Abstract
Life-history trade-offs between components of fitness arise because reproduction entails both gains and costs. Costs of reproduction can be divided into ecological and physiological costs. The latter have been rarely studied yet are probably a dominant component of the effect. A deeper understanding of life-history evolution will only come about once these physiological costs are better understood. Physiological costs may be direct or indirect. Direct costs include the energy and nutrient demands of the reproductive event, and the morphological changes that are necessary to facilitate achieving these demands. Indirect costs may be optional ‘compensatory costs’ whereby the animal chooses to reduce investment in some other aspect of its physiology to maximize the input of resource to reproduction. Such costs may be distinguished from consequential costs that are an inescapable consequence of the reproductive event. In small mammals, the direct costs of reproduction involve increased energy, protein and calcium demands during pregnancy, but most particularly during lactation. Organ remodelling is necessary to achieve the high demands of lactation and involves growth of the alimentary tract and associated organs such as the liver and pancreas. Compensatory indirect costs include reductions in thermogenesis, immune function and physical activity. Obligatory consequential costs include hyperthermia, bone loss, disruption of sleep patterns and oxidative stress. This is unlikely to be a complete list. Our knowledge of these physiological costs is currently at best described as rudimentary. For some, we do not even know whether they are compensatory or obligatory. For almost all of them, we have no idea of exact mechanisms or how these costs translate into fitness trade-offs.
Keywords: energy, protein, calcium, pregnancy, lactation, life-history
1. Introduction
The theory of evolution by natural selection assumes that an individual's attributes result from their historical attempts to maximize fitness—their genetic contribution to the future gene pool. Fitness, however, is not a singular concept, but rather consists of a number of components. The life history of an organism is a reflection of how the animal blends together these components into an integrated life-history ‘strategy’. A fundamental axiom of our understanding and interpretation of the evolution of life-history strategies is that trade-offs exist between the various life-history components (Fisher 1930). For example, an increase in current reproductive output by maximizing fecundity might occur only at the expense of future survivorship or future reproductive output (Williams 1966; Stearns 1992; Charnov 1993; Charlesworth 1994). We assume that animals cannot combine together the fitness components in a strategy that simultaneously maximizes them all, and must therefore trade-off the components against each other (but see Johnston et al. 2006).
The idea that there must be trade-offs in fitness components stems from the idea that reproductive events generate fitness benefits in terms of viable offspring, but that reproduction also entails costs as well. These costs drive the nature of the trade-off between the fitness components (Reznick et al. 1990; Roff 1992). There are several different types of trade-off among components of current reproductive effort, for example, trading off the size against number of offspring (Smith & Fretwell 1974) and also trade-offs between current reproduction and components of future fitness: either survival or fecundity. Stearns (1992) identified 10 different fitness components or life-history ‘traits’ and detailed a matrix of potential trade-offs between them. For each trade-off, there must be a mechanism that links the fitness components together. This mechanism is the ‘cost’ that is attached to the reproductive event. Previous investigators have identified that these costs can be further partitioned into physiological costs, and costs that are mediated via ecology (e.g. Zera & Harshman 2001). An example of an ecological cost is the increased risk of predation that is associated with foraging while acquiring energy and nutrients, like protein and calcium, for the reproductive event. Historically, the study of life histories has been rooted in the determination of the magnitude of trade-offs with little concern for their mechanistic basis. However, it is becoming increasingly recognized that distinguishing the importance of ecological and physiological mechanisms is a key goal if we are to understand the evolution of life-history patterns (Zera & Harshman 2001; McNamara & Houston 2008). Nevertheless, it should be borne in mind that ecological and physiological components strongly interact and cannot be completely isolated from each other. Despite the fact that it is widely recognized that physiological costs underpin all life-history trade-offs (e.g. Stearns 1992; Ricklefs & Wikelski 2002), the physiological contribution to most, if not all, trade-offs remains very poorly studied. In this paper, I propose to review our understanding of some of the physiological costs that are attached to the process of reproduction. I have focused the review on small mammals where there is at least some depth of study, but I also confess that this betrays a bias towards a group of animals that I have studied personally.
Physiological costs of reproduction can be divided into two different types. The first is direct costs that stem from satisfying the demands of the reproductive event itself. These demands at their simplest level are the energy and nutrients that the parental animal needs to acquire to successfully reproduce. In the previous paragraph, I identified the predation risk that attends the extra foraging required to collect the energy or nutrients required for reproduction as an ecological cost. The direct physiological cost of reproduction is that energy and nutrient demand. It is probable that these direct costs have deeper layers of complexity to them. Animals not only require energy and the major macronutrients (like protein) but also essential amino and fatty acids, as well as intakes of vitamins and micronutrients. Our knowledge of many of these physiological requirements is almost non-existent. I have also included in this ‘direct physiological cost’ the physiological and anatomical modifications that are necessary for the animal to achieve these demands. For example, if an animal needs to double or treble its rate of food intake to satisfy the demand for energy during reproduction, it cannot simply do that without some modifications to its alimentary tract and the machinery that processes ingested food. These necessary modifications are a second form of direct cost.
The second type of cost of reproduction is indirect. Like direct costs, we can also subdivide the indirect costs into two sub-types. First, to meet the direct physiological costs of reproduction, an animal may cut corners in other components of its physiology. Many animals probably inhabit environments where it is simply not possible for their intake to be increased adequately to cover their reproductive demands. In these circumstances, the animals may make compensatory adjustments in aspects of their physiology to save energy that can then be devoted to reproduction. Even where food is sufficiently available, there may be ecological costs involved in obtaining it (e.g. predation risks), which are greater than the physiological costs of cutting down the expenditure on other components of physiological functioning. These compensatory physiological costs are an indirect cost of the reproductive event. A distinguishing feature of these costs is that they have a degree of optionality about them. The animal is choosing to modify its physiology to invest more in reproduction, and it can choose to make this allocation of resource or not. The costs derive from the fact that resource is limited and animals must therefore choose how to allocate this limited resource between competing functions. We can distinguish these optional ‘compensatory costs’ from other indirect costs that are an inevitable physiological consequence of the reproductive event. For example, reproduction might involve the production of a toxic by-product that elevates the risk of the animal developing a fatal disease like cancer. This would be a cost of reproduction that would not be optional. The animal could not choose to reproduce without this negative consequence.
2. Direct costs
(a) Increased demand for energy and nutrients
(i) Energy
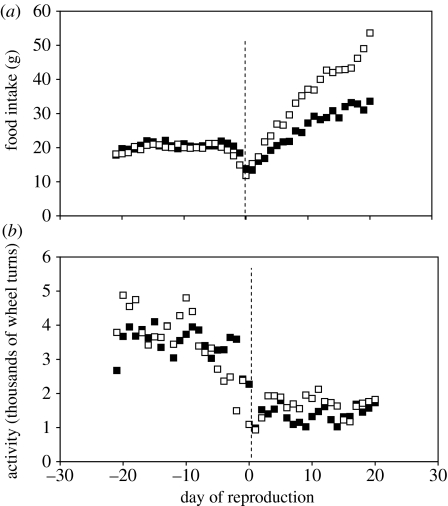
Probably the most detailed data available for the energy demands of reproduction are derived from domesticated rodents. This is primarily because these animals are easily kept, and even quite invasive measurements can be made on them without the risk that they will desert their offspring. The time course of food intake, at 21°C, throughout reproduction in a strain of laboratory mouse (MF1) that we have been studying is shown in figure 1a (Johnson et al. 2001a). Food intake increased during pregnancy to approximately 8 g, compared with 5.5 g in the non-breeding females prior to reproduction. Although the pattern of foetal growth is essentially exponential between conception and birth, the pattern of food intake does not mirror this, but rather rises to a peak approximately 2–3 days earlier and then declines slightly before the day of parturition. The reason for this pattern is uncertain, but one hypothesis is that the expanding foetal mass competes for space with the alimentary tract in the abdomen, limiting the food intake. This may suggest that resources to support the gestation become limited in late pregnancy. Under such limitation, competing demands may have detrimental effects on the success of the pregnancy. One such competing demand may be the level of basal energy requirements (basal metabolic rate, BMR). We have recently shown in another strain of mouse (the C57BL/6 black mouse) that mice with higher BMR have a greater likelihood of mass anomalies occurring during pregnancy. These mass anomalies probably reflect foetal resorption events. Because BMR is correlated with body mass, this relationship might only be an artefact of both resorption rate and BMR being affected by mass, but the effect of BMR is also evident if the effect of mass is removed statistically (Johnston et al. 2007). This effect supports the hypothesis that resource intake in late pregnancy may be limited, perhaps by space competition in the abdomen between the alimentary tract and the developing foetuses.
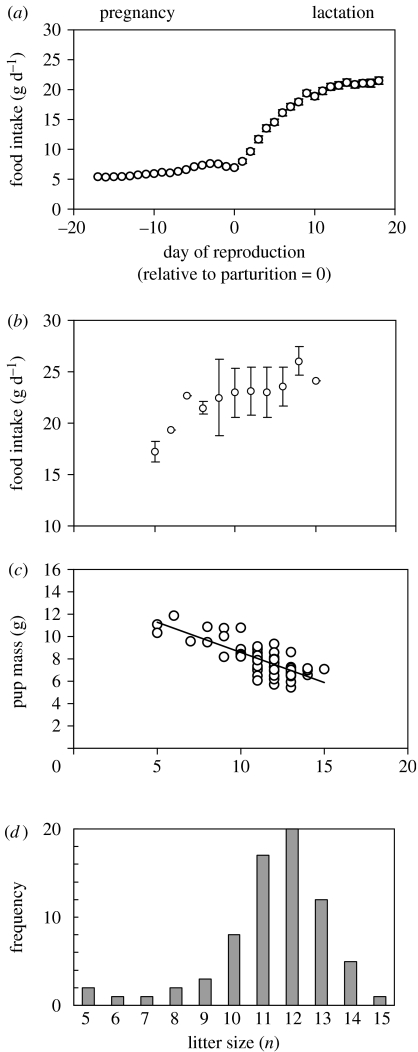
Figure 1.
(a) Food intake each day throughout pre-breeding, pregnancy and lactation phases for MF1 mice. Data averaged across 71 litters. (b) Daily food intake averaged over the last days of lactation (10–18) plotted against litter size in MF1 mice. (c) Pup mass in relation to litter size in MF1 mice. (d) Histogram showing the frequency at birth of different litter sizes across 71 litters of MF1 mice.
The most dramatic increase in food intake occurred during lactation. During the initial 10 days, this increase was linear, but then it reached a plateau at approximately 23 g of food per day. Food intake at the plateau (between days 10 and 18) was related to litter size. Small litters reached plateau intakes of less than 23 g (figure 1b), but as the litter size increased food intake also increased to a plateau at approximately 23 g of food per day. The mice seemed to reach a limit in their food intake at this level. Although litter sizes increased, food intake did not increase in parallel.
We have shown that this limit in food intake is not mediated via the aspects of the cage the animals live in (Speakman & Krol 2005), and therefore appears to be a physiological feature intrinsic to the animals themselves. This apparent physiological limit in the capacity of the mouse to ingest food at peak lactation may underpin an important life-history trait (maximum litter size) and an important life-history trade-off. Since the maximum asymptotic food intake at peak lactation is fixed, the energy that can be devoted to milk is also fixed. As the litter size increases, this milk must be divided between more and more offspring and, consequently, the pups wean at progressively smaller body masses (figure 1c; Johnson et al. 2001a). A physiological limit in the capacity to ingest food therefore appears to underpin the life-history trade-off between the number and size of offspring. Presumably, there is a minimum size of offspring at weaning, which would stand any chance of survival. Where the declining relationship between pup size and litter size intersects, this theoretical minimum viable pup size may define the maximum litter size. A distribution of natural litter sizes in this mouse strain is shown in figure 1d. It is noticeable that the asymptotic intakes for the mice that raised 14 and 15 pups were actually higher than the average 23 g limit (figure 1d). Perhaps these females were only able to raise such large litters because they were individuals that were capable of above-average intakes, and therefore still able to wean their pups at a pup mass of 7.5 g. Natural litter sizes of 16 and above in this strain may not be feasible owing to the interaction between the limit on maximal intake rate and the minimum viable pup size (this minimum viable pup size at weaning may also be physiologically mediated).
Understanding the physiological basis of this maximal intake limit is therefore of critical importance. Two hypotheses concerning the nature of this limit were proposed in the early 1990s (Peterson et al. 1990; Weiner 1992; Hammond & Diamond 1997). The first hypothesis was that the limit was imposed by the capacity of the alimentary tract to absorb food (energy) and process it into a form for mobilization. This was called the ‘central limit hypothesis’. The second hypothesis was that the limit is imposed at the peripheral site where the centrally supplied energy is used: the mammary glands. This was called the peripheral limit hypothesis.
Hammond & Diamond (1992) manipulated litters of Swiss Webster mice and found that females did not elevate their food intake when given up to 23 pups. Similarly, we have also shown that MF1 mice given up to 19 pups also could not breach the 23 g limit that they reached during unmanipulated lactations with litters of greater than 10 offspring (Johnson et al. 2001a). Faced with this problem of a litter size that is ‘too large’, females will often cull their offspring rather than eat more food (Johnson et al. 2001a; Gandelman & Simon 1978). In a separate experiment, Hammond & Diamond (1994) prevented pups from weaning at their normal weaning age and hence their increasing growth demands needed to be supplied by the mother until the pups were 24 days old. In these conditions, the mothers also could not upregulate their food intake. The absence of an increase in the intake when pup numbers are increased or lactation extended confirms there is a physiologically imposed limit, but does not separate between the peripheral and central limits hypotheses.
Giving female mice additional energy-demanding tasks during late lactation can better separate the alternative hypotheses. The basis of these experiments is that, if the system is centrally limited, the total energy available will be fixed. Using some of this energy to perform another task will reduce the amount available to support milk production and the consequence will be a diminution of reproductive output. Alternatively, if the system is peripherally limited, the animals will increase their intakes to meet the combined demands, and reproductive output will remain unaffected. Three manipulations have been performed to test these ideas: lactating mice have been forced to exercise to obtain their food; they have been made simultaneously pregnant, combining the demands detailed in figure 1a, and they have been exposed to cold.
Perrigo (1987) compared the reproductive strategies of house mice Mus domesticus and deer mice Peromyscus maniculatus by forcing females to run a preset number of revolutions (between 75 and 275) on a wheel to obtain each pellet of food. Despite the combined demands of lactation and locomotor activity, neither house nor deer mice exceeded the upper limit of food intake compared with unmanipulated mothers, given free access to food. As a result of the decreased amount of energy available for reproduction, the wheel-running house mice routinely killed some of the offspring throughout the first 12 days of lactation, whereas deer mice extended lactation well beyond normal weaning age.
Johnson et al. (2001b) followed the intakes of mice that had been mated immediately post-partum and found that the mice concurrently lactating and pregnant did not respond to the increased energy burden by elevating their food intake. Instead, they delayed implantation at the start of the second pregnancy and the length of this delay was directly related to the numbers of pups. The animals therefore ‘avoided’ overlapping their energy demands, perhaps because they could not elevate their total intake at peak lactation to accommodate both. Similar observations were made in Rockland-Swiss mice (Biggerstaff & Mann 1992), and in rats (Rattus norvegicus: Koiter et al. 1999), when food intake in late lactation was actually reduced in those rats that carried a simultaneous pregnancy, relative to rats just lactating. Together, these activity and pregnancy studies indicate that the limits are centrally, rather than peripherally, mediated; although, in case of concurrent pregnancy, the evidence is less strong as the animals avoided the problem.
However, when Hammond et al. (1994) exposed lactating Swiss Webster mice to 8°C (approx. 22°C below the lower critical temperature), they found that food intake increased dramatically beyond the supposed centrally imposed limit. Similar observations have since been made in deer mice (Hammond & Kristan 2000), MF1 mice (Johnson & Speakman 2001) and cotton rats (Sigmodon hispidus; Rogowitz 1998). The capacity of the mice to elevate their food intake in the cold was completely at odds with the central limitation hypothesis. To test whether the limit was imposed peripherally, Hammond et al. (1996) experimentally manipulated female mice by surgically removing some of their mammary glands during late lactation. The rationale behind this experiment was that if the capacity of the mammary glands was limited, then, if the mammary tissue was halved in size, the remaining tissue would be unable to compensate by elevating its milk production. However, if the capacity of the tissue was flexible and limited only by the centrally controlled supply of energy, then it would respond to the absence of half the tissue by expanding its capacity. They found that productivity in the halved glands did not increase, suggesting that the mammary gland was indeed the point at which the system was peripherally limited.
The above experiments suggested that there is a physiological limit in the capacity of the mammary tissue to secrete milk which underlies the asymptotic food intake in late lactation, and thus the trade-off between litter size and pup size and potentially defines the maximal litter size. When animals were manipulated at room temperature by giving them more pups to raise, food intake did not increase because milk production was limited by the capacity of the mammary glands. When exposed to cold conditions, food intake did increase (demonstrating a lack of central limitation) owing to the combined demands for milk production (at maximal capacity) and increased thermogenesis.
Several other studies have been performed, which also indicate that the limits to intake at peak lactation are not centrally mediated. These studies involve manipulation of the energy density of the food. If the energy density of food is decreased, but animals have a central processing capacity limit, they should be unable to upregulate their intake to compensate. Speakman et al. (2001) fed MF1 mice a diet that provided 25% less digestible energy than their normal food and then mated them. Food intake in the mice fed on the low energy density food increased at peak lactation on average of 3.8 g (from 23.1 to 26.9 g per day). Similar data are available for brown hares (Lepus europaeus; Hacklander et al. 2002). When fed on a diet with lower energy content, asymptotic food intake in late lactation increased from 230–250 to 280–300 g per day. Consequently, milk production was stable across the dietary treatments at approximately 35 g per day for females raising single offspring and 70 g per day for females raising twins. Conversely, when energy density was manipulated in the opposite direction, there was no indication that energy intake was elevated. In another example, rats fed a high energy density diet during lactation decreased their food intake to sustain energy intake constant (Denis et al. 2003).
An important aspect of the peripheral limitation idea is that milk production levels remain constant across the different manipulations—reflecting the fact that mammary glands are working at capacity. Several studies have measured milk production and support this prediction. Drummond et al. (2000) studied milk production in rabbits (Oryctolagus cuniculus) and observed that, following natural deaths of some offspring, the flow of milk was unaltered. Fink et al. (2001) studied lactation in captive mink (Mustela vision) and showed that in mothers raising litters of three, six and nine offspring, milk production did not increase when litters increased from six to nine offspring. Rogowitz (1998) demonstrated in cotton rats that levels of milk production in rats at 21 and 8°C were similar—consistent with the mammary glands working at maximal capacity. However, other studies have failed to find this consistency (Johnson & Speakman 2001; Król & Speakman 2003a,b; Król et al. 2003a,b). We have now studied food intake and milk production in mice at peak lactation at three different temperatures: 30, 21 and 8°C. As predicted by the peripheral limitation hypothesis, food intake increased across these measurements as temperature declined. However, unexpectedly, milk production and, consequently, pup growth were not constant across the different temperatures, but followed the pattern of food intake. Mice exported 88 kJ energy in milk per day at 30°C, 167 kJ at 21°C and 288 kJ at 8°C (Król & Speakman 2003b). Moreover, the weaning masses of pups at 30, 21 and 8°C averaged 6.1, 7.0 and 7.3 g, respectively (Król & Speakman 2003a). Consequently, the colder it got the more food the mice ate, the more milk they produced and the heavier the pups were at weaning.
These data are fundamentally inconsistent with both the suggestion that the limits are imposed by the capacity of the alimentary tract to process ingested energy and the suggestion that the limits reside in the milk production capacity of the mammary gland. There are a number of new ideas about the nature of the limit in food intake during lactation, which have been recently summarized (Speakman & Krol 2005). For example, one of these is that the limits are imposed by aspects of the neuroendocrinological system that regulates food intake rates—the ‘neural saturation hypothesis’. An alternative, suggested by (Król & Speakman 2003a,b), is that the capacity to expend energy during lactation at 21°C might be limited by the ability of the female mouse to dissipate heat. Hence, manipulations at 21°C—which aim to stimulate both food intake and milk production—notably increasing litter size (Hammond & Diamond 1992), extending lactation (Hammond & Diamond 1994), making them simultaneously pregnant (Biggerstaff & Mann 1992; Koiter et al. 1999; Johnson et al. 2001b) and making them exercise (Perrigo 1987)—all failed to increase either food intake or milk production, because the animals could not increase their heat production without risking fatal hyperthermia (see below). In effect, this hypothesis is a central limitation idea, but is focused around the ability to dissipate heat rather than assimilation from the gut. Under the heat dissipation limit hypothesis, when mice are exposed to the cold, this is not an additional demand, but a relaxation of the heat dissipation limit, allowing the animals to elevate not only their food intake but also their milk production and thus the size of their offspring. Similarly, when mice were placed in the hot, this reduced their capacity to dissipate heat, restricted their food intake and milk production, and led to smaller pups being weaned.
There are two putative mechanisms for how the capacity to dissipate heat may influence lactation performance. At high ambient temperatures, lactating mice may continuously face difficulties dissipating heat. This would lead to a perpetually elevated body temperature (see below) which might influence the regulation of milk production. It is well established that endogenous opioids in the preoptic anterior hypothalamus are of key importance in the regulation of body temperature, and are reduced during lactation (Kim et al. 1997). Projections from this area terminate in the paraventricular nucleus (PVN), where the magnocellular cells synthesize oxytocin. Rayner et al. (1988) showed that intracerebroventricular (ICV) administration of morphine inhibits oxytocin production in the PVN. Thus, elevated endogenous opioids under hyperthermia might directly reduce oxytocin secretion. Morphine administration also disrupts maternal suckling behaviour (Cox et al. 1976; Bridges & Grimm 1982). In addition, thyroid hormone, which may be regulated by differences in body temperature, is an important modulator of prolactin production. Continual maternal hyperthermia in relation to external ambient temperatures (or differential capacity to dissipate body heat) may therefore directly inhibit oxytocin and prolactin secretion, thereby reducing milk production. Finally, there may be a more direct link between hyperthermia and milk production. A response to hyperthermia might be to direct blood flow away from the mammary glands to other peripheral areas to dissipate heat by vasodilatation (Black et al. 1993). Blood flow in the mammary glands has been shown to have a direct effect on milk production (Vernon & Flint 1983).
A second mechanism is that suckling schedules of the mice may be influenced by the development of maternal hyperthermia in the nest. Although pups act as heat sinks early in their development (Scribner & Wynne-Edwards 1994a,b), in late lactation offspring are capable of considerable heat production. The suckling unit of mother and pups, therefore, may generate heat that leads to maternal hyperthermia, ultimately forcing the female to discontinue suckling (Croskerry et al. 1978). The progress to hyperthermia would be more rapid as the capacity to dissipate heat declines. The sucking stimulus is one of the primary factors stimulating oxytocin release and milk let down, and also feeds back onto prolactin release, thereby regulating milk production. Continual disruption of suckling, due to intermittent hyperthermia, would be a second mechanism linking heat dissipation capacity to lactation performance.
Many studies have examined the pattern of change in food intake across the reproductive cycle among other small non-domesticated mammals. Some examples of these measurements are in table 1. This is not an exhaustive review. Using the data for rodents and converting estimated food intakes into energy for those species where only food intakes are reported in the original papers, there was a significant effect of body mass (F=42.6, p<.001) and a significant effect of reproductive status (F=30.6, p<.001), but no significant interaction between these two variables (F=1.55, p=0.225; figure 2a). Using pairwise Tukey comparisons, the non-breeding energy demands did not differ significantly from those in pregnancy, but the demands in lactation were significantly elevated above both the non-breeding and pregnancy demands. This analysis reveals that the energy costs of pregnancy are relatively trivial. This does not mean that demands in pregnancy do not impose limits on reproduction (see above arguments regarding foetal volume competing for abdominal space with the alimentary tract). Nevertheless, the pattern observed in laboratory mice and rats that the costs of reproduction are substantially greater during lactation than in pregnancy appears to be very broadly applicable.
Table 1.
Some examples of peak energy intakes of non-domesticated small (less than 1 kg) mammals at different phases of the reproduction cycle. LS is litter size. Under P this is litter size at birth; under L, this is litter size at weaning. Where original paper does not state which, it is entered under both. Status: NB, non-breeding; P, pregnant; L, lactating. × NB is the intake expressed as a multiple of the non-breeding intake. In all cases (except *), the animals were studied in the laboratory at standard room temperatures (21–24°C) with ad libitum access to food. ** animals kept at 5°C * animals kept at 10°C. In some cases, the original data were quoted relative to body mass0.75, and in these instances the actual intakes have been recalculated using the cited body mass. In other papers, only mass of food ingested is cited and these have been converted assuming a dry mass energy content for the food of 20.9 kJ g−1, unless a different value was cited in the paper. In yet other studies, average daily metabolic rate (ADMR) is quoted. I have converted this to energy intake assuming a digestive efficiency of 80%. Genera: Rodents C. = Clethrionomys, P. = Peromyscus, M. = Microtus, Me. = Meriones, A. = Acomys, Ca. = Cavia, S. = Sigmodon, Sc. = Sciurus. Insectivores, S. = Sorex, C. = Crocidura, Ec. = Echinops, E. = Erinaceus. Bats P. = Plecotus, T. = Tadarida, E. = Eptesicus, M. = Myotis, R. = Rousettus. ^ estimates relative to NB calculated using the NB in reference 7. $ estimates expressed as difference to unquoted NB level in original ms.
| species | mass | status | LS | food intake | energy (kJ d−1) | × NB | refa |
|---|---|---|---|---|---|---|---|
| rodents | |||||||
| P. polionotus | 13.4 | NB | 54.0 | 1 | |||
| L | 3.64 | 94.0 | 1.74 | 1 | |||
| C. gapperi | 18.8 | NB | 3.80 | 2 | |||
| 34.0 | P | 5.58 | 6.39 | 1.68 | 2 | ||
| 27.0 | L | 12.0 | 3.15 | 2 | |||
| P. maniculatus | 14.5 | NB | 58.4 | 1 | |||
| L | 4.30 | 144.6 | 2.47 | 1 | |||
| P. maniculatus | 20.1 | NB | 5.14 | 3.5 | 3 | ||
| 25.8 | L | — | 9.6 | 2.70 | 3 | ||
| P. leucopus | 21.0 | NB | 72.45 | 1 | |||
| L | 3.91 | 143.64 | 1.98 | 1 | |||
| P. eremicus | 21.5 | NB | 52.0 | 1 | |||
| L | 2.42 | 104.9 | 2.02 | 1 | |||
| M. pennsylvanicus | 24.0 | NB | 4.82 | 2 | |||
| 38.2 | P | 5.05 | 6.57 | 1.36 | 2 | ||
| 31.0 | L | 15.5 | 3.21 | 2 | |||
| C. glareolus | 24.5 | NB | 73.2 | 4 | |||
| — | P | 5.0 | 99.2 | 1.35 | 4 | ||
| — | L | 4.0 | 162.8 | 2.22 | 4 | ||
| P. leucopus | 25.0 | NB | 4.0 | 3.2 | 5 | ||
| L | 6.0 | 1.875 | 5 | ||||
| 24.5 | NB** | 4.0 | 5.5 | 5 | |||
| L** | 8.7 | 1.58 | 5 | ||||
| M. arvalis | 25.3 | NB | — | 62.3 | 6 | ||
| 33.9 | P | 4.25 | 66.5 | 1.07 | 6 | ||
| — | L | 4.00 | 175.7 | 2.82 | 6 | ||
| P. leucopus | 25.8 | NB | 62.8 | 7 | |||
| 25.8 | L | 5.0 | 8.23 | 150.1 | 2.39 | 7 | |
| Phodopus sungorus | 30.0 | NB | 56.0 | 8 | |||
| 41.0 | P | 5.6 | 68.0 | 1.21 | 8 | ||
| 35.0 | L | 104.3 | 1.93 | 8 | |||
| P. floridanus | 42.0 | NB | 94.5 | 1 | |||
| L | 2.25 | 144.0 | 1.54 | 1 | |||
| Onchomys leucogaster | 45.3 | NB | 4.15 | 72.8 | 9 | ||
| 50.7 | L | 4.0 | 9.49 | 166.0 | 2.28 | 9 | |
| A. cahirinus | 49.4 | NB | 45.8 | 10 | |||
| 65.8 | P | 2.0 | 60.9 | 1.33 | 10 | ||
| 46.1 | L | 2.0 | 63.1 | 1.38 | 10 | ||
| M. brandtii | 59.3 | NB | 94.54 | 11 | |||
| 83.6 | P | 8.5 | 105.22 | 1.11 | 11 | ||
| 51.5 | L | 8.5 | 334.2 | 3.53 | 11 | ||
| Me. crassus | 93.6 | NB | 113.1 | 12 | |||
| 90.7 | L | 3.75 | 120.1 | 1.05 | 12 | ||
| 88.8 | NB | 112.5 | 12 | ||||
| 90.6 | L | 4.0 | 121.5 | 1.08 | 12 | ||
| 92.0 | NB | 147.9 | 12 | ||||
| 92.5 | L | 4.18 | 153.9 | 1.04 | 12 | ||
| S. hispidus | 125.8 | NB | 146.8 | 13 | |||
| 180.2 | P | 5.0 | 233.9 | 1.60 | 13 | ||
| 125.0 | L | 5.0 | 193.7 | 1.30 | 13 | ||
| S. hispidus | 175 | NB | 158.5 | 14 | |||
| 241 | P | 7.0 | 240 | 1.51 | 14 | ||
| 169 | L | 6.9 | 450 | 2.84 | 14 | ||
| S. hispidus | 145.7 | L* | 5.0 | 390.4 | 2.65^ | 15 | |
| 127.5 | L | 5.0 | 269.3 | 1.83^ | 15 | ||
| Ca. porcellus | 402.6 | P | 1.7 | +160$ | 16 | ||
| 550.5 | L | 1.7 | +340 | 16 | |||
| 615.8 | P | 2.5 | +200 | 16 | |||
| 628.8 | L | 2.5 | +380 | 16 | |||
| Sc. niger | 880 | NB | 1516 | 17 | |||
| L | 4.0 | 3579 | 2.36 | 17 | |||
| primates | |||||||
| Callithrix jacchus | 418.1 | NB | 34.58 | ||||
| P | 45.71 | 1.27 | 18 | ||||
| 375.7 | L | 64.28 | 1.85 | 18 | |||
| insectivores | |||||||
| S. minutus | 4.9 | NB | 43.0 | 19 | |||
| P | 9 | 48.0 | 1.12 | 19 | |||
| L | 9 | 173.0 | 4.02 | 19 | |||
| Geogale aurita | 5.9 | NB | 9.67 | 20 | |||
| 9.6 | P | 11.6 | 1.20 | 20 | |||
| S. araneus | 9.3 | NB | 61.8 | 21 | |||
| 14.8 | P | 6.9 | 85.3 | 1.38 | 21 | ||
| 10.7 | L | 4.3 | 175.4 | 2.83 | 21 | ||
| S. coronatus | 10.3 | NB | 46.6 | 19 | |||
| P | 5.1 | 60.0 | 1.29 | 19 | |||
| L | 5.1 | 191.0 | 4.09 | 19 | |||
| C. russula | 13.8 | NB | 45.3 | 19 | |||
| P | 4.4 | 50.2 | 1.09 | 19 | |||
| L | 4.4 | 128.0 | 2.82 | 19 | |||
| C. viaria | 17.1 | NB | 45.9 | 19 | |||
| P | 3.5 | 48.5 | 1.06 | 19 | |||
| L | 3.5 | 106 | 2.31 | 19 | |||
| C. olivieri | 39.5 | NB | 47.4 | 19 | |||
| P | 3.8 | 47.5 | 1.00 | 19 | |||
| L | 3.8 | 132 | 2.78 | 19 | |||
| Ec. telfairi | 174.8 | NB | 49.6 | 22 | |||
| 279 | P | 1.5 | 121.0 | 2.44 | 22 | ||
| 262.6 | L | 1.5 | 151.0 | 3.04 | 22 | ||
| E. europaeus | 750 | NB | 87.2 | 23 | |||
| 850 | L | 3.0 | 187.5 | 2.15 | 23 | ||
| bats | |||||||
| M. lucifugus | 9.0 | P | 1 | 33.7 | 24 | ||
| 6.7 | L | 1 | 60.3 | 1.79 | 24 | ||
| P. auritus | 7.35 | NB | 1.8 | 48.0 | 25 | ||
| 8.53 | L | 1 | 2.0 | 53.0 | 1.10 | 25 | |
| T. brasiliensis | 13.4 | P | 57.0 | 26 | |||
| 11.4 | L | 1 | 114.0 | 2.0 | 26 | ||
| E. fuscus | 20.84 | P | 1 | 48.9 | 2.15 | 27 | |
| 17.4 | L | 1 | 105.1 | 2.15 | 27 | ||
| R. aegyptiacus | NB | 200.0 | 28 | ||||
| P | 271.0 | 1.35 | 28 | ||||
| L | 360.0 | 1.80 | 28 | ||||
| marsupials | |||||||
| Caluromys philander | 304 | NB | 2544 | 29 | |||
| 308 | L | 4124 | 1.62 | 29 |
References: 1. Glazier (1985), 2. Innes & Millar (1981, 1985), 3. Millar (1985), Millar & Innes (1985), 4. Kaczmarski (1966), 5. Hammond & Kristan (2000), 6. Migula (1969), 7. Millar (1978), 8. Weiner (1987), 9. Sikes (1995) 27, 10. Degen et al. (2002), 11. Liu et al. (2003), 12. Kam et al. (2003), 13. Randolph et al. (1977), 14. Mattingly & McClure (1982), 15. Rogowitz (1998), 16. Ku¨nkele (2000), 17 Havera (1979), 18 Nievergelt & Martin (1999), 19. Genoud & Vogel (1990), 20. Stephenson & Racey (1993b), 21. Poppitt et al. (1993), 22. Poppitt et al. (1994), 23. Król (1985), 24. Kurta et al. (1989), 25. Mclean & Speakman (1999), 26. Kunz et al. (1995), 27. Kurta et al. (1990), 28. Korine et al. (2004), 29. Atramentowicz (1992).
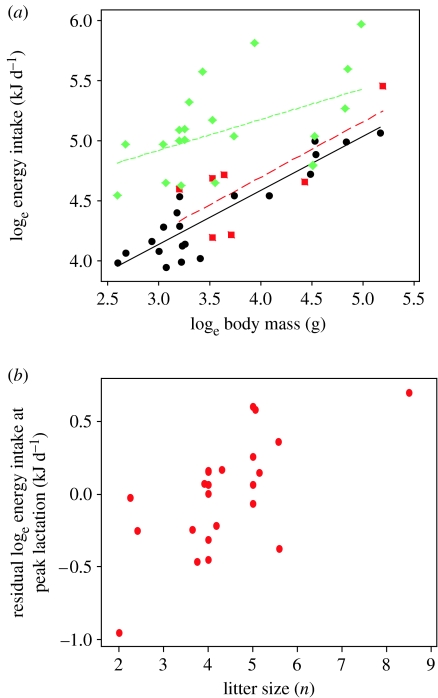
Figure 2.
(a) Peak energy intake of female small rodents, when not breeding (black), pregnant (red) and lactating (green). There was no significant elevation in intake during pregnancy, but intake during lactation was significantly elevated compared with both non-breeders and pregnant individuals. (b) Residual energy intake in lactation was significantly associated with litter size. Data from small rodents are given in table 1.
There was a wide variation in the energy demands at peak lactation (table 1; figure 2a). The residual variation, once the effects of body mass had been removed, was significantly associated with litter size (figure 2b), which explained 49.3% of the residual variance (p=0.0103). Although litter size and body size explain much of the variation in energy intake at peak lactation, there is still considerable variation in the energy intakes across species. One factor that may be of importance in this variation is the developmental strategy. Studies of the patterns of development in eutherian mammals have identified two separate developmental strategies (reviewed in Martin & MacLarnon 1985; Martin 1989), with some species having prolonged periods of gestation followed by birth of relatively well-developed offspring that rapidly become independent of the mother and are capable of feeding themselves relatively quickly after birth (precocial development). In contrast, other species have a relatively short gestation that is followed by a more protracted period of lactation during which they are completely dependent on nutrient supply from the mother (altricial development). The use of these strategies is not independent of body mass with mammals weighing more than 100 kg using only the precocial strategy, while species weighing less than 100 g use the altricial strategy almost exclusively, although some species follow what has been called an ‘intermediate’ strategy (defined in Martin & MacLarnon 1985), e.g. Acomys caharinus (Degen et al. 2004). As anticipated from their size, mice follow the altricial strategy. Between 100 g and 100 kg species are found that follow either precocial or altricial strategies with a few ‘intermediates’ (Martin & MacLarnon 1985; Martin 1989).
Clearly, this dichotomy in developmental strategy may impact on the levels of energy investment by the mother during lactation (Oftedal 1984; Hill 1992; Kam et al. 2006). Since it is the rarer strategy, relatively few studies have addressed the levels of food intake of small rodents following the clearly precocial strategy (but see, for example, the studies of the guinea pig Cavia porcellus; Kunkele & Trillmich 1997) with many more studies following the patterns of investment during lactation of species raising clearly altricial or intermediate offspring. Gestation period (days) reflecting position on the altricial–precocial continuum was not an additional significant factor influencing peak energy demands (p=0.13). The absence of an effect of this dichotomy might be expected given the selection of species that were either altricial or intermediate strategists. The only rodent with clear precocial development in the data reviewed in table 1 was the guinea pig (Krebs 1950), but the manner in which the data were presented in that paper relative to unstated levels in non-breeding animals precluded its inclusion in the data analysis.
The residual variation once the effects of litter size and body mass were removed to an extent follow different strategies for coping with the direct costs of reproduction. Hence, the low values in the cotton rat (Sigmodon hispidus) and the hamster (Phodopus sungorus) reflect the fact that these animals deposit fat stores during the early phase of reproduction, which are withdrawn in lactation and reduce the need to supply all the energy from food intake. Low demands in other species may reflect the use of compensatory mechanisms that reduce expenditure on other components. These strategies will be discussed in detail below.
Expressing the peak lactation energy intake as a multiple of the intake of non-breeding animals, the average across all the rodents in table 1 was 2.1 (s.d. =0.72, n=21). This is considerably lower than the ratio in MF1 mice at 21°C, which equalled 4.2. However, the intake of the MF1 mice at this temperature is within the 95% confidence limits of the prediction from the fitted regression equation from the non-domesticated rodents, given the body size and litter size for this animal.
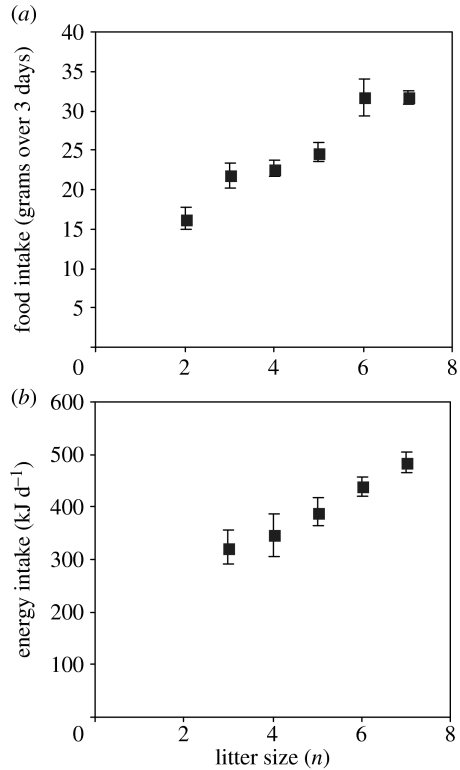
The importance of the differences in magnitude between the peak levels of intake in wild rodents and laboratory mice and rats remain uncertain. In most of the species studied in table 1, and in other species, peak intake of food during lactation is dependent on litter size. Generally, however, these patterns do not reach an asymptotic level as observed in the MF1 mice (figure 1b). Two examples are shown in figure 3. However, despite this absence of an asymptote in both species (and others), there is still an inverse relationship between the litter size and the mean offspring size, indicating that increases in investment were insufficient to match elevated demand. These data suggest that the trade-off between litter size and pup mass is more complex than the simple model of the mother reaching an asymptotic intake at which point a fixed investment is divided between increasing numbers of offspring. Part of this equation, however, may be capacity limits in the offspring themselves and how these depend on litter size. Pup–pup competition may be a key element of the impact of litter size on their growth efficiency.
Figure 3.
(a) Food intake at peak lactation (grams over 3 days) in relation to litter size in Peromyscus leucopus (drawn from tabulated data in Millar 1978). (b) Energy intake during lactation (kJ d−1) in relation to litter size in Sigmodon hispidus (drawn from tabulated data in Rogowitz 1998).
(ii) Protein and calcium
Previous discussion of limits on lactational performance has focused almost entirely on energy. Yet growing offspring also require large amounts of protein, calcium and other micronutrients. At weaning, an MF1 mouse has produced on average 12 pups each weighing 7.5 g: a total of 96 g of wet tissue. Of this tissue, approximately 13.5 g is protein and 1.8 g is calcium, both of which must be supplied by the mother. The diet we feed our mice on contains 22.45% crude protein (RM3 breeder diet SDS diet services, Witham Essex). Thus, over the last 8 days of lactation when they are taking in 23.2 g each day, their total protein intake amounts to 41.6 g of protein—substantially more than ultimately appears in the pups. This rough balance suggests that in the lactating MF1 mouse system, protein supply is unlikely to exert limits on the animals. Several studies have been performed, however, where the level of protein in the diet is much lower.
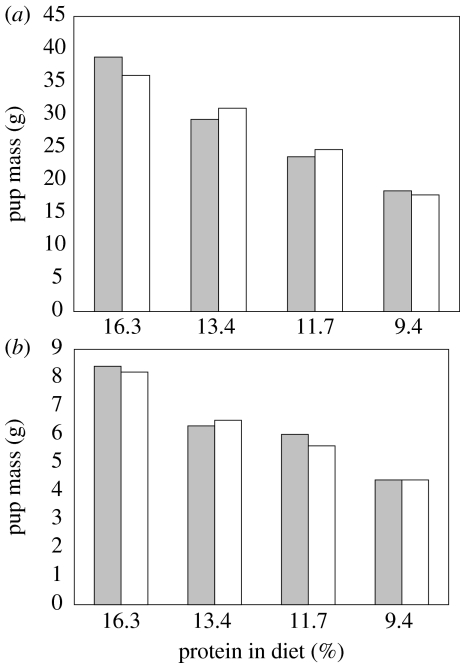
Goettsch (1960) examined the effects of feeding nursing rats and mice on four diets containing 16.3, 13.6, 11.4 and 9.4% protein. Food intake in lactation was broadly independent of the protein content of the diets, except that it was reduced in mice feeding on the lowest protein diet and ‘highly variable’ in rats on this diet. However, the growth of offspring was directly affected in both rats and mice, with offspring size at weaning being positively related to the protein content of the diet (figure 4a,b). Maternal mean protein intake in these mice throughout lactation averaged only 0.5 g per day in the lowest protein group which even accounting for the mean litter size difference between the strains (12 in our work and 7.1–7.5 in Goettsch 1960) is still substantially lower than that for our mice (5.2 g/day at peak lactation). There are several additional studies which show that low maternal dietary protein levels affect pup growth. The mothers appear constrained, however, owing to the maximal amounts of energy that they can process—perhaps defined by the heat dissipation limit. Hence, this energetic limit prevents them from simply eating more food to meet their protein demands. These data indicate that energy limitation imposes a primary constraint on the animals under low protein supply.
Figure 4.
Mean mass at weaning of male (grey) and female (white) pups of (a) rats and (b) mice when fed on diets of varying protein content (from 16.3% to 9.4% protein). Drawn from data in Goettsch (1960).
If low protein in the maternal diet imposes a restriction on the growth of offspring then, perhaps, protein content of the diet is the key factor that links food intake to pup growth. When we exposed lactating females to hot and cold conditions, we may have manipulated the constraint on their total energy intake that was imposed by their capacity to dissipate heat (Krol & Speakman 2003a,b). However, the impact on pup growth may have been mediated via the consequences of these different levels of energy intake for levels of protein intake—indeed the mice at 8°C were ingesting approximately 7.2 g of protein each day, those at 21°C only 5.2 g per day and those at 30°C only 2.91 g per day. Perhaps the trade-off between litter size and pup size (figure 1c) may move depending on dietary protein contents—to the right when protein in the diet is elevated and to the left when it is reduced. Supporting these ideas, Hitchcock (1927) fed nursing rats a base diet that contained 29% protein, and additionally supplemented some of the animals with raw meat. Litter size in both groups was the same yet the offspring from the mothers fed meat daily weighed 61.8 g at weaning, compared with only 47.3 g in the rats not given the meat ration.
A similar situation probably pertains to calcium supply. The diet we use in our studies of mouse lactation contains 1.24% Ca. Thus, in the last 8 days of lactation, the mice ingest 2.30 g of calcium, compared with an estimated calcium content of the pups of 1.8 g. Hence, as long as calcium and protein levels in diets are above a certain limit, the constraint on maximal energy intake is unlikely to severely impact offspring in terms of their protein and calcium status.
For wild animals, the situation where calcium and protein contents of the diet are below the critical point, where the energy limit becomes restrictive, may be more routinely breached. One situation that has attracted considerable attention is in microchiropteran bats. Because microchiropteran bats are almost exclusively nocturnal, a constraint probably imposed by ecological factors such as predation risk and competition (Speakman 1990; 1991a,b; Rydell & Speakman 1995; Speakman et al. 2000), they do not routinely get any exposure to sunlight. This may influence their production of vitamin D (Cavaleros et al. 2003), which is an essential component of calcium physiology. Moreover, most microchiropteran species are predominantly insectivores and these prey are very low in calcium content. Their inability to manoeuvre effectively on the ground restricts them from finding additional mineral sources of calcium that may be available to other animals. Insectivorous bats have very low litter sizes for their body sizes (Kurta & Kunz 1987) and one interpretation of these low rates of productivity is that they are primarily constrained by their capacity to obtain calcium (Barclay 1994).
(b) Organ remodelling to meet these demands
The alimentary tract has a more limited capacity to process energy than the mouth has capability to supply it. This is why the gut includes storage organs like the crop and stomach at the entry to the tract to take on board the intake at this elevated rate. If an animal requires to increase its food intake from approximately 5 g per day, prior to breeding, to 23 g per day at peak lactation, it could probably achieve this intake immediately, at the level of the mouth. There are no obvious changes in the morphology of the mouth as the animals progress through reproduction. However, while a non-breeding mouse might be capable of eating 23 g each day, its alimentary tract would probably be unable to process the intake. Hence, animals need to modify their internal architecture to cope with the altered demands. That lactating animals modify their tracts and associated organs in this manner has been known for at least 45 years. During lactation, there is an increase in the sizes of both the liver (Kennedy et al. 1958) and the pancreas (Jolicoeur et al. 1980). The most dramatic changes however are in the alimentary tract itself, which involve major morphological increases in the absorptive surface of the intestinal mucosa and also growth in the length of the tract (Boyne et al. 1966; Fell et al. 1963; Campbell & Fell 1964; Craft 1970; Cripps & Williams 1975; Burdett & Reek 1979; Prieto et al. 1994; Speakman & McQueenie 1996; Hammond 1997). These changes are paralleled by alterations in the transport capacity at the cellular level (Larradale et al. 1966; Dugas et al. 1970). The growth of the mucosal layer of the alimentary tract in lactation includes both hypertrophic (cell expansion) and hyperplastic (cell proliferation) responses (Fell et al. 1963; Cairnie & Bentley 1967; Prieto et al. 1994). These changes effectively allow the lactating female to continue to extract the same amount (%) of energy from the ingested food independent of the rate of intake (e.g. Campbell & Fell 1964; Hammond et al. 1994).
Studies of wild rodents reveal similar patterns of change, although generally including more modest increases (e.g. Myrcha 1964; Chlethrionomys glareolus; Myrcha 1965 Apodemus flavicollis; Gebczynska & Gebczynski 1971 Clethrionomys oeconomus) reflective of the lower level of increase in food intake between non-breeding and lactation states (table 1). This is consistent with the fact that the extent of modification of the intestine is related to inter-individual variations in litter size in mice.
Several hypotheses have been advanced about how the growth in the gut is stimulated. The first is that the hormones that underpin the increase in food intake during lactation (reviewed in Speakman & Krol 2005), including elevated prolactin and reduced leptin, directly stimulate the changes in the alimentary organs. Alternatively, the food intake itself may result in the production of local growth factors stimulating tissue proliferation (Datta et al. 1995). Finally, hormones linked to milk production, e.g. oxytocin, may stimulate the growth. Unfortunately, separating these effects is difficult. Injection of hormones, like prolactin, affect food intake (Noel & Woodside 1993), so eliminating a secondary local effect stimulated by the elevated food intake would need animals to be pair-fed with non-injected controls, and these critical experiments have not yet been performed. Hammond (1997), however, noted that hypertrophy of the gut is a generalized response to elevated nutritional demands (such as cold exposure) and the hormonal profile in these circumstances is completely different from that in lactation, favouring the hypothesis that changes are primarily stimulated by a local response, stimulated directly by the food intake.
Many other morphological changes occur during pregnancy and lactation, not least of which is the growth of mammary tissue during late pregnancy to facilitate milk production during the subsequent lactation. As might be expected, considerable attention has been paid to this growth process and its hormonal basis, particularly the roles of sex steroids progesterone and 17β-oestradiol (reviewed in Lamote et al. 2004). Not all changes in the lactating animal, however, include expansion of tissue sizes. In particular, there is a large reduction in the size of the adipose tissue stores (Speakman & McQueenie 1996; Vernon & Pond 1997). These reductions in the size of adipose tissue stores have generally been interpreted as withdrawal of stored energy to support energy delivery during the lactation event. Certainly, in some species (like the cotton rat: Randolph et al. 1977 and the Siberian hamster: Weiner 1987), there is accumulation of fat during pregnancy that is withdrawn later in lactation and this strategy appears to reduce the peak food intake demands in lactation (see above and table 1). However, in other animals, the contribution of fat to the overall energy budget is relatively trivial. In mice, for example, the fat stores decline by approximately 2 g during lactation (Johnson et al. 2001c) equivalent to approximately 80 kJ of energy, compared with a metabolizable energy intake over the last 10 days of lactation of approximately 2600 kJ.
The increasing recognition that adipose tissue is not only an energy store but also an endocrine regulator suggests an alternative explanation of the reduction in fat mass may be that this reduces production of leptin (and possibly other adipokines) which then act to stimulate food intake. That low levels of leptin during lactation are involved in stimulating food intake has been demonstrated by repleting leptin levels using miniosmotic pumps. This provision of exogenous leptin blunts the level of increase in food intake during lactation (Stocker et al. 2004). Decreased leptin levels may also be important in signalling reductions in the UCP-1 gene expression in brown adipose tissue of lactating rodents, the significance of which will be addressed below. Elevated levels of circulating adipokines are often inferred to underlie the links between obesity and disease. For example, high leptin levels, independent of adiposity, are an increased risk factor for the development of the metabolic syndrome in humans (Franks et al. 2005). By reducing the levels of production of these compounds, lactating animals may actually derive an advantage relative to non-lactating individuals.
It is often suggested that a direct impact of this remodelling of the internal architecture during lactation is an increase in the resting metabolic rate relative to non-breeding animals. This occurs in theory because the animal expands the size of tissues that have high rates of metabolism (e.g. the liver and alimentary tract; Field et al. 1939; Krebs 1950), while simultaneously decreasing the size of other tissues with low energy demands. This is the direct energy cost linked to the lactation process of making these morphological modifications. When comparisons are made of the resting or basal energy expenditure in lactating small mammals compared with non-breeding individuals, many studies have reported that the RMR of lactating animals is higher (Camas et al. 1982; Stephenson & Racey 1993a,b; Garton et al. 1994; Poppitt et al. 1994; Harder et al. 1996; Speakman & McQueenie 1996; McLean & Speakman 2000; Johnson et al. 2001b; Zenuto et al. 2002; Krol et al. 2003a,b). Although other studies, particularly in non-domestic species, have reported minor or even no changes (e.g. Dryden et al. 1974; Turbill & Geiser 2006), this might be anticipated in those groups where the increase in food intake in lactation is more modest (table 1) and thus requires correspondingly minor adjustments in morphology. However, if the theoretical basis of the effect is that organs such as the alimentary tract and liver require greater maintenance costs (Field et al. 1939, Krebs 1950), then one would anticipate that differences between lactating individuals in the sizes of these organs would have impacts on metabolism, and corresponding impacts on food intake and lactation performance. However, when such associations have been sought, they have generally not been found (reviewed in Speakman et al. 2004). It remains uncertain why these expected trends are not observed, but this lack of an association calls into question the cost of the tissue remodelling.
3. Indirect costs
(a) Optional compensatory costs
(i) Thermoregulatory demands
During lactation, many species of small mammals experience large morphological and biochemical changes in their interscapular brown adipose tissue (BAT). These modifications include reductions in the amount of BAT in mice, rats, ground squirrels and hamsters (Agius & Williamson 1980; Wade et al. 1986 Johnson et al. 2001b). In addition to reductions in overall tissue mass, there are also reductions in BAT mitochondrial mass (Trayhurn et al. 1982; Trayhurn & Jennings 1987a,b). In late lactation, mitochondria-specific content of uncoupling protein 1 (UCP-1) is reduced to only 8% of the level found in non-breeding mice (Trayhurn & Jennings 1987a,b, 1988) and to 26% in ground squirrels (Nizielski et al. 1993). GDP binding, which is a measure of mitochondrial thermogenic capacity, is also reduced in mice and rats (Trayhurn et al. 1982) and ground squirrels (Nizielski et al. 1993), but not in hamsters (Wade et al. 1986). Brown adipose tissue is the key thermogenic organ in small rodents (Cannon & Nedergaard 2004) and the changes observed in lactating rodents mediate a reduction in the noradrenaline-induced non-shivering thermogenesis (Trayhurn et al. 1982; Trayhurn 1983), which is rapidly reversed upon weaning (Trayhurn & Jennings 1987a,b, 1988). In rats, the extent of decrease in thermogenic capacity is related to litter size (Isler et al. 1984), but this does not appear to be the case in mice (Trayhurn & Wusterman 1987a,b). These morphological, physiological and biochemical changes in BAT appear to be controlled by reduced sympathetic activity in lactation (Trayhurn & Wusterman 1987a,b), which may be responsive to elevated corticosteroid levels (Vernon & Flint 1983). Treatment of lactating rats with exogenous leptin completely reversed the downregulation of UCP-1 gene expression in BAT (Xiao et al. 2004). Changes in other aspects of BAT physiology are also apparent during lactation, including reduction in the activity of iodothyronine 5’-deiodinase, which catalyses conversion of thyroxine (T4) to triiodothyronine (Giralt et al. 1986). All these changes are consistent with small lactating animals attempting to reduce obligatory heat production from BAT. Trayhurn (1989) interpreted this reduction as an energy-saving mechanism that increased the efficiency of milk production. However, in the light of our studies of limits to food intake in lactating mice described above, an alternative interpretation is that this downregulation does not save energy which can be used for milk production, but rather reduces the heat burden on the animal, allowing it to elevate milk production.
In recent years, a number of additional uncoupling proteins have been described (UCP-2 to UCP-5). These uncoupling proteins have different tissue distributions, with UCP-2 being very widespread, UCP-3 being restricted to BAT and skeletal muscle, and both UCP-4 and UCP-5 being found only in the brain. The role of these UCPs in resting and thermogenic heat production has been an issue of debate (e.g. Erlanson-Albertsson 2002, 2003). Studies of the UCP-1 knockout mouse indicate that the other UCPs cannot reverse the lack of thermogenic capacity brought about by the absence of UCP-1 (Enerbäck et al. 1997; Golozoubova et al. 2001; Nedergaard et al. 2001). Although UCP-3 cannot be facultatively upregulated to replace the function of UCP-1, when it is transgenically overexpressed, the resultant mice have elevated resting metabolism (Clapham et al. 2000). Surprisingly, given the suggested absence of any role for natural levels of UCP-3 in thermogenesis, it has recently been shown that UCP-3 is also downregulated enormously in BAT during lactation, and this is reflected in reduced protein levels as well (Pedraza et al. 2000, 2001; Xiao et al. 2004), but UCP-2 is unchanged (Pedraza et al. 2001). Levels of UCP-3 gene expression in muscle are also decreased in lactation (Xiao et al. 2004). These effects appear to reflect circulating levels of free fatty acids (Pedraza et al. 2000). Possibly, UCP-3 is thermogenically insignificant but it is incidentally downregulated because the mechanisms for regulating UCP-1 and UCP-3 are similar. There may be consequences of this downregulation, which the animal cannot avoid (Cadenas et al. 2002), and will be discussed below under ‘consequential costs’.
Many small mammals are able to make profound savings of energy by relaxing thermoregulation at normothermic levels and entering periods of torpor, during which body temperature is commonly reduced to levels just above ambient (Heldmaier et al. 2004). While bringing significant benefits in terms of energy saving, torpor is fundamentally incompatible with some of the processes of reproduction as was first demonstrated in a series of elegant experiments in the early 1970s, where bats were forced into torpor for varying periods with the consequence that gestation period was extended by the exact same period that the mice had been made to spend in torpor (Racey 1973). Foetal growth therefore halts when bats enter torpor. The significance of this effect was demonstrated in wild populations of bats during the early 1980s, when it was shown that adverse weather conditions during early pregnancy slowed foetal growth in wild bat populations resulting in significant year-to-year differences in the duration of pregnancy (Racey & Swift 1981). These findings seem to be at odds with the fact that hibernating bears gestate and lactate. However, studies of body temperature in bears during lactation reveal that while metabolism is downregulated to a similar extent as in small mammals, body temperatures only cool to approximately 30–32°C. If the interruption of foetal growth is temperature mediated, this difference in body temperatures during torpor may explain how bears are able to combine their reproductive functions with hibernation. Despite these apparently negative impacts of torpor in pregnancy, it is used frequently in some species of marsupial (e.g. dunnarts (Sminthopsis macroura): Geiser et al. 2005; and mulgaras (Dasycercus cristicauda): Geiser & Masters 1994), although in these animals the body temperature is still defended at approximately 14°C.
The impact of torpor on lactation is less clear. Many species appear to use periodic entry into torpor as an energy-saving mechanism during lactation (Geiser 1994; Turbill et al. 2003) and energy budgeting indicates that this effect may be sufficiently great that the species concerned only need to make minor increases in food intake during lactation to achieve an overall energy balance. For example, the mean dry food consumption of non-reproductive brown long-eared bats averaged 1.8 g/day (48 kJ d−1) while lactating bats ate 2.0 g each day (53 kJ), yet the bats in lactation were able to export 22 kJ d−1 of this intake as milk (Mclean & Speakman 1999) while deriving only on average 1.2 kJ from stored fat. Physiological compensation of energy budgets in this manner, so that food intake requirements are unaltered, may clearly have profound effects on the ecological costs of reproduction. Brown long-eared bats in the wild do not increase their flight times between pregnancy and lactation (Entwistle et al. 1996), a pattern repeated in other species such as the common pipistrelle (Pipistrellus pipistrellus (=pygmaeus); Swift 1980) and long-tailed bats (Chalinolobus tuberculatus; O'Donnell 2002). If predation risk during reproduction is a simple function of time spent flying, then for these animals there may be no ecological cost at all. This pattern however may be peculiar to bats under particular energy stress (e.g. Turbill et al. 2003) or at the margins of their distributions (Speakman et al. 1991), since studies of other bat species more centrally in their distributions yield different patterns. Big brown bats (Eptesicus fuscus) and little brown bats (Myotis lucifugus), for example, have increased food intake during lactation (Kurta et al. 1989, 1990) and a decreased tendency to enter torpor during lactation (Audet & Fenton 1988; Hamilton & Barclay 1994; Grinevitch et al. 1995; Lausen & Barclay 2003). Flight time in northern bats in Sweden is almost doubled in lactation compared with non-breeding individuals (Rydell 1993).
The fact that not all bats use torpor during lactation suggests that there are significant costs associated with its use. One possibility is that torpor is incompatible with milk synthesis. Studies using explants of mammary tissue from bats confirm that they have no special protection from reduced milk synthesis as temperature declines compared with explants of rodent mammary tissue (Wilde et al. 1999). Using torpor in lactation by bats at the margins of their distributions may be a necessity forced on them by the short duration of the night which constrains their capacity to extend flight times.
In spite of the energetic benefits and widespread use of torpor by bats in lactation, some groups that are otherwise capable of torpor appear to avoid using it during lactation. For example, longitudinal records of body temperature in free-ranging female short-beaked echidna (Tachyglossus aculeatus) indicate that they defend a continuous but low body temperature throughout the summer unless they lose their offspring (Nicol et al. 2005). Although many species of rodent are known to hibernate and use daily torpor, no species of rodent has ever been reported to use torpor during lactation. Whether torpor and lactation are mutually incompatible in rodents was addressed by Stamper et al. (1998) who administered 2-deoxy-D-glucose (2-DG), a glucose analogue that interferes with cellular glycolysis, to lactating and non-lactating Siberian hamsters. 2-DG induced torpor in both groups, although the duration of torpor tended to be shorter in lactating animals. Evidence suggested that pups were able to obtain milk from torpid mothers, but whether any milk synthesis occurred was uncertain. Although apparently physiologically capable of entering torpor females that were subjected either to a combination of brief food deprivation and food restriction or just food restriction failed to display torpor, but instead killed one or more of their pups (Stamper et al. 1998).
(ii) Physical activity
In a classic paper, Slonaker (1924) detailed the changes in physical activity that occurred in female rats during reproduction. Plots of some of the meticulously reported data are shown in figure 5. These data show that rats have similar patterns of food intake to mice during pregnancy and lactation, and that wheel-running activity is dramatically suppressed during lactation to approximately one-third to one-half of that in pregnancy. The suppression of activity however was not proportional to litter size—since rats with small litters (n<6 offspring) ran about the same amount as those with large litters (n>6 offspring) (figure 5). Mice (MF1) also show similar reductions in spontaneous physical activity during lactation (Speakman et al. 2001). Rabbits also increase the time spent resting as lactation progresses (Fernandez-Carmona et al. 2005).
Figure 5.
(a) Food intake and (b) wheel-running activity of rats during pregnancy and lactation. Days are expressed relative to the day of parturition (day =0) also indicated by the dotted line. Data are split between those raising large (n>6; open squares) and those raising small (n<6; filled squares) litters. Note how food intake in the larger litters increases dramatically a few days before weaning probably due to intake by the offspring (plots from data presented in tables 2 and 3 of Slonaker 1924).
Equivalent data on physical activity for non-domestic animals are scarce. This is probably because most activity in wild animals concerns foraging activity, which is generally increased during lactation owing to the elevated food intake requirements (see above). In domestic animals, physical activity and food intake are not inescapably linked, so modulations of activity without affecting food intake are feasible. However, animals engage in other activities that are not associated with foraging, e.g. grooming. We have monitored the behaviour of bats during lactation and found that brown long-eared bats (Plecotus auritus) significantly reduce the amount of time spent grooming when compared with non-breeding females occupying the same roost. On average non-lactating bats were observed grooming in 26.9% of the behaviour records, while lactating bats were only observed grooming in 11.1% of records. Similar high levels of time spent grooming in non-lactating bats have been observed in some other species (Shen & Lee 2000; Fleming et al. 1998). However, such high levels are not universal (Winchell & Kunz 1996) and reductions in grooming during lactation are also not observed in these species (Winchell & Kunz 1996). Female long-eared bats groom their offspring during lactation but this time allocation was relatively small and even when this was taken into account, the lactating females we studied still spent less than half of the time spent grooming by non-breeding individuals (McLean & Speakman 1997). Grooming behaviour by bats is energetically expensive (Giorgi et al. 2001) and plays a role in the removal of ectoparasites. By reducing the time spent grooming, lactating bats may release significant amounts of energy for lactation, but may pay a price in terms of elevated ectoparasite burden. In the wild, in the mouse-eared bat (Myotis myotis), ectoparasite burden increases during pregnancy and lactation (Christe et al. 2000) when it is also related to body mass—suggesting a role for energy balance in driving ectoparasite burden. However, potential interactions here are complex. Neuhaus (2003) experimentally removed ectoparasites (mainly fleas) from female Columbian ground squirrels using a commercially available insecticide. Removing parasites led to an increase in female body condition during lactation and at weaning and an increase in weaned litter size. Hence, removing ectoparasites may be costly, but not removing them may impose other costs (see also Khokhlova et al. 2002).
(iii) Immune system
The adaptive interplay between immune function and reproduction has been extensively studied in birds (Cichoń et al. 2001; Martin et al. 2008). Relatively little attention has been paid to this trade-off in small mammals. There is some evidence that particularly energetically costly phases of reproduction in small mammals lead to impaired immune function. This effect can be magnified if the reproductive event coincides with a period of food shortage. If rats are experimentally malnourished, for example, during lactation there is a marked increase in the numbers of binucleate lymphocytes in the maternal spleen (Ortiz et al. 1995), increasing from 11.5% of cells in non-lactating controls to 21.5% in the malnourished lactating animals. Since the spleen is an important lymphopoetic organ in rodents, this direct cell damage during energy restriction in lactation may have important negative impacts on the mother’s immune system. Direct evidence of this impact of restriction was the experimental observation that malnourished rats in lactation had an impaired response to cholera toxin (Flo´ et al. 1994).
Even during normal pregnancy, there are significant effects on the immune system. Committed B-lymphocyte precursors in the bone marrow of pregnant mice decline by 6.5 days into the pregnancy and by parturition they are down to only 10% of control levels (Medina et al. 1993). Newly formed B cells were also reduced, but cells carrying myeloid and erythroid markers were not. This indicates the production and export of B cells during pregnancy is much reduced. These effects could be mimicked by oestrogen treatment—suggesting an endocrine sex steroid-based mechanism of control (Medina et al. 1993).
However, the reasons for this effect are unclear, because the energy demands of maintaining the immune system are generally regarded to be trivial (Derting & Compton 2003). One possibility is that the reduction in body fatness at peak lactation itself mediates reduced immunity, since studies in voles and hamsters have shown that surgical removal of body fat decreases immune capability (Demas et al. 2003; Demas 2004). A mechanism for this effect might involve changes in circulating levels of leptin, as it has direct effects on immune cells stimulating T-cell immunity, phagocytosis, cytokine production and haemopoiesis, resulting in attenuated susceptibility to infectious insults (Ingvartsen & Boisclair 2001). In that case, the immunocompetence cost of reproduction may not be a compensatory cost but an obligatory consequential cost of the reduced fat content, which may itself be part of the mechanism of endocrine control of lactational food intake or manipulation of heat dissipation limits by effects on UCPs (see above).
(b) Obligatory consequential costs
(iii) Hyperthermia
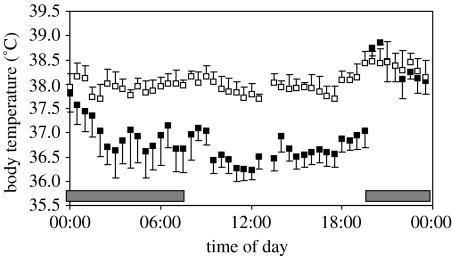
It has been known for at least 50 years that high ambient temperatures and humidity, or exposure to solar radiation, all negatively affect milk production in dairy cows (Bos taurus; Cobble & Herman 1951; Brody et al. 1958). Similar negative effects of high ambient temperatures have been reported in other large domestic animals such as pigs (Sus scrofa: Black et al. 1993; Quiniou & Noblet 1999; Renaudeau & Noblet 2001; Renaudeau et al. 2003) and sheep (Ovis aries: Abdalla et al. 1993). Many of these larger animals also exhibit chronic hyperthermia during lactation (e.g. sows, Ulmershakibaei & Plonait 1992). The risk of hyperthermia in lactation therefore appears to be a significant obligatory cost in larger mammals. Heat dissipation difficulties found in large domestic animals may be far less significant in small mammals owing to their more favourable surface-to-volume ratios. Nevertheless, direct measurements of maternal body temperatures (figure 6) confirm that lactating mice are continuously hotter than their non-reproducing equivalents. Similar effects have been observed in rats (Croskerry et al. 1978; Leon et al. 1978, 1985; Kittrell & Satinoff 1988) and Siberian hamsters Phodopus sungorus (Scribner & Wynne Edwards 1994a). Rabbits also show negative effects of high temperatures on food intake and pup growth (Marai et al. 2001).
Figure 6.
Body temperatures of a single female MF1 mouse measured at 1-minute intervals using an implanted body temperature transmitter (e-mitter Minimitter Inc) for 7 days prior to breeding and for 7 days at peak lactation. Data are screened to include only times when the animals are at rest, and then averaged over 30-minute periods within each day. The error bars refer to the s.d. for day-to-day variability (n=7 for each data set). During lactation, the body temperature is consistently elevated approximately 1.5°C higher than that prior to breeding during the light phase and much of the dark phase. Dark bar indicates period when lights out (J. R. Speakman, K. Drerrer, F. J. Munro & C. T. Troup 2005, unpublished data).
Studies of blood flow during lactation indicate that there is no major redirection of blood away from the mammary glands to facilitate heat loss (cattle, Lough et al. 1990; goats Capra hircus, Sano et al. 1985; rabbits, Lublin & Wolfenson 1996). There is a substantial body of behavioural literature following the pioneering work of Leon et al. (1978), which suggests that suckling behaviour of small mammals may be influenced by the risks of maternal hyperthermia. These studies included observations that rats terminated suckling bouts when their body temperatures rose. Similar effects have been reported in larger lactating animals that have contact with their offspring such as sows (Renaudeau & Noblet 2001), where elevated temperature led to reduced durations and greater frequencies of suckling bouts coupled with lowered milk production and piglet growth. Perhaps the best evidence comes from direct experimental manipulations of body temperature and examination of the subsequent effects on suckling behaviour. These experiments have only been performed on rats, but have involved two separate manipulations. Direct heating of the preoptic area of the brain, which led to premature termination of suckling bouts (Woodside & Leon 1980), and injection of rats with morphine elevates body temperature and causes disruption of maternal suckling behaviour (Bridges & Grimm 1982). However, rats are an order of magnitude larger than mice, so perhaps of greater relevance are the studies of Siberian hamsters, which show that during the daytime, the time spent with the litter in late lactation may be constrained by ambient temperature (Scribner & Wynne Edwards 1994b). Interestingly, the two subspecies of Siberian hamster (P. sungorus sungorus and P. sungorus campbelli) differ in the rates at which the offspring develop their own thermoregulatory capacities—faster in P. s. campbelli (Newkirk et al. 1998). This difference is reflected in greater and earlier problems in maintaining body temperature during suckling bouts by female P. s. campbelli (Scribner & Wynne Edwards 1994b) and consequent negative effects on pup growth in this subspecies (Newkirk et al. 1998).
Despite a large literature indicating that there are direct effects of the litter on maternal hyperthermia, there are some contradictory data. For example, the body temperatures at which rats discontinue suckling are generally lower than the levels they tolerate while exercising outside the nest (Kittrell & Satinoff 1988). Perhaps more important are experimental inductions of reduced body temperature using sodium salicylate, which did not extend suckling bouts (Bates et al. 1985). Although treatment of lactating rats with morphine simultaneously elevates body temperature and disrupts maternal suckling (above), the negative effect of morphine appears to be independent of its effects on body temperature. This is shown by experiments in which morphine was administered with naloxone, an opioid receptor antagonist. Blocking morphine with naloxone reversed the negative effects on maternal behaviour (Bridges & Grimm 1982) but not maternal hyperthermia (Cox et al. 1976). Rats with experimentally induced increases in body temperature using morphine and naloxone combinations (Stern & Azzara 2002) did not shorten their suckling bouts. However, this latter manipulation was performed only 7 days into lactation, and it would be instructive to know whether a similar absence of the effects of combined morphine/naloxone treatment was apparent later in lactation when pup heat stress is more profound. Finally, rats fed low-quality protein in their diets did not show reduced performance when raised at 30°C compared with 20°C (Jansen & Binard 1991), even though their litters probably generated similar heat dissipation problems. Overall, there is a definite negative effect of ambient temperature on lactation performance of large animals, but the significance of these effects in smaller animals like mice remains uncertain.
(iv) Disruption of sleep patterns
Anyone who has had a child of their own is aware that one of the key features of sharing ones life with a small neonate is the disruption of adult sleep patterns to accommodate suckling demands of the offspring (Shinkoda et al. 1999). Only approximately 23% of infants sleep through entire nights (defined as from 0000 to 0500 h) by eight weeks of age (Pinilla & Birch 1993). Breast feeding mothers exhibit stronger delta and theta power spectra during NREM sleep (Nishihara et al. 2004), but this pattern is the same on all nights whether sleep is disrupted or not to attend to their offspring, suggesting that there is an association between the sleep patterns and the hormonal changes in lactation. This is supported by observations that slow wave sleep duration is approximately three times longer in females who breast feed compared with age-matched and body condition-matched individuals who bottle fed their offspring (Blyton et al. 2002). Effects of disruption of sleep patterns as a physiological cost of lactation in animals has been virtually unstudied. In black tufted ear marmosets (Callithrix kuhlii), the presence of young infants significantly impacted the sleep of mothers who were awake more than three times as often as mothers without infants (Fite et al. 2003). Surprisingly, there was no impact on the sleep patterns of fathers—despite the fact that callitrichid mothers relinquish the care of their offspring to fathers and alloparents very early in infant life. The impact of the disruption of sleep on lactating female animals is unknown.
(v) Bone loss
During pregnancy and lactation, the female animal must deliver sufficient calcium to her offspring to ensure adequate bone development. During pregnancy, females increase their absorption of calcium in the alimentary tract (Halloran & DeLuca 1980). Although urinary losses also increase (Omi & Ezawa 2001), the elevated absorption leads to elevated calcium levels in the blood (hypercalciuria), which persist throughout reproduction (Sarli et al. 2005). In the first trimester of pregnancy, this extra uptake may actually exceed the foetal growth requirements. Consequently, there is a phase of bone accretion in the earliest part of pregnancy (Zeni et al. 1999; Omi & Ezawa 2001), which is characterized by increases in markers of bone turnover (like osteocalcin and alkaline phosphatase levels; Sengupta et al. 2005). This bone accretion appears to act as a temporary storage of calcium that can be withdrawn as the pregnancy proceeds and the foetal demands for calcium increase. By the end of pregnancy, most studies report significantly reduced bone mineral densities relative to controls, although Leopold et al. (1995) indicated that levels were still elevated at day 20 in rats.
In lactation, the requirements for calcium are much greater, in line with the much larger offspring (as is also the case for energy and protein requirements). Lactating mothers reduce their levels of urinary calcium excretion (Sarli et al. 2005) to facilitate secretion into the milk. Although food intake in lactation is greatly elevated, lactating animals derive a large amount of the calcium that is exported in milk from their own skeletons. This leads to a substantial reduction in the thickness of bones from across the whole body (Miller & Bowman 2004). An additional direct effect of the calcium withdrawal from bone is a reduction in dentine and enamel mineralization in the teeth of lactating rats (Ozbek et al. 2004). On the face of it, this use of skeletal calcium to provide the requirements for pup development may appear to indicate a shortfall in the available calcium from the diet, relative to the energy intake. However, three factors suggest that this is not the case. First, simple budgets of calcium availability relative to how much appears in the offspring (see above) indicate that it should not be limiting on most unsupplemented diets. Second, supplementing the dietary intake with calcium has no impact on the extent of calcium withdrawal from bone (Weisstaub et al. 2003; Kovacs & Fuleihan 2006). Finally, in rats the extent of withdrawal is independent of litter size (at least comparing two to six pups), and hence neonatal calcium requirements (Sengupta et al. 2005), although Tojo et al. (1998) did suggest that litter size correlated with the extent of withdrawal when data for non-lactating animals (litter size=0) were included in the regression. In spite of this, the diet supplementation data strongly suggest that the loss of calcium from bone is not driven by a shortfall in dietary intake.
Although supplementing calcium in the diet does not impact on bone resorption, providing less dietary calcium increases the rate of bone turnover (Gruber & Stover 1994). This is indicated indirectly by increases in the markers of turnover (Sengupta et al. 2005) and also directly from labelling studies. For example, by pre-labelling bone tissue of lactating female rats with 45Ca, it was shown that under a calcium-deficient diet, 40% of the label was lost from the whole skeleton over the course of a normal pregnancy and lactation cycle—with losses in some bones like the femur being 75% (Wang et al. 1994). Under situations where the diet naturally contains low calcium levels (e.g. fruit and insects), lactating and pregnant individuals may seek out other sources to keep their calcium supply high (e.g. fruit bats, Ruby et al. 2000, Nelson et al. 2005 and insectivorous bats, Adams et al. 2003).
A primary hypothesis therefore is that the endocrine mechanisms that promote the secretion of calcium into the milk are incompatible with the retention of calcium in bone. Several lines of evidence support this hypothesis. First, prolactin, which is one of the key hormones necessary to support successful milk production in the lactating female, and the prolactin receptor, are also found in the synovial fluid (Ogueta et al. 2002). Studies of the impact of prolactin on mesenchymal stem cells strongly implicate it as playing a role in bone and cartilage formation and repair processes. Prolactin receptor KO mice (Kelly et al. 2001) have greatly elevated prolactin concentrations and reduced bone formation. Prolactin secretion to support lactation may therefore influence bone resorption.
Ovariectomized rats also lose bone mass (Miller & Wronski 1993). Consequently, absence of oestrogen may be a contributory factor in lactation (Miller & Bowman 1998). This is supported by observations that oestrogen treatment of ovariectomized lactating rats improved bone retention in lactation by 15% (Garner et al. 1991), but the mechanism here is confused because these mothers also ate less and their pups grew less—so presumably other hormones related to lactation (e.g. prolactin) were also at lower levels. Another hormone in addition to prolactin that appears important in the supply of calcium to the foetuses is the parathyroid hormone-related peptide (PTHrP; Yamamoto et al. 1991). PTHrP has strong sequence similarities to parathyroid hormone (PTH) and both seem to interact with parathyroid hormone receptors located on osteoclasts, which they inhibit. PTHrP increases during late pregnancy and remains high throughout lactation (Sarli et al. 2005) where it is produced in mammary epithelial cells. This promotes maternal bone resorption allowing calcium release for export in the milk. Production of mammary PTHrP appears to be sensitive to extracellular calcium levels acting through the calcium-sensing receptor. Involvement of PTH itself in the calcium withdrawal process seems unlikely since parathyroidectomy had no impact on the extent of bone resorption in lactation (Hodnett et al. 1992).
In addition to hormones that support milk production having impacts on bone, there is also evidence of actions working in the reverse direction. For example, the osteoprotegerin ligand (OPGL) is a known osteoclast differentiation and activation factor that is essential for successful bone remodelling. Transgenic mice lacking OPGL (or its receptor) fail to develop lobular alveolar structures in their mammary glands resulting in an inability to secrete milk successfully. This effect can be reversed by recombinant OPGL treatment (Fata et al. 2000). Mammary and bone function therefore appears to be intimately associated and this association, which may not necessarily be functional, leads to the obligatory bone loss during lactation. Following weaning, there is a rapid increase in osteoblast proliferation and cancellous bone deposition (Miller et al. 2005). This osteoprogenitor pool may be primed during the late phase of lactation for this post-weaning activity. Peak bone formation in rats occurs approximately four weeks after the end of lactation (Miller & Bowman 2004), restoring the bone lost during the lactation event (Nishiwaki et al. 1999; Bowman et al. 2002) and its strength (Vajda et al. 2001).
The physiological mechanism of lactation therefore exposes the lactating female to an elevated risk of osteoporosis, which may extend for at least twice the duration of the actual lactation event before levels are normalized. The direct effect of this calcium withdrawal from bone is a reduction in bone strength and this increases the risks of bone fracture. Direct measurements of bone physical properties support this interpretation. By the end of pregnancy, rat tibiae were not significantly different in their strength from controls, but by the end of lactation, bone strength was substantially reduced in both cortical and cancellous bone, paralleled by the decline in bone volume (Vajda et al. 2001).
There are no direct measures of the increased risk of fracture during lactation in small mammals. However, in humans, there are reports of case studies where lactation-induced osteoporosis did lead to limb fracture (Clemetson et al. 2004) suggesting that its effects may be significant. Paradoxically repeated bouts of lactation appear to have a protective effect in humans on later life risks of fracture attached to osteoporosis (Hoffman et al. 1993; Jacobsen et al. 1998; Michaelsson et al. 2001; Petersen et al. 2002; Naves et al. 2005). This suggests that in the post-weaning period regrowth of bone is overcompensated. There is some direct evidence to support this suggestion (Akesson et al. 2004).
(vi) Oxidative stress
Animals during lactation have high rates of metabolism, which may result in elevated generation of free radicals. These free radicals may cause oxidative damage to macromolecules if the mice do not simultaneously upregulate their oxidative defence and repair mechanisms. Wiersma et al. (2004) showed that birds feeding nestlings, which is the most energetically expensive phase in avian reproduction, do not upregulate the levels of superoxide dismutase, catalase and glutathione peroxidase, the main defences against radical oxygen species. However, that study did not include measures of oxidative damage—so the lack of elevation of defence mechanisms may have been because there was no increase in free radical production to defend against. Indeed, the links between radical oxygen species production and metabolic rate are not straightforward (Speakman et al. 2002; Speakman 2003), and in some cases increased metabolism may actually reduce oxidative stress (Speakman et al. 2004). However, the reduction in levels of UCP-1 and UCP-3 in lactation (above) would suggest that mitochondria in lactating animals are more closely coupled—supporting the suggestion that this may be a time of elevated oxidative stress (Demin et al. 1998a,b; Brand 2000; Speakman 2003). Studies of antioxidant protection levels and damage during reproduction are needed.
4. Conclusion
The physiological costs of reproduction are probably the most significant component underlying life-history trade-offs. In this review, I have summarized some of the work that has been performed to measure physiological costs in small mammals. Physiological costs can be divided into two different aspects. The first is direct costs of the reproductive event. These include the energy and nutrient costs of reproduction and the changes in organ morphology necessary to meet these demands. Considerable progress in elucidating the limits on energy budgets in small domesticated rodents have been made in the last 15 years or so. However, even in this area of intensive activity, we are still far short of being able to translate this knowledge into an understanding of a simple life-history trade-off between the number and size of offspring. Knowledge of these demands among non-domesticated species is very sparse, physiological information gleaned in a wild context is virtually non-existent. To these direct costs must be added indirect costs of reproduction. These include optional compensatory adjustments that animals make to divert more resource into reproduction. Such compensations include reductions in thermoregulation, physical activity and immunocompetence. In some species, these adjustments can be sufficient that total energy requirements during reproduction are no greater than experienced by non-breeding animals. This may annul any ecological impact of reproduction mediated, for example, via increased predation risk due to extra time spent foraging. Their long-term impacts have however never been explored. To these compensatory costs must be added obligatory costs, which are an inevitable side effect of reproduction that cannot be avoided. For small mammals these include hyperthermia, bone loss, disruption of sleep patterns and oxidative stress. Knowledge of all these effects is presently rudimentary. Indeed, whether some changes are optional or obligatory (like immunocompetence changes) is uncertain, and the list of costs presented here is unlikely to be complete.
Acknowledgments
Work performed by my own group described here was performed under UK Home Office regulations following ethical review and complied with local ethical guidelines for animal experiments.
This paper was generated as part of the fitness function of food intake project funded by the Scottish Executive Environment and Rural Affairs Department (SEERAD).
The work on MF1 mice described in this paper was supported by BBSRC and particularly included work by Ela Krol and Maria Johnson. Many undergraduate and postgraduate students have also contributed to this work. I am grateful to Ela Krol for her comments on the earlier drafts of this paper.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Abdalla E.B, Kotby E.A, Johnson H.D. Physiological responses to heat-induced hyperthermia of pregnant and lactating ewes. Small Ruminant Res. 1993;11:125–134. doi:10.1016/0921-4488(93)90145-8 [Google Scholar]
- Adams R.A, Pedersen S.C, Thibault K.M, Jadin J, Petru B. Calcium as a limiting resource to insectivorous bats: can water holes provide a supplemental mineral source? J. Zool. 2003;260:189–194. doi:10.1017/S0952836903003613 [Google Scholar]
- Akesson A, Vahter M, Berglund M, Eklof T, Bremme K, Bjellerup P. Bone turnover from early pregnancy to postweaning. Acta Obstet. Gyn. Scan. 2004;83:1049–1055. doi: 10.1111/j.0001-6349.2004.00428.x. doi:10.1111/j.0001-6349.2004.00428.x [DOI] [PubMed] [Google Scholar]
- Agius L, Williamson D.H. Lipogenesis in interscapular brown adipose tissue of virgin, pregnant and lactating rats. The effects of intragastric feeding. Biochem. J. 1980;190:477–480. doi: 10.1042/bj1900477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atramentowicz M. Optimal litter size—does it cost more to raise a large litter in Caluromys philander? Can. J. Zool. 1992;70:1511–1515. [Google Scholar]
- Audet D, Fenton M.B. Heterothermy and the use of torpor by the bat Eptesicus fuscus (Chiroptera: Vespertilionidae): a field study. Physiol. Zool. 1988;61:197–204. [Google Scholar]
- Barclay R.M.R. Constraints on reproduction by flying vertebrates—energy and calcium. Am. Nat. 1994;144:1021–1031. doi:10.1086/285723 [Google Scholar]
- Bates A, Adels L, Leon M. Thermal control of maternal contact bouts: the interbout interval. Physiol. Behav. 1985;34:835–837. doi: 10.1016/0031-9384(85)90386-5. doi:10.1016/0031-9384(85)90386-5 [DOI] [PubMed] [Google Scholar]
- Biggerstaff S, Mann M. Consummatory behaviors and weight regulation in pregnant, lactating, and pregnant-lactating mice. Physiol. Behav. 1992;52:485–491. doi: 10.1016/0031-9384(92)90335-y. doi:10.1016/0031-9384(92)90335-Y [DOI] [PubMed] [Google Scholar]
- Black J.L, Mullan B.P, Lorschy M.L, Giles L.R. Lactation in the sow during heat-stress. Livest. Prod. Sci. 1993;35:153–170. doi:10.1016/0301-6226(93)90188-N [Google Scholar]
- Blyton D.M, Sullivan C.E, Edwards N. Lactation is associated with an increase in slow-wave sleep in women. J. Sleep Res. 2002;11:297–303. doi: 10.1046/j.1365-2869.2002.00315.x. doi:10.1046/j.1365-2869.2002.00315.x [DOI] [PubMed] [Google Scholar]
- Bowman B.M, Siska C.C, Miller S.C. Greatly increased cancellous bone formation with rapid improvements in bone structure in the rat maternal skeleton after lactation. J. Bone Mineral Res. 2002;17:1954–1960. doi: 10.1359/jbmr.2002.17.11.1954. doi:10.1359/jbmr.2002.17.11.1954 [DOI] [PubMed] [Google Scholar]
- Boyne R, Fell B.F, Robb I. The surface area of the intestinal mucosa in the lactating rat. J. Physiol. 1966;183:570–576. doi: 10.1113/jphysiol.1966.sp007884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M.D. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp. Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. doi:10.1016/S0531-5565(00)00135-2 [DOI] [PubMed] [Google Scholar]
- Bridges R.S, Grimm C.T. Reversal of morphine disruption of maternal behavior by concurrent treatment with the opiate antagonist naloxone. Science. 1982;218:166–168. doi: 10.1126/science.7123227. doi:10.1126/science.7123227 [DOI] [PubMed] [Google Scholar]
- Brody S, Ragsdale A.C, Yeck R.G, Worstell D. Environmental physiology and shelter engineering with special reference to domestic animals XXXIII. Milk production, feed and water consumption and body weight of Jersey and Holstein cows in relation to several diurnal temperature rhythms. Mo AES Res. Bull. 1958;1958:578. [Google Scholar]
- Burdett K, Reek C. Adaptation of the small intestine during pregnancy and lactation in the rat. Biochem. J. 1979;184:245–251. doi: 10.1042/bj1840245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas S, et al. The basal proton conductance of skeletal muscle mitochondria from transgenic mice overexpressing or lacking uncoupling protein-3. J. Biol. Chem. 2002;277:2773–2778. doi: 10.1074/jbc.M109736200. doi:10.1074/jbc.M109736200 [DOI] [PubMed] [Google Scholar]
- Cairnie A.B, Bentley R.E. Cell proliferation studies in the intestinal epithelium of the rat. Hyperplasia during lactation. Exper. Cell Res. 1967;46:428–440. doi: 10.1016/0014-4827(67)90079-1. doi:10.1016/0014-4827(67)90079-1 [DOI] [PubMed] [Google Scholar]
- Camas R, Romero V.J, Baldwin R.L. Maintenance energy requirements during lactation in rats. J. Nutr. 1982;112:1876–1880. doi: 10.1093/jn/112.10.1876. [DOI] [PubMed] [Google Scholar]
- Campbell R.M.S, Fell B.F. Gastro-intestinal hypertrophy in the lactating rat in relation to its food intake. J. Physiol. 1964;171:90–97. doi: 10.1113/jphysiol.1964.sp007363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. doi:10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- Cavaleros M, Buffenstein R, Ross F.P, Pettifor J.M. Vitamin D metabolism in a frugivorous nocturnal mammal, the Egyptian fruit bat (Rousettus aegyptiacus) Gen. Comp. Endocrinol. 2003;133:109–117. doi: 10.1016/s0016-6480(03)00150-3. doi:10.1016/S0016-6480(03)00150-3 [DOI] [PubMed] [Google Scholar]
- Charlesworth B.C. 2nd edn. Cambridge University Press; Cambridge, UK: 1994. Evolution in age-structured populations. [Google Scholar]
- Charnov E.L. Oxford University Press; Oxford, UK: 1993. Life history invariants: some explorations of symmetry in evolutionary ecology. [Google Scholar]
- Christe P, Arlettaz R, Vogel P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis) Ecol. Lett. 2000;3:207–212. doi:10.1046/j.1461-0248.2000.00142.x [Google Scholar]
- Cichoń M, Dubiec A, Chadzińska M. The effect of elevated reproductive effort on humoral immune function in collared flycatcher females. Acta Oecol. 2001;22:71–76. doi:10.1016/S1146-609X(00)01094-8 [Google Scholar]
- Clapham J.C, et al. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–418. doi: 10.1038/35019082. doi:10.1038/35019082 [DOI] [PubMed] [Google Scholar]
- Clemetson I.A, Popp A, Lippuner K, Ballmer F, Anderson S.E. Postpartum osteoporosis associated with proximal tibial stress fracture. Skeletal Radiol. 2004;33:96–98. doi: 10.1007/s00256-003-0721-2. doi:10.1007/s00256-003-0721-2 [DOI] [PubMed] [Google Scholar]
- Cobble J.W, Herman H.A. The influence of environmental temperature on the composition of milk of the dairy cow. Mo AES Res. Bull. 1951:485. [Google Scholar]
- Cox B, Ary M, Chesarkek W, Lomax P. Morphine hyperthermia in the rat: an action on the central thermostats. Eur. J. Pharmacol. 1976;36:33–39. doi: 10.1016/0014-2999(76)90253-3. doi:10.1016/0014-2999(76)90253-3 [DOI] [PubMed] [Google Scholar]
- Craft I.L. The influence of pregnancy and lactation on the morphology and absorptive capacity of the rat small intestine. Clin. Sci. 1970;38:287–295. doi: 10.1042/cs0380287. [DOI] [PubMed] [Google Scholar]
- Cripps A.W, Williams V.J. The effect of pregnancy and lactation on food intake, gastrointestinal anatomy and the absorptive capacity of the small intestine of the albino rat. Br. J. Nutr. 1975;33:17–32. doi: 10.1079/bjn19750005. [DOI] [PubMed] [Google Scholar]
- Croskerry P.G, Smith G.K, Leon M. Thermoregulation and the maternal behaviour of the rat. Nature. 1978;273:299–300. doi: 10.1038/273299a0. doi:10.1038/273299a0 [DOI] [PubMed] [Google Scholar]
- Datta U.K, Datta A.N, Mukherjee S. Role of hyperphagia in structural changes of small intestine during lactation. Indian J. Physiol. Pharmacol. 1995;39:259–262. [PubMed] [Google Scholar]
- Degen A.A, Khokhlova I.S, Kam M, Snider I. Energy requirements during reproduction in female common spiny mice (Acomys cahirinus) J. Mammal. 2002;83:645–651. doi:10.1644/1545-1542(2002)083<0645:ERDRIF>2.0.CO;2 [Google Scholar]
- Degen A.A, Khokhlova I.S, Kam M, Snider I. Water budget during reproduction in female common spiny mice (Acomys cahirinus) J. Mammal. 2004;85:1106–1110. doi:10.1644/BRG-211.1 [Google Scholar]
- Demas G.E. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Horm. Behav. 2004;45:173–180. doi: 10.1016/j.yhbeh.2003.11.002. doi:10.1016/j.yhbeh.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Demas G.E, Drazen D.L, Nelson R.J. Reductions in total body fat decrease humoral immunity. Proc. R. Soc. B. 2003;270:905–911. doi: 10.1098/rspb.2003.2341. doi:10.1098/rspb.2003.2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demin O.V, Kholodenko B.N, Skulachev V.P. A model of O2 generation in the complex III of the electron transport chain. Mol. Cell. Biochem. 1998a;184:21–33. doi:10.1023/A:1006849920918 [PubMed] [Google Scholar]
- Demin O.V, Westerhoff H.V, Kholodenko B.N. Mathematical modelling of superoxide generation with the bc(1) complex of mitochondria. Biochemistry (Mosc) 1998b;63:634–649. [PubMed] [Google Scholar]
- Denis R.G, Williams G, Vernon R.G. Regulation of serum leptin and its role in the hyperphagia of lactation in the rat. J. Endocrinol. 2003;176:193–203. doi: 10.1677/joe.0.1760193. doi:10.1677/joe.0.1760193 [DOI] [PubMed] [Google Scholar]
- Derting T.L, Compton S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus) Physiol. Biochem. Zool. 2003;76:744–752. doi: 10.1086/375662. doi:10.1086/375662 [DOI] [PubMed] [Google Scholar]
- Dryden G.L, Gebczynski M, Douglas E.L. Oxygen consumption by nursling and adult musk shrews. Acta Theriol. 1974;19:453–461. [Google Scholar]
- Drummond H, Vazquez E, Sanchez-Colon S, Martinez-Gomez M, Hudson R. Competition for milk in the domestic rabbit: survivors benefit from littermate deaths. Ethology. 2000;106:511–526. doi:10.1046/j.1439-0310.2000.00554.x [Google Scholar]
- Dugas M.C, Hazelwood R.L, Lawrence A.L. Influence of pregnancy and/or exercise on intestinal transport of amino acids in rats. Proc. Soc. Exptl Biol. Med. 1970;135:127–128. doi: 10.3181/00379727-135-35003. [DOI] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson E.M, Guerra C, Yamashita H, Harper M.E, Kozak L.P. Mice lacking mitochondrial uncoupling protein are cold- sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. doi:10.1038/387090a0 [DOI] [PubMed] [Google Scholar]
- Entwistle A.C, Racey P.A, Speakman J.R. Habitat exploitation by a gleaning bat, Plecotus auritus. Phil. Trans. R. Soc. B. 1996;351:921–931. doi:10.1098/rstb.1996.0085 [Google Scholar]
- Erlanson-Albertsson C. Uncoupling proteins—a new family of proteins with unknown function. Nutr. Neurosci. 2002;5:1–11. doi: 10.1080/10284150290007038. doi:10.1080/10284150290007038 [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C. The role of uncoupling proteins in the regulation of metabolism. Acta Physiol. Scand. 2003;178:405–412. doi: 10.1046/j.1365-201X.2003.01159.x. doi:10.1046/j.1365-201X.2003.01159.x [DOI] [PubMed] [Google Scholar]
- Fata J.E, et al. The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell. 2000;103:41–50. doi: 10.1016/s0092-8674(00)00103-3. doi:10.1016/S0092-8674(00)00103-3 [DOI] [PubMed] [Google Scholar]
- Fell B.F, Smith A, Campbell R.M. Hypertrophic and hyperplastic changes in the alimentary canal of the lactating rat. J. Pathol. Bact. 1963;85:179–188. doi: 10.1002/path.1700850117. doi:10.1002/path.1700850117 [DOI] [PubMed] [Google Scholar]
- Fernandez-Carmona J, et al. The behaviour of farm rabbit does around parturition and during lactation. World Rabbit Sci. 2005;13:253–277. [Google Scholar]
- Field 2nd J, Belding H.S, Martin A.W. An analysis of the relation between basal metabolism and summated tissue respiration in the rat. I. The post-pubertal albino rat. J. Cell. Comp. Physiol. 1939;14:143–157. doi:10.1002/jcp.1030140202 [Google Scholar]
- Fink R, Tauson A.H, Hansen K.B, Wamberg S, Kristensen N.B. Energy intake and milk production in mink (Mustela vison)—effect of litter size. Arch. Anim. Nutr. 2001;55:221–242. doi: 10.1080/17450390109386194. [DOI] [PubMed] [Google Scholar]
- Fisher R.A. Clarendon Press; Oxford, UK: 1930. The genetical theory of natural selection. [Google Scholar]
- Fite J.E, French J.A, Patera K.J, Hopkins E.C, Rukstalis M, Jensen H.A, Ross C.N. Nighttime wakefulness associated with infant rearing in Callithrix kuhlii. Int. J. Primatol. 2003;24:1267–1280. doi:10.1023/B:IJOP.0000005992.72026.e6 [Google Scholar]
- Fleming T.H, Nelson A.A, Dalton V.M. Roosting behavior of the lesser long-nosed bat, Leptonycteris curasoae. J. Mammal. 1998;79:147–155. doi:10.2307/1382849 [Google Scholar]
- Flo´ J, Elías F, Massouh E, Roux M.E. Impairment of B and T cell maturation in gut associated lymphoid tissues due to malnutrition during lactation. Devel. Comparat. Immunol. 1994;18:543–555. doi: 10.1016/s0145-305x(06)80008-x. doi:10.1016/S0145-305X(06)80008-X [DOI] [PubMed] [Google Scholar]
- Franks P.W, Brage S, Luan J, Ekelund U, Rahman M, Farooqi I.S, Halsall I, O'Rahilly S, Wareham N.J. Leptin predicts a worsening of the features of the metabolic syndrome independently of obesity. Obes. Res. 2005;13:1476–1484. doi: 10.1038/oby.2005.178. [DOI] [PubMed] [Google Scholar]
- Gandelman R, Simon N.G. Spontaneous pup-killing by mice in response to large litters. Dev. Psychobiol. 1978;11:235–241. doi: 10.1002/dev.420110307. doi:10.1002/dev.420110307 [DOI] [PubMed] [Google Scholar]
- Garner S.C, Anderson J.J.B, Mar M.-H, Parikh I. Estrogens reduce bone loss in the ovariectomized, lactating rat model. Bone Mineral. 1991;15:19–31. doi: 10.1016/0169-6009(91)90108-c. doi:10.1016/0169-6009(91)90108-C [DOI] [PubMed] [Google Scholar]
- Garton D.W, Hsu M.J, Harder J.D. Environmental temperature and metabolic rates during gestation and lactation in golden-hamsters (Mesocricetus auratus) Physiol. Zool. 1994;67:497–514. [Google Scholar]
- Gebczynska Z, Gebczynski M. Length and weight of the alimentary tract of the root vole. Acta Theriol. 1971;26:359–369. [Google Scholar]
- Geiser F. Hibernation and daily torpor in marsupials—a review. Aust. J. Zool. 1994;42:1–16. doi:10.1071/ZO9940001 [Google Scholar]
- Geiser F, Masters P. Torpor in relation to reproduction in the mulgara, Dasycercus cristicauda (Dasyuridae: Marsupialia) J. Therm. Biol. 1994;19:33–40. doi:10.1016/0306-4565(94)90007-8 [Google Scholar]
- Geiser F, McAllan B.M, Brigham R. Daily torpor in a pregnant dunnart (Sminthopsis macroura Dasyuridae: Marsupialia) Mamm. Biol. 2005;70:117–121. doi:10.1016/j.mambio.2004.06.003 [Google Scholar]
- Genoud M, Vogel P. Energy-requirements during reproduction and reproductive effort in shrews (Soricidae) J. Zool. 1990;220:41–60. [Google Scholar]
- Giorgi M.S, Arlettaz R, Christe P, Vogel P. The energetic grooming costs imposed by a parasitic mite (Spinturnix myoti) upon its bat host (Myotis myotis) Proc. R. Soc. B. 2001;268:2071–2075. doi: 10.1098/rspb.2001.1686. doi:10.1098/rspb.2001.1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt M, Villarroya F, Mampel T, Iglesias R. Impaired basal and noradrenaline induced isodothyrionine 5′—deiodinase activity in brown adipose tissue form pregnant and lactating rats. Biochem. Biophys. Res. Commun. 1986;138:1315–1321. doi: 10.1016/s0006-291x(86)80426-0. doi:10.1016/S0006-291X(86)80426-0 [DOI] [PubMed] [Google Scholar]
- Glazier D.S. Energetics of litter size in five species of peromyscus with generalisations for other mammals. J. Mamm. 1985;66:629–642. doi:10.2307/1380789 [Google Scholar]
- Goettsch M. Comparative protein requirement of the rat and mouse for growth, reproduction and lactation using casein diets. J. Nutr. 1960;70:307–312. [Google Scholar]
- Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:U327–U340. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- Grinevitch L, Holroyd S.L, Barclay R.M.R. Sex-differences in the use of daily torpor and foraging time by big brown bats (Eptesicus fuscus) during the reproductive season. J. Zool. 1995;235:301–309. [Google Scholar]
- Gruber H.E, Stover S.J. Maternal and weanling bone—the influence of lowered calcium intake and maternal dietary history. Bone. 1994;15:167–176. doi: 10.1016/8756-3282(94)90704-8. doi:10.1016/8756-3282(94)90704-8 [DOI] [PubMed] [Google Scholar]
- Hacklander K, Tataruch F, Ruf T. The effect of dietary fat content on lactation energetics in the European hare (Lepus europaeus) Physiol. Biochem. Zool. 2002;75:19–28. doi: 10.1086/324770. doi:10.1086/324770 [DOI] [PubMed] [Google Scholar]
- Halloran B.P, DeLuca H.F. Calcium transport in small intestine during pregnancy and lactation. Am. J. Physiol. 1980;239:E64–E68. doi: 10.1152/ajpendo.1980.239.1.E64. [DOI] [PubMed] [Google Scholar]
- Hamilton I.M, Barclay R.M.R. Patterns of daily torpor and day-roost selection by male and female big brown bats (Eptesicus fuscus) Can. J. Zool. 1994;72:744–749. [Google Scholar]
- Hammond K.A. Adaptation of the maternal intestine during lactation. J. Mammary Gland Biol. Neoplasia. 1997;2:243–252. doi: 10.1023/a:1026332304435. doi:10.1023/A:1026332304435 [DOI] [PubMed] [Google Scholar]
- Hammond K.A, Diamond J. An experimental test for a ceiling on sustained metabolic rate in lactating mice. Physiol. Zool. 1992;65:952–977. [Google Scholar]
- Hammond K.A, Diamond J. Limits to dietary nutrient uptake and intestinal nutrient uptake in lactating mice. Physiol. Zool. 1994;67:282–303. [Google Scholar]
- Hammond K.A, Diamond J. Maximal sustained energy budgets in humans and animals. Nature. 1997;386:457–462. doi: 10.1038/386457a0. doi:10.1038/386457a0 [DOI] [PubMed] [Google Scholar]
- Hammond K.A, Kristan D.M. Responses to lactation and cold exposure by deer mice (Peromyscus maniculatus) Physiol. Biochem. Zool. 2000;73:547–556. doi: 10.1086/317757. doi:10.1086/317757 [DOI] [PubMed] [Google Scholar]
- Hammond K.A, Konarzewski M, Torres R.M, Diamond J. Metabolic ceilings under a combination of peak energy demands. Physiol. Zool. 1994;67:1479–1506. [Google Scholar]
- Hammond K.A, Lloyd K.C.K, Diamond J. Is mammary output capacity limiting to lactational performance in mice? J. Exp. Biol. 1996;199:337–349. doi: 10.1242/jeb.199.2.337. [DOI] [PubMed] [Google Scholar]
- Harder J.D, Hsu M.J, Garton D.W. Metabolic rates and body temperature of the gray short-tailed opossum (Monodelphis domestica) during gestation and lactation. Physiol. Zool. 1996;69:317–339. [Google Scholar]
- Havera S.P. Energy and nutrient cost of lactation in fox squirrels. J. Wildlife Manage. 1979;43:958–965. doi:10.2307/3808281 [Google Scholar]
- Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Resp. Physiol. Neurobiol. 2004;141:317–329. doi: 10.1016/j.resp.2004.03.014. doi:10.1016/j.resp.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Hill R.W. The altricial/precocial contrast in the thermal relations and energetics of small mammals. In: Tomasi T.E, Horton T.H, editors. Mammalian energetics: interdiciplinary views of metabolism and reproduction. Cornell University Press; Ithaca, New York, NY: 1992. pp. 122–159. [Google Scholar]
- Hitchcock F.A. The total energy requirement of the albino rat for growth and activity. Am. J. Physiol. 1927;83:28–36. [Google Scholar]
- Hodnett D.W, Deluca H.F, Jorgensen N.A. Bone-mineral loss during lactation occurs in absence of parathyroid tissue. Am. J. Physiol. 1992;262:E230–E233. doi: 10.1152/ajpendo.1992.262.2.E230. [DOI] [PubMed] [Google Scholar]
- Hoffman S, Grisso J.A, Kelsey J.L, Gammon M.D, O'Brien L.A. Parity, lactation and hip fracture. Osteoporosis Int. 1993;3:171–176. doi: 10.1007/BF01623672. doi:10.1007/BF01623672 [DOI] [PubMed] [Google Scholar]
- Ingvartsen K.L, Boisclair Y.R. Leptin and the regulation of food intake, energy homeostasis and immunity with special focus on the periparturient ruminants. Domest. Anim. Endocrinol. 2001;21:215–250. doi: 10.1016/s0739-7240(02)00119-4. doi:10.1016/S0739-7240(02)00119-4 [DOI] [PubMed] [Google Scholar]
- Innes D.G, Millar J.S. Body weight, litter size and energetics of reproduction in Clethrionomys gapperi and Microtus pennsylvanicus. Can. J. Zool. 1981;59:785–789. [Google Scholar]
- Isler D, Trayhurn P, Lunn P.G. Brown adipose-tissue metabolism in lactating rats—the effect of litter size. Ann. Nutr. Metab. 1984;28:101–109. doi: 10.1159/000176789. [DOI] [PubMed] [Google Scholar]
- Jacobsen B.K, Nilssen S, Heuch I, Kvale G. Reproductive factors and fatal hip fractures. A Norwegian prospective study of 63 000 women. J. Epidemiol. Commun. Health. 1998;52:645–650. doi: 10.1136/jech.52.10.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G.R, Binard R. Effect of dietary protein and environmental factors on lactation performance in rats. Physiol. Behav. 1991;50:297–304. doi: 10.1016/0031-9384(91)90069-z. doi:10.1016/0031-9384(91)90069-Z [DOI] [PubMed] [Google Scholar]
- Johnson M.S, Speakman J.R. Limits to sustained energy intake V. Effect of cold exposure during lactation in Mus musculus. J. Exp. Biol. 2001;204:1967–1977. doi: 10.1242/jeb.204.11.1967. [DOI] [PubMed] [Google Scholar]
- Johnson M.S, Thomson S.C, Speakman J.R. Limits to sustained energy intake I. Lactation in the laboratory mouse Mus musculus. J. Exp. Biol. 2001a;204:1925–1935. doi: 10.1242/jeb.204.11.1925. [DOI] [PubMed] [Google Scholar]
- Johnson M.S, Thomson S.C, Speakman J.R. Limits to sustained energy intake III. Effects of concurrent pregnancy and lactation in Mus musculus. J. Exp. Biol. 2001b;204:1947–1956. doi: 10.1242/jeb.204.11.1947. [DOI] [PubMed] [Google Scholar]
- Johnson M.S, Thomson S.C, Speakman J.R. Limits to sustained energy intake II. Inter-relationships between resting metabolic rate, life-history traits and morphology in Mus musculus. J. Exp. Biol. 2001c;204:1937–1946. doi: 10.1242/jeb.204.11.1937. [DOI] [PubMed] [Google Scholar]
- Johnston S.L, et al. Having it all: historical energy intakes do not generate the anticipated trade-offs in fecundity. Proc. R. Soc. B. 2006;273:1369–1374. doi: 10.1098/rspb.2005.3456. doi:10.1098/rspb.2005.3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S.L, Souter D.M, Erwin S.S, Tolkamp B.J, Yearsley J.M, Gordon I.J, Illius A.W, Kyriazakis I, Speakman J.R. Associations between basal metabolic rate and reproductive performance in C57BL/6J mice. J. Exp. Biol. 2007;210:65–74. doi: 10.1242/jeb.02625. doi:10.1242/jeb.02625 [DOI] [PubMed] [Google Scholar]
- Jolicoeur L, Asselin J, Morisset J. Trophic effects of gestation and lactation on rat pancreas. Biomed. Res. 1980;1:482–488. [Google Scholar]
- Kaczmarski F. Bioenergetics of pregnancy and lactation in the bank vole. Acta Theriol. XI. 1966;19:409–417. [Google Scholar]
- Kam M, Cohen-Gross S, Khokhlova I.S, Degen A.A, Geffen E. Average daily metabolic rate, reproduction and energy allocation during lactation in the Sundevall jird Meriones crassus. Funct. Ecol. 2003;17:496–503. doi:10.1046/j.1365-2435.2003.00754.x [Google Scholar]
- Kam M, Khokhlova I.S, Degen A.A. Partitioning of metabolizable energy intake in sucking altricial and precocial rodent pups. J. Zool. 2006;269:502–505. doi:10.1111/j.1469-7998.2006.00088.x [Google Scholar]
- Kelly P.A, Binart N, Freemark M, Lucas B, Goffin V, Bouchard B. Prolactin receptor signal transduction pathways and actions determined in prolactin receptor knockout mice. Biochem. Soc. Trans. 2001;29:48–52. doi: 10.1042/0300-5127:0290048. doi:10.1042/BST0290048 [DOI] [PubMed] [Google Scholar]
- Kennedy G.C, Pearce K.M, Parrott D.M.V. Liver growth in the lactating rat. J. Endocrinol. 1958;17:158–160. doi: 10.1677/joe.0.0170158. [DOI] [PubMed] [Google Scholar]
- Khokhlova I.S, Krasnov B.R, Kam M, Burdelova N, Degen A.A. Energy cost of ectoparasitism: the flea Xenopsylla ramesis on the desert gerbil Gerbillus dasyurus. J. Zool. 2002;258:349–354. doi:10.1017/S0952836902001498 [Google Scholar]
- Kim E.M, Kotz C.M, Welch C.C, Grace M.K, Billington C.J, Levine A.S. Lactation decreases mRNA levels of opioid peptides in the arcuate nucleus of the rat. Brain Res. 1997;769:303–308. doi: 10.1016/s0006-8993(97)00722-1. doi:10.1016/S0006-8993(97)00722-1 [DOI] [PubMed] [Google Scholar]
- Kittrell E.M.W, Satinoff E. Diurnal rhythms of body temperature, drinking and activity over reproductive cycles. Physiol. Behav. 1988;42:477–484. doi: 10.1016/0031-9384(88)90180-1. doi:10.1016/0031-9384(88)90180-1 [DOI] [PubMed] [Google Scholar]
- Koiter T.R, Moes H, Valkhof N, Wijkstra S. Interaction of late pregnancy and lactation in rats. J. Reprod. Fertil. 1999;115:341–347. doi: 10.1530/jrf.0.1150341. doi:10.1530/jrf.0.1150341 [DOI] [PubMed] [Google Scholar]
- Korine C, Speakman J.R, Arad Z. Reproductive energetics of captive and free-ranging Egyptian fruit bats (Rousettus aegyptiacus) Ecology. 2004;85:220–230. doi:10.1890/02-0632 [Google Scholar]
- Kovacs C.S, Fuleihan G.E.-H. Calcium and bone disorders during pregnancy and lactation. Endocrinol. Metabol. Clinics North America. 2006;35:21–27. doi: 10.1016/j.ecl.2005.09.004. doi:10.1016/j.ecl.2005.09.004 [DOI] [PubMed] [Google Scholar]
- Krebs H.A. Body size and tissue respiration. Biochim. Biophys. Acta. 1950;4:249–269. doi: 10.1016/0006-3002(50)90032-1. doi:10.1016/0006-3002(50)90032-1 [DOI] [PubMed] [Google Scholar]
- Król E. Reproductive energy budgets of hedgehogs during lactation. Zesz. Nauk. Filii UW Biol. 1985;10:105–117. [Google Scholar]
- Król E, Speakman J.R. Limits to sustained energy intake VI. Energetics of lactation in laboratory mice at thermoneutrality. J. Exp. Biol. 2003a;206:4255–4266. doi: 10.1242/jeb.00674. doi:10.1242/jeb.00674 [DOI] [PubMed] [Google Scholar]
- Król E, Speakman J.R. Limits to sustained energy intake VII. Milk energy output in laboratory mice at thermoneutrality. J. Exp. Biol. 2003b;206:4267–4281. doi: 10.1242/jeb.00675. doi:10.1242/jeb.00675 [DOI] [PubMed] [Google Scholar]
- Król E, Johnson M, Speakman J.R. Limits to sustained energy intake VII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J. Exp. Biol. 2003a;206:4267–4281. doi: 10.1242/jeb.00676. doi:10.1242/jeb.00674 [DOI] [PubMed] [Google Scholar]
- Król E, Johnson M.S, Speakman J.R. Limits to sustained energy intake VIII. Resting metabolic rate and organ morphology of laboratory mice lactating at thermoneutrality. J. Exp. Biol. 2003b;206:4283–4291. doi: 10.1242/jeb.00676. doi:10.1242/jeb.00676 [DOI] [PubMed] [Google Scholar]
- Ku¨nkele J. Effects of litter size on the energetics of reproduction in a highly precocial rodent, the guinea pig. J. Mammal. 2000;81:691–700. doi:10.1644/1545-1542(2000)081<0691:EOLSOT>2.3.CO;2 [Google Scholar]
- Kunkele J, Trillmich F. Are precocial young cheaper? Lactation energetics in the guinea pig. Physiol. Zool. 1997;70:589–596. doi: 10.1086/515863. [DOI] [PubMed] [Google Scholar]
- Kunz T.H, Whitaker J.O, Wadanoli M.D. Dietary energetics of the insectivorous Mexican free-tailed bat (Tadarida brasiliensis) during pregnancy and lactation. Oecologia. 1995;101:407–415. doi: 10.1007/BF00329419. doi:10.1007/BF00329419 [DOI] [PubMed] [Google Scholar]
- Kurta A, Kunz T.H. Size of bats at birth and maternal investment during pregnancy. Symp. Zool. Soc. 1987;57:79–107. [Google Scholar]
- Kurta A, Bell G.P, Nagy K.A, Kunz T.H. Energetics of pregnancy and lactation in free-ranging little brown bats (Myotis lucifugus) Physiol. Zool. 1989;62:804–818. [Google Scholar]
- Kurta A, Kunz T.H, Nagy K.A. Energetics and water flux of free-ranging big brown bats (Eptesicus fuscus) during pregnancy and lactation. J. Mammal. 1990;71:59–65. doi:10.2307/1381316 [Google Scholar]
- Lamote I, Meyer E, Massart-Leen A.M, Burvenich C. Sex steroids and growth factors in the regulation of mammary gland proliferation, differentiation, and involution. Steroids. 2004;69:145–159. doi: 10.1016/j.steroids.2003.12.008. doi:10.1016/j.steroids.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Larradale J, Fernandez-Ortero P, Gonzalez M. Increased active transport of glucose through the intestine during pregnancy. Nature. 1966;209:1356–1357. doi: 10.1038/2091356b0. doi:10.1038/2091356b0 [DOI] [PubMed] [Google Scholar]
- Lausen C.L, Barclay R.M.R. Thermoregulation and roost selection by reproductive female big brown bats (Eptesicus fuscus) roosting in rock crevices. J. Zool. 2003;260:235–244. doi:10.1017/S0952836903003686 [Google Scholar]
- Leon M, Croskerry P.G, Smith G. Thermal control of mother–young contact in rats. Physiol. Behav. 1978;21:793–811. doi: 10.1016/0031-9384(78)90021-5. doi:10.1016/0031-9384(78)90021-5 [DOI] [PubMed] [Google Scholar]
- Leon M, Adels L, Coppersmith R. Thermal limitation of mother-young contact in Norway rats. Dev. Psychobiol. 1985;18:85–105. doi: 10.1002/dev.420180202. doi:10.1002/dev.420180202 [DOI] [PubMed] [Google Scholar]
- Leopold S.S, Boskey A.L, Doty S.B, Gertner J.M, Peterson M.G.E, Torzilli P.A. Diminished material properties and altered bone structure in rat femora during pregnancy. J. Orthopaed. Res. 1995;13:41–49. doi: 10.1002/jor.1100130108. doi:10.1002/jor.1100130108 [DOI] [PubMed] [Google Scholar]
- Liu H, Wang D.H, Wang Z.W. Energy requirements during reproduction in female Brandt's voles (Microtus brandtii) J. Mammal. 2003;84:1410–1416. doi:10.1644/BRG-030 [Google Scholar]
- Lough D.S, Beede D.L, Wilcox C.J. Effects of feed intake and thermal stress on mammary blood-flow and other physiological measurements in lactating dairy cows. J. Dairy Sci. 1990;73:325–332. doi: 10.3168/jds.S0022-0302(90)78677-8. [DOI] [PubMed] [Google Scholar]
- Lublin A, Wolfenson D. Lactation and pregnancy effects on blood flow to mammary and reproductive systems in heat-stressed rabbits. Comp. Biochem. Physiol. A. 1996;115:277–285. doi: 10.1016/s0300-9629(96)00060-6. doi:10.1016/S0300-9629(96)00060-6 [DOI] [PubMed] [Google Scholar]
- McNamara J.M, Houston A.I. Optimal annual routines: behaviour in the context of physiology and ecology. Phil. Trans. R. Soc. 2008;363:301–319. doi: 10.1098/rstb.2007.2141. doi:10.1098/rstb.2007.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marai I.F.M, Ayyat M.S, Abd El-Monem U.M. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop. Anim. Health Prod. 2001;33:451–462. doi: 10.1023/a:1012772311177. [DOI] [PubMed] [Google Scholar]
- Martin L.B, Weil Z.M, Nelson R.J. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil. Trans. R. Soc. B. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. doi:10.1098/rstb.2007.2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.D. Size, shape and evolution. In: Keynes M, editor. Evolutionary studies—a centenary celebration of the life of Julian Huxley. Eugenics Society; London, UK: 1989. pp. 96–141. [Google Scholar]
- Martin R.D, MacLarnon A.M. Gestation period, neonatal size and maternal investment in placental mammals. Nature. 1985;313:220–223. doi:10.1038/313220a0 [Google Scholar]
- Mattingly D.K, McClure P.A. Energetics of reproduction in large littered cotton rats (Sigmodon hispidus) Ecology. 1982;63:183–195. doi:10.2307/1937043 [Google Scholar]
- Mclean J.A, Speakman J.R. Non-nutritional maternal support in the brown long-eared bat. Anim. Behav. 1997;54:1193–1204. doi: 10.1006/anbe.1997.0498. [DOI] [PubMed] [Google Scholar]
- Mclean J.A, Speakman J.R. Energy budgets of lactating and non-reproductive brown long-eared bats (Plecotus auritus) suggest females use compensation in lactation. Funct. Ecol. 1999;13:360–372. doi:10.1046/j.1365-2435.1999.00321.x [Google Scholar]
- McLean J.A, Speakman J.R. Effects of body mass and reproduction on the basal metabolic rate of brown long-eared bats (Plecotus auritus) Physiol. Biochem. Zool. 2000;73:112–121. doi: 10.1086/316715. doi:10.1086/316715 [DOI] [PubMed] [Google Scholar]
- Medina K.L, Smithson G, Kincade P.W. Supression of B lymphopoiesis during normal pregnancy. J. Exp. Med. 1993;178:1507–1515. doi: 10.1084/jem.178.5.1507. doi:10.1084/jem.178.5.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelsson K, Baron J.A, Farahmand B.Y, Ljunghall S. Influence of parity and lactation on hip fracture risk. Am. J. Epidemiol. 2001;153:1166–1172. doi: 10.1093/aje/153.12.1166. doi:10.1093/aje/153.12.1166 [DOI] [PubMed] [Google Scholar]
- Migula P. Bioenergetics of pregnancy and lactation in the common vole. Acta Theriol. 1969;13:167–179. [Google Scholar]
- Millar J.S. Energetics of reproduction in Peromyscus leucopus: the cost of lactation. Ecology. 1978;59:1055–1061. doi:10.2307/1938558 [Google Scholar]
- Millar J.S. Life cycle characteristics of Peromyscus maniculatus nebrascensis. Can. J. Zool. 1985;63:1280–1284. [Google Scholar]
- Millar J.S, Innes D.G. Breeding by Peromyscus maniculatus over an elevational gradient. Can. J. Zool. 1985;63:124–129. [Google Scholar]
- Miller S.C, Wronski T.J. Long-term osteopenic changes in cancellous bone structure in ovariectomized rats. Anat. Rec. 1993;236:433–441. doi: 10.1002/ar.1092360303. doi:10.1002/ar.1092360303 [DOI] [PubMed] [Google Scholar]
- Miller S.C, Bowman B.M. Rapid improvements in cortical bone dynamics and structure after lactation in established breeder rats. Anat. Rec. A—Disc. Molec. Cell. Evol. Biol. 2004;276A:143–149. doi: 10.1002/ar.a.10138. doi:10.1002/ar.a.10138 [DOI] [PubMed] [Google Scholar]
- Miller S.C, Anderson B.L, Bowman B.M. Weaning initiates a rapid and powerful anabolic phase in the rat maternal skeleton. Biol. Reprod. 2005;73:156–162. doi: 10.1095/biolreprod.105.039610. doi:10.1095/biolreprod.105.039610 [DOI] [PubMed] [Google Scholar]
- Myrcha A. Variations in the length and weight of the alimentary tract of Clethrionomys glareolus (Schreber, 1780) Acta Theriol. IX. 1964;10:139–148. [Google Scholar]
- Myrcha A. Length and weight of the alimentary tract of Apodemus flavicollis. Acta Theriol. 1965;10:225–228. [Google Scholar]
- Naves M, Diaz-Lopez J.B, Gomez C, Rodriguez-Rebollar A, Cannata-Andia J.B. Determinants of incidence of osteoporotic fractures in the female Spanish population older than 50. Osteoporosis Int. 2005;16:2013–2017. doi: 10.1007/s00198-005-1983-4. doi:10.1007/s00198-005-1983-4 [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Golozoubova V, Matthias A, Shabalina I, Ohba K.I, Ohlson K, Jacobsson A, Cannon B. Life without UCPI: mitochondrial, cellular and organismal characteristics of the UCP1-ablated mice. Biochem. Soc. Trans. 2001;29:756–763. doi: 10.1042/0300-5127:0290756. doi:10.1042/BST0290756 [DOI] [PubMed] [Google Scholar]
- Nelson S.L, Kunz T.H, Humphrey S.R. Folivory in fruit bats: leaves provide a natural source of calicum. J. Chem. Ecol. 2005;31:1683–1691. doi: 10.1007/s10886-005-5920-y. doi:10.1007/s10886-005-5920-y [DOI] [PubMed] [Google Scholar]
- Neuhaus P. Parasite removal and its impact on litter size and body condition in Columbian ground squirrels (Spermophilus columbianus) Proc. R. Soc. B. 2003;270(Suppl.):S213–S215. doi: 10.1098/rsbl.2003.0073. doi:10.1098/rsbl.2003.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk K.D, Cheung B.L.W, Scribner S.J, Wynne-Edwards K.E. Earlier thermoregulation and consequences for pup growth in the Siberian versus Djungarian dwarf hamster (Phodopus) Physiol. Behav. 1998;63:435–443. doi: 10.1016/s0031-9384(97)00465-4. doi:10.1016/S0031-9384(97)00465-4 [DOI] [PubMed] [Google Scholar]
- Nicol, S., Vedel-Smith, C. & Andersen, N. A. 2005 Behaviour, body temperature and hibernation in Tasmanian echidnas (Tachyglossus aculeatus). In Life in the cold. Evolution, mechanisms, adaptation and application. Twelfth international hibernation symposium, (eds B. M. Barnes & H. V. Carey). Biological papers of the University of Alaska, number 27. Fairbanks, Alaska USA, Institute of Arctic Biology, University of Alaska.
- Nievergelt C.M, Martin R.D. Energy intake during reproduction in captive common marmosets (Callithrix jacchus) Physiol. Behav. 1999;65:849–854. doi: 10.1016/s0031-9384(98)00249-2. doi:10.1016/S0031-9384(98)00249-2 [DOI] [PubMed] [Google Scholar]
- Nishihara K, Horiuchi S, Eto H, Uchida S, Honda M. Delta and theta power spectra of night sleep EEG are higher in breast-feeding mothers than in non-pregnant women. Neurosci. Lett. 2004;368:216–2202. doi: 10.1016/j.neulet.2004.07.021. doi:10.1016/j.neulet.2004.07.021 [DOI] [PubMed] [Google Scholar]
- Nishiwaki M, Yasumizu T, Hoshi K, Ushijima H. Effect of pregnancy, lactation and weaning on bone mineral density in rats as determined by dual-energy X-ray absorptiometry. Endocr. J. 1999;46:711–716. doi: 10.1507/endocrj.46.711. [DOI] [PubMed] [Google Scholar]
- Nizielski S.E, Billington C.J, Levine A.S. Bat thermogenic activity and capacity are reduced during lactation in ground squirrels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993;264:R16–R21. doi: 10.1152/ajpregu.1993.264.1.R16. [DOI] [PubMed] [Google Scholar]
- Noel M.B, Woodside B. Effects of systemic and central prolactin injections on food intake, weight gain, and estrous cyclicity in female rats. Physiol. Behav. 1993;54:151–154. doi: 10.1016/0031-9384(93)90057-m. doi:10.1016/0031-9384(93)90057-M [DOI] [PubMed] [Google Scholar]
- O'Donnell C.F.J. Influence of sex and reproductive status on nocturnal activity of long-tailed bats (Chalinolobus tuberculatus) J. Mammal. 2002;83:794–803. doi:10.1644/1545-1542(2002)083<0794:IOSARS>2.0.CO;2 [Google Scholar]
- Oftedal O.T. Body size and reproductive strategy as correlates of milk energy output in lactating mammals. Acta Zool. Fenn. 1984;171:183–186. [Google Scholar]
- Ogueta S, Muñoz J, Obregon E, Delgado-Baeza E, García-Ruiz J.P. Prolactin is a component of the human synovial liquid and modulates the growth and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol. Cell. Endocrinol. 2002;190:51–63. doi: 10.1016/s0303-7207(02)00013-8. doi:10.1016/S0303-7207(02)00013-8 [DOI] [PubMed] [Google Scholar]
- Omi N, Ezawa I. Change in calcium balance and bone mineral density during pregnancy in female rats. J. Nutr. Sci. Vitaminol. 2001;47:195–200. doi: 10.3177/jnsv.47.195. [DOI] [PubMed] [Google Scholar]
- Ortiz R, Cortés E, González C, Pérez L, Betancourt M. Micronucleus frequency in spleen lymphocytes from severely malnourished rats during lactation. Env. Mol. Mutagen. 1995;26:55–59. doi: 10.1002/em.2850260108. doi:10.1002/em.2850260108 [DOI] [PubMed] [Google Scholar]
- Ozbek M, Kanli A, Dural S, Sahin I, Gonen E, Tulunoglu I. Effects of pregnancy and lactation on the microhardness of rat incisor dentine and enamel. Arch. Oral Biol. 2004;49:607–612. doi: 10.1016/j.archoralbio.2004.02.003. doi:10.1016/j.archoralbio.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Pedraza N, Solanes G, Carmona M.C, Iglesias R, Vinas O, Mampel T, Vazquez M, Giralt M, Villarroya F. Impaired expression of the uncoupling protein-3 gene in skeletal muscle during lactation: Fibrates and troglitazone reverse lactation-induced downregulation of the uncoupling protein-3 gene. Diabetes. 2000;49:1224–1230. doi: 10.2337/diabetes.49.7.1224. doi:10.2337/diabetes.49.7.1224 [DOI] [PubMed] [Google Scholar]
- Pedraza N, Solanes G, Iglesias R, Vazquez M, Giralt M, Villarroya F. Differential regulation of expression of genes encoding uncoupling proteins 2 and 3 in brown adipose tissue during lactation in mice. Biochem. J. 2001;355:105–111. doi: 10.1042/0264-6021:3550105. doi:10.1042/0264-6021:3550105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrigo G. Breeding and feeding strategies in deer mice and house mice when females are challenged to work for their food. Anim. Behav. 1987;35:1298–1316. doi:10.1016/S0003-3472(87)80002-7 [Google Scholar]
- Petersen H.C, Jeune B, Vaupel J.W, Christensen K. Reproduction life history and hip fractures. Ann. Epidemiol. 2002;12:257–263. doi: 10.1016/s1047-2797(01)00275-7. doi:10.1016/S1047-2797(01)00275-7 [DOI] [PubMed] [Google Scholar]
- Peterson C.C, Nagy K.A, Diamond J. Sustained metabolic scope. Proc. Natl Acad. Sci. USA. 1990;87:2324–2328. doi: 10.1073/pnas.87.6.2324. doi:10.1073/pnas.87.6.2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto R.M.S, Ferrer M, Fe J.M, Rayo J.M, Tur J. Morphological adaptative changes of small intestinal tract regions due to pregnancy and lactation in rats. Ann. Nutr. Metab. 1994;38:295–300. doi: 10.1159/000177824. [DOI] [PubMed] [Google Scholar]
- Pinilla T, Birch L.L. Help me make it through the night—behavioral entrainment of breast-fed infants sleep patterns. Pediatrics. 1993;91:436–444. [PubMed] [Google Scholar]
- Poppitt S.D, Speakman J.R, Racey P.A. The energetics of reproduction in the common shrew (Sorex araneus)—a comparison of indirect calorimetry and the doubly labeled water method. Physiol. Zool. 1993;66:964–982. [Google Scholar]
- Poppitt S.D, Speakman J.R, Racey P.A. Energetics of reproduction in the lesser hedgehog tenrec, Echinops telfairi (Martin) Physiol. Zool. 1994;67:976–994. [Google Scholar]
- Quiniou N, Noblet J. Influence of high ambient temperatures on performance of multiparous lactating sows. J. Anim. Sci. 1999;77:2124–2134. doi: 10.2527/1999.7782124x. [DOI] [PubMed] [Google Scholar]
- Racey P.A. Environmental factors influencing the length of gestation in heterothermic bats. J. Reprod. Fertil. Supp. 1973;19:175–189. [PubMed] [Google Scholar]
- Racey P.A, Swift S.M. Variations in gestation length in a colony of pipistrelle bats (Pipistrellus pipistrellus) from year to year. J. Reprod. Fertil. 1981;61:123–129. doi: 10.1530/jrf.0.0610123. doi:10.1530/jrf.0.0610123 [DOI] [PubMed] [Google Scholar]
- Randolph P.A, Randolph J.C, Mattingly K, Foster M.M. Energy costs of reproduction in the cotton rat (Sigmodon hispidus) Ecology. 1977;58:31–45. doi:10.2307/1935106 [Google Scholar]
- Rayner V.C, Robinson I.C.A.F, Russell J.A. Chronic intracerebroventricular morphine and lactation in rats—dependence and tolerance in relation to oxytocin neurons. J. Physiol. 1988;396:319–347. doi: 10.1113/jphysiol.1988.sp016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau D, Noblet J. Effects of exposure to high ambient temperature and dietary protein level on sow milk production and performance of piglets. J. Anim. Sci. 2001;79:1540–1548. doi: 10.2527/2001.7961540x. [DOI] [PubMed] [Google Scholar]
- Renaudeau D, Noblet J, Dourmad J.Y. Effect of ambient temperature on mammary gland metabolism in lactating sows. J. Anim. Sci. 2003;81:217–231. doi: 10.2527/2003.811217x. [DOI] [PubMed] [Google Scholar]
- Reznick D.N, Bryga H, Endler J.A. Experimentally induced life history evolution in natural population. Nature. 1990;346:357–359. doi:10.1038/346357a0 [Google Scholar]
- Ricklefs R.E, Wikelski M. The physiology–life history nexus. Trends Ecol. Evol. 2002;17:462–468. doi:10.1016/S0169-5347(02)02578-8 [Google Scholar]
- Roff D.A. Chapman & Hall; New York, NY: 1992. The evolution of life histories: theory and analysis. [Google Scholar]
- Rogowitz G.L. Limits to milk flow and energy allocation during lactation in the hispod cotton rat (Sigmodon hispidus) Physiol. Zool. 1998;71:312–320. doi: 10.1086/515923. [DOI] [PubMed] [Google Scholar]
- Ruby J, Nathan P.T, Balasingh J, Kunz T.H. Chemical composition of fruits and leaves eaten by short-nosed fruit bat, Cynopterus sphinx. J. Chem. Ecol. 2000;26:2825–2841. doi:10.1023/A:1026446011693 [Google Scholar]
- Rydell J. Variation in foraging activity of an aerial insectivorous bat during reproduction. J. Mammal. 1993;74:503–509. doi:10.2307/1382411 [Google Scholar]
- Rydell J, Speakman J.R. Evolution of nocturnality in bats—potential competitors and predators during their early history. Biol. J. Linn. Soc. 1995;54:183–191. doi:10.1006/bijl.1995.0011 [Google Scholar]
- Sano H, Ambo K, Tsuda T. Blood glucose kinetics in whole body and mammary gland of lactating goats exposed to heat. J. Dairy Sci. 1985;68:2557–2564. doi: 10.3168/jds.S0022-0302(85)81137-1. [DOI] [PubMed] [Google Scholar]
- Sarli M, Hakim C, Rey P, Zanchetta J. Osteoporosis during pregnancy and lactation. Medicina—Buenos Aires. 2005;65:533–540. [PubMed] [Google Scholar]
- Scribner S.J, Wynne Edwards K.E. Disruption of body temperature and behavior rhythms during reproduction in dwarf hamster (Phodopus) Physiol. Behav. 1994a;55:361–369. doi: 10.1016/0031-9384(94)90147-3. doi:10.1016/0031-9384(94)90147-3 [DOI] [PubMed] [Google Scholar]
- Scribner S.J, Wynne Edwards K.E. Thermal constraints on maternal behavior during reproduction in dwarf hamsters (Phodopus) Physiol. Behav. 1994b;55:897–903. doi: 10.1016/0031-9384(94)90077-9. doi:10.1016/0031-9384(94)90077-9 [DOI] [PubMed] [Google Scholar]
- Sengupta S, Arshad M, Sharma S, Dubey M, Singh M.M. Attainment of peak bone mass and bone turnover rate in relation to estrous cycle, pregnancy and lactation in colony-bred Sprague–Dawley rats: suitability for studies on pathophysiology of bone and therapeutic measures for its management. J. Steroid Biochem. Mol. Biol. 2005;94:421–429. doi: 10.1016/j.jsbmb.2004.12.039. doi:10.1016/j.jsbmb.2004.12.039 [DOI] [PubMed] [Google Scholar]
- Shen H.-P, Lee L.-L. Mother–young interactions in a maternity colony of Myotis formosus. J. Mammol. 2000;81:726–733. doi:10.1644/1545-1542(2000)081<0726:MYIIAM>2.3.CO;2 [Google Scholar]
- Shinkoda H, Matsumoto K, Park Y.M. Changes in sleep–wake cycle during the period from late pregnancy to puerperium identified through the wrist actigraph and sleep logs. Psychiatry Clin. Neurosci. 1999;53:133–135. doi: 10.1046/j.1440-1819.1999.00518.x. doi:10.1046/j.1440-1819.1999.00518.x [DOI] [PubMed] [Google Scholar]
- Sikes R.S. Costs of lactation and optimal litter size in northern grasshopper mice (Onychomys leucogaster) J. Mammal. 1995;76:348–357. [Google Scholar]
- Slonaker J.R. The effect of copulation, pregnancy, pseudopregnancy and lactation on the voluntary activity and food consumption of the albino rat. Am. J. Physiol. 1924;71:362–394. [Google Scholar]
- Smith C.C, Fretwell S.D. The optimal balance between size and number of offspring. Am. Nat. 1974;108:499–506. doi:10.1086/282929 [Google Scholar]
- Speakman J.R. The function of daylight flying in British bats. J. Zool. 1990;220:101–113. [Google Scholar]
- Speakman J.R. Why do insectivorous bats in Britain not fly in daylight more frequently. Funct. Ecol. 1991a;5:518–524. doi:10.2307/2389634 [Google Scholar]
- Speakman J.R. The impact of predation by birds on bat populations in the British-isles. Mamm. Rev. 1991b;21:123–142. [Google Scholar]
- Speakman, J. R. 2003 Oxidative phosphorylation, mitochondrial proton cycling, free-radical production and aging. In Energy metabolism and lifespan determination (ed. M. P. Mattson). Elsevier series advances in cell aging and gerontology, pp. 35–68. Amsterdam, The Netherlands: Elsevier.
- Speakman J.R, McQueenie J. Limits to sustained metabolic rate: the link between food intake, basal metabolic rate, and morphology in reproducing mice, Mus musculus. Physiol. Zool. 1996;69:746–769. [Google Scholar]
- Speakman J.R, Krol E. Limits to sustained energy intake IX: a review of hypotheses. J. Comp. Physiol. B. 2005;175:375–394. doi: 10.1007/s00360-005-0013-3. doi:10.1007/s00360-005-0013-3 [DOI] [PubMed] [Google Scholar]
- Speakman J.R, Racey P.A, Catto C.M.C, Webb P.I, Swift S.M, Burnett A.M. Minimum summer population and densities of bats in N.E. Scotland near the northern borders of their distributions. J. Zool. 1991;225:327–345. [Google Scholar]
- Speakman J.R, Rydell J, Webb P.I, Hayes J.P, Hays G.C, Hulbert I.A.R, McDevitt R.M. Activity patterns of insectivorous bats and birds in northern Scandinavia (69 degrees N), during continuous midsummer daylight. Oikos. 2000;88:75–86. doi:10.1034/j.1600-0706.2000.880109.x [Google Scholar]
- Speakman J.R, Gidney A, Bett J, Mitchell I.P, Johnson M.S. Limits to sustained energy intake IV. Effect of variation in food quality on lactating mice Mus musculus. J. Exp. Biol. 2001;204:1957–1965. doi: 10.1242/jeb.204.11.1957. [DOI] [PubMed] [Google Scholar]
- Speakman J.R, Selman C, McLaren J.S, Harper E.J. Living fast, dying when? The link between aging and energetics. J. Nutr. 2002;132:1583S–1597S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- Speakman J.R, et al. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. doi:10.1111/j.1474-9728.2004.00097.x [DOI] [PubMed] [Google Scholar]
- Stamper J.L, Zucker I, Lewis D.A, Dark J. Torpor in lactating Siberian hamsters subjected to glucoprivation. Am. J. Physiol.—Reg. Int. Compar. Physiol. 1998;274:R46–R51. doi: 10.1152/ajpregu.1998.274.1.R46. [DOI] [PubMed] [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
- Stephenson P.J, Racey P.A. Reproductive energetics of the Tenrecidae (Mammalia: Insectivora). II. The shrew-tenrecs, Microgale spp. Physiol. Zool. 1993a;66:664–685. [Google Scholar]
- Stephenson P.J, Racey P.A. Reproductive energetics of the Tenrecidae (Mammalia: Insectivora). I. The large-eared tenrec, Geogale aurita. Physiol. Zool. 1993b;66:643–663. [Google Scholar]
- Stern J.M, Azzara A.V. Thermal control of mother–young contact revisited: Hyperthermic rats nurse normally. Physiol. Behav. 2002;77:11–18. doi: 10.1016/s0031-9384(02)00798-9. doi:10.1016/S0031-9384(02)00798-9 [DOI] [PubMed] [Google Scholar]
- Stocker C, et al. Modulation of susceptibility to weight gain and insulin resistance in low birthweight rats by treatment of their mothers with leptin during pregnancy and lactation. Int. J. Obesity. 2004;28:129–136. doi: 10.1038/sj.ijo.0802476. doi:10.1038/sj.ijo.0802476 [DOI] [PubMed] [Google Scholar]
- Swift S.M. Activity patterns of pipistrelle bats (Pipistrellus pipistrellus) in north-east scotland. J. Zool. 1980;190:285–295. [Google Scholar]
- Tojo Y, Kurabayashi T, Honda A, Yamamoto Y, Yahata T, Takakuwa K, Tanaka K. Bone structural and metabolic changes at the end of pregnancy and lactation in rats. Am. J. Obstetrics Gynecol. 1998;178:180–185. doi: 10.1016/s0002-9378(98)70649-0. doi:10.1016/S0002-9378(98)70649-0 [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Decreased capacity for non-shivering thermogenesis during lactation in mice. Pflügers Archiv. 1983;398:264–265. doi: 10.1007/BF00657164. doi:10.1007/BF00657164 [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Thermogenesis and the energetics of pregnancy and lactation. Can. J. Physiol. Pharmacol. 1989;67:370–375. doi: 10.1139/y89-060. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Jennings G. Functional atrophy of brown adipose-tissue during lactation in mice—effects of lactation and weaning on mitochondrial gdp binding and uncoupling protein. Biochem. J. 1987a;248:273–276. doi: 10.1042/bj2480273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Wusterman M.C. Sympathetic activity in brown adipose tissue during lactation in mice. Proc. Nutr. Soc. 1987b;46:A27. [Google Scholar]
- Trayhurn P, Jennings G. Non-shivering thermogenesis and the thermogenic capacity of brown fat in fasted/re-fed mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1988;254:R11–R16. doi: 10.1152/ajpregu.1988.254.1.R11. [DOI] [PubMed] [Google Scholar]
- Trayhurn P, Douglas J.B, McGuckin M.M. Brown adipose tissue thermogenesis is suppressed during lactation in mice. Nature. 1982;298:59–60. doi: 10.1038/298059a0. doi:10.1038/298059a0 [DOI] [PubMed] [Google Scholar]
- Turbill C, Geiser F. Thermal physiology of pregnant and lactating female and male long-eared bats, Nyctophilus geoffroyi and N. gouldi. J. Comp. Physiol. B. 2006;176:165–172. doi: 10.1007/s00360-005-0038-7. doi:10.1007/s00360-005-0038-7 [DOI] [PubMed] [Google Scholar]
- Turbill C, Kortner G, Geiser F. Natural use of heterothermy by a small, tree-roosting bat during Summer. Physiol. Biochem. Zool. 2003;76:868–876. doi: 10.1086/378915. doi:10.1086/378915 [DOI] [PubMed] [Google Scholar]
- Ulmershakibaei C, Plonait H. Studies of lactational hyperthermia in sows. Tierarztl. Umschau. 1992;47:605–611. [Google Scholar]
- Vajda E.G, Bowman B.M, Miller S.C. Cancellous and cortical bone mechanical properties and tissue dynamics during pregnancy, lactation, and postlactation in the rat. Biol. Reprod. 2001;65:689–695. doi: 10.1095/biolreprod65.3.689. doi:10.1095/biolreprod65.3.689 [DOI] [PubMed] [Google Scholar]
- Vernon R.G, Flint D.G. Control of fatty acid synthesis during lactation. Proc. Nutr. Soc. 1983;42:315–331. doi: 10.1079/pns19830035. doi:10.1079/PNS19830035 [DOI] [PubMed] [Google Scholar]
- Vernon R.L, Pond C.M. Adaptations of maternal adipose tissue to lactation. J. Mam. Gland Biol. Neoplasia. 1997;2:231–241. doi: 10.1023/a:1026380220364. doi:10.1023/A:1026380220364 [DOI] [PubMed] [Google Scholar]
- Wade G.N, Jennings G, Trayhurn P. Energy balance and brown adipose tissue thermogenesis during pregnancy in Syrian hamsters. Am. J. Physiol. 1986;250:R845–R850. doi: 10.1152/ajpregu.1986.250.5.R845. [DOI] [PubMed] [Google Scholar]
- Wang C.H, Brown S, Bhattacharyya M.H. Effect of cadmium on bone calcium and 45Ca in mouse dams on a calcium-deficient diet: evidence of Itai-Itai-like syndrome. Toxicol. Appl. Pharmacol. 1994;127:320–330. doi: 10.1006/taap.1994.1168. doi:10.1006/taap.1994.1168 [DOI] [PubMed] [Google Scholar]
- Weiner J. Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770) Symp. Zool. Soc. Lond. 1987;57:167–187. [Google Scholar]
- Weiner J. Physiological limits to sustainable energy budgets in birds and mammals: ecological implications. Trends Ecol. Evol. 1992;7:384–388. doi: 10.1016/0169-5347(92)90009-Z. doi:10.1016/0169-5347(92)90009-Z [DOI] [PubMed] [Google Scholar]
- Weisstaub A, de Ferrer P.R, Zeni S, de Portela M.L. Influence of low dietary calcium during pregnancy and lactation on zinc levels in maternal blood and bone in rats. J. Trace Elements Med. Biol. 2003;17:27–32. doi: 10.1016/s0946-672x(03)80042-1. doi:10.1016/S0946-672X(03)80042-1 [DOI] [PubMed] [Google Scholar]
- Wiersma P, Selman C, Speakman J.R, Verhulst S. Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. B. 2004;271:S360–S363. doi: 10.1098/rsbl.2004.0171. doi:10.1098/rsbl.2004.0171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde C.J, Knight C.R, Racey P.A. Influence of torpor on milk protein composition and secretion in lactating bats. J. Exp. Zool. 1999;284:35–41. doi: 10.1002/(sici)1097-010x(19990615)284:1<35::aid-jez6>3.0.co;2-z. doi:10.1002/(SICI)1097-010X(19990615)284:1<35::AID-JEZ6>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection. [Google Scholar]
- Winchell J.M, Kunz T.H. Day-roosting activity budgets of the Eastern pipistrelle bat, Pipistrellus subflavus (Chiroptera: Vespertilionidae) Can. J. Zool. 1996;74:431–441. [Google Scholar]
- Woodside B, Leon M. Thermoendocrine influences on maternal nesting behavior in rats. J. Comp. Physiol. Psychol. 1980;94:41–60. doi: 10.1037/h0077652. doi:10.1037/h0077652 [DOI] [PubMed] [Google Scholar]
- Xiao X.Q, Grove K.L, Grayson B.E, Smith M.S. Inhibition of uncoupling protein expression during lactation: Role of leptin. Endocrinology. 2004;145:830–838. doi: 10.1210/en.2003-0836. doi:10.1210/en.2003-0836 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, et al. Suckling-mediated increases in urinary phosphate and 3′,5′-cyclic adenosine-monophosphate excretion in lactating rats—possible systemic effects of parathyroid hormone-related protein. Endocrinology. 1991;129:2614–2622. doi: 10.1210/endo-129-5-2614. [DOI] [PubMed] [Google Scholar]
- Zeni S.N, Di Gregorio S, Mautalen C. Bone mass changes during pregnancy and lactation in the rat. Bone. 1999;25:681–685. doi: 10.1016/s8756-3282(99)00228-8. doi:10.1016/S8756-3282(99)00228-8 [DOI] [PubMed] [Google Scholar]
- Zenuto R.R, Antinuchi C.D, Busch C. Bioenergetics of reproduction and pup development in a subterranean rodent (Ctenomys talarum) Physiol. Biochem. Zool. 2002;75:469–478. doi: 10.1086/344739. doi:10.1086/344739 [DOI] [PubMed] [Google Scholar]
- Zera A.J, Harshman L.G. The physiology of life history trade-offs in animals. Ann. Rev. Ecol. Syst. 2001;32:95–126. doi:10.1146/annurev.ecolsys.32.081501.114006 [Google Scholar]