birth) animals (Weil et al. 2006d).
6. Proximate mechanisms mediating trade-offs
Altogether, the above examples indicate that the costliness of immune activity probably drives some of the seasonal variation in this physiological system. Oftentimes, changes in immune activity are driven by short- and long-term fluctuations in hormones. In fact, the immune, endocrine and nervous systems are intricately connected and serve to communicate information about the internal and external environments to the control systems of the body. These relationships probably change seasonally, as hormone concentrations and receptor abundances and distributions vary as animals progress through different life stages (e.g. breeding, overwintering, moult, etc.). Below, we outline how several hormones influence the immune system, emphasizing those that probably mediate seasonal variation in immune function.
(a) Melatonin
Melatonin is thought to be a primary mediator of seasonality in the immune and reproductive systems (Guerrero & Reiter 2002; Hotchkiss & Nelson 2002). As a general principle, melatonin has negative effects on the reproductive axis, but its effects on the immune system are varied and depend on the specific arm of the immune system tested, the species studied and the timing of its delivery. One well-documented function of melatonin is to transduce day-length information into a physiological signal (Goldman & Nelson 1993). Pineal melatonin production occurs at night, but daylight suppresses its synthesis. It is the duration of the melatonin secretion rather than the amplitude that is the critical feature for the measurement of day length (Goldman 2001).
Melatonin, an indoleamine, is synthesized in a two-step enzymatic reaction from serotonin (Moore & Klein 1974). The rate-limiting enzyme (N-acetyltransferase) is produced via activation of sympathetic afferent nerves of the pineal gland (Moore 1996). Pineal-derived melatonin is critical in transducing day-length information to the mammalian reproductive system. Pinealectomy prevented photoperiod-induced regression of the gonads in Siberian hamsters, and both appropriately timed infusions and implantation of melatonin can induce the winter phenotype in long days (Bartness et al. 1993). This discovery led to the principle that melatonin is anti-gonadotrophic in mammals. Many avian species also use photoperiod to time reproduction. However, pineal melatonin does not appear to be the critical physiological cue to time reproduction for birds (Juss et al. 1993). The role of pineal melatonin in avian seasonality is difficult to generalize, in part, owing to the abundance of extra-pineal melatonin (Dawson et al. 2001). However, melatonin concentrations in birds show a similar circadian pattern of secretion that varies over the year. The persistence of this signal in birds suggests that it may be important for providing seasonal information for non-reproductive functions, including immune activity.
The effects of melatonin on the immune system are well established (Guerrero & Reiter 2002; Hotchkiss & Nelson 2002). Receptors for melatonin are found on mammalian and avian splenocytes, thymocytes and lymphocytes (Nelson & Demas 1996). In general, melatonin is associated with enhanced immune function in most laboratory animals. In domestic mice, pinealectomy reduced antibody-dependent cellular toxicity, antibody production and mitogen-stimulated splenocyte proliferation (Nelson & Demas 1996). However, house mice are not reproductively photoperiodic, and many strains have a genetic defect that prevents synthesis of melatonin (Reppert & Weaver 1995). Therefore, it is difficult to draw definitive conclusions regarding seasonality of immune activity from studies of domesticated mice.
Strong evidence exists that melatonin mediates many of the effects of photoperiod on the immune system in non-domesticated species. Generally, exogenous melatonin reproduces the immunological effects of short day lengths. Short-day deer mice, for example, enhanced in vitro cell-mediated immunity; exogenous melatonin produced the same effect in long days (Demas et al. 1996). Similarly, short photoperiods or exogenous melatonin prevented deer mice (Peromyscus maniculatus) from developing chemically induced tumours (Nelson & Blom 1994). However, melatonin can also inhibit certain aspects of the immune system. For example, Siberian hamsters in contrast to other photoperiodic rodents reduced in vitro cell-mediated immune activity and attenuated antibody production in short days when compared with long days (Prendergast et al. 2001b). Addition of physiological concentrations of melatonin to cultured lymphocytes reduced proliferative responses in long but not in short days. Pinealectomy also blocked the short-day reduction in antibody responses to sheep red blood cells (Yellon et al. 1999). Further, pineal melatonin can affect some components of the innate immune system in hamsters (Yellon et al. 2005), but this part of the immune system has been understudied when compared with the adaptive arm in this context.
The effects of photoperiod and melatonin on the immune system are temporally similar to the effects on the reproductive system. Photoperiodic rodents become refractory to short day lengths after prolonged exposure (approx. 20–24 weeks) and then ‘spontaneously’ regrow their testes. Melatonin does not affect immune activity in vivo or in vitro in refractory rodents (Prendergast & Nelson 2001; Prendergast et al. 2002b). Latitude of origin is related to responsiveness to photoperiod as deer mice from a high-latitude population, but not mice from a low-latitude population, responded reproductively to photoperiod and exogenous melatonin (Bronson 1985). The same pattern emerged for immune responses; animals from the high-latitude population, but not the low-latitude population, increased in vitro cell-mediated immune function in response to both melatonin and short day lengths (Demas et al. 1996).
It is important to note that manipulation of melatonin in the whole animal is associated with alterations in several neuroendocrine and physiological systems. Thus, it is difficult to determine whether immunological effects of melatonin are direct or indirect (Hotchkiss & Nelson 2002). Some evidence suggests that melatonin can directly affect immune cells and tissues. For instance, addition of melatonin to splenocyte cultures enhanced mitogen-stimulated proliferation in house mice and prairie voles; treatment with luzindole, a melatonin receptor antagonist, attenuated this effect (Drazen et al. 2000a; Drazen & Nelson 2001). Studies of animals that fail to respond reproductively to melatonin have illuminated the direct effects of this hormone on immune activity. European starlings (Sturnus vulgaris) are seasonal breeders that become refractory to long day lengths and require exposure to short days before they can again respond to long days. Exogenous melatonin prevented the decrease in splenocyte proliferation associated with photostimulation in castrated versus intact birds (Bentley et al. 1998).
(b) Glucocorticoids
Glucocorticoids are the end product, primary effectors and principal negative regulators of an important neuroendocrine axis (hypothalamus–pituitary–adrenal (HPA) axis). Originally described for their role in energy mobilization, glucocorticoids are now recognized as powerful mediators of many physiological processes including reproduction and immune activity. Unlike other hormones, glucocorticoids tend to suppress both reproduction and immune function. This observation has led to the principles that elevated glucocorticoids promote physiological and behavioural responses that (i) favour immediate survival at the expense of other processes (i.e. the emergency life-history stage hypothesis; Wingfield et al. 1998) and (ii) maintain homeostasis in the face of environmental changes (i.e. allostasis; McEwen & Wingfield 2003).
Interaction between glucocorticoids and the immune system is complex and bidirectional. Stressor-induced elevated glucocorticoid concentrations can modulate immune activity; however, activation of the immune system can also drive the production of glucocorticoids (Turnbull & Rivier 1996; McEwen et al. 1997). Because glucocorticoids tend to suppress inflammation but be induced by proinflammatory stimuli, they have been conceptualized as ‘brakes’ on the immune system, having evolved to prevent runaway inflammation and promote fine-tuning of the immune response (Sapolsky et al. 2000). A wealth of information demonstrates how glucocorticoids suppress immune function (McEwen et al. 1997), which led to the conjecture that glucocorticoids are largely responsible for decrements in immune activity in free-living animals in winter (Nelson et al. 2002).
Now there is compelling evidence that in certain contexts glucocorticoids can enhance aspects of immune function. In many cases, immunosuppression may be immunoredistribution in disguise (Braude et al. 1999). From an adaptationist perspective, one might predict that animals would enhance immune function in parts of the body at times when injury is probable, such as during a territorial dispute or failed predation event. Recent data in mice and rats support part of this prediction; in response to acute stressors, circulating leucocytes do not die, but instead they leave the bloodstream and move into peripheral tissues (skin, gut and lymph nodes). In the absence of injury or infection, these cells return to the general circulation quickly (Dhabhar et al. 1995). One of the best examples of these stress-induced immunoredistributions comes from work on delayed-type hypersensitivity (DTH). The DTH response is characterized by T-cell-mediated trafficking of immune cells into the skin (Dhabhar & McEwen 1997). Thus, if acute stress truly enhances front line immune defences, then DTH responses should be enhanced in response to short-term stressors. Laboratory rats (R. rattus) restrained daily for three weeks prior to antigenic challenge displayed the expected suppression of DTH responses. In contrast, when animals were stressed briefly just before immune challenge, they enhanced responses relative to non-stressed control animals (Dhabhar et al. 1996). These stress-induced alterations in the DTH response were directly mediated by corticosterone.
Photoperiod affects the character of these stress-induced immunological redistributions, suggesting that these hormones probably influence seasonal changes in immune activity in wild animals. Siberian hamsters acutely stressed prior to immune challenge had elevated DTH responses, and this response was significantly augmented in short versus long day animals (Bilbo et al. 2002). This enhancement in skin immune function was associated with an enhanced glucocorticoid response and expedited movement of leucocytes out of the blood during restraint stress in short versus long day-housed hamsters (Bilbo et al. 2002). As with photoperiod, latitude of origin can also influence corticosteroid effects on the immune system. Tropical-dwelling, but not temperate, house sparrows failed to show immunosuppression in response to chronically elevated corticosterone (Martin et al. 2005).
(c) Androgens
Androgens, primarily testosterone (T), are necessary for the organization and expression of many male sexual behaviours and morphological traits. In contrast, androgens have long been considered immunosuppressive, particularly because females and gonadectomized males tend to have stronger immune responses than intact males (Klein 2000). Antagonistic effects of androgens on the immune system were suspected as early as 1898 when it was reported that rabbits castrated pre-pubertally had larger thymuses than intact animals (Calzolari 1898). In recent years, similar observations led to the immunocompetence handicap hypothesis (ICHH), a mechanistic version of the Hamilton–Zuk handicap hypothesis of sexual selection (Hamilton & Zuk 1982; Folstad & Karter 1992). This model, which provided one of the first attempts to link immune activity and reproduction in an ecological context, has recently been challenged because the effects of androgens on the immune system are apparently less consistent than once believed (Owen-Ashley et al. 2004; Roberts et al. 2004). In the laboratory, males of seasonally breeding rodent species housed in long days tend to maintain higher androgen concentrations in circulation and have weaker immune responses than conspecifics maintained in short days (Nelson et al. 2002; Nelson 2005). Hence, an early explanation of short day increases in immune activity involved the reduction in circulating androgens detected in these conditions (Nelson & Demas 1996).
It was soon recognized, however, that this explanation could not account for short-day increases in immune function seen in females, as T concentrations are generally low in females at all times of year. Further, because oestrogens tend to enhance immune activity, if photoperiodic changes in immune function are mainly due to fluctuations in sex steroid hormones, then females in short days that have low oestrogen concentrations should show reduced immune function when compared with long-day animals (or at least not exhibit male-like increases). Because these predictions were not substantiated in male and female deer mice (Demas & Nelson 1998) and gonadectomy and exogenous testosterone administration did not alter short-day enhancement of the DTH response in Siberian hamsters (Prendergast et al. 2005), this hypothesis has fallen out of favour.
Still, it is apparent that the immune and reproductive systems are intimately interconnected and that androgens are important components of these interactions. Indeed, the immune system can be modulated by androgens in some cases; conversely, activation of the immune system, particularly the innate arm, is associated with suppression of the reproductive neuroendocrine axis (Turnbull & Rivier 1997). Further, in domestic fowl, birds selected for strong antibody responses have smaller combs, sexually selected traits that are testosterone dependent and lower testosterone titres (Verhulst et al. 1999). Conversely, chickens infected with an intestinal parasite exhibited similar concentrations of testosterone and similar-sized combs as uninfected birds (Johnsen & Zuk 1998). As evidenced by these studies, trade-offs between reproduction and immune function may be mediated directly via communication between the reproductive and immune systems in certain contexts (Avitsur & Yirmiya 1999; Weil et al. 2006b), although these interactions tend to be complex.
The apparent immunosuppressive effects of androgens may sometimes be due to glucocorticoids (Evans et al. 2000; Berger et al. 2005), at least in certain experimental contexts. For example, captive dark-eyed juncos (Junco hyemalis) implanted with testosterone had reduced PHA responses when compared with those without implants. This effect could not be attributed directly to testosterone, as birds with implants also had higher circulating corticosterone (Casto et al. 2001). Similarly, house sparrows implanted with testosterone had decreased antibody response but increased corticosterone concentrations post-implantation (Evans et al. 2000). In this study, after controlling for corticosterone concentrations statistically, testosterone was positively associated with antibody production. In a related study, house sparrows implanted with testosterone and held in short days showed no suppression of cell-mediated immune activity, indicating that testosterone is not obligatorily immunosuppressive year-round in this species (Greenman et al. 2005).
(d) Prolactin
Prolactin is a peptide hormone released from the anterior pituitary (Goffin et al. 1999) that influences reproduction, growth and development, as well as water and electrolyte balance, maintenance of integumentary structures and components of the immune system (Yu-Lee 2002; Nelson 2005). In mammals, prolactin is regulated positively by hypothalamic thyrotrophin releasing hormone (TRH) and negatively by dopamine (Freeman et al. 2000). In birds, prolactin release is controlled by hypothalamic vasoactive intestinal peptide (el Halawani et al. 1996). Despite these regulatory differences, the short-day reduction in circulating prolactin concentrations is a nearly ubiquitous phenomenon that occurs even in short-day breeding species (Goldman & Nelson 1993).
The relationship between pituitary prolactin and immune function was suggested as early as 1930 when hypophysectomized (anterior pituitary removed) rats were observed to undergo thymic involution (Smith 1930). More recent support comes from studies showing deficits in cell-mediated and humoral immunity after hypophysectomy that are reversible via exogenous prolactin (Reber 1993). Prolactin receptors are expressed in primary lymphoid organs such as the thymus, lymph nodes, bone marrow and spleen and on peripheral T-cells, B-cells and macrophages (Leite De Moraes et al. 1995). Most studies of the effects of prolactin on the immune system have indicated enhancive effects (Yu-Lee 2002). Prolactin stimulated in vitro proliferative responses of natural killer cells, as well as T- and B-cells, to multiple mitogens (Matera et al. 1992). However, high concentrations of prolactin can be detrimental as evidenced by reduced proliferative responses to mitogens, reduced tumoricidal activity of natural killer cells and increased susceptibility to autoimmune diseases (Gerli et al. 1987; Matera et al. 1992; Vera-Lastra et al. 2002). Interestingly, prolactin opposes glucocorticoid-induced cell death in vitro (Fletcher-Chiappini et al. 1993) and improves macrophage function after trauma-haemorrhage or infection (Zellweger et al. 1996). These results and work in Snell dwarf mice, which are deficient in anterior pituitary hormones including prolactin, led to the hypothesis that prolactin may be part of a physiological system that opposes glucocorticoids and some inflammatory mediators by maintaining immunological homeostasis during stress (Dorshkind & Horseman 2000, 2001).
Few studies have directly tested the effects of prolactin on the immune system in a seasonal context in small mammals; some work on this subject has been conducted on larger mammals however (Auchtung & Dahl 2004). Furthermore in laboratory studies, prolactin treatment tends to be negatively associated with immune function in seasonal breeders. For instance, nearly 90% of female deer mice housed in long days and injected with the chemical carcinogen 9, 10-dimethyl-1, 2-benzanthracene developed squamous cell carcinomas; no mice housed in short days developed tumours. When long-day mice were treated with the prolactin release inhibitor, bromocriptine, the incidence of tumours declined by nearly 50% (Nelson & Blom 1994). This study suggests that the elevated prolactin during long days may decrease immune function to favour reproduction and/or somatic growth. In sum, characteristics of prolactin secretion indicate that it may be an important mediator of seasonal changes in immune activity, but insufficient evidence exists as yet to support this possibility.
(e) Leptin
The adipocyte-derived hormone leptin was originally discovered as the product of the ob gene (Zhang et al. 1994). Mice deficient in leptin are obese due to both overeating and decreased energy expenditure (Coleman 1978). Thus, early research on leptin focused on its role in body weight regulation and metabolism. However, leptin may also be an important regulator of the reproductive and immune systems and their interactions, as it communicates adiposity and satiety. Although there is no universal relationship between day length and leptin, many seasonal animals undergo annual variation in food intake and adiposity. Not surprisingly, seasonal variation in leptin has been reported in a variety of species including woodchucks (Marmota monax) and Siberian hamsters (Klingenspor et al. 1996; Concannon et al. 2001; Marie et al. 2001).
Leptin interacts with the immune system both directly via receptors on immune cells and indirectly by increasing metabolic fuel availability. Obese ob-/- mice have fewer T-cells, reduced macrophage responsiveness and impaired wound healing (Lord et al. 1998). Similar outcomes were induced in wild-type mice following severe food restriction (leading to decreased leptin production); these effects could be reversed by exogenous leptin treatment (Lord et al. 1998). In genetically unmodified mice, leptin enhanced phagocytosis and T-cell proliferation (Baumann et al. 1996). Inflammatory stimuli such as LPS or recombinant proinflammatory cytokines, including IL-1β and tumour necrosis factor alpha (TNFα), acutely increased leptin concentrations and may be involved in the anorexia associated with inflammation (Grunfeld et al. 1996).
Siberian hamsters have stronger humoral immune responses in long versus short days. Treatment with exogenous leptin eliminated the short-day reduction in humoral immunity, body mass and food intake, but it did not affect these measures in long-day animals. In addition, leptin did not enhance immune function in short days when food intake was limited to levels consumed by short-day hamsters not supplemented with leptin (Drazen et al. 2001). Previous studies have indicated a photoperiod-induced differential sensitivity to leptin in this species (Klingenspor et al. 2000). A certain proportion of Siberian hamsters, as other photoperiodic animals, do not respond to short day lengths with reproductive regression (Prendergast et al. 2001a). Such animals produced anti-KLH IgG concentrations reflective of their photoperiodic exposure, not their reproductive state; that is, immune function of the reproductive non-responders was not different from reproductive responders when leptin was provided. However, leptin concentrations of the short day non-responders resembled that of long-day hamsters (Drazen et al. 2000b). A recent study demonstrated that leptin directly regulates humoral immunity in response to energy stores in Siberian hamsters. Hamsters undergoing lipectomy and treated with leptin did not display the decreased antibody responsiveness to a novel antigen that lipectomized, vehicle-treated hamsters did (Demas & Sakaria 2005). Taken together, these data indicate that leptin may be an important signal driving seasonal counterbalances between immune function and reproduction, but as with prolactin further study is necessary.
7. Future prospects
A recent effort has been made to delineate the best way to study the immune systems of animals in ecologically relevant contexts (Norris & Evans 2000). A recent comparative study in birds indicated that single measures of immune activity do not adequately characterize inter- and intraspecific variation in immune defence (Matson et al. 2006). If these data hold for other taxa, then single measures of immune activity are probably insufficient to characterize immunocompetence. To resolve problems associated with measuring something as complicated and dynamic as immunocompetence, some have suggested a shift in focus to measuring disease resistance because only such measures reliably indicate whether changes in immune defence are directly relevant to fitness (Adamo 2004). A related proposal suggests that immune responses be ignored altogether and the fitness consequences of immune challenges or disease resistance endpoints be favoured (Viney et al. 2005). Although these approaches may be appropriate in certain contexts, a third approach may be more realistic given the difficulties associated with characterizing resistance to multiple pathogens and/or measuring lifetime reproductive success, be more amenable to using the techniques currently available and be more relevant in studies in which the immune system itself is the subject of interest. This approach, the immune defence component model (IDCM; Schmid-Hempel & Ebert 2003), suggests that consideration of the general characteristics of the immune system can achieve these ends.
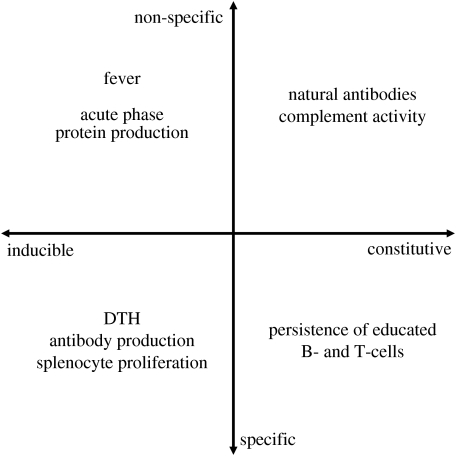
The IDCM model (Schmid-Hempel & Ebert 2003) separates the immune system into four quadrants composed of two intersecting axes: one a continuum of specific to non-specific defence, the other a continuum of constitutive to inducible defences (figure 2). These categorizations do not necessarily match the conventional ‘adaptive’ versus ‘innate’ categorizations of immune activity (Janeway et al. 2004); rather, they account for more basic features of immune defences that the conventional classifications lack. For instance, the IDCM recognizes that some animals may favour constitutive over inducible defence if they live in habitats where parasite threats are high. Organisms in disease-depauperate environments might allocate resources solely to inducible defence mechanisms and invest more in physiological systems promoting reproduction. Likewise, the high costs of generating lymphocyte diversity may be prohibitively costly for short-lived species (Ricklefs 1992; Martin et al. 2006a). The short-lived species may favour cheaper non-specific immune defences, especially if they are vagile and subsequently rarely participate in host–parasite arms races (Klasing 2004). Altogether, the IDCM leads to a new perspective for ecological immunology; instead of looking for changes in ‘immunocompetence’ over an animal's lifetime, attention should be focused on potential redistribution among different physiological components, including aspects of the immune system. In other words, trade-offs between reproduction and immune defence may be just that, or they may represent reallocations within the immune system itself (perhaps in a cost-effective manner) that would not be detected unless multiple, particular aspects of immune activity were measured (Martin et al. 2006c).
Figure 2.
The immune defence component model (IDCM). This conceptualization of the immune system, originally proposed by Schmid-Hempel & Ebert (2003) separates immune defences into four quadrants representing the four types of defences available to animals. One axis represents a continuum from non-specific to specific defences, whereas the other axis represents static (constitutive) versus dynamic (induced) defences. In the figure, we categorize several of the assays currently favoured in ecological immunology (see table 1 for details of each assay). Adapted from Schmid-Hempel & Ebert (2003).
An additional benefit of this model is that unlike the conventional ‘innate–adaptive’ dichotomy, the IDCM is as applicable to other animal species as it is to mammals and birds. Additionally, the IDCM is amenable to the immunological techniques currently available to ecologists, although it emphasizes incorporation of potentially beneficial prophylactic defences (e.g. behavioural avoidance, grooming). Behavioural defences may be important as these defences may often alleviate the need for strong downstream investments in fever and antibody production, but they are rarely studied in this context.
A further critical requisite of the IDCM is that the immune assays that ecological immunologists currently favour should be understood as more than simple measures of immunocompetence. For example, PHA-induced wing-web swelling cannot be coarsely characterized as a cell-mediated immune index, as the immune activity underlying the swelling response involves more than just T-cells (Martin et al. 2006a). Moreover, care must be taken when initially categorizing immune measures ecologists commonly use. Figure 2 depicts our assessments of some favoured immunological assays, but these categorizations are open to debate. According to the IDCM, antibody production should be classified as a specific inducible defence. However, B-cells and constitutively expressed cells of the innate immune system can both influence antibody production. Likewise, DTH responses are typified as cell-mediated assays because T-cells orchestrate local inflammation even though macrophages and granulocytes are responsible for much of the swelling. For these reasons, the development of new immune assays and more in-depth studies of currently favoured assays is imperative. Years of successful research in other fields indicate that ecologists need not abandon favoured techniques just yet (Adamo 2004; Viney et al. 2005); they need only to appreciate that immune function is no more a monolithic entity than nervous system function (Matson et al. 2006).
8. Summary
Research in the past decade has revealed that changes in the way organisms invest in immune defence over their lifetimes may help explain long-standing unresolved phenomena in evolutionary ecology. Refinements of current approaches in ecological immunology, particularly more attention to the nature of immune defence itself, may expand our appreciation of linkages among disease resistance, immune function and fitness. Studies in domestic chickens have shown that selection for antibody responsiveness can impinge on growth and reproductive output (Norris & Evans 2000). Conversely, lines of chickens selected for rapid growth versus prolific egg production use very distinct innate immune defences when given bacterial challenges (Leshchinsky & Klasing 2001). The natural extension of these studies in terms of seasonality in reproduction and immune activity in small birds and mammals might involve selective breeding. Such research could identify how immune investments impinge on life-history characters in an evolutionary sense, and they could intimate the endocrine mechanisms that drive these relationships.
In addition, emphasis on pre-emptive (e.g. constitutive) defences may help us better understand resistance or control of infection. Sickness behaviour, for example, is a strategy common to most vertebrates, but only recently have the adaptive benefits of these behaviours been proposed (Hart 1988). Finally, the physiological mechanisms by which immune challenges affect fitness remain unresolved. Are trade-offs between reproduction and immunity predominantly mediated through activation of acute phase responses (Klasing 2004)? If so, then proinflammatory cytokines (in the periphery and the brain) should modulate many important outcomes, and research on this level would lead to identification of the molecular mechanisms, which to this point in ecological immunology are generally unknown. Altogether, it is becoming apparent that variation in immune defence in vertebrates is the rule more than the exception. It is also apparent, however, that we are only at the threshold of a new and enormously interactive realm of biology.
Acknowledgments
All studies cited from our laboratory comply with US NIH guidelines for animal research and were approved by the appropriate institutional animal care and use committees.
The authors thank Michaela Hau, Kristen Navara, Leah Pyter and Brian Trainor for their comments on previous drafts. Preparation of this manuscript and studies cited from our laboratory were supported by NIH grants MH57535 and MH66144 and NSF grant IBN 04-16897.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Adamo S.A. How should behavioural ecologists interpret measurements of immunity? Anim. Behav. 2004;68:1443–1449. doi:10.1016/j.anbehav.2004.05.005 [Google Scholar]
- Ardia D.R. Tree swallows trade off immune function and reproductive effort differently across their range. Ecology. 2005;86:2040–2046. doi:10.1890/04-1619 [Google Scholar]
- Ardia D.R, Schat K.A, Winkler D.W. Reproductive effort reduces long-term immune function in breeding tree swallows (Tachycineta bicolor) Proc. R. Soc. B. 2003;270:1679–1683. doi: 10.1098/rspb.2003.2424. doi:10.1098/rspb.2003.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav. Immun. 1997;11:107–118. doi: 10.1006/brbi.1997.0485. doi:10.1006/brbi.1997.0485 [DOI] [PubMed] [Google Scholar]
- Auchtung T.L, Dahl G.E. Prolactin mediates photoperiodic immune enhancement: effects of administration of exogenous prolactin on circulating concentrations, receptor expression, and immune function in steers. Biol. Reprod. 2004;71:1913–1918. doi: 10.1095/biolreprod.104.031005. doi:10.1095/biolreprod.104.031005 [DOI] [PubMed] [Google Scholar]
- Avitsur R, Yirmiya R. The immunobiology of sexual behavior: gender differences in the suppression of sexual activity during illness. Pharmacol. Biochem. Behav. 1999;64:787–796. doi: 10.1016/s0091-3057(99)00165-3. doi:10.1016/S0091-3057(99)00165-3 [DOI] [PubMed] [Google Scholar]
- Bartness T.J, Powers J.B, Hastings M.H, Bittman E.L, Goldman B.D. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. doi:10.1111/j.1600-079X.1993.tb00903.x [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella K.K, White D.W, Dembski M, Bailon P.S, Kim H, Lai C.F, Tartaglia L.A. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl Acad. Sci. USA. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. doi:10.1073/pnas.93.16.8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayyari G.R, Huff W.E, Rath N.C, Balog J.M, Newberry L.A, Villines J.D, Skeeles J.K, Anthony N.B, Nestor K.E. Effect of the genetic selection of turkeys for increased body weight and egg production on immune and physiological responses. Poult. Sci. 1997;76:289–296. doi: 10.1093/ps/76.2.289. [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Demas G.E, Nelson R.J, Ball G.F. Melatonin, immunity and cost of reproductive state in male European starlings. Proc. R. Soc. B. 1998;265:1191–1195. doi: 10.1098/rspb.1998.0418. doi:10.1098/rspb.1998.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Martin L.B, Wikelski M, Romero L.M, Kalko E.K.V, Vitousek M.N, Rodl T. Corticosterone suppresses immune activity in territorial Galapagos marine iguanas during reproduction. Horm. Behav. 2005;47:419–429. doi: 10.1016/j.yhbeh.2004.11.011. doi:10.1016/j.yhbeh.2004.11.011 [DOI] [PubMed] [Google Scholar]
- Bilbo S.D, Nelson R.J. Melatonin regulates energy balance and attenuates fever in Siberian hamsters. Endocrinology. 2002;143:2527–2533. doi: 10.1210/endo.143.7.8922. [DOI] [PubMed] [Google Scholar]
- Bilbo S.D, Dhabhar F.S, Viswanathan K, Saul A, Yellon S.M, Nelson R.J. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc. Natl Acad. Sci. USA. 2002;99:4067–4072. doi: 10.1073/pnas.062001899. doi:10.1073/pnas.062001899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am. Nat. 2003;161:367–379. doi: 10.1086/346134. doi:10.1086/346134 [DOI] [PubMed] [Google Scholar]
- Brainard G.C, Knobler R.L, Podoin P.I, Lavasa M, Lubin F.D. Neuroimmunology: modulation of the hamster immune system by photoperiod. Life Sci. 1987;40:1319–1326. doi: 10.1016/0024-3205(87)90589-3. doi:10.1016/0024-3205(87)90589-3 [DOI] [PubMed] [Google Scholar]
- Braude S, Tang-Martinez Z, Taylor G.T. Stress, testosterone, and the immunoredistribution hypothesis. Behav. Ecol. 1999;10:345–350. doi:10.1093/beheco/10.3.345 [Google Scholar]
- Bronson F.H. Mammalian reproduction: an ecological perspective. Biol. Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. doi:10.1095/biolreprod32.1.1 [DOI] [PubMed] [Google Scholar]
- Bronson F.H. Mammalian reproductive strategies—genes, photoperiod and latitude. Reprod. Nutr. Dev. 1988;28:335–347. doi: 10.1051/rnd:19880301. [DOI] [PubMed] [Google Scholar]
- Calzolari A. Recherches experimentales sur un rapport probable entre la function du thymus et celle des testicules. Arch. Ital. Biol. 1898;30:71–77. [Google Scholar]
- Casto J.M, Nolan V, Ketterson E.D. Steroid hormones and immune function: experimental studies in wild and captive dark-eyed juncos (Junco hyemalis) Am. Nat. 2001;157:408–420. doi: 10.1086/319318. doi:10.1086/319318 [DOI] [PubMed] [Google Scholar]
- Christe P, Arlettaz R, Vogel P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis) Ecol. Lett. 2000;3:207–212. doi:10.1046/j.1461-0248.2000.00142.x [Google Scholar]
- Cichon M. Costs of incubation and immunocompetence in the collared flycatcher. Oecologia. 2000;125:453–457. doi: 10.1007/s004420000461. doi:10.1007/s004420000461 [DOI] [PubMed] [Google Scholar]
- Cohn M, Langman R.E. The Protecton—the unit of humoral immunity selected by evolution. Immunol. Re. 1990;115:7–147. doi: 10.1111/j.1600-065x.1990.tb00783.x. doi:10.1111/j.1600-065X.1990.tb00783.x [DOI] [PubMed] [Google Scholar]
- Coleman D. Obese and diabetes: two mutant genes causing diabetes–obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. doi:10.1007/BF00429772 [DOI] [PubMed] [Google Scholar]
- Concannon P, Levac K, Rawson R, Tennant B, Bensadoun A. Seasonal changes in serum leptin, food intake, and body weight in photoentrained woodchucks. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R951–R959. doi: 10.1152/ajpregu.2001.281.3.R951. [DOI] [PubMed] [Google Scholar]
- Dark J, Johnston P.G, Healy M, Zucker I. Latitude of origin influences photoperiodic control of reproduction of deer mice (Peromyscus maniculatus) Biol. Reprod. 1983;28:213–220. doi: 10.1095/biolreprod28.1.213. doi:10.1095/biolreprod28.1.213 [DOI] [PubMed] [Google Scholar]
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. doi:10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Deerenberg C, Arpanius V, Daan S, Bos N. Reproductive effort decreases antibody responsiveness. Proc. R. Soc. B. 1997;264:1021–1029. doi:10.1098/rspb.1997.0141 [Google Scholar]
- Demas G.E. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm. Behav. 2004;45:173–180. doi: 10.1016/j.yhbeh.2003.11.002. doi:10.1016/j.yhbeh.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Demas G.E, Nelson R.J. Short-day enhancement of immune function is independent of steroid hormones in deer mice (Peromyscus maniculatus) J. Comp. Physiol. B. 1998;168:419–426. doi: 10.1007/s003600050161. doi:10.1007/s003600050161 [DOI] [PubMed] [Google Scholar]
- Demas G.E, Nelson R.J. Lack of immunological responsiveness to photoperiod in a tropical rodent, Peromyscus aztecus hylocetes. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2003;173:171–176. doi: 10.1007/s00360-002-0325-5. [DOI] [PubMed] [Google Scholar]
- Demas G.E, Sakaria S. Leptin regulates energetic tradeoffs between body fat and humoural immunity. Proc. R. Soc. B. 2005;272:1845–1850. doi: 10.1098/rspb.2005.3126. doi:10.1098/rspb.2005.3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas G.E, Klein S.L, Nelson R.J. Reproductive and immune responses to photoperiod and melatonin are linked in Peromyscus subspecies. J. Comp. Physiol. A. 1996;179:819–825. doi: 10.1007/BF00207360. doi:10.1007/BF00207360 [DOI] [PubMed] [Google Scholar]
- Demas G.E, Chefer V, Talan M.I, Nelson R.J. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am. J. Physiol. Regul. Integrat. Compar. Physiol. 1997;42:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Demas G.E, Drazen D.L, Nelson R.J. Reductions in total body fat decrease humoral immunity. Proc. R. Soc. B. 2003;270:905–911. doi: 10.1098/rspb.2003.2341. doi:10.1098/rspb.2003.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derting T.L, Compton S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus) Physiol. Biochem. Zool. 2003;76:744–752. doi: 10.1086/375662. doi:10.1086/375662 [DOI] [PubMed] [Google Scholar]
- Dhabhar F.S, McEwen B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav. Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. doi:10.1006/brbi.1997.0508 [DOI] [PubMed] [Google Scholar]
- Dhabhar F.S, Miller A.H, McEwen B.S, Spencer R.L. Effects of stress on immune cell distribution—dynamics and hormonal mechanisms. J. Immunol. 1995;154:5511–5527. [PubMed] [Google Scholar]
- Dhabhar F.S, Miller A.H, McEwen B.S, Spencer R.L. Stress-induced changes in blood leukocyte distribution—role of adrenal steroid hormones. J. Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- Dorshkind K, Horseman N.T. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr. Rev. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. doi:10.1210/er.21.3.292 [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Horseman N.D. Anterior pituitary hormones, stress, and immune system homeostasis. BioEssays. 2001;23:288–294. doi: 10.1002/1521-1878(200103)23:3<288::AID-BIES1039>3.0.CO;2-P. doi:10.1002/1521-1878(200103)23:3<288::AID-BIES1039>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- Drazen D.L, Nelson R.J. Melatonin receptor subtype MT2 (Mel 1b) and not mt1 (Mel 1a) is associated with melatonin-induced enhancement of cell-mediated and humoral immunity. Neuroendocrinology. 2001;74:178–184. doi: 10.1159/000054684. doi:10.1159/000054684 [DOI] [PubMed] [Google Scholar]
- Drazen D.L, Klein S.L, Yellon S.M, Nelson R.J. In vitro melatonin treatment enhances splenocyte proliferation in prairie voles. J. Pineal Res. 2000a;28:34–40. doi: 10.1034/j.1600-079x.2000.280105.x. doi:10.1034/j.1600-079x.2000.280105.x [DOI] [PubMed] [Google Scholar]
- Drazen D.L, Kriegsfeld L.J, Schneider J.E, Nelson R.J. Leptin, but not immune function, is linked to reproductive responsiveness to photoperiod. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000b;278:R1401–R1407. doi: 10.1152/ajpregu.2000.278.6.R1401. [DOI] [PubMed] [Google Scholar]
- Drazen D.L, Demas G.E, Nelson R.J. Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus) Endocrinology. 2001;142:2768–2775. doi: 10.1210/endo.142.7.8271. doi:10.1210/en.142.7.2768 [DOI] [PubMed] [Google Scholar]
- Drazen D.L, Trasy A, Nelson R.J. Photoperiod differentially affects energetics of immunity in pregnant and lactating Siberian hamsters (Phodopus sungorus) Can. J. Zool.-Revue Canadienne De Zoologie. 2003;81:1406–1413. doi:10.1139/z03-120 [Google Scholar]
- Drent R.H, Daan S. The prudent parent—energetic adjustments in avian breeding. Ardea. 1980;68:225–252. [Google Scholar]
- Dubiec A, Cichon M. Seasonal decline in nestling cellular immunocompetence results from environmental factors - an experimental study. Can. J. Zool. -Revue Canadienne De Zoologie. 2005;83:920–925. doi:10.1139/z05-076 [Google Scholar]
- Ebert D. Virulence and local adaptation of a horizontally transmitted parasite. Science. 1994;265:1084–1086. doi: 10.1126/science.265.5175.1084. doi:10.1126/science.265.5175.1084 [DOI] [PubMed] [Google Scholar]
- el Halawani M.E, Pitts G.R, Sun S, Silsby J.L, Sivanandan V. Active immunization against vasoactive intestinal peptide prevents photo-induced prolactin secretion in turkeys. Gen. Comp. Endocrinol. 1996;104:76–83. doi: 10.1006/gcen.1996.0143. doi:10.1006/gcen.1996.0143 [DOI] [PubMed] [Google Scholar]
- Eraud C, Duriez O, Chastel O, Faivre B. The energetic cost of humoral immunity in the Collared Dove, Streptopelia decaocto: is the magnitude sufficient to force energy-based trade-offs? Funct. Ecol. 2005;19:110–118. doi:10.1111/j.0269-8463.2005.00934.x [Google Scholar]
- Evans M.R, Goldsmith A.R, Norris S.R.A. The effects of testosterone on antibody production and plumage coloration in male house sparrows (Passer domesticus) Behav. Ecol. Sociobiol. 2000;47:156–163. doi:10.1007/s002650050006 [Google Scholar]
- Fair J.M, Myers O.B. The ecological and physiological costs of lead shot and immunological challenge to developing western bluebirds. Ecotoxicology. 2002;11:199–208. doi: 10.1023/a:1015474832239. doi:10.1023/A:1015474832239 [DOI] [PubMed] [Google Scholar]
- Fitzgerald S.D, Fitzgerald A.L, Reed W.M, Burnstein T. Immune function in pheasants experimentally infected with marble spleen disease virus. Avian Dis. 1992;36:410–414. doi:10.2307/1591521 [PubMed] [Google Scholar]
- Fletcher-Chiappini S, Compton M, La Voie H, Day E, Witorsch R. Glucocorticoid-prolactin interactions in Nb2 lymphoma cells: antiproliferative versus anticytolytic effects. Proc. Soc. Exp. Biol. Med. 1993;202:345–352. doi: 10.3181/00379727-202-43545. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter A.J. Parasites, bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi:10.1086/285346 [Google Scholar]
- Freeman M.E, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol. Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Garamszegi L.Z, Moller A.P, Torok J, Michl G, Peczely P, Richard M. Immune challenge mediates vocal communication in a passerine bird: an experiment. Behav. Ecol. 2004;15:148–157. doi:10.1093/beheco/arg108 [Google Scholar]
- Gerli R, Riccardi C, Nicoletti I, Orlandi S, Cernetti C, Spinozzi F, Rambotti P. Phenotypic and functional abnormalities of T lymphocytes in pathological hyperprolactinemia. J. Clin. Immunol. 1987;7:463–470. doi: 10.1007/BF00915056. doi:10.1007/BF00915056 [DOI] [PubMed] [Google Scholar]
- Goffin V, et al. From the molecular biology of prolactin and its receptor to the lessons learned from knockout mice models. Genet. Anal. 1999;15:189–201. doi: 10.1016/s1050-3862(99)00025-x. [DOI] [PubMed] [Google Scholar]
- Goldman B.D. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. doi:10.1177/074873001129001980 [DOI] [PubMed] [Google Scholar]
- Goldman B.D, Nelson R.J. Melatonin and seasonality in mammals. In: Reiter R.J, editor. Melatonin: biosynthesis, physiological effects, and clinical applications. CRC; Boca Raton, FL: 1993. pp. 225–252. [Google Scholar]
- Gonzalez G, Sorci G, de Lope F. Seasonal variation in the relationship between cellular immune response and badge size in male house sparrows (Passer domesticus) Behav. Ecol. Sociobiol. 1999;46:117–122. doi:10.1007/s002650050600 [Google Scholar]
- Greenman C.G, Martin L.B, Hau M. Reproductive state, but not testosterone, reduces immune function in male house sparrows (Passer domesticus) Physiol. Biochem. Zool. 2005;78:60–68. doi: 10.1086/425194. doi:10.1086/425194 [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold K.R. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero J.M, Reiter R.J. Melatonin–immune system relationships. Curr. Top. Med. Chem. 2002;2:167–179. doi: 10.2174/1568026023394335. doi:10.2174/1568026023394335 [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Nordling D, Andersson M.S, Sheldon B.C, Qvarnstrom A. Infectious-diseases, reproductive effort and the cost of reproduction in birds. Phil. Trans. R. Soc. B. 1994;346:323–331. doi: 10.1098/rstb.1994.0149. doi:10.1098/rstb.1994.0149 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circannual rhythms in birds. Curr. Opin. Neurobiol. 2003;13:770–778. doi: 10.1016/j.conb.2003.10.010. doi:10.1016/j.conb.2003.10.010 [DOI] [PubMed] [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds—a role for parasites. Science. 1982;218:384–387. doi: 10.1126/science.7123238. doi:10.1126/science.7123238 [DOI] [PubMed] [Google Scholar]
- Hanssen S.A, Hasselquist D, Folstad I, Erikstad K.E. Costs of immunity: immune responsiveness reduces survival in a vertebrate. Proc. R. Soc. B. 2004;271:925–930. doi: 10.1098/rspb.2004.2678. doi:10.1098/rspb.2004.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen S.A, Hasselquist D, Folstad I, Erikstad K.E. Cost of reproduction in a long-lived bird: incubation effort reduces immune function and future reproduction. Proc. R. Soc. B. 2005;272:1039–1046. doi: 10.1098/rspb.2005.3057. doi:10.1098/rspb.2005.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. doi:10.1016/S0149-7634(88)80004-6 [DOI] [PubMed] [Google Scholar]
- Hemborg C, Merila¨ J. Reproductive investment and moult-breeding overlap in the collared flycatcher Ficedula albicollis: an experimental approach. Annales Zoologici Fennici. 1999;36:1–9. [Google Scholar]
- Horak P, Saks L, Ots I, Kullissaar T, Kollist H, Zilmer M. Physiological effects of immune challenge in captive greenfinches (Carduelis chloris) Can. J. Zool.-Revue Canadienne De Zoologie. 2003;81:371–379. doi:10.1139/z03-020 [Google Scholar]
- Hotchkiss A.K, Nelson R.J. Melatonin and immune function: hype or hypothesis? Critic. Rev. Immunol. 2002;22:351–371. [PubMed] [Google Scholar]
- Ilmonen P, Taarna T, Hasselquist D. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc. R. Soc. B. 2000;267:665–670. doi: 10.1098/rspb.2000.1053. doi:10.1098/rspb.2000.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmonen P, Taarna T, Hasselquist D. Are incubation costs in female pied flycatchers expressed in humoral immune responsiveness or breeding success? Oecologia. 2002;130:199–204. doi: 10.1007/s004420100804. [DOI] [PubMed] [Google Scholar]
- Janeway C.A, Travers P, Walport M, Schlomchik M.J. Immunobiology. Garland Science; New York: NY: 2004. [Google Scholar]
- Johnsen T.S, Zuk M. Parasites, morphology, and blood characters in male Red Jungle Fowl during development. Condor. 1998;100:749–752. doi:10.2307/1369760 [Google Scholar]
- Juss T.S, Meddle S.L, Servant R.S, King V.M. Melatonin and photoperiodic time measurement in Japanese quail (Coturnix coturnix japonica) Proc. R. Soc. B. 1993;254:21–28. doi: 10.1098/rspb.1993.0121. doi:10.1098/rspb.1993.0121 [DOI] [PubMed] [Google Scholar]
- Kilpimaa J, Alatalo R.V, Siitari H. Trade-offs between sexual advertisement and immune function in the pied flycatcher (Ficedula hypoleuca) Proc. R. Soc. B. 2004;271:245–250. doi: 10.1098/rspb.2003.2568. doi:10.1098/rspb.2003.2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey S.G, Prendergast B.J, Nelson R.J. Photoperiod and stress affect wound healing in Siberian hamsters. Physiol. Behav. 2003;78:205–211. doi: 10.1016/s0031-9384(02)00967-8. doi:10.1016/S0031-9384(02)00967-8 [DOI] [PubMed] [Google Scholar]
- Klasing K.C. Nutritional modulation of resistance to infectious diseases. Poult. Sci. 1998;77:1119–1125. doi: 10.1093/ps/77.8.1119. [DOI] [PubMed] [Google Scholar]
- Klasing K.C. The costs of immunity. Acta Zoologica Sinica. 2004;50:961–969. [Google Scholar]
- Klasing K.C, Peng R.K. Influence of cell sources, stimulating agents, and incubation conditions on release of interleukin-1 from chicken macrophages. Dev. Comp. Immunol. 1987;11:385–394. doi: 10.1016/0145-305x(87)90082-6. doi:10.1016/0145-305X(87)90082-6 [DOI] [PubMed] [Google Scholar]
- Klein S.L. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci. Biobehav. Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. doi:10.1016/S0149-7634(00)00027-0 [DOI] [PubMed] [Google Scholar]
- Klingenspor M, Dickopp A, Heldmaier G, Klaus S. Short photoperiod reduces leptin gene expression in white and brown adipose tissue of Djungarian hamsters. FEBS Lett. 1996;399:290–294. doi: 10.1016/s0014-5793(96)01343-9. [DOI] [PubMed] [Google Scholar]
- Klingenspor M, Niggemann H, Heldmaier G. Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus. J. Comp. Physiol. B. 2000;170:37–43. doi: 10.1007/s003600050005. doi:10.1007/s003600050005 [DOI] [PubMed] [Google Scholar]
- Korytko A.I, Dluzen D.E, Blank J.L. Photoperiod and steroid-dependent adjustments in hypothalamic gonadotropic hormone-releasing hormone, dopamine, and norepinephrine content in male deer mice. Biol. Reprod. 1997;56:617–624. doi: 10.1095/biolreprod56.3.617. doi:10.1095/biolreprod56.3.617 [DOI] [PubMed] [Google Scholar]
- Ksiazek A, Konarzewski M, Chadzinska M, Cichon M. Costs of immune response in cold-stressed laboratory mice selected for high and low basal metabolism rates. Proc. R. Soc. B. 2003;270:2025–2031. doi: 10.1098/rspb.2003.2474. doi:10.1098/rspb.2003.2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara S, et al. Seasonal changes in the hypothalamo-pituitary-testes axis of the Japanese wood mouse (Apodemus speciosus) Anat. Rec. 2000;260:366–372. doi: 10.1002/1097-0185(20001201)260:4<365::AID-AR50>3.0.CO;2-M. doi:10.1002/1097-0185(20001201)260:4<365::AID-AR50>3.0.CO;2-M [DOI] [PubMed] [Google Scholar]
- Lee K.A, Klasing K.C. A role for immunology in invasion biology. Trends Ecol. Evol. 2004;19:523–529. doi: 10.1016/j.tree.2004.07.012. doi:10.1016/j.tree.2004.07.012 [DOI] [PubMed] [Google Scholar]
- Lee K.A, Martin L.B, Wikelski M.C. Responding to inflammatory challenges is less costly for a successful avian invader, the house sparrow (Passer domesticus), than its less-invasive congener. Oecologia. 2005;145:244–251. doi: 10.1007/s00442-005-0113-5. doi:10.1007/s00442-005-0113-5 [DOI] [PubMed] [Google Scholar]
- Leite De Moraes M.C, Touraine P, Gagnerault M.C, Savino W, Kelly P.A, Dardenne M. Prolactin receptors and the immune system. Ann. Endocrinol. (Paris) 1995;56:567–570. [PubMed] [Google Scholar]
- Leshchinsky T.V, Klasing K.C. Divergence of the inflammatory response in two types of chickens. Dev. Comp. Immunol. 2001;25:629–638. doi: 10.1016/s0145-305x(01)00023-4. doi:10.1016/S0145-305X(01)00023-4 [DOI] [PubMed] [Google Scholar]
- Lifjeld J.T, Dunn P.O, Whittingham L.A. Short-term fluctuations in cellular immunity of tree swallows feeding nestlings. Oecologia. 2002;130:185–190. doi: 10.1007/s004420100798. doi:10.1007/s004420100798 [DOI] [PubMed] [Google Scholar]
- Lindström K.M, Foufopoulos J, Parn H, Wikelski M. Immunological investments reflect parasite abundance in island populations of Darwin's finches. Proc. R. Soc. B. 2004;271:1513–1519. doi: 10.1098/rspb.2004.2752. doi:10.1098/rspb.2004.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi:10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Lochmiller R.L, Vestey M.R, Boren J.C. Relationship between protein nutritional-status and immunocompetence in northern bobwhite chicks. Auk. 1993;110:503–510. [Google Scholar]
- Lochmiller R.L, Vestey M.R, McMurry S.T. Temporal variation in humoral and cell-mediated immune-response in a Sigmodon hispidus population. Ecology. 1994;75:236–245. doi:10.2307/1939397 [Google Scholar]
- Lord G.M, Matarese G, Howard J.K, Baker R.J, Bloom S.R, Lechler R.I. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. doi:10.1038/29795 [DOI] [PubMed] [Google Scholar]
- Lozano G.A, Lank D.B. Seasonal trade-offs in cell-mediated immunosenescence in ruffs (Philomachus pugnax) Proc. R. Soc. B. 2003;270:1203–1208. doi: 10.1098/rspb.2002.2309. doi:10.1098/rspb.2002.2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano G.A, Ydenberg R.C. Transgenerational effects of maternal immune challenge in tree swallows (Tachycineta bicolor) Can. J. Zool.-Revue Canadienne De Zoologie. 2002;80:918–925. doi:10.1139/z02-063 [Google Scholar]
- Lynch G.R, Heath H.W, Johnston C.M. Effect of geographical origin on the photoperiodic control of reproduction in the white-footed mouse, Peromyscus leucopus. Biol. Reprod. 1981;25:475–480. doi: 10.1095/biolreprod25.3.475. doi:10.1095/biolreprod25.3.475 [DOI] [PubMed] [Google Scholar]
- Marie M, Findlay P.A, Thomas L, Adam C.L. Daily patterns of plasma leptin in sheep: effects of photoperiod and food intake. J. Endocrinol. 2001;170:277–286. doi: 10.1677/joe.0.1700277. doi:10.1677/joe.0.1700277 [DOI] [PubMed] [Google Scholar]
- Martin L.B. Trade-offs between molt and immune activity in two populations of house sparrows (Passer domesticus) Can. J. Zool.-Revue Canadienne De Zoologie. 2005;83:780–787. [Google Scholar]
- Martin S.M, Malkinson T.J, Veale W.L, Pittman Q.J. Fever in pregnant parturient, and lactating rats. Am. J. Physiol. -Regul. Integr. Comp. Physiol. 1995;37:R919–R923. doi: 10.1152/ajpregu.1995.268.4.R919. [DOI] [PubMed] [Google Scholar]
- Martin L.B, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. B. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. doi:10.1098/rspb.2002.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.B, Pless M, Svoboda J, Wikelski M. Immune activity in temperate and tropical house sparrows: A common-garden experiment. Ecology. 2004;85:2323–2331. doi:10.1890/03-0365 [Google Scholar]
- Martin L.B, Gilliam J, Han P, Lee K, Wikelski M. Corticosterone suppresses cutaneous immune function in temperate but not tropical house sparrows, Passer domesticus. Gen. Comp. Endocrinol. 2005;140:126–135. doi: 10.1016/j.ygcen.2004.10.010. doi:10.1016/j.ygcen.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Martin L.B, Hasselquist D, Wikelski M. Immune investments are linked to pace of life in house sparrows. Oecologia. 2006a;147:565–575. doi: 10.1007/s00442-005-0314-y. doi:10.1007/s00442-005-0314-y [DOI] [PubMed] [Google Scholar]
- Martin L.B, Han P, Kwong J, Hau M. Cutaneous immune activity varies with physiological state in female house sparrows (Passer domesticus) Physiol. Biochem. Zool. 2006b;79:775–783. doi: 10.1086/504608. [DOI] [PubMed] [Google Scholar]
- Martin L.B, Weil Z.M, Kuhlman J.R, Nelson R.J. Trade-offs between cutaneous immune responses in female white-footed mice (Peromyscus maniculatus) Funct. Ecol. 2006c;20:630–636. [Google Scholar]
- Martin L.B, Navara K.J, Weil Z.M, Nelson R.J. Immunological memory is compromised by food restriction in deer mice, Peromyscus maniculatus. Am. J. Physiol. Regul. Integrat. Compar. Physiol. 2007;292:R316–R320. doi: 10.1152/ajpregu.00386.2006. [DOI] [PubMed] [Google Scholar]
- Martinez J, Merino S, Rodriguez-Caabeiro F. Physiological responses to Trichinella spiralis infection in Wistar rats: is immune response costly? Helminthologia. 2004;41:67–71. [Google Scholar]
- Matera L, Cesano A, Bellone G, Oberholtzer E. Modulatory effect of prolactin on the resting and mitogen-induced activity of T, B, and NK lymphocytes. Brain Behav. Immun. 1992;6:409–417. doi: 10.1016/0889-1591(92)90039-q. doi:10.1016/0889-1591(92)90039-Q [DOI] [PubMed] [Google Scholar]
- Matson K.D, Cohen A.A, Klasing K.C, Ricklefs R.E, Scheuerlein A. No simple answers for ecological immunology: relationships among immune indices at the individual level break down at the species level in waterfowl. Proc. R. Soc. B. 2006;273:815–822. doi: 10.1098/rspb.2005.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S, Wingfield J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. doi:10.1016/S0018-506X(02)00024-7 [DOI] [PubMed] [Google Scholar]
- McEwen B.S, et al. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. doi:10.1016/S0165-0173(96)00012-4 [DOI] [PubMed] [Google Scholar]
- Monaghan P, Haussmann M.F. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 2006;21:47–53. doi: 10.1016/j.tree.2005.11.007. doi:10.1016/j.tree.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Monaghan P, Nager R.G. Why don't birds lay more eggs? Trends Ecol. Evol. 1997;12:270–274. doi: 10.1016/s0169-5347(97)01094-x. doi:10.1016/S0169-5347(97)01094-X [DOI] [PubMed] [Google Scholar]
- Moore R.Y. Neural control of the pineal gland. Behav. Brain Res. 1996;73:125–130. doi: 10.1016/0166-4328(96)00083-6. doi:10.1016/0166-4328(96)00083-6 [DOI] [PubMed] [Google Scholar]
- Moore R.Y, Klein D.C. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974;71:17–33. doi: 10.1016/0006-8993(74)90188-7. doi:10.1016/0006-8993(74)90188-7 [DOI] [PubMed] [Google Scholar]
- Moreno J, Sanz J.J, Arriero E. Reproductive effort and T-lymphocyte cell-mediated immunocompetence in female pied flycatchers Ficedula hypoleuca. Proc. R. Soc. B. 1999;266:1105–1109. doi:10.1098/rspb.1999.0750 [Google Scholar]
- Nelson R.J. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. doi:10.1016/j.it.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Nelson R.J. Sinauer Associates; Sunderland, MA: 2005. An introduction to behavioral endocrinology. [Google Scholar]
- Nelson R.J, Blom J.M. Photoperiodic effects on tumor development and immune function. J. Biol. Rhythms. 1994;9:233–249. doi: 10.1177/074873049400900305. doi:10.1177/074873049400900305 [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Demas G.E. Seasonal changes in immune function. Q. Rev. Biol. 1996;71:511–548. doi: 10.1086/419555. doi:10.1086/419555 [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Demas G.E, Klein S.L, Kriegsfeld L.J. Cambridge University Press; New York, NY: 2002. Seasonal patterns of stress, immune function, and disease. [Google Scholar]
- Nilsson J.A, Svensson E. The cost of reproduction: a new link between current reproductive effort and future reproductive success. Proc. R. Soc. B. 1996;263:711–714. doi:10.1098/rspb.1996.0106 [Google Scholar]
- Nordling D, Andersson M, Zohari S, Gustafsson L. Reproductive effort reduces specific immune response and parasite resistance. Proc. R. Soc. B. 1998;265:1291–1298. doi:10.1098/rspb.1998.0432 [Google Scholar]
- Norris K, Evans M.R. Ecological immunology: life history trade-offs and immune defense in birds. Behav. Ecol. 2000;11:19–26. doi:10.1093/beheco/11.1.19 [Google Scholar]
- Ots I, Kerimov A.B, Ivankina E.V, IIyina T.A, Horak P. Immune challenge affects basal metabolic activity in wintering great tits. Proc. R. Soc. B. 2001;268:1175–1181. doi: 10.1098/rspb.2001.1636. doi:10.1098/rspb.2001.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen-Ashley N.T, Wingfield J.C. Seasonal modulation of sickness behavior in free-living northwestern song sparrows (Melospiza melodia morphna) J. Exptl. Biol. 2006;209:3062–3070. doi: 10.1242/jeb.02371. doi:10.1242/jeb.02371 [DOI] [PubMed] [Google Scholar]
- Owen-Ashley N.T, Hasselquist D, Wingfield J.C. Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am. Nat. 2004;164:490–505. doi: 10.1086/423714. doi:10.1086/423714 [DOI] [PubMed] [Google Scholar]
- Palacios M.G, Martin T.E. Incubation period and immune function: a comparative field study among coexisting birds. Oecologia. 2006;146:505–512. doi: 10.1007/s00442-005-0220-3. doi:10.1007/s00442-005-0220-3 [DOI] [PubMed] [Google Scholar]
- Paramithiotis E, Ratcliffe M.J.H. Survivors of bursal B-cell production and emigration. Poult. Sci. 1994;73:991–997. doi: 10.3382/ps.0730991. [DOI] [PubMed] [Google Scholar]
- Parmentier H.K, Nieuwland M.G.B, Rijke E, Reilingh G.D, Schrama J.W. Divergent antibody responses to vaccines and divergent body weights of chicken lines selected for high and low humoral responsiveness to sheep red blood cells. Avian Dis. 1996;40:634–644. doi:10.2307/1592275 [PubMed] [Google Scholar]
- Piersma T. Do global patterns of habitat use and migration strategics co-evolve with relative investments in immunocompetence due to spatial variation in parasite pressure? Oikos. 1997;80:623–631. doi:10.2307/3546640 [Google Scholar]
- Pilorz V, Jackel M, Knudsen K, Trillmich F. The cost of a specific immune response in young guinea pigs. Physiol. Behav. 2005;85:205–211. doi: 10.1016/j.physbeh.2005.04.008. doi:10.1016/j.physbeh.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Nelson R.J. Spontaneous “regression” of enhanced immune function in a photoperiodic rodent Peromyscus maniculatus. Proc. R. Soc. B. 2001;268:2221–2228. doi: 10.1098/rspb.2001.1784. doi:10.1098/rspb.2001.1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast B.J, Kriegsfeld L.J, Nelson R.J. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q. Rev. Biol. 2001a;76:293–325. doi: 10.1086/393989. doi:10.1086/393989 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Yellon S.M, Tran L.T, Nelson R.J. Photoperiod modulates the inhibitory effect of in vitro melatonin on lymphocyte proliferation in female Siberian hamsters. J. Biol. Rhythms. 2001b;16:224–233. doi: 10.1177/074873040101600305. doi:10.1177/074873001129001935 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Freeman D.A, Zucker I, Nelson R.J. Periodic arousal from hibernation is necessary for initiation of immune responses in ground squirrels. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002a;282:R1054–R1062. doi: 10.1152/ajpregu.00562.2001. [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Wynne-Edwards K.E, Yellon S.M, Nelson R.J. Photorefractoriness of immune function in male Siberian hamsters (Phodopus sungorus) J. Neuroendocrinol. 2002b;14:318–329. doi: 10.1046/j.1365-2826.2002.00781.x. doi:10.1046/j.1365-2826.2002.00781.x [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Hotchkiss A.K, Bilbo S.D, Nelson R.J. Peripubertal immune challenges attenuate reproductive development in male Siberian hamsters (Phodopus sungorus) Biol. Reprod. 2004;70:813–820. doi: 10.1095/biolreprod.103.023408. doi:10.1095/biolreprod.103.023408 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Bilbo S.D, Nelson R.J. Short day lengths enhance skin immune responses in gonadectomised Siberian hamsters. J. Neuroendocrinol. 2005;17:18–21. doi: 10.1111/j.1365-2826.2005.01273.x. doi:10.1111/j.1365-2826.2005.01273.x [DOI] [PubMed] [Google Scholar]
- Promislow D.E.L, Harvey P.H. Living fast and dying young—a comparative-analysis of life-history variation among mammals. J. Zool. 1990;220:417–437. [Google Scholar]
- Pyter L.M, Neigh G.N, Nelson R.J. Social environment modulates photoperiodic immune and reproductive responses in adult male white-footed mice (Peromyscus leucopus) Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005a;288:R891–R896. doi: 10.1152/ajpregu.00680.2004. [DOI] [PubMed] [Google Scholar]
- Pyter L.M, Weil Z.M, Nelson R.J. Latitude affects photoperiod-induced changes in immune response in meadow voles (Microtus pennsylvanicus) Can. J. Zool.-Revue Canadienne De Zoologie. 2005b;83:1271–1278. doi:10.1139/z05-121 [Google Scholar]
- Råberg L, Grahn M, Hasselquist D, Svensson E. On the adaptive significance of stress-induced immunosuppression. Proc. R. Soc. B. 1998;265:1637–1641. doi: 10.1098/rspb.1998.0482. doi:10.1098/rspb.1998.0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Nilsson J.A, Ilmonen P, Stjernman M, Hasselquist D. The cost of an immune response: vaccination reduces parental effort. Ecol. Lett. 2000;3:382–386. doi:10.1046/j.1461-0248.2000.00154.x [Google Scholar]
- Råberg L, Vestberg M, Hasselquist D, Holmdahl R, Svensson E, Nilsson J.A. Basal metabolic rate and the evolution of the adaptive immune system. Proc. R. Soc. B. 2002;269:817–821. doi: 10.1098/rspb.2001.1953. doi:10.1098/rspb.2001.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber P.M. Prolactin and immunomodulation. Am. J. Med. 1993;95:637–644. doi: 10.1016/0002-9343(93)90360-2. doi:10.1016/0002-9343(93)90360-2 [DOI] [PubMed] [Google Scholar]
- Reppert S.M, Weaver D.R. Melatonin madness. Cell. 1995;83:1059–1062. doi: 10.1016/0092-8674(95)90131-0. doi:10.1016/0092-8674(95)90131-0 [DOI] [PubMed] [Google Scholar]
- Reynaud C.A, Weill J.C. Immunology and developmental biology of the chicken. vol. 212. 1996. Postrearrangement diversification processes in gut-associated lymphoid tissues. pp. 7–15. [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E. Embryonic-development period and the prevalence of avian blood parasites. Proc. Natl Acad. Sci. USA. 1992;89:4722–4725. doi: 10.1073/pnas.89.10.4722. doi:10.1073/pnas.89.10.4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs R.E, Wikelski M. The physiology/life-history nexus. Trends Ecol. Evol. 2002;17:462–468. doi:10.1016/S0169-5347(02)02578-8 [Google Scholar]
- Roberts M.L, Buchanan K.L, Evans M.R. Testing the immunocompetence handicap hypothesis: a review of the evidence. Anim. Behav. 2004;68:227–239. doi:10.1016/j.anbehav.2004.05.001 [Google Scholar]
- Romero L.M. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen. Comp. Endocrinol. 2002;128:1–24. doi: 10.1016/s0016-6480(02)00064-3. doi:10.1016/S0016-6480(02)00064-3 [DOI] [PubMed] [Google Scholar]
- Saino N, Canova L, Fasola M, Martinelli R. Reproduction and population density affect humoral immunity in bank voles under field experimental conditions. Oecologia. 2000;124:358–366. doi: 10.1007/s004420000395. doi:10.1007/s004420000395 [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M, Romero L.M, Munck A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. doi:10.1210/er.21.1.55 [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. B. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. doi:10.1098/rspb.2002.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel P, Ebert D. On the evolutionary ecology of specific immune defence. Trends Ecol. Evol. 2003;18:27–32. doi:10.1016/S0169-5347(02)00013-7 [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. doi:10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Sidky Y.A, Hayward J, Ruth R.F. Seasonal variations of the immune response of ground squirrels kept at 22–24°C. Can. J. Physiol. Pharmacol. 1972;50:203–206. doi: 10.1139/y72-031. [DOI] [PubMed] [Google Scholar]
- Sinclair J.A, Lochmiller R.L. Resistance to a pathogenic challenge lacks seasonal specificity in prairie voles (Microtus ochrogaster) and cotton rats (Sigmodon hispidus) J. Mamm. 1999;80:1331–1335. doi:10.2307/1383183 [Google Scholar]
- Sinclair J.A, Lochmiller R.L. The winter immunoenhancement hypothesis: associations among immunity, density, and survival in prairie vole (Microtus ochrogaster) populations. Can. J. Zool.-Revue Canadienne De Zoologie. 2000;78:254–264. doi:10.1139/cjz-78-2-254 [Google Scholar]
- Smith P.E. The effect of hypophysectomy upon the involution of the thymus in the rat. Anat. Rec. 1930;47:119–129. doi:10.1002/ar.1090470110 [Google Scholar]
- Speakman J.R. Advances in ecological research. vol. 30. 2000. The cost of living: field metabolic rates of small mammals. pp. 177–297. [Google Scholar]
- Speakman J.R. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. doi:10.1098/rstb.2007.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Svensson E, Råberg L, Koch C, Hasselquist D. Energetic stress, immunosuppression and the costs of an antibody response. Funct. Ecol. 1998;12:912–919. doi:10.1046/j.1365-2435.1998.00271.x [Google Scholar]
- Tella J.L, Blanco G, Forero M.G, Gajon A, Donazar J.A, Hiraldo F. Habitat, world geographic range, and embryonic development of hosts explain the prevalence of avian Hematozoa at small spatial and phylogenetic scales. Proc. Natl Acad. Sci. USA. 1999;96:1785–1789. doi: 10.1073/pnas.96.4.1785. doi:10.1073/pnas.96.4.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella J.L, Scheuerlein A, Ricklefs R.E. Is cell-mediated immunity related to the evolution of life-history strategies in birds? Proc. R. Soc. B. 2002;269:1059–1066. doi: 10.1098/rspb.2001.1951. doi:10.1098/rspb.2001.1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S.D, Nicoll M.E. Basal metabolic-rate and energetics of reproduction in therian mammals. Nature. 1986;321:690–693. doi: 10.1038/321690a0. doi:10.1038/321690a0 [DOI] [PubMed] [Google Scholar]
- Turnbull A.V, Rivier C. Corticotropin–releasing factor, vasopressin, and prostaglandins mediate, and nitric oxide restrains, the hypothalamic–pituitary–adrenal response to acute local inflammation in the rat. Endocrinology. 1996;137:455–463. doi: 10.1210/endo.137.2.8593789. doi:10.1210/en.137.2.455 [DOI] [PubMed] [Google Scholar]
- Turnbull A, Rivier C. Inhibition of gonadotropin-induced testosterone secretion by the intracerebroventricular injection of interleukin-1 beta in the male rat. Endocrinology. 1997;138:1008–1013. doi: 10.1210/endo.138.3.5019. doi:10.1210/en.138.3.1008 [DOI] [PubMed] [Google Scholar]
- Vera-Lastra O, Jara L, Espinoza L.R. Prolactin and autoimmunity. Autoimmun. Rev. 2002;1:360–364. doi: 10.1016/s1568-9972(02)00081-2. doi:10.1016/S1568-9972(02)00081-2 [DOI] [PubMed] [Google Scholar]
- Verhulst S, Dieleman S.J, Parmentier H.K. A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Proc. Natl Acad. Sci. USA. 1999;96:4478–4481. doi: 10.1073/pnas.96.8.4478. doi:10.1073/pnas.96.8.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst S, Riedstra B, Wiersma P. Brood size and immunity costs in zebra finches Taeniopygia guttata. J. Avian Biol. 2005;36:22–30. doi:10.1111/j.0908-8857.2005.03342.x [Google Scholar]
- Viney M.E, Riley E.M, Buchanan K.L. Optimal immune responses: immunocompetence revisited. Trends Ecol. Evol. 2005;20:665–669. doi: 10.1016/j.tree.2005.10.003. doi:10.1016/j.tree.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Weil Z.M, Bowers S.L, Dow E.R, Nelson R.J. Maternal aggression persists following lipopolysaccharide-induced activation of the immune system. Physiol. Behav. 2006a;87:694–699. doi: 10.1016/j.physbeh.2006.01.005. doi:10.1016/j.physbeh.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Weil Z.M, Bowers S.L, Pyter L.M, Nelson R.J. Social interactions alter proinflammatory cytokine gene expression and behavior following endotoxin administration. Brain Behav. Immun. 2006b;20:72–79. doi: 10.1016/j.bbi.2005.05.001. doi:10.1016/j.bbi.2005.05.001 [DOI] [PubMed] [Google Scholar]
- Weil Z.M, Martin L.B, Workman J, Nelson R.J. Immune challenge retards seasonal reproductive regression: evidence for terminal investment? Biol. Lett. 2006c;2:393–396. doi: 10.1098/rsbl.2006.0475. doi:10.1098/rsbl.2006.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil Z.M, Pyter L.M, Martin L.B, Nelson R.J. Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus) Am. J. Physiol.- Regul. Integr. Comp. Physiol. 2006d;280:R1714–1719. doi: 10.1152/ajpregu.00869.2005. [DOI] [PubMed] [Google Scholar]
- Wiersma P, Selman C, Speakman J.R, Verhulst S. Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. B. 2004;271:S360–S363. doi: 10.1098/rsbl.2004.0171. doi:10.1098/rsbl.2004.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.D, Christians J.K, Aiken J.J, Evanson M. Enhanced immune function does not depress reproductive output. Proc. R. Soc. B. 1999;266:753–757. doi:10.1098/rspb.1999.0701 [Google Scholar]
- Wingfield J.C, Maney D.L, Breuner C.W, Jacobs J.D, Lynn S, Ramenofsky M, Richardson R.D. Ecological bases of hormone–behavior interactions: the “emergency life history stage”. Am. Zool. 1998;38:191–206. [Google Scholar]
- Yellon S.M, Teasley L.A, Fagoaga O.R, Nguyen H.C, Truong H.N, Nehlsen-Cannarella L. Role of photoperiod and the pineal gland in T cell-dependent humoral immune reactivity in the Siberian hamster. J. Pineal Res. 1999;27:243–248. doi: 10.1111/j.1600-079x.1999.tb00622.x. doi:10.1111/j.1600-079X.1999.tb00622.x [DOI] [PubMed] [Google Scholar]
- Yellon S.M, Kim K, Hadley A.R, Tran L.T. Time course and role of the pineal gland in photoperiod control of innate immune cell functions in male Siberian hamsters. J. Neuroimmunol. 2005;161:137–144. doi: 10.1016/j.jneuroim.2004.12.008. doi:10.1016/j.jneuroim.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Yu-Lee L.Y. Prolactin modulation of immune and inflammatory responses. Recent Prog. Horm. Res. 2002;57:435–455. doi: 10.1210/rp.57.1.435. doi:10.1210/rp.57.1.435 [DOI] [PubMed] [Google Scholar]
- Zellweger R, Zhu X, Wichmann M, Ayala A, DeMaso C, Chaudry I. Prolactin administration following hemorrhagic shock improves macrophage cytokine release capacity and decreases mortality from subsequent sepsis. J. Immunol. 1996;157:5748–5754. [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. doi:10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- Zuk M, Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160:S9–S22. doi: 10.1086/342131. doi:10.1086/342131 [DOI] [PubMed] [Google Scholar]