Abstract
Endogenous circannual clocks are found in many long-lived organisms, but are best studied in mammal and bird species. Circannual clocks are synchronized with the environment by changes in photoperiod, light intensity and possibly temperature and seasonal rainfall patterns. Annual timing mechanisms are presumed to have important ultimate functions in seasonally regulating reproduction, moult, hibernation, migration, body weight and fat deposition/stores. Birds that live in habitats where environmental cues such as photoperiod are poor predictors of seasons (e.g. equatorial residents, migrants to equatorial/tropical latitudes) rely more on their endogenous clocks than birds living in environments that show a tight correlation between photoperiod and seasonal events. Such population-specific/interspecific variation in reliance on endogenous clocks may indicate that annual timing mechanisms are adaptive. However, despite the apparent adaptive importance of circannual clocks, (i) what specific adaptive value they have in the wild and (ii) how they function are still largely untested. Whereas circadian clocks are hypothesized to be generated by molecular feedback loops, it has been suggested that circannual clocks are either based upon (i) a de-multiplication (‘counting’) of circadian days, (ii) a sequence of interdependent physiological states, or (iii) one or more endogenous oscillators, similar to circadian rhythms. We tested the de-multiplication of days (i) versus endogenous regulation hypotheses (ii) and (iii) in captive male and female house sparrows (Passer domesticus). We assessed the period of reproductive (testicular and follicular) cycles in four groups of birds kept either under photoperiods of LD 12 L : 12 D (period length: 24 h), 13.5 L : 13.5 D (27 h), 10.5 L : 10.5 D (23 h) or 12 D : 8 L : 3 D : 1 L (24-h skeleton photoperiod), respectively, for 15 months. Contrary to predictions from the de-multiplication hypothesis, individuals experiencing 27-h days did not differ (i.e. did not have longer) annual reproductive rhythms than individuals from the 21- or 24-h day groups. However, in line with predictions from endogenous regulation, birds in the skeleton group had significantly longer circannual period lengths than all other groups. Birds exposed to skeleton photoperiods experienced fewer light hours per year than all other groups (3285 versus 4380) and had a lower daily energy expenditure, as tested during one point of the annual cycle using respirometry. Although our results are tantalizing, they are still preliminary as birds were only studied over a period of 15 months. Nevertheless, the present data fail to support a ‘counting of circadian days’ and instead support hypotheses proposing whole-organism processes as the mechanistic basis for circannual rhythms. We propose a novel energy turnover hypothesis which predicts a dependence of the speed of the circannual clock on the overall energy expenditure of an organism.
Keywords: endogenous rhythms, frequency de-multiplication hypothesis, photoperiod, melatonin, circannual rhythms
1. Circannual rhythms: patterns and possible adaptive significance
Circannual rhythms are self-sustained endogenous rhythms with a period length of roughly 1 year that affect the morphology, physiology and behaviour of an organism. The first robust avian circannual rhythm was reported in migration behaviour and moult of the willow warbler (Phylloscopus trochilus) by Gwinner (1967, 1968). Gwinner was initially puzzled by the precise annual timing of fat deposition and the onset of spring migration of these birds in their equatorial wintering quarters. What cues would trigger a northward departure in a place where environmental variation, especially in terms of day length, is minimal over much of the year? When he took birds into captivity and kept them under a constant photoperiod and temperature, individuals showed persistent rhythms in migratory restlessness (zugunruhe) and moult for up to 3 years, suggesting that the timing of spring migration may be controlled by an endogenous circannual clock, rather than being triggered by external factors (Gwinner 1977). Since then, endogenous circannual rhythms have been experimentally demonstrated in a wide range of species, including more than 20 migratory and resident bird species from both tropical and temperate regions (e.g. Gwinner 1986; Gwinner & Dittami 1990; Holberton & Able 1992; Cadee et al. 1996; Styrsky et al. 2004; Newton 2007).
The most obvious overt expressions of circannual rhythms in vertebrates include seasonal changes in reproduction (Hamner & Stocking 1970; Gwinner & Dittami 1990), moult (Craig 1985), body mass, hibernation (Pengelley & Fisher 1963; Kondo et al. 2006) and migratory restlessness (Gwinner 1967, 1968, 1996a,b; Pohl 1971) under constant captive conditions. Circannual rhythms possess all properties expected of self-sustained, free-running oscillations, including persistence in isolation, a period length deviating from 12 months (usually shorter) under constant conditions, entrainment to synchronizing cues (zeitgebers) such as photoperiod, and transient changes in response to zeitgebers (Gwinner 1986). In general, circannual rhythms tend to be relatively weakly self-sustained, i.e. they typically show considerable inter-individual variation and can be entrained to a wide range of period lengths (Gwinner 1986; Goldmann et al. 2004; Helm 2006). Further, they are usually expressed only under a relatively narrow range of permissive day length conditions (Gwinner 1986; Lincoln et al. 2006). The circannual organization of animals shows great diversity. At one extreme are rhythms that persist for many years under constant light–dark schedules, constant dim light and in exceptional cases even under naturally changing day length (e.g. Holberton & Able 1992; Heideman & Bronson 1994; Loudon 1994; Gwinner 1996a,b; Kondo et al. 2006). At the other extreme are cycles that damp rapidly in amplitude or require a recurring seasonal signal for sustained rhythmicity. The observed variation has been classified into two types of annual rhythms, one being truly circannual (Type II) and another displaying a mix of endogenous and exogenous characteristics (Type I; Mrosovsky 1978; Nelson et al. 2001; Prendergast et al. 2002; Goldmann et al. 2004; Lincoln et al. 2006).
The diversity of circannual patterns parallels the great natural diversity in seasonal behaviours. Animals occupy habitats that are diverse with respect to the amplitude and the predictability of environmental fluctuations (e.g. Wingfield et al. 1992). Animals also differ in the geographical ranges they inhabit over the course of their lives. Accordingly, free-living vertebrates, and birds in particular, differ greatly in the timing and sequence of life-cycle stages (e.g. Nelson et al. 2001; Newton 2007).
(a) Interactions of circannual rhythms with environmental cues
Circannual rhythms need to function in interaction with the environment and may be best understood as ‘seasonally changing dispositions to respond to environmental cues’ (Gwinner 1999; Helm 2006). In free-living animals, circannual rhythms, like circadian rhythms, are normally entrained to temporal information provided by the environment (Loudon 1994; Gwinner 2003; Newton 2007; but see, e.g. Heideman & Bronson 1994). The most important temporal cue in the wild and zeitgeber for circannual rhythms is photoperiod, i.e. the light fraction of the day (e.g. Hahn & McDougall-Shackleton 2008). Environmental factors other than light may also influence seasonal timing. While there is only tentative evidence for non-photic cues as zeitgebers in the synchronization of circannual rhythms (Gwinner & Scheuerlein 1998; Scheuerlein & Gwinner 2002), factors like temperature can clearly affect circannual behaviour, notably in hibernating mammals (Mrosovsky 1977, 1978, 1986). In free-living animals, non-photic environmental factors are thought to function mainly as supplemental and modifying cues (Hamner & Stocking 1970; Mrosovsky 1986; Hahn et al. 1992, 1997; Hau et al. 2000, 2004; Helm et al. 2006). These cues provide fine adjustment of life-cycle stages to the particular ecological circumstances of an animal.
(b) Functional significance of circannual rhythms: intuitive, but untested in the wild
The fact that circannual rhythms are normally entrained to environmental information makes it hard to test directly for fitness benefits of relying upon an endogenous circannual rhythm in the wild (Gwinner 1996a,b). There seem to be only few examples of avian species that do not entrain their annual cycles to the external year (e.g. Chapin 1954). In a natural setting, manipulations of circannual rhythms are inherently difficult (Scheuerlein & Gwinner 2002; see also Gwinner & Scheuerlein 1999) and effects of endogenous rhythms cannot easily be separated from direct responses to the environment. Furthermore, it is difficult to monitor individual free-living animals over long periods of time (but see Cooke et al. 2004). In addition, output from circannual clocks can be obscured by inter-annual effects in birds (Marra et al. 1998; Webster et al. 2002; Buehler & Piersma 2008). Thus, the adaptive significance of circannual rhythms has so far only been indirectly tested by comparing endogenous rhythms in captivity with behaviour in the wild. The implicit rationale is that endogenous clocks help animals to keep time in the wild (e.g. Aschoff 1958; Gwinner 1986; DeCoursey 2004; Roenneberg et al. 2005; Helm 2006; Menaker 2006).
(c) Variations in circannual rhythms of populations or species with different life histories
(i) Latitudinal variation in circannual organization
Captive stonechats (Saxicola torquata) from equatorial Africa display persistent circannual cycles of moult and gonadal activation for several years. In contrast, stonechats from higher latitudes show repeated but irregular bouts of seasonal behaviours under constant conditions in the laboratory (Gwinner 1991, 1996a,b; Helm 2006). Circannual rhythms of zugunruhe in strongly migratory species, such as the willow warbler, persist for many cycles without major damping or loss of precision, while cycles of the sibling species, the chiffchaff (Phylloscopus collybita), a shorter-distance migrant, show greater inter-individual variability and a stronger tendency to damp (Gwinner 1967, 1972a). Furthermore, long-distance migrants that habitually experience a wide range of day length differ from other species by a greater range of conditions under which circannual rhythms are expressed. Sylviid warblers show persistent circannual cycles of zugunruhe over a wide day length range from 10 to 16 h. Their circannual clock is not temporarily arrested, as evidenced by a progressively faster response to photostimulation in late winter and spring (Gwinner et al. 1988). In contrast, the European starling (Sturnus vulgaris; Dawson et al. 2001) and the African stonechat (Gwinner 1996b) show circannual rhythms only under day lengths close to 12 h. Under longer or shorter photoperiods, the rhythms of these birds may become arrested in either active or regressed reproductive states (Gwinner 2003). In addition to these differences under constant conditions, birds from different latitudes differ in the way they respond to day length cues. Their responses can be characterized by population-specific reaction norms to photoperiod that differ in overall timing as well as plasticity to day length information (Silverin et al. 1993; Helm & Gwinner 1999, 2001, 2006; Gwinner & Helm 2003; Helm et al. 2005; Hahn & McDougall-Shackleton 2008).
(ii) Reproductive timing
Reproductive timing, thought to be under particularly strong natural selection, differs between species and even local populations. Characteristics of reproductive timing are often maintained under constant conditions. For instance, blackcap warblers (Sylvia atricapilla) go through one annual breeding cycle in central Europe but on some climatically more mild Atlantic islands, breeding occurs biannually and is only interrupted by a post-breeding moult (Berthold & Querner 1993; for further examples, see Hahn et al. 1997). This natural diversity is paralleled in circannual patterns of these birds. Captive blackcap warblers from the Cape Verde Islands undergo two distinct annual gonadal cycles whereas European populations show only one cycle (Berthold & Querner 1993). Hybrids show a roughly intermediate pattern.
(iii) Moult
Similar observations relate to the timing of another highly important life-cycle stage in birds, moult (Barta et al. 2008). Free-living willow warblers and closely related chiffchaffs differ not only in the duration of zugunruhe, but also in schedules of reproduction and moult: while chiffchaffs show the typical pattern of only one annual complete moult, willow warblers have evolved a rare pattern of a biannual complete moult (Weber et al. 2005). These differences persist under constant conditions (Gwinner 1971, 1972b). In-depth documentation of moult rhythms also comes from different subspecies of stonechats (Gwinner et al. 1983, 1995; Gwinner 1995; Helm et al. 2005). European stonechats depart from their birthplaces at a leisurely pace in late summer and thus have a relatively long time available for concluding their pre-migratory moult. Time constraints for moulting are even more relaxed in African stonechats that do not migrate at all. However, Siberian stonechats have to condense their pre-migratory moult to terminate before conditions deteriorate in late summer. These differences in moult schedules are maintained in young birds in captivity under constant short day length, such that Siberian stonechats start moulting at 30 days of age and finish within 21 days. European stonechats start moulting at about 38 days of age and need 55 days, whereas African stonechats start to moult at 72 days of age and need 91 days to complete it. Hybrids again show intermediate time patterns (Helm & Gwinner 1999, 2001). In addition to this variation in moult schedule, stonechats differ in the way they respond to day length cues. Again, one might expect that such population differences in endogenous moult timing are very likely to have ultimate consequences in nature (Barta et al. 2008).
(iv) Migration
Perhaps the best explored life-cycle stage that is controlled by circannual rhythms is migration. Circannual rhythms have been documented for a suite of migratory traits, including timing of zugunruhe, fat deposition, orientation direction and modulation of nocturnal melatonin patterns (Gwinner et al. 1993; Bairlein & Gwinner 1994; Gwinner 1996a). Rhythms and timing programmes are thought to be especially important for first-year migrants that cannot rely on prior experience (Gwinner 1986, 1996a; Berthold 2001; Mouritsen 2003). In long-distance migrants, circannual timing of the onset of zugunruhe generally matches species-specific patterns. Zugunruhe in captive birds shows distinct responsiveness to photoperiod, typically in accordance with calendar responses noted in the wild (Gwinner 1968, 1971, 1972a,b, 1988). Thus, in various species, including sylviid warblers and stonechats, captive birds initiate fall migratory restlessness at similar day length conditions as free-living conspecifics (Gwinner, Helm et al. 2005; Helm 2006). Circannual rhythms and programmed responses to photoperiod have been found for many other migration-related traits, including migratory direction (Gwinner 1986; Berthold 2001; Mouritsen 2003). A clock-and-compass mechanism could have important adaptive advantages, for example for young birds which, upon reaching Gibraltar (coastal Spain), need to change their migratory heading from SW to SE, to avoid flying out over the Atlantic. It is still unclear whether migrating birds in the wild truly rely upon their endogenous information, or instead follow informed conspecifics (Chernetsov et al. 2004; Couzin et al. 2005) to determine their direction, speed and overall timing.
In summary, there exists a compelling correspondence between the functioning of circannual rhythms in captivity and behaviour shown in the field, but these findings cannot be taken as conclusive evidence for an adaptive value of circannual rhythms. Any adaptive role of circannual rhythms in the wild still requires confirmation. As an additional cautionary note, patterns in the field and laboratory are not always perfectly matched (Gwinner 1986; Helm 2006). For instance, zugunruhe of many species reflects gradients in migratory behaviour, yet even resident populations display some zugunruhe (Berthold 2001; Helm 2006; Helm & Gwinner 2006). Although these findings caution against a direct parallelism of laboratory and field studies, they point to the possibility of an extended repertoire of life-history strategies that may only be expressed under a given set of conditions.
In general, the most important adaptive function of circannual clocks will be to provide an internal representation of time. As such, circannual rhythms may help to improve the consistency of seasonal timing, the ability to respond in a programmed way to particularly important cues, and buffer against misleading environmental information. In particular, circannual clocks may make the timing of seasonal activities in relatively constant environments more precise. At the same time, precise and consistent circannual rhythms could help organisms living in areas with unpredictable climates to determine whether, e.g. a severe rainfall is indicative of the coming rainy season or just a random rainfall during a prolonged dry season (cf. Shine & Brown 2008). These benefits also apply to animals that need to anticipate environmental conditions they cannot directly assess, e.g. long-distance migrants or hibernating mammals, or that need to keep local environmental cues from influencing their seasonal schedules.
(d) Proximate mechanisms underlying circannual rhythms
On the mechanistic level very little is known about how circannual clocks work. While research on the proximate control of circadian rhythms has made enormous progress during the past decade (e.g. Brandstätter et al. 2001; Yamaguchi et al. 2001), the mechanisms underlying circannual rhythms are only beginning to be understood (Lincoln et al. 2003, 2006; Kondo et al. 2006). Gwinner (1986) wrote: ‘The problems [with understanding circannual rhythms] stem partly from our almost complete ignorance of the physiological processes involved in generating circannual rhythmicity.’ Over 20 years later, we are still searching for the mechanisms of circannual rhythms.
Several models have been suggested to provide the basis for circannual rhythms (Mrosovsky 1978; Gwinner 1986). One hypothesis is the frequency de-multiplication hypothesis (FDH). According to the FDH, an animal derives a yearly rhythm from the de-multiplication of a series of circadian rhythms (Gwinner 1973; Farner & Follett 1979). In other words, animals may count days to derive an annual cycle. The FDH thus posits that circannual rhythms are intimately related to an individual's circadian rhythm. Thus, individuals with circadian rhythms shorter than 24 h will exhibit circannual rhythms with a shorter period length, and vice versa. Several experimental tests of this hypothesis were inconclusive: for instance, animals exposed to constant photoperiods with shortened period lengths (e.g. 21 h, 10.5 L : 10.5 D) did not differ in the period of their circannual rhythms in gonad size or moult from control animals held under photoperiods of 24 h (12 L : 12 D) or from those exposed to artificially lengthened photoperiods of 27 h (13.5 L 0: 13.5 D; Gwinner 1973, 1981; Kenagy 1981; Carmichael & Zucker 1986). Thus to date, there is no supporting evidence for the FDH.
Another hypothesis explaining circannual rhythmicity invokes a ‘sustained hourglass’ mechanism (Mrosovsky 1970, 1978). Dawson et al. (2001) suggest that, at least under 12 h photoperiods, circannual rhythmicity may represent a simple consequence of a life-cycle adaptation to the annual cycle. That is, animals go through a sequence of physiological stages whose summed duration creates an approximately 12-month rhythm. For birds, Jacobs & Wingfield (2000) suggested that this temporal sequence of life-cycle stages is analogous to a ‘finite state machine’ whereby wintering birds progress uni-directionally into a state of vernal migration and from there to breeding, moulting and autumn migration states (Buehler & Piersma 2008). As each phase of the annual cycle is tuned to make full use of the time available for it, such a ‘clock’ could run indefinitely simply based upon the subsequent transitions between life-history stages (Mrosovsky 1970). Dawson et al. (2001) conclude that ‘such a system could still be defined as a clock, but one operating at a macrolevel whose ‘clockworks’ are the physiological processes of the entire organism rather than a mechanism operating at the molecular or cellular level, as is the case in circadian clocks’.
Although parsimonious and plausible in many cases, the model of a sequence-of-states cannot sufficiently explain observations from animals with strong (i.e. Type II) circannual rhythms. For example, circannual rhythmicity is often restricted to particular life-cycle stages. In various species kept under constant conditions, one life-cycle stage may show persistent cycling while others are not expressed (e.g. Lofts 1964; Pengelley 1968; Gwinner 1986; Helm 2006; Lincoln et al. 2006). For instance, white-crowned sparrows (Zonotrichia leucophrys) show persistent cycles in testicular size but not moult under a range of day lengths (e.g. King 1968; Farner et al. 1980), suggesting that the expression of gonadal rhythms does not directly depend on an intermittent moult. Such patterns have been explained by the existence of specific permissive conditions for the rhythmic expression of different life-cycle stages (e.g. Farner et al. 1980; Gwinner 1986; Lincoln et al. 2006). Further, in species in which all major life-cycle stages are expressed under constant conditions, these stages can get out of phase with each other. Post-breeding moult can overlap or precede migratory restlessness and gonadal activation (e.g. Lofts 1964; Gwinner & Dorka 1976; Holberton & Able 1992; Helm 2006; Newton 2007). Thus, annual rhythms in a given seasonal behaviour cannot solely be a consequence of a preceding life-cycle stage.
Divergent circannual cycles within an individual are better explained by a third set of hypotheses which assumes truly self-sustained endogenous circannual rhythms, possibly generated by multiple oscillators. This idea has been proposed on theoretical grounds (e.g. Pengelley 1968; Pengelley et al. 1976; Gwinner 1986) and has recently found empirical support in studies of mammalian circannual rhythms (Lincoln et al. 2006). Lincoln and colleagues described the occurrence of free-running circannual rhythms driven by a pacemaker that is separate from the regulation of reproductive activity by studying prolactin rhythms in sheep. The authors suggest that ‘long-term feed-back’ processes are responsible for the continued oscillation. Their findings together with circannual patterns observed in birds support the notion of multiple sites or ‘systems’ of circannual oscillations (Lincoln et al. 2003, 2006), possibly similar to the situation observed for circadian rhythms (e.g. Menaker 2006). As in circadian rhythms, such separate units could be synchronized by a master clock and/or entrainment to zeitgebers. As a cautionary note, we acknowledge that all of the above ideas still lack conclusive evidence, and that in view of the diversity of seasonal behaviours, there is little ground to expect one mechanism to provide a full explanation (Mrosovsky 1970, 1978; Gwinner 1986; Prendergast et al. 2002; Goldmann et al. 2004).
2. A first test of the energy turnover hypothesis as a circannual mechanism
We wish to build upon the ideas discussed above and propose a new approach to unravel the mechanisms involved in annual timing. The FDH hypothesis differs from the other discussed models in being restricted to ‘counting’ the incidence of day light events. In contrast, both sequence-of-stage and true oscillation involve physiological processes that may depend on other physiological properties, e.g. metabolic activity (Gwinner 1986). We suggest that in both the cases, the turnover of energy may provide a parsimonious, quantifiable and simple mechanism for tracking the passage of time (see Mrosovsky 1977, 1980b). We propose that this energy turnover hypothesis (ETH) could provide a mechanistic explanation for circannual rhythms in birds, and perhaps other vertebrates as well. The ETH rests on the following assumptions. (i) The average energy allocation to a given seasonal behaviour, e.g. reproductive activation, is more or less fixed. That is, a given life-cycle stage requires (and is allocated) a relatively specific amount of energy. When resources are available ad libitum, growing a gonad supposedly takes a fairly invariant amount of time (Wingfield & Farner 1993). (ii) As a consequence, the duration of a given life-cycle stage is related to, and possibly determined by, the amount of energy turnover that occurs during that stage. (iii) After completion, the time until reinitiation of the life-cycle stage is also influenced by energy turnover, for example via long-term feed-back processes (e.g. Lincoln et al. 2006).
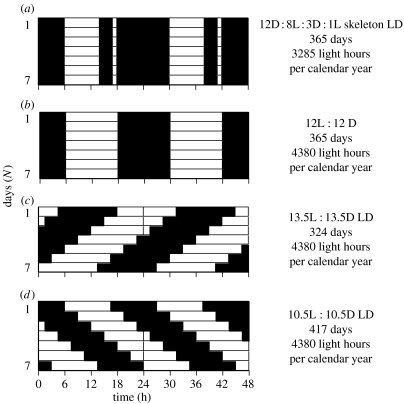
Here we report on a first preliminary test of the ETH versus the FDH in an experimental set-up that predicts different outcomes for each hypothesis. In principle, we repeated previous experiments testing the FDH by using photoperiods with zeitgeber periods of 24, 21 and 27 h. However, in our experiment, we added a new fourth group that was exposed to a zeitgeber period of 24 h but with a photoperiod in which the day was interrupted by a 4 h period of darkness (skeleton photoperiod). Birds perceive a skeleton photoperiod as a continuous light period. Our aim in using a skeleton photoperiod was to expose birds to the same number of days in a calendar year as the 24 h group, but reduce energy turnover by inducing a resting phase by turning the lights off. The FDH states that circannual rhythms progress at similar rates regardless of the photoperiod (control and skeleton), whereas zeitgeber periods of 21 h should lead to shorter and 27 h zeitgeber periods to longer circannual rhythms. If the FDH was correct, individuals under the skeleton photoperiod should show a similar circannual period length as the 24 h control group irrespective of the energy turnover. In contrast, the ETH postulates that only the amount of energy turnover determines the period length of a circannual rhythm. Under this hypothesis, the experimental group with the lowest overall energy expenditure should have the longest circannual rhythm. Hence, the ETH predicts that birds in the skeleton photoperiod should have slower circannual rhythms if they had lower total energy turnover compared to 24 h photoperiod controls (figure 1).
Figure 1.
Schematic of the four photoperiod schedules birds were exposed to during the experiment. (a–d) The 24-h skeleton photoperiod, the control 24-h day, the 27- and the 21-h day. The descriptions on the right-hand side indicate the number of subjective days per calendar year, as well as the number of light hours. Note that double plots over 48 h are shown.
As a potential alternative to the ETH, we considered that circannual rhythms could be related to the total amount of melatonin that birds in the different groups experience. The hormone melatonin is secreted by all vertebrates during darkness (Gwinner & Dittami 1980). Because the house sparrows exposed to the skeleton photoperiod experience more dark hours than any of the other groups, this group might be exposed to higher cumulative melatonin levels.
3. Material and methods
(a) Animals
In March and April 2000, 87 house sparrows of mixed sex were captured in Champaign, IL, divided into four approximately equally sized groups with similar sex ratios in aviaries of 2×1×1 m, and exposed to four different photoperiods. The 24 h group (10 males, 11 females) was kept under a light : dark (LD) cycle of 12 L : 12 D, the 21-h group (11 males, 11 females) under LD 10.5 L : 10.5 D, the 27 h group (10 males, 10 females) under LD 13.5 L : 13.5 D. A fourth group (12 males, 12 females) was kept under a skeleton photoperiod that was selected to mimic a LD 12 L : 12 D photoperiod, but designed to have fewer light hours (12 D : 8 L : 3 D : 1 L; see figure 1) such that birds could not eat as much and expended less energy (see below), when compared with the 24-h group. We consider it a confounding but uncontrollable effect in our experiment that birds captured from the wild in central Illinois already experienced about 12–13 h of daylight in March/April, thus potentially experienced a shortening of the daylight period when entering the experiment. It is possible that an initial decrease in photoperiod prevented complete gonadal regression in the subsequent winter (figure 2; cf. Hahn & Ball 1995).
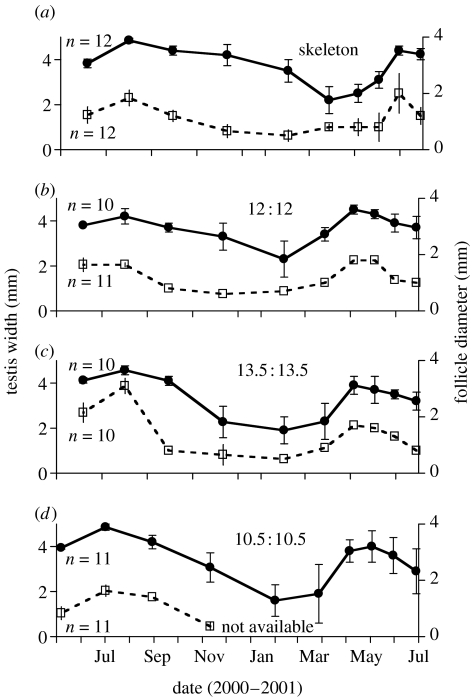
Figure 2.
Temporal (approx. bimonthly) changes in gonad (circle) and follicle (square) sizes of house sparrows exposed to the photoperiods as indicated (see explanation in figure 1). Data are means±1s.e. Sample sizes for each group are indicated in (a–d). Data for females in the 21-h day group are missing after November 2000 due to equipment failure.
Birds were transported in aviaries in a closed truck by M.W. from Illinois to Princeton, NJ, on 4 August 2000, and kept under their respective light regime during the transport. Upon arrival, birds were immediately transferred into separate aviaries whose light cycle matched each group's continued light cycle. Temperature was kept at 21±1°C and each aviary was illuminated by two 60 W fluorescent light tubes. Due to feeding equipment failure, seven female sparrows in the 21-h photoperiod group died in December 2000. Therefore, we do not use data for peak-to-peak follicle sizes for females in this group.
At regular intervals (see figure 2), we measured the testis and follicle sizes in all birds by unilateral laparotomy (Hau et al. 1998). Birds were captured from their cages and subsequently anaesthesized using an isoflurane–air mixture. A small incision was made between the two most distal ribs which provides visual access to reproductive organs. We measured the length and width of the left testes in males to the nearest 0.1 mm below 1 mm total size, and to the nearest 0.2 mm above that size. Follicle sizes in females were assessed as the largest single follicle measured to the nearest 0.2 mm. Incisions were then closed with surgical glue and birds allowed to recover in an opaque bag for 20 min. All birds tolerated this procedure well.
(b) Respirometry
During early July 2001, we conducted continuous respirometry measurements for 24 h on seven random males from each group to determine their energy expenditure. We decided that one respirometry measurement per year at a time when birds had large gonads might give a representative estimate of the relative energy expenditures of the various experimental groups against each other (Klaassen 1995; Wikelski et al. 2003). We do not want to suggest that the absolute values we measured sufficiently represent the annual energy expenditure of the sparrows, however. We used an open-flow, push-through respirometry system (Martin et al. 2003). Birds were placed in 2 l plastic metabolic chambers in a climate chamber at 25°C and exposed to their respective photoperiod. Each chamber contained a small dish of water as well as no-waste bird seeds ad libitum. In the chamber, birds could freely move and hop around. External, naturally humid air (85–95% humidity) was pumped through a mass flow controller (Sable systems, Nevada) and a multiplexer (V2-0, Sable systems) into the metabolic and a reference chamber. The flow rate was 700 ml min−1 and the flow controller was calibrated prior to use with a bubble meter. A factory-calibration post-experiment indicated that flow rate errors were less then 1.2%. Air leaving the chambers was dehumidified using a Peltier-effect condenser (Pc-1; Sable systems) and CO2 concentration was measured from a subsample of the outlet flow (Ca-1b, Sable systems). Before oxygen concentration was determined (using Fc-1b, Sable systems), drierite (Fisher) was used to scrub the potential remaining water from the air. We measured each bird for 10 min, then switched to the next bird, waited for 3 min to flush latent gases out of the tubing system, and continued the measurements. This sequence was repeated continuously throughout the 24 h period, thus providing 16 measurement periods of 10 min duration for each individual. The rates of oxygen consumption were calculated using Default 3b in Withers (1977; using Datacan software, Sable systems) after adjusting flow rate for the amount of water due to humidity (Labanalyst, Riverside).
(c) Melatonin
In early June 2001, we captured six individuals randomly from each group at five different times during their subjective night (early night, midnight, late night) and two time points during their subjective day (indicated in figure 6). For the skeleton photoperiod group, we included one sampling time during the 3-h dark period in the later part of their subjective daytime (figure 4). For each individual bird, we waited at least 3 days between successive samplings. When sampling during the subjective night, we used dimmed headlamps with additional dark blue gauze for the entire procedure so that the birds' melatonin-producing circadian rhythm would not be impaired by light (Hau et al. 2002). We can, however, not assess to what extent sleep rhythms may have been disrupted by the sampling procedure. We collected 150 μl blood from each bird's alar vein, held it on ice until it was centrifuged (within 4 h). We then collected plasma and stored it at −20°C until analysis.
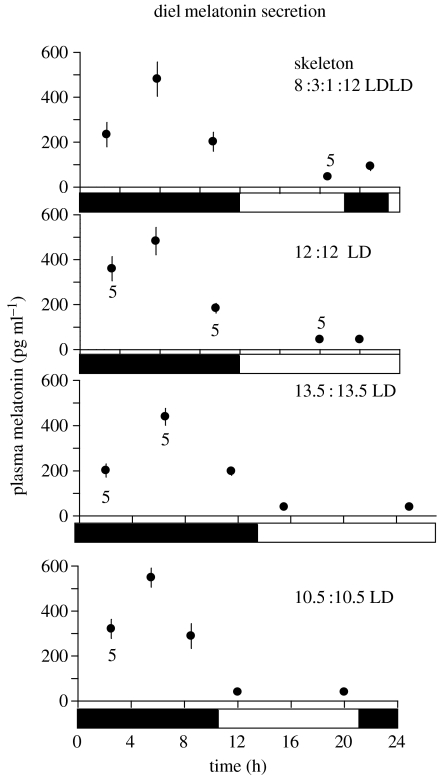
Figure 6.
Graphs showing temporal (within 24 h) changes in plasma concentrations of the hormone melatonin, for house sparrows exposed to the photoperiods as indicated (see explanation in figure 1). Data show means±1s.e., sample sizes are N=6 except when indicated, black bars indicate dark periods, light bars indicate light periods.
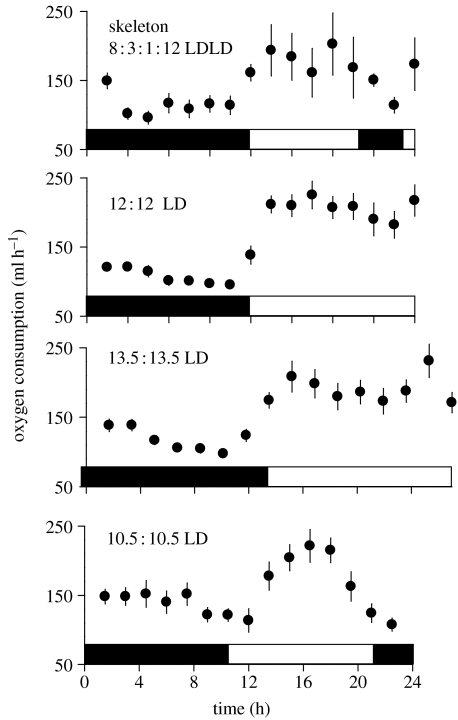
Figure 4.
Temporal (within 24 h) changes in energy expenditure of house sparrows exposed to the photoperiods as indicated (see explanation in figure 1). Data are means±1s.e. (N=7), black bars indicate dark periods, light bars indicate light periods.
Plasma samples were analysed for melatonin content by radioimmunoassay (Hau et al. 2002). In short, melatonin was extracted from plasma with chloroform containing 1 M NaOH. Samples were then aspirated, dried under N2, dissolved in buffer and washed with petroleum to remove lipids. They were then incubated with melatonin anti-serum at room temperature for 30 min and subsequently with 3H-labelled melatonin at 4°C for 19 h. After separating the free, labelled melatonin from the bound one, radioactivity was counted and melatonin concentration determined. The lower detection limit of the assay was at 40 pg melatonin per ml, intra-assay variation was 11.4%. All samples were run in a single assay.
(d) Data analysis
Data were processed with SPSS v.10 (1991) for Windows. Two-tailed test statistics were used. Data are shown as means±s.e. (if not indicated otherwise). The normal distribution of data and residuals was tested before applying parametric tests. To estimate the average melatonin concentrations per subjective day, we interpolated hourly melatonin averages from the population averages shown in figure 4 and assumed that the melatonin concentrations measured during two random time points during the day were representative of overall daytime levels. We then used a general linear model (GLM) followed by Scheffe post hoc tests to identify differences between groups. To determine the period lengths of circannual rhythms, we measured the time (days) between the peaks of gonad sizes for each individual. We then conducted a GLM to determine differences between groups (figure 3). To compare the average energy expenditure between treatments, we first averaged the VO2 values for each of the seven individuals during the respirometry trials and normalized the values to 24 h by giving equal weight to average nocturnal and diurnal energy expenditures. We then used a GLM to determine whether there were differences in the overall average energy expenditure for a 24 h period for each group.
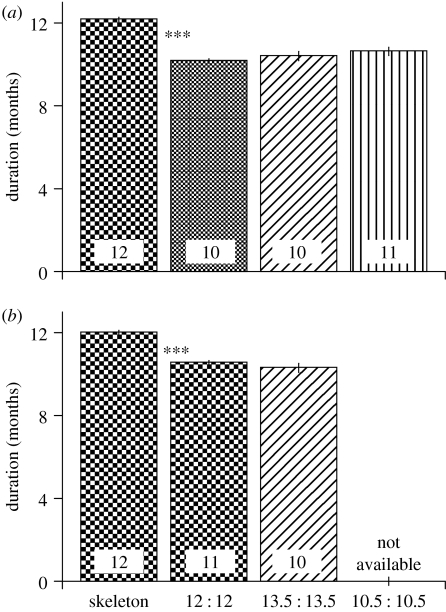
Figure 3.
Circannual periodicities of gonad (a) and follicle (b) sizes of house sparrows exposed to various photoperiods (explained in figure 1). Data show mean±1s.e., sample sizes are indicated in the columns. Stars denote significant differences as determined by Scheffe post hoc tests.
4. Results
(a) Testis and follicle sizes
As an overt expression of circannual rhythms, we determined the development of reproductive organs, i.e. sizes of testes and follicles. Among the males of the four treatment groups, we found a significant difference in the number of months between testis peaks. Birds under the skeleton photoperiod had longer peak-to-peak intervals than the birds under the three other photoperiods (GLM, F3,44=32, p<0.001; Scheffe post hoc test significant for skeleton photoperiod birds compared to all others; figures 2 and 3). Likewise, among females, there was a significant difference in the duration of time between peak-to-peak follicle sizes. Again, the skeleton photoperiod group showed a longer period length than the control or the 27-h photoperiod group (GLM, F2,32=27, p<0.001; Scheffe post hoc test significant for skeleton photoperiod birds compared to all others; figures 2 and 3). Figure 3 shows a summary of the findings, indicating that all groups except the skeleton photoperiod group had free-running circannual period lengths of approximately 10 months, whereas the skeleton photoperiod group had a period length of around 12 months.
(b) Energy expenditure
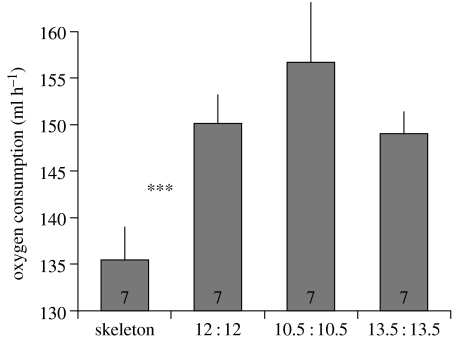
The hourly average oxygen consumption of sparrows, a measure of overall energy expenditure, showed a typical circadian pattern, with lower levels at night than during the day (figure 4). When we calculated the overall average energy expenditure for a 24-h period, however, we found that the skeleton photoperiod group had a lower energy expenditure than all other groups, which were indistinguishable from each other (GLM, F3,26=4, p=0.021; Scheffe post hoc test significant for skeleton photoperiod birds compared to all others; figure 5).
Figure 5.
Summary data on energy expenditure of house sparrows exposed to various photoperiods (explained in figure 1). Data are normalized to 21-, 24- and 27-h days and show mean±s.e., sample sizes are indicated in the columns. Stars denote significant differences as determined by Scheffe post hoc tests. Note that the y-axis does not start from zero.
(c) Melatonin levels
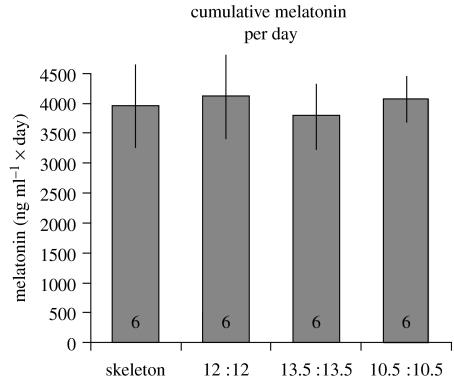
All birds had a typical diel pattern of melatonin secretion, with an increase during the dark phase in the skeleton photoperiod group. We found no differences among photoperiodic treatment groups in the average cumulative melatonin concentrations when normalized to a 21-, 24- or 27-h day (GLM, F3, 21, p=0.62; figures 6 and 7). Since we found no difference in this conservative test, we did not conduct a time-series analysis of the melatonin values.
Figure 7.
Summary data on average melatonin concentrations of house sparrows exposed to various photoperiods (explained in figure 1). Averages are roughly approximated from data shown in figure 6 and calculated per day. Data indicate summed means±s.e., sample sizes are indicated in the columns (missing data were replaced by the population averages). We found no significant differences between the groups.
5. Discussion
Our experiment was designed to distinguish between two hypotheses for the mechanistic basis of circannual rhythms: if house sparrows count the approximate number of days to compose 1 year, as suggested by the FDH, birds exposed to diel zeitgeber periods of 24 h duration, even a skeleton photoperiod, should have circannual rhythms with similar period lengths. Birds exposed to either shorter or longer zeitgeber periods (such as 21 or 27 h), however, should have circannual rhythms that are correspondingly shorter or longer compared to the 24 h birds. Although this hypothesis was not supported in three previous tests, it could not be fully rejected by such simple negative evidence (Gwinner 1973, 1986). Thus, alternative ideas such as macro-level timing mechanisms or true oscillators have remained plausible alternatives (Gwinner 1986; Dawson et al. 2001). We hypothesized that the birds’ circannual clocks may involve tracking of the total amount of energy expenditure they expend over a year. Thus, we suggest that by measuring or accounting for energy turnover (by a yet unknown mechanism), individuals can tell what time of year it is independent of environmental conditions.
One critical assumption for such a metabolically based timing mechanism is that each particular life-cycle stage has a specific energy budget. Based on field studies (Nagy et al. 1999; McNab 2003), such an assumption seems valid. Furthermore, animals presumably attempt to expend a constant amount of energy per day, as evidenced by energy compensation mechanisms (Deerenberg et al. 1998; Wikelski et al. 1999) or other physiological trade-offs individuals employ if they are put to hard work (cf. Martin et al. 2008; Speakman 2008). If our ETH is correct, we should see no difference in circannual rhythms of individuals exposed to 21-, 24- or 27-h days given that days have equal durations of light and dark periods. Under these conditions, all individuals would experience the same number of daylight hours in a year, and thus presumably expend similar amounts of energy. This assumption about energy expenditure was found to be true in our experiment as the 24-, 21- and 27-h groups had similar energy expenditures. However, individuals exposed to a 24-h skeleton photoperiod that received 25% fewer light hours per year had circannual rhythms with a longer period length. It is yet unclear whether the decreased energy turnover itself was responsible for slower circannual rhythmicity, or whether birds perceived the skeleton photoperiod as a decrease in light intensity by a mechanism that integrates photons over the photophase (Bentley et al. 1998). The circannual clock could be differentially sensitive to energy turnover in a phase-dependent manner, in the following way: some species, for example golden-mantled ground squirrels (Spermophilus lateralis), responded to different temperature regimes in captivity by changes in circannual behaviour (Mrosovsky 1986, 1990). These responses were phase-specific and differed between the sexes and also between related species. Thus, an alternative explanation for a delayed (slower running) clock could be that the rhythm was phase-shifted, i.e. phase-delayed, producing a longer overt period length. It is also not yet clear if energy expenditure is actually part of the circannual clockwork (the ‘gears’ of the clock), or whether it is simply an input component. If it is just an input component, other mechanisms such as changes in hormone secretion may be the primary gear of the clock.
One potential mechanistic explanation underlying the ETH, that the total levels of the hormone melatonin are related to the speed of the circannual clock, was not supported. Thus, it appears that absolute melatonin concentrations may have no direct relationship to the running of the circannual clock in sparrows. A lack of a connection between melatonin levels and circannual clocks is not too surprising, as Gwinner (1981) likewise did not find a clear mechanistic connection between melatonin and circannual clocks. However, melatonin could affect clocks indirectly, for instance by influencing phase-relationships of oscillators, photosensitivity or via other hormones, for instance GnIH (e.g. Gwinner & Dittami 1980; Gwinner 1981; Ubuka et al. 2005). Other hormones, such as leptin, orexin, ghrelin and thyroxines could influence circannual rhythmicity, but so far the evidence for a causal involvement in the generation of these long-term clocks is missing (Yoshimura et al. 2003; Mustonen et al. 2005; Kondo et al. 2006).
The ETH is falsifiable as it proposes that whole-animal changes in energy expenditure are related to the speed of circannual clocks. Based on this prediction we propose several additional experiments that could test the ETH. Some of these experiments have already been partially conducted in the past (Pengelley 1968; Heller & Poulson 1970; Mrosovsky & Fisher 1970; Mrosovsky 1980a,b). For example, hibernating animals expend very little energy during deep hibernation. We suggest that preventing hibernators from entering a low-energy state should significantly speed up their circannual clock (cf. Mrosovsky & Lang 1971). Mrosovsky (1980a, 1986, 1990) showed that at least in golden-mantled ground squirrels (S. lateralis), a low ambient temperature produced longer cycles and cold pulses caused a phase delay in circannual rhythms, apparently contradicting our above expectations. However, low temperature could also induce additional endogenous heat production (i.e. energy expenditure) in hibernators, and thus support the predictions of a phase delay according to the ETH. Another test would be to put animals to hard work over long periods of time without allowing them to compensate for their high energy expenditure, which should result in faster circannual clocks (Loudon 1994). Moreover, individuals that are continuously housed below thermoneutrality and kept unable to acclimate energetically to such conditions should have faster circannual clocks than controls.
These and many other experiments have shown that circannual clocks, like circadian clocks (e.g. Pengelley & Fisher 1963; Aschoff 1979; Thomas et al. 1993; Sawyer et al. 1997; Rensing & Ruof 2002), are not entirely temperature-compensated. That is, if environmental temperatures induce changes in energy turnover in some individuals, these individuals should have faster or slower endogenous circannual clocks. Alternatively, rather than speeding up or slowing down the clock, environmental temperatures might adjust rhythm phase from year to year by some non-clock mechanism (Mrosovsky 1986, 1990). The ETH also predicts that circannual clocks of captive animals, i.e. individuals that are fully fed and able to expend as much energy as they can ingest, should have a shorter period length than one year. In fact, most circannual rhythms are shorter than 12 months, which is different from circadian rhythms that are about as likely to be shorter or longer than 24 h. All of these hypotheses are currently speculative, but we want to provide a flavour of the potential explanatory power of the ETH, as well as methods for experimental falsification.
The study of circannual rhythms is notoriously difficult, mostly owing to the unfavourable ‘ratio of the period length of a single circannual cycle to the length of the productive life of the biologist’ (Menaker 1974), and to the funding cycles of most funding agencies. Nevertheless, we suggest that the ETH may provide biologists with a new impetus for addressing a mechanistic basis for circannual clocks, both in the laboratory and in the wild.
Acknowledgments
All animal experiments adhere to the standards of the American Ornithologists Union on the use of wild birds in research, and were permitted by the Animal Care Committees of the University of Illinois and Princeton University, as well as by local and state authorities.
We thank Mary Guimond and Vilma Zolynas for logistical help, the Princeton Eco-phys lab, particularly Laura Spinney, as well as Herbert Biebach and Dustin Rubenstein for discussions, and Mary Grabowski for help in capturing and caring for house sparrows. Heinz Wikelski constructed the sparrow cages and helped in the bird transfer from Illinois to New Jersey. Unfortunately, the circannual research field lost one of its main proponents with the untimely death of Ebo Gwinner. This study was supported by the University of Illinois at Urbana-Champaign, Princeton University and the US National Science Foundation (IRCEB- 0212587) to M.W. and M.H.
Footnotes
One contribution of 14 to a Theme Issue ‘Adaptation to the annual cycle’.
References
- Aschoff J. Tierische Periodik unter dem Einfluß von Zeitgebern. Zeitschrift für Tierpsychologie. 1958;15:1–30. [Google Scholar]
- Aschoff J. Circadian rhythms: Influences of internal and external factors on the period measured in constant conditions. Zeitschrift für Tierpsychologie. 1979;49:225–249. doi: 10.1111/j.1439-0310.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Bairlein F, Gwinner E. Nutritional mechanisms and temporal control of migratory energy accumulation in birds. Annu. Rev. Nutr. 1994;14:187–215. doi: 10.1146/annurev.nu.14.070194.001155. doi:10.1146/annurev.nu.14.070194.001155 [DOI] [PubMed] [Google Scholar]
- Barta Z, McNamara J.M, Houston A.I, Weber T.P, Hedenstro¨m A, Feró O. Optimal moult strategies in migratory birds. Phil. Trans. R. Soc. B. 2008;363:211–229. doi: 10.1098/rstb.2007.2136. doi:10.1098/rstb.2007.2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley G.E, Goldsmith A.R, Dawson A, Briggs C, Pemberton M. Decreased light intensity alters the perception of daylength by male European starlings (Sturnus vulgaris) J. Biol. Rhythms. 1998;13:140–150. doi: 10.1177/074873098128999998. doi:10.1177/074873098128999998 [DOI] [PubMed] [Google Scholar]
- Berthold P. Oxford University; Oxford, UK: 2001. Bird migration. [Google Scholar]
- Berthold B, Querner U. Genetic and photoperiodic control of an avian reproductive cycle. Experientia. 1993;49:342–344. doi:10.1007/BF01923418 [Google Scholar]
- Brandstätter R, Abraham U, Abrecht U. Initial demonstration of rhythmic per gene expression in the hypothalamus of a non-mammalian vertebrate, the house sparrow. Neuroreport. 2001;12:1167–1170. doi: 10.1097/00001756-200105080-00023. doi:10.1097/00001756-200105080-00023 [DOI] [PubMed] [Google Scholar]
- Buehler D.M, Piersma T. Travelling on a budget: predictions and ecological evidence for bottlenecks in the annual cycle of long-distance migrants. Phil. Trans. R. Soc. B. 2008;363:247–266. doi: 10.1098/rstb.2007.2138. doi:10.1098/rstb.2007.2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadee N, Piersma T, Daan S. Endogenous circannual rhythmicity in a non-passerine migrant, the knot Calidris canutus. Ardea. 1996;84:75–84. [Google Scholar]
- Chapin J.P. The calendar of Wideawake Fair. Auk. 1954;71:1–15. [Google Scholar]
- Carmichael M.S, Zucker I. Circannual rhythms of ground squirrels: a test of the frequency demultiplication hypothesis. J. Biol. Rhythms. 1986;1:277–284. doi: 10.1177/074873048600100402. doi:10.1177/074873048600100402 [DOI] [PubMed] [Google Scholar]
- Chernetsov N, Berthold P, Querner U. Migratory orientation of first-year white storks (Ciconia ciconia): inherited information and social interactions. J. Exp. Biol. 2004;207:937–943. doi: 10.1242/jeb.00853. doi:10.1242/jeb.00853 [DOI] [PubMed] [Google Scholar]
- Cooke S.J, Hinch S.G, Wikelski M, Andrews R.D, Kuchel L.J, Wolcott T.G, Butler P.J. Biotelemetry: a mechanistic approach to ecology. Trends Ecol. Evol. 2004;19:334–343. doi: 10.1016/j.tree.2004.04.003. doi:10.1016/j.tree.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Couzin I.D, Krause J, Franks N.R, Levin S.A. Effective leadership and decision making in animal groups on the move. Nature. 2005;433:513–516. doi: 10.1038/nature03236. doi:10.1038/nature03236 [DOI] [PubMed] [Google Scholar]
- Craig A.J.F.K. Breeding condition of male red bishops under artificial photoperiods. Ostrich. 1985;56:74–78. [Google Scholar]
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. doi:10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- DeCoursey P. The behavioral ecology and evolution of timing systems. In: Dunlap J.C, Loros J, DeCoursey P, editors. Chronobiology. Biological timekeeping. Sinauer; Sunderland, MA: 2004. pp. 27–65. [Google Scholar]
- Deerenberg C, Overkamp G.J.F, Visser G.H, Daan S. Compensation in resting metabolism for experimentally increased activity. J. Comp. Physiol. B. 1998;168:507–512. doi:10.1007/s003600050171 [Google Scholar]
- Farner D.S, Follett B.K. Reproductive periodicity in birds. In: Barrington E.J.W, editor. Hormones and evolution. Academic Press; London, UK: 1979. pp. 129–148. [Google Scholar]
- Farner D.S, Donham R.S, Moore M.C, Lewis R.A. The temporal relationship between the cycle of testicular development and molt in the white-crowned sparrows, Zonotrichia leucophrys gambelii. Auk. 1980;97:63–75. [Google Scholar]
- Goldmann B, Gwinner E, Karsch F, Saunders D, Zucker I, Ball G. Circannual rhythms and photoperiodism. In: Dunlap J.C, Loros J, DeCoursey P, editors. Chronobiology. Biological timekeeping. Sinauer; Sunderland, MA: 2004. pp. 107–142. [Google Scholar]
- Gwinner E. Circannuale Periodik der Mauser und der Zugunruhe bei einem Vogel. Naturwissenschaften. 1967;54:447. doi: 10.1007/BF00603157. doi:10.1007/BF00603157 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Artspezifische Muster der Zugunruhe bei Laubsängern und ihre mögliche Bedeutung für die Beendigung des Zuges im Winterquartier. Z. Tierpsychol. 1968;25:843–853. [Google Scholar]
- Gwinner E. A comparative study of circannual rhythms in warblers. In: Menaker M, editor. Biochronometry. National Academy of Sciences; Washington, DC: 1971. pp. 404–427. [Google Scholar]
- Gwinner, E. 1972a Adaptive functions of circannual rhythms in warblers. In Proc. XVth Internatl Ornithol. Congr, The Hague, The Netherlands 30 August–5 September 1970, pp. 218–236. Leiden, The Netherlands: E. J. Brill.
- Gwinner E. Endogenous timing factors in bird migration. In: Galler S.R, editor. Animal orientation and navigation. National Academy of Sciences; Washington, DC: 1972b. pp. 321–338. [Google Scholar]
- Gwinner E. Circannual rhythyms in birds: their interaction with circadian rhythms and environmental photoperiod. J. Reprod. Fertil. 1973;19:51–65. [PubMed] [Google Scholar]
- Gwinner E. Circannual rhythms in bird migration. Annu. Rev. Ecol. Syst. 1977;8:381–405. doi:10.1146/annurev.es.08.110177.002121 [Google Scholar]
- Gwinner E. Circannual rhythms in animals and their photoperiodic synchronization. Naturwissenschaften. 1981;68:542–551. doi: 10.1007/BF00401662. doi:10.1007/BF00401662 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Springer; Heidelberg, Germany: 1986. Circannual rhythms. [Google Scholar]
- Gwinner, E. 1988 Photorefractoriness in equatorial migrants. In Acta XIX Congressus Internationalis Ornithologicus, Ottawa, Canada June 1986, pp. 626–633. Ottawa, Canada: University of Ottawa Press.
- Gwinner E. Circannual rhythms in tropical and temperate-zone stonechats: a comparison of properties under constant conditions. Ökol. Vögel (Ecol. Birds) 1991;13:5–14. [Google Scholar]
- Gwinner E. Circannual rhythms in tropical stonechats bred and raised under constant conditions. J. Ornithol. 1995;136:79–82. doi:10.1007/BF01647212 [Google Scholar]
- Gwinner E. Circadian and circannual programmes in avian migration. J. Exp. Biol. 1996a;199:39–48. doi: 10.1242/jeb.199.1.39. [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circannual clocks in avian reproduction and migration. Ibis. 1996b;138:47–63. [Google Scholar]
- Gwinner E. Rigid and flexible adjustments to a periodic environment: role of circadian and circannual programs. In: Adams N, Slotow R, editors. Proc. XXII Int. Ornithol. Congr. Durban. BirdLife South Africa; Johannesburg, South Africa: 1999. pp. 2366–2378. [Google Scholar]
- Gwinner E. Circannual rhythms in birds. Curr. Opin. Neurobiol. 2003;13:770–778. doi: 10.1016/j.conb.2003.10.010. doi:10.1016/j.conb.2003.10.010 [DOI] [PubMed] [Google Scholar]
- Gwinner E, Dittami J. Pinealectomy affects the circannual testicular rhythm in European starlings (Sturnus vulgaris) J. Comp. Physiol. 1980;136:345–348. doi:10.1007/BF00657355 [Google Scholar]
- Gwinner E, Dittami J. Endogenous reproductive rhythms in a tropical bird. Science. 1990;249:906–908. doi: 10.1126/science.249.4971.906. doi:10.1126/science.249.4971.906 [DOI] [PubMed] [Google Scholar]
- Gwinner, E. & Dorka, V. 1976 Endogenous control of annual reproductive rhythm in birds. In Proc. XIVth Internatl Ornithol. Congr., Oxford, UK 24–30 July 1966 pp. 223–234. Oxford and Edinburgh, UK: Blackwell Scientific Publications.
- Gwinner E, Helm B. Circannual and circadian contributions to the timing of avian migration. In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian migration. Springer; Heidelberg, Germany: 2003. pp. 81–95. [Google Scholar]
- Gwinner E, Scheuerlein A. Seasonal changes in day-light intensity as a potential zeitgeber of circannual rhythms in equatorial Stonechats. J. Ornithol. 1998;139:407–412. doi:10.1007/BF01653467 [Google Scholar]
- Gwinner E, Scheuerlein A. Photoperiodic responsiveness of equatorial and temperate-zone stonechats. Condor. 1999;101:347–359. doi:10.2307/1369998 [Google Scholar]
- Gwinner E, Dittami J, Gwinner H. Postjuvenile molt in East African and Central European stonechats (Saxicola torquata axillaris, S.t. rubicula) and its modification by photoperiod. Oecologia. 1983;60:66–70. doi: 10.1007/BF00379321. doi:10.1007/BF00379321 [DOI] [PubMed] [Google Scholar]
- Gwinner E, Dittami J, Beldhuis H.J. The seasonal development of photoperiodic responsiveness in an equatorial migrant, the garden warbler (Sylvia borin) J. Comp. Physiol. A. 1988;162:389–396. doi:10.1007/BF00606125 [Google Scholar]
- Gwinner E, Schwabl-Benzinger I, Schwabl H, Dittami J. Twenty-four hour melatonin profiles. Gen. Comp. Endocrinol. 1993;90:119–124. doi: 10.1006/gcen.1993.1066. doi:10.1006/gcen.1993.1066 [DOI] [PubMed] [Google Scholar]
- Gwinner E, König S, Zeman M. Endogenous gonadal Lh and moult rhythms in tropical stonechats—effect of pair bond on period, amplitude, and pattern of circannual cycles. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 1995;177:73–79. doi: 10.1007/BF00243399. [DOI] [PubMed] [Google Scholar]
- Hahn T.P, Ball G.F. Changes in brain Gnrh associated with photorefractoriness in-house sparrows (Passer domesticus) Gen. Comp. Endocrinol. 1995;99:349–363. doi: 10.1006/gcen.1995.1119. doi:10.1006/gcen.1995.1119 [DOI] [PubMed] [Google Scholar]
- Hahn T.P, MacDougall-Shackleton S.A. Adaptive specialization, conditional plasticity, and phylogenetic history in the reproductive cue response systems of birds. Phil. Trans. R. Soc. B. 2008;363:267–286. doi: 10.1098/rstb.2007.2139. doi:10.1098/rstb.2007.2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Swingle J, Wingfield J, Ramenofsky M. Adjustments of the prebasic molt schedule in birds. Ornis. Scand. 1992;23:314–321. doi:10.2307/3676655 [Google Scholar]
- Hahn T, Boswell T, Wingfield J, Ball G. Temporal flexibility in avian reproduction. Patterns and mechanisms. In: Nolan V, Ketterson E, editors. Current ornithology. vol. 14. Plenum; New York, NY: 1997. pp. 39–80. [Google Scholar]
- Hamner W.H, Stocking J. Why don't bobolinks breed in Brazil? Ecology. 1970;51:743–751. doi:10.2307/1934060 [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proc. R. Soc. B. 1998;265:89–95. doi:10.1098/rspb.1998.0268 [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. Visual and nutritional food cues fine-tune timing of reproduction in a neotropical rainforest bird. J. Exp. Zool. 2000;286:494–504. doi:10.1002/(SICI)1097-010X(20000401)286:5<494::AID-JEZ7>3.0.CO;2-3 [PubMed] [Google Scholar]
- Hau M, Romero L.M, Brawn J.D, Van't Hof T.J. Effect of polar day on plasma profiles of melatonin, testosterone, and estradiol in high-Arctic Lapland longspurs. Gen. Comp. Endocrinol. 2002;126:101–112. doi: 10.1006/gcen.2002.7776. doi:10.1006/gcen.2002.7776 [DOI] [PubMed] [Google Scholar]
- Hau M, Wikelski M, Gwinner H, Gwinner E. Timing of reproduction in a Darwin's finch: temporal opportunism under spatial constraints. Oikos. 2004;106:489–500. doi:10.1111/j.0030-1299.2004.13206.x [Google Scholar]
- Heideman P.D, Bronson F.H. An endogenous circannual rhythm of reproduction in a tropical bat, Anoura geoffroyi, is not entrained by photoperiod. Biol. Reprod. 1994;50:607–614. doi: 10.1095/biolreprod50.3.607. doi:10.1095/biolreprod50.3.607 [DOI] [PubMed] [Google Scholar]
- Heller H.C, Poulson T.L. Circannian rhythms. 2. Endogenous and exogenous factors controlling reproduction and hibernation in chipmunks (Eutamias) and ground squirrels (Spermophilus) Comp. Biochem. Physiol. 1970;33:357–383. doi:10.1016/0010-406X(70)90356-7 [Google Scholar]
- Helm B. Point of view: Zugunruhe of migratory and non-migratory birds in a circannual context. J. Avian Biol. 2006;37:533–540. doi:10.1111/j.2006.0908-8857.03947.x [Google Scholar]
- Helm B, Gwinner E. Timing of postjuvenal moult in African (Saxicola torquata axillaris) and European (Saxicola torquata rubicola) stonechats: effects of genetic and environmental factors. Auk. 1999;116:589–603. [Google Scholar]
- Helm B, Gwinner E. Nestling growth and post-juvenile moult under a tight seasonal schedule in stonechats Saxicola torquata maura from Kazakhstan. Avian Sci. 2001;1:31–42. [Google Scholar]
- Helm B, Gwinner E. Migratory restlessness in an equatorial non-migratory bird. PLoS Biol. 2006;4:611–614. doi: 10.1371/journal.pbio.0040110. doi:10.1371/journal.pbio.0040110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm B, Gwinner E, Trost L. Flexible seasonal timing and migratory—behavior results from stonechat breeding programs. Ann. NY Acad. Sci. 2005;1046:216–227. doi: 10.1196/annals.1343.019. [DOI] [PubMed] [Google Scholar]
- Helm B, Piersma T, Van der Jeugd H. Sociable schedules: interplay between avian social and seasonal behavior. Anim. Behav. 2006;72:245–262, 1215. doi:10.1016/j.anbehav.2005.12.007 [Google Scholar]
- Holberton R.L, Able K.P. Persistence of circannual cycles in a migratory bird held in constant dim light. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 1992;171:477–481. [Google Scholar]
- Jacobs J.D, Wingfield J.C. Endocrine control of life-cycle stages: a constraint on response to the environment? Condor. 2000;102:35–51. doi:10.1650/0010-5422(2000)102[0035:ECOLCS]2.0.CO;2 [Google Scholar]
- Kenagy G.J. Endogenous annual rhythm of reproductive function in the non-hibernating desert ground squirrel, Ammospermophilus leucurus. J. Comp. Physiol. 1981;142:251–258. doi:10.1007/BF00605743 [Google Scholar]
- King J. Cycles of fat deposition and molt in white-croned sparrows in constant environmental conditions. Comp. Biochem. Physiol. 1968;24:827–837. doi: 10.1016/0010-406x(68)90794-9. doi:10.1016/0010-406X(68)90794-9 [DOI] [PubMed] [Google Scholar]
- Klaassen M. Molt and basal metabolic costs in males of 2 subspecies of stonechats—the European Saxicola torquata rubicula and the East-African Saxicola torquata axillaris. Oecologia. 1995;104:424–432. doi: 10.1007/BF00341339. doi:10.1007/BF00341339 [DOI] [PubMed] [Google Scholar]
- Kondo N, Sekijima T, Kondo J, Takamatsu N, Tohya K, Ohtsu T. Circannual control of hibernation by HP complex in the brain. Cell. 2006;125:161–172. doi: 10.1016/j.cell.2006.03.017. doi:10.1016/j.cell.2006.03.017 [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Andersson H, Loudon A. Clock genes in calendar cells as the basis of annual timekeeping in mammals—a unifying hypothesis. J. Endocrinol. 2003;179:1–13. doi: 10.1677/joe.0.1790001. doi:10.1677/joe.0.1790001 [DOI] [PubMed] [Google Scholar]
- Lincoln G.A, Clarke I.J, Hut R.A, Hazlerigg D. Characterizing a mammalian circannual pacemaker. Science. 2006;314:1941–1944. doi: 10.1126/science.1132009. doi:10.1126/science.1132009 [DOI] [PubMed] [Google Scholar]
- Lofts B. Evidence of an autonomous reproductive rhythm in an equatorial bird (Quelea quelea) Nature. 1964;201:523–524. doi: 10.1038/201523b0. doi:10.1038/201523b0 [DOI] [PubMed] [Google Scholar]
- Loudon A.S.I. Photoperiod and the regulation of annual and circannual cycles of food-intake. Proc. Nutr. Soc. 1994;53:495–507. doi: 10.1079/pns19940060. doi:10.1079/PNS19940060 [DOI] [PubMed] [Google Scholar]
- Marra P.P, Hobson K.A, Holmes R.T. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science. 1998;282:1884–1886. doi: 10.1126/science.282.5395.1884. doi:10.1126/science.282.5395.1884 [DOI] [PubMed] [Google Scholar]
- Martin L.B, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. B. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. doi:10.1098/rspb.2002.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.B, Weil Z.M, Nelson R.J. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil. Trans. R. Soc. B. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. doi:10.1098/rstb.2007.2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab B.K. Metabolism—ecology shapes bird bioenergetics. Nature. 2003;426:620–621. doi: 10.1038/426620b. doi:10.1038/426620b [DOI] [PubMed] [Google Scholar]
- Menaker M. Circannual rhythms in circadian perspective. In: Pengelley E.T, editor. Circannual clocks. Academic; New York, NY: 1974. pp. 507–518. [Google Scholar]
- Menaker M. Circadian organization in the real world. Proc. Natl Acad. Sci. USA. 2006;103:3015–3016. doi: 10.1073/pnas.0600360103. doi:10.1073/pnas.0600360103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H. Spatiotemporal orientation strategies of long-distance migrants. In: Berthold P, Gwinner E, Sonnenschein E, editors. Avian migration. Springer; Heidelberg, Germany: 2003. pp. 493–514. [Google Scholar]
- Mrosovsky N. Mechanism of hibernation cycles in ground squirrels: circannian rhythm or sequence of stages? Pennsyl. Acad. Sci. 1970;44:172–175. [Google Scholar]
- Mrosovsky N. Hibernation and body weight in dormice: a new type of endogenous cycle. Science. 1977;196:902–903. doi: 10.1126/science.860123. doi:10.1126/science.860123 [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. Circannual cycles in hibernators. In: Wang L.C.H, Hudson J.W, editors. Strategies in the cold: natural torpidity and thermogenesis. Academic Press; New York, NY: 1978. pp. 21–65. [Google Scholar]
- Mrosovsky N. Circannual cycles in golden-mantled ground squirrels: phase shift produced by low temperatures. J. Comp. Physiol. 1980a;136:349–353. doi:10.1007/BF00657356 [Google Scholar]
- Mrosovsky N. Circannual cycles in golden-mantled ground squirrels: experiments with food deprivation and effects of temperature on periodicity. J. Comp. Physiol. 1980b;136:355–360. doi:10.1007/BF00657357 [Google Scholar]
- Mrosovsky N. Thermal effects on the periodicity, phasing, and persistence of circannual cycles. In: Heller H.C, Musacchia X.J, Wang L.C.H, editors. Living in the cold: physiological biochemical adaptations. Elsevier; New York, NY: 1986. pp. 403–410. [Google Scholar]
- Mrosovsky N. Circannual cycles in golden-mantled squirrels: fall and spring cold pulses. J. Comp. Physiol. A. 1990;167:683–689. doi:10.1007/BF00192662 [Google Scholar]
- Mrosovsky N, Fisher K.C. Sliding set points for body weight in ground squirrels during hibernation season. Can. J. Zool. 1970;48:241–247. doi: 10.1139/z70-040. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Lang K. Disturbances in the annual weight and hibernation cycles of thirteen-lined ground squirrels kept in constant conditions and the effect of temperature changes. J. Interdiscipl. Cycle Res. 1971;2:79–90. [Google Scholar]
- Mustonen A.M, Pyykonen T, Asikainen J, Hanninen S, Mononen J, Nieminen P. Circannual leptin and ghrelin levels of the blue fox (Alopex lagopus) in reference to seasonal rhythms of body mass, adiposity, and food intake. J. Exp. Zool. 2005;303:26–36. doi: 10.1002/jez.a.125. doi:10.1002/jez.a.125 [DOI] [PubMed] [Google Scholar]
- Nagy K.A, Girard I.A, Brown T.K. Energetics of free-ranging mammals, reptiles, and birds. Annu. Rev. Nutr. 1999;19:247–277. doi: 10.1146/annurev.nutr.19.1.247. doi:10.1146/annurev.nutr.19.1.247 [DOI] [PubMed] [Google Scholar]
- Nelson R.J, Demoas G, Klein S.L, Kriegsfeld L.J. Cambridge University Press; New York, NY: 2001. Seasonal cycles in immune function and disease proscesses. [Google Scholar]
- Newton, I. 2007 The ecology of bird migration London, UK: Academic Press.
- Pengelley E.T. Interrelationships of circannian rhythms in ground squirrel Citellus lateralis. Comp. Biochem. Physiol. 1968;24:915–919. doi: 10.1016/0010-406x(68)90803-7. doi:10.1016/0010-406X(68)90803-7 [DOI] [PubMed] [Google Scholar]
- Pengelley E.T, Fisher K. The effect of temperature and photoperiod on the yearly hibernating behavior of captive golden-mantled ground squirrels (Citellus lateralis tescorum) Can. J. Zool. 1963;41:1104–1120. [Google Scholar]
- Pengelley E.T, Asmundson S.J, Aloia R.C, Barnes B. Circannual rhythmicity in a non-hibernating ground squirrel, Citellus leucurus. Comp. Biochem. Physiol. A. 1976;54:233–237. doi: 10.1016/s0300-9629(76)80103-x. doi:10.1016/S0300-9629(76)80103-X [DOI] [PubMed] [Google Scholar]
- Pohl H. Circannuale Periodik beim Bergfinken. Naturwissenschaften. 1971;58:572–573. doi:10.1007/BF00598733 [Google Scholar]
- Prendergast B.J, Nelson R.J, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff D.W, editor. Hormones, brain and behavior. Vol. 2. Elsevier Science; Amsterdam, The Netherlands: 2002. pp. 93–156. [Google Scholar]
- Rensing L, Ruof P. Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol. Int. 2002;19:807–864. doi: 10.1081/cbi-120014569. doi:10.1081/CBI-120014569 [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Tan Y, Dragovic Z, Ricken J, Kuehnle T, Merrow M. Chronoecology from fungi to humans. In: Honma K, Honma S, editors. Biological Rhythms. Proc. Tenth Sapporo Symp. on Biological Rhythm. Hokkaido University Press; Sapporo, Japan: 2005. pp. 73–90. [Google Scholar]
- Sawyer L, Hennessy J.M, Peixoto A.A, Rosato E, Parkinson H, Costa R, Kyriacou C. Natural variation in a Drosophila clock gene and termperature compensation. Science. 1997;278:2117–2120. doi: 10.1126/science.278.5346.2117. doi:10.1126/science.278.5346.2117 [DOI] [PubMed] [Google Scholar]
- Scheuerlein A, Gwinner E. Is food availability a circannual zeitgeber in tropical birds? A field experiment on stonechats in tropical Africa. J. Biol. Rhythms. 2002;17:171–180. doi: 10.1177/074873002129002465. doi:10.1177/074873002129002465 [DOI] [PubMed] [Google Scholar]
- Silverin B, Massa R, Stokkan K. Photoperiodic adaptation to breeding at different latitudes in great tits. Gen. Comp. Endocrinol. 1993;90:14–22. doi: 10.1006/gcen.1993.1055. doi:10.1006/gcen.1993.1055 [DOI] [PubMed] [Google Scholar]
- Shine R, Brown G.P. Adapting to the unpredictable: reproductive biology of vertebrates in the Australian wet–dry tropics. Phil. Trans. R. Soc. B. 2008;363:363–373. doi: 10.1098/rstb.2007.2144. doi:10.1098/rstb.2007.2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J.R. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B. 2008;363:375–398. doi: 10.1098/rstb.2007.2145. doi:10.1098/rstb.2007.2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS Inc. 2nd edn. SPSS Inc.; Chicago, IL: 1991. SPSS statistical algorithms. [Google Scholar]
- Styrsky J.D, Berthold P, Robinson W.D. Endogenous control of migration and calendar effects in an intratropical migrant, the yellow–green vireo. Anim. Behav. 2004;67:1141–1149. doi:10.1016/j.anbehav.2003.07.012 [Google Scholar]
- Thomas E, Jewett M, Zucker I. Torpor shortens the period of Siberian hamster circadian rhythms. Am. J. Physiol. 1993;265:R951–R956. doi: 10.1152/ajpregu.1993.265.4.R951. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Bentley G, Ukena K, Wingfield J.C, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc. Natl Acad. Sci. USA. 2005;102:3052–3057. doi: 10.1073/pnas.0403840102. doi:10.1073/pnas.0403840102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T.P, Borgudd J, Hedenström A, Persson K, Sandberg R. Resistance of flight feathers to mechanical fatigue covaries with moult strategy in two warbler species. Biol. Lett. 2005;1:27–30. doi: 10.1098/rsbl.2004.0244. doi:10.1098/rsbl.2004.0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M.S, Marra P.P, Haig S.M, Bensch S, Holmes R.T. Links between worlds: unraveling migratory connectivity. Trends Ecol. Evol. 2002;17:76–83. doi:10.1016/S0169-5347(01)02380-1 [Google Scholar]
- Wikelski M, Lynn S, Breuner C, Wingfield J.C, Kenagy G.J. Energy metabolism, testosterone and corticosterone in white-crowned sparrows. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 1999;185:463–470. doi:10.1007/s003590050407 [Google Scholar]
- Wikelski M, Spinney L, Schelsky W, Scheuerlein A, Gwinner E. Slow pace of life in tropical sedentary birds: a common-garden experiment on four stonechat populations from different latitudes. Proc. R. Soc. B. 2003;270:2383–2388. doi: 10.1098/rspb.2003.2500. doi:10.1098/rspb.2003.2500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J.C, Farner D.S. Endocrinology of reproduction in wild species. In: Farner D.S, King J.R, Parkes K.C, editors. Avian biology. vol. IX. Academic Press; London, UK: 1993. pp. 163–327. [Google Scholar]
- Wingfield J.C, Hahn T.P, Levin R, Honey P. Environmental predictability and control of gonadal cycles in birds. J. Exp. Zool. 1992;261:214–231. doi:10.1002/jez.1402610212 [Google Scholar]
- Withers P.C. Measurement of VO2 VCO2 and evaporative water loss with a flow-through mask. J. Appl. Physiol. 1977;42:120–123. doi: 10.1152/jappl.1977.42.1.120. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Kobayashi M, Mitsui S, Ishida Y, van der Horst G.T.J, Suzuki M, Shibatall S, Okamura H. View of a mouse clock gene ticking. Nature. 2001;409:684. doi: 10.1038/35055628. doi:10.1038/35055628 [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. doi:10.1038/nature02117 [DOI] [PubMed] [Google Scholar]