Abstract
Pre-photosynthetic niches were meagre with a productivity of much less than 10−4 of modern photosynthesis. Serpentinization, arc volcanism and ridge-axis volcanism reliably provided H2. Methanogens and acetogens reacted CO2 with H2 to obtain energy and make organic matter. These skills pre-adapted a bacterium for anoxygenic photosynthesis, probably starting with H2 in lieu of an oxygen ‘acceptor’. Use of ferrous iron and sulphide followed as abundant oxygen acceptors, allowing productivity to approach modern levels. The ‘photobacterium’ proliferated rooting much of the bacterial tree. Land photosynthetic microbes faced a dearth of oxygen acceptors and nutrients. A consortium of photosynthetic and soil bacteria aided weathering and access to ferrous iron. Biologically enhanced weathering led to the formation of shales and, ultimately, to granitic rocks. Already oxidized iron-poor sedimentary rocks and low-iron granites provided scant oxygen acceptors, as did freshwater in their drainages. Cyanobacteria evolved dioxygen production that relieved them of these vicissitudes. They did not immediately dominate the planet. Eventually, anoxygenic and oxygenic photosynthesis oxidized much of the Earth's crust and supplied sulphate to the ocean. Anoxygenic photosynthesis remained important until there was enough O2 in downwelling seawater to quantitatively oxidize massive sulphides at mid-ocean ridge axes.
Keywords: Archaean, soils and weathering, anoxygenic photosynthesis, cyanobacteria, continental tectonics
1. Introduction
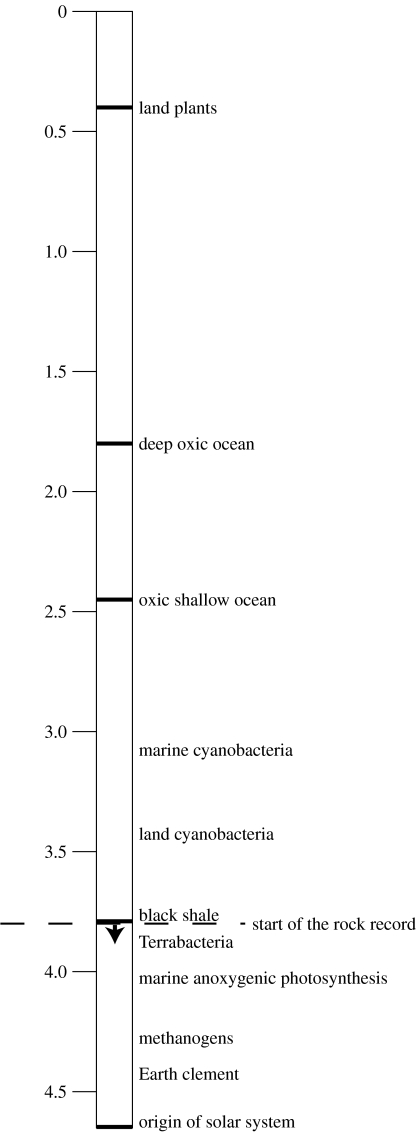
Oxygenic photosynthesis maintains our atmosphere far from equilibrium with crustal and mantle rocks (Lovelock 1979). Almost all life on the modern Earth depends ultimately on photosynthetic primary producers (e.g. Holser et al. 1988). Yet, these situations did not always prevail (figure 1). Studies of mass-independent fractionation of sulphur isotopes in ancient sediments demonstrate that dioxygen was present, in at most trace amounts, before ca 2.45 Ga (e.g. Farquhar et al. 2007; Kaufman et al. 2007). As one goes further back in time the tree of life roots in non-photosynthetic microbes (e.g. Woese et al. 1990; Pace 1991, 1997; Reysenbach & Shock 2002; Nealson & Rye 2004; Olson & Blankenship 2004). The early geological record of life is meagre as geologists have found no supracrustal rocks older than ca 3.8 Ga (e.g. Rosing & Frei 2004).
Figure 1.
Time scale in billion years before present (Ga) for events relevant to the evolution of photosynthesis. Dark lines on the column indicate well-constrained dates. The other events are in order, but their absolute ages are less well constrained. The oldest rocks (marked as ‘start of the rock record’) include black shales, which is evidence for photosynthetic life and weathering of rocks on land. The downward arrow indicates that the black shales existed on the Earth before they were preserved in the currently available rock record. We thus place events inferred to occur before these biological innovations at more ancient times.
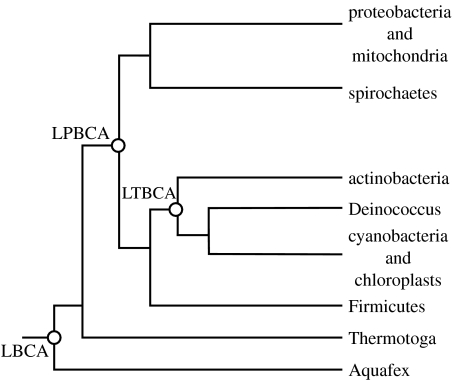
Still we have life witnesses. Molecular biochemists have interrogated the descendents of the survivors. Studies of the molecular genetics have defined candidate trees of life (figure 2). With regard to photosynthesis, two recent hypotheses are relevant. (i) Bacterial photosystems I and II are related, implying that bacterial photosynthesis originated just once (e.g. Sadekar et al. 2006; Barber 2008). Cyanobacteria evolved later by the fusion of two microbes: one with photosystem I and the other with photosystem II (Xiong et al. 2000; Baymann et al. 2001; Allen & Martin 2007). (ii) More controversially, cyanobacteria are related to actinobacteria that inhabit soil, implying that cyanobacteria, and hence oxygenic photosynthesis, originally evolved on land (Battistuzzi et al. 2004; Bern & Goldberg 2005).
Figure 2.
Tree of life for bacteria from the last bacteria common ancestor (LBCA) simplified from Battistuzzi et al. (2004). The last photobacterial common ancestor (LPBCA) developed efficient photosynthesis and evolved into the common clades of bacteria. The last Terrabacterial common ancestor (LTBCA) was a successful colonist on land.
We do not attempt to appraise molecular biology beyond noting that these inferences are near the current limits of resolution. In particular, we tentatively accept the nodes in the bacterial tree of Battistuzzi et al. (2004) at face value for purposes of discussion. However, we do not use the molecular-clock dates of Battistuzzi et al. (2004). (See Graur & Martin (2004) for a critic of such methods.) Rather, we use the geological record with Battistuzzi et al.'s (2004) sequence of nodes to constrain the absolute times of evolutionary events.
That is, we address questions that can be productively evaluated by the Earth scientists: for example, what do these evolutionary paths of metabolic processes tell us about environmental conditions and ecology on early Earth? Conversely, do the implied events in these evolutionary trees (figure 2) make geological, and hence ecological, sense? What geological events and processes on our planet modulated this biological evolution, and ultimately to what extent did biological evolution modulate the geochemical evolution of the Earth's crust and fluid envelopes, as well as its interior? Canfield (2005), Canfield et al. (2006) and Rollinson (2007) provided excellent reviews on geological aspects of dioxygen and photosynthesis. Buick (2008), Kirschvink & Kopp (2008) and Nisbet & Nisbet (2008) discussed the geological record and the timing of evolutionary events.
Here we consider these questions geochronologically as a sequence of interlinked geological and biological events in the Earth's evolution (figure 1). We begin the discussion at a point in geological time where cellular organisms have evolved and colonized our planet. The origin of life, survival of life after asteroid impacts and interplanetary transfer of life are not explicitly considered.
For clarity, we note that geological and molecular biological terminologies have evolved separately. In the present paper we focus on general issues and define other terms as they arise.
Strict biological definitions in terms of modern chemical pathways are somewhat inapplicable to the early Earth before (ca 3 Ga) as no suitably preserved rock record exists. They are likely to be inappropriate to other planets. We thus take a functional approach using the net chemical effects of biological innovations. For example, we include ‘light gathering’ within ‘photosynthesis’ (see Rothschild 2008). There is no standard term and no convenient candidate for the chemical species that is oxidized during anoxygenic photosynthesis. Geologists pre-empt ‘sink’ to indicate a region of geological scale that sequesters material for geological times. We realize that molecular biologists use ‘acceptor’ in a different manner (see below).
We distinguish oxygen that is bound in compounds with elements that have only one oxidation state in geological environments (i.e. Na2O, MgO, Al2O3, SiO2, K2O, CaO) from the oxygen that is available for biological redox reactions. Large geological reservoirs of available oxygen include atmospheric and dissolved aqueous dioxygen, O2 and the oxygen bonded to iron and sulphur in their various oxidation states.
Finally, we nowhere imply a purpose-driven ‘teleological’ approach to evolution. Below we identify selective environmental pressures that might drive natural selection, and functional pre-adaptations for photosynthesis where they are evident. For example, Tabita et al. (2008) discussed non-photosynthetic biochemistry that evolved along one line of descent to RubisCO. However, we do not attempt to find actual molecular sequences of ancient biological innovations.
2. Pre-photosynthetic ecology
Canfield et al. (2006) and Sleep & Bird (2007) evaluated the nature of pre-photosynthetic niches on the early Earth. Tectonics and volcanism maintained reliable sources of available chemical (Gibbs) energy to sustain chemoautotrophic ecosystems. A key point for evolution is that the global primary productivity was small. Sleep & Bird (2007), as discussed below, estimated primary productivity of 0.06 Tmol yr−1 of carbon (1 Tmol=1012 mol). This rate is small compared with the current photosynthetic productivity of approximately 104 Tmol yr−1 (e.g. Holser et al. 1988). In addition, it is small relative to the overall turnover rate of surface carbon on the modern Earth, i.e. the current organic carbon burial rate of 10 Tmol yr−1 and the current inorganic carbon burial rate of 40 Tmol yr−1 (e.g. Holser et al. 1988). For visualization, we note that each carbon atom goes through photosynthesis and decay approximately 200 times (104 Tmol yr−1/(10 Tmol yr−1+40 Tmol yr−1)) before it is buried on the modern Earth (e.g. Sleep & Bird 2007). In the pre-photosynthetic biosphere, only 1 carbon atom in approximately 700 became organic matter during its sojourn in surface environments. This vast productivity difference between photosynthetic and pre-photosynthetic ecosystems implies that even the earliest photosynthetic systems were far more productive (probably by factors of thousands) than the ecosystems they supplanted.
We use productivity as a quantifiable (rate) proxy for the gross (standing crop) qualities of biomass, abundance, geographical distribution and taxonomic diversity, which influence at a given time whether a clade of organisms leaves descendants. In particular, the metabolic evolution of serviceable photosynthesis probably led to a population explosion of the innovative organism(s) followed by their adaptive radiation. With these concepts in mind, we begin by evaluating pre-photosynthetic niches to illustrate environmental conditions that pre-adapted life for photosynthesis.
(a) Available non-photosynthetic niches
Metabolic processes of the pre-photosynthetic biosphere most likely involved energy derived by redox disequilibrium involving Fe, S, C, H2 and O2 (Walker 1977a,b). On a thermodynamic basis, successful niches were formed in regions where tectonic and fluid movement brought together combinations of these elements with incompatible oxidation states (Shock et al. 1995; Nisbet & Sleep 2001; Nisbet & Fowler 2004; Kharecha et al. 2005; Canfield et al. 2006; Sleep & Bird 2007). Hydrogen generated by oxidation of Fe(II) minerals provides an important chemical niche (McCollom & Shock 1997; McCollom 1999; Hoehler 2005; McCollom & Amend 2005). The common example is hydrothermal circulation through basalt (Wetzel & Shock 2000; Canfield 2005; Sleep & Bird 2007) and serpentinite (Berndt et al. 1996; McCollum & Seewald 2001; Charlou et al. 2000; Seyfried et al. 2004; Sleep et al. 2004; Kelley et al. 2005), which release hydrogen to aqueous solutions by water–rock reactions expressed in general form as
| (2.1) |
Microbes use H2 in two generalized ways. Methanogens reduce CO2 by H2 in a dissimilative reaction
| (2.2) |
Methanogenic reduction of CO2 by H2 provides energy for reactions such as the synthesis of ATP from ADP and inorganic phosphate. Acetogens reduce CO2 in an assimilative reaction
| (2.3) |
which provides complex organic matter (idealized formula CH2O) for the cell, as well as energy if the reactants CO2 and H2 are abundant (e.g. Hoehler et al. 1998). By analogy with modern microbes, most of the available hydrogen was probably used for producing energy in reaction (2.2), rather than organic matter in reaction (2.3) (Canfield et al. 2006; Sleep & Bird 2007).
Arc volcanoes (which form about zones of crustal subduction; Fujiyama and Mount St Helens provide modern examples) were a source for SO2 and hydrogen gases that were probably used by pre-photosynthetic organisms (e.g. Canfield et al. 2006; Sleep & Bird 2007). Microbes obtain energy by reactions that have the net effect of disproportionating sulphite
| (2.4) |
The reaction may have occurred in two steps with photolysis disproportionating SO2 into native sulphur and sulphate and then microbes disproportionating the native sulphur (Philippot et al. 2007). Microbes also react sulphite and sulphate with organic matter and hydrogen to obtain energy.
Photolysis from UV light decomposes atmospheric methane into carbon monoxide
| (2.5) |
Photolysis also produces complex organic haze (Pavlov et al. 2001)
| (2.6) |
The biological consequence of an atmosphere that retains hydrogen is that the rate of photolysis of methane controls productivity (see Tian et al. 2005; Canfield et al. 2006). However, photolytically produced hydrogen gas probably escaped to space (Kharecha et al. 2005; Catling 2006), and hence did not provide substrate for methanogens and acetogens. Concentrating on that possibility, carbon monoxide (reaction 2.5) and organic haze (reaction 2.6) return to the surface where they provide a substrate for organisms that eventually produce methane and carbon dioxide (the reverse of reactions 2.5 and 2.6). Some modern microbes use CO as an energy source (Rother & Metcalf 2004; Sokolova et al. 2004) and as an assimilatory source of carbon in organic matter (Rother & Metcalf 2004; Ferry & House 2006). The amount of hydrogen available from geological processes limits the productivity of niches that use it directly, as well as niches that use the products of photolysis.
(b) Pre-adaptations for photosynthesis
An essential pre-adaptation for photosynthesis is the ability to tolerate sunlight in surface environments. Photolytic products, together with hydrogen and SO2 from arc volcanoes, provided potential metabolic energy sources in surface environments. These included shallow marine water, lakes, land and even clouds. Being able to tolerate slightly more sunlight than one's competition was ultimately a selective advantage when competing for atmospheric photochemical products.
The acetogenesis reaction (2.3) is the basis for a modern form of anoxygenic photosynthesis (Ehrenreich & Widdel 1994; Zaar et al. 2003). This metabolic process is a form of photocatalysis, as the reaction yields energy (except in extremely hydrogen-poor environments). The energetics of reaction (2.3) are advantageous for the evolution of efficient photosynthesis, with every intermediate modification beneficial to the organism. That is, an organism with an inept ability to obtain a slight metabolic benefit by using available sunlight may gradually evolve by greatly speeding up the rate at which it uses hydrogen through photosynthetic energy fixation. This innovation increased the fraction of the available H2 that produced organic matter (reaction 2.3) versus methane (reaction 2.2), and hence total productivity (Jim Kasting 2007, personal communication). However, hydrogen-based photosynthesis does not provide for an ecosystem with large productivity unless the atmosphere retains sufficient quantities of H2. The products of hydrogen-based photosynthesis have a lower energy than the reactants, unless the hydrogen concentration is very low (see Lee & Zinder 1988; Kotsyurbenko et al. 2001). There is thus no heterotrophic niche with a back reaction that recycles the initial substrates. The productivity of the ecosystem depends on the rate that geological processes irreversibly produce H2 (Sleep et al. 2004). We note that Tice & Lowe (2004, 2006) have suggested, based on mineralogical and trace element distributions, that thin carbonaceous laminations preserved in shallow-water facies of the ca 3.4 Ga Buck Reef Chert represent bacterial mats that used aqueous H2 as a reductant for photosynthesis.
3. Efficient anoxygenic photosynthesis
We begin this section with the idealized chemistry of a bacterium that uses ferrous iron and/or sulphide as oxygen acceptors. We continue with the consequences of life becoming abundant after those evolutionary innovations. Obtaining energy from reactions involving sulphur and iron is an obvious pre-adaptation along with hydrogen-based photosynthesis. In this regard, Jiao et al. (2005) discussed a ferrous-iron-using photosynthetic microbe that can also use H2 for photosynthesis as well as to increase its rate of Fe-based photosynthesis.
Idealized reactions for anoxygenic photosynthesis are
| (3.1) |
(e.g. Grassineau et al. 2001) and
| (3.2) |
(e.g. Ehrenreich & Widdel 1994; Kappler & Newman 2004). The reverse of reaction (3.1) with sulphate (Canfield & Raiswell 1999; Nisbet & Fowler 1996; Xiong et al. 2000) and reaction (3.2) with ferric iron (Schroder et al. 2003; Luu & Ramsey 2003) yields usable energy and forms the metabolic basis for modern heterotrophic biota.
The metabolic innovation of sulphur- or iron-based photosynthesis freed a bacterium of its dependence on redox disequilibrium in geological processes as a source of available energy. Battistuzzi et al. (2004) proposed that this organism (their node I, last photobacterial common ancestor (LPBCA) in our figure 2) is the ancestor of common bacterial clades. Battistuzzi et al. (2004) did not formally name this clade; here we use ‘photobacteria’ as an obvious informal term (figure 2). This inference is compatible with the finding that photosystems I and II are related with a common ancestor that evolved the trait just once (e.g. Sadekar et al. 2006; Barber 2008).
The photobacterial root to common bacteria makes ecological sense. Even a slow rate of iron- or sulphur-based photosynthesis would add to an organism's productivity. Note that ferrous iron and sulphide form complex ions, so both components can be present in seawater at the same time (Saito et al. 2003). Once it had evolved to the point that it had no obligate need for H2, the organism found abundant ferrous iron and sulphide in seawater as substrates. Its descendents soon became the dominant organism(s) on the Earth. As already noted, a productivity of even a few percentage of that of the modern Earth is thousands of times the pre-photosynthetic productivity. Adaptive radiation began on a scale of years to centuries as atmospheric and oceanic currents redistributed the organism. It is biochemically unlikely that another microbe species would have independently evolved non-hydrogen-based photosynthesis during this geologically brief time.
In general, the existence of heterotrophs increases the productivity of an ecosystem. Today, each carbon atom exposed by geological processes is cycled through photosynthesis and decay approximately 200 times before it is buried as carbonate or organic carbon (e.g. Sleep & Bird 2007). That is, modern productivity is approximately 200 times higher than it would be if each exhumed organic carbon atom went through photosynthesis and decay just once. The extreme case that organic matter is always buried and never consumed as an energy source for heterotrophs would result over a period of 108 years (cycle time of sedimentary carbon from Holser et al. 1988) in all the crustal carbon being sequestered in sedimentary rocks in its reduced oxidation state.
In the case of anoxygenic photosynthesis, heterotrophs recycle both the biomass and the oxygen provided by ferric iron oxides/hydroxides and sulphates. The first photobacterium may very well have been able to use dead organic matter as an energy source. Sulphur-based organisms have this ability (Canfield & Raiswell 1999; Nisbet & Fowler 1996; Xiong et al. 2000), but apparently iron-based photosynthetic organisms do not (Dianne Newman 2007, personal communication). This facultative skill is advantageous to organisms that encountered prolonged periods of darkness within their geographical niches. If so, the photosynthetic organism probably evolved into its own diverse consortium of symbionts and heterotrophs as implied by the photobacterial root hypothesis.
Before continuing with bacteria evolution, we discuss two analogous situations where the evolutionary innovation of photosynthesis benefited an organism and started a population explosion. Woese (1977) discussed the first event to which we do not attempt to assign an absolute age. He proposed that mitochondria are the descendants of an organelle that once acted as an anoxygenic photosynthetic ‘chloroplast’. The eukaryote's last common ancestor captured a purple sulphur bacterium. The combined organism was larger and occupied a different niche than smaller photosynthetic microbes. It prospered, adaptively radiated and roots extant eukaryotes. The second occurred ca 10 000 years ago (Colledge et al. 2004). Alert members of our species observed that plants grow from seeds. The resultant agriculturists freed humans from depending on the vagaries of what they could hunt and gather. It has led to a massive increase in human population, and the Early Anthropogenic Hypothesis of Ruddiman (2003), which posits that agricultural activity since ca 6000 BC produced quantities of greenhouse gases sufficient to counteract declining insolation and prevent global glacial expansion. This event, however, occurred too recently for much human adaptive genetic radiation to have taken place.
4. Colonization of land and the origin of soil
Waves, tides, sea spray and storms transported countless photosynthetic microbes from their marine ecosystem onto land. The proposed grouping of actinobacteria (soil bacteria), radiation-tolerant Deinococcus and cyanobacteria into the clade Terrabacteria bears on the nature of the successful colonist (Battistuzzi et al. 2004; Bern & Goldberg 2005). The first Terrabacterium on land faced vicissitudes. It could not avoid UV light and desiccation in exposed environments, driving selection for efficient DNA repair. It also needed to obtain nutrients that were ubiquitous in seawater and to rid itself of oxygen as in reactions (3.1) and (3.2) to perform anoxygenic photosynthesis. With regard to oxygen acceptors, we note that sulphur (as sulphide) and ferrous iron are soluble in water, they are readily available to react with oxygen, and as solutes or as components of colloidal matter tend to get flushed from open drainages or swept downwards by ground water, eventually reaching the ocean.
Mass balance bears on auspicious chemical paths for land-based anoxygenic photosynthetic microbes. Ferrous iron is the most abundant potential oxygen acceptor in common rocks. Mafic and ultramafic rocks are about 10% FeO by mass. There is approximately 7% FeO in andesite from arc volcanoes. By contrast, sulphide is typically present in minor amounts, approximately 0.1% S in basalt (e.g. Krauskopf & Bird 1995). Sulphur oases did exist in the oceanic and continental environments as the element concentrates into massive sulphide deposits at mid-ocean ridges, and when obducted and eroded on land (e.g. Peterson 1988; Gustin 1990; Slack et al. 2007). It could build up into a sulphuretum within water bodies in closed drainages including the ocean. The potential oxygen acceptor, MnO, is present in minor amounts (0.18% in basalt, e.g. Krauskopf & Bird 1995). Other potential acceptors are present only in trace amounts in common rock types.
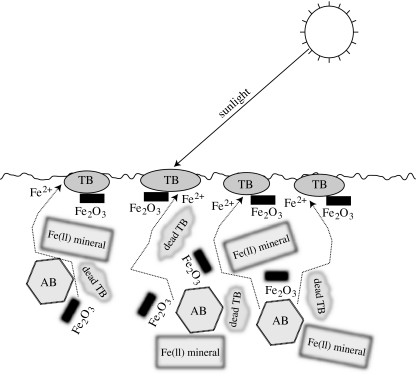
An iron-based photosynthetic ecosystem (i.e. reaction 3.2) faces the limitation that Fe(III) is largely insoluble in water, and hence immobile (e.g. Walker 1977a,b). Soils and lake bottoms are efficient traps for the products of iron-based photosynthesis. Dead photosynthetic individuals get washed downwards or buried, sequestering organic carbon (figure 3). Formation of low-solubility oxides/hydroxide of Fe(III) and Mn(IV/III) will sequester available oxygen, as well as biologically important metals and phosphorus, by surface sorption processes (Crowe et al. 2007a,b). Extant organisms have evolved complex mechanisms to turn the buried mixture of oxidants, nutrients and organic matter in soils from inconvenience to bounty (Crowe et al. 2007a,b). They react immobile Fe- and Mn-oxides and hydroxides with organic matter and hydrogen, mobilizing nutrient elements in the process. Organisms can even use well-crystallized materials, such as specular haematite (Gonzalez-Gil et al. 2005). Some organisms have biological electrically conducting ‘wires’ that connect the same or different species (Gorby et al. 2006). The soil then acts as a battery where the biological wires transmit a current from which the organisms extract energy. The process increases the macroscopic electrical conductivity of the soil (Davis et al. 2006). Overall, these processes accomplish long-range (centimetre scale) chemical and energy transport in soils and lakebeds. That is, their function is analogous to that of roots.
Figure 3.
Schematic of soil with ecosystem based on anoxygenic photosynthesis. Photosynthetic Terrabacteria (TB) produce organic matter and Fe(III) oxide and/or oxyhydroxide minerals (marked as Fe2O3) from atmospheric CO2 and Fe2+ dissolved in the soil water (reaction 3.2). Actinobacteria (AB) in the soil obtain energy by reacting organic matter (including dead photosynthetic microbes) with Fe(III), the reverse of reaction (3.2), producing aqueous Fe2+ (arrows). The soil bacteria also weather Fe(II)-bearing minerals, releasing Fe(II) into solution, which increases the productivity of the ecosystem.
Overall, we avoid the tendency to regard land on the ancient Earth as barren or even sterile. We observe barren slopes today only in harsh environments such as deserts where land plants do poorly and land recently denuded by acid from industrial smelters or agricultural clearing. These localities are not good analogues for well-watered low-relief ancient land environments.
5. Shale, granite and cyanobacteria
Soil microbes use energy ultimately from photosynthesis to weather exposed rock and form soil. By contrast, pre-photosynthetic microbes subsisted on a much smaller amount of available energy from the rock. Land ecosystems of photosynthetic and soil bacteria thus had productivities far beyond those of pre-photosynthetic land ecosystems. Biology began to modulate geological processes by harvesting solar energy and channelling it into the geochemical cycle. Subsequently, biological forcing of weathering and diagenetic rock alteration affected the geological evolution of the Earth's crust (Rosing et al. 2006).
Anoxygenic photosynthetic ecosystems benefit from efficient weathering. The primary producers acquire ferrous iron as an oxygen acceptor. Minerals and volcanic glass chemically hold this compound. To obtain it, microbes chemically decompose much of the rock (figure 3). This process leaves soil with an aluminium hydroxide-rich clay fraction, soluble hydroxides of sodium, potassium, magnesium and calcium, and silica. All these materials move downstream as solids and in solution. Their eventual deposition yields the well-known partition of sedimentary rocks into shale, sandstones and chemical precipitates. Sand forms from quartz-bearing igneous and metamorphic rocks and from recycled chert.
We note that the existence of anoxygenic photosynthesis provides an explanation for the observation of pronounced chemical weathering in some Archaean paleosols (Retallack 2001, pp. 241–242) and Archaean sediments (Corcoran et al. 1998, 1999; Lowe & Tice 2007). Soil microbes in an anoxygenic ecosystem benefit by rapidly weathering Fe(II)-bearing silicates as the availability of this component in reaction (3.2) limits productivity. That is, the soil microbes in essence farm photosynthetic microbes by providing their substrate ferrous iron.
Elevated weathering temperatures have been proposed as an alternative explanation for enhanced Archaean chemical weathering. Lowe & Tice (2007) preferred 50–73°C for ocean temperatures, while Corcoran et al. (1998, 1999) stated that the land surface temperatures were as high as 80°C. For reference, rocks on the modern Earth weather at approximately 25°C except in extremely year-round cold environments (Ekart et al. 1999). Elevated concentrations of CO2 in the atmosphere would both directly speed chemical weathering and maintain an effective greenhouse climate (Lowe & Tice 2007).
These hypotheses are potentially testable. The hierarchical order through which different minerals weather and the relative mobilization of different trace elements should depend on whether an anoxygenic ecosystem, high temperatures or high CO2 speeds weathering. In particular, iron is merely one of many nutrients in a modern (or putative ancient) ecosystem based on oxygenic photosynthesis. Non-Fe(II)-bearing minerals carry other nutrients, e.g. potassium in mica and feldspar. There is thus no obvious modern advantage for soil microbes to rapidly weather Fe(II)-bearing minerals compared to other minerals. Further consideration of these complex issues is beyond the scope of this paper.
(a) Weathering and tectonics
The presence of detrital and chemical sedimentary rocks and their deformation in orogens is pervasive on the Earth. Nineteenth-century geologists recognized vast thicknesses of these deposits and their subsequent deformation in ‘geosynclines’, as well as the genesis of granites being an indirect result of weathering following the melting of hydrous minerals in metamorphosed sediments (e.g. Marvin 1973). The accumulation of granitic rocks modulated tectonics by stabilizing continents, increasing land-based niches (Rosing et al. 2006). Thick stratigraphic sequences of shale (and granitic and metamorphic rocks derived from it) are thus weak biosignatures. Black shales and metamorphic rocks formed from them (including diamond-bearing gneiss, Searle et al. 2001, and some kimberite diamonds, Nisbet et al. 1994) are strong biosignatures for photosynthesis. In an environment without the accumulation of carbon from photosynthesis, shales are unlikely to accumulate organic carbon. Their minerals are already weathered and near chemical equilibrium with near surface environments. Hence, they provide no biochemical resource for chemotrophs. Conversely, buried organic carbon became voluminous only after photosynthesis.
The effects of weathering on tectonics provide durable biosignatures for the early Earth and other planets. Following Perry et al. (2006a), organic matter accumulates in black shales with high concentrations of the heat-producing radioactive element uranium. Thick uranium-rich deposits formed along passive continental margins (modern analogues include deltas of the Mississippi and Niger Rivers) became parts of orogens (regions of continent–continent collisions that become mountain belts; the Alps and the Himalayas are modern examples). Continental collisions carried the sedimentary rocks to crustal depths with locally elevated temperatures. After the orogeny, the metamorphic products of this process remain as crustal scale terrains of uranium-rich rocks within stable continents. Geophysical measurements have provided information on the regional scale of such terrains with elevated crustal heat production. In general, higher than average heat flow is associated with metasedimentary rocks in orogenic terrains (Perry et al. 2006a).
Ultimately, the biologically induced high concentrations of uranium in metamorphosed shales modulated subsequent continental tectonics because geotherms are steeper than average in these areas. The elevated temperature from radioactive heat generation at depth makes the lithosphere somewhat weaker than average. That is, the physics is like that of a chain, the weakest link; here the region with an elevated geotherm fails. Perry et al. (2006b) discussed an example in the Canadian Shield where thrusting occurred in such a region of elevated geotherm, although not the one associated with black shales.
(b) Biological response to low-Fe terrains
Weathering, however, led to hostile land and freshwater environments for anoxygenic photosynthesis. In low-relief areas with slow erosion, the aqueous Fe2+ from weathering eventually flowed downstream leaving the soil depleted in iron. Exposed sedimentary rocks including shales, many carbonates, sandstone, chert and quartzite are strongly depleted in iron to begin with. Granitic rocks are also a meagre source of iron in crustal environments. That is, with the exception of basalt, most regional lithological terrains were not major sources of iron for metabolic processes.
The idealized reaction for oxygenic photosynthesis is
| (5.1) |
The ability to use this process had a selective advantage to microbes inhabiting Fe-poor environments on land. Production of even small quantities of O2 increased the total productivity of an Fe-based photosynthetic organism in ferrous-iron-poor environments, as did the ability to use meagre MnO in the soil as an oxygen acceptor. Hence, it is thus reasonable that cyanobacteria evolved on land from Terrabacteria. The complicated molecular evolutionary steps that led to oxygenic photosynthesis are beyond the scope of the paper (see Barber 2008).
Once cyanobacteria had innovated a complicated biochemistry for oxygenic photosynthesis, their probable niches were near the top of the soils or in shallow waters. Innovation of dioxygen tolerance became initially valuable on land. On the ancient Earth, cyanobacteria easily produced dioxygen oases in poorly ventilated endolithic and pond environments cornering the photosynthetic resource for themselves. (In analogy, microbes saturate modern swamp water with methane in gross disequilibrium with the atmosphere.) Dioxygen-based heterotrophy became a valuable innovation in such micro-ecosystems.
6. Barriers to oxygenating the atmosphere
Burial of organic carbon sequesters it from reacting with dioxygen. There is an intuitive tendency to regard this process as key for the 109 year scale build-up of atmospheric dioxygen on the Earth. However, uplift and erosion exhume buried carbon on a scale of 108 years where it once again enters the surface ecosystems of heterotrophy and photosynthesis (e.g. Holser et al. 1988). Thus, burial of organic carbon does not explain the rise of O2 in the air.
(a) Mass balance of oxygen reservoirs
Conservation of mass provides a basis for evaluating the build-up of atmospheric dioxygen over geological time. It is important to note that atmospheric oxygen is only a modest reservoir of available oxygen (38×1018 mol, Holser et al. 1988) when compared with the large reservoirs represented by marine (80×1018 mol, Holser et al. 1988) and sedimentary sulphates (280×1018 mol, Holser et al. 1988). The excess oxygen in ferric iron in ‘crystalline’ continental crustal rocks is 2000×1018 mol as O2 (Lecuyer & Ricard 1999). (We use the term ‘excess’ to denote that much of this reservoir lies below the depths normally exhumed by erosion. It is thus not ‘available’ to life on a time scale of 108 years. Sedimentary rocks including banded iron formations are much smaller reservoirs of excess oxygen (Holser et al. 1988).) This quantity is predicted by the difference between the observed molar ratio of iron in Fe(III) to total Fe in crustal rocks and the ratio in the mantle-derived rocks that ultimately formed the crust. As a practical matter, Fe(III)/(total Fe)=∼0 in the mantle-derived rocks, so that its uncertainty is small compared with that of estimates of the global mass of oxidized crustal material (Sleep 2005).
Both excess oxygen (2000×1018 mol) and available oxygen (400×1018 mol) are likely to be ultimately products of photosynthesis. However, their sum (2400×1018 mol) is twice the ‘visible’ reduced reservoir from photosynthesis, buried carbon (1200×1018 mol, Holser et al. 1988). One must thus postulate a ‘hidden’ reduced reservoir. Subduction of organic-rich sediments is one possibility. Catling et al. (2001) suggested an alternate mechanism involving methane ultimately derived from photosynthesis. That is, photolysis decomposes methane by reactions (2.5) and (2.6) and hydrogen escapes to space. The hydrogen ultimately comes from water, so the net effect is
| (6.1) |
This process can occur only when there is negligible dioxygen in the air. Overall, anoxygenic photosynthesis could have supplied the sulphate and excess ferric iron reservoirs (reactions 3.1 and 3.2). Neither anoxygenic photosynthesis nor hydrogen escape from methane photolysis could have supplied the Earth's atmosphere with dioxygen.
(b) Chronology of dioxygen
Geological processes that consume available oxygen and produce excess oxygen continue to operate. Dioxygen can persist in the atmosphere and the ocean only if its net rate of production balances its geological loss (e.g. Sleep 2005). Three major sinks are evident. (i) Ferrous iron in continental rocks is oxidized during weathering, producing over geological time a large reservoir of excess oxygen in the Earth's crust. (ii) The water in modern ‘black smoker’ vents is quantitatively depleted in sulphate and carries abundant sulphide. Downwelling seawater feeds these hydrothermal systems (figure 4). Approximately, nine-tenths of the dissolved marine sulphate precipitates in the shallow oceanic crust as anhydrite CaSO4. This mineral later redissolves into the ocean and is not a net sulphur or available oxygen sink. The rest, approximately one-tenth of the marine sulphate, reacts with ferrous iron in basalt to form sulphide. The flux from this process is equivalent to approximately 0.5 Tmol yr−1 of O2 on the modern Earth (e.g. Sleep 2005) compared with the organic carbon burial rate of 10 Tmol yr−1 (e.g. Holser et al. 1988). (iii) Sulphide in basalt and that formed by the reduction of marine sulphate dissolve into the hot fluid. Massive sulphide deposits form when the fluid quenches near vents, and today dissolved dioxygen in the seawater oxidizes the available sulphide to sulphate (figure 4). The flux from massive sulphide oxidation is approximately 0.5 Tmol yr−1 of O2 on the modern Earth (e.g. Sleep 2005).
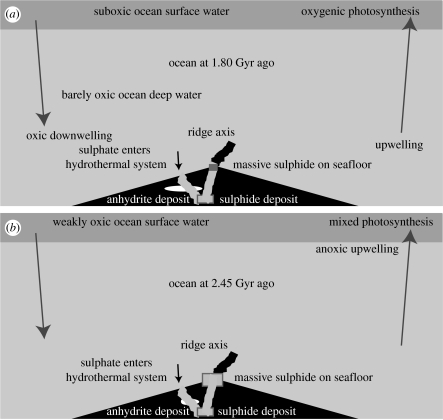
Figure 4.
Schematic of (a) the ocean and atmosphere at 1.80 Ga when the deep ocean became barely oxic (much oxygen (800 ppm) in the air) and (b) at 2.45 Ga when the shallow ocean and atmosphere became barely oxic (some oxygen (2 ppm) in the air). At 1.80 Ga, there was enough dioxygen in downwelling water to just oxidize massive sulphide deposits on the seafloor. Upwellings and the deep ocean were barely oxic. The dioxygen flux from oxidizing seafloor sulphides has remained independent of the dioxygen concentration in the ocean and the atmosphere since that time. At 2.45 Ga, the sulphate concentration in seawater was enough that anhydrite precipitated in hydrothermal systems. The amount of sulphide precipitated at depth by hydrothermal circulation became independent of the seawater sulphate concentration after that time. The amount of anhydrite that precipitates and later dissolves has increased with time as the seawater sulphate concentration increased.
The three processes involving oxygen have evolved differently over geological time (figure 5). The rate that excess oxygen accumulates into the Earth's continental crust, and perhaps the rate that tectonics expose fresh rock with ferrous iron, has decreased over time. There is also less ferrous iron in the crust remaining to be oxidized. We have not identified any obvious transition in the geological record that we can associate with oxidation of ferrous to ferric iron in the crystalline rocks of the Earth's crust (see Holland (2002) and Kump & Barley (2007) for a hypothesis involving the change of the composition of volcanic gases over time). The flux of sulphate into high-temperature hydrothermal systems at mid-ocean ridges depends on its concentration within the downwelling seawater, which was much lower in the early ocean than at present (e.g. Canfield et al. 2006). At first, the concentration was below approximately one-tenth of the current level. Anhydrite did not form in the downwelling limbs of axial hydrothermal systems; rather sulphate entering hydrothermal systems was quantitatively reduced so that the sulphur and oxygen fluxes were proportional to the sulphate concentration in seawater. Hydrothermal circulation vented sulphide to seawater. Most of this material precipitated on the seafloor as massive sulphides that were not oxidized. Some sulphide remained in solution in the photic zone. Anoxygenic photosynthesis oxidized the dissolved sulphide directly to sulphate (reaction 3.1). Dissolved dioxygen could not increase above trace levels in the ocean and atmosphere, as long as significant dissolved sulphide remained, which consumed dioxygen as soon as it was produced by photosynthesis.
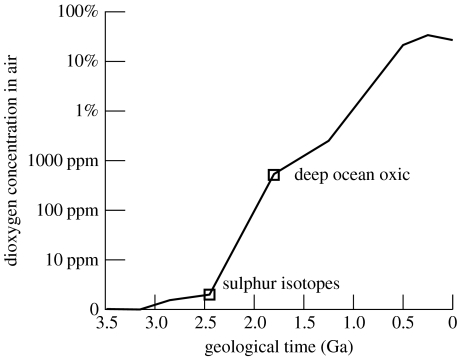
Figure 5.
Schematic history of the dioxygen concentration in the air over geological time on a truncated logarithmic scale. Sulphur isotope systematics and the transition to oxic conditions in the deep ocean provide data. (See text for details, and Canfield (2005), Rollinson (2007) and Kump (2008) for other estimates of the dioxygen history.)
Eventually, the concentration of marine sulphate built up and exceeded approximately one-tenth of the present day level. Anhydrite formed from reaction of downwelling fluids with basalts marginal to submarine hydrothermal systems at ridge axes (figure 4). The available oxygen flux into the oceanic crust was no longer dependent upon sulphate concentration, which was constrained by local equilibrium with anhydrite. Sulphate continued to build up in seawater. Formation of anhydrite as an evaporite is currently an effective but episodic reservoir for marine sulphate.
The shallow ocean was (sub)oxic after that time. The dynamic equilibrium of dissolved dioxygen in the shallow ocean with the air makes this transition observable from studies of mass-independent sulphur fractionation. There was significant dioxygen in the atmosphere at 2.45 Ga (figure 5; e.g. Kaufman et al. 2007; Farquhar et al. 2007). Downwelling surface waters for the first time carried dissolved dioxygen into the deep ocean, which was anoxic (e.g. Saito et al. 2003; Canfield et al. 2006). Circulating seawater oxidized part of the exposed massive sulphide deposits (figure 4). This quantitatively exhausted the dioxygen in the water.
The inference that dioxygen in downwelling seawater oxidized much of the available massive sulphide deposits provides a straightforward way to estimate the dioxygen concentration in seawater at that time (figure 4). Photosynthesis oxygenated seawater while it was in the photic zone. The flux of shallow water into the deep ocean was the mass of the ocean Mocean divided by its circulation time tc. The dioxygen flux was this quantity times the concentration of dioxygen XO2 in shallow water. Conservation of mass requires this flux to be equal to the dioxygen flux derived from oxidizing massive sulphides (FO2). Solving for the dioxygen concentration yields
| (6.2) |
The terms on the right-hand side of equation (6.2) are grossly constrained by their modern geological values. For an example calculation that strives for a factor of a few accuracy, we let the flux of the dioxygen sink (FO2) to be 1 Tmol yr−1, the circulation time of the ocean is assumed to be 3000 years and the mass of the ocean is taken as the present value, 13.7×1020 kg. The process buffered dissolved dioxygen in shallow water at a minor, but finite concentration of approximately 2 μM, approximately 0.4% of the current level. The O2 concentration in the air in equilibrium with the shallow ocean was 840 ppm (figure 5, plotted at 1.8 Ga, see below for details).
Conversely, dissolved sulphide in upwelling seawater was oxidized ultimately by photosynthesis. Thus, it became a minor species in seawater with its amount determined by the flux of sulphide from vents during the circulation time of the ocean, crudely scaling with the O2 concentration in shallow water. During this epoch, anoxygenic photosynthesis continued to prosper. Upwellings were anoxic and nutrient rich. Cyanobacteria had to compete with anoxygenic photosynthetic microbes in the regions of oceanic upwellings, or persist within oxygenated water on their flanks. Biochemical studies show that at least some clades of cyanobacteria evolved in anoxic marine water (Saito et al. 2003).
Eventually, there was enough dioxygen in the downwelling marine water to quantitatively oxidize exposed massive sulphides. The dioxygen flux from sulphide oxidation depended only on the rate that ridge axes produced massive sulphides and became independent of the dissolved dioxygen concentration in downwelling seawater. The deep ocean became oxic and upwellings no longer carried dissolved sulphide and ferrous iron to the surface. The end of significant dissolved iron is observed in the geological record (marked as ‘deep oxic ocean’ in figure 1); widespread banded iron formation deposits end at ca 1.8 Ga (e.g. Slack et al. 2007). There is also a marked change in marine Fe isotopes systematics at this time ca 1.8 Ga (Rouxel et al. 2005). Studies of deep-sea deposits indicate that the deep ocean was suboxic (less than 5 μM of O2) after approximately 1.8 until at least ca 1.24 Ga (Slack et al. 2007; figure 5). This upper limit on O2 concentration in the deep ocean is in gross agreement with our mass balance estimate of 2 μM at 1.8 Ga.
Thallium isotope studies provide a very durable biosignature for oxic conditions in the deep ocean, as well as a measure of the pervasive effects of photosynthesis on the interior of the Earth (Nielsen et al. 2006a). Circulating oxic seawater leaches thallium from the shallow oceanic crust (layer 2A). This thallium accumulates in manganese nodules on the seafloor, and the process is recorded by thallium isotope fractionations (Nielsen et al. 2006b). This fractionation in ancient rocks is evidence that the deep ocean was oxic. Nielsen et al. (2006a) detected thallium isotope variations and coupled chemical variations in Hawaiian basalts. This implies that the source region of the basalts contained ancient subducted deep-sea sediments with vestiges of manganese nodules that have been transported into the deep mantle and then ascended to shallow depths within the Hawaiian plume.
Falkowski & Godfrey (2008) discussed additional topics related to the transition from anoxic to oxic air and water with emphasis on the nitrogen cycle. We do not consider here the later history of dioxygen in the air and ocean, as excellent reviews are provided by Anbar & Knoll (2002), Berner et al. (2003), Hansen & Wallmann (2003) and Kump (2008).
7. Conclusions
Throughout the Earth's history, biology has interacted with surface geological processes, and even modulated tectonics and the geochemical evolution of the crust (cf. Rosing et al. 2006; Rollinson 2007). Natural selection thus operated on the level of global ecosystems as well as from the more familiar scales of local ecosystems, symbiotic consortia, species, individuals and genes.
Early life was dependent on energy sources provided by redox disequilibria generated by abiotic irreversible mass transfer of geological processes (Canfield et al. 2006; Sleep & Bird 2007 and references therein). It was advantageous for life to harvest the far more bountiful energy in sunlight (see Rosing et al. 2006). The metabolic innovation of photosynthesis probably developed from a series of evolutionary pre-adaptations to the environmental conditions of pre-photosynthetic niches. The evolutionary ability to survive near the Earth's surface gave microbes immediate access to ‘out-of-equilibrium’ products of volcanism and photolysis. Hydrogen-based photosynthesis probably evolved gradually from photocatalysis, and the ability to metabolize iron and sulphur compounds pre-adapted organisms for use of sulphide and ferric iron as bountiful oxygen acceptors. This ability allowed the development of complex ecosystems where the reverse reactions provided heterotrophic niches.
Molecular genetics indicate that bacteria evolved photosynthesis just once (Sadekar et al. 2006) and that the innovative photobacterium roots the tree of common bacterial clades (Battistuzzi et al. 2004). This result is not unexpected, as the productivity of a photosynthetic ecosystem is many orders of magnitude greater than a non-photosynthetic one.
At a very early stage, the photosynthetic ecosystem began to produce a geological record (Rosing 1999; Rosing & Frei 2004; Tice & Lowe 2006). As already noted, black shale (and its metamorphic products) is a strong biosignature for photosynthesis (Sleep & Bird 2007). Geologists find this rock type in the oldest preserved supracrustal rocks at ca 3.8 Ga (M. T. Rosing 2007, personal communication). That is, geologists find no record of the abiotic or pre-photosynthetic epoch on the Earth's surface.
Efficient weathering is a valuable skill essential to land ecosystems. It provides both ferrous iron for photosynthesis and nutrient elements. The successful land colonists, Terrabacteria, evolved into ecosystems of photosynthetic organisms and actinobacteria that weathered the soil. As already noted, shale, the product of efficient weathering, appears in the oldest supracrustal sequences.
Weathering modified the surface of the Earth fouling much of the land environment for anoxygenic photosynthesis. Shale melted at depth within subduction zones to form low-Fe granite. Sandstone and some carbonates also contained little iron. The need for an oxygen acceptor limited ecosystems occupying terrains with these rock types. In order to feed themselves in this energy-limited ecological environment, cyanobacteria evolved the complex biochemistry required for oxygenic photosynthesis.
It is difficult to ascertain when cyanobacteria evolved. First, an ecosystem with trace dioxygen recycles organic matter just like the modern one. (The ability to use minute concentrations of dioxygen may have evolved much earlier with microbes consuming O2 from abiotic photolysis; e.g. Canfield 2005.) We know that biology functions with necessary gases present in only trace quantities. For example, photosynthesis operates well today with 300 ppm of CO2 in the air. Second, cyanobacteria started on land. It was easy for them to generate local and transient dioxygen oases.
Lead isotope studies in ca 3.8 Ga rocks in Greenland may indicate land weathering in the presence of dioxygen (Rosing & Frei 2004). The lead isotopes 206 and 207 form by decay of 238U and 235U. 238Pb forms by decay of 232Th. Abiotic geological processes do not usually separate U from Th on the scale of the source terrain for a shale deposit. Weathering in the presence of dioxygen mobilized uranium in aqueous solution but leaves thorium behind. Lead isotope systematics shows that the Greenland shales and turbidites had a high U : Th ratio, indicative for this type of weathering (Rosing & Frei 2004). It is possible that an ecosystem based on anoxygenic photosynthesis could also do this.
Dioxygen remained a minuscule component of the atmosphere as long as sulphide was a significantly dissolved species in the shallow ocean. This transition in an oxic shallow ocean did not occur until the net long-term production of available oxygen exceeded its loss by geological processes. Excess ferric iron in the continental crust sequesters about twice as much oxygen as would be released by burial of organic matter. Part of the available oxygen reservoir is balanced by hydrogen escape to space. Anoxygenic photosynthesis produced the ferric iron and sulphate (reactions 3.1 and 3.2). Decomposing organic matter produced methane. The methane carried hydrogen into the upper atmosphere where it escaped after photolysis.
As the transition to an oxic shallow ocean occurred, photosynthesis increased the dissolved sulphate concentration in the ocean to where the compound was not quantitatively removed during high-temperature hydrothermal circulation. Once dioxygen was present in seawater, downwelling surface waters oxidized massive sulphide deposits on the seafloor. Dioxygen could not build up above modest levels until there was enough of it to quantitatively consume the exposed massive sulphide deposits.
Acknowledgments
We appreciate constructive comments on earlier versions of this manuscript from Kevin Zahnle and two anonymous reviewers. Olivier Rouxel, Dianne Newman and Jim Kasting promptly answered questions. Kevin Zahnle pointed out the analogy of the rapid increase of human populations once they bought photosynthetic organisms under their control with ancient geological events. We benefited from questions and discussions with John Allen, Roger Buick, Paul Falkowski, Joe Kirshvink, Bill Martin, Euan Nisbet and Minik Rosing. We acknowledge support form the US National Science Foundation grants NSF EAR-0406658 (N.H.S.) and EAR-0408690 (D.K.B.). This work was performed as part of our collaboration with the NASA Astrobiology Institute Virtual Planetary Laboratory Lead Team, Kevin Zahnle and Paul Wallace. The Stanford Department of Geological and Environmental Sciences Endowment Fund provided support.
Footnotes
One contribution of 15 to a Discussion Meeting Issue ‘Photosynthetic and atmospheric evolution’.
Supplementary Material
References
- Allen J.F, Martin W. Evolutionary biology—out of thin air. Nature. 2007;445:610–612. doi: 10.1038/445610a. doi:10.1038/445610a [DOI] [PubMed] [Google Scholar]
- Anbar A.D, Knoll A.H. Proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science. 2002;297:1137–1142. doi: 10.1126/science.1069651. doi:10.1126/science.1069651 [DOI] [PubMed] [Google Scholar]
- Barber, J. 2008 Photosynthetic generation of oxygen. Phil. Trans. R. Soc. B363, 2665–2674. (doi:10.1098/rstb.2008.0047) [DOI] [PMC free article] [PubMed]
- Battistuzzi F.U, Feijao A, Hedges S.B. A genomic timescale of prokaryote evolution: insights into the origin of methanogenesis, phototrophy, and the colonization of land. Evol. Biol. 2004;4:44. doi: 10.1186/1471-2148-4-44. doi:10.1186/1471-2148-4-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baymann F, Brugna M, Muehlenhoff U, Nitschke W. Daddy, where did (PS)I come from? Biochim. Biophys. Acta. 2001;1507:291–310. doi: 10.1016/s0005-2728(01)00209-2. doi:10.1016/S0005-2728(01)00209-2 [DOI] [PubMed] [Google Scholar]
- Bern M, Goldberg D. Automatic selection of representative proteins for bacterial phylogeny. BMC Evol. Biol. 2005;5:34. doi: 10.1186/1471-2148-5-34. doi:10.1186/1471-2148-5-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt M.E, Allen D.E, Seyfried W.E. Reduction of CO2 during serpentinization of olivine at 300 degrees C and 500 bar. Geology. 1996;24:671–671. doi:10.1130/0091-7613(1996)024<0351:ROCDSO>2.3.CO;2 [Google Scholar]
- Berner R.A, Beerling D.J, Dudley R, Robinson J.M, Wildman R.A. Phanerozoic atmospheric oxygen. Annu. Rev. Earth Planet. Sci. 2003;31:105–134. doi:10.1146/annurev.earth.31.100901.141329 [Google Scholar]
- Buick, R. 2008 When did oxygenic photosynthesis evolve? Phil. Trans. R. Soc. B363, 2731–2743. (doi:10.1098/rstb.2008.0041) [DOI] [PMC free article] [PubMed]
- Canfield D.E. The early history of atmospheric oxygen: homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 2005;33:1–36. doi:10.1146/annurev.earth.33.092203.122711 [Google Scholar]
- Canfield D.E, Raiswell R. The evolution of the sulfur cycle. Am. J. Sci. 1999;299:697–723. doi:10.2475/ajs.299.7-9.697 [Google Scholar]
- Canfield D.E, Rosing M.T, Bjerrum C. Early anaerobic metabolisms. Phil. Trans. R. Soc. B. 2006;361:1819–1836. doi: 10.1098/rstb.2006.1906. doi:10.1098/rstb.2006.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catling D.C. Comment on ‘A hydrogen-rich early Earth atmosphere’. Science. 2006;311:38. doi: 10.1126/science.1118412. doi:10.1126/science.1117827 [DOI] [PubMed] [Google Scholar]
- Catling D.C, Zahnle K.J, McKay C.P. Biogenic methane, hydrogen escape, and the irreversible oxidation of early Earth. Science. 2001;293:839–843. doi: 10.1126/science.1061976. doi:10.1126/science.1061976 [DOI] [PubMed] [Google Scholar]
- Charlou J.L, Donval J.P, Douville E, Jean-Baptiste P, Radfor-Knoery J, Fouquet Y, Dapoigny A, Stievenard M. Compared geochemical signatures and the evolution of the Menez Gwen (37°50′N) and Lucky Strike (37°17′N) hydrothermal fluids, south of the Azores Triple Junction on the Mid-Atlantic Ridge. Chem. Geol. 2000;171:49–75. doi:10.1016/S0009-2541(00)00244-8 [Google Scholar]
- Colledge S, Conolly J, Shennan S. Archaeobotanical evidence for the spread of farming in the eastern mediterranean. Curr. Anthropol. 2004;45:S35–S58. doi:10.1086/422086 [Google Scholar]
- Corcoran P.L, Mueller W.U, Chown E.H. Climatic and tectonic influences on fan deltas and wave- to tide-controlled shoreface deposites: evidence from the Archaean Kesharrah Formation, Slave Province, Canada. Sediment. Geol. 1998;120:125–152. doi:10.1016/S0037-0738(98)00030-X [Google Scholar]
- Corcoran P.L, Mueller W.U, Padgham W.A. Influence of tectonism and climate on lithofacies distribution and sandstone and conglomerate composition in the Archean Beaulieu Rapids Formation, Northwest Terretories, Canada. Precambrian Res. 1999;94:175–204. doi:10.1016/S0301-9268(98)00114-4 [Google Scholar]
- Crowe S.A, O'Neill A.H, Kulczycki E, Weisener C.G, Roberts J.A, Fowle D.A. Reductive dissolution of trace metals from sediments. Geomicrobiol. J. 2007a;24:157–165. doi:10.1080/01490450701457329 [Google Scholar]
- Crowe S.A, Roberts J.A, Weisener C.G, Fowle D.A. Alteration of iron-rich lacustrine sediments by dissimilatory iron-reducing bacteria. Geobiology. 2007b;5:63–73. doi: 10.1111/j.1472-4669.2006.00086.x. doi:10.1111/j.1472-4669.2006.00086.x [DOI] [PubMed] [Google Scholar]
- Davis C.A, Atekwana E, Atekwana E, Slater L.D, Rossbach S, Mormile M.R. Microbial growth and biofilm formation in geologic media is detected with complex conductivity measurements. Geophys. Res. Lett. 2006;33:L18403. doi:10.1029/2006GL027312 [Google Scholar]
- Ehrenreich A, Widdel F. Anaerobic oxidation of ferrous iron by purple bacteria, a new type of phototrophic metabolism. Appl. Environ. Microbiol. 1994;60:4517–4526. doi: 10.1128/aem.60.12.4517-4526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekart D.D, Cerling T.E, Montañez I.P, Tabor N.J. A 400 million year carbon isotopic record of pedogenic carbonate: implications for paleoatmospheric carbon dioxide. Am. J. Sci. 1999;299:805–827. doi:10.2475/ajs.299.10.805 [Google Scholar]
- Falkowski, P. G. & Godfrey, L. V. 2008 Electrons, life and the evolution of Earth's oxygen cycle. Phil. Trans. R. Soc. B363, 2705–2716. (doi:10.1098/rstb.2008.0054) [DOI] [PMC free article] [PubMed]
- Farquhar J, Peters M, Johnston D.T, Strauss H, Masterson A, Wiechert U, Kaufman A.J. Isotopic evidence for Mesoarchaean anoxia and changing atmospheric sulphur chemistry. Nature. 2007;449:706–709. doi: 10.1038/nature06202. doi:10.1038/nature06202 [DOI] [PubMed] [Google Scholar]
- Ferry J.G, House C.H. The stepwise evolution of early life driven by energy conservation. Mol. Biol. Evol. 2006;23:1286–1292. doi: 10.1093/molbev/msk014. doi:10.1093/molbev/msk014 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gil G, Amonette J.E, Romine M.F, Gorby Y.A, Geesey G.G. Bioreduction of natural specular hematite under flow conditions. Geochim. Cosmochim. Acta. 2005;69:1145–1155. doi:10.1016/j.gca.2004.08.014 [Google Scholar]
- Gorby Y.A, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl Acad. Sci. 2006;103:11 358–11 363. doi: 10.1073/pnas.0604517103. doi:10.1073/pnas.0604517103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassineau N.V, Nisbet E.G, Bickle M.J, Fowler C.M.R, Lowry D, Mattey D.P, Abell P, Martin A. Antiquity of the biological sulphur cycle: evidence from sulphur and carbon isotopes in 2700 million-year-old rocks of the Belingwe Belt, Zimbabwe. Proc. R. Soc. B. 2001;268:113–119. doi: 10.1098/rspb.2000.1338. doi:10.1098/rspb.2000.1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graur D, Martin W. Reading the entrails of chickens: molecular timescales of evolution and the illusion of precision. Trends Genet. 2004;20:80–86. doi: 10.1016/j.tig.2003.12.003. doi:10.1016/j.tig.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Gustin M.S. Stratigraphy and alteration of the host rocks, United-Verde massive sulfide deposit, Jerome, Arizona. Econ. Geol. 1990;85:29–49. [Google Scholar]
- Hansen K.W, Wallmann K. Cretaceous and Cenozoic evolution of seawater composition, atmospheric O2 and CO2: a model perspective. Am. J. Sci. 2003;303:94–148. doi:10.2475/ajs.303.2.94 [Google Scholar]
- Hoehler T.M. Biochemistry of dihydrogen (H2) In: Sigel A, Sigel H, Sigel R.K.O, editors. Metal ions in biological systems, biogeocehmical cycles of elements. vol. 43. Taylor and Francis; Boca Raton, FL: 2005. pp. 9–48. [DOI] [PubMed] [Google Scholar]
- Hoehler T.M, Alperin M.J, Albert D.B, Martens C.S. Thermodynamic control on H2 concentrations in an anoxic marine sediment. Geochim. Cosmochim. Acta. 1998;62:1745–1756. doi:10.1016/S0016-7037(98)00106-9 [Google Scholar]
- Holland H.D. Volcanic gases, black smokers, and the great oxidation event. Geochim. Cosmochim. Acta. 2002;66:3811–3826. doi:10.1016/S0016-7037(02)00950-X [Google Scholar]
- Holser W.T, Schidlowski M, Mackenzie F.T, Maynard J.B. Geochemical cycles of carbon and sulfur. In: Gregor C.B, Garrels R.M, Mackenzie F.T, Maynard J.B, editors. Chemical cycles in the evolution of the Earth. Wiley; New York, NY: 1988. pp. 105–173. [Google Scholar]
- Jiao Y.Y.Q, Kappler A, Croal L.R, Newman D.K. Isolation and characterization of a genetically tractable photo autotrophic Fe(II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl. Environ. Microbiol. 2005;71:4487–4496. doi: 10.1128/AEM.71.8.4487-4496.2005. doi:10.1128/AEM.71.8.4487-4496.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler A, Newman D.K. Formation of Fe(III) minerals by Fe(II)-oxidizing photoautotrophic bacteria. Geochim. Cosmochim. Acta. 2004;68:1217–1226. doi:10.1016/j.gca.2003.09.006 [Google Scholar]
- Kaufman A.J, et al. Late Archean biospheric oxygenation and atmospheric evolution. Science. 2007;317:1900–1903. doi: 10.1126/science.1138700. doi:10.1126/science.1138700 [DOI] [PubMed] [Google Scholar]
- Kelley D.S, et al. A serpentine-hosted ecosystem: the lost city hydrothermal field. Science. 2005;307:1428–1422. doi: 10.1126/science.1102556. doi:10.1126/science.1102556 [DOI] [PubMed] [Google Scholar]
- Kharecha P, Kasting J.F, Siefert J. A coupled atmosphere-ecosystem model of the early Archean biosphere. Geobiology. 2005;3:53–76. doi:10.1111/j.1472-4669.2005.00049.x [Google Scholar]
- Kirschvink, J. L. & Kopp. R. E. 2008 Palaeoproterozoic ice houses and the evolution of oxygen-mediating enzymes: the case for a late origin of photosystem II. Phil. Trans. R. Soc. B363, 2755–2765. (doi:10.1098/rstb.2008.0024) [DOI] [PMC free article] [PubMed]
- Kotsyurbenko O.R, Glagolev M.V, Noxhevnikova A.N, Conrad R. Competition between homoactogenic bacteria and methanogenic Archaea for hydrogen at low temperature. FEMS Microbiol. Ecol. 2001;38:153–159. doi:10.1111/j.1574-6941.2001.tb00893.x [Google Scholar]
- Krauskopf, K. B. & Bird, D. K. 1995 Introduction to geochemistry, p. 647, 3rd edn. New York, NY: McGraw-Hill.
- Kump L.R. The rise of atmospheric oxygen. Nature. 2008;451:277–278. doi: 10.1038/nature06587. doi:10.1038/nature06587 [DOI] [PubMed] [Google Scholar]
- Kump L.R, Barley M.E. Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature. 2007;448:1033–1036. doi: 10.1038/nature06058. doi:10.1038/nature06058 [DOI] [PubMed] [Google Scholar]
- Lecuyer C, Ricard Y. Long-term fluxes and budget of ferric iron: implication for the redox states of the Earth's mantle and atmosphere. Earth Planet. Sci. Lett. 1999;165:197–211. doi:10.1016/S0012-821X(98)00267-2 [Google Scholar]
- Lee M.J, Zinder S.H. Isolation and characterization of a thermophilic bacterium which oxidizes acetate in syntrophic association with a methanogen and which grows acetogenically on hydrogen-carbon dioxide. Appl. Environ. Microbiol. 1988;54:124–129. doi: 10.1128/aem.54.1.124-129.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelock J.E. Oxford University Press; Oxford, UK: 1979. Gaia. A new look at life on Earth. 157 p. [Google Scholar]
- Lowe D.R, Tice M.M. Tectonic controls on atmospheric, climatic, and biological evolution 3.5–2.4 Ga. Precambrian Res. 2007;158:177–197. doi:10.1016/j.precamres.2007.04.008 [Google Scholar]
- Luu Y.-S, Ramsey J.A. Review: microbial mechanisms of accessing insoluble Fe(III) as an energy source. World J. Microbiol. Biotechnol. 2003;19:215–225. doi:10.1023/A:1023225521311 [Google Scholar]
- Marvin U.B. Smithsonian Institution Press; Washington, DC: 1973. Continental drift, the evolution of a concept. 239 p. [Google Scholar]
- McCollom T.M. Methanogenesis as a potential source of chemical energy for primary biomass production by autotrophic organisms in hydrothermal systems on Europa. J. Geophys. Res. 1999;E12:300 729–331 742. [Google Scholar]
- McCollom T.M, Amend J.P. A thermodynamic assessment of energy requirements for biomass synthesis by chemolithoautotrophic micro-organisms in oxic and anoxic environments. Geobiology. 2005;3:135–144. doi:10.1111/j.1472-4669.2005.00045.x [Google Scholar]
- McCollom T.M, Seewald J.S. A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine. Geochim. Cosmochim. Acta. 2001;65:3769–3778. doi:10.1016/S0016-7037(01)00655-X [Google Scholar]
- McCollom T.M, Shock E.L. Geochemical constraints on chemolithoautotrophic metabolism by microorganisms in seafloor hydrothermal systems. Geochim. Cosmochim. Acta. 1997;61:4375–4391. doi: 10.1016/s0016-7037(97)00241-x. doi:10.1016/S0016-7037(97)00241-X [DOI] [PubMed] [Google Scholar]
- Nealson, R. K. & Rye, R. 2004 Evolution of metabolism. In Biogeochemistry (ed. W. H. Schlesinger). Treatise on Geochemistry, vol. 8, pp. 41–61. Oxford, UK: Elsevier.
- Nielsen S.G, Rehkamper M, Norman M.D, Halliday A.N, Harrison D. Thallium isotopic evidence for ferromanganese sediments in the mantle source of Hawaiian basalts. Nature. 2006a;7074:314–317. doi: 10.1038/nature04450. doi:10.1038/nature04450 [DOI] [PubMed] [Google Scholar]
- Nielsen S.G, Rehkamper M, Teagle D.A.H, Butterfield D.A, Alt J.C, Halliday A.N. Hydrothermal fluid fluxes calculated from the isotopic mass balance of thallium in the ocean crust. Earth Planet. Sci. Lett. 2006b;251:120–133. doi:10.1016/j.epsl.2006.09.002 [Google Scholar]
- Nisbet, E. G. & Fowler, C. M. R. 1996 The hydrothermal imprint on life; did heat-shock proteins, metalloproteins and photosynthesis begin around hydrothermal vents? In Tectonic and biological segmentation of mid-ocean ridges, vol. 188 (eds C. J. MacLeod, P. A. Tyler & C. L. Walker), pp. 239–251. London, UK: Geological Society of London.
- Nisbet E.G, Fowler C.M.R. The early history of life. In: Schlesinger W.H, editor. Biogeochemistry. Treatise on Geochemistry. vol. 8. Elsevier; Oxford, UK: 2004. pp. 1–39. [Google Scholar]
- Nisbet, E. G & Nisbet, R. E. R. 2008 Methane, oxygen, photosynthesis, rubisco, and the regulation of the air through time. Phil. Trans. R. Soc. B363, 2745–2754. (doi:10.1098/rstb.2008.0057) [DOI] [PMC free article] [PubMed]
- Nisbet E.G, Sleep N.H. The habitat and nature of early life. Nature. 2001;401:1083–1091. doi: 10.1038/35059210. doi:10.1038/35059210 [DOI] [PubMed] [Google Scholar]
- Nisbet E.G, Mattey D.P, Lowry D. Can diamonds be dead bacteria? Nature. 1994;367:694–694. doi:10.1038/367694b0 [Google Scholar]
- Olson J.M, Blankenship R.E. Thinking about the evolution of photosynthesis. Photosyn. Res. 2004;80:373–386. doi: 10.1023/B:PRES.0000030457.06495.83. doi:10.1023/B:PRES.0000030457.06495.83 [DOI] [PubMed] [Google Scholar]
- Pace N.R. Origin of life: facing up to the physical. Cell. 1991;65:531–533. doi: 10.1016/0092-8674(91)90082-a. doi:10.1016/0092-8674(91)90082-A [DOI] [PubMed] [Google Scholar]
- Pace N.R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. doi:10.1126/science.276.5313.734 [DOI] [PubMed] [Google Scholar]
- Pavlov A.A, Kasting J.F, Eigenbrode J.L, Freeman K.H. Organic haze in Earth's early atmosphere: source of low-C-13 Late Archean kerogens? Geology. 2001;29:1003–1006. doi:10.1130/0091-7613(2001)029<1003:OHIESE>2.0.CO;2 [Google Scholar]
- Perry H.K.C, Jaupart C, Mareschal J.-C, Bienfait G. Crustal heat production in the superior province, Canadian shield, and in North America inferred from heat flow data. J. Geophys. Res. 2006a;111:B04401. doi:10.1029/2005JB003893 [Google Scholar]
- Perry H.K.C, Mareschal J.C, Jaupart C. Variations of strength and localized deformation in cratons: the 1.9 Ga Kapuskasing uplift, Superior Province, Canada. Earth Planet. Sci. Lett. 2006b;249:216–228. doi:10.1016/j.epsl.2006.07.013 [Google Scholar]
- Peterson J.A. Distribution of selected trace and major elements around the massive sulfide deposit at the Penn Mine, California. Econ. Geol. 1988;83:419–427. [Google Scholar]
- Philippot P, Van Zuilen M, Leopt K, Thomazo C, Farquhar J, Van Kranendonk M.J. Early Archaean microorganisms preferred elemental sulfur, not sulfate. Science. 2007;317:1534–1537. doi: 10.1126/science.1145861. doi:10.1126/science.1145861 [DOI] [PubMed] [Google Scholar]
- Retallack G.J. Blackwell Science; Oxford, UK: 2001. Soils of the past: an introduction to paleopedology. 404 p. [Google Scholar]
- Reysenbach A.L, Shock E. Merging genomes with geochemistry in hydrothermal ecosystems. Science. 2002;296:1077–1082. doi: 10.1126/science.1072483. doi:10.1126/science.1072483 [DOI] [PubMed] [Google Scholar]
- Rollinson H.R. Blackwell; Malden, MA: 2007. Early Earth systems: a geochemical approach. 285 p. [Google Scholar]
- Rosing M.T. C-13-depleted carbon microparticles in >3700-Ma sea-floor sedimentary. Science. 1999;283:674–676. doi: 10.1126/science.283.5402.674. doi:10.1126/science.283.5402.674 [DOI] [PubMed] [Google Scholar]
- Rosing M.T, Frei R. U-rich Archaean sea-floor sediments from Greenland—indications of >3700 Ma oxygenic photosynthesis. Earth Planet. Sci. Lett. 2004;217:237–244. doi:10.1016/S0012-821X(03)00609-5 [Google Scholar]
- Rosing M.T, Bird D.K, Sleep N.H, Glassley W, Albarede F. The rise of continents—an essay on the geologic consequences of photosynthesis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006;232:99–113. doi:10.1016/j.palaeo.2006.01.007 [Google Scholar]
- Rother M, Metcalf W.W. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: an unusual way of life for a methanogenic archaeon. Proc. Natl Acad. Sci. 2004;101:16 929–16 934. doi: 10.1073/pnas.0407486101. doi:10.1073/pnas.0407486101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild, L. J. 2008 The evolution of photosynthesis…again? Phil. Trans. R. Soc. B363, 2787–2801. (doi:10.1098/rstb.2008.0056) [DOI] [PMC free article] [PubMed]
- Rouxel O, Bekker J.A, Edwards K.J. Iron isotope constraints on the Archean and Paleoproterozoic ocean redox state. Science. 2005;307:1088–1090. doi: 10.1126/science.1105692. doi:10.1126/science.1105692 [DOI] [PubMed] [Google Scholar]
- Ruddiman W.F. The Anthropogenic Era began thousands of years ago. Climatic Change. 2003;61:261–293. doi:10.1023/B:CLIM.0000004577.17928.fa [Google Scholar]
- Sadekar S, Raymond J, Blankenship R.E. Conservation of distantly related membrane proteins: photosynthetic reaction centers share a common structural core. Mol. Biol. Evol. 2006;23:2001–2007. doi: 10.1093/molbev/msl079. doi:10.1093/molbev/msl079 [DOI] [PubMed] [Google Scholar]
- Saito M.A, Sigman D.M, Morel F.M.M. The bioinorganic chemistry of the ancient ocean: the co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean-Proterozoic boundary? Inorgan. Chim. Acta. 2003;356:308–318. doi:10.1016/S0020-1693(03)00442-0 [Google Scholar]
- Schroder I, Johnson E, de Vries S. Microbial ferric iron reductases. FEMS Microbiol. Rev. 2003;27:427–447. doi: 10.1016/S0168-6445(03)00043-3. doi:10.1016/S0168-6445(03)00043-3 [DOI] [PubMed] [Google Scholar]
- Searle M, Hacker B.R, Bilham R. The Hindu Kush seismic zone as a paradigm for the creation of ultrahigh-pressure diamond- and coesite-bearing continental rocks. J. Geol. 2001;109:143–153. doi:10.1086/319244 [Google Scholar]
- Seyfried W.E, Foustoukos D.I, Allen D.E. Ultramafic-hosted hydrothermal systems at mid-ocean ridges: chemical and physical controls on pH, redox and carbon reduction reactions. In: German C.R, Lin J, Parson L.M, editors. Mid-ocean ridges: interactions between lithosphere and oceans. Geophysical Monograph Series. vol. 148. American Geophysical Union; Washington, DC: 2004. pp. 267–284. [Google Scholar]
- Shock E.L, McCollom T, Schulte M.D. Geochemical constraints on chemolithoautotrophic reaction in hydrothermal systems. Orig. Life Evol. Biosph. 1995;25:141–159. doi: 10.1007/BF01581579. doi:10.1007/BF01581579 [DOI] [PubMed] [Google Scholar]
- Slack J.F, Grenne T, Bekker A, Rouxel O.J, Lindberg P.A. Suboxic deep seawater in the late Paleoproterozoic: evidence from hematitic chert and iron formation related to seafloor-hydrothermal sulfide deposits, central Arizona, USA. Earth Planet. Sci. Lett. 2007;255:243–256. doi:10.1016/j.epsl.2006.12.018 [Google Scholar]
- Sleep N.H. Dioxygen over geological time. In: Sigel A, Sigel H, Sigel R.K.O, editors. Metal ions in biological systems, biogeocehmical cycles of elements. vol. 43. Taylor and Francis; Boca Raton, FL: 2005. pp. 49–73. [DOI] [PubMed] [Google Scholar]
- Sleep N.H, Bird D.K. Niches of the pre-photosynthetic biosphere and geologic preservation of Earth's earliest ecology. Geobiology. 2007;5:101–117. doi:10.1111/j.1472-4669.2007.00105.x [Google Scholar]
- Sleep N.H, Meibom A, Fridriksson T, Coleman R.G, Bird D.K. H2-rich fluids from serpentinization: geochemical and biotic implications. Proc. Natl Acad. Sci. 2004;101:12 818–12 823. doi: 10.1073/pnas.0405289101. doi:10.1073/pnas.0405289101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova T.G, Jeanthon C, Kostrikina N.A, Chernyh N.A, Lebedinsky A.V, Stackebrandt E, Bonch-Osmolovskaya E.A. The first evidence of anaerobic CO oxidation couple with H2 production by a hyperthermophile archaeon isolated from a deep-sea hydrothermal vent. Extremophiles. 2004;8:317–323. doi: 10.1007/s00792-004-0389-0. doi:10.1007/s00792-004-0389-0 [DOI] [PubMed] [Google Scholar]
- Tabita, F. R., Hanson, T. E., Satagopan, S., Witte, B. H. & Kreel, N. E. 2008 Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Phil. Trans. R. Soc. B363, 2629–2640. (doi:10.1098/rstb.2008.0023) [DOI] [PMC free article] [PubMed]
- Tian F, Toon O.B, Pavlov A.A, De Sterck H. A hydrogen-rich early Earth atmosphere. Science. 2005;308:1014–1017. doi: 10.1126/science.1106983. doi:10.1126/science.1106983 [DOI] [PubMed] [Google Scholar]
- Tice M.M, Lowe D.R. Photosynthetic microbial mats in the 3.416-Myr-old ocean. Nature. 2004;43:549–552. doi: 10.1038/nature02888. doi:10.1038/nature02888 [DOI] [PubMed] [Google Scholar]
- Tice M.M, Lowe D.R. Hydrogen-based carbon fixation in the earliest known photosynthetic organisms. Geology. 2006;34:37–40. doi:10.1130/G22012.1 [Google Scholar]
- Walker J.C.G. Macmillan; New York, NY: 1977a. The evolution of the atmosphere. 318 pp. [Google Scholar]
- Walker J.C.G. Was the Archaean biosphere upside down? Nature. 1977b;329:710–712. doi: 10.1038/329710a0. doi:10.1038/329710a0 [DOI] [PubMed] [Google Scholar]
- Wetzel L.R, Shock E.L. Distinguishing ultramafic- from basalt-hosted submarine hydrothermal systems by comparing calculated vent fluid compositions. J. Geophys. Res. 2000;105:8319–8340. doi:10.1029/1999JB900382 [Google Scholar]
- Woese C.R. Endosymbionts and mitochondrial origins. J. Mol. Evol. 1977;10:93–96. doi: 10.1007/BF01751802. doi:10.1007/BF01751802 [DOI] [PubMed] [Google Scholar]
- Woese C.R, Kandler O, Wheelis M.L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc. Natl Acad. Sci. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. doi:10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Fischer W.M, Inoue K, Nakahara M, Bauer C.E. Molecular evidence for the early evolution of photosynthesis. Science. 2000;289:1724–1730. doi: 10.1126/science.289.5485.1724. doi:10.1126/science.289.5485.1724 [DOI] [PubMed] [Google Scholar]
- Zaar A, Fuchs G, Golecki J.R, Overmann J. A new purple sulfur bacterium isolated from a littoral microbial mat, Thiorhodococcus drewsii sp. nov. Arch. Microbiol. 2003;179:174–183. doi: 10.1007/s00203-002-0514-3. doi:10.1007/s00203-002-0514-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.