Abstract
Animals have evolved many season-specific behavioural and physiological adaptations that allow them to both cope with and exploit the cyclic annual environment. Two classes of endogenous annual timekeeping mechanisms enable animals to track, anticipate and prepare for the seasons: a timer that measures an interval of several months and a clock that oscillates with a period of approximately a year. Here, we discuss the basic properties and biological substrates of these timekeeping mechanisms, as well as their reliance on, and encoding of environmental cues to accurately time seasonal events. While the separate classification of interval timers and circannual clocks has elucidated important differences in their underlying properties, comparative physiological investigations, especially those regarding seasonal prolactin secretions, hint at the possibility of common substrates.
Keywords: seasonality, photoperiodism, circannual, interval timers
While the Earth remaineth, seedtime and harvest, and cold and heat, and summer and winter, and day and night shall not cease. Genesis 8: 22, King James Version (1611)
As the Earth makes its yearly orbit around the Sun, the planet's 23.5° axial tilt leads to the cyclical environmental changes that we call the seasons. Recurrent challenges to the survival of organisms and their offspring arise with the dawning of each new season, and animals have evolved seasonally induced changes in phenotype that adapt them to these predictable events. For example, in many temperate environments, winter is generally characterized by severe decreases in ambient temperature and food availability, leading to increased energetic demands at a time when resources are diminished. Because energy requirements of female mammals typically increase markedly during lactation (Bronson 1989) and newly weaned offspring are particularly vulnerable to environmental perturbations (Hill 1992), raising offspring during this time of year is often futile. Thus, many temperate species have evolved winter-specific behaviours and physiology to conserve energy (e.g. hibernation, a more insulative pelage, huddling) or provide for escape (e.g. migration). Many species also increase the chances of offspring survival by timing their mating behaviours so that parturition occurs during energetically favourable times of year.
In this review, we highlight the mechanisms animals use to achieve seasonally appropriate adjustments. Although reference is mostly made to studies of reproduction and to particular species, the conclusions derived probably apply to a broad spectrum of animals and to non-reproductive traits. Not covered here are remarkable recent developments in identifying some of the genes responsible for seasonal flowering in plants (Yanovsky & Kay 2003).
The simplest solution for seasonal adaptations would be a mechanism by which environmental cues directly trigger the required changes (sometimes referred to as a type 3 mechanism or rhythm; Zucker et al. 1991). In the absence of the external stimulus, the adaptive response would be lost. As an example, cloud forest mice, Peromyscus nudipes, do not give birth during the dry season in Costa Rica, although they breed year-round. Restricted food and water availability during the dry months are the stimuli that appear to prevent implantation or lead to reabsorption of the embryo (Heideman & Bronson 1992). Type 3 annual rhythms in human births have also been reported, attributable to social taboos on copulation during the yam growing season in a village in Papua New Guinea (Scaglion 1978) and to end of the year tax breaks in the United States (Dickert-Conlin & Chandra 1999).
Of course, there are limitations to a type 3 mechanism. Many seasonal changes require several weeks to complete (e.g. moult to a thicker pelage, deposition of large amounts of white adipose tissue, completion of spermatogenesis) and would occur too late if they were a direct response to an ultimate environmental factor. For example, if ground squirrels waited until the onset of winter conditions to initiate weight gain or food storage, they would have insufficient energy reserves upon entry into hibernation to survive the winter. Species often use predictive cues to forecast the coming season—primarily changing day lengths in non-equatorial regions—but even this strategy might not be sufficiently reliable under all conditions. Migrating birds experience complex, dynamic environments and photoperiods, and animals that overwinter in burrows do not have access to most seasonal cues for much of the year.
An internal seasonal timekeeping mechanism, either one that measures the duration of time from an antecedent cue (like an hourglass) or one that oscillates endogenously with an annual period (like a clock), would enable animals to track, anticipate and prepare for the seasons even when predictive cues are noisy or not immediately present. Such timing mechanisms (sometimes referred to as type 1 and type 2 mechanisms or rhythms, respectively; Zucker et al. 1991) are widespread among seasonal species, even those with continuous access to relatively noise-free predictors (e.g. sheep, deer). Currently there is no satisfactory explanation for their prevalence in these latter species (for discussion see Gwinner 1981a,b).
1. Annual timekeeping mechanisms
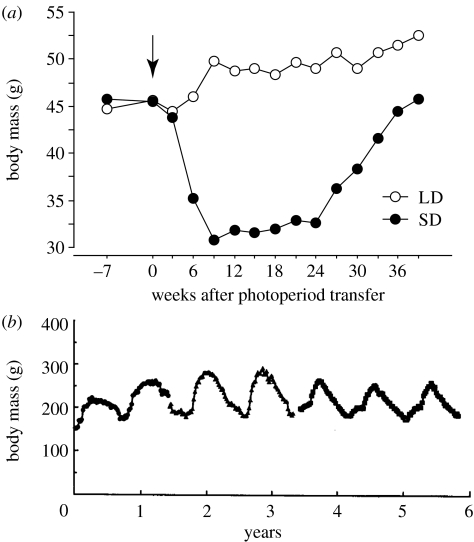
Both Siberian hamsters (Phodopus sungorus) and golden-mantled ground squirrels (Spermophilus lateralis) undergo seasonal cycles for several traits, including reproduction, body mass and heterothermy (Figala et al. 1973; Heldmaier & Steinlechner 1981; Zucker 2001). Laboratory experiments have revealed that seasonal adaptations in these two species are governed by different timing mechanisms (figure 1). Here, we illustrate this concept with reference to annual cycles of gonadal size and function, which are in breeding condition for both squirrels and hamsters during the spring and for hamsters in the summer as well (McKeever 1964; Figala et al. 1973). A similar argument can be made, however, using other seasonal traits of these species (figure 2; Pengelley & Fisher 1957; Pengelley & Asmundson 1974; Prendergast et al. 2002b).
Figure 1.
Annual timekeeping mechanisms. (a) Interval timer of the Siberian hamster. (1) Decreasing day lengths trigger the interval timer/induce the winter phenotype. (2) The timer runs to completion. (3) Refractoriness/spontaneous reversion to the spring phenotype. (4) Prolonged exposure to long day lengths breaks refractoriness/resets the interval timer. (b) Circannual clock of the golden-mantled ground squirrel. Successive oscillations between the summer and winter phenotypes are driven by an endogenous clock rather than exogenous factors.
Figure 2.
(a) Body mass records of two Siberian hamsters, one maintained continuously in a long day length (LD; 14 h light per day) and the other transferred to a short day length (SD; 10 h light per day) as indicated by arrow. SDs initially induce a marked decrease in body mass, but after 24 weeks body mass spontaneously reverts to LD values, even though there has been no change in environmental conditions. At this point, the hamster is unresponsive to SDs and is considered photorefractory. In contrast, maintenance in a LD results in uninterrupted body mass increases (M. J. Paul 2006, unpublished data). (b) Circannual body mass rhythm of an individual golden-mantled ground squirrel housed in a fixed photoperiod of 14 h light per day, then 12 h light per day, then constant light for the last 11 months. At year 3, the ambient temperature was changed from 23 to 6.5°C. The rhythm in body mass persists under all housing conditions (modified from Ruby et al. 1998).
(a) Interval timers
Siberian hamsters housed under long photoperiods (more than 13 h light per day, 13L; Hoffmann 1982a) display the spring/summer phenotype of large, functional gonads indefinitely. Upon transfer to short photoperiods (less than 13L; Hoffmann 1982a), the gonads undergo regression, reproductive hormone concentrations decrease and gametogenesis gradually ceases over the following 6–10 weeks (Bergmann 1987). If maintained in a short photoperiod for more than 20 weeks, hamsters spontaneously revert to the reproductively competent phenotype (Hoffmann 1979). The loss of responsiveness to short day lengths (photorefractoriness) is governed by a process with hourglass properties designated an interval timer (Goldman 2001). This timer lacks calendar properties but adequately measures the lapse of time since the advent of short days.
Growth of the testes in Siberian hamsters is not affected by the increase in day length between the winter solstice and the vernal equinox (Gorman & Zucker 1995). The regrowth process is initiated endogenously by the interval timer, all other environmental factors being held constant. Field studies of meadow voles (Microtus pennsylvanicus) and white-footed mice (Peromyscus leucopus) suggest that the interval timer governs testicular growth in the wild: spermatogenesis recommences in most males in early December in Pennsylvania (Christian 1980), when day lengths are still decreasing and short in absolute terms. Initiation of gonadal development long before the advent of stimulatory long day lengths allows animals to take advantage of favourable breeding conditions in early spring. The duration of the interval timer differs both interspecifically (e.g. Siberian and Syrian hamsters, Mesocricetus auratus) and between different populations of the same species (Gram et al. 1982); it is presumably shaped by the selection pressures of the local habitat to favour appropriately timed reproduction.
Photorefractory Siberian hamsters housed in short days do not undergo a second gonadal regression unless they are first exposed for six or more weeks to a long photoperiod (Kauffman et al. 2003; Watson-Whitmyre & Stetson 1988). The interpolation of long days breaks refractoriness and resets the interval timer. Under simulated natural photoperiods, the decrease in day lengths in late summer initiates, and the long spring/summer day lengths reset, the interval timer resulting in successive annual cycles (Gorman & Zucker 1995).
(b) Circannual clocks
By contrast, golden-mantled ground squirrels housed under constant conditions (including static photoperiods) undergo several successive annual cycles of reproductive competence/incompetence with a period of approximately 10.5 months (Pengelley & Asmundson 1974). The largest variation in period for a given squirrel in this study was 29 days over four cycles, and in one squirrel there was only a 4-day variation in period. The generation of the ground squirrel's annual rhythm, in contrast to that of the Siberian hamster, does not require environmental input but rather is a manifestation of an endogenous circannual (approx. annual) clock. Under unchanging environmental conditions, this rhythm is said to free-run, with its period reflecting that of the underlying oscillator. Because the free-running rhythm does not match the 12-month annual cycle, it must be adjusted via environmental cues to prevent ‘out-of-season’ behaviours.
How a circannual clock can run with such a long period is not understood. It does not require timed feedback from its physiological outputs (see Gwinner 1981b) and can track seasonal time even when its physiological expression is suppressed. Manipulations that either prevent the usual increase or accelerate the decrease in body mass have little or no effect on the onset or termination of the hibernation season, the period length between successive hibernation seasons or the onset of circannual weight gain in golden-mantled ground squirrels (Pengelley 1968; Heller & Poulson 1970; Pengelley & Asmundson 1974). Similar results have also been obtained in 13-lined ground squirrels (Spermophilus tridecemlineatus), whose energetic demands are increased by induced arousal from hibernation bouts without available food; with the return of food, the squirrels regain the proper body mass for that time of year (Mrosovsky & Fisher 1970). In this volume, Wikelski and colleagues revisit a possible role of energy turnover in circannual timekeeping mechanisms in an avian model.
(c) Timers versus clocks
It has been proposed that annual timekeeping mechanisms have multiple evolutionary origins (Farner 1985), so it is not surprising that different species have evolved distinct mechanisms for tracking seasonal time, an hourglass interval timer that needs to be reset or a self-sustaining circannual clock. Natural selection apparently has acted on different underlying substrates to solve the problem of seasonal timing. That circannual clocks are favoured by a number of long-lived species, however, argues for a functional explanation for the evolution of these two distinct classes of timekeeping mechanisms. Species that rarely live longer than a year in the wild would have no need for a mechanism that repeatedly generates annual cycles. An interval timer might also provide a selective advantage over a circannual clock in short-lived species, which tend to breed opportunistically (Bronson 1989). Anecdotally, it has been reported that out-of-season breeding is more common among interval timing than circannual species, suggesting that the timer is more easily overridden than the clock (Prendergast et al. 2001). A flexible annual timekeeping mechanism that can be ignored is probably a better option for species that stand to benefit from a risky breeding strategy.
Species without reliable access to noise-free environmental cues might require a circannual clock (e.g. species that migrate or overwinter in burrows). If isolation from noise-free environmental cues is sufficiently long enough, these animals would probably opt for an annual timekeeping mechanism that is less reliant on the environment. Tropical species might also lack sufficient environmental cues for interval timing. At and around the equator, changes in photoperiod are minimal at best, and other environmental cues may not occur at the correct time with respect to ultimate factors. For example, rainfall may occur too close to the onset of available food (i.e. insects and vegetation), so this signal would not provide sufficient time for reproductive development. A circannual clock may prevent erratic environmental fluctuations from altering the seasonal rhythms in species or traits that cannot exploit small variations in seasonal onsets. For example, because gestation in sheep lasts several months, an extra month of unseasonable warmth at the end of summer would not provide an opportunity for births of additional progeny. Circannual species tend to produce but one litter a year, more or less on the same calendar date, whereas those that deploy an interval timer typically have a more extended breeding season, multiple litters and more variable times of reproduction.
2. On the role of environmental cues
(a) Photoperiod
Without some kind of external input, interval timers would not cycle and circannual clocks would drift out of phase with the environment; both timekeeping mechanisms require environmental cues to accurately time seasonal events. Day length, the most accurate natural predictor of annual phase, is the predominant cue for timers and clocks (Gorman et al. 2001; Zucker 2001; Gwinner 2003). However, the functional role of photoperiod differs for the two mechanisms.
An interval timer relies on photoperiod to both trigger and reset the hourglass mechanism. In several small rodents, the interval timer is triggered by short day lengths and reset by long day lengths (Gorman et al. 2001). In the majority of birds, however, long day lengths first trigger the interval timer, after which prolonged exposure results in refractoriness to long days and a reversion to the winter phenotype (Dawson et al. 2001). Subsequent exposure to short day lengths is then required to break refractoriness. It has been proposed that a critical day length exists that defines all shorter photoperiods as winter and all longer photoperiods as summer (reviewed in Gorman et al. 2001). Relatively sharp inflection day lengths have been reported for several species (Gaston & Menaker 1967; Elliott 1976; Hoffmann 1982a; Rhodes 1989). Although a simple critical day length model is sufficient to account for seasonal cycles of laboratory animals held in and moved between static photoperiods, it is not sufficient to explain seasonal changes of animals in the wild. Each day length, except for those at the solstices, occurs twice each year. Thus, intermediate day lengths around the equinoxes could signal either the coming winter or the coming summer depending on whether they are preceded by decreasing or increasing day lengths. The response to these ‘ambiguous’ day lengths must vary according to the season, whether late summer or early spring. To solve this problem, photoperiodic animals store information about preceding photoperiods that they compare to the current day length (Prendergast et al. 2000). Siberian hamsters transferred from 16L or 8L to the identical 14L photoperiod undergo gonadal involution or recrudescence, respectively (Hoffmann et al. 1986). Such experiments suggest that an animal's photoperiodic history allows it to track the natural changes in day length, enabling it to unambiguously predict the upcoming season and initiate/reset the interval timer at the appropriate time of year (Gorman et al. 2001). Studies of Siberian hamsters in simulated natural photoperiods have revealed that both absolute day length and incremental changes in natural day lengths contribute to the timing of seasonal behaviour and physiology (Gorman & Zucker 1995; Butler et al. 2007).
On the other hand, a circannual clock requires photoperiod to synchronize its endogenous free-running period to the sidereal year, analogous to the photic entrainment of a circadian clock to the 24 h day by the light/dark cycle. Thus, circannual researchers have adopted the circadian terminologies of entrainment and zeitgeber (an environmental factor that shifts the phase of the endogenous oscillator; German for ‘time giver’). Central to entrainment theory is the observation that the oscillator is differentially sensitive to the phase of zeitgeber presentation. This can be assessed by constructing a ‘phase response curve’, i.e. by plotting the phase shifts that occur in a measured rhythm when zeitgeber pulses are applied at different phase points across the free-running circadian or circannual cycle. Owing to the long time scales involved (a typical experiment lasts a minimum of 2 years), reports of complete circannual phase response curves are uncommon. The circannual body mass rhythm of golden-mantled ground squirrels exposed to a three-month block of short days (8L) in late summer is phase advanced for 2 years, whereas the identical day length manipulation has no effect when given in the spring (Zucker 2001). A pseudophase response curve has been generated for rainbow trout (Oncorhynchus mykiss) exposed to blocks of constant light at different phases of the circannual rhythm (Randall et al. 1998), but the fishes were returned to natural day lengths rather than free-running conditions after zeitgeber presentation. Only recently in the varied carpet beetle (Anthrenus verbasci) has a complete phase response curve to photoperiod been generated for the circannual pupation rhythm (Miyazaki et al. 2005). Additional data supporting the concept of a photoperiod-entrainable circannual clock include the gradual resynchronization of annual rhythmicity after artificially reversing the photoperiod (woodchucks, Marmota monax: Concannon et al. 1993; sika deer, Cervus nippon: Goss 1969a, 1980); and entrainment, within limits, to photoperiodic cycles that differ from 12 months (e.g. 3, 6 or 24 months; sika deer: Goss 1969a; European starlings, Sturnus vulgaris: Gwinner 1977; golden-mantled ground squirrels: Lee & Zucker 1991).
(b) Other environmental factors
In the winter breeding California vole (Microtus californicus) short photoperiods suppress and long photoperiods promote testicular growth and spermatogenesis (Nelson et al. 1983). Evidently some non-photoperiodic seasonal factor(s) must override the normal photoperiodic input in the wild. Food, water, ambient temperature and social cues have all been shown to alter seasonal traits (Whitsett & Lawton 1982; Desjardins & Lopez 1983; Eskes 1983; Nelson et al. 1983; Pearce & Oldham 1988; Schneider & Wade 1989; Wayne et al. 1989; Honrado & Fleming 1996; Hegstrom & Breedlove 1999; Kriegsfeld et al. 2000a,b; Larkin et al. 2001, 2002; Reynolds et al. 2003; Schoech et al. 2004; Genin et al. 2005), including a single plant compound identified in newly grown vegetation that stimulates reproduction in wild montane voles (Microtus montanus) during the winter months (Berger et al. 1981).
Reliance on non-photoperiodic cues for seasonal timing appears to be highly species specific. Wingfield and colleagues (1992) have proposed a method for predicting the relative contributions of non-photoperiodic factors in seasonal timing for a given species. Their quantitative analysis is beyond the scope of this review, but in general, they argue that species with short but highly predictable seasonal events (e.g. breeding seasons) rely mostly on photoperiod because this cue should be sufficient; in fact, it might be beneficial for such species to ignore inhibitory environmental factors because the narrow seasonal window confers limited opportunities. On the other hand, non-photoperiodic cues probably provide useful information to species that inhabit seasonal environments with more year-to-year variability and a longer seasonal window. Thus, as the certainty of the seasonal event decreases, the importance of photoperiod, timers and clocks decreases, whereas that of non-photoperiodic, supplementary cues increases. These predictions have been tested in three subspecies of white-crowned sparrows (Zonotrichia leucophrys). Z. l. gambelli breed in Alaska with a short but highly predictable breeding season; Z. l. oriantha and Z. l. pugetensis breed in the temperate latitudes with longer breeding seasons of more variable onset and offset. Supporting Wingfield et al.'s (1992) view, ambient temperature modifies photoperiodic responses of prolactin and gonadal development in Z. l. oriantha and Z. l. pugetensis but not in Z. l. gambelli (Wingfield et al. 1996, 1997, 2003; Maney et al. 1999).
Tropical species also might be expected to rely more heavily on non-photoperiodic cues given the minimal changes in day length in their natural habitat. Two populations of equatorial rufous-collared sparrows (Zonotrichia capensis) living 25 km apart at the same latitude breed seasonally and out of phase with each other even though the amplitude of the annual day length cycle is only approximately 3 min (Moore et al. 2005). Few studies have investigated the non-photoperiodic cues that might regulate seasonality in the tropics, but food intake and other food-related cues are probably important (Hau et al. 2000; Scheuerlein & Gwinner 2002). Surprisingly, some tropical birds can respond to small changes in photoperiod in the laboratory (African stonechats, Saxicola torquata axillaries: Gwinner 2003; spotted antbird, Hylophylax n. naevioides: Hau et al. 1998); a 17 min increase in photoperiod is sufficient to stimulate some gonadal growth in the spotted antbird (Hau et al. 1998). Whether or not these species attend to day length in the wild is unknown (Gwinner 2003). Recently, it has been suggested that equatorial birds time seasonal events by tracking seasonal changes in daytime light intensity during the dry and rainy seasons; African stonechats maintained in a constant 12.5L photoperiod synchronize their reproductive and moult rhythms to 10-month cycles of light intensity (Gwinner 2003). Notably, both light intensity (Bentley et al. 1998b) and small changes in photoperiod (Dawson 2007) can affect the day length perception of European starlings, a temperate avian species, indicating that these phenomena are not restricted to equatorial species.
Some individuals of photoperiodic rodent species do not express the typical winter phenotype upon transfer to short day lengths (Prendergast et al. 2001), but such photo-non-responders may undergo gonadal involution if short days are combined with low temperatures and/or food restriction (Desjardins & Lopez 1983; Prendergast et al. 2001). Thus, the reliance on non-photoperiodic cues can vary intraspecifically. In this case, it is interesting to note that these cues are necessary for the response itself rather than the timing of the response.
Most studies do not explicitly assess whether non-photoperiodic cues act by directly driving or suppressing expression of the trait (‘masking’) or instead by altering an underlying timekeeping mechanism; in general, the results are more in line with the former possibility. This issue has been addressed in a recent field study of African stonechats in which provision of supplemental food advanced the initial breeding season without changing the onsets of the post-nuptial moult or of the following breeding season (Scheuerlein & Gwinner 2002). This result suggests that the food-induced advance did not perturb the circannual clock, although it is possible that other uncontrolled zeitgebers (i.e. photoperiod) might have compensated for the advance prior to the onset of later seasonal changes. Although most non-photoperiodic cues probably act by masking, at least one (extended intervals of exposure to low ambient temperatures) appears to phase shift the circannual body mass and reproductive rhythms of golden-mantled ground squirrels (Mrosovsky 1980).
Jacobs & Wingfield (2000) have proposed a ‘finite state machine’ model to predict the impact of environmental signals and their dependence on the state of the organism, determined by a finite number of inputs and outputs. The model emphasizes that individual cues such as day length may be sufficient in some circumstances, but in others they combine with temperature and food availability signals to affect seasonally appropriate responses. Laboratory-based scientists would do well to take seriously their caution that ‘isolating a single cue and presenting it to the organism may result in responses not normally expressed in the field’ (p. 45).
Wild animals almost certainly attend to multiple environmental cues. At sites with considerable year-to-year variability in the onset of seasonally favourable conditions, cues such as food availability and soil temperature can fine-tune annual rhythms. Perhaps photoperiod acts to grossly time seasonal changes by triggering, resetting and entraining interval timers and circannual clocks, while other environmental cues adjust precise rhythm onsets each year by a masking mechanism.
3. Photoperiodic time measurement
(a) Models
In 1936, Erwin Bünning suggested that plants use the circadian timekeeping system to measure day length (for review see Saunders 2005). He proposed the existence of a circadian-controlled photo-inducible phase; a sufficiently long day length would illuminate this phase and trigger a long-day response. In this scheme, light both entrains and illuminates the photo-inducible phase for photoperiodic time measurement (PTM). Colin Pittendrigh later extended this ‘external coincidence’ PTM model and proposed an alternative ‘internal coincidence’ or dual oscillator model. The latter postulates two circadian oscillators: one accelerated by light and synchronized to dawn (morning oscillator, M), and the other decelerated by light and synchronized to dusk (evening oscillator, E). As day length changes, the changing phase relation between the two oscillators encodes seasonal time. Several variations of these models have been developed more recently to account for findings in insects (see Vaz Nunes & Saunders 1999).
Several unnatural photoperiod treatments have been used to test the circadian basis for PTM. Animals housed in a short photoperiod but given a brief light pulse during the dark portion of the light/dark cycle (scotophase) respond as though they were housed in a long photoperiod, even though the total duration of light provided falls far below the critical day length (Follett & Sharp 1969; Hoffmann 1982b; Underwood & Hyde 1990). In Syrian hamsters, two 1 s light pulses administered daily 14 h apart maintain the reproductive system in its long day mode (Earnest & Turek 1983), whereas 11 h of continuous light exposure sustains the short-day reproductive phenotype (Elliott 1976). Thus, PTM in these cases is not a simple quantitative measure of the hours of light, hours of darkness or the ratio of light to darkness.
While the skeleton photoperiods described above suggest that the circadian system is used for PTM, they do not rule out the use of other non-circadian timing mechanisms. Nanda–Hamner, Bunsow and bi-stability experiments provide the best evidence for circadian involvement in PTM (Vaz Nunes & Saunders 1999). The Nanda–Hamner protocol, also referred to as resonance photocycles, has been used extensively (see also Elliott 1976). In such studies, groups of animals are exposed to photocycles with the same duration photophase (e.g. 6L) followed by varying durations of darkness (e.g. 18, 30, 42 and 54 h). Animals display a short-day response if the period of the total photocycle (T= light plus darkness) is a multiple of 24 h, whereas those in non-24 h T cycles display a long-day response. These results are compatible with a circadian mechanism in which light in the non-24 h photocycles falls during the postulated photo-inductive phase every few cycles or alters the phase relation between morning and evening circadian oscillators. Circadian involvement in PTM has been implicated in insects (Vaz Nunes & Saunders 1999), fishes (Baggerman 1972; Bromage et al. 1990), reptiles (Underwood & Hyde 1990), birds (Hamner 1963, 1964; Follett & Sharp 1969) and mammals (Elliott et al. 1972; Almeida & Lincoln 1982; Nelson et al. 1982) and is believed to be ubiquitous among birds and mammals. In insects, reliance on the circadian system for PTM is more variable, with some species using an hourglass mechanism (Vaz Nunes & Saunders 1999). Evidence in other insects is conflicting and depends on the type of test given or even the ambient temperature during the test. Nonetheless, with notable exceptions, the circadian system is implicated in PTM in the majority of insects tested.
Recent experiments in European starlings have questioned the validity of the external coincidence model. Starlings maintained in 18L photophases of different light intensities do not exhibit the same photoperiodic response; lower light intensities are interpreted as a less stimulatory photoperiod (less than 18L, but greater than the critical day length) as determined by the rate of photostimulation of testicular development and onset of photorefractoriness (Bentley et al. 1998b). This appears to contradict the external coincidence model of PTM; if detectable light illuminates the same proportion of the photo-inductive phase, then the same long-day response should ensue. Yet as the authors point out, differing light intensities differentially affect the underlying circadian oscillator, which could alter timing of the photo-inducible phase. Thus, even though all birds were maintained in 18L, the lower light intensities may have resulted in a later photo-inductive phase and thus less overlap with light. Similarly, differing light intensities could affect the phase relation between morning and evening oscillators of an internal coincidence model via differing entrainment strengths.
(b) Melatonin
Only in mammals has the physiological basis for PTM been elucidated. An intact pineal gland is necessary for seasonal responses to photoperiod; pinealectomy prevents seasonal responses in most interval-timing species and in the majority of cases results in a persistent expression of the long-day phenotype (Goldman 2001). The indolamine, melatonin, is produced in the pineal during the night by the rhythmic activity of the rate-limiting enzyme N-acetyltransferase (NAT); this rhythm is controlled by a light-entrainable circadian pacemaker in the hypothalamus, the suprachiasmatic nucleus (SCN; Schwartz et al. 2001). A multi-synaptic pathway from the SCN communicates lighting information to the pineal gland (Moore 1996; Perreau-Lenz et al. 2003); the paraventricular nucleus (PVN) of the hypothalamus receives extensive SCN projections, and PVN efferents, including some to pre-ganglionic neurons of the sympathetic nervous system, relay to post-ganglionic neurons that ultimately innervate the pinealocytes. The SCN exhibits endogenous circadian rhythms that are photoperiod dependent in pinealectomized animals (Sumová & Illnerová 1996; Jacob et al. 1997). In the Syrian hamster and rat SCN, rhythms of photosensitivity (Sumová et al. 1995; Vuillez et al. 1996), electrical activity (Mrugala et al. 2000; Schaap et al. 2003) and gene expression (see Schwartz et al. 2001 and de la Iglesia et al. 2004 for references) display intervals of high activity that expand and contract in concert with changes in the ambient photophase and scotophase. Light, by both entraining the circadian pacemaker in the SCN and suppressing NAT activity in the pineal, restricts melatonin secretion to the scotophase. The contraction of the melatonin signal during short summer nights and its expansion in long winter nights provides an accurate endocrine representation of night length. Indeed, exogenous administration of short and long duration melatonin infusions to pinealectomized mammals induces the summer and winter phenotypes, respectively (Bittman et al. 1983; Carter & Goldman 1983a,b). The circadian basis for PTM is thus realized by its role in the generation of a hormonal signal proportional to night length. The induction of the summer/winter phenotype by exotic lighting schedules (resonance and night-interruption protocols) can now be referred to their effects on melatonin secretion (Illnerová 1991).
Although the duration of nightly melatonin is sufficient to mimic photoperiodic responses, gonadal development can be attenuated in pinealectomized Siberian hamsters infused with melatonin for only 1 h per day, provided the hormone is administered just before or at the onset of the scotophase (Gunduz & Stetson 2001a,b). This raises the possibility that long duration melatonin infusions induce short-day responses because they overlap with a phase of target tissue sensitivity to melatonin. This view is compatible with the contention that the phase of melatonin sensitivity is entrained by the pattern of endogenous melatonin secretion (Stetson & Watson-Whitmyre 1986; Pitrosky & Pévet 1997). However, these studies infused supraphysiological doses of melatonin and the effects on the reproductive axis were less robust than those obtained with long duration melatonin infusions within the physiological range. Importantly, the putative phase of melatonin sensitivity in pinealectomized hamsters (around the onset of the scotophase) would correspond to a time of low endogenous melatonin secretion (Shaw & Goldman 1995; Goldman et al. 1984). At present, the evidence is insufficient to invalidate the current melatonin duration hypothesis for PTM.
Because light pulses independently phase shift the evening onset and morning offset of pineal NAT activity, the search for the dual M and E oscillators of the ‘internal coincidence’ model has focused on the SCN. There is some evidence to suggest that the SCN is composed of two oscillating components differentially affected by changes in the antecedent photoperiod (Sumová & Illnerová 1998; Jagota et al. 2000; Hazlerigg et al. 2005) and independently shifted by zeitgebers in a phase-dependent manner (Jagota et al. 2000). Several authors have proposed that M and E might be represented by the differently phased transcription of SCN ‘clock’ genes, the cyclic expression of which are thought to underlie the autoregulatory feedback loops that constitute the core of the circadian oscillatory machinery (Daan et al. 2001; see also Hastings & Herzog 2004 for a current review of circadian clock genes). Data on Period 1 (Per1) and Per2 expression patterns of mutant mice in different photoperiods suggest that the two genes might be anchored to morning and evening, respectively (Steinlechner et al. 2002). It is not known if photoperiodic effects are a property of individual SCN cells, with their activities compressed and decompressed, or instead emerge from intercellular interactions, with the cells assuming variable phase relations as a function of day length. Recent analyses hint that the latter may be the case (Schaap et al. 2003; Rohling et al. 2006).
The duration of nocturnal melatonin secretion also entrains circannual clocks. In both golden-mantled ground squirrels and Suffolk sheep (Ovies aries), pinealectomy prevents entrainment to annual photoperiodic regimens (Woodfill et al. 1991; Hiebert et al. 2000), and melatonin administration phase shifts and entrains circannual reproductive rhythms in pinealectomized ground squirrels (Zucker 2001) and sheep (Woodfill et al. 1991), respectively. In sheep, long duration infusions during the summer result in the most reliable entrainment compared to melatonin profiles mimicking other seasons, and winter infusions are ineffective (Woodfill et al. 1994; Barrell et al. 2000). The winter melatonin profile, although neither necessary nor sufficient for entrainment of circannual rhythms of reproduction in ewes, is probably the short-day physiological signal that regulates the duration of the breeding season (Malpaux et al. 1988; Malpaux & Karsch 1990).
(c) Physiological basis of PTM in non-mammalian vertebrates
Much of the research into PTM in non-mammalian vertebrates has focused on whether the roles of the pineal gland and melatonin elaborated for mammals are also characteristic of other vertebrates, but the results obtained often differ depending upon the species tested (see Mayer et al. 1997). The pineal gland of many non-mammalian vertebrates contains both photoreceptors and a circadian pacemaker (Underwood & Goldman 1987); it thereby has direct access to day length and circadian information, placing it in an ideal position for PTM. As in mammals, the daily pineal and/or plasma melatonin profile of reptiles and birds is entrained by the light/dark cycle with peak values occurring during the scotophase, and in some species, changes in day length have been shown to alter the waveform (duration, amplitude or phase; Underwood & Goldman 1987; Mayer et al. 1997 and references therein). Cultured pineal glands from house sparrows (Passer domesticus) housed in either long or short day lengths maintain their differing durations of melatonin secretion for several cycles under constant darkness (Brandstätter et al. 2000), indicating that the house sparrow pineal itself is capable of storing a photoperiodic ‘memory’, at least for a few cycles.
The pineal gland and melatonin play a significant role in regulating the reproductive axis of at least some snakes and lizards. In the chequered water snake (Natrix piscator), pinealectomy is antigonadal during the autumn testicular recrudescence phase, but progonadal during the winter and spring regression and inactive phases, respectively (Haldar & Pandey 1989). Constant release melatonin implants, as well as both morning and evening melatonin injections, administered to pineal-intact water snakes decrease testicular size at all phases of the annual reproductive cycle except when testes are already fully regressed (Haldar & Pandey 1988). In the iguanid lizard (Anolis carolinensis), pinealectomy stimulates gonadal growth at certain times of year, and subcutaneous melatonin implants block these progonadal effects (Underwood 1985a).
It is not known if these effects of melatonin involve the transduction of photoperiodic cues. Although melatonin secretion in non-mammalian vertebrates is restricted to the night, there is some question whether melatonin duration conveys day length information. In Anolis, the phase, rather than the duration or amplitude, of the melatonin signal correlates with seasonal responses in various nightbreak, T cycle and resonance photocycles (Hyde & Underwood 1993). Whether phase differences mediate seasonal changes have not been tested. In the ruin lizard (Podarcis sicula), the amplitude of the melatonin signal differs in long and short days, but again, a causal relation between the amplitude of melatonin secretion and seasonal characteristics has not been established (Bertolucci et al. 2002; Bertolucci et al. 2003).
In some ectothermic species, temperature alters the amplitude of the daily melatonin rhythm (iguanid lizard: Menaker & Wisner 1983; diamondback water snake, Nerodia rhombifera: Tilden & Hutchinson 1993; box turtle, Terrapene carolina triunguis: Vivien-Roels et al. 1988) suggesting that the daily rhythm in circulating melatonin may also integrate seasonal temperature cues (Vivien-Roels 1985). In the iguanid lizard, temperature cycles (approx. 9 h at 20°C, approx. 15 h at 32°C in constant dim light) entrain the daily pineal melatonin rhythm, with peak concentrations occurring during the cold phase (Underwood 1985b). In fact, when these lizards are exposed to ‘cold days’ and ‘warm nights’ (20°C during photophase, 32°C during scotophase), the melatonin profile entrains to the temperature cycle with peak values occurring in the light. Post-hibernation courtship behaviours of male red-sided garter snakes (Thamnophis sirtalis parietalis) are thought to be regulated by the characteristic environmental temperature changes of winter and spring (Hawley & Aleksiuk 1975; Crews 1990). Pinealectomy in the autumn, but not spring, eliminates courtship behaviours in the majority of males (Mendonca et al. 1996a,b). Pinealectomized males that do not court females exhibit a disrupted daily melatonin profile, whereas those that continue to court have normal daily melatonin rhythms (Mendonca et al. 1996a,b). These correlative data suggest that a normal daily rhythm of melatonin in the autumn is necessary to trigger springtime courtship behaviours; in some individuals, however, extra-pineal sources of melatonin may be sufficient to maintain this daily rhythm.
In birds, the effects of pinealectomy and melatonin administration are more varied, in part because tissues other than the pineal, mainly the eyes, contribute to melatonin concentrations in the general circulation (Underwood & Goldman 1987). Results in a few species, however, have led to the view that neither the pineal gland nor circulating melatonin plays a role in photoperiodic regulation of the reproductive axis. Blinded and pinealectomized American tree sparrows (Spizella arborea) continue to display seasonal gonadal cycles in response to changes in photoperiod (Wilson 1991). In the Japanese quail (Coturnix coturnix japonica), melatonin injections that extend the nocturnal melatonin profile do not mimic short-day suppressive effects on gonadal growth (Juss et al. 1993). A recent investigation, however, has revived a possible role for melatonin in photoperiodic regulation of reproduction in Japanese quail via control of gonadotrophin-inhibitory hormone (GnIH; Ubuka et al. 2005), a hypothalamic peptide known to suppress pituitary luteinizing hormone (LH) secretion (Tsutsui et al. 2000; Osugi et al. 2004) and male gonadal development (Ubuka et al. 2006) when administered exogenously. GnIH mRNA and protein content in the diencephalon of Japanese quail increase after three weeks of short-day exposure (Ubuka et al. 2005). Pinealectomy combined with bilateral ocular enucleation, which markedly decreases plasma and neural melatonin, decreases GnIH mRNA and protein content, whereas melatonin implants restore these levels in a dose-dependent fashion. The results of Juss et al. (1993), however, suggest that altered melatonin secretion alone is insufficient to drive seasonal alterations of gonadal function in Japanese quail. It also remains possible that the effects of melatonin on GnIH represent pharmacological actions with a role for endogenous melatonin yet to be demonstrated.
In addition to being species specific, a photoperiodic role for the avian melatonin rhythm may be trait specific. Pinealectomy prevents the long day-induced increase in nesting behaviour of male ring doves (Streptopelia risoria; McDonald 1982). In castrated European starlings, constant release melatonin implants prevent photorefractory-induced changes in the immune system (Bentley et al. 1998a) and long day-induced increases in the high vocal centre (HVc), a brain nucleus important for birdsong (Bentley et al. 1999). Again, these effects may be of pharmacological rather than physiological significance. To our knowledge, long-term melatonin infusions that mimic the endogenous secretory profile have not yet been conducted in either birds or reptiles.
A pineal- and melatonin-independent mechanism of PTM and its subsequent transduction has been proposed for Japanese quail, via local regulation of thyroid hormone in the medial basal hypothalamus (MBH; Yasuo et al. 2006). The MBH has long been proposed to regulate photoperiodic responses of the reproductive system. Immediate early gene expression is induced within this area after exposure to a single long day (Meddle & Follett 1995, 1997). Lesions within the MBH block photoperiodic responses of Japanese quail (Sharp & Follett 1969), and electrical stimulation induces LH secretion (Konishi et al. 1987) and testicular growth (Ohta et al. 1984). Photoperiodic cues may act directly on the MBH via deep brain photoreceptors within the infundibular nucleus (Oliver & Bayle 1982; Silver et al. 1988). Selective daily illumination of the MBH for 16 h per day stimulates gonadal growth in quail housed under 10L conditions (Ohta et al. 1984). Type 2 iodothyronine deiodinase gene expression in the MBH is elevated under long compared to short day lengths (Yoshimura et al. 2003). This enzyme, which converts thyroxine (T4) to 3,5,3′-triiodothyronine (T3), implicates local changes in thyroid hormone concentrations in the photoperiodic stimulation of the testes. Lending support to this idea, intracerebroventricular (ICV) infusions of T3 induce gonadal growth in quail housed in a short day length, whereas infusions of iopanoic acid, a type 2 iodothyronine deiodinase inhibitor, attenuate gonadal growth in long day lengths (Yoshimura et al. 2003). Interestingly, MBH mRNA levels of type 3 iodothyronine deiodinase, an enzyme that degrades both T3 and T4, are decreased under long compared with short day lengths, a photoperiodic pattern opposite to that of type 2 iodothyronine deiodinase (Yasuo et al. 2005).
Thyroid hormones (Vriend 1985; Dawson 1998; Wilson & Reinert 2000; Watanabe et al. 2004; Revel et al. 2006b; Freeman et al. 2007) and the MBH/premammillary bodies (Maywood & Hastings 1995; Malpaux et al. 1998; Lewis et al. 2002) have also been implicated in the transduction of day length information in several other birds and mammals, and melatonin has been shown to regulate MBH type 2 iodothyronine deiodinase expression in several rodents (Watanabe et al. 2004; Revel et al. 2006b; Yasuo et al. 2007). Thus, a role for thyroid hormones within the MBH may be an evolutionarily conserved photoperiodic mechanism in birds and mammals, directly regulated by light impinging on deep brain and possibly retinal photoreceptors in the former and indirectly by light affecting the duration of nocturnal melatonin secretion in the latter.
It is important to note that the precise role for thyroid hormones in seasonality remains uncertain. In some species, the hormones appear to act downstream of photoperiodic transduction. Thyroidectomy prevents the development of refractoriness to unchanging photoperiods in sheep (Moenter et al. 1991; Parkinson & Follett 1994) and the transition from the breeding to the non-breeding condition in red deer (Cervus elaphus) maintained in natural day lengths (Shi & Barrell 1992). In sheep, the effect of thyroid hormones on refractoriness also appears to be mediated in the MBH/premammillary region (Anderson et al. 2003). In European starlings, different experimental procedures have shown that thyroidectomy can prevent both the photoperiodic responses (Dawson 1993) and the development of refractoriness (Goldsmith & Nicholls 1984). The full suite of seasonal responses (photoinduction and photorefractoriness) can be restored in thyroidectomized American tree sparrows by icv infusions of thyroid hormones (Wilson & Reinert 2000). At least in starlings, thyroid hormones appear to act permissively (Bentley et al. 1997a), involving changes in hypothalamic GnRH synthesis and/or secretion (Dawson et al. 2001, 2002), possibly mediated by nerve growth factor (Bentley et al. 1997b). The direction of thyroid hormone action on photorefractoriness, however, may not be consistent across all species; thyroidectomy hastens the onset of gonadal recrudescence in short day-housed Siberian hamsters (Prendergast et al. 2002a; see also Dawson & Thapliyal 2001).
(d) Other factors implicated in photoperiodic transduction
Thyroid hormones are not the only hypothalamically controlled factors involved in phenotypic changes to photoperiod. In Japanese quail, transforming growth factor α has been implicated in photoperiodic stimulation of the reproductive axis and appears to act through a separate signalling mechanism from that of type 2 iodothyronine deiodinase (Takagi et al. 2007). The search for additional neural substrates involved in the transduction of day length information is underway and recent investigations have uncovered novel hypothalamic candidates.
The recently discovered hypothalamic peptide, kisspeptin, which stimulates gonadotrophin secretion, is regulated by photoperiod in the arcuate and anteroventral periventricular nuclei of Siberian hamsters and in the arcuate nucleus of Syrian hamsters (Revel et al. 2006a; Greives et al. 2007). In sheep, the levels of kisspeptin expression in the arcuate nucleus are altered according to season (Smith et al. 2007). Photoperiodic differences in hypothalamic kisspeptin expression in male Syrian hamsters persist in short-day hamsters given testosterone implants, but are blocked by pinealectomy, indicating that these effects are not the result of changes in gonadal hormone secretion but more likely reflect changes in the duration of nocturnal melatonin secretion (Revel et al. 2006a). Exogenous administration of kisspeptin stimulates LH secretion in short-day-housed Siberian hamsters and testosterone secretion and testis growth in short-day-housed Syrian hamsters (Revel et al. 2006a; Greives et al. 2007), probably by direct actions on GnRH neurons (for reviews see Navarro et al. 2007; Smith & Clarke 2007). Kisspeptin also appears to convey feeding-related information to the reproductive axis (Castellano et al. 2005), suggesting that this peptide integrates reproductive inputs from various environmental stimuli.
Other studies point to the dorsal medial posterior arcuate nucleus and the ependymal layer as candidate sites involved in photoperiodic regulation of body mass and/or reproduction in Siberian hamsters. Transcription of several genes within the dorsal medial posterior arcuate nucleus (VGF, histamine H3 receptor and several retinoic acid related genes) and the ependymal layer (cellular retinoic acid-binding protein 1, G-protein-coupled orphan receptor 50, nestin, type 2 iodothyronine deiodinase and type 3 iodothyronine deiodinase) is regulated by photoperiod (Barrett et al. 2005, 2006; Watanabe et al. 2004, 2007; Ross et al. 2005). Changes in the expression of VGF, histamine H3 receptor and cellular retinoic acid-binding protein 2 within the dorsal medial posterior arcuate nucleus occur before any detectable changes in body mass or gonadal size, raising the possibility that these genes play a role in the induction of photoperiodic changes in behaviour and physiology (Ross et al. 2005).
4. On the location of interval timers and circannual clocks
The neural circuitry that mediates different seasonal traits remains largely unspecified. The search for biological substrates of annual timekeeping mechanisms has been largely restricted to mammals and focused on neural structures that might logically function as targets of melatonin action. In fact, the distribution of tissues that exhibit melatonin binding and receptor expression is highly species specific; the pars tuberalis (PT) of the pituitary gland is thought to be the only melatonin target tissue common to all mammals (Bittman 1993; Morgan et al. 1994). Melatonin implants directed at various targets (including the anterior hypothalamus, SCN, nucleus reuniens, paraventricular nucleus of the thalamus or MBH) in sheep, Siberian hamsters or white-footed mice induce seasonal changes in various traits (Glass & Lynch 1982; Dowell & Lynch 1987; Badura & Goldman 1992; Lincoln & Maeda 1992; Malpaux et al. 1998; Freeman & Zucker 2001). Although these studies provide evidence that melatonin can act at multiple structures to effect seasonal changes, they do not specify the precise role of the hormone at each site.
(a) Multiple sites for timers and clocks
Long duration daily melatonin infusions via microdialysis to the SCN, paraventricular nucleus of the thalamus or the nucleus reuniens prevents gonadal development of juvenile hamsters housed in constant light (Badura & Goldman 1992). Melatonin implants directed at these same structures of adult pinealectomized Siberian hamsters each first induced gonadal regression, followed some weeks later by gonadal recrudescence indicative of refractoriness to melatonin (Freeman & Zucker 2001). Even though each of these individual tissues was rendered refractory to melatonin, subsequent subcutaneous long duration melatonin infusions induced a second testicular regression. This result establishes that the induction of refractoriness in a single tissue is not associated with refractoriness in all other melatonin targets. The interval timing mechanism that culminates in refractoriness to melatonin may be a feature common to multiple melatonin target tissues. These results and those of Badura & Goldman (1992) point to a highly redundant system for seasonal control of gonadal function. Alternatively, these brain nuclei may regulate different aspects of the melatonin-based photoperiodic reproductive response (Teubner & Freeman 2007). Melatonin signalling to the nucleus reuniens in Siberian hamsters appears to be critical for photostimulation by long day lengths and development of a photoperiodic history. Interrupting the melatonin signal in the SCN, however, only affects the photoperiodic history response, whereas the same treatment in the paraventricular nucleus of the thalamus has no effect on photostimulation or photoperiodic history.
Whether seasonal rhythms in several different seasonal traits (e.g. food intake, sex behaviour, aggressive encounters, body mass) are selectively regulated by one or more melatonin targets are presently unknown. Separation of function and multiple seasonal mechanisms is inferred from a study of garden warblers (Sylvia borin), in which at least two circannual clocks control the annual rhythms of gonadal function and moulting; under constant conditions, free-running circannual rhythms of testis size and moult dissociate and oscillate with different periods (Gwinner & Dorka 1976). Analogously, T3 injections delay the onset of gonadal, but not somatic, recrudescence in Siberian hamsters (Freeman et al. 2007). In hamsters and sheep, melatonin differentially controls seasonal variations in prolactin and gonadotrophin secretion through its actions on the PT and MBH, respectively. In the Syrian hamster, MBH lesions that block photoperiodic responses in gonadal function do not compromise short day-induced decreases in prolactin secretion (Maywood & Hastings 1995; Maywood et al. 1996; Lewis et al. 2002). Hypothalamo–pituitary surgical disconnection in Soay rams disrupts photoperiodic changes in food intake, body weight and metabolic hormones, but seasonal changes in prolactin secretion persist (Lincoln & Clarke 1994; Lincoln et al. 2001). Thus, an annual timekeeping mechanism that suffices to control prolactin is critically dependent on the pituitary, whereas other seasonal traits are mediated by neural tissues, probably within the hypothalamus and thalamus (Lincoln & Clarke 1997).
The PT, situated on the pituitary stalk between the median eminence of the hypothalamus and the pars distalis of the pituitary gland, is currently the best understood annual timekeeping tissue and probably contains an interval timer (in hamsters) and a circannual clock (in sheep) that regulates seasonal prolactin secretion. Soay rams whose pituitary is surgically isolated from direct hypothalamic inputs continue to show long-term seasonal changes (i.e. refractoriness) and circannual cycles of prolactin secretion under constant photoperiod exposure (Lincoln & Clarke 1997, 2000; Lincoln et al. 2003a, 2006). This tissue provides a powerful model system for elucidating the melatonin decoding and annual timekeeping mechanisms that contribute to the seasonal regulation of prolactin secretion.
Melatonin is believed to act directly on PT cells to regulate photoperiodic changes in prolactin secretion (Lincoln & Clarke 1994); high melatonin receptor binding is seen in the PT of several species, whereas little to no binding is found in the pars distalis of adult sheep, Siberian hamsters, Syrian hamsters, rats and mice (Bittman & Weaver 1990; Morgan et al. 1994). Current evidence suggests that melatonin-sensitive cells in the PT secrete a prolactin releasing factor (‘tuberalin’) that acts in a paracrine fashion to stimulate lactotrophs in the pars distalis (reviewed in Johnston 2004). Melatonin binds to melatonin 1a (MT1) receptors coupled to the Gi class of G proteins in PT cells, inhibiting adenylyl cyclase activity and cyclic AMP production and somehow altering the synthesis and/or release of tuberalin. The chemical identity of tuberalin remains unknown.
Prolactin mRNA and protein content of the pars distalis decrease in short-day-housed hamsters but rebound to approximate long-day levels in short-day refractory animals, indicating neuroendocrine refractory state of these cells (Bockers et al. 1997; Johnston et al. 2003). The endocrine cells of the PT of Soay sheep and Siberian hamsters also become refractory as assessed by the α-glycoprotein hormone subunit (Bockers et al. 1997; Lincoln et al. 2005). In PT cells from refractory Siberian hamsters, melatonin receptor kinetics and the cAMP response to melatonin continue to reflect the photosensitive phenotype (Weaver et al. 1991), indicating that the substrate of the refractory state must lie downstream from melatonin signal transduction. On the other hand, PT cell cultures from refractory Syrian hamsters can induce long-day release of prolactin from pars distalis co-cultures, so the refractory state in PT must lie upstream of tuberalin secretion (Johnston et al. 2003).
(b) Circadian substrates beyond PTM
Several investigations have questioned whether the circadian system might be a part of the annual timekeeping mechanism, in addition to and downstream from its well-known role in PTM. At least in the golden-mantled ground squirrel, the data indicate that the circadian pacemaker in the SCN is not the location of the circannual clock. SCN lesions that induce circadian arrhythmicity of wheel-running behaviour do not disrupt circannual rhythms in body mass, hibernation and reproduction in the majority of squirrels (Zucker et al. 1983; Ruby et al. 1998). Circannual rhythms were disrupted in a subset of squirrels, but this effect did not correlate with lesion size or location (Zucker et al. 1983; Dark et al. 1985; Ruby et al. 1998). Interestingly, when lesioned animals were housed in the cold, a significant number hibernated indefinitely, displaying torpor bouts throughout the 2.5 year experiment, in contrast to the normal hibernation season in this species, which is approximately five to six months (Ruby et al. 1996). Thus the SCN, which remains functional at low ambient temperatures (Kilduff et al. 1990; Ruby & Heller 1996), may play a role in regulating the duration of the hibernation season, but it is not the site of an indispensable circannual clock.
Additional data argue against a role for the circadian system in setting the duration of the interval timer or the oscillation of the circannual clock. Circannual timing is not achieved by ‘counting’ circadian cycles, the so-called frequency-demultiplication model (Gwinner 1973). According to this hypothesis, animals entrained to light dark cycles of various circadian periods (T cycles) should have different circannual periods with respect to real time; animals housed in short T cycles (e.g. 23 h) will experience a greater number of circadian cycles in a given interval and should have shorter circannual cycles than those housed in longer T cycles. Yet the circannual periods of golden-mantled ground squirrels and European starlings entrained to different T cycles (squirrels T=23, 24 or 25 h; starlings T=22 or 24 h) are indistinguishable (Gwinner 1981b; Carmichael & Zucker 1986). The frequency demultiplication hypothesis also failed to gain support in Syrian hamsters, an interval-timing species. In the free-running tau mutant Syrian hamster, the shortened circadian period (20 h in homozygotes) does not alter the duration of the seasonal interval timer relative to that of free-running wild-type hamsters with approximately a 24 h circadian period (Lucas et al. 2000).
Whereas the circadian system does not appear to underlie the annual timekeeping mechanism of timers and clocks, the data regarding its role in decoding melatonin duration are conflicting. On one hand are studies in pinealectomized Syrian hamsters that do not support this possibility; melatonin infusions in such animals are effective at all phases of the circadian cycle (Maywood et al. 1990) and at frequencies outside the limits of entrainment of the circadian locomotor activity rhythm (once every 20 h; Grosse et al. 1993). Infusions provided once every 16 or 28 h do not result in the winter phenotype, demonstrating the limits to non-circadian intervals that are effective (Grosse et al. 1993). Lesions of the SCN have been reported to prevent photoperiodic responses to melatonin infusions in Siberian hamsters (Bartness et al. 1991), but similar findings have not been obtained in SCN-ablated mink (Mustela vision), spotted skunks (Spilogale putorius latifrons) or Syrian hamsters (Bittman et al. 1979; Berria et al. 1988; Bonnefond et al. 1990; Maywood et al. 1990). Curiously, the SCN may play some role in decoding a series of melatonin signals in Syrian hamsters; SCN-intact, but not SCN-ablated, pinealectomized Syrian hamsters are capable of responding to long duration melatonin infusions presented in a random fashion (Grosse & Hastings 1996). The relevance of such random infusions for understanding normal function is not clear.
On the other hand, a few studies of tau mutant Syrian hamsters hint at a possible circadian involvement in decoding melatonin signal duration. Melatonin infusions presented to this mutant at 16 h (but not at 24 h) intervals induce the short day phenotype (Stirland et al. 1996). This 4 h shift in the range of effective melatonin frequencies from that of wild-type hamsters correlates well with the shortened circadian period of the tau mutant. Tau mutant hamsters also have shorter critical day lengths and more robust short-day responses to an intermediate duration melatonin infusion (6.67 h) than do wild-type hamsters; this circadian mutation does affect the threshold duration of day length and melatonin required for short-day responses (Stirland et al. 1995; Shimomura et al. 1997). Perhaps decoding the melatonin signal, while not using a circadian mechanism, must resonate temporally with underlying daily physiological processes that are regulated by the circadian system. Alternatively, reading the melatonin signal may involve extra-SCN circadian oscillators within the target tissues.
(c) Circadian ‘clock’ genes and the seasons
Whereas prior investigations had suggested that extra-SCN circadian oscillators might exist, only recently have a variety of cultured brain regions (Abe et al. 2002), peripheral tissues (including the pituitary gland: Yamazaki et al. 2000; Abe et al. 2002) and even immortalized fibroblasts (Balsalobre et al. 1998) been shown to exhibit circadian oscillations in the expression of circadian clock genes. These in vitro oscillations are sustained (Granados-Fuentes et al. 2004; Yoo et al. 2004), although overall amplitude may dampen as the rhythms of individual cells desynchronize (Nagoshi et al. 2004; Welsh et al. 2004). The physiological significance of the cycling of such genes in extra-SCN tissues is not known, but they may be autonomous ‘slave’ oscillators whose phase is set by the ‘master’ oscillator within the SCN. Conceivably, such a multi-site circadian network could allow for the independent adjustment of multiple rhythm phases to each other and to the ambient photoperiod; hypothetically, extra-SCN tissues might co-opt the expression of circadian clock genes for a ‘calendar’ mechanism that decodes melatonin signal duration (Lincoln et al. 2003b).
Such genes are rhythmically expressed in the PT (Lincoln et al. 2002) and at least the Per1 rhythm is melatonin dependent. The rhythm is absent in pinealectomized Syrian hamsters (Messager et al. 2001), as well as in inbred strains of mice that do not synthesize melatonin (Sun et al. 1997) or lack functional melatonin 1a receptors (von Gall et al. 2002). Peaks of Per1 and Per2 mRNA in the early photophase appear phase-locked to the suppression of melatonin secretion by light onset at dawn. Acute melatonin administration inhibits Per1 mRNA expression while chronic treatments appear to sensitize it to some other stimulatory factor in the PT (Morgan et al. 1998; Messager et al. 1999, 2000; von Gall et al. 2002). In contrast, melatonin stimulates Cryptochrome 1 (Cry1) mRNA expression (Dardente et al. 2003; Johnston et al. 2006), and peaks of Cry1 and Cry2 mRNA in the early scotophase appear phase locked to the activation of melatonin synthesis by light offset at dusk. Thus, a differential phase relation between Per and Cry levels in the PT could act to transduce day length and melatonin duration into seasonal changes in prolactin secretion (see Lincoln et al. 2003b). However, immunoreactive PER and CRY protein levels have yet to be measured and a causal link between clock gene expression and tuberalin activity has not been demonstrated.
Researchers have also questioned whether clock gene expression contributes to the annual timekeeping mechanism itself, specifically whether their phase relations in refractory animals would no longer reflect the ambient photoperiod. Yet mRNA rhythms of such genes continue to faithfully represent the ambient photoperiod and melatonin profile in short-day refractory Syrian hamsters and long-day refractory sheep (Johnston et al. 2003; Lincoln et al. 2005).
5. A possible synthesis: are circannual clocks constructed from interval timers?
Seasonal regulation of prolactin secretion appears to be an ancient system (Lincoln 2000) highly conserved between species using either an interval timer (hamsters) or circannual clock (sheep; Lincoln et al. 2003a). Goldman et al. (2004) proposed that the circannual clock is an interval timer whose refractoriness is spontaneously broken, i.e. the stimulus for resetting the interval timer in a circannual species is endogenous and no longer relies on the environment. Interval timing species such as the Siberian hamster only display successive cycles when exposed to alternating short and long photoperiods because they require short days to trigger the timer and long days to reset it. Circannual species may have evolved a completely endogenous mechanism for triggering and resetting the interval timer.
This concept may help account for species that express endogenous annual cycles but only under a specific set of photoperiodic conditions (‘permissive photoperiods’). European starlings (and possibly European hamsters, Cricetus cricetus; see Canguilhem et al. 1988a,b; Canguilhem 1989) display circannual cycles when housed in 12L, but not in other photoperiods (Schwab 1976; Dawson 2007). In sika deer, 12L is the only photoperiod in which the circannual rhythm in antler growth is not expressed (Goss 1969b; Goss 1984). Rams exhibit a robust circannual rhythm of prolactin secretion under long photoperiods, but rhythmicity is weak or absent in short photoperiods (Howles et al. 1982; Langford et al. 1987; Lincoln & Clarke 2000). In contrast, obligate circannual species such as the golden-mantled ground squirrel display circannual cycles under all photoperiodic conditions tested, including constant light and constant darkness (Pengelley & Asmundson 1970; Zucker & Boshes 1982; Zucker et al. 1983). Permissive photoperiods may reveal the sequential activation of interval timers that is otherwise suppressed. Circannual cycles in European starlings and European hamsters might result from sequential interpretation of 12L first as a long day and then as a short day (Gwinner 2003), thereby triggering and resetting the interval timer. Turkish hamsters (Mesocricetus brandti) could provide an interesting opportunity to demonstrate circannual cycles in a species traditionally considered to use an interval timer. Because a 20L photoperiod can both initiate seasonal gonadal regression and break refractoriness to short day lengths in Turkish hamsters (Hong et al. 1986; Hong & Stetson 1988), circannual rhythmicity might be predicted in 20L if the interval timer is both triggered and reset by this day length.
In theory, there are many ways in which seasonally appropriate adjustments can be assured, but the limited data available have been interpreted as evidence for just two conceptual mechanisms, despite the probable independent evolution of seasonality several times (Farner 1985) and the assertion that natural selection selects for outcomes, not mechanisms (Mayr 1982). The rigid classification of timing mechanisms as either timers or clocks may be physiologically misleading despite its heuristic value. It seems probable that the timekeeping mechanism of the ‘circannual’ golden-mantled ground squirrel is more similar to that of the ‘interval-timing’ Syrian hamster than to that of ‘circannual’ birds or even ungulates. In addition to the existence of true timers and clocks in some animals, other species may rely on mechanisms that lie on a continuum between timer-like and clock-like classifications.
Acknowledgments
This work is supported by the National Institute of Neurological Disorders and Stroke (NINDS) grants RO1 NS46605 and T32 NS007366 and the National Institute of Mental Health (NIMH) RO1 MH61171. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NINDS or NIMH.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Abe M, Herzog E.D, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block G.D. Circadian rhythms in isolated brain regions. J. Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida O.F, Lincoln G.A. Photoperiodic regulation of reproductive activity in the ram: evidence for the involvement of circadian rhythms in melatonin and prolactin secretion. Biol. Reprod. 1982;27:1062–1075. doi: 10.1095/biolreprod27.5.1062. doi:10.1095/biolreprod27.5.1062 [DOI] [PubMed] [Google Scholar]
- Anderson G.M, Hardy S.L, Valent M, Billings H.J, Connors J.M, Goodman R.L. Evidence that thyroid hormones act in the ventromedial preoptic area and the premammillary region of the brain to allow the termination of the breeding season in the ewe. Endocrinology. 2003;144:2892–2901. doi: 10.1210/en.2003-0322. doi:10.1210/en.2003-0322 [DOI] [PubMed] [Google Scholar]
- Badura L.L, Goldman B.D. Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res. 1992;598:98–106. doi: 10.1016/0006-8993(92)90172-6. doi:10.1016/0006-8993(92)90172-6 [DOI] [PubMed] [Google Scholar]
- Baggerman B. Photoperiodic responses in the stickleback and their control by a daily rhythms of photosensitivity. Gen. Comp. Endocrinol. Suppl. 1972;3:466–476. doi:10.1016/0016-6480(72)90177-3 [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. doi:10.1016/S0092-8674(00)81199-X [DOI] [PubMed] [Google Scholar]
- Barrell G.K, Thrun L.A, Brown M.E, Viguie C, Karsch F.J. Importance of photoperiodic signal quality to entrainment of the circannual reproductive rhythm of the ewe. Biol. Reprod. 2000;63:769–774. doi: 10.1095/biolreprod63.3.769. doi:10.1095/biolreprod63.3.769 [DOI] [PubMed] [Google Scholar]
- Barrett P, et al. Photoperiodic regulation of histamine H3 receptor and VGF messenger ribonucleic acid in the arcuate nucleus of the Siberian hamster. Endocrinology. 2005;146:1930–1939. doi: 10.1210/en.2004-1452. doi:10.1210/en.2004-1452 [DOI] [PubMed] [Google Scholar]
- Barrett P, et al. Photoperiodic regulation of cellular retinoic acid-binding protein 1, GPR50 and nestin in tanycytes of the third ventricle ependymal layer of the Siberian hamster. J. Endocrinol. 2006;191:687–698. doi: 10.1677/joe.1.06929. doi:10.1677/joe.1.06929 [DOI] [PubMed] [Google Scholar]
- Bartness T.J, Goldman B.D, Bittman E.L. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am. J. Physiol. 1991;260:R102–R112. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Goldsmith A.R, Dawson A, Glennie L.M, Talbot R.T, Sharp P.J. Photorefractoriness in European starlings (Sturnus vulgaris) is not dependent upon the long-day-induced rise in plasma thyroxine. Gen. Comp. Endocrinol. 1997a;107:428–438. doi: 10.1006/gcen.1997.6941. doi:10.1006/gcen.1997.6941 [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Goldsmith A.R, Juss T.S, Dawson A. The effects of nerve growth factor and anti-nerve growth factor antibody on the neuroendocrine reproductive system in the European starling Sturnus vulgaris. J. Comp. Physiol. A. 1997b;181:133–141. doi: 10.1007/s003590050100. doi:10.1007/s003590050100 [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Demas G.E, Nelson R.J, Ball G.F. Melatonin, immunity and cost of reproductive state in male European starlings. Proc. Biol. Sci. 1998a;265:1191–1195. doi: 10.1098/rspb.1998.0418. doi:10.1098/rspb.1998.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley G.E, Goldsmith A.R, Dawson A, Briggs C, Pemberton M. Decreased light intensity alters the perception of day length by male European starlings (Sturnus vulgaris) J. Biol. Rhythms. 1998b;13:148–158. doi: 10.1177/074873098128999998. doi:10.1177/074873098128999998 [DOI] [PubMed] [Google Scholar]
- Bentley G.E, Van't Hof T.J, Ball G.F. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc. Natl Acad. Sci. USA. 1999;96:4674–4679. doi: 10.1073/pnas.96.8.4674. doi:10.1073/pnas.96.8.4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger P.J, Negus N.C, Sanders E.H, Gardner P.D. Chemical triggering of reproduction in Microtus montanus. Science. 1981;214:69–70. doi: 10.1126/science.7025210. doi:10.1126/science.7025210 [DOI] [PubMed] [Google Scholar]
- Bergmann M. Photoperiod and testicular function in Phodopus sungorus. Adv. Anat. Embryol. Cell Biol. 1987;105:1–76. doi: 10.1007/978-3-642-71930-1. [DOI] [PubMed] [Google Scholar]
- Berria M, DeSantis M, Mead R.A. Effects of suprachiasmatic nuclear ablation and melatonin on delayed implantation in the spotted skunk. Neuroendocrinology. 1988;48:371–375. doi: 10.1159/000125037. [DOI] [PubMed] [Google Scholar]
- Bertolucci C, Foa A, Van't Hof T.J. Seasonal variations in circadian rhythms of plasma melatonin in ruin lizards. Horm. Behav. 2002;41:414–419. doi: 10.1006/hbeh.2002.1781. doi:10.1006/hbeh.2002.1781 [DOI] [PubMed] [Google Scholar]
- Bertolucci C, Wagner G, Foa A, Gwinner E, Brandstatter R. Photoperiod affects amplitude but not duration of in vitro melatonin production in the ruin lizard (Podarcis sicula) J. Biol. Rhythms. 2003;18:63–70. doi: 10.1177/0748730402239677. doi:10.1177/0748730402239677 [DOI] [PubMed] [Google Scholar]
- Bittman E.L. The sites of consequences of melatonin binding in mammals. Am. Zool. 1993;33:200–211. [Google Scholar]
- Bittman E.L, Weaver D.R. The distribution of melatonin binding sites in neuroendocrine tissues of the ewe. Biol. Reprod. 1990;43:986–993. doi: 10.1095/biolreprod43.6.986. doi:10.1095/biolreprod43.6.986 [DOI] [PubMed] [Google Scholar]
- Bittman E.L, Goldman B.D, Zucker I. Testicular responses to melatonin are altered by lesions of the suprachiasmatic nuclei in golden hamsters. Biol. Reprod. 1979;21:647–656. doi: 10.1095/biolreprod21.3.647. doi:10.1095/biolreprod21.3.647 [DOI] [PubMed] [Google Scholar]
- Bittman E.L, Dempsey R.J, Karsch F.J. Pineal melatonin secretion drives the reproductive response to daylength in the ewe. Endocrinology. 1983;113:2276–2283. doi: 10.1210/endo-113-6-2276. [DOI] [PubMed] [Google Scholar]
- Bockers T.M, Bockmann J, Salem A, Niklowitz P, Lerchl A, Huppertz M, Wittkowski W, Kreutz M.R. Initial expression of the common alpha-chain in hypophyseal pars tuberalis-specific cells in spontaneous recrudescent hamsters. Endocrinology. 1997;138:4101–4108. doi: 10.1210/endo.138.10.5423. doi:10.1210/en.138.10.4101 [DOI] [PubMed] [Google Scholar]
- Bonnefond C, Martinet L, Monnerie R. Effects of timed melatonin infusions and lesions of the suprachiasmatic nuclei on prolactin and progesterone secretions in pregnant or psuedopregnant mink (Mustela vision) J. Neuroendocrinol. 1990;2:583–591. doi: 10.1111/j.1365-2826.1990.tb00451.x. doi:10.1111/j.1365-2826.1990.tb00451.x [DOI] [PubMed] [Google Scholar]
- Brandstätter R, Kumar V, Abraham U, Gwinner E. Photoperiodic information acquired and stored in vivo is retained in vitro by a circadian oscillator, the avian pineal gland. Proc. Natl Acad. Sci. USA. 2000;97:12 324–12 328. doi: 10.1073/pnas.200354997. doi:10.1073/pnas.200354997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromage N, Duston J, Randall C, Brook A, Thrush M, Carrillo M, Zanuy S. Photoperiodic control of teleost reproduction. Prog. Clin. Biol. Res. 1990;342:620–626. [PubMed] [Google Scholar]
- Bronson F.H. University of Chicago Press; Chicago, IL: 1989. Mammalian reproductive biology. [Google Scholar]
- Butler, M. P., Turner, K. W., Park, J. H., Butler, J. P., Trumbull, J. J., Dunn, S. P., Villa, P. & Zucker, I. 2007 Simulated natural day lengths synchronize seasonal rhythms of asynchronously born male Siberian hamsters, Am. J. Physiol. Regul. Integr. Comp. Physiol. (doi:10.1152/ajpregu.00146.2007) [DOI] [PubMed]
- Canguilhem B. External and endogenous control of body weight rhythm in the European hamster, Cricetus cricetus. In: Malan A, Canguilhem B, editors. Living in the cold II. John Libbey Eurotext Ltd; Paris, France: 1989. pp. 25–32. [Google Scholar]
- Canguilhem B, Masson-Pévet M, Koehl C, Pévet P, Bentz I. Non-gonadal mediated effect of photoperiod on hibernation and body weight cycles of the European hamster. Comp. Biochem. Physiol. A. 1988a;89:575–578. doi: 10.1016/0300-9629(88)90835-3. doi:10.1016/0300-9629(88)90835-3 [DOI] [PubMed] [Google Scholar]
- Canguilhem B, Vaultier J.P, Pévet P, Coumaros G, Masson-Pévet M, Bentz I. Photoperiodic regulation of body mass, food intake, hibernation, and reproduction in intact and castrated male European hamsters, Cricetus cricetus. J. Comp. Physiol. A. 1988b;163:549–557. doi: 10.1007/BF00604908. doi:10.1007/BF00604908 [DOI] [PubMed] [Google Scholar]
- Carmichael M.S, Zucker I. Circannual rhythms of ground squirrels: a test of the frequency demultiplication hypothesis. J. Biol. Rhythms. 1986;1:277–284. doi: 10.1177/074873048600100402. doi:10.1177/074873048600100402 [DOI] [PubMed] [Google Scholar]
- Carter D.S, Goldman B.D. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983a;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Carter D.S, Goldman B.D. Progonadal role of the pineal in the Djungarian hamster (Phodopus sungorus sungorus): mediation by melatonin. Endocrinology. 1983b;113:1268–1273. doi: 10.1210/endo-113-4-1268. [DOI] [PubMed] [Google Scholar]
- Castellano J.M, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. doi:10.1210/en.2005-0337 [DOI] [PubMed] [Google Scholar]
- Christian J.J. Regulation of annual rhythms in temperate small rodents. In: Steinberger A, Steinberger E, editors. Testicular development, structure, and function. Raven Press; New York, NY: 1980. pp. 367–380. [Google Scholar]
- Concannon P, Roberts P, Baldwin B, Erb H, Tennant B. Alteration of growth, advancement of puberty, and season-appropriate circannual breeding during 28 months of photoperiod reversal in woodchucks (Marmota monax) Biol. Reprod. 1993;48:1057–1070. doi: 10.1095/biolreprod48.5.1057. doi:10.1095/biolreprod48.5.1057 [DOI] [PubMed] [Google Scholar]
- Crews D. Neuroendocrine adaptations. In: Balthazart J, editor. Hormones, brain, and behavior in vertebrates. vol. 9. Karger; Basel, Germany: 1990. pp. 1–14. [Google Scholar]
- Daan S, Albrecht U, van der Horst G.T, Illnerová H, Roenneberg T, Wehr T.A, Schwartz W.J. Assembling a clock for all seasons: are there M and E oscillators in the genes? J. Biol. Rhythms. 2001;16:105–116. doi: 10.1177/074873001129001809. doi:10.1177/074873001129001809 [DOI] [PubMed] [Google Scholar]
- Dardente H, Menet J.S, Poirel V.J, Streicher D, Gauer F, Vivien-Roels B, Klosen P, Pévet P, Masson-Pévet M. Melatonin induces Cry1 expression in the pars tuberalis of the rat. Brain Res. Mol. Brain Res. 2003;114:101–106. doi: 10.1016/s0169-328x(03)00134-7. doi:10.1016/S0169-328X(03)00134-7 [DOI] [PubMed] [Google Scholar]
- Dark J, Pickard G.E, Zucker I. Persistence of circannual rhythms in ground squirrels with lesions of the suprachiasmatic nuclei. Brain Res. 1985;332:201–207. doi: 10.1016/0006-8993(85)90589-x. doi:10.1016/0006-8993(85)90589-X [DOI] [PubMed] [Google Scholar]
- Dawson A. Thyroidectomy progressively renders the reproductive system of starlings (Sturnus vulgaris) unresponsive to changes in daylength. J. Endocrinol. 1993;139:51–55. doi: 10.1677/joe.0.1390051. [DOI] [PubMed] [Google Scholar]
- Dawson A. Thyroidectomy of house sparrows (Passer domesticus) prevents photo-induced testicular growth but not the increased hypothalamic gonadotrophin-releasing hormone. Gen. Comp. Endocrinol. 1998;110:196–200. doi: 10.1006/gcen.1998.7065. doi:10.1006/gcen.1998.7065 [DOI] [PubMed] [Google Scholar]
- Dawson A. Seasonality in a temperate zone bird can be entrained by near equatorial photoperiods. Proc. Biol. Sci. 2007;274:721–725. doi: 10.1098/rspb.2006.0067. doi:10.1098/rspb.2006.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, Thapliyal A. The thyroid and photoperiodism. In: Dawson A, Chaturvedi C.M, editors. Avian endocrinology. Narosa Publishing House; New Delhi, India: 2001. pp. 141–151. [Google Scholar]
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. doi:10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Dawson A, Talbot R.T, Dunn I.C, Sharp P.J. Changes in basal hypothalamic chicken gonadotropin-releasing hormone-I and vasoactive intestinal polypeptide associated with a photo-induced cycle in gonadal maturation and prolactin secretion in intact and thyroidectomized starlings (Sturnus vulgaris) J. Neuroendocrinol. 2002;14:533–539. doi: 10.1046/j.1365-2826.2002.00807.x. doi:10.1046/j.1365-2826.2002.00807.x [DOI] [PubMed] [Google Scholar]
- de la Iglesia H.O, Meyer J, Schwartz W.J. Using per gene expression to search for photoperiodic oscillators in the hamster suprachiasmatic nucleus. Brain Res. Mol. Brain Res. 2004;127:121–127. doi: 10.1016/j.molbrainres.2004.05.018. doi:10.1016/j.molbrainres.2004.05.018 [DOI] [PubMed] [Google Scholar]
- Desjardins C, Lopez M.J. Environmental cues evoke differential responses in pituitary–testicular function in deer mice. Endocrinology. 1983;112:1398–1406. doi: 10.1210/endo-112-4-1398. [DOI] [PubMed] [Google Scholar]
- Dickert-Conlin S, Chandra A. Taxes and the timing of births. J. Polit. Econ. 1999;107:161–177. doi:10.1086/250054 [Google Scholar]
- Dowell S.F, Lynch G.R. Duration of the melatonin pulse in the hypothalamus controls testicular function in pinealectomized mice (Peromyscus leucopus) Biol. Reprod. 1987;36:1095–1101. doi: 10.1095/biolreprod36.5.1095. doi:10.1095/biolreprod36.5.1095 [DOI] [PubMed] [Google Scholar]
- Earnest D.J, Turek F.W. Effect of one-second light pulses on testicular function and locomotor activity in the golden hamster. Biol. Reprod. 1983;28:557–565. doi: 10.1095/biolreprod28.3.557. doi:10.1095/biolreprod28.3.557 [DOI] [PubMed] [Google Scholar]
- Elliott J.A. Circadian rhythms and photoperiodic time measurement in mammals. Fed. Proc. 1976;35:2339–2346. [PubMed] [Google Scholar]
- Elliott J.A, Stetson M.H, Menaker M. Regulation of testis function in golden hamsters: a circadian clock measures photoperiodic time. Science. 1972;178:771–773. doi: 10.1126/science.178.4062.771. doi:10.1126/science.178.4062.771 [DOI] [PubMed] [Google Scholar]
- Eskes G.A. Gonadal responses to food restriction in intact and pinealectomized male golden hamsters. J. Reprod. Fertil. 1983;68:85–90. doi: 10.1530/jrf.0.0680085. [DOI] [PubMed] [Google Scholar]
- Farner D.S. Annual rhythms. Annu. Rev. Physiol. 1985;47:65–82. doi: 10.1146/annurev.ph.47.030185.000433. doi:10.1146/annurev.ph.47.030185.000433 [DOI] [PubMed] [Google Scholar]
- Figala J, Hoffmann K, Goldau G. Zur Jahresperiodik beim Dsungarischen Zwerghamster Phodopus sungorus PALLAS. Oecologia. 1973;12:89–118. doi: 10.1007/BF00345511. doi:10.1007/BF00345511 [DOI] [PubMed] [Google Scholar]
- Follett B.K, Sharp P.J. Circadian rhythmicity in photoperiodically induced gonadotrophin release and gonadal growth in the quail. Nature. 1969;223:968–971. doi: 10.1038/223968b0. doi:10.1038/223968b0 [DOI] [PubMed] [Google Scholar]
- Freeman D.A, Zucker I. Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc. Natl Acad. Sci. USA. 2001;98:6447–6452. doi: 10.1073/pnas.111140398. doi:10.1073/pnas.111140398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman D.A, Teubner B.J, Smith C.D, Prendergast B.J. Exogenous T3 mimics long day lengths in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R2368–R2372. doi: 10.1152/ajpregu.00713.2006. doi:10.1152/ajpregu.00713.2006 [DOI] [PubMed] [Google Scholar]
- Gaston S, Menaker M. Photoperiodic control of hamster testis. Science. 1967;158:925–928. doi: 10.1126/science.158.3803.925. doi:10.1126/science.158.3803.925 [DOI] [PubMed] [Google Scholar]
- Genin F, Schilling A, Perret M. Social inhibition of seasonal fattening in wild and captive gray mouse lemurs. Physiol. Behav. 2005;86:185–194. doi: 10.1016/j.physbeh.2005.07.002. doi:10.1016/j.physbeh.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Glass J.D, Lynch G.R. Evidence for a brain site of melatonin action in the white-footed mouse, Peromyscus leucopus. Neuroendocrinology. 1982;34:1–6. doi: 10.1159/000123269. [DOI] [PubMed] [Google Scholar]
- Goldman B.D. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. doi:10.1177/074873001129001980 [DOI] [PubMed] [Google Scholar]
- Goldman B.D, Darrow J.M, Yogev L. Effects of timed melatonin infusions on reproductive development in the Djungarian hamster (Phodopus sungorus) Endocrinology. 1984;114:2074–2083. doi: 10.1210/endo-114-6-2074. [DOI] [PubMed] [Google Scholar]
- Goldman, B. D., Gwinner, E., Karsch, F. J., Saunders, D., Zucker, I. & Ball G. 2004 Circannual rhythms and photoperiodicity. In Chronobiology: biological time keeping (eds J. C. Dunlap, J. J. Loros & P. J. De Coursey), pp. 107–144. Sunderland, M. A.: Sinauer Associates.
- Goldsmith A.R, Nicholls T.J. Thyroidectomy prevents the development of photorefractoriness and the associated rise in plasma prolactin in starlings. Gen. Comp. Endocrinol. 1984;54:256–263. doi: 10.1016/0016-6480(84)90179-5. doi:10.1016/0016-6480(84)90179-5 [DOI] [PubMed] [Google Scholar]
- Gorman M.R, Zucker I. Seasonal adaptations of Siberian hamsters. II. Pattern of change in daylength controls annual testicular and body weight rhythms. Biol. Reprod. 1995;53:116–125. doi: 10.1095/biolreprod53.1.116. doi:10.1095/biolreprod53.1.116 [DOI] [PubMed] [Google Scholar]
- Gorman M.R, Goldman B.D, Zucker I. Mammalian photoperiodism. In: Takahashi J.S, Turek F.W, Moore R.Y, editors. Handbook of behavioral neurobiology: circadian clocks. vol. 12. Kluwer Academic/Plenum Publishers; New York, NY: 2001. pp. 481–508. [Google Scholar]
- Goss R.J. Photoperiodic control of antler cycles in deer. I. Phase shift and frequency changes. J. Exp. Zool. 1969a;170:311–324. doi:10.1002/jez.1401700308 [Google Scholar]
- Goss R.J. Photoperiodic control of antler cycles in deer. II. Alterations in amplitude. J. Exp. Zool. 1969b;171:223–234. doi:10.1002/jez.1401710210 [Google Scholar]
- Goss R.J. Photoperiodic control of antler cycles in deer. V. Reversed seasons. J. Exp. Zool. 1980;211:101–111. doi: 10.1002/jez.1402110111. doi:10.1002/jez.1402110111 [DOI] [PubMed] [Google Scholar]
- Goss R.J. Photoperiodic control of antler cycles in deer. VI. Circannual rhythms on altered day lengths. J. Exp. Zool. 1984;230:265–271. doi: 10.1002/jez.1402300212. doi:10.1002/jez.1402300212 [DOI] [PubMed] [Google Scholar]
- Gram W.D, Heath H.W, Wichman H.A, Lynch G.R. Geographic variation in Peromyscus leucopus: short-day induced reproductive regression and spontaneous recrudescence. Biol. Reprod. 1982;27:369–373. doi: 10.1095/biolreprod27.2.369. doi:10.1095/biolreprod27.2.369 [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Saxena M.T, Prolo L.M, Aton S.J, Herzog E.D. Olfactory bulb neurons express functional, entrainable circadian rhythms. Eur. J. Neurosci. 2004;19:898–906. doi: 10.1111/j.0953-816x.2004.03117.x. doi:10.1111/j.0953-816X.2004.03117.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greives T.J, Mason A.O, Scotti M.A, Levine J, Ketterson E.D, Kriegsfeld L.J, Demas G.E. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. doi:10.1210/en.2006-1249 [DOI] [PubMed] [Google Scholar]
- Grosse J, Hastings M.H. A role for the circadian clock of the suprachiasmatic nuclei in the interpretation of serial melatonin signals in the Syrian hamster. J. Biol. Rhythms. 1996;11:317–324. doi: 10.1177/074873049601100405. doi:10.1177/074873049601100405 [DOI] [PubMed] [Google Scholar]
- Grosse J, Maywood E.S, Ebling F.J, Hastings M.H. Testicular regression in pinealectomized Syrian hamsters following infusions of melatonin delivered on non-circadian schedules. Biol. Reprod. 1993;49:666–674. doi: 10.1095/biolreprod49.4.666. doi:10.1095/biolreprod49.4.666 [DOI] [PubMed] [Google Scholar]
- Gunduz B, Stetson M.H. A test of the coincidence and duration models of melatonin action in Siberian hamsters. II. The effects of 4- and 8-hr melatonin infusions on testicular development of pinealectomized juvenile Siberian hamsters (Phodopus sungorus) J. Pineal Res. 2001a;30:56–64. doi: 10.1034/j.1600-079x.2001.300108.x. doi:10.1034/j.1600-079X.2001.300108.x [DOI] [PubMed] [Google Scholar]
- Gunduz B, Stetson M.H. A test of the coincidence and duration models of melatonin action in Siberian hamsters: the effects of 1-hr melatonin infusions on testicular development in intact and pinealectomized prepubertal Phodopus sungorus. J. Pineal Res. 2001b;30:97–107. doi: 10.1034/j.1600-079x.2001.300205.x. doi:10.1034/j.1600-079X.2001.300205.x [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circannual rhythms in birds: their interaction with circadian rhythms and environmental photoperiod. J. Reprod. Fertil. Suppl. 1973;19:51–65. [PubMed] [Google Scholar]
- Gwinner E. Photoperiodic synchronization of circannual rhythms in the European starling (Sturnus vulgaris) Naturwissenschaften. 1977;64:44–45. doi: 10.1007/BF00439901. doi:10.1007/BF00439901 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Annual rhythms: perspective. In: Aschoff J, editor. Handbook of behavioral neurobiology: biological rhythms. vol. 4. Plenum Press; New York, NY: 1981a. pp. 381–389. [Google Scholar]
- Gwinner E. Circannual systems. In: Aschoff J, editor. Handbook of behavioral neurobiology: biological rhythms. vol. 4. Plenum Press; New York, NY: 1981b. pp. 391–410. [Google Scholar]
- Gwinner E. Circannual rhythms in birds. Curr. Opin. Neurobiol. 2003;13:770–778. doi: 10.1016/j.conb.2003.10.010. doi:10.1016/j.conb.2003.10.010 [DOI] [PubMed] [Google Scholar]
- Gwinner, E. & Dorka, V. 1976 Endogenous control of annual reproductive rhythms in birds. In Proc. 16th Int. Ornithological Congress, pp. 223–234.
- Haldar C, Pandey R. Effect of melatonin and 5-methoxytryptamine administration on the testis and pineal gland activity of the fresh-water snake, Natrix piscator. Arch. Anat. Histol. Embryol. 1988;71:85–95. [PubMed] [Google Scholar]
- Haldar C, Pandey R. Effect of pinealectomy on annual testicular cycle of Indian chequered water snake, Natrix piscator. Gen. Comp. Endocrinol. 1989;76:214–222. doi: 10.1016/0016-6480(89)90152-4. doi:10.1016/0016-6480(89)90152-4 [DOI] [PubMed] [Google Scholar]
- Hamner W.M. Diurnal rhythms and photoperiodism in testicular recrudescence of the house finch. Science. 1963;142:1294–1295. doi: 10.1126/science.142.3597.1294. doi:10.1126/science.142.3597.1294 [DOI] [PubMed] [Google Scholar]
- Hamner W.M. Circadian control of photoperiodism in the house finch demonstrated by interrupted-night experiments. Nature. 1964;203:1400–1401. doi:10.1038/2031400a0 [Google Scholar]
- Hastings M.H, Herzog E.D. Clock genes, oscillators, and cellular networks in the suprachiasmatic nuclei. J. Biol. Rhythms. 2004;19:400–413. doi: 10.1177/0748730404268786. doi:10.1177/0748730404268786 [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proc. R. Soc. B. 1998;265:89–95. doi:10.1098/rspb.1998.0268 [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. Visual and nutritional food cues fine-tune timing of reproduction in a neotropical rainforest bird. J. Exp. Zool. 2000;286:494–504. doi:10.1002/(SICI)1097-010X(20000401)286:5<494::AID-JEZ7>3.0.CO;2-3 [PubMed] [Google Scholar]
- Hawley A.W.L, Aleksiuk M. Thermal regulation of spring mating behavior in the red-sided garter snake (Thamnophis sirtalis parietalis) Can. J. Zool. 1975;53:768–776. [Google Scholar]
- Hazlerigg D.G, Ebling F.J, Johnston J.D. Photoperiod differentially regulates gene expression rhythms in the rostral and caudal SCN. Curr. Biol. 2005;15:R449–R450. doi: 10.1016/j.cub.2005.06.010. doi:10.1016/j.cub.2005.06.010 [DOI] [PubMed] [Google Scholar]
- Hegstrom C.D, Breedlove S.M. Social cues attenuate photoresponsiveness of the male reproductive system in Siberian hamsters (Phodopus sungorus) J. Biol. Rhythms. 1999;14:54–61. doi: 10.1177/074873099129000443. doi:10.1177/074873099129000443 [DOI] [PubMed] [Google Scholar]
- Heideman P.D, Bronson F.H. A pseudoseasonal reproductive strategy in a tropical rodent, Peromyscus nudipes. J. Reprod. Fertil. 1992;95:57–67. doi: 10.1530/jrf.0.0950057. [DOI] [PubMed] [Google Scholar]
- Heldmaier G, Steinlechner S. Seasonal control of energy requirements for thermoregulation in the Djungarian hamster (Phodopus sungorus), living in natural photoperiod. J. Comp. Physiol. 1981;142:429–437. [Google Scholar]
- Heller H.C, Poulson T.L. Circannian rhythms II. Endogenous and exogenous factors controlling reproduction and hibernation in chipmunks (Eutamias) and ground squirrels (Spermophilus) Comp. Biochem. Physiol. 1970;33:357–383. doi:10.1016/0010-406X(70)90356-7 [Google Scholar]
- Hiebert S.M, Thomas E.M, Lee T.M, Pelz K.M, Yellon S.M, Zucker I. Photic entrainment of circannual rhythms in golden-mantled ground squirrels: role of the pineal gland. J. Biol. Rhythms. 2000;15:126–134. doi: 10.1177/074873040001500207. [DOI] [PubMed] [Google Scholar]
- Hill R.W. The altricial/precocial contrast in the thermal relations and energetics of small mammals. In: Tomasi T.E, Horton T.H, editors. Mammalian energetics. Cornell University Press; Ithaca, NY: 1992. pp. 122–159. [Google Scholar]
- Hoffmann K. Photoperiod, pineal, melatonin and reproduction in hamsters. Prog. Brain Res. 1979;52:397–415. doi: 10.1016/S0079-6123(08)62946-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. The critical photoperiod in the Djungarian hamster Phodopus sungorus. In: Aschoff J, Daan S, Groos G.A, editors. Vertebrate circadian systems. Springer; Berlin, Germany: 1982a. pp. 297–304. [Google Scholar]
- Hoffmann K. The effect of brief light pulses on the photoperiodic reaction in the Djungarian hamster Phodopus sungorus. J. Comp. Physiol. 1982b;148:529–534. doi:10.1007/BF00619790 [Google Scholar]
- Hoffmann K, Illnerová H, Vanecek J. Change in duration of the nighttime melatonin peak may be a signal driving photoperiodic responses in the Djungarian hamster (Phodopus sungorus) Neurosci. Lett. 1986;67:68–72. doi: 10.1016/0304-3940(86)90210-7. doi:10.1016/0304-3940(86)90210-7 [DOI] [PubMed] [Google Scholar]
- Hong S.M, Stetson M.H. Termination of gonadal refractoriness in Turkish hamsters, Mesocricetus brandti. Biol. Reprod. 1988;38:639–643. doi: 10.1095/biolreprod38.3.639. doi:10.1095/biolreprod38.3.639 [DOI] [PubMed] [Google Scholar]
- Hong S.M, Rollag M.D, Stetson M.H. Maintenance of testicular function in Turkish hamsters: interaction of photoperiod and the pineal gland. Biol. Reprod. 1986;34:527–531. doi: 10.1095/biolreprod34.3.527. doi:10.1095/biolreprod34.3.527 [DOI] [PubMed] [Google Scholar]
- Honrado G.I, Fleming A.S. Chemical and behavioral stimulation from females accelerates recrudescence in male Syrian hamsters exposed to short days. J. Biol. Rhythms. 1996;11:103–112. doi: 10.1177/074873049601100203. doi:10.1177/074873049601100203 [DOI] [PubMed] [Google Scholar]
- Howles C.M, Craigon J, Haynes N.B. Long-term rhythms of testicular volume and plasma prolactin concentrations in rams reared for 3 years in constant photoperiod. J. Reprod. Fertil. 1982;65:439–446. doi: 10.1530/jrf.0.0650439. [DOI] [PubMed] [Google Scholar]
- Hyde L.L, Underwood H. Effects of nightbreak, T-cycle, and resonance lighting schedules on the pineal melatonin rhythm of the lizard Anolis carolinensis: correlations with the reproductive response. J. Pineal Res. 1993;15:70–80. doi: 10.1111/j.1600-079x.1993.tb00512.x. doi:10.1111/j.1600-079X.1993.tb00512.x [DOI] [PubMed] [Google Scholar]
- Illnerová H. The suprachiasmatic nucleus and rhythmic pineal melatonin production. In: Klein D.C, Moore R.Y, Reppert S.M, editors. Suprachiasmatic nucleus: the mind's clock. Oxford University Press; New York, NY: 1991. pp. 197–216. [Google Scholar]
- Jacob N, Vuillez P, Pévet P. Photoperiod does not act on the suprachiasmatic nucleus photosensitive phase through the endogenous melatonin, in the Syrian hamster. Neurosci. Lett. 1997;229:117–120. doi: 10.1016/s0304-3940(97)00428-x. doi:10.1016/S0304-3940(97)00428-X [DOI] [PubMed] [Google Scholar]
- Jacobs J.D, Wingfield J.C. Endocrine control of life-cycle stages: a constraint on response to the environment? Condor. 2000;102:35–51. doi:10.1650/0010-5422(2000)102[0035:ECOLCS]2.0.CO;2 [Google Scholar]
- Jagota A, de la Iglesia H.O, Schwartz W.J. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat. Neurosci. 2000;3:372–376. doi: 10.1038/73943. doi:10.1038/73943 [DOI] [PubMed] [Google Scholar]
- Johnston J.D. Photoperiodic regulation of prolactin secretion: changes in intra-pituitary signalling and lactotroph heterogeneity. J. Endocrinol. 2004;180:351–356. doi: 10.1677/joe.0.1800351. doi:10.1677/joe.0.1800351 [DOI] [PubMed] [Google Scholar]
- Johnston J.D, Cagampang F.R, Stirland J.A, Carr A.J, White M.R, Davis J.R, Loudon A.S. Evidence for an endogenous per1- and ICER-independent seasonal timer in the hamster pituitary gland. Faseb J. 2003;17:810–815. doi: 10.1096/fj.02-0837com. doi:10.1096/fj.02-0837com [DOI] [PubMed] [Google Scholar]
- Johnston J.D, Tournier B.B, Andersson H, Masson-Pévet M, Lincoln G.A, Hazlerigg D.G. Multiple effects of melatonin on rhythmic clock gene expression in the mammalian pars tuberalis. Endocrinology. 2006;147:959–965. doi: 10.1210/en.2005-1100. doi:10.1210/en.2005-1100 [DOI] [PubMed] [Google Scholar]
- Juss T.S, Meddle S.L, Servant R.S, King V.M. Melatonin and photoperiodic time measurement in Japanese quail (Coturnix coturnix japonica) Proc. R. Soc. B. 1993;254:21–28. doi: 10.1098/rspb.1993.0121. doi:10.1098/rspb.1993.0121 [DOI] [PubMed] [Google Scholar]
- Kauffman A.S, Freeman D.A, Zucker I. Termination of neuroendocrine refractoriness to melatonin in Siberian hamsters (Phodopus sungorus) J. Neuroendocrinol. 2003;15:191–196. doi: 10.1046/j.1365-2826.2003.00966.x. doi:10.1046/j.1365-2826.2003.00966.x [DOI] [PubMed] [Google Scholar]
- Kilduff T.S, Miller J.D, Radeke C.M, Sharp F.R, Heller H.C. 14C-2-deoxyglucose uptake in the ground squirrel brain during entrance to and arousal from hibernation. J. Neurosci. 1990;10:2463–2475. doi: 10.1523/JNEUROSCI.10-07-02463.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Foster R.G, Follett B.K. Evidence for a daily rhythmicity in the acute release of luteinizing hormone in response to electrical stimulation in the Japanese quail. J. Comp. Physiol. A. 1987;161:315–319. doi: 10.1007/BF00615251. doi:10.1007/BF00615251 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L.J, Ranalli N.J, Bober M.A, Nelson R.J. Photoperiod and temperature interact to affect the GnRH neuronal system of male prairie voles (Microtus ochrogaster) J. Biol. Rhythms. 2000a;15:306–316. doi: 10.1177/074873000129001413. doi:10.1177/074873000129001413 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld L.J, Trasy A.G, Nelson R.J. Temperature and photoperiod interact to affect reproduction and GnRH synthesis in male prairie voles. J. Neuroendocrinol. 2000b;12:553–558. doi: 10.1046/j.1365-2826.2000.00485.x. doi:10.1046/j.1365-2826.2000.00485.x [DOI] [PubMed] [Google Scholar]
- Langford G.A, Ainsworth L, Marcus G.J, Shrestha J.N. Photoperiod entrainment of testosterone, luteinizing hormone, follicle-stimulating hormone, and prolactin cycles in rams in relation to testis size and semen quality. Biol. Reprod. 1987;37:489–499. doi: 10.1095/biolreprod37.2.489. doi:10.1095/biolreprod37.2.489 [DOI] [PubMed] [Google Scholar]
- Larkin J.E, Freeman D.A, Zucker I. Low ambient temperature accelerates short-day responses in Siberian hamsters by altering responsiveness to melatonin. J. Biol. Rhythms. 2001;16:76–86. doi: 10.1177/074873040101600109. [DOI] [PubMed] [Google Scholar]
- Larkin J.E, Jones J, Zucker I. Temperature dependence of gonadal regression in Syrian hamsters exposed to short day lengths. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R744–R752. doi: 10.1152/ajpregu.00299.2001. [DOI] [PubMed] [Google Scholar]
- Lee T.M, Zucker I. Suprachiasmatic nucleus and photic entrainment of circannual rhythms in ground squirrels. J. Biol. Rhythms. 1991;6:315–330. doi: 10.1177/074873049100600403. doi:10.1177/074873049100600403 [DOI] [PubMed] [Google Scholar]
- Lewis D, Freeman D.A, Dark J, Wynne-Edwards K.E, Zucker I. Photoperiodic control of oestrous cycles in Syrian hamsters: mediation by the mediobasal hypothalamus. J. Neuroendocrinol. 2002;14:294–299. doi: 10.1046/j.1365-2826.2002.00779.x. doi:10.1046/j.1365-2826.2002.00779.x [DOI] [PubMed] [Google Scholar]
- Lincoln G, Messager S, Andersson H, Hazlerigg D. Temporal expression of seven clock genes in the suprachiasmatic nucleus and the pars tuberalis of the sheep: evidence for an internal coincidence timer. Proc. Natl Acad. Sci. USA. 2002;99:13 890–13 895. doi: 10.1073/pnas.212517599. doi:10.1073/pnas.212517599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln G.A. Melatonin modulation of prolactin and gonadotropin secretion. Systems ancient and modern. In: Olcese J, editor. Melatonin after four decades. Kluwer Academic/Plenum Publishers; New York, NY: 2000. pp. 137–153. [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Maeda K.I. Reproductive effects of placing micro-implants of melatonin in the mediobasal hypothalamus and preoptic area in rams. J. Endocrinol. 1992;132:201–215. doi: 10.1677/joe.0.1320201. [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Clarke I.J. Photoperiodically-induced cycles in the secretion of prolactin in hypothalamo–pituitary disconnected rams: evidence for translation of the melatonin signal in the pituitary gland. J. Neuroendocrinol. 1994;6:251–260. doi: 10.1111/j.1365-2826.1994.tb00580.x. doi:10.1111/j.1365-2826.1994.tb00580.x [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Clarke I.J. Refractoriness to a static melatonin signal develops in the pituitary gland for the control of prolactin secretion in the ram. Biol. Reprod. 1997;57:460–467. doi: 10.1095/biolreprod57.2.460. doi:10.1095/biolreprod57.2.460 [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Clarke I.J. Role of the pituitary gland in the development of photorefractoriness and generation of long-term changes in prolactin secretion in rams. Biol. Reprod. 2000;62:432–438. doi: 10.1095/biolreprod62.2.432. doi:10.1095/biolreprod62.2.432 [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Rhind S.M, Pompolo S, Clarke I.J. Hypothalamic control of photoperiod-induced cycles in food intake, body weight, and metabolic hormones in rams. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R76–R90. doi: 10.1152/ajpregu.2001.281.1.R76. [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Andersson H, Hazlerigg D. Clock genes and the long-term regulation of prolactin secretion: evidence for a photoperiod/circannual timer in the pars tuberalis. J. Neuroendocrinol. 2003a;15:390–397. doi: 10.1046/j.1365-2826.2003.00990.x. doi:10.1046/j.1365-2826.2003.00990.x [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals—a unifying hypothesis. J. Endocrinol. 2003b;179:1–13. doi: 10.1677/joe.0.1790001. doi:10.1677/joe.0.1790001 [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Johnston J.D, Andersson H, Wagner G, Hazlerigg D.G. Photorefractoriness in mammals: dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology. 2005;146:3782–3790. doi: 10.1210/en.2005-0132. doi:10.1210/en.2005-0132 [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Clarke I.J, Hut R.A, Hazlerigg D.G. Characterizing a mammalian circannual pacemaker. Science. 2006;314:1941–1944. doi: 10.1126/science.1132009. doi:10.1126/science.1132009 [DOI] [PubMed] [Google Scholar]
- Lucas R.J, Stirland J.A, Mohammad Y.N, Loudon A.S. Postnatal growth rate and gonadal development in circadian tau mutant hamsters reared in constant dim red light. J. Reprod. Fertil. 2000;118:327–330. doi: 10.1530/jrf.0.1180327. doi:10.1530/reprod/118.2.327 [DOI] [PubMed] [Google Scholar]
- Malpaux B, Karsch F.J. A role for short days in sustaining seasonal reproductive activity in the ewe. J. Reprod. Fertil. 1990;90:555–562. doi: 10.1530/jrf.0.0900555. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Robinson J.E, Brown M.B, Karsch F.J. Importance of changing photoperiod and melatonin secretory pattern in determining the length of the breeding season in the Suffolk ewe. J. Reprod. Fertil. 1988;83:461–470. doi: 10.1530/jrf.0.0830461. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Daveau A, Maurice-Mandon F, Duarte G, Chemineau P. Evidence that melatonin acts in the premammillary hypothalamic area to control reproduction in the ewe: presence of binding sites and stimulation of luteinizing hormone secretion by in situ microimplant delivery. Endocrinology. 1998;139:1508–1516. doi: 10.1210/endo.139.4.5879. doi:10.1210/en.139.4.1508 [DOI] [PubMed] [Google Scholar]
- Maney D.L, Hahn T.P, Schoech S.J, Sharp P.J, Morton M.L, Wingfield J.C. Effects of ambient temperature on photo-induced prolactin secretion in three subspecies of white-crowned sparrow, Zonotrichia leucophrys. Gen. Comp. Endocrinol. 1999;113:445–456. doi: 10.1006/gcen.1998.7219. doi:10.1006/gcen.1998.7219 [DOI] [PubMed] [Google Scholar]
- Mayer I, Bornestaf C, Borg B. Melatonin in non-mammalian vertebrates: physiological role in reproduction? Comp. Biochem. Physiol. A. 1997;118:515–531. doi:10.1016/S0300-9629(96)00468-9 [Google Scholar]
- Mayr E.The growth of biological thought1982Harvard University Press; Cambridge, MA [Google Scholar]
- Maywood E.S, Hastings M.H. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136:144–153. doi: 10.1210/endo.136.1.7828525. doi:10.1210/en.136.1.144 [DOI] [PubMed] [Google Scholar]
- Maywood E.S, Buttery R.C, Vance G.H, Herbert J, Hastings M.H. Gonadal responses of the male Syrian hamster to programmed infusions of melatonin are sensitive to signal duration and frequency but not to signal phase nor to lesions of the suprachiasmatic nuclei. Biol. Reprod. 1990;43:174–182. doi: 10.1095/biolreprod43.2.174. doi:10.1095/biolreprod43.2.174 [DOI] [PubMed] [Google Scholar]
- Maywood E.S, Bittman E.L, Hastings M.H. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol. Reprod. 1996;54:470–477. doi: 10.1095/biolreprod54.2.470. doi:10.1095/biolreprod54.2.470 [DOI] [PubMed] [Google Scholar]
- McDonald P.A. Influence of pinealectomy and photoperiod on courtship and nest-building in male doves. Physiol. Behav. 1982;29:813–818. doi: 10.1016/0031-9384(82)90330-4. doi:10.1016/0031-9384(82)90330-4 [DOI] [PubMed] [Google Scholar]
- McKeever S. The biology of the golden-mantled ground squirrel, Citellus lateralis. Ecol. Monagr. 1964;34:383–401. doi:10.2307/2937069 [Google Scholar]
- Meddle S.L, Follett B.K. Photoperiodic activation of fos-like immunoreactive protein in neurones within the tuberal hypothalamus of Japanese quail. J. Comp. Physiol. A. 1995;176:79–89. doi: 10.1007/BF00197754. doi:10.1007/BF00197754 [DOI] [PubMed] [Google Scholar]
- Meddle S.L, Follett B.K. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J. Neurosci. 1997;17:8909–8918. doi: 10.1523/JNEUROSCI.17-22-08909.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaker M, Wisner S. Temperature-compensated circadian clock in the pineal of Anolis. Proc. Natl Acad. Sci. USA. 1983;80:6119–6121. doi: 10.1073/pnas.80.19.6119. doi:10.1073/pnas.80.19.6119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca M.T, Tousignant A.J, Crews D. Courting and noncourting male red-sided garter snakes (Thamnophis sirtalis parietalis: plasma melatonin levels and the effects of pinealectomy) Horm. Behav. 1996a;30:176–185. doi: 10.1006/hbeh.1996.0022. doi:10.1006/hbeh.1996.0022 [DOI] [PubMed] [Google Scholar]
- Mendonca M.T, Tousignant A.J, Crews D. Pinealectomy, melatonin, and courtship behavior in male red-sided garter snakes (Thamnophis sirtalis parietalis) J. Exp. Zool. 1996b;274:63–74. doi: 10.1002/(SICI)1097-010X(19960101)274:1<63::AID-JEZ7>3.0.CO;2-D. doi:10.1002/(SICI)1097-010X(19960101)274:1<63::AID-JEZ7>3.0.CO;2-D [DOI] [PubMed] [Google Scholar]
- Messager S, Ross A.W, Barrett P, Morgan P.J. Decoding photoperiodic time through Per1 and ICER gene amplitude. Proc. Natl Acad. Sci. USA. 1999;96:9938–9943. doi: 10.1073/pnas.96.17.9938. doi:10.1073/pnas.96.17.9938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Hazlerigg D.G, Mercer J.G, Morgan P.J. Photoperiod differentially regulates the expression of Per1 and ICER in the pars tuberalis and the suprachiasmatic nucleus of the Siberian hamster. Eur. J. Neurosci. 2000;12:2865–2870. doi: 10.1046/j.1460-9568.2000.00174.x. doi:10.1046/j.1460-9568.2000.00174.x [DOI] [PubMed] [Google Scholar]
- Messager S, Garabette M.L, Hastings M.H, Hazlerigg D.G. Tissue-specific abolition of Per1 expression in the pars tuberalis by pinealectomy in the Syrian hamster. Neuroreport. 2001;12:579–582. doi: 10.1097/00001756-200103050-00029. doi:10.1097/00001756-200103050-00029 [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Nisimura T, Numata H. A phase response curve for circannual rhythm in the varied carpet beetle Anthrenus verbasci. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2005;191:883–887. doi: 10.1007/s00359-005-0012-6. doi:10.1007/s00359-005-0012-6 [DOI] [PubMed] [Google Scholar]
- Moenter S.M, Woodfill C.J, Karsch F.J. Role of the thyroid gland in seasonal reproduction: thyroidectomy blocks seasonal suppression of reproductive neuroendocrine activity in ewes. Endocrinology. 1991;128:1337–1344. doi: 10.1210/endo-128-3-1337. [DOI] [PubMed] [Google Scholar]
- Moore R.Y. Neural control of the pineal gland. Brain Res. 1996;73:125–130. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- Moore I.T, Bonier F, Wingfield J.C. Reproductive asynchrony and population divergence between two tropical bird populations. Behav. Ecol. 2005;16:755–762. doi:10.1093/beheco/ari049 [Google Scholar]
- Morgan P.J, Barrett P, Howell H.E, Helliwell R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem. Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. doi:10.1016/0197-0186(94)90100-7 [DOI] [PubMed] [Google Scholar]
- Morgan P.J, Ross A.W, Graham E.S, Adam C, Messager S, Barrett P. Per1 is an early response gene under photoperiodic regulation in the ovine pars tuberalis. J. Neuroendocrinol. 1998;10:319–323. doi: 10.1046/j.1365-2826.1998.00232.x. doi:10.1046/j.1365-2826.1998.00232.x [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Circannual cycles in golden-mantled ground squirrels: phase shift produced by low temperatures. J. Comp. Physiol. 1980;136:349–353. doi:10.1007/BF00657356 [Google Scholar]
- Mrosovsky N, Fisher K.C. Sliding set points for body weight in ground squirrels during the hibernation season. Can. J. Zool. 1970;48:241–247. doi: 10.1139/z70-040. [DOI] [PubMed] [Google Scholar]
- Mrugala M, Zlomanczuk P, Jagota A, Schwartz W.J. Rhythmic multiunit neural activity in slices of hamster suprachiasmatic nucleus reflect prior photoperiod. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R987–R994. doi: 10.1152/ajpregu.2000.278.4.R987. [DOI] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Navarro V.M, Castellano J.M, Garcia-Galiano D, Tena-Sempere M. Neuroendocrine factors in the initiation of puberty: the emergent role of kisspeptin. Rev. Endocr. Metab. Disord. 2007;8:11–20. doi: 10.1007/s11154-007-9028-2. doi:10.1007/s11154-007-9028-2 [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Bamat M.K, Zucker I. Photoperiodic regulation of testis function in rats: mediation by a circadian mechanism. Biol. Reprod. 1982;26:329–335. doi: 10.1095/biolreprod26.2.329. doi:10.1095/biolreprod26.2.329 [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Dark J, Zucker I. Influence of photoperiod, nutrition and water availability on reproduction of male California voles (Microtus californicus) J. Reprod. Fertil. 1983;69:473–477. doi: 10.1530/jrf.0.0690473. [DOI] [PubMed] [Google Scholar]
- Ohta M, Wada M, Homma K. Induction of rapid testicular growth in Japanese quail by phasic electrical stimulation of the hypothalamic photosensitive area. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1984;154:583–589. doi:10.1007/BF00610171 [Google Scholar]
- Oliver J, Bayle J.D. Brain photoreceptors for the photo-induced testicular response in birds. Experientia. 1982;38:1021–1029. doi: 10.1007/BF01955346. doi:10.1007/BF01955346 [DOI] [PubMed] [Google Scholar]
- Osugi T, Ukena K, Bentley G.E, O'Brien S, Moore I.T, Wingfield J.C, Tsutsui K. Gonadotropin-inhibitory hormone in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J. Endocrinol. 2004;182:33–42. doi: 10.1677/joe.0.1820033. doi:10.1677/joe.0.1820033 [DOI] [PubMed] [Google Scholar]
- Parkinson T.J, Follett B.K. Effect of thyroidectomy upon seasonality in rams. J. Reprod. Fertil. 1994;101:51–58. doi: 10.1530/jrf.0.1010051. [DOI] [PubMed] [Google Scholar]
- Pearce G.P, Oldham C.M. Importance of non-olfactory ram stimuli in mediating ram-induced ovulation in the ewe. J. Reprod. Fertil. 1988;84:333–339. doi: 10.1530/jrf.0.0840333. [DOI] [PubMed] [Google Scholar]
- Pengelley E.T. Interrelationships of circannian rhythms in the ground squirrel, Citellus lateralis. Comp. Biochem. Physiol. 1968;24:915–919. doi: 10.1016/0010-406x(68)90803-7. doi:10.1016/0010-406X(68)90803-7 [DOI] [PubMed] [Google Scholar]
- Pengelley E.T, Fisher K.C. Onset and cessation of hibernation under constant temperature and light in the golden-mantled ground squirrel (Citellus lateralis) Nature. 1957;180:1371–1372. doi:10.1038/1801371b0 [Google Scholar]
- Pengelley E.T, Asmundson S.J. The effect of light on the free running circannual rhythm of the golden-mantled ground squirrel, Citellus lateralis. Comp. Biochem. Physiol. 1970;32:155–160. doi: 10.1016/0010-406x(70)90930-8. doi:10.1016/0010-406X(70)90930-8 [DOI] [PubMed] [Google Scholar]
- Pengelley E.T, Asmundson S.J. Circannual rhythmicity in hibernating mammals. In: Pengelley E.T, editor. Circannual clocks. Academic Press, Inc; New York, NY: 1974. pp. 95–160. [Google Scholar]
- Perreau-Lenz S, Kalsbeek A, Garidou M.L, Wortel J, van der Vliet J, van Heijningen C, Simonneaux V, Pévet P, Buijs R.M. Suprachiasmatic control of melatonin synthesis in rats: inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003;17:221–228. doi: 10.1046/j.1460-9568.2003.02442.x. doi:10.1046/j.1460-9568.2003.02442.x [DOI] [PubMed] [Google Scholar]
- Pitrosky B, Pévet P. The photoperiodic response in Syrian hamsters depends upon a melatonin-driven rhythm of sensitivity to melatonin. Biol. Signals. 1997;6:264–271. doi: 10.1159/000109137. [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Gorman M.R, Zucker I. Establishment and persistence of photoperiodic memory in hamsters. Proc. Natl Acad. Sci. USA. 2000;97:5586–5591. doi: 10.1073/pnas.100098597. doi:10.1073/pnas.100098597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast B.J, Kriegsfeld L.J, Nelson R.J. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q. Rev. Biol. 2001;76:293–325. doi: 10.1086/393989. doi:10.1086/393989 [DOI] [PubMed] [Google Scholar]
- Prendergast B.J, Mosinger B, Jr, Kolattukudy P.E, Nelson R.J. Hypothalamic gene expression in reproductively photoresponsive and photorefractory Siberian hamsters. Proc. Natl Acad. Sci. USA. 2002a;99:16 291–16 296. doi: 10.1073/pnas.232490799. doi:10.1073/pnas.232490799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast B.J, Nelson R.J, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff D.W, Arnold A.P, Etgen A.M, Fahrbach S.E, Rubin R.T, editors. Hormones, brain and behavior. vol. 2. Elsevier Science; New York, NY: 2002b. pp. 93–156. [Google Scholar]
- Randall C.F, Bromage N.R, Duston J, Symes J. Photoperiod-induced phase-shifts of the endogenous clock controlling reproduction in the rainbow trout: a circannual phase-response curve. J. Reprod. Fertil. 1998;112:399–405. doi: 10.1530/jrf.0.1120399. [DOI] [PubMed] [Google Scholar]
- Revel F.G, Saboureau M, Masson-Pévet M, Pévet P, Mikkelsen J.D, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr. Biol. 2006a;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. doi:10.1016/j.cub.2006.07.025 [DOI] [PubMed] [Google Scholar]
- Revel F.G, Saboureau M, Pevet P, Mikkelsen J.D, Simonneaux V. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology. 2006b;147:4680–4687. doi: 10.1210/en.2006-0606. doi:10.1210/en.2006-0606 [DOI] [PubMed] [Google Scholar]
- Reynolds S.J, Schoech S.J, Bowman R. Nutritional quality of prebreeding diet influences breeding performance of the Florida scrub-jay. Oecologia. 2003;134:308–316. doi: 10.1007/s00442-002-1126-y. [DOI] [PubMed] [Google Scholar]
- Rhodes D.H. The influence of multiple photoperiods and pinealectomy on gonads, pelage and body weight in male meadow voles, Microtus pennsylvanicus. Comp. Biochem. Physiol. A. 1989;93:445–449. doi: 10.1016/0300-9629(89)90064-9. doi:10.1016/0300-9629(89)90064-9 [DOI] [PubMed] [Google Scholar]
- Rohling J, Wolters L, Meijer J.H. Simulation of day-length encoding in the SCN: from single-cell to tissue-level organization. J. Biol. Rhythms. 2006;21:301–313. doi: 10.1177/0748730406290317. doi:10.1177/0748730406290317 [DOI] [PubMed] [Google Scholar]
- Ross A.W, Bell L.M, Littlewood P.A, Mercer J.G, Barrett P, Morgan P.J. Temporal changes in gene expression in the arcuate nucleus precede seasonal responses in adiposity and reproduction. Endocrinology. 2005;146:1940–1947. doi: 10.1210/en.2004-1538. doi:10.1210/en.2004-1538 [DOI] [PubMed] [Google Scholar]
- Ruby N.F, Heller H.C. Temperature sensitivity of the suprachiasmatic nucleus of ground squirrels and rats in vitro. J. Biol. Rhythms. 1996;11:126–136. doi: 10.1177/074873049601100205. doi:10.1177/074873049601100205 [DOI] [PubMed] [Google Scholar]
- Ruby N.F, Dark J, Heller H.C, Zucker I. Ablation of suprachiasmatic nucleus alters timing of hibernation in ground squirrels. Proc. Natl Acad. Sci. USA. 1996;93:9864–9868. doi: 10.1073/pnas.93.18.9864. doi:10.1073/pnas.93.18.9864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby N.F, Dark J, Heller H.C, Zucker I. Suprachiasmatic nucleus: role in circannual body mass and hibernation rhythms of ground squirrels. Brain Res. 1998;782:63–72. doi: 10.1016/s0006-8993(97)01263-8. doi:10.1016/S0006-8993(97)01263-8 [DOI] [PubMed] [Google Scholar]
- Saunders D.S. Erwin Bünning and Tony Lees, two giants of chronobiology, and the problem of time measurement in insect photoperiodism. J. Insect Physiol. 2005;51:599–608. doi: 10.1016/j.jinsphys.2004.12.002. doi:10.1016/j.jinsphys.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Scaglion R. Seasonal births in a Western Abelam village, Papua New Guinea. Hum. Biol. 1978;50:313–323. [PubMed] [Google Scholar]
- Schaap J, Albus H, VanderLeest H.T, Eilers P.H, Détari L, Meijer J.H. Heterogeneity of rhythmic suprachiasmatic nucleus neurons: implications for circadian waveform and photoperiodic encoding. Proc. Natl Acad. Sci. USA. 2003;100:15 994–15 999. doi: 10.1073/pnas.2436298100. doi:10.1073/pnas.2436298100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuerlein A, Gwinner E. Is food availability a circannual zeitgeber in tropical birds? A field experiment on stonechats in tropical Africa. J. Biol. Rhythms. 2002;17:171–180. doi: 10.1177/074873002129002465. doi:10.1177/074873002129002465 [DOI] [PubMed] [Google Scholar]
- Schneider J.E, Wade G.N. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science. 1989;244:1326–1328. doi: 10.1126/science.2734610. doi:10.1126/science.2734610 [DOI] [PubMed] [Google Scholar]
- Schoech S.J, Bowman R, Reynolds S.J. Food supplementation and possible mechanisms underlying early breeding in the Florida Scrub-Jay (Aphelocoma coerulescens) Horm. Behav. 2004;46:565–573. doi: 10.1016/j.yhbeh.2004.06.005. doi:10.1016/j.yhbeh.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Schwab R.G. Circannian testicular periodicity in the European starling in the absence of photoperiodic change. In: Menaker M, editor. Biochronometry. National Academy of Sciences; Washington, DC: 1976. pp. 428–447. [Google Scholar]
- Schwartz W.J, de la Iglesia H.O, Zlomanczuk P, Illnerová H. Encoding le quattro stagioni within the mammalian brain: photoperiodic orchestration through the suprachiasmatic nucleus. J. Biol. Rhythms. 2001;16:302–311. doi: 10.1177/074873001129002024. doi:10.1177/074873001129002024 [DOI] [PubMed] [Google Scholar]
- Sharp P.J, Follett B.K. The effect of hypothalamic lesions on gonadotrophin release in Japanese quail (Coturnix coturnix japonica) Neuroendocrinology. 1969;5:205–218. doi: 10.1159/000121861. [DOI] [PubMed] [Google Scholar]
- Shaw D, Goldman B.D. Gender differences in influence of prenatal photoperiods on postnatal pineal melatonin rhythms and serum prolactin and follice-stimulating hormone in the Siberian hamster (Phodopus Sungorus) Endocrinology. 1995;136:4237–4246. doi: 10.1210/endo.136.10.7664641. doi:10.1210/en.136.10.4237 [DOI] [PubMed] [Google Scholar]
- Shi Z.D, Barrell G.K. Requirement of thyroid function for the expression of seasonal reproductive and related changes in red deer (Cervus elaphus) stags. J. Reprod. Fertil. 1992;94:251–259. doi: 10.1530/jrf.0.0940251. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Nelson D.E, Ihara N.L, Menaker M. Photoperiodic time measurement in tau mutant hamsters. J. Biol. Rhythms. 1997;12:423–430. doi: 10.1177/074873049701200504. [DOI] [PubMed] [Google Scholar]
- Silver R, Witkovsky P, Horvath P, Alones V, Barnstable C.J, Lehman M.N. Coexpression of opsin- and VIP-like-immunoreactivity in CSF-contacting neurons of the avian brain. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. doi:10.1007/BF00221754 [DOI] [PubMed] [Google Scholar]
- Smith J.T, Clarke I.J. Kisspeptin expression in the brain: Catalyst for the initiation of puberty. Rev. Endocr. Metab. Disord. 2007;8:1–9. doi: 10.1007/s11154-007-9026-4. doi:10.1007/s11154-007-9026-4 [DOI] [PubMed] [Google Scholar]
- Smith J.T, Clay C.M, Caraty A, Clarke I.J. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. doi:10.1210/en.2006-1435 [DOI] [PubMed] [Google Scholar]
- Steinlechner S, Jacobmeier B, Scherbarth F, Dernbach H, Kruse F, Albrecht U. Robust circadian rhythmicity of Per1 and Per2 mutant mice in constant light, and dynamics of Per1 and Per2 gene expression under long and short photoperiods. J. Biol. Rhythms. 2002;17:202–209. doi: 10.1177/074873040201700303. doi:10.1177/07430402017003003 [DOI] [PubMed] [Google Scholar]
- Stetson M.H, Watson-Whitmyre M. Effects of exogenous and endogenous melatonin on gonadal function in hamsters. J. Neural Trans. Supp. 1986;21:55–80. [PubMed] [Google Scholar]
- Stirland J.A, Grosse J, Loudon A.S, Hastings M.H, Maywood E.S. Gonadal responses of the male tau mutant Syrian hamster to short-day-like programmed infusions of melatonin. Biol. Reprod. 1995;53:361–367. doi: 10.1095/biolreprod53.2.361. doi:10.1095/biolreprod53.2.361 [DOI] [PubMed] [Google Scholar]
- Stirland J.A, Hastings M.H, Loudon A.S, Maywood E.S. The tau mutation in the Syrian hamster alters the photoperiodic responsiveness of the gonadal axis to melatonin signal frequency. Endocrinology. 1996;137:2183–2186. doi: 10.1210/endo.137.5.8612567. doi:10.1210/en.137.5.2183 [DOI] [PubMed] [Google Scholar]
- Sumová A, Illnerová H. Endogenous melatonin signal does not mediate the effect of photoperiod on the rat suprachiasmatic nucleus. Brain Res. 1996;725:281–283. doi: 10.1016/0006-8993(96)00408-8. [DOI] [PubMed] [Google Scholar]
- Sumová A, Illnerová H. Photic resetting of intrinsic rhythmicity of the rat suprachiasmatic nucleus under various photoperiods. Am. J. Physiol. 1998;274:R857–R863. doi: 10.1152/ajpregu.1998.274.3.R857. [DOI] [PubMed] [Google Scholar]
- Sumová A, Trávnícková Z, Peters R, Schwartz W.J, Illnerová H. The rat suprachiasmatic nucleus is a clock for all seasons. Proc. Natl Acad. Sci. USA. 1995;92:7754–7758. doi: 10.1073/pnas.92.17.7754. doi:10.1073/pnas.92.17.7754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.S, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee C.C. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90:1003–1011. doi: 10.1016/s0092-8674(00)80366-9. doi:10.1016/S0092-8674(00)80366-9 [DOI] [PubMed] [Google Scholar]
- Takagi T, Yamamura T, Anraku T, Yasuo S, Nakao N, Watanabe M, Iigo M, Ebihara S, Yoshimura T. Involvement of transforming growth factor α in the photoperiodic regulation of reproduction in birds. Endocrinology. 2007;148:2788–2792. doi: 10.1210/en.2007-0112. doi:10.1210/en.2007-0112 [DOI] [PubMed] [Google Scholar]
- Teubner B.J, Freeman D.A. Different neural melatonin-target tissues are critical for encoding and retrieving day length information in Siberian hamsters. J. Neuroendocrinol. 2007;19:102–108. doi: 10.1111/j.1365-2826.2006.01511.x. doi:10.1111/j.1365-2826.2006.01511.x [DOI] [PubMed] [Google Scholar]
- Tilden A.R, Hutchinson V.H. Influence of photoperiod and temperature on serum melatonin in the diamondback water snake, Nerodia rhombifera. Gen. Comp. Endocrinol. 1993;92:347–354. doi: 10.1006/gcen.1993.1172. doi:10.1006/gcen.1993.1172 [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp P.J. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem. Biophys. Res. Commun. 2000;275:661–667. doi: 10.1006/bbrc.2000.3350. doi:10.1006/bbrc.2000.3350 [DOI] [PubMed] [Google Scholar]
- Ubuka T, Bentley G.E, Ukena K, Wingfield J.C, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl Acad. Sci. USA. 2005;102:3052–3057. doi: 10.1073/pnas.0403840102. doi:10.1073/pnas.0403840102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubuka T, Ukena K, Sharp P.J, Bentley G.E, Tsutsui K. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology. 2006;147:1187–1194. doi: 10.1210/en.2005-1178. doi:10.1210/en.2005-1178 [DOI] [PubMed] [Google Scholar]
- Underwood H. Annual testicular cycle of the lizard Anolis carolinensis: effects of pinealectomy and melatonin. J. Exp. Zool. 1985a;233:235–242. doi: 10.1002/jez.1402330210. doi:10.1002/jez.1402330210 [DOI] [PubMed] [Google Scholar]
- Underwood H. Pineal melatonin rhythms in the lizard Anolis carolinensis: effects of light and temperature cycles. J. Comp. Physiol. A. 1985b;157:57–65. doi: 10.1007/BF00611095. doi:10.1007/BF00611095 [DOI] [PubMed] [Google Scholar]
- Underwood H, Goldman B.D. Vertebrate circadian and photoperiodic systems: role of the pineal gland and melatonin. J. Biol. Rhythms. 1987;2:279–315. doi: 10.1177/074873048700200404. doi:10.1177/074873048700200404 [DOI] [PubMed] [Google Scholar]
- Underwood H, Hyde L.L. A circadian clock measures photoperiodic time in the male lizard Anolis carolinensis. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 1990;167:231–243. [Google Scholar]
- Vaz Nunes M, Saunders D. Photoperiodic time measurement in insects: a review of clock models. J. Biol. Rhythms. 1999;14:84–104. doi: 10.1177/074873049901400202. doi:10.1177/074873099129000470 [DOI] [PubMed] [Google Scholar]
- Vivien-Roels B. Interactions between photoperiod, temperature, pineal and seasonal reproduction in non-mammalian vertebrates. In: Mess B, Rúzsás C, Tima L, Pévet P, editors. The pineal gland: current state of pineal research. Elsevier; Amsterdam, The Netherlands: 1985. pp. 187–209. [Google Scholar]
- Vivien-Roels B, Pévet P, Claustrat B. Pineal and circulating melatonin rhythms in the box turtle, Terrapene carolina triunguis: effect of photoperiod, light pulse, and environmental temperature. Gen. Comp. Endocrinol. 1988;69:163–173. doi: 10.1016/0016-6480(88)90002-0. doi:10.1016/0016-6480(88)90002-0 [DOI] [PubMed] [Google Scholar]
- von Gall C, et al. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat. Neurosci. 2002;5:234–238. doi: 10.1038/nn806. doi:10.1038/nn806 [DOI] [PubMed] [Google Scholar]
- Vriend J. Effects of melatonin and thyroxine replacement on thyrotropin, luteinizing hormone, and prolactin in male hypothyroid hamsters. Endocrinology. 1985;117:2402–2407. doi: 10.1210/endo-117-6-2402. [DOI] [PubMed] [Google Scholar]
- Vuillez P, Jacob N, Teclemariam-Mesbah R, Pévet P. In Syrian and European hamsters, the duration of sensitive phase to light of the suprachiasmatic nuclei depends on the photoperiod. Neurosci. Lett. 1996;208:37–40. doi: 10.1016/0304-3940(96)12535-0. doi:10.1016/0304-3940(96)12535-0 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Yasuo S, Watanabe T, Yamamura T, Nakao N, Ebihara S, Yoshimura T. Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology. 2004;145:1546–1549. doi: 10.1210/en.2003-1593. doi:10.1210/en.2003-1593 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, Ebihara S, Yoshimura T. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R568–R572. doi: 10.1152/ajpregu.00521.2006. [DOI] [PubMed] [Google Scholar]
- Watson-Whitmyre M, Stetson M.H. Reproductive refractoriness in hamsters: environmental and endocrine etiologies. In: Stetson M.H, editor. Processing of environmental information in vertebrates. Springer; New York, NY: 1988. pp. 219–249. [Google Scholar]
- Wayne N.L, Malpaux B, Karsch F.J. Social cues can play a role in timing onset of the breeding season of the ewe. J. Reprod. Fertil. 1989;87:707–713. doi: 10.1530/jrf.0.0870707. [DOI] [PubMed] [Google Scholar]
- Weaver D.R, Provencio I, Carlson L.L, Reppert S.M. Melatonin receptors and signal transduction in photorefractory Siberian hamsters (Phodopus sungorus) Endocrinology. 1991;128:1086–1092. doi: 10.1210/endo-128-2-1086. [DOI] [PubMed] [Google Scholar]
- Welsh D.K, Yoo S.H, Liu A.C, Takahashi J.S, Kay S.A. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. doi:10.1016/j.cub.2004.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J.M, Lawton A.D. Social stimulation of reproductive development in male deer mice housed on a short-day photoperiod. J. Comp. Physiol. Psychol. 1982;96:416–422. doi: 10.1037/h0077891. doi:10.1037/h0077891 [DOI] [PubMed] [Google Scholar]
- Wilson F.E. Neither retinal nor pineal photoreceptors mediate photoperiodic control of seasonal reproduction in the American tree sparrow (Spizella arborea) J. Exp. Zool. 1991;259:117–127. doi:10.1002/jez.1402590114 [Google Scholar]
- Wilson F.E, Reinert B.D. Thyroid hormone acts centrally to programme photostimulated male American tree sparrows (Spizella arborea) for vernal and autumnal components of seasonality. J. Neuroendocrinol. 2000;12:87–95. doi: 10.1046/j.1365-2826.2000.00437.x. doi:10.1046/j.1365-2826.2000.00437.x [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hahn T.P, Levin R, Honey P. Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 1992;261:214–231. doi:10.1002/jez.1402610212 [Google Scholar]
- Wingfield J.C, Hahn T.P, Wada M, Astheimer L.B, Schoech S. Interrelationship of day length and temperature on the control of gonadal development, body mass, and fat score in white-crowned sparrows, Zonotrichia leucophrys gambelii. Gen. Comp. Endocrinol. 1996;101:242–255. doi: 10.1006/gcen.1996.0027. doi:10.1006/gcen.1996.0027 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hahn T.P, Wada M, Schoech S.J. Effects of day length and temperature on gonadal development, body mass, and fat depots in white-crowned sparrows, Zonotrichia leucophrys pugetensis. Gen. Comp. Endocrinol. 1997;107:44–62. doi: 10.1006/gcen.1997.6894. doi:10.1006/gcen.1997.6894 [DOI] [PubMed] [Google Scholar]
- Wingfield J.C, Hahn T.P, Maney D.L, Schoech S.J, Wada M, Morton M.L. Effects of temperature on photoperiodically induced reproductive development, circulating plasma luteinizing hormone and thyroid hormones, body mass, fat deposition and molt in mountain white-crowned sparrows, Zonotrichia leucophrys oriantha. Gen. Comp. Endocrinol. 2003;131:143–158. doi: 10.1016/s0016-6480(02)00648-2. doi:10.1016/S0016-6480(02)00648-2 [DOI] [PubMed] [Google Scholar]
- Woodfill C.J, Robinson J.E, Malpaux B, Karsch F.J. Synchronization of the circannual reproductive rhythm of the ewe by discrete photoperiodic signals. Biol. Reprod. 1991;45:110–121. doi: 10.1095/biolreprod45.1.110. doi:10.1095/biolreprod45.1.110 [DOI] [PubMed] [Google Scholar]
- Woodfill C.J, Wayne N.L, Moenter S.M, Karsch F.J. Photoperiodic synchronization of a circannual reproductive rhythm in sheep: identification of season-specific time cues. Biol. Reprod. 1994;50:965–976. doi: 10.1095/biolreprod50.4.965. doi:10.1095/biolreprod50.4.965 [DOI] [PubMed] [Google Scholar]
- Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. doi:10.1126/science.288.5466.682 [DOI] [PubMed] [Google Scholar]
- Yanovsky M.J, Kay S.A. Living by the calendar: how plants know when to flower. Nat. Rev. Mol. Cell Biol. 2003;4:265–275. doi: 10.1038/nrm1077. doi:10.1038/nrm1077 [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Nakao N, Takagi T, Follett B.K, Ebihara S, Yoshimura T. The reciprocal switching of two thyroid hormone-activating and -inactivating enzyme genes is involved in the photoperiodic gonadal response of Japanese quail. Endocrinology. 2005;146:2551–2554. doi: 10.1210/en.2005-0057. doi:10.1210/en.2005-0057 [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Iigo M, Yamamura T, Nakao N, Takagi T, Ebihara S, Yoshimura T. Molecular mechanism of photoperiodic time measurement in the brain of Japanese quail. Chronobiol. Int. 2006;23:307–315. doi: 10.1080/07420520500521913. doi:10.1080/07420520500521913 [DOI] [PubMed] [Google Scholar]
- Yasuo S, Watanabe M, Iigo M, Nakamura T.J, Watanabe T, Takagi T, Ono H, Ebihara S, Yoshimura T. Differential response of type 2 deiodinase gene expression to photoperiod between photoperiodic Fischer 344 and nonphotoperiodic Wistar rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1315–R1319. doi: 10.1152/ajpregu.00396.2006. [DOI] [PubMed] [Google Scholar]
- Yoo S.H, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl Acad. Sci. USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. doi:10.1073/pnas.0308709101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. doi:10.1038/nature02117 [DOI] [PubMed] [Google Scholar]
- Zucker I. Circannual rhythms. In: Takahashi J.S, Turek F.W, Moore R.Y, editors. Handbook of behavioral neurobiology: circadian clocks. vol. 12. Kluwer Academic/Plenum Publishers; New York, NY: 2001. pp. 509–527. [Google Scholar]
- Zucker I, Boshes M. Circannual body weight rhythms of ground squirrels: role of gonadal hormones. Am. J. Physiol. 1982;243:R546–R551. doi: 10.1152/ajpregu.1982.243.5.R546. [DOI] [PubMed] [Google Scholar]
- Zucker I, Boshes M, Dark J. Suprachiasmatic nuclei influence circannual and circadian rhythms of ground squirrels. Am. J. Physiol. 1983;244:R472–R480. doi: 10.1152/ajpregu.1983.244.4.R472. [DOI] [PubMed] [Google Scholar]
- Zucker I, Lee T.M, Dark J. The suprachiasmatic nucleus and the annual rhythms of mammals. In: Klein D.C, Moore R.Y, Reppert S.M, editors. Suprachiasmatic nucleus: the mind's clock. Oxford University Press; New York, NY: 1991. pp. 246–259. [Google Scholar]