Abstract
In the wet–dry tropics of northern Australia, temperatures are high and stable year-round but monsoonal rainfall is highly seasonal and variable both annually and spatially. Many features of reproduction in vertebrates of this region may be adaptations to dealing with this unpredictable variation in precipitation, notably by (i) using direct proximate (rainfall-affected) cues to synchronize the timing and extent of breeding with rainfall events, (ii) placing the eggs or offspring in conditions where they will be buffered from rainfall extremes, and (iii) evolving developmental plasticity, such that the timing and trajectory of embryonic differentiation flexibly respond to local conditions. For example, organisms as diverse as snakes (Liasis fuscus, Acrochordus arafurae), crocodiles (Crocodylus porosus), birds (Anseranas semipalmata) and wallabies (Macropus agilis) show extreme annual variation in reproductive rates, linked to stochastic variation in wet season rainfall. The seasonal timing of initiation and cessation of breeding in snakes (Tropidonophis mairii) and rats (Rattus colletti) also varies among years, depending upon precipitation. An alternative adaptive route is to buffer the effects of rainfall variability on offspring by parental care (including viviparity) or by judicious selection of nest sites in oviparous taxa without parental care. A third type of adaptive response involves flexible embryonic responses (including embryonic diapause, facultative hatching and temperature-dependent sex determination) to incubation conditions, as seen in squamates, crocodilians and turtles. Such flexibility fine-tunes developmental rates and trajectories to conditions–-especially, rainfall patterns–-that are not predictable at the time of oviposition.
Keywords: adaptation, embryo, predictability, reproduction, reptile, seasonal
1. Introduction
Textbooks in ecology and evolution abound with examples of how reproductive cycles of organisms have become modified in ways that adapt them to their local environment and to annual cycles within that environment (e.g. Pianka 1986; Krebs & Davies 1987). However, those examples disproportionately involve particular kinds of organisms (notably, endothermic vertebrates) and environments (notably, temperate zone habitats rather than tropical or arid areas). Although much has been learnt about the reproductive adaptations of ectothermic taxa in the tropics, knowledge of such taxa still lags far behind that available for cooler-climate organisms. This situation is unfortunate, because tropical ectotherms comprise the majority of all living species, and many of the most pressing conservation and management issues are centred on tropical areas (Caughley 1994). Accordingly, we urgently need a better understanding of the way that annual cycles of abiotic factors in the tropics have shaped the reproductive biology of organisms in these regions.
The challenge is a formidable one, partly because the tropics encompass such a diversity of climatic regimes. Thus, the simple fact that an area lies within the tropics tells us little about its climate. In this paper, we focus on a particular (and widespread) type of tropical climate: one in which temperatures are high year-round, but precipitation is highly seasonal and often erratic. Although these ‘wet–dry’ tropics cover large areas on several continents, research has been conducted only sporadically over much of this enormous area. One of the most intensively studied regions within the wet–dry tropics worldwide involves the area around the city of Darwin in Australia's Northern Territory (12°25′ S 130°50′ E). Over several decades, researchers from a variety of institutions have gathered information on reproductive biology of major components of the fauna of Australia's ‘Top End’ (Haynes et al. 1991). For example, we and our collaborators have conducted detailed studies on snakes over a period of more than 20 years on the Adelaide River flood plain 50 km east of Darwin, yielding the most extensive datasets yet gathered on tropical reptiles (e.g. Madsen & Shine 1996a,b, 1998a,b, 1999a–c, 2000a–c, 2001, 2002; Brown et al. 2002; Brown & Shine 2002, 2004a,b). This information on the ecology of the Australian wet–dry tropics thus provides an opportunity to examine the nature of challenges imposed by this climatic type and the ways in which vertebrates have responded to those challenges.
2. Climatic variation in the wet-dry tropics
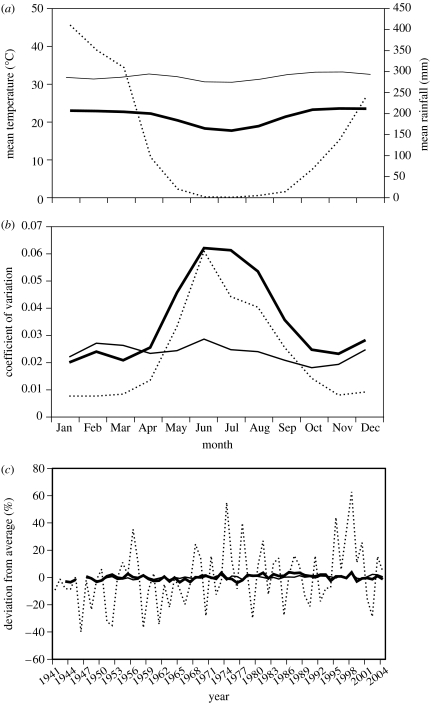
Data on rainfall and ambient temperatures have been recorded in Darwin for many years, facilitating analysis of seasonal and annual variation (Taylor & Tulloch 1985; McDonald & McAlpine 1991). The pattern is very clear: maximum monthly temperatures remain above 30°C year-round with remarkably little variation, whereas minimum (overnight) air temperatures are sharply lower mid-year (figure 1a). These lower air temperatures reflect clear skies at this time of year, compared to heavy cloud cover during the annual wet season. More than 75% of Darwin's annual rainfall comes in less than four months, peaking in the period from January to March (figure 1a). These monsoonal rains induce profound changes in the landscape, with extensive inundation of low-lying areas (flood plains) and rapid growth of ephemeral vegetation (Dunlop & Webb 1991). Following cessation of the rains, the landscape progressively reverts to semi-arid conditions. Thus, although the dominant trees (eucalypts) show little overt seasonal change, deciduous forest trees respond dramatically to the onset of monsoonal rains, as does the understorey of grasses and shrubs (Dunlop & Webb 1991).
Figure 1.
Analyses of ambient temperature and rainfall at Darwin Airport (12°25′ S 130°52′ E), in the Australian ‘wet–dry tropics’, based on data from 1941 to 2004 (from the Australian Bureau of Meteorology). (a) Mean annual values for monthly air temperatures and precipitation. Maximum temperatures (thin solid line) are similar among months, minimum temperatures (thick solid line) fall mid-year and rainfall (thin broken line) is concentrated in a brief wet season; (b) monthly mean coefficients of variation from the overall (long-term) annual mean monthly value for each trait. Maximum temperatures (thin solid line) are less variable than are minimum temperatures (thick solid line). Variation in both temperature and rainfall levels is high during the dry season, but is low for all weather variables otherwise. (c) Degree of year-to-year variation in the deviation of these traits from the long-term average, to clarify the extent to which weather conditions are predictable from one year to the next. To do this, we plot the absolute value of the deviation from the long-term average for each variable for each month. Temperatures (solid lines) are predictable, but rainfall (broken line) is not.
These broad seasonal shifts in precipitation regimes presumably are critical to organisms living in this area, but the nature of any effects will depend heavily upon the predictability of these events. Extensive research in cold-climate systems has shown that organisms can adapt to extreme seasonal changes in weather conditions, as long as those changes occur at consistent times from one year to the next. For example, mammals such as marmots and bears lay down fat seasonally as a prelude to hibernation, while many birds undergo regular annual migrations, over vast distances, to exploit seasonally available food resources and avoid lethally low or energetically costly temperatures (Dingle 1980; Wikelski et al. 2003; also see Wikelski et al. 2008 and Barta et al. 2008). However, the nature of that challenge changes dramatically, if there is substantial year-to-year variation in the timing of significant weather events. Such variation can occur for both precipitation and average temperature, and the rising threat of global climate change has stimulated extensive research to understand the factors driving year-to-year weather variation (e.g. Gutzler 1992).
In Darwin, thermal variation is relatively minor from month to month (figure 1b) as well as from year to year (figure 1c). However, rainfall is highly stochastic (Taylor & Tulloch 1985). For example, the total wet season (January to March) rainfall in Darwin has varied from 589 to 1954 mm over the last 65 years and has spanned most of that range even in comparisons between successive years. During our own research, for example, our study area on the Adelaide River flood plain received 1000 mm during January to March in 2004 but only 549 mm during the same period the following year (January to March 2005). Also, there is massive annual variation at the times of onset and the time of cessation of the monsoonal rains. For example, over the last 30 years, the date by which 100 mm of rain has accumulated on the Adelaide River flood plain has ranged from 9 October to 9 December (R. Shine & G. Brown 2006, unpublished data). In summary, temperatures in the Australian wet–dry tropics are high and fairly stable (over both short-term and long-term time-scales), whereas rainfall shows immense stochastic variation over many time-scales (figure 1b,c).
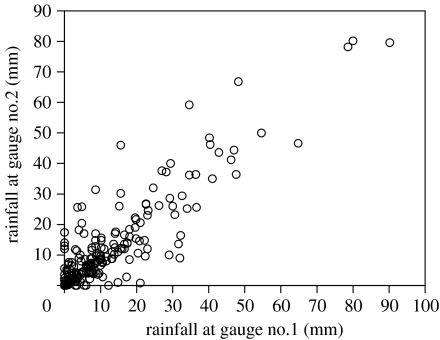
Allied to this temporal variation is equivalent spatial variation: rainstorms can be very localized even at the height of the wet season, so that areas separated by only a few kilometres can receive very different levels of precipitation. For example, on 9 January 2002 we recorded rainfall at two weather stations less than 1 km apart on the Adelaide River flood plain. One gauge registered 28.2 mm within a few hours, whereas the other registered no rainfall at all. Figure 2 provides more extensive data comparing the readings from these two sites. Unsurprisingly, precipitation records were highly correlated at the two nearby sites (for the data in figure 2, r2=0.84), but on many occasions one site received heavy rain whereas the other did not (figure 2). Thus, although temperatures are relatively invariant and highly predictable on both temporal and spatial scales, precipitation is highly variable and unpredictable along both of these dimensions.
Figure 2.
Spatial variation in rainfall events over a small area on Beatrice Hill Farm, 50 km east of Darwin. The precipitation records are from two rain gauges situated 1 km apart. The data plotted are daily readings during the rainy season (October–May) over 2 consecutive years (2000–2001, 2001–2002). Note that although the two sets of readings are highly correlated, it is not unusual for heavy rain to fall in one part of the farm but miss a nearby area almost completely.
For these reasons, the most important climatic influences on the seasonal timing of reproduction for an organism living in such an area are likely to involve rainfall regimes rather than ambient temperatures. Temperatures are relatively benign year-round whereas precipitation—the primary determinant of vegetation growth, levels of flooding, etc.—is highly seasonal. There is a strong contrast in this respect with many (perhaps most) cool-climate habitats. In many temperate zone systems, seasonal variation in ambient temperatures is the primary driving force for seasonality in reproduction. This pattern is particularly clear in ectothermic animals, which typically synchronize the seasonal production of eggs or embryos with the availability of temperatures high enough to sustain embryogenesis (Vitt 1991; Vitt & Caldwell 1994). The seasonal march of temperatures is relatively predictable, allowing adaptive linking of reproductive processes to correlated factors such as photoperiod (Hau et al. 1998; Wikelski et al. 2003; also see Wikelski et al. 2008 and Paul et al. 2008). The challenge is very different if rainfall rather than ambient temperature is the primary determinant of optimal seasonal reproductive timing and levels of reproductive output. Thus, organisms in the wet–dry tropics are faced with the challenge of linking their reproductive biology to a factor (rainfall) that is highly unpredictable in space and time. This situation is not unique to the wet–dry tropics, but the absence of confounding effects from seasonal thermal variation facilitates the detection of adaptive responses to rainfall variation in this system. Precipitation probably is critical in many other systems also, notably in deserts (e.g. Pianka 1986; Hau et al. 2004), but reproductive activity of organisms in many of these latter areas is constrained also by seasonal thermal variation.
3. Adapting to unpredictability
The broad direction of an effective adaptive response to link reproduction to a stochastic variable (such as rainfall in the wet–dry tropics) is clear-cut: organisms should evolve the ability to respond rapidly, in a facultative fashion, depending upon local conditions (Hau et al. 2000, 2004). Canalized (‘hard-wired’) traits are unlikely to enhance organismal fitness in such a system, because there is no single optimal time to reproduce, or nest-site to select, or reproductive effort to allocate. Instead, the optimal value for any reproduction-associated trait probably varies from year to year and place to place. Such a selective regime is likely to favour the ability of organisms to modify their reproductive output on a facultative basis, in response to cues from the immediate environment, rather than exhibiting temporally and spatially consistent (invariant) patterns in reproductive traits. If such a linkage is impossible, the only remaining option in evolutionary time would be to evolve mechanisms (such as nest-site selection, parental care or viviparity) that function to buffer the offspring from climatic extremes during the period when they are most vulnerable to such fluctuations.
Adaptation of reproductive traits to precipitation regimes may take many forms and can involve either parental traits or offspring characteristics. The most obvious candidates for adaptive responses involve attributes of the reproducing adults, either to synchronize reproductive ‘decisions’ with rainfall events (such as a flexible preparedness to reproduce at high rates whenever favourable conditions are encountered) or to buffer their offspring from the effects of variable precipitation (by nest-site selection or parental care). Others involve traits of the offspring themselves, after parental provisioning and/or care has ceased, such that major events in development (initiation of embryogenesis, sex determination and timing of escape from the egg) either are buffered from environmental variation or are tied to environmental cues that influence optimal developmental trajectories or timing. Below, we interpret diverse reproductive attributes of vertebrates from the Australian wet–dry tropics within this framework. Reflecting our own research backgrounds, we emphasize examples from reptiles (especially snakes) rather than other vertebrate groups.
4. Adaptations to synchronize reproductive decisions with unpredictable rainfall events
(a) To reproduce or not to reproduce?
The first critical determinant of reproductive output for any adult organism is the ‘decision’ as to whether or not to reproduce. Although most adult females may reproduce in most years in some natural populations (especially in endothermic vertebrates), reproductive rates of females in many ectotherm populations are much lower and more variable (Jonsson 1997; Bonnet et al. 1998; see McNamara & Houston 2008). This is true even in cold-climate populations; for example, female vipers and rattlesnakes frequently reproduce on biennial or triennial cycles, reflecting the long periods of time required for females to replenish their energy reserves after giving birth to the previous litter (Fitch 1960; Brown 1991). However, infrequent reproduction is not simply enforced by low ambient temperatures: rates of energy replenishment may be low in tropical taxa as well, and thus less-than-annual reproduction may be the norm in many tropical as well as temperate zone reptiles (Madsen & Shine 1996a,b).
The relationship between annual rainfall and reproductive output has been intensively studied in two species of tropical snakes in our study area: water pythons Liasis fuscus and file snakes Acrochordus arafurae. On average, adult female pythons reproduce about once every 2 years (Madsen & Shine 1996a), and adult female file snakes about once every 3–4 years (Madsen & Shine 2000b). However, there is great temporal variation in these rates. For example, the proportion of reproductive adult female file snakes within the study population varied from 0 to 60% over a 10-year period (Madsen & Shine 2000b). Despite the phylogenetic and ecological differences between pythons and file snakes, the factors generating this variation are similar in the two snake species. In both cases, rainfall patterns over the preceding wet seasons influence the abundance of prey (rats for pythons, fishes for file snakes) and thus, feeding rates of female snakes. Since females do not initiate vitellogenesis until they have accumulated sufficient energy reserves, these rain-dependent feeding rates generate substantial annual variation in the duration of delays between successive clutches by the same female (Madsen & Shine 2000b). For both of these taxa, we also know that the critical variable driving prey availability (and thus, feeding rates and in turn, reproduction) is not simply total wet season rainfall. Instead, prey (rat, fish) abundance depends upon the duration of inundation of the flood plain. Monsoonal rains generally begin in December but the timing of their cessation is more variable from year to year (figure 1c; McDonald & McAlpine 1991). Thus, rainfall late in the wet season (February to March) is the critical variable determining the amount of time that the flood plain remains inundated. These relationships are so consistent that major demographic descriptors (proportion of reproductive female snakes within a population, age structure, etc.) can be predicted from the timing and intensity of wet season rainfall over the preceding few years (Madsen & Shine 2000b; Madsen et al. 2006).
The snakes of these tropical flood plains are not unusual in showing clear relationships between rainfall and breeding activity. For example, the proportion of adult females that breed varies with wet season rainfall not only in snakes, but also in native rats (Rattus colletti: Madsen & Shine 1999c), agile wallabies (Macropus agilis: Williams & Newsome 1991), magpie geese (Anseranas semipalmata: Frith & Davies 1961; Morton & Brennan 1991) and saltwater crocodiles (Crocodylus porosus: Webb 1991). For example, the proportion of anoestrous females in populations of agile wallabies (M. agilis) varies among years, depending upon the rate at which monsoonal rains induce flooding. Years with a rapid onset of flooding result in a dramatic reduction in the proportion of reproductive female wallabies, whereas a gradual onset of the monsoons allows many females to continue reproducing (Williams & Newsome 1991).
(b) How much energy to allocate to reproduction?
Reproduction is not an all-or-none decision: animals can modify their total allocation of resources such that more or less is devoted to reproduction rather than to the competing demands of maintenance, growth and energy storage (Congdon et al. 2001; Roff 2002). Again, some of the most detailed information comes from water pythons and file snakes. The relatively simple morphology of snakes means that their body condition (mass relative to length) offers a robust index of their energy stores (accumulated fat bodies: Weatherhead & Brown 1996). Studies on temperate zone snakes have identified threshold values for body condition required before a female will initiate vitellogenesis; and importantly, these values remain constant through time and across a wide range of maternal body sizes (Saint Girons 1957; Naulleau & Bonnet 1996). In contrast, both water pythons and file snakes flexibly modify the body condition threshold required to initiate reproduction. Following ‘bad’ (low-rainfall) years, females reproduce even when their reserves are well below the levels that would be required to initiate reproduction following ‘good’ years (Madsen & Shine 1999a, 2000b). The adaptive significance of this flexibility may lie in shifting probabilities of maternal survival (Madsen & Shine 1999a; McNamara et al. 2004). The end result is that reproductive effort per litter is reduced in such years, reflected in a reduced clutch size in water pythons (Madsen & Shine 1999a). Offspring (egg) size is unaffected, presumably reflecting strong stabilizing selection on this trait (Shine & Madsen 1997; Madsen & Shine 1999a).
Detailed studies on the most common kangaroo of the Top End reveal a similar dependence upon rainfall patterns. Female agile wallabies (M. agilis) can produce up to three young within a 2-year period, but reproductive output is heavily influenced by rainfall patterns (Williams & Newsome 1991). The facultative response contrasts with the more strictly seasonal reproductive schedules of most other macropod species (except arid zone taxa), a difference that Williams & Newsome (1991) interpret as an adaptive response to the high variability in timing and duration of the monsoons.
(c) When to initiate reproduction?
In any environment with temporal variation in the resources needed for reproduction or offspring survival, we expect selection for the ability to synchronize the timing of reproductive events with the availability of those resources (Hau et al. 2000; Hau 2001; Satake et al. 2001). Since the timing and intensity of rainfall events are difficult to predict, we would expect the Top End fauna to flexibly modify reproductive timing to take advantage of unpredictable rainfall events. However, the seasonal timing of rainfall is sufficiently consistent that we may see reproductive consistencies also at this broader temporal scale (e.g. wet season versus dry season breeders).
The strongest temporal links between rainfall and reproduction will come from taxa that depend upon water to breed. Anurans that spawn in temporary ponds are the clearest example; we expect (and see) that adults of both sexes develop mature gametes long before reproduction occurs, and are able to breed as soon as falling rain creates suitable habitat for their eggs and tadpoles (Tyler & Crook 1987). A similar link may be important with fishes that depend upon shallowly inundated areas for breeding, but the temporal and spatial correlation between rainfall and breeding will be weaker; that is, rain in one area may cause flooding many kilometres downstream and it may take prolonged monsoonal rains before billabongs burst their banks (McDonald & McAlpine 1991). Nonetheless, the link with flooding (rather than rainfall) may be tight: Bishop & Forbes (1991) note that many species of fishes in the Alligator Rivers region (100 km east of Darwin) depend upon the first floods of the wet season (which can come at any time from mid-December to February: McDonald & McAlpine 1991) for the initiation and induction of spawning.
One important difference between fishes and frogs on the one hand, and other vertebrates on the other, is the prevalence of external fertilization in the former group versus internal fertilization in the latter group. This difference means that mating and oviposition must perforce occur simultaneously in most fishes and frogs, but not in reptiles and endothermic vertebrates. Thus, any rainfall-driven patterns in breeding times must apply to mating as well as oviposition in externally fertilizing taxa. In contrast, internal fertilization allows for sperm storage within the female reproductive tract, and hence a temporal dissociation between the time of mating and the time of oviposition. Accordingly, we might expect reproductive cycles in males of externally fertilizing species to be more highly responsive to rainfall cues than would be true of internally fertilizing species, or any of the amniotic vertebrate lineages. Mating seems to occur over much of the year in many of these latter groups, although it is highly seasonal in some. For example, all python taxa in the Top End seem to display similar schedules of reproductive activity, with mating in July to August, ovulation in October and hatching of eggs in December to January (Barker & Barker 1994; R. Shine 2006, unpublished data). In the case of water pythons (L. fuscus), the mating aggregations occur at sites where reproductive females will later lay their eggs (Madsen & Shine 1998b). The consequent concentration of reproductive females in a small area may enhance mate encounter rates for male pythons.
The seasonal time of production of offspring shows remarkable diversity among the vertebrates of the wet–dry tropics, sometimes even between closely related taxa. For example, freshwater crocodiles (Crocodylus johnstoni) nest in sandy burrows during the dry season, whereas saltwater crocodiles (C. porosus) oviposit in specially constructed mounds of vegetation early in the wet season (Webb 1991). In other groups, phylogenetic conservatism is stronger; for example, tropical representatives of lizard lineages from arid areas generally nest in the dry season, whereas moist forest lineages tend to nest during the wet season (James & Shine 1985). This conservatism in reproductive timing might reflect an underlying conservatism in embryonic physiology, with eggs and embryos best able to develop successfully under incubation conditions similar to those experienced by ancestral taxa from different climatic conditions (James & Shine 1985).
One of the best-understood taxa in this regard is the keelback (Tropidonophis mairii), a small short-lived non-venomous (colubrid) snake species. Females of this species lay one to four clutches per year in shallow soil nests during the dry season (Brown & Shine 2002). Experimental studies show that eggs cannot survive immersion in water, but benefit from incubation in moist soil (Brown & Shine 2004b). High moisture content in the incubation substrate allows the embryo to fully use the yolk store contained within the egg, and thus hatch at a larger size (Shine & Brown 2002). Larger hatchlings have a higher probability of survival, providing a direct fitness benefit to females that lay their eggs in moist but not in flooded soil (Brown & Shine 2004b).
Is the fitness benefit of oviposition in moist but not in flooded nests the main determinant of seasonal schedules of oviposition in keelbacks? Other potential explanations for reproductive seasonality in this taxon are less consistent with available data. For example, the timing of nesting does not minimize predation on eggs, nor maximize food availability or survival rates for hatchlings (Brown & Shine 2006). Instead, female keelbacks nest most intensely soon after the cessation of monsoonal rains when soils are moist enough to sustain optimal embryogenesis but are unlikely to become waterlogged (Shine & Brown 2002).
In taxa with a high capacity for dispersal, one of the most effective facultative responses to spatial heterogeneity in rainfall may be migration to favourable sites. Mobility of this kind is widespread among birds in the Top End, including species from a diverse array of phylogenetic lineages and a wide variety of feeding habits. For example, year-to-year variation in rainfall patterns, and thus in resource availability, generates corresponding diversity in the spatial distribution of honeyeaters across the tropical landscape (Morton & Brennan 1991). Other resources are more predictable in space, especially those in and around waterbodies. Nonetheless, even birds that rely upon such habitats frequently display temporal and spatial variation in the seasonal onset of breeding activities. For example, magpie geese (A. semipalmata) defer laying their eggs until the water is deep enough and aquatic vegetation is sufficiently dense (Morton & Brennan 1991).
(d) When to cease reproduction?
The time of cessation as well as the time of onset of breeding can be driven by stochastic variation in rainfall patterns. For example, the number of generations of dusky rats (R. colletti) produced each year depends upon the duration of the breeding season, which varied from one to five months over the 4-year period studied by Madsen & Shine (1999c). That variation was generated by annual variation in rainfall late in the wet season; the rats depend upon moist soil for burrowing to obtain corms and other food, and thus cease reproducing when the flood plain begins to dry out. Under favourable conditions in captivity, this species breeds year-round (Taylor & Horner 1973). A causal link between annual climatic (rainfall) variation and the duration of rodent breeding is supported by the observation of synchronized across-year variation in the duration of rat breeding across widely separated flood plains (Williams & Newsome 1991).
5. Adaptations to buffer embryonic development against unpredictable rainfall events
(a) Where to lay your eggs?
Heavy rainfall in the wet–dry tropics modifies not only hydric conditions in the soil, but also temperatures: in particular, the onset of drenching monsoonal rains causes a significant and rapid drop in mean temperatures near the soil surface (Webb 1991; Shine & Brown 2002; Brown & Shine 2004b). Thus, an animal that lays its eggs in a soil nest cannot predict the thermal and hydric regimes to which those eggs will be exposed prior to hatching. This unpredictability will be especially important if the incubation conditions in nest sites can substantially affect the developmental rates and phenotypic traits of offspring, as occurs in many kinds of oviparous animals. For example, the sex, size, shape and behaviour of hatchling reptiles can be profoundly affected by minor fluctuations in incubation conditions (Ji & Brana 1999; Deeming 2004). Embryogenesis can be affected by shifts in diel or weekly variance as well as in mean temperature, and by more gradual heating and cooling during incubation (Shine 2002, 2004). This sensitivity to apparently minor aspects of the nest environment means that reproducing animals may be under strong selection either to buffer their developing offspring from such fluctuations, or to evolve developmental pathways that enable effective development under a wide range of abiotic conditions.
Judicious selection of appropriate nest sites can act as an effective buffer, because aspects such as shading, soil type and local drainage will generate substantial spatial heterogeneity in the degree to which conditions in a given nest site fluctuate in response to ambient stochasticity. One particularly effective option is to select nest sites that will be virtually unaffected by rainfall events, by laying the eggs in tree hollows rather than on the ground. This tactic is used by the slatey-grey snake Stegonotus cucullatus, and allows this species to reproduce during the wet season when soil nests would be under significant risk of flooding (Brown et al. 2005). Saltwater crocodiles (C. porosus) and magpie geese (A. semipalmata) also lay their eggs at the time when flooding is probable, but escape inundation by constructing their nests on floating grass mats that remain above water level at all times (Frith & Davies 1961; Webb 1991).
Another mechanism to buffer embryos from environmental fluctuation is for one or both parents to remain with the clutch after oviposition, and regulate conditions in the nest by behavioural means. Parental care is virtually ubiquitous among the endothermic vertebrates (including those of the Top End) but is more sporadically distributed among ectotherms. Hence, it is of interest to note that parental care is widespread among the reptiles and fishes of the wet–dry tropics. Thus, female pythons (a specious group in this area) typically remain with their eggs and coil tightly around them throughout incubation. This maternal behaviour can not only reduce desiccation and thus enhance hatching success and offspring size (Aubret et al. 2003) but also heat the clutch through shivering thermogenesis (rhythmic muscular contractions) of the brooding female (Harlow & Grigg 1984; Slip & Shine 1988). In keeping with the idea that reproducing animals flexibly adjust their reproductive biology to local conditions, female water pythons (L. fuscus) remain with eggs laid in cool nests, but abandon eggs laid in warmer nests (Shine et al. 1997; Madsen & Shine 1999b).
Viviparity is perhaps the most extreme development of this ability to buffer incubation conditions against ambient fluctuations. By retaining the developing offspring within her body, the mother can maintain virtually constant hydric and thermal conditions (in endotherms) or constant hydric conditions and relatively invariant thermal conditions (in thermoregulating ectotherms). For example, pregnant death adders (Acanthophis praelongus) regulate their body temperatures more precisely than do non-pregnant females of the same species. Experiments in which pregnant females were kept under these two types of thermal regimes showed that thermostability significantly enhances offspring size; and a mark–recapture study showed that offspring fitness is increased by this larger size at birth (Webb et al. 2006). Various kinds of parental care (buccal incubation, guarding of nests) as well as viviparity also are seen in a high proportion of fish species in the wet–dry tropics (Bishop & Forbes 1991). These authors speculate that the high incidence of parental protection ‘may have evolved in response to the variable conditions imposed by the extreme wet and dry seasons’ (Bishop & Forbes 1991, p. 103).
6. Adaptations of embryonic biology
The preceding discussion has dealt entirely with adaptations of reproducing adults. However, organisms can adapt to unpredictability also by modifying their developmental programmes (Tinkle & Gibbons 1977). In the wet–dry tropics, we might expect to see three types of modifications those that (i) buffer embryogenesis against unpredictable environmental fluctuations, (ii) synchronize developmental timing with rainfall events or their consequences, and (iii) flexibly modify the trajectory of embryogenesis depending upon local conditions during incubation.
(a) When to initiate embryonic development?
In most species, embryonic development begins soon after the ovum is fertilized. However, a phylogenetically diverse array of taxa have evolved embryonic diapause, whereby development is arrested at an early stage until an environmental cue triggers resumption of growth and differentiation (Ewert 1979; Braby & Jones 1995; Tauber et al. 1998; Andrews & Donoghue 2004). Clearly, such a mechanism can permit synchronization of developmental timing with external conditions. A freshwater turtle (Chelodina rugosa) from the wet–dry tropics provides a remarkable example of such flexibility. Uniquely among amniotic vertebrates (so far as is known), these turtles lay their eggs underwater (Kennett et al. 1993; Kennett 1999). Oviposition occurs late in the wet season, in low-lying areas, and embryonic development does not commence until receding water levels expose the eggs to higher oxygen levels in air spaces between the soil particles (Kennett et al. 1993). Thus, the initiation of embryogenesis is tied to the timing of drying out of the flood plain, which can vary considerably from one year to the next (Taylor & Tulloch 1985).
(b) When to hatch?
Similar flexibility is evident at the other end of embryogenesis. Most turtle species hatch from the eggs when development is complete, although fully developed embryos of some temperate zone taxa overwinter inside their eggs prior to hatching (Goode & Russell 1968). In the pignose turtle (Carettochelys insculpta), full-term embryos go into diapause until they experience a rapid fall in oxygen availability. This happens when the first floods of the wet season inundate the nesting beaches, thus stimulating explosive hatching of the young turtles coincident with flooding, when survival chances of hatchling turtles may be maximized (Doody et al. 2001).
(c) How to match offspring phenotypes to incubation conditions?
Developmental trajectories as well as timing (above) can be modified by environmental cues. As noted above (see ‘Where to lay your eggs?’), the phenotypes of hatchling reptiles are sensitive to incubation temperature and moisture. Those reaction norms presumably have been shaped by selection, such that embryos pursue developmental pathways best suited to the incubation conditions that they encounter in the nest. Perhaps the clearest examples involve sex determination. By delaying the time in embryogenesis when sex is determined, and by linking that decision to local conditions, organisms may be able to produce the sex that can benefit most from the time it hatches within the season, or from the phenotype that will result from those incubation conditions. For example, temperature-dependent sex determination (TSD) is seen in representatives of all the major reptilian lineages in the wet–dry tropics, including the turtle C. insculpta (Georges 1989), the lizards Lophognathus gilberti and Chlamydosaurus kingii (Harlow & Shine 1999; Harlow 2004) and both crocodile species (C. johnstoni and C. porosus: Webb et al. 1987). By tying sex determination to incubation temperature, these animals might benefit either because of sex-specific advantages to early versus late season hatching combined with precipitation-induced seasonal shifts in mean nest temperature, or because incubation temperature directly affects hatchling phenotypic traits that contribute differentially to the subsequent fitness of male versus female hatchlings (Charnov & Bull 1977; Shine 1999). The same kinds of fitness benefits might favour the evolution of reaction norms concerning phenotypic traits other than sex (Brown & Shine 2006). An alternative pathway to deal with unpredictable fluctuations in incubation conditions involves buffering: impossible over any prolonged period for thermal factors, but possible for moisture levels. Thus, the eggs of keelback snakes (T. mairii) are able to deal with wide fluctuations in water availability throughout incubation, taking up water whenever the substrate is moist and retaining it successfully during subsequent drier periods (Brown & Shine 2005).
Although data on embryonic flexibility are most extensive for reptiles, analogous phenomena presumably occur in other ectothermic vertebrates. For example, one consequence of facultative breeding by anurans in response to monsoonal rainfall will be that many sympatric frog species are likely to breed in the same places at the same time, thus intensifying interspecific competition. We might expect that such synchrony would exert powerful selection for character displacement in species-specific mate recognition systems, to avoid the fitness penalties associated with hybrid matings (Tyler & Crook 1987; Ryan & Wilcczynski 1991). Thus, for example, we might see clear interspecific partitioning of traits such as calling sites or the characteristics of the advertisement calls given by male frogs. To our knowledge, this prediction has not been tested. Similarly, we might expect selection to favour larval traits that enhance success in mixed-species assemblages within waterbodies. The high frequency of cannibal morphotypes in taxa such as Cyclorana (Tyler & Crook 1987) may have evolved under this scenario.
7. Discussion
We do not suggest that selection for the ability to exploit unpredictable events is unique to the fauna of the wet–dry tropics, nor that we are the first to suggest that adaptations to unpredictability may be important in this system (Williams & Newsome 1991; Flannery 1994). The present review is innovative only in that it proposes a conceptual framework for interpreting such features, and attempts to integrate the results of recent (and some older) studies within this framework. The high and relatively aseasonal ambient temperatures of the wet–dry tropics facilitate interpretation in this respect, by removing the severe seasonal thermal constraints on reproductive seasonality that operate in many other systems. That is, the wet–dry tropics provide an unusually clear example of a widespread phenomenon—adaptations of vertebrate reproduction to factors that are broadly unpredictable in space and time.
Nonetheless, we emphasize that the kinds of mechanisms that we have described can also play a significant role in other environments. Virtually all environments on Earth have some degree of long-term predictability on which is superimposed some degree of unpredictability. This is certainly true for the wet–dry tropics, where the broad seasonal distribution of rainfall events is more highly predictable than for many other parts of Australia (Taylor & Tulloch 1985). Environments (and the specific resources within them) fall along a continuum from extremely predictable to extremely unpredictable, and at some point along that continuum the level of unpredictability presumably reaches a point at which qualitatively different adaptive mechanisms are needed for successful reproduction. These ideas have been explored in detail with reference to the bird fauna of the temperate zone, and some of the conceptual schemes that have been developed to quantify predictability might usefully be applied to tropical environments as well (Wingfield et al. 1992, 1993; Hahn et al. 1995, 1997).
The link between rainfall events and facultative reproduction in the wet–dry tropics is direct in some cases, for example, in anurans that breed in temporary pools created by the first monsoonal rains. In other cases, the link is less direct. For example, fish breeding in response to flooding (Bishop & Forbes 1991) involves an integration of rainfall patterns over longer timescales and greater spatial scales. Yet another link in the causal chain comes when predator populations respond to fluctuations in prey populations that have themselves responded to the timing and duration of annual inundation of the flood plain. Nonetheless, despite the greater complexity of the causal links between abiotic variation and biotic responses in such a case, the end result can be a close correlation between rainfall patterns and predator demography (as in water pythons: Madsen et al. 2006). In species that breed infrequently, females may accumulate energy stores for several years prior to reproducing and hence, reproductive rates will depend upon rainfall patterns integrated over an even longer timescale (e.g. file snakes: Madsen & Shine 2000b).
A second common complication in the wet–dry tropics is that although air temperatures are high year-round, incubation temperatures of eggs in natural nests may be significantly modified by precipitation events. Thus, rainfall may affect the fitness consequences of alternative reproductive schedules via an influence on nest thermal regimes rather than rainfall per se. For example, wet-season rainfall may play a critical role in cooling the eggs within nests of saltwater crocodiles; in years without rain at this time, eggs may experience lethally high temperatures (Webb 1991). Similarly, rainfall early in the wet season cools natural nests of dragon lizards with TSD, thereby generating (possibly adaptive) seasonal shifts in sex ratio of the hatchlings (Harlow 2004).
The different pathways by which rainfall influences organismal fitness have different timescales. For example, cooling of the soil by rainfall occurs virtually instantaneously, whereas enhanced prey availability due to increased vegetation growth has a much greater lag time. Thus, the time of year at which rain falls is important, but the spatial and temporal scales over which that importance is determined will differ considerably among taxa. Heavy but brief and local showers early in the wet season may be the most significant events for pond-breeding frogs and for sex determination in agamid lizards, whereas monthly rainfall late in the wet season (and thus, the rate of drying out of the flood plain) may be the critical issue for reproduction in rodents, fishes and the animals that prey upon them. Hence, although these species are also subject to the same environmental conditions, specific features of their reproductive biology mean that they are affected by unpredictable rainfall events at very different scales and different times of year. That interspecific diversity, combined with the fact that only a small proportion of vertebrate species in the wet–dry tropics have so far attracted detailed study, suggests that future work is likely to discover a far richer array of reproductive adaptations to the annual cycle.
Acknowledgments
We thank the many researchers whose studies have provided both the inspiration and the information for this review. In particular, we have benefited from discussions with G. Bedford, K. Christian, W. Freeland, M. Greenlees, B. Phillips, T. Madsen, C. Shilton, G. J. W. Webb and J. K. Webb. Thanks also to the staff of Beatrice Hill Farm for access to rainfall data. Our studies have been supported (in the case of R.S., for more than 30 years) by the Australian Research Council (ARC), and we are deeply indebted to the ARC for this long-term investment.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Andrews R.M, Donoghue S. Effects of temperature and moisture on embryonic diapause of the veiled chameleon (Chamaeleo calyptratus) J. Exp. Zool. A. 2004;301:629–635. doi: 10.1002/jez.a.56. doi:10.1002/jez.a.56 [DOI] [PubMed] [Google Scholar]
- Aubret F, Bonnet X, Shine R, Maumelat S. Clutch size manipulation, hatching success and offspring phenotype in the ball python (Python regius, Pythonidae) Biol. J. Linn. Soc. 2003;78:263–272. doi:10.1046/j.1095-8312.2003.00169.x [Google Scholar]
- Barker D.G, Barker T.M. Australia. vol. 1. Advanced Vivarium Systems; Lakeside, CA: 1994. Pythons of the world. [Google Scholar]
- Barta Z, McNamara J.M, Houston A.I, Weber T.P, Hedenstro¨m A, Fero´ O. Optimal moult strategies in migratory birds. Phil. Trans. R. Soc. B. 2008;363:211–229. doi: 10.1098/rstb.2007.2136. doi:10.1098/rstb.2007.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop K.A, Forbes M.A. The freshwater fishes of northern Australia. In: Haynes C.D, Ridpath M.G, Williams M.A.J, editors. Monsoonal Australia. Landscape, ecology and man in the northern lowlands. A. A. Balkema; Rotterdam, The Netherlands: 1991. pp. 79–108. [Google Scholar]
- Bonnet X, Bradshaw D, Shine R. Capital versus income breeding: an ectothermic perspective. Oikos. 1998;83:333–342. doi:10.2307/3546846 [Google Scholar]
- Braby M.F, Jones R.E. Reproductive patterns and resource allocation in tropical butterflies—influence of adult diet and seasonal phenotype on fecundity, longevity and egg size. Oikos. 1995;72:189–204. doi:10.2307/3546221 [Google Scholar]
- Brown W.S. Female reproductive ecology in a northern population of the timber rattlesnake, Crotalus horridus. Herpetologica. 1991;47:101–115. [Google Scholar]
- Brown G.P, Shine R. Reproductive ecology of a tropical natricine snake Tropidonophis mairii (Colubridae) J. Zool. (Lond.) 2002;258:63–72. [Google Scholar]
- Brown G.P, Shine R. Effects of reproduction on the antipredator tactics of snakes (Tropidonophis mairii Colubridae) Behav. Ecol. Sociobiol. 2004a;56:257–262. [Google Scholar]
- Brown G.P, Shine R. Maternal nest-site choice and offspring fitness in a tropical snake (Tropidonophis mairii Colubridae) Ecology. 2004b;85:1627–1634. doi:10.1890/03-0107 [Google Scholar]
- Brown G.P, Shine R. Do changing moisture levels during incubation influence phenotypic traits of hatchling snakes (Tropidonophis mairii)? Physiol. Biochem. Zool. 2005;78:524–530. doi: 10.1086/430231. doi:10.1086/430231 [DOI] [PubMed] [Google Scholar]
- Brown G.P, Shine R. Why do most tropical animals reproduce seasonally? Testing alternative hypotheses on the snake Tropidonophis mairii (Colubridae) Ecology. 2006;87:133–143. doi: 10.1890/04-1882. doi:10.1890/04-1882 [DOI] [PubMed] [Google Scholar]
- Brown G.P, Shine R, Madsen T. Responses of three sympatric snake species to tropical seasonality in northern Australia. J. Trop. Ecol. 2002;18:549–558. doi:10.1017/S0266467402002365 [Google Scholar]
- Brown G.P, Shine R, Madsen T. Spatial ecology of slatey-grey snakes (Stegonotus cucullatus, Colubridae) on a tropical Australian floodplain. J. Trop. Ecol. 2005;21:605–612. doi:10.1017/S0266467405002671 [Google Scholar]
- Caughley G. Directions in conservation biology. J. Anim. Ecol. 1994;63:215–244. doi:10.2307/5542 [Google Scholar]
- Charnov E.L, Bull J.J. When is sex environmentally determined? Nature. 1977;266:828–830. doi: 10.1038/266828a0. doi:10.1038/266828a0 [DOI] [PubMed] [Google Scholar]
- Congdon J.D, Dunham A.E, Hopkins W.A, Rowe C.L, Hinton T.G. Resource allocation-based life histories: a conceptual basis for studies of ecological toxicology. Environ. Toxicol. Chem. 2001;20:1698–1703. doi:10.1897/1551-5028(2001)020<1698:RABLHA>2.0.CO;2 [PubMed] [Google Scholar]
- Deeming D.C. Post-hatching phenotypic effects of incubation on reptiles. In: Deeming D.C, editor. Reptilian incubation. Environment, evolution and behaviour. Nottingham University Press; Nottingham, UK: 2004. pp. 229–251. [Google Scholar]
- Dingle H. Ecology and evolution of migration. In: Gauthreaux S.A.J, editor. Animal migration, orientation and navigation. Academic Press; New York, NY: 1980. pp. 1–103. [Google Scholar]
- Doody J.S, Georges A, Young J.E. Embryonic aestivation and emergence behaviour in the pig-nosed turtle Carettochelys insculpta. Can. J. Zool. 2001;79:1062–1072. doi:10.1139/cjz-79-6-1062 [Google Scholar]
- Dunlop C.R, Webb L.J. Flora and vegetation. In: Haynes C.D, Ridpath M.G, Williams M.A.J, editors. Monsoonal Australia. Landscape, ecology and man in the northern lowlands. A. A. Balkema; Rotterdam, The Netherlands: 1991. pp. 41–60. [Google Scholar]
- Ewert M.A. The embryo and its egg: development and natural history. In: Harless M, Morlock H, editors. Turtles: perspectives and research. Wiley; New York, NY: 1979. pp. 333–413. [Google Scholar]
- Fitch H.S. Autecology of the copperhead. Univ. Kansas Publ. Mus. Nat. Hist. 1960;13:85–288. [Google Scholar]
- Flannery T.F. Reed Books; Sydney, Australia: 1994. The future eaters. [Google Scholar]
- Frith H.J, Davies S.J.J.F. Ecology of the magpie goose, Anseranas semipalmata Latham (Anatidae) Wildl. Res. 1961;6:91–141. doi:10.1071/CWR9610091 [Google Scholar]
- Georges A. Female turtles from hot nests: is it duration of incubation or proportion of development at high temperatures that matters. Oecologia. 1989;81:323–328. doi: 10.1007/BF00377078. doi:10.1007/BF00377078 [DOI] [PubMed] [Google Scholar]
- Goode J, Russell J. Incubation of eggs of three species of chelid tortoises, and notes on their embryological development. Aust. J. Zool. 1968;16:749–761. doi:10.1071/ZO9680749 [Google Scholar]
- Gutzler D.S. Climatic variability of temperature and humidity over the tropical western pacific. Geophys. Res. Lett. 1992;19:1595–1598. [Google Scholar]
- Hahn T.P, Wingfield J.C, Mullen R, Deviche P.J. Endocrine bases of spatial and temporal opportunism in Arctic-breeding birds. Am. Zool. 1995;35:259–273. [Google Scholar]
- Hahn T.P, Boswell T, Wingfield J.C, Ball G.F. Temporal flexibility in avian reproduction: patterns and mechanisms. Curr. Ornithol. 1997;14:39–80. [Google Scholar]
- Harlow P. Temperature-dependent sex determination in lizards. In: Valenzuela N, Lance V.A, editors. Temperature-dependent sex determination in vertebrates. Smithsonian Books; Washington, DC: 2004. pp. 42–52. [Google Scholar]
- Harlow P.S, Grigg G. Shivering thermogenesis in a brooding diamond python, Python spilotes spilotes. Copeia. 1984;1984:959–965. doi:10.2307/1445340 [Google Scholar]
- Harlow P, Shine R. Temperature-dependent sex determination in the frillneck lizard, Chlamydosaurus kingii (Agamidae) Herpetologica. 1999;55:205–212. [Google Scholar]
- Hau M. Timing of breeding in variable environments: tropical birds as model systems. Horm. Behav. 2001;40:281–290. doi: 10.1006/hbeh.2001.1673. doi:10.1006/hbeh.2001.1673 [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proc. R. Soc. B. 1998;265:89–95. doi:10.1098/rspb.1998.0268 [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. Visual and nutritional food cues fine-tune timing of reproduction in a neotropical rainforest bird. J. Exp. Zool. 2000;286:494–504. doi:10.1002/(SICI)1097-010X(20000401)286:5<494::AID-JEZ7>3.0.CO;2-3 [PubMed] [Google Scholar]
- Hau M, Wikelski M, Gwinner H, Gwinner E. Timing of reproduction in a Darwin's finch: temporal opportunism under spatial constraints. Oikos. 2004;106:489–500. doi:10.1111/j.0030-1299.2004.13206.x [Google Scholar]
- Haynes C.D, Ridpath M.G, Williams M.A.J. Landscape, ecology and man in the northern lowlands. A.A. Balkema; Rotterdam, The Netherlands: 1991. Monsoonal Australia. [Google Scholar]
- James C, Shine R. The seasonal timing of reproduction: a tropical–temperate comparison in Australian lizards. Oecologia. 1985;67:464–474. doi: 10.1007/BF00790016. doi:10.1007/BF00790016 [DOI] [PubMed] [Google Scholar]
- Ji X, Brana F. The influence of thermal and hydric environments on embryonic use of energy and nutrients, and hatchling traits, in the wall lizards (Podarcis muralis) Comp. Biochem. Physiol. A. 1999;124:205–213. doi:10.1016/S1095-6433(99)00111-7 [Google Scholar]
- Jonsson K.I. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos. 1997;78:57–66. doi:10.2307/3545800 [Google Scholar]
- Kennett R. Reproduction of two species of freshwater turtle, Chelodina rugosa and Elseya dentata, from the wet-dry tropics of northern Australia. J. Zool. (Lond.) 1999;247:457–473. [Google Scholar]
- Kennett R, Georges A, Palmer-Allen M. Early developmental arrest during immersion of eggs of a tropical freshwater turtle Chelodina rugosa (Testudinata: Chelidae), from northern Australia. Aust. J. Zool. 1993;41:37–45. doi:10.1071/ZO9930037 [Google Scholar]
- Krebs J.R, Davies N.B. Blackwell Scientific Publications; Oxford, UK: 1987. An introduction to behavioural ecology. [Google Scholar]
- Madsen T, Shine R. Determinants of reproductive output in female water pythons (Liasis fuscus, Pythonidae) Herpetologica. 1996a;52:146–159. [Google Scholar]
- Madsen T, Shine R. Seasonal migration of predators and prey: pythons and rats in tropical Australia. Ecology. 1996b;77:149–156. doi:10.2307/2265663 [Google Scholar]
- Madsen T, Shine R. Quantity or quality? Determinants of maternal reproductive success in tropical pythons (Liasis fuscus) Proc. R. Soc. B. 1998a;265:1521–1525. doi:10.1098/rspb.1998.0467 [Google Scholar]
- Madsen T, Shine R. Spatial subdivision within a population of tropical pythons (Liasis fuscus) in a superficially homogeneous habitat. Aust. J. Ecol. 1998b;23:340–348. doi:10.1111/j.1442-9993.1998.tb00739.x [Google Scholar]
- Madsen T, Shine R. The adjustment of reproductive threshold to prey abundance in a capital breeder. J. Anim. Ecol. 1999a;68:571–580. doi:10.1046/j.1365-2656.1999.00306.x [Google Scholar]
- Madsen T, Shine R. Life history consequences of nest-site variation in tropical pythons (Liasis fuscus) Ecology. 1999b;80:989–997. [Google Scholar]
- Madsen T, Shine R. Rainfall and rats: climatically-driven dynamics of a tropical rodent population. Aust. J. Ecol. 1999c;24:80–89. doi:10.1046/j.1442-9993.1999.00948.x [Google Scholar]
- Madsen T, Shine R. Energy versus risk: costs of reproduction in free-ranging pythons in tropical Australia. Austral Ecol. 2000a;25:670–675. doi:10.1046/j.1442-9993.2000.01067.x [Google Scholar]
- Madsen T, Shine R. Rain, fish and snakes: climatically driven population dynamics of Arafura filesnakes in tropical Australia. Oecologia. 2000b;124:208–215. doi: 10.1007/s004420050008. doi:10.1007/s004420050008 [DOI] [PubMed] [Google Scholar]
- Madsen T, Shine R. Silver spoons and snake sizes: prey availability early in life influences long-term growth rates of free-ranging pythons. J. Anim. Ecol. 2000c;69:952–958. doi:10.1046/j.1365-2656.2000.00477.x [Google Scholar]
- Madsen T, Shine R. Conflicting conclusions from long-term versus short-term studies on growth and reproduction of a tropical snake. Herpetologica. 2001;57:147–156. [Google Scholar]
- Madsen T, Shine R. Short and chubby or long and thin? Food intake, growth and body condition in free-ranging pythons. Austral Ecol. 2002;27:672–680. doi:10.1046/j.1442-9993.2002.01228.x [Google Scholar]
- Madsen T, Ujvari B, Shine R, Olsson M, Loma J. Rain, rats and pythons: climate-driven population dynamics of predators and prey in tropical Australia. Austral Ecol. 2006;31:30–37. doi:10.1111/j.1442-9993.2006.01540.x [Google Scholar]
- McDonald N.S.M, McAlpine J. Floods and droughts: the northern climate. In: Haynes C.D, Ridpath M.G, Williams M.A.J, editors. Monsoonal Australia. Landscape, ecology and man in the northern lowlands. A. A. Balkema; Rotterdam, The Netherlands: 1991. pp. 19–30. [Google Scholar]
- McNamara J.M, Houston A.I. Optimal annual routines: behaviour in the context of physiology and ecology. Phil. Trans. R. Soc. B. 2008;363:301–319. doi: 10.1098/rstb.2007.2141. doi:10.1098/rstb.2007.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J.M, Welham R.K, Houston A.I, Daan S, Tinbergen J.M. The effects of background mortality on optimal reproduction in a seasonal environment. Theor. Popul. Biol. 2004;65:361–372. doi: 10.1016/j.tpb.2003.10.006. doi:10.1016/j.tpb.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Morton S.R, Brennan K.G. Birds. In: Haynes C.D, Ridpath M.G, Williams M.A.J, editors. Monsoonal Australia. Landscape, ecology and man in the northern lowlands. A. A. Balkema; Rotterdam, The Netherlands: 1991. pp. 133–150. [Google Scholar]
- Naulleau G, Bonnet X. Body condition threshold for breeding in a viviparous snake. Oecologia. 1996;107:301–306. doi: 10.1007/BF00328446. doi:10.1007/BF00328446 [DOI] [PubMed] [Google Scholar]
- Paul M.J, Zucker I, Schwartz W.J. Tracking the seasons: the internal calendars of vertebrates. Phil. Trans. R. Soc. B. 2008;363:341–361. doi: 10.1098/rstb.2007.2143. doi:10.1098/rstb.2007.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka E.R. Princeton University Press; Princeton, NJ: 1986. Ecology and natural history of desert lizards. [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Ryan M.J, Wilcczynski W. Evolution of intraspecific variation in the advertisement call of a cricket frog Acris crepitans. Biol. J. Linn. Soc. 1991;44:249–271. [Google Scholar]
- Saint Girons H. Croissance et fécondité de Vipera aspis (L) Vie et Milieu. 1957;8:265–286. [Google Scholar]
- Satake A, Sasaki A, Iwasa Y. Variable timing of reproduction in unpredictable environments: adaptation of flood plain plants. Theor. Popul. Biol. 2001;60:1–15. doi: 10.1006/tpbi.2001.1528. doi:10.1006/tpbi.2001.1528 [DOI] [PubMed] [Google Scholar]
- Shine R. Why is sex determined by nest temperature in many reptiles? Trends Ecol. Evol. 1999;14:186–189. doi: 10.1016/s0169-5347(98)01575-4. doi:10.1016/S0169-5347(98)01575-4 [DOI] [PubMed] [Google Scholar]
- Shine R. Eggs in autumn: responses to declining incubation temperatures by the eggs of montane lizards. Biol. J. Linn. Soc. 2002;76:71–77. doi:10.1046/j.1095-8312.2002.00049.x [Google Scholar]
- Shine R. Seasonal shifts in nest temperature can modify the phenotypes of hatchling lizards, regardless of overall mean incubation temperature. Funct. Ecol. 2004;18:43–49. doi:10.1046/j.0269-8463.2004.00806.x [Google Scholar]
- Shine R, Brown G.P. Effects of seasonally varying hydric conditions on hatchling phenotypes of keelback snakes (Tropidonophis mairii, Colubridae) from the Australian wet–dry tropics. Biol. J. Linn. Soc. 2002;76:339–347. doi:10.1046/j.1095-8312.2002.00068.x [Google Scholar]
- Shine R, Madsen T. Prey abundance and predator reproduction: rats and pythons on a tropical Australian floodplain. Ecology. 1997;78:1078–1086. [Google Scholar]
- Shine R, Madsen T.R.L, Elphick M.J, Harlow P.S. The influence of nest temperatures and maternal thermogenesis on hatchling phenotypes of water pythons. Ecology. 1997;78:1713–1721. [Google Scholar]
- Slip D.J, Shine R. Reptilian endothermy: a field study of thermoregulation by brooding diamond pythons. J. Zool. (Lond.) 1988;216:367–378. [Google Scholar]
- Tauber M.J, Tauber C.A, Nyrop J.P, Villani M.G. Moisture, a vital but neglected factor in the seasonal ecology of insects: hypotheses and tests of mechanisms. Environ. Entomol. 1998;27:523–530. [Google Scholar]
- Taylor J.M, Horner B.E. Reproductive characteristics of wild native Australian Rattus (Rodentia: Muridae) Aust. J. Zool. 1973;21:437–475. doi: 10.1071/zo9730437. doi:10.1071/ZO9730437 [DOI] [PubMed] [Google Scholar]
- Taylor J.A, Tulloch D. Rainfall in the wet–dry tropics: extreme events at Darwin and similarities between years during the period 1870–1983. Aust. J. Ecol. 1985;10:281–295. doi:10.1111/j.1442-9993.1985.tb00890.x [Google Scholar]
- Tinkle D.W, Gibbons J.W. The distribution and evolution of viviparity in reptiles. Misc. Publ. Mus. Zool., Univ. Michigan. 1977;154:1–55. [Google Scholar]
- Tyler M.J, Crook G.A. Technical Memorandum 19, Supervising Scientist for the Alligator Rivers Region. Australian Government Publishing Service; Canberra, Australia: 1987. Frogs of the Magela Creek system. [Google Scholar]
- Vitt L.J. An introduction to the ecology of Cerrado lizards. J. Herpetol. 1991;25:79–90. doi:10.2307/1564798 [Google Scholar]
- Vitt L.J, Caldwell J.P. Resource utilization and guild structure of small vertebrates in the Amazon forest leaf-litter. J. Zool. (Lond.) 1994;234:463–476. [Google Scholar]
- Weatherhead P.J, Brown G.P. Measurement versus estimation of condition in snakes. Can. J. Zool. 1996;74:1617–1621. [Google Scholar]
- Webb G.J.W. The influence of season on Australian crocodiles. In: Haynes C.D, Ridpath M.G, Williams M.A.J, editors. Monsoonal Australia. Landscape, ecology and man in the northern lowlands. A. A. Balkema; Rotterdam, The Netherlands: 1991. pp. 125–132. [Google Scholar]
- Webb G.J.W, Beal A.M, Manolis S.C, Dempsey K.E. The effects of incubation temperature on sex determination and embryonic development rate in Crocodylus johnstoni and C. porosus. In: Webb G.J.W, Manolis S.C, Whitehead P.J, editors. Wildlife management: crocodiles and alligators. Surrey Beatty and Sons Limited; Chipping Norton, Australia: 1987. pp. 507–531. [Google Scholar]
- Webb J, Christian K, Shine R. The adaptive significance of reptilian viviparity in the tropics: testing the maternal manipulation hypothesis. Evolution. 2006;60:115–122. doi:10.1111/j/0014-3820.2006.tb01087.x [PubMed] [Google Scholar]
- Wikelski M, Tarlow E.M, Raim A, Diehl R.H, Larkin R.P, Visser G.H. Costs of migration in free-flying songbirds. Nature. 2003;423:704. doi: 10.1038/423704a. doi:10.1038/423704a [DOI] [PubMed] [Google Scholar]
- Wikelski M, Martin L.B, Scheuerlein A, Robinson M.T, Robinson N.D, Helm B, Hau M, Gwinner A. Avian circannual clocks: adaptive significance and possible involvement of energy turnover in their proximate control. Phil. Trans. R. Soc. B. 2008;363:411–423. doi: 10.1098/rstb.2007.2147. doi:10.1098/rstb.2007.2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.K, Newsome A.E. Adaptation in native mammals. In: Haynes C.D, Ridpath M.G, Williams M.A.J, editors. Monsoonal Australia. Landscape, ecology and man in the northern lowlands. A. A. Balkema; Rotterdam, The Netherlands: 1991. pp. 151–167. [Google Scholar]
- Wingfield J.C, Hahn T.P, Levin R, Honey P. Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 1992;261:214–231. doi:10.1002/jez.1402610212 [Google Scholar]
- Wingfield J.C, Hahn T.P, Doak D. Integration of environmental factors regulating transitions of physiological state, morphology and behaviour. In: Sharp P.J, editor. Avian endocrinology. Journal of Endocrinology, Ltd; Bristol, UK: 1993. pp. 111–122. [Google Scholar]