Abstract
Phytoplankton growth and productivity relies on light, multiple nutrients and temperature. These combined factors constitute the ‘integrated growth environment’. Since their emergence in the Archaean ocean, phytoplankton have experienced dramatic shifts in their integrated growth environment and, in response, evolved diverse mechanisms to maximize growth by optimizing the allocation of photosynthetic resources (ATP and NADPH) among all cellular processes. Consequently, co-limitation has become an omnipresent condition in the global ocean. Here we focus on evolved phytoplankton populations of the contemporary ocean and the varied energetic pathways they employ to solve the optimization problem of resource supply and demand. Central to this discussion is the allocation of reductant formed through photosynthesis, which we propose has the following three primary fates: carbon fixation, direct use and ATP generation. Investment of reductant among these three sinks is tied to cell cycle events, differentially influenced by specific forms of nutrient stress, and a strong determinant of relationships between light-harvesting (pigment), photosynthetic electron transport and carbon fixation. Global implications of optimization are illustrated by deconvolving trends in the 10-year global satellite chlorophyll record into contributions from biomass and physiology, thereby providing a unique perspective on the dynamic nature of surface phytoplankton populations and their link to climate.
Keywords: phytoplankton, photosynthesis, carbon fixation, nutrients, climate
1. Introduction
Ocean biology has had a profound effect on atmospheric chemistry and global climate since the emergence of phototrophic life on the Earth more than 3 Gyr ago. Climate is, in turn, a powerful controlling factor for ocean biology. The biology–climate link functions primarily through the uptake and release of radiatively important compounds (e.g. CO2, CH4, dimethyl sulphide). The active interface between the atmosphere and deep sea is the surface photic layer, where sufficient light penetrates to support net phytoplankton primary production. This thin veneer of the ocean exports billions of tons of carbon to the subphotic zone each year, prevents significant degassing of CO2 to the atmosphere in upwelled waters, releases sulphur compounds that play an important role in cloud formation, emits powerful greenhouse gases and alters the distribution of solar energy within the water column, to name just a few important functions. All of these impacts are traceable to the biomass and activity of this upper layer's biology. Climate controls this distribution and activity of biomass through its effect on growth-limiting factors for phytoplankton (light, temperature and nutrients). The climate–biology link functions through cloud-driven changes in incident light, spatio-temporal variability in vertical nutrient transport (wind mixing, eddy pumping, upwelling, etc.) and fluctuations in atmospheric dust deposition that alter ocean iron budgets and nitrogen fixation, again to name just a few mechanisms.
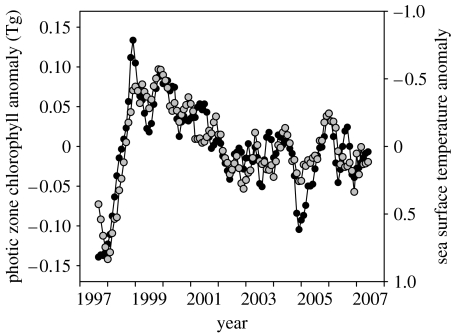
The intimate connection between the biology of the photic zone and the climate is becoming ever more apparent with the availability of accurate satellite ocean colour data, which allow a limited set of biological properties to be derived on a regular basis, globally. An example of this relationship is shown in figure 1, where monthly anomalies in globally integrated water-column chlorophyll concentrations (i.e. integrated to the 1% light level) for the permanently stratified oceans are compared with simultaneous changes in low-latitude sea-surface temperatures (SST). The striking aspect of this figure is that a single climatically driven physical property can capture nearly all of the net chlorophyll variability over the 10-year record. This relationship has been further expanded to show that variations in chlorophyll and SST are also spatially correlated and that the time-series changes in SST are coupled to parallel changes in upper ocean thermal stratification (Behrenfeld et al. 2006a). An important aspect to recognize about global chlorophyll distributions, however, is that they express variability in both biomass and physiology. Distinction between these two sources of variability is a prerequisite for fully understanding the biology–climate link and its implications on primary production, ecosystems and carbon cycling.
Figure 1.
SeaWiFS-based monthly anomalies in photic zone chlorophyll for the permanently stratified oceans (average temperature greater than 15°C) between 1997 and 2007 (black circles) and coincident low-latitude SST anomalies (°C; grey circles). See Behrenfeld et al. (2005) for details on data analysis.
As with the other articles in this issue, our contribution focuses on photosynthetic organisms, their immediate physical environment and their atmosphere–climate link. In our case, however, the perspective is the present-day ocean, rather than the deep past, and our focus is the question, ‘After billions of years of evolution, what physiological strategies emerged in phytoplankton allowing their contemporary proliferation and what role does physiology play in observed climate–ocean biology relationships (e.g. figure 1)?’ At the very heart of this question is the issue of how phytoplankton respond to changes in the key growth-limiting environmental factors: nutrients and light. We address this issue by first considering characteristics of the upper ocean growth environment and then, building from specific laboratory results, identify fundamental photosynthetic and metabolic pathways evolved in response to these conditions. These physiological adaptations and acclimations strongly influence relationships between pigment (chlorophyll) concentrations, photosynthesis and carbon fixation. Accordingly, we conclude this discussion by revisiting the satellite chlorophyll record with an expanded interpretation encompassing climate-driven changes in upper ocean growth conditions and concurrent physiological responses.
Important terminology. Throughout this article, ‘photosynthesis’ is distinguished from ‘carbon fixation’. We consider photosynthesis to be the production of ATP and NADPH by the photosynthetic electron transport (PET) chain and refer to these two products as ‘photosynthate’. By contrast, carbon fixation will refer to the use of photosynthate by the Calvin cycle to produce simple organic carbon products. Also, we generally refer to NADPH and NADH as ‘reductant’, but recognize that in many metabolic reactions electrons can be contributed from other forms. Likewise, ‘ATP’ can be substituted by, or is replaced by, other phosphorylated compounds (e.g. GTP).
2. The integrated growth environment and optimization
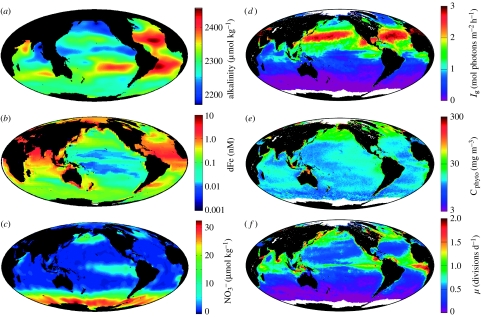
Phytoplankton growth in the photic zone is never limited by a single environmental factor, although in many cases a particular resource can be identified as overwhelmingly more important than all others (e.g. light at depth or iron in high-nutrient, low-chlorophyll—HNLC—regions). Lately, recognition of simultaneous stresses has popularized discussions on ‘co-limitation’ (Price & Morel 1991; Morel et al. 1994; Bertrand et al. 2007; Saito et al. 2008). In a sense though, phytoplankton growth is always concurrently constrained by every essential environmental resource that requires energy and/or reductant to acquire or concentrate. The global distribution of four such growth constraints is illustrated in figure 2. Each of these resources can exist at levels in nature that place demands on photosynthate supplies. For example, while we do not often think of carbon as being growth limiting, energy is expended even at the average environmental concentration to elevate intracellular CO2 to levels that allow the key Calvin cycle enzyme, rubisco, to operate primarily as a carboxylase rather than as an oxygenase. Likewise, reduced nitrogen forms are often subsaturating for growth and require resources to acquire, concentrate, reduce and assimilate. Physiological strategies for acquiring iron can place additional demands on photosynthates. Consequently, artificial enhancement of any photosynthate-requiring resource must infallibly stimulate growth, although in many cases such responses are too small to easily detect. Nevertheless, the universal condition of co-limitation implies that phytoplankton everywhere are challenged with balancing photosynthate supplies with variable demands. The summed influence of these factors comprises the ‘integrated growth environment’ and it is to this multifactor environment that natural phytoplankton populations are acclimated.
Figure 2.
Global distributions of (a) annual average total alkalinity as an index of inorganic carbon concentration (data from Key et al. 2004), (b) boreal summer mixed layer dissolved iron (dFe) concentration (data from Moore & Braucher 2007), (c) July surface nitrate (Levitus climatology), (d) July median mixed layer growth irradiance (Ig), (e) July surface phytoplankton carbon (Cphyto) biomass, and (f) July surface phytoplankton growth rates (μ). Data for (d–f) are from http://web.science.oregonstate.edu/ocean.productivity/.
In addition to the physical and chemical factors regulating phytoplankton growth, ecosystem properties also play a vital role through the selective consumption of phytoplankton and subsequent recycling of nutrients. Indeed, the entire phytoplankton carbon biomass of the global ocean is lost (grazing, sinking, lysis) on average every 2–6 days (Behrenfeld & Falkowski 1997) and, for many species in the permanently stratified oceans, life expectancy of an individual is often only a day. Given this impending demise, reproduction before death is certainly the penultimate metric of success, and in the often nutrient-impoverished surface layer of the ocean this implies that physiological strategies that either reduce division time (e.g. through changes in stoichiometry or efficiency) or extend life expectancy (e.g. morphological changes to reduce predation) are of supreme evolutionary value.
Together, the integrated growth environment and ‘ecological pressure to divide’ give rise to the ‘optimization problem’, which can be thought of as a resource cost-benefit issue where the resource of interest is photosynthate (ATP and NADPH) and the objective is cell division. Optimization is easy enough to conceptualize, at least to first order. If a cell invests too much resource into photosynthetic machinery and cellular components for acquiring nutrients from the environment, then too little remains for growth and division. Conversely, if all new resources are dedicated to growth and division, the rate of nutrient and light acquisition will be slow and thus an impediment to division. Through a regulated investment approach, the optimization solution(s) emerge(s) as the strategy (or set of strategies) that minimize(s) the time increment between divisions. This concept of resource allocation is fundamental to many current models of steady-state and dynamic photoacclimation (Shuter 1979; Geider et al. 1996, 1998; Flynn 2001) and has profound implications on elemental stoichiometry (Sterner & Elser 2002).
Beyond this rather cursory view, optimization solutions evolved over the millennia are as diverse as the growth constraints they address. Some solutions are macroscopic, involving changes in cell size, shape or vacuolization. Others have been revolutionary biochemical advances, such as the evolution of high-affinity iron transporters, carbon-concentrating mechanisms and the C4 pathway. More often, optimization involves subtle single-component substitutions that improve efficiency or reduce cellular demands for a particular limiting factor (e.g. flavodoxin replacing ferredoxin). Also important have been new metabolic pathways for converting reductant (NADPH, NADH, etc.) into ATP. Our objective here is not to review each and every one of the mechanisms involved in optimization, but rather to investigate specifically how adaptations to the integrated growth environment influence the coordination of metabolic and photosynthetic activities and are ultimately expressed through variations in photosynthesis–carbon fixation relationships.
3. Patterns in photosynthesis
Phytoplankton net primary production (NPP) can be defined as diurnal photosynthesis minus diel respiration or, alternatively, as the daily production of organic carbon formed through photosynthesis and available for heterotrophic consumption (Lindeman 1942). NPP is an essential ecological property related to the uptake of CO2 from the environment and the sustenance and carrying capacity of higher trophic level biomass. The best assessment of NPP is the product of phytoplankton carbon biomass and growth rate. Unfortunately, these two variables are not readily determined with sufficient accuracy in the field, so alternative measures have been developed. The most important of these techniques is the 14C method first introduced by Einer Steemann-Nielsen in 1951. Relating 14C-based carbon fixation rates to ocean NPP, however, requires an understanding of how physiological acclimations to the integrated growth environment are expressed in 14C-uptake variability.
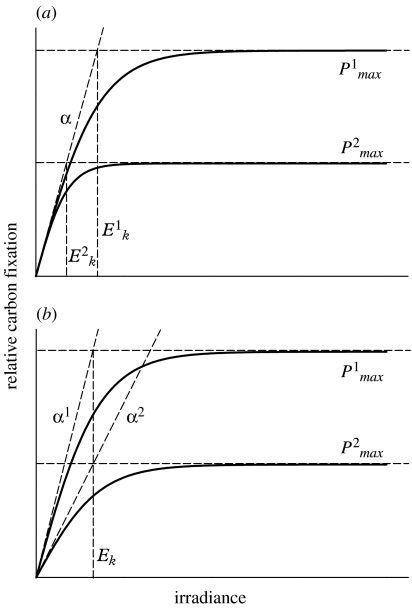
14C measurements in the field take two basic forms, 12- to 24-h in situ or simulated in situ incubations and short-term (20 min–2 h) carbon fixation–irradiance (CI) measurements. From a physiological perspective, the latter of these two is generally more insightful. Measured CI relationships exhibit a few common features (e.g. figure 3a). At low light levels, carbon fixation increases linearly with increasing light at a rate (α) proportional to the concentration of photosynthetically active pigment in the sample (Steemann Nielsen & Jørgensen 1968). At higher light, carbon fixation achieves a light-saturated maximum rate (Pmax) that reflects electron transport capacities downstream of oxygen-evolving photosystem II (PSII). The ratio of Pmax to α is referred to as the ‘light-saturation index’ (Ek: units of irradiance). Evaluation of field CI data reveal two primary categories of behaviour, termed ‘Ek-dependent’ (figure 3a) and ‘Ek-independent’ (figure 3b) variability (Behrenfeld et al. 2004). Ek-dependent variability results from uncoupled adjustments in Pmax and α that alter Ek. The physiological mechanisms responsible for this behaviour are generally well understood and involve acclimation strategies aimed at maximizing growth under variable light conditions. Ek-independent variability involves parallel changes in Pmax and α that essentially leaves Ek unaltered. The mechanistic basis for Ek-independent behaviour is largely unresolved.
Figure 3.
Two basic categories of variability in CI relationships. (a) Ek-dependent behaviour involves uncoupled changes in the light-saturated (Pmax) and light-limited (α) rates of carbon fixation, such that the light-saturation index, Ek, changes. In (b) Ek-independent behaviour, changes in Pmax and α are coupled, such that Ek is constant.
Lack of any consistent explanation for Ek-independent behaviour represents a critical shortfall in our overall understanding of phytoplankton ecophysiology, as this behaviour often dominates CI variability across vast ocean areas and over a wide temporal range (hours to seasons; Behrenfeld et al. 2004). Nutrient stress has been implicated as an important driver for Ek-independent variability at the broader space and time scales (Côté & Platt 1983; Platt et al. 1992; Behrenfeld et al. 2004), although other factors have been suggested (Côté & Platt 1983; Claustre et al. 1997). What is missing is a mechanism by which nutrients, or any other factors, cause Ek-independent behaviour. In §§4–6, we propose such a mechanism.
4. Plants are not animals
The title of this section may seem inexcusably obvious, but when it comes to understanding phytoplankton metabolism and optimization it makes an important point: the photoautotrophic lifestyle is much different from a heterotroph's in terms of sources for reductant (sunlight and water versus preformed organic material) and energy (photosynthetic and respiratory electron transport versus respiration alone). Perhaps most importantly and in contrast to heterotrophs, metabolic activities in plants that transform pre-existing carbon substrates into other forms (e.g. synthesizing lipids from carbohydrates, nucleic acids from amino acids, etc.) do not have to rely on ATP and/or reductant produced from the oxidation of organic matter, but rather can draw directly from solar energy using the principal products of PET. In other words, photosynthate (ATP and NADPH) does not have to pass through a carbon form before being used by the cell. While this concept is not novel, it is often overlooked and is proposed here as a key to understanding Ek-independent behaviour.
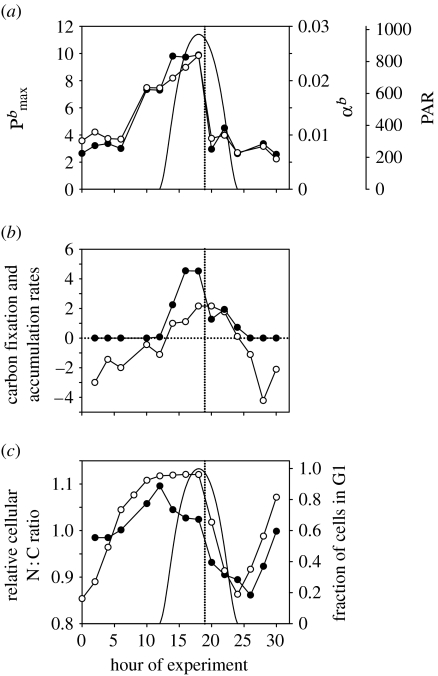
To begin this discussion, we revisit the results from an earlier laboratory experiment (ProMolec) with the globally relevant, oceanic oxyphotobacterium, Prochlorococcus (strain PCC 9511; Steglich et al. 2001; Behrenfeld et al. 2004; Bruyant et al. 2005; see part I of the electronic supplementary material). The ProMolec study involved cyclostat cultures acclimated for 15 days to a smooth 12-h diel light cycle with a peak noon irradiance of 970 μmol quanta m−2 s−1. 14C-based CI relationships (20-min incubations) revealed strong and highly correlated cycles in both the light-limited and light-saturated rates of carbon fixation (i.e. Ek-independent behaviour; figure 4a). The most striking feature was a sudden and drastic (64% and 71%) drop in carbon fixation capacity immediately after noon (figure 4a). This shift did not correspond to any significant change in electron transport rates within the photosynthetic membranes (Behrenfeld et al. 2004). Photosynthesis was light saturated throughout all but the first and last hours of the photoperiod (average Ek=220 μmolquanta m−2 s−1). Diurnal downregulation of PSII was a smooth inverse function of incident light (Bruyant et al. 2005). This ‘photoinhibition’ phenomenon lacked any pronounced noon feature and was of sufficiently small magnitude (of the order of 20–30%) to be easily compensated by increased turnover rates within the remaining functional PSII pool (Kok 1956; Weinbaum et al. 1979; Heber et al. 1988; Leverenz et al. 1990; Behrenfeld et al. 1998; see part II of the electronic supplementary material). The ProMolec results thus reveal that Ek-independent behaviour is not due to processes at the level of the photosystems, but rather to the varied fate of photosynthates (ATP and NADPH) once formed.
Figure 4.
ProMolec results. (a) Diel cycles in incident irradiance (PAR; μmol quanta m−2 s−1; solid line, offset right axis), chlorophyll-specific light-saturated carbon fixation (; mg C mg chl−1 h−1; filled circles, left axis), and the chlorophyll-specific light-limited slope for carbon fixation (αb; mg C m2 s (mg Chl h μmol quanta)−1; open circles, right axis). αb has been corrected for midday photoinhibition effects using variable fluorescence data to demonstrate more clearly the strong correlation in the fraction of light-limited and light-saturated photosynthate used for carbon fixation. (b) Diel carbon fixation rate (fg C cell−1 h−1; filled circles) calculated from CI data and incident light (a) and cellular carbon accumulation rate (fg C cell−1 h−1; open circles). (c) Diel cycles in cellular N:C ratios normalized to the average value measured at midnight (filled circles) and fraction of cells in G1 phase (open circles, right axis). Solid line indicates PAR. Vertical dotted line in each figure demarks period when carbon fixation dropped by greater than 60% (see text).
Data shown in figure 4a represent carbon-fixing capacities, not actual carbon fixation rates. Carbon fixation rates are calculated by applying the CI data to ambient light levels (filled circles in figure 4b). These carbon fixation rates can then be compared with cellular carbon accumulation rates calculated for each 2-h measurement period using CHN and flow cytometric data (open circles in figure 4b; Behrenfeld et al. 2004). In this comparison, carbon accumulation rate is positive during the day (i.e. net production) and negative during the night (first due to cell division and then mobilization of carbon reserves; figure 4b). The critical feature in figure 4b is that 14C-based carbon fixation is, on average, 62% higher than the rates of carbon accumulation for the first half of the photoperiod and then matches carbon accumulation only after the drastic midday drop. The obvious question is, ‘Why?’
To answer this question, we first need a bit more information regarding the ProMolec culture. The 15-day pre-acclimation period resulted in a highly synchronized culture with respect to the cell cycle, with cells dividing during the first half of the night (Bruyant et al. 2005). This synchronization led to a highly ordered sequence of metabolic events, initiated during the second half of the night by Calvin cycle gene transcription (e.g. rbcL; Behrenfeld et al. 2004) and subsequent translation, as reflected by enhanced carbon-fixing capacities prior to sunrise (figure 4a) and contributing to the rise in cellular N : C ratios from the consumption of carbon-rich storage products (filled circles in figure 4c). Between sunrise and noon, photosynthates were primarily directed towards carbon fixation to support amino acid synthesis. After noon, photosynthetically fixed carbon was redirected to carbohydrate synthesis (Behrenfeld et al. 2004), resulting in a lowering of N : C ratios (figure 4c). Concurrently, the dominant cell cycle phase transitioned from G1 to S (chromosome replication), which persisted through the afternoon (i.e. S-phase duration is relatively conserved in Prochlorococcus at 4.7±1.1 hours, followed by a G2 phase of the order of 2 hours; Burbage & Binder 2007; figure 4c). With this additional information, we can now evaluate the metabolic link between the cell cycle, the midday drop in carbon fixation rates and the divergent relationships between carbon fixation and accumulation.
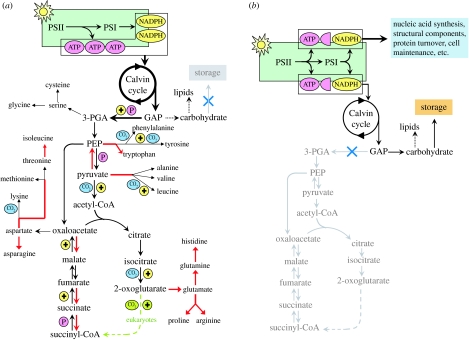
Linear PET generates ATP and NADPH in a 3 : 2 or slightly lower ratio (figure 5). Formation of glyceraldehyde-3-phosphate (‘GAP’ or ‘triosephosphate’), the export product of the Calvin cycle, consumes ATP and NADPH at a ratio of 3 : 2. While one might marvel at this beautiful match between product formation and consumption, it actually presents a serious problem to the cell: it leaves no ATP or NADPH for any other cellular activities. The ProMolec data reveal that the solution to this ‘ATP–reductant crisis’ varies with the dominant metabolic activities occurring at a given time. And this is the key to the question posed above regarding matches and mismatches between carbon fixation and cellular carbon accumulation.
Figure 5.
Comparison of dominant metabolic pathways during (a) amino acid and (b) carbohydrate synthesis. Photosynthesis generates ATP and NADPH (green box). (a) During amino acid synthesis, most of this photosynthate enters the Calvin cycle to produce glyceraldehyde-3-phosphate (GAP), which is the initial substrate for the 20 amino acids. The process generates reductant (yellow circles with ‘+’), ATP (pink circles with ‘P’) and CO2 (blue circles), while some steps also consume ATP and reductant (red arrows). In prokaryotes, the citric acid cycle is incomplete between 2-oxoglutarate and succinyl-CoA (green dashed arrow). Some lipids are also produced during amino acid synthesis but not storage carbohydrates. (b) During carbohydrate synthesis, the glycolytic pathway is downregulated and photosynthate is used directly for other cell activities (blue box). 3-PGA, 3-phosphoglyceric acid; PEP, phosphoenolpyruvate. For simplicity, histidine, which is synthesized from PRPP (5-phosphoribosyl-1-pyrophosphate), ATP and glutamine, is depicted in (a) as deriving from glutamine. PRPP derives from ribose-5-phosphate which is primarily formed by the pentose phosphate pathway. The pentose phosphate pathway is not shown in this figure, but is another important pathway for generating ATP and reductant at the expense of GAP, while releasing CO2.
During the first half of the photoperiod of the ProMolec experiment, amino acid synthesis was the dominant metabolic activity. Amino acids are created from five intermediate products of glycolysis and the citric acid cycle (figure 5a; Coruzzi & Last 2000). GAP is the initial substrate for amino acid synthesis in the light and the process yields a net production of ATP and NAD(P)H (see part III of the electronic supplementary material). Amino acid synthesis also liberates CO2, such that only a fraction of the carbon originally fixed by the Calvin cycle is ultimately retained in the final amino acid products (figure 5a). This CO2 release causes cellular carbon to accumulate more slowly than the rate at which CO2 is fixed, but it does not fully account for the divergence between carbon fixation and accumulation observed during ProMolec in the first half of the photoperiod (figure 4b). This discrepancy implies that some of the GAP formed in the morning is fully oxidized, probably by the pentose phosphate pathway, to augment ATP and reductant supplies. While the relative fraction of GAP dedicated to these different pathways may differ between prokaryotic and eukaryotic phytoplankton, their combined ATP and reductant provides the fuel necessary for all other cell activities and thus solves the ATP–reductant crisis for Prochlorococcus in the morning.
The drastic midday drop in carbon fixation capacity during ProMolec corresponded to the second phase of the cell cycle and a switch in the dominant product of carbon fixation from amino acids to carbohydrates (figure 4). Carbon products accumulated during G1 are used during the S-phase (e.g. polymerization of nucleotides). These activities require significant ATP and reductant, but no de novo amino acid synthesis (Vaulot 1995 and references therein). The simultaneous shift to carbohydrate synthesis creates a problem, however, as it generates neither ATP and NADH nor CO2 (figure 5b). This new ATP–reductant crisis is solved by redirecting photosynthate away from the Calvin cycle and using it directly (figure 5b). The ProMolec data indicate that this direct photosynthate sink can be remarkably large, amounting to at least 65% of the photosynthate produced during the afternoon (this is a ‘minimum’ estimate because it assumes that all photosynthate is used for carbon fixation during the morning). Dedicating the remaining 35% of photosynthate to carbohydrate synthesis has an additional important ramification. Since none of the carbon in GAP is respired, carbohydrate synthesis yields a close match between rates of carbon fixation and cellular carbon accumulation (figure 4b).
From the above considerations, an explanation emerges that Ek-independent variability at the diel scale arises in part from temporal shifts in the allocation of photosynthate between carbon fixation and direct use and from differences between dominant synthetic pathways in the fraction of carbon retained from the new Calvin cycle products (GAP). Pathways with low carbon retention have high ATP and reductant yields, allowing photosynthate to be heavily invested into carbon fixation. When these pathways dominate, carbon fixation (filled circles in figure 4b) correlates with total photosynthesis, but is poorly correlated with cellular carbon accumulation (open circles in figure 4b). Conversely, pathways with high carbon retention allow only a fraction of total photosynthate to be invested in carbon fixation. When these pathways dominate, carbon fixation is well correlated with carbon accumulation, but not total photosynthesis (figure 4b).
5. Supply and demand
Section 4 draws attention to an important aspect of a photoautotroph's supply and demand problem: photosynthesis must supply ATP and reductant for all cellular activities, not just carbon fixation. This problem is solved by either generating ATP and NAD(P)H from GAP metabolism (e.g. during amino acid synthesis) or by investing only a fraction of photosynthate into carbon fixation and directly using the remaining photosynthate for other activities. The second important supply and demand problem not addressed by these solutions is matching the production ratio of ATP and reductant to cellular requirements. As suggested above, this production ratio is a function of both the yield from linear PET and the yield from parallel carbon metabolism. In the case of amino acid synthesis, ATP and reductant are provided at a ratio closer to 1 : 1 than 3 : 2. On the other hand, during carbohydrate formation, these products are provided directly from photosynthesis at the 3 : 2 ratio, with no additional input from the metabolism of new carbon compounds. In neither case does this production ratio necessarily match cellular needs. To balance overall supply and demand, therefore, phytoplankton have evolved multiple mechanisms for generating ATP, often at the expense of reductant. These processes contribute to Ek-independent behaviour.
The ATP : reductant supply-to-demand ratio is easily balanced at night by respiratory electron transport, which uses NAD(P)H to form ATP. In prokaryotes, photosynthetic membranes are employed for this respiration, while in eukaryotes it occurs in both the mitochondria and the chloroplasts. During daylight hours when light is saturating (i.e. most of the day in the surface ocean), photosynthetic membranes are less available for respiratory electron flow, creating a particular problem for prokaryotes. Specifically, PET is limited downstream of PSII either by a particular PET component (plastoquinone (PQ), cytochrome b6f, plastocyanin (or cyt 553), photosystem I (PSI)) or by the supply of substrate (NADP+). Under such conditions, PSII turnover is limited by the oxidation rate of plastoquinol (PQH2), causing light absorption by PSII to exceed use for photochemistry and creating a condition favourable to photoinhibition. Any additional respiratory electron flow to the PQ pool from NAD(P)H would consequently exacerbate the PSII turnover problem. We propose that multiple thylakoid pathways are engaged that balance ATP :reductant supply and demand while bypassing any need for respiratory electron flow into the PQ pool.
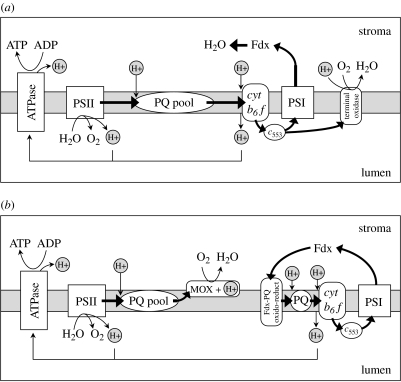
One of the simplest solutions for enhancing ATP : reductant yields is a thylakoid water–water cycle, whereby electrons generated from water by PSII are returned to water after being used for proton pumping across the thylakoid membrane (figure 6a). The most thoroughly studied water–water cycle is the Mehler reaction (Asada 1999), which transfers electrons derived from water by PSII back to water on the downstream side of PSI through an intermediate step involving ferredoxin and creates a trans-thylakoid proton gradient for ATP synthesis (figure 6a). However, the Mehler reaction can create damaging oxygen radicals (Miyake & Asada 2003). A ‘safer’ water–water cycle in prokaryotes might thus entail the direct transfer of electrons from plastocyanin to water via the same membrane-bound terminal oxidase as involved in night-time respiration (figure 6a).
Figure 6.
Thylakoid pathways for enhancing ATP production. (a) Water–water cycles involving the respiratory terminal oxidase in prokaryotes and the Mehler reaction. (b) Decoupled electron flow where electrons from PSII are transferred back to water by a midstream terminal oxidase and electron flow around PSI is cyclic. Proton pumping for ATP generation is accomplished in this scheme through water splitting at PSII and cyclic electron flow around PSI by PQH2 oxidation and Q-cycle pumping at cytochrome b6f.
Another potentially important pathway for generating ATP in the light using PET components is cyclic electron flow around PSI. In this pathway, ferredoxin reduced by PSI transfers electrons to the PQ pool through a membrane-bound Fdx-PQ oxido-reductase, which then transfers them to cyctochrome b6f, plastocyanin and back to PSI (Malkin & Niyogi 2000; figure 6b). Like respiratory electron flow, this pathway too represents a flow of electrons into the PQ pool that exacerbates the PSII turnover problem under saturating light. We propose that, in addition to downregulating PSII and upregulating non-photochemical quenching, photoautotrophs have evolved a profitable solution to this condition: they employ ‘midstream’ terminal oxidases (MOXs) to augment ATP synthesis using electron flow from PSII. Two examples of these midstream PQ oxidases are PTOX and cytochrome bd, and they transfer electrons directly from the PQ pool to water (Pils & Schmetterer 2001; Berry et al. 2002; Peltier & Cournac 2002; Hart et al. 2005; Bailey et al. in press).1 This short water–water cycle reduces PSII photoinhibitory stress and contributes to a transmembrane proton gradient for ATP synthesis through water splitting at PSII (note, protons entering the PQ pool do not contribute to a transmembrane gradient because they are used by MOX for O2 reduction; figure 6b). In this view, therefore, ATP synthesis from PSI cyclic electron flow is only half the story. The more complete version is that low ATP : reductant demands favour coordinated linear electron flow through PSII and PSI, while high ATP : reductant demands decouple PSII and PSI electron flow to create two distinct pathways, each supporting ATP production. The basic nature of this ‘decoupled ATP configuration’ is illustrated in figure 6b.
In eukaryotes, an additional group of mechanisms exists that generate ATP from NADPH and are referred to as ‘substrate shuttles’. These pathways transfer reducing power from the chloroplast, through the cytosol, and into the mitochondria in a sort of ‘bucket brigade’ manner. One such shuttle involves the substrate couple, malate and oxaloacetate (Heber 1974; Heldt et al. 1990; Raghavendra et al. 1994; Hoefnagel et al. 1998; Peltier & Cournac 2002). Currently, substrate shuttles are viewed as a minor component in overall ATP synthesis.
6. Basic principles and implications
Earlier we stated that variability in 14C-based CI relationships can be divided into two categories: Ek-dependent and Ek-independent behaviours (§3). We then proposed that the formerly unresolved basis for Ek-independent behaviour can now be attributed to specific metabolic processes that decouple carbon fixation from photosynthesis. In summary, cellular pigment concentration for a given light climate is proportional to the summed demand for photosynthetically generated ATP and reductant, which itself represents the optimization solution for a particular integrated growth environment. Carbon fixation, on the other hand, is only one of multiple sinks for this ATP and reductant. The relative fraction of photosynthate dedicated to carbon fixation is determined by the following four primary factors: (i) the demand for new carbon products relative to ATP and reductant demand, (ii) the ATP and reductant yield of parallel carbon metabolism, (iii) the requirement for storage products, and (iv) the differences in the production and demand ratios for ATP and reductant. Ek-independent behaviour, therefore, is predominantly the consequence of these four factors causing changes in the ratio of carbon fixation to photosynthesis or, equivalently, carbon fixation per unit pigment.
The primary factors driving Ek-independent behaviour are themselves functions of multiple cellular and environmental determinants. In §4, we used results from the laboratory ProMolec experiment to describe how phases of the cell cycle and switching between amino acid and carbohydrate synthesis influence ATP and reductant production and use over the diurnal cycle. These concepts can be extended to the much broader space and time scales under which Ek-independent behaviour is expressed by considering the wide variety of integrated growth conditions that exist in the global oceans, for example:
Growth rate. Increased growth rate involves shortening of the G1 cell cycle phase, while the duration of and energetic demands for division (S+G2+M) remain relatively conserved. The optimization solution for rapid growth is to increase carbohydrate synthesis during the division phase to provide ATP and reductant for the subsequent G1 phase (i.e. a short-lived carbon pool). Slow growth allows photosynthetic capacity to be closely matched to division phase requirements, while prolonged G1 allows a greater fraction of photosynthate to be consumed directly. At the extreme, cell maintenance consumes essentially all photosynthates as growth rate (and by definition, NPP) approaches zero.
Resource acquisition. Variations in external bioavailable essential resources (e.g. figure 2a–d) alter requirements for membrane-bound transporters (amino acid demanding components) and ATP demands for operating these transporters (i.e. changing the ATP–reductant demand ratio).
Photoperiod. Increased day length decreases demands for storage products and increases direct consumption of photosynthates.
Additional factors contributing to Ek-independent behaviour include unique physiological responses to specific types of nutrient stress (see below) and the influence of accessory pigments on chlorophyll-specific carbon fixation rates. This later taxonomically dependent factor, however, is generally not a major source of Ek-independent variability in the field (Côté & Platt 1983; Behrenfeld et al. 2004).
Our proposed basis for Ek-independent behaviour focuses on the relative role of carbon fixation in overall cellular metabolism and photosynthesis; considers unique attributes of energy and reductant acquisition involved in the photoautotrophic lifestyle; and provides a mechanism through which nutrient constraints contribute to observed variability in carbon fixation efficiencies (e.g. via growth rate and resource acquisition). Important practical implications of this interpretation are the following:
14C-uptake does not measure NPP. The relationship between 14C uptake and NPP is dependent on the duration of the 14C incubation and the four factors responsible for Ek-independent behaviour identified above. In general, deviation of 14C uptake from NPP increases as growth rate increases.
14C-uptake does not measure photosynthesis. Photosynthesis is the light-driven production of ATP and reductant by the PET chain. The fraction of photosynthate invested into carbon fixation varies with the four factors controlling Ek-independent behaviour. In general, deviation of 14C uptake from photosynthesis increases as growth rate decreases.
Light-saturated photosynthesis is not determined by the Calvin cycle capacity. It is often assumed that the light-saturated rate of photosynthesis is limited by the enzymatic capacity of the Calvin cycle, particularly rubisco (e.g. Sukenik et al. 1987; MacIntyre & Geider 1996), but carbon fixation is only one of multiple sinks for photosynthate. Instead, photosynthetic capacity is tuned to the cell's overall capacity to use photosynthate and thus substrate turnover (NADP+ and ADP) limits light-saturated photosynthesis.
Field carbon biomass and growth rate data are needed. NPP is best measured as the product of phytoplankton carbon biomass and growth rate, but such data are difficult, at best, to collect in the field and highly controversial. Resolving methodological and technological issues to allow routine phytoplankton carbon and growth rate measurements will contribute greatly to our understanding of ocean productivity.
7. Nutrients
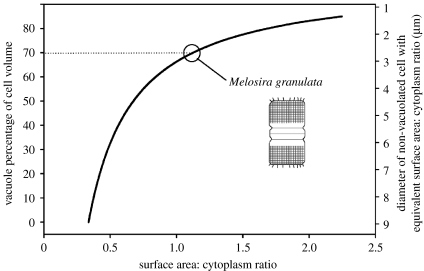
Availability of nutrients (C, N, P, Si, Fe, etc.) in the surface ocean has varied tremendously over geological time scales and continues to vary widely over space and time in the contemporary ocean. Phytoplankton have evolved diverse strategies to cope with this variability, some physiological and some more ‘holistic’. At the community level, for example, low nutrient environments favour species with high surface-to-cytoplasm ratios, both giving an advantage to small cells and creating an evolutionary motive for adopting non-spherical shapes and internal vacuoles (figure 7). In addition to increasing surface area : cytoplasm ratios, vacuolization has the added benefits of influencing grazing pressure (a larger cell is less suitable for micrograzer consumption) and enabling luxury nutrient uptake, which can stabilize growth rates when nutrient supply is episodic or prolong population expansion when external nutrients diminish.
Figure 7.
Change in the surface area : cytoplasm ratio for a cylindrical cell as the vacuole percentage of cell volume increases from 0 to 85% (left axis), and the equivalent diameter of a spherical, non-vacuolated cell with the same surface area : cytoplasm ratio (right axis). As a specific example, the diatom Melosira granulata (inset line drawing) was described by Sicko-Goad et al. (1984) as having an average cell volume of 5130 μm3 (approximate dimensions: 30 μm×15 μm) and a vacuole occupying 70% of the cell, giving a surface area : cytoplasm ratio of approximately 1.1. The diameter of a non-vacuolated spherical cell with an equivalent surface area : cytoplasm ratio is 2.7 μm (top x-axis).
In §6, we highlighted some of the general physiological consequences of nutrient stress that give rise to Ek-independent variability across the full spatio-temporal spectrum. In this section, we expand upon this role of nutrients by considering some unique aspects of nitrogen, carbon and iron availability.
(a) Nitrogen
Fixed inorganic nitrogen in the Archaean and early Protozeroic oceans was scarce and only increased substantially following the proliferation of nitrogen-fixing diazotrophs. From this point until the explosion of oxygenic photosynthesis, reduced nitrogen available to phytoplankton was primarily in the form of ammonium (Falkowski & Raven 1997). Nitrification evolved after the formation of free O2 in the ocean and yielded and as the dominant inorganic nitrogen forms (Falkowski & Raven 1997). In the contemporary ocean, multiple forms of reduced nitrogen are available to phytoplankton (, , and organic nitrogen), but their summed concentration is highly variable over space and time and an important determinant of phytoplankton biomass globally.
Physiologically, the total concentration of nitrogen is not as important as its form and daily allotment per cell (which is independent of biomass or total N concentration). is the primary nitrogen form supporting phytoplankton in the recycling-based communities of permanently stratified ocean regions, while is essential in regions supporting significant algal blooms. Energetically, is preferential to , as the former requires only one NAD(P)H or ferredoxin and one ATP for assimilation into glutamate, while the latter requires nine reductants and one ATP (Coruzzi & Last 2000). During the day, these substrates are provided directly from photosynthesis and thus the form of nitrogen used influences both the ATP : reductant demand ratio and the photosynthesis : carbon fixation ratio, thereby contributing to Ek-independent variability.
(b) Carbon
Carboxylation of ribulose-1,5-biphosphate (RuBp) by rubisco in the early Earth was probably unlimited by the availability of CO2, leading to the evolution of a rubisco with particularly low substrate affinity (Km=20–100 μM; Cooper et al. 1969; Badger et al. 1998). rubisco, however, can also catalyse the oxidation of RuBp and this reaction, termed ‘photorespiration’, became significant following the development of oxygenic photosynthesis. Loss of valuable carbon and ATP to photorespiration created an evolutionary motive for improving the CO2 affinity of rubisco, but even in extant forms these improvements are minor and oxygenase activity persists (Badger et al. 1998; Tortell et al. 2000). Consequently, all rapidly growing oxygenic photoautotrophs now employ CO2-concentrating mechanisms (CCMs) that elevate intracellular CO2 to levels where rubisco functions primarily as a carboxylase, not an oxygenase. CCMs require resources to construct and ATP to operate. Thus, while inorganic carbon is generally not a primary limiting factor for phytoplankton growth, it is a resource-requiring aspect of the integrated growth environment that influences optimization.
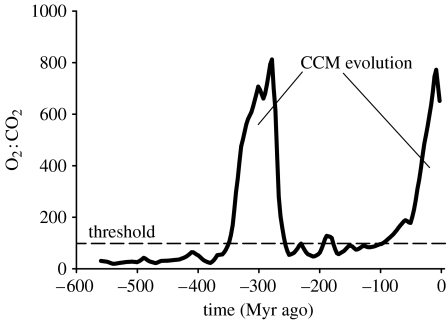
The requirement for CCMs has fluctuated over the Earth's history and occurs when CO2 concentrations are low relative to O2. Prior to the Phanerozoic (ca 542 Myr ago), CO2 was probably only low during extreme glaciation events (snowball Earth) of the Cryogenian and Ediacaran (ca 850–542 Myr ago). During the Phanerozoic, the O2 : CO2 ratio exceeded the theoretical threshold predicating CCM evolution in photosynthetic organisms (estimated by von Caemmerer & Furbank 1999) on two occasions (figure 8). While there is no direct evidence that either phytoplankton or vascular plants evolved CCMs during the Carboniferous (359–299 Myr ago), an increase in the 13C signature of bulk marine organic matter in the late Eocene (ca 50 Myr ago) suggests reduced photosynthetic discrimination and is consistent with the operation of CCMs (Falkowski et al. 2005).
Figure 8.
Changes in the atmospheric O2 : CO2 ratio over the Phanerozoic. The peak at 300 Myr ago coincides with the evolution of vascular plants. Horizontal dashed line indicates O2 : CO2 ratio of 100 estimated by von Caemmerer & Furbank (1999) as the selection pressure threshold for developing a CCM. The O2 : CO2 ratios were calculated from Falkowski et al. (2005) and Berner (2006) (see part IV of the electronic supplementary material).
In phytoplankton, concentrating CO2 involves either the biophysical accumulation of bicarbonate (via ion pumping or facilitated CO2 transport) or the biochemical production of an organic four carbon intermediate (i.e. C4 photosynthesis; Reinfelder et al. 2004). Whole-cell kinetic studies show that prokaryotic and eukaryotic species accumulate inorganic carbon to intracellular concentrations 10–1000 times greater than their external milieu (Burns & Beardall 1987; Dixon & Merrett 1988; Merret 1990; Colman & Rotatore 1995; Rotatore et al. 1995; Korb et al. 1997; Nimer et al. 1998; Wolf-Gladrow et al. 1999). In most prokaryotes, CCMs include both an ATP-requiring active transport system for bicarbonate (via a uniport and a Na+/ symport) and an NADH-requiring CO2 uptake system that converts CO2 to bicarbonate intracellularly, although some species appear to lack this latter system (e.g. Prochlorococcus; Badger & Price 2003). Bicarbonate accumulation in the cytoplasm is thought to have an ATP : or NADH : CO2 cost ratio of 1 (but see Ritchie et al. 1996). This ATP demand for CCM operation can be supplied directly by thylakoid pathways described in §5 (figure 6), including the pseudocyclic pathway (Mehler; Miller et al. 1988; Sültemeyer et al. 1993). Rubisco levels are also elevated under low CO2 conditions to overcome slow catalytic rates (Tortell et al. 2000), representing an additional resource cost to the cell.
The widespread occurrence of CCMs and their mechanistic diversity indicates that inorganic carbon availability is a physiologically relevant property of the integrated growth environment. While the resource requirements for CCMs logically imply that extracellular CO2 levels impact phytoplankton growth rates and optimization, these effects have not been consistently observed in the few field manipulation studies conducted (Tortell et al. 1997, 2000). For example, growth rates of the diatom Skeletonema increased 40% upon doubling pCO2 in one experiment, while the co-occurring diatom Nitzschia exhibited no apparent response (Moore et al. 2006). In Synechococcus, doubling pCO2 (380–750 ppm) increased growth rates by 15% and increased inventories of cellular carbon and nitrogen (Fu et al. 2007). Similar results were obtained for the N2-fixing cyanobacterium, Trichodesmium, where both growth and nitrogen fixation rates increased when pCO2 doubled (Hutchins et al. 2007).
(c) Iron
In the primordial ocean, iron availability was not a problem. In the contemporary ocean, it is. Iron was readily available in the reducing environment of early Earth and is an effective redox agent, so it was widely integrated into core electron transport components (Raven 1988, 1990; Falkowski & Raven 1997). For example, a single copy of each PET component involved in linear electron transport from water to NADPH requires 24 Fe atoms (Michel & Pistorius 2004). The advent of this incredible oxygenic photosynthesis system, however, had the unfortunate side effect of altering the Earth so profoundly that it threatened not only the existence of its inventor, but all other life. Oxygen is toxic to anaerobic organisms, while oxygenation of the ocean drastically reduced the solubility of Fe. In combination, these two consequences of oxygenic photosynthesis surely created one of the strongest biologically instigated evolutionary drivers in the Earth's history.
Iron remains a primary limiting factor for phytoplankton production over broad ocean regions (Martin & Fitzwater 1988; Coale et al. 1996; Boyd et al. 2000; Tsuda et al. 2003; Behrenfeld et al. 2006b) and may restrict nitrogen fixation (Falkowski 1997). Physiological responses to low iron include employment of photosynthate-demanding high-affinity iron transporters, substitution of mobile iron-requiring PET components (plastocyanin for cytochrome c553 and flavodoxin for ferredoxin; Geider & La Roche 1994; Straus 1994) and downregulation of membrane-bound PET components in rough proportion to their iron requirement (Sandmann 1985; Vassiliev et al. 1995; Ivanov et al. 2000). This later response, termed ‘retrenchment’, thus reduces PSI more severely than cytochrome b6f, which itself is diminished relative to PSII. Such stoichiometric shifts alter the rate-limiting step for PET (Sandmann 1985; Greene et al. 1992; Vassiliev et al. 1995; Belkhodja et al. 1998; Ivanov et al. 2000; Morales et al. 2001), have the overall net effect of making iron stress equivalent to limitation by photosynthate availability, and decrease pigment-specific efficiencies for photosynthetic reductant formation. Under high macronutrient levels, iron stress has the additional important consequence of stimulating the synthesis of special pigment–protein complexes that appear largely disconnected from PSII or PSI (Reithman & Sherman 1988; Vassiliev et al. 1995; Park et al. 1999; Morales et al. 2001; Sandström et al. 2002; Michel & Pistorius 2004; Behrenfeld et al. 2006b). Accordingly, these complexes lower chlorophyll-specific photosynthetic and carbon fixation efficiencies and represent a specific nutrient-stress response that yields Ek-independent variability through changes in the pigment : photosynthesis relationship, rather than the ATP/reductant-based changes in photosynthesis : carbon fixation relationship discussed in §§4–6.
A final intriguing consequence of iron stress that deserves attention is the peculiar stoichiometric shift in PSII and PSI. Specifically, retrenchment invariably causes an apparent overexpression of PSII. A potential explanation for this is that PSI can achieve substantially faster turnover rates than PSII, so the iron stress response simply implies a shift in relative turnover for the two photosystems. This explanation, however, raises the question of why PSI (and cytochrome b6f) is overexpressed when iron is not limiting? One possibility is that the additional PSI allows enhanced cyclic electron transport when iron is abundant. But this idea raises its own issue of how phytoplankton deal with balancing ATP : reductant supply and demand when iron is low. As an additional complication, field investigations of diel cycles in variable fluorescence properties indicate that PET is rate limited at or downstream of cytochrome b6f under iron-limiting conditions (Behrenfeld et al. 2006b). Consequently, overexpression of PSII not only appears as a waste of iron (each functional PSII requires three Fe atoms) but also risks severe photoinhibition when light is saturating, yet phytoplankton in HNLC waters often exhibit lower midday photoinhibition relative to non iron-stressed cells under similar light levels (Behrenfeld & Kolber 1999).
One potential explanation for this long list of apparent contradictions is that the enhanced PSII levels under low iron actually serve an important function, namely ATP synthesis. As described in §5, midstream PQ-oxidases exist in thylakoid membranes that create a short water–water cycle allowing ATP synthesis without the iron-demanding downstream components, cytochrome b6f and PSI. Thus, the classical retrenchment effect of elevated PSII : PSI ratios may simply represent a mechanism by which phytoplankton capitalize on the low Fe requirements of PSII and the MOXs (two Fe per complex) to balance ATP supply and demand in the absence of normal PSI levels. If this is the case, might it not also be that these PQ-oxidases (e.g. PTOX, cyt bd) represent an evolutionary consequence of vanishing iron during the original ‘oxygen crisis’?
8. Light
Photoadaptation is the evolutionarily driven, genetically encoded response of plants to light quality and quantity, and it encompasses forms ranging from environmentally regulated pigmentation changes, protective mechanisms for high light exposure and morphological adjustments. Photoacclimation, on the other hand, is the dynamic employment of a subset of these mechanisms at lifetime scales to effectively ‘smooth out’ short-term fluctuations in incident light and ensure that light harvesting matches photosynthate demands in an optimal manner. A comprehensive treatment of photoacclimation is far beyond the scope of this article, but a brief discussion is provided here because light is one of the key environmental resources subject to climate variability and it influences investment strategies for optimization that strongly impact relationships between pigment content, photosynthesis and carbon fixation. The Ek-dependent expression of photoacclimation also contrasts with Ek-independent phenomena discussed above.
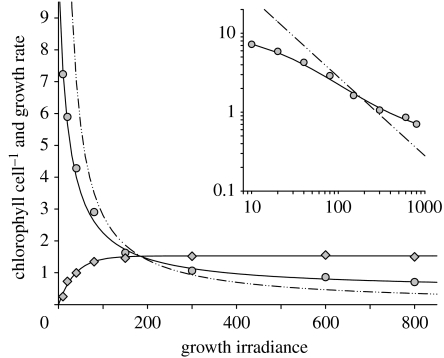
Our primary photoacclimation response of interest is the change in cellular pigmentation with changing growth irradiance (Ig; figure 9). Increases in pigment level with decreasing light function to minimize the influence of Ig on growth rate. The response is imperfect, though, and growth rate eventually decreases at sufficiently low light (figure 9). This ‘imperfection’ exists because it is simply impossible to increase chlorophyll sufficiently to prevent it. Specifically, maintaining a constant growth rate at all light levels would require cellular chlorophyll to increase minimally (i.e. ignoring self-shading; Duysens 1956) as a function of 1/Ig (dot-dashed line in figure 9), leading to the solution that chlorophyll concentration goes to infinity as light goes to zero. Instead, phytoplankton optimize investments in light-harvesting machinery to maximize growth at subsaturating Ig (Shuter 1979; Geider et al. 1996, 1998; Flynn 2001), with losses in growth rate roughly proportional to the difference between the adopted chlorophyll–Ig relationship and the 1/Ig curve (figure 9).
Figure 9.
Cellular chlorophyll concentration (pg cell−1; circles) and growth rate (divisions d−1; diamonds) of Dunaliella tertiolecta acclimated to a range of growth irradiance (Ig) levels (μmol quanta m−2 s−1). Dot-dashed line is 1/Ig scaled for comparison with chlorophyll data. Inset is same chlorophyll data as the main figure except with log-log axes to better show deviation between acclimated chlorophyll values and the 1/Ig relationship. Cells were grown under constant light in nutrient-replete (F/2) medium.
In stark contrast to low light conditions, cellular chlorophyll concentrations exceed the 1/Ig curve at high light and often exhibit a clear and significant plateau (figure 9; Behrenfeld et al. 2002). This minimum chlorophyll level (Chlmin) under saturating light is dictated by the following three factors: (i) the total cellular demand for photosynthate (Pmax, electrons time−1), (ii) the minimum electron turnover time of PSII () and PSI (), and (iii) the minimal pigment content necessary for effective light absorption and energy transfer (),
Light-driven adjustments in cellular pigmentation (figure 9) cause Ek-dependent changes in CI relationships because they target light-harvesting capacities while having minimal impacts on light-saturated rates of photosynthesis. In addition, photoacclimation in surface phytoplankton is keyed to the median mixed layer light level, which can vary widely. Consequently, photoacclimation strongly impacts global surface chlorophyll concentrations and is a major contributor to variability in chlorophyll-specific light-saturated photosynthetic rates.
9. Phytoplankton and climate
A central theme in this discussion has been the influence of the integrated growth environment on physiological strategies in phytoplankton and we have assigned distinct CI behaviours to nutrient effects (Ek-independent) and light effects (Ek-dependent). These two key attributes of the surface growth environment are intimately connected to climate. For example, inorganic carbon varies with SST and salinity, surface nitrate depends on stratification, upwelling, eddy pumping and planetary wave action, iron is uniquely influenced by aeolian dust deposition, and growth irradiance varies with incident light, mixing depth and vertical light attenuation. With these dependencies in mind, we now return to where we started: the satellite chlorophyll record (figure 1).
A path for deconvolving contributions of phytoplankton biomass variability and physiology to global distributions and trends in surface chlorophyll concentrations has recently emerged. In the initial analysis of Behrenfeld et al. (2005) and subsequent study by Westberry et al. (in press), particulate backscattering coefficients derived from satellite ocean colour data using the Garver–Siegel–Maritorena (GSM) inversion algorithm (Garver & Siegel 1997; Maritorena et al. 2002; Siegel et al. 2002) were used to estimate global phytoplankton carbon concentrations. From these data, a direct index of phytoplankton physiology is provided through changes in chlorophyll : carbon (Chl : C) ratios, which we can further dissect into light versus nutrient-temperature effects using global fields of surface mixing depths, attenuation coefficients, incident light levels and a photoacclimation response function similar to figure 9 (Behrenfeld et al. 2005). Results from such an interrogation of the satellite data are shown in figure 10.
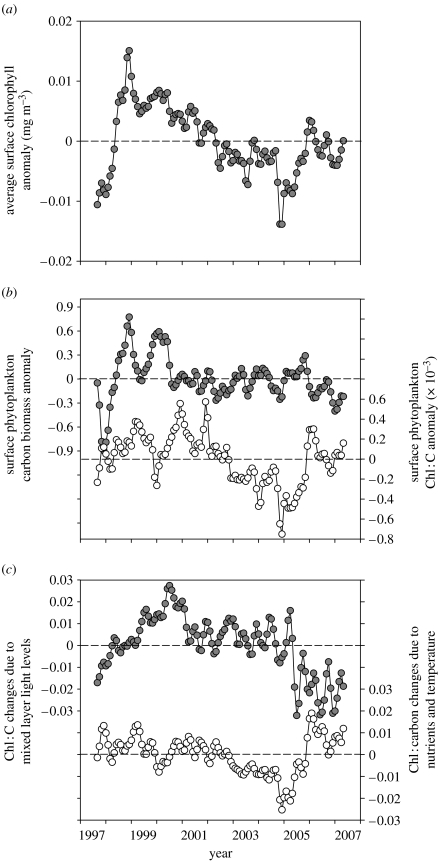
Figure 10.
(a) Monthly anomalies in surface chlorophyll concentration (mg m−3) for the permanently stratified ocean (greater than 15°C) over the first 10 years of the SeaWiFS record. (b) Monthly anomalies in phytoplankton carbon biomass (mg C m−3; filled circles, left axis) and chlorophyll : carbon (Chl : C) ratios (mg : mg×10−3; open circles, right axis). (c) Monthly anomalies in the relative contribution of photoacclimation (filled circles, left axis) and nutrient-temperature stress (open circles, right axis) to total Chl : C variability. Anomalies were calculated as the difference between a given month's value and the average value for that month for the entire record. Horizontal dashed lines in each figure indicate zero values for each y-axis. Data are based on the GSM satellite algorithm.
Monthly anomalies in GSM-based surface chlorophyll concentrations (m−3) for the permanently stratified ocean (figure 10a) exhibit similar temporal patterns as the photic zone integrated values from the standard SeaWiFS chlorophyll algorithm (figure 1). Comparison of these data to monthly carbon anomalies (figure 10b) reveals that the initial increase and subsequent decrease in surface chlorophyll from September 1997 to December 2000 (figure 10b—filled circles, left axis) was largely due to changes in phytoplankton biomass. Since 2001, however, phytoplankton biomass remained essentially constant, while the continued trends in surface chlorophyll (figure 10a) were predominantly due to intracellular changes in pigmentation (figure 10b—open circles, right axis). This remarkable result emphasizes the importance of physiology at the global scale. But we can go a step further. Separating Chl : C variability (figure 10b) into light and nutrient-temperature effects reveals that physiological adjustments in pigmentation between 1997 and 2004 were dominated by photoacclimation responses to varying mixed layer light levels, while the nutrient-temperature component exhibited its largest feature between 2005 and 2007 (figure 10c). This analysis thus illustrates in rich detail global responses of natural phytoplankton populations to climate variations over a decadal period, a complexity hidden from view when analysing chlorophyll data alone. Distinguishing these diverse responses is critical to the accurate assessment of global ocean NPP and assessment of climate change effects as surface nutrient and light conditions are further altered.
10. Deep time to the present
The tireless ubiquitous activities of microscopic photoautotrophs in the global ocean have left an indelible signature on the Earth's atmosphere. Many of the major events coupling photosynthetic and atmospheric evolution in the distant past are detailed in accompanying articles of this issue. Here, we have looked at the present Earth system from cellular to global scales. We have focused on evolved optimization strategies that maximize growth through the judicious allocation of resources and contrasted nutrient-related strategies with those for light acclimation. We then explored the global expression of nutrient and light effects using the satellite data, illustrating both the significance of physiological variability in the chlorophyll record and the importance of new satellite analysis techniques for resolving this variability and its link to climate.
From an even broader perspective, global distributions of phytoplankton biomass and growth rates (e.g. figure 2e,f) can be seen as representing two very different ecological properties: the former is the consequence of evolution and the latter the impetus for evolution. From deep time to the present, the stage upon which the story of evolution unfolds is at the level of resource acquisition and allocation, where novel solutions to old problems emerge. These solutions alter interspecific competition for resources at the level of the individual. The severity of this competition is expressed far more clearly in patterns of phytoplankton growth rates (figure 2f) than in biomass (figure 2e), but their eventual consequence is to alter biomass yields for a given pool of resources. Biomass, then, is viewed as an expression of the current winners in this struggle for resources. The challenge today is to turn this sequence around and use our understanding of phytoplankton physiology to interpret global-scale changes in observed biological properties. This understanding requires insights into the mechanisms employed by evolved phytoplankton populations that enable survival, and even proliferation, within the integrated growth environments of our contemporary seas.
Acknowledgments
We thank Kieth Moore for providing surface dissolved iron data, Toby Westberry and Robert O'Malley for assistance with figures 1, 2 and 10, Kirby Worthington for data in figure 9, and Dr Marcel Babin, Dr Flavienne Bruyant, Dr Wolfgand Hess and Dr Claudia Steglich for ProMolec data. Special thanks to Dr Karl Banse for encouragement. This work was supported by National Science Foundation grant no. 0550502 and two grants from the Ocean Biology and Biogeochemistry programme at the National Aeronautics and Space Administration (EOS programme and Oceans and Ice programme).
One contribution of 15 to a Discussion Meeting Issue ‘Photosynthetic and atmospheric evolution’.
Endnote
During final revisions of this manuscript, we received a preprint from Mackey et al. (in press) demonstrating that electron flow through ‘midstream’ oxidases can represent a large fraction of total photosynthetic electron flow in light-saturated natural mixed populations of marine phytoplankton.
Supplementary Material
Text and figures for SI parts I through IV
References
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. doi:10.1146/annurev.arplant.50.1.601 [DOI] [PubMed] [Google Scholar]
- Badger M.R, Price G.D. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J. Exp. Bot. 2003;54:609–622. doi: 10.1093/jxb/erg076. doi:10.1093/jxb/erg076 [DOI] [PubMed] [Google Scholar]
- Badger M.R, Andrews T.J, Whitney S.M, Ludwig M, Yellowlees D.C, Leggat W, Price G.D. The diversity and coevolution of rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 1998;76:1052–1071. doi:10.1139/cjb-76-6-1052 [Google Scholar]
- Bailey, S. et al In press. Photosynthesis in marine Synechococcus and the critical nature of electron flow to oxygen. Biochim. Biophys. Acta [DOI] [PubMed]
- Behrenfeld M.J, Falkowski P.G. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 1997;42:1–20. [Google Scholar]
- Behrenfeld M.J, Kolber Z.S. Widespread iron limitation of phytoplankton in the South Pacific Ocean. Science. 1999;283:840–843. doi: 10.1126/science.283.5403.840. doi:10.1126/science.283.5403.840 [DOI] [PubMed] [Google Scholar]
- Behrenfeld M.J, Prasil O, Kolber Z.S, Babin M, Falkowski P.G. Compensatory changes in photosystem II electron turnover rates protect photosynthesis from photoinhibition. Photosyn. Res. 1998;58:259–268. doi:10.1023/A:1006138630573 [Google Scholar]
- Behrenfeld M.J, Maranon E, Siegel D.A, Hooker S.B. Photoacclimation and nutrient-based model of light-saturated photosynthesis for quantifying oceanic primary production. Mar. Ecol. Prog. Ser. 2002;228:103–117. doi:10.3354/meps228103 [Google Scholar]
- Behrenfeld M.J, Prasil O, Babin M, Bruyant F. In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis. J. Phycol. 2004;40:4–25. [Google Scholar]
- Behrenfeld M.J, Boss E, Siegel D.A, Shea D.M. Carbon-based ocean productivity and phytoplankton physiology from space. Global Biogeochem. Cycles. 2005;19:1–14. doi:10.1029/2004GB002299 [Google Scholar]
- Behrenfeld M.J, et al. Climate-driven trends in contemporary ocean productivity. Nature. 2006a;444:752–755. doi: 10.1038/nature05317. doi:10.1038/nature05317 [DOI] [PubMed] [Google Scholar]
- Behrenfeld M.J, Worthington K, Sherrell R.M, Chavez F.P, Strutton P, McPhaden M, Shea D.M. Controls on tropical Pacific Ocean productivity revealed through nutrient stress diagnostics. Nature. 2006b;442:1025–1028. doi: 10.1038/nature05083. doi:10.1038/nature05083 [DOI] [PubMed] [Google Scholar]
- Belkhodja R, Morales F, Quilez R, Lopez-Millan A.F, Abadia A, Abadia J. Iron deficiency causes changes in chlorophyll fluorescence due to the reduction in the dark of the photosystem II acceptor side. Photosyn. Res. 1998;56:265–276. doi:10.1023/A:1006039917599 [Google Scholar]
- Berner R.A. GEOCARBSULF: a combined model for Phanerozoic atmospheric O2 and CO2. Geochim. Cosmochim. Acta. 2006;70:5653–5664. doi:10.1016/j.gca.2005.11.032 [Google Scholar]
- Berry S, Schneider D, Vermaas W.F.J, Rogner M. Electron transport routes in whole cells of Synechocystis sp. strain PCC 6803: the role of the cytochrome bd-type oxidase. Biochemistry. 2002;41:3422–3429. doi: 10.1021/bi011683d. doi:10.1021/bi011683d [DOI] [PubMed] [Google Scholar]
- Bertrand E.M, Saito M.A, Rose J.M, Riesselman C.R, Lohan M.C, Noble A.E, Lee P.A, DiTullio G.R. Vitamin B-12 and iron colimitation of phytoplankton growth in the Ross Sea. Limnol. Oceanogr. 2007;52:1079–1093. [Google Scholar]
- Boyd P.W, et al. A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature. 2000;407:695–702. doi: 10.1038/35037500. doi:10.1038/35037500 [DOI] [PubMed] [Google Scholar]
- Bruyant F, et al. Diel variations in the photosynthetic parameters of Prochlorococcus strain PCC 9511: combined effects of light and cell cycle. Limnol. Oceanogr. 2005;50:850–863. [Google Scholar]
- Burbage C.D, Binder B.J. Relationship between cell cycle and light-limited growth rate in oceanic Prochlorococcus (MIT9312) and Synechococcus (WH8103) (cyanobacteria) J. Phycol. 2007;43:266–274. doi:10.1111/j.1529-8817.2007.00315.x [Google Scholar]
- Burns B.D, Beardall J. Utilization of inorganic carbon by marine microalgae. J. Exp. Mar. Biol. Ecol. 1987;107:75–86. doi:10.1016/0022-0981(87)90125-0 [Google Scholar]
- Claustre H, Moline M.A, Prezelin B.B. Sources of variability in the column photosynthetic cross section for Antarctic coastal waters. J. Geophys. Res. Oceans. 1997;102:25 047–25 060. doi:10.1029/96JC02439 [Google Scholar]
- Coale K.H, et al. A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean. Nature. 1996;383:495–501. doi: 10.1038/383495a0. doi:10.1038/383495a0 [DOI] [PubMed] [Google Scholar]
- Colman B, Rotatore C. Photosynthetic inorganic carbon uptake and accumulation in two marine diatoms. Plant Cell Environ. 1995;18:919–924. doi:10.1111/j.1365-3040.1995.tb00601.x [Google Scholar]
- Cooper T.G, Filmer D, Wishnick M, Lane M.D. The active species of ‘CO2’ utilized by ribulose diphosphate carboxylase. J. Biol. Chem. 1969;244:1081–1083. [PubMed] [Google Scholar]
- Coruzzi G, Last R. Amino acids. In: Buchannan B, Gruissem W, Jones R, editors. Biochemistry and molecular biology of plants. American Society of Plant Physiologists; Rockville, MD: 2000. pp. 358–410. [Google Scholar]
- Côté B, Platt T. Day-to-day variations in the spring–summer photosynthetic parameters of coastal marine phytoplankton. Limnol. Oceanogr. 1983;28:320–344. [Google Scholar]
- Dixon G.K, Merrett M.J. Bicarbonate utilization by the marine diatom Phaeodactylum tricornutum Bohlin. New Phytol. 1988;109:47–51. doi:10.1111/j.1469-8137.1988.tb00217.x [Google Scholar]
- Duysens L.N.M. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim. Biophys. Acta. 1956;19:1–12. doi: 10.1016/0006-3002(56)90380-8. doi:10.1016/0006-3002(56)90380-8 [DOI] [PubMed] [Google Scholar]
- Falkowski P.G. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. doi:10.1038/387272a0 [Google Scholar]
- Falkowski P.G, Raven J.A. Blackwell Scientific; Malden, MA: 1997. Aquatic photosynthesis. [Google Scholar]
- Falkowski P.G, Katz M.E, Milligan A.J, Fennel K, Cramer B.S, Aubry M.P, Berner R.A, Novacek M.J, Zapol W.M. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science. 2005;309:2202–2204. doi: 10.1126/science.1116047. doi:10.1126/science.1116047 [DOI] [PubMed] [Google Scholar]
- Flynn K.J. A mechanistic model for describing dynamic multi-nutrient, light, temperature interactions in phytoplankton. J. Plankton Res. 2001;23:977–997. doi:10.1093/plankt/23.9.977 [Google Scholar]
- Fu F.-X, Warner M.E, Zhang Y, Feng Y, Hutchins D.A. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (Cyanobacteria) J. Phycol. 2007;43:485–496. doi:10.1111/j.1529-8817.2007.00355.x [Google Scholar]
- Garver S.A, Siegel D.A. Inherent optical property inversion of ocean color spectra and its biogeochemical interpretation. 1. Time series from the Sargasso Sea. J. Geophys. Res. Oceans. 1997;102:18 607–18 625. doi:10.1029/96JC03243 [Google Scholar]
- Geider R.J, La Roche J. The role of iron in phytoplankton photosynthesis, and the potential for iron-limitation of primary productivity in the sea. Photosyn. Res. 1994;39:275–301. doi: 10.1007/BF00014588. doi:10.1007/BF00014588 [DOI] [PubMed] [Google Scholar]
- Geider R.J, MacIntyre H.L, Kana T.M. A dynamic model of photoadaptation in phytoplankton. Limnol. Oceanogr. 1996;41:1–15. [Google Scholar]
- Geider R.J, MacIntyre H.L, Kana T.M. A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol. Oceanogr. 1998;43:679–694. [Google Scholar]
- Greene R.M, Geider R.J, Kolber Z, Falkowski P.G. Iron-induced changes in light harvesting and photochemical energy conversion processes in eukaryotic marine algae. Plant Physiol. 1992;100:565–575. doi: 10.1104/pp.100.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart S.E, Schlarb-Ridley B.G, Bendall D.S, Howe C.J. Terminal oxidases of cyanobacteria. Biochem. Soc. Trans. 2005;33:832–835. doi: 10.1042/BST0330832. doi:10.1042/BST0330832 [DOI] [PubMed] [Google Scholar]
- Heber U. Metabolite exchange between chloroplasts and cytoplasm. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1974;25:393–421. [Google Scholar]
- Heber U, Neimanis S, Dietz K.-J. Fractional control of photosynthesis by the QB protein, the cytochrome b6/f complex and other components of the photosynthetic apparatus. Planta. 1988;173:267–274. doi: 10.1007/BF00403020. doi:10.1007/BF00403020 [DOI] [PubMed] [Google Scholar]
- Heldt H.W, Heineke D, Heupel R, Krömer S, Riens B. Transfer of redox equivalents between subcellular compartments of a leaf cell. In: Batscheffsky M, editor. Current research in photosynthesis. Kluwer Academic; Dordrecht, The Netherlands: 1990. pp. 151–157. [Google Scholar]
- Hoefnagel M.H.N, Atkin O.K, Wiskich J.T. Interdependence between chloroplasts and mitochondria in the light and the dark. Biochim. Biophys. Acta. 1998;1366:235–255. doi:10.1016/S0005-2728(98)00126-1 [Google Scholar]
- Hutchins D.A, Fu F.X, Zhang Y, Warner M.E, Feng Y, Portune K, Bernhardt P.W, Mulholland M.R. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: implications for past, present, and future ocean biogeochemistry. Limnol. Oceanogr. 2007;52:1293–1304. [Google Scholar]
- Ivanov A.G, Park Y.I, Miskiewicz E, Raven J.A, Huner N.P.A, Oquist G. Iron stress restricts photosynthetic intersystem electron transport in Synechococcus sp. PCC 7942. FEBS Lett. 2000;485:173–177. doi: 10.1016/s0014-5793(00)02211-0. doi:10.1016/S0014-5793(00)02211-0 [DOI] [PubMed] [Google Scholar]
- Key R.M, et al. A global ocean carbon climatology: results from Global Data Analysis Project (GLODAP) Global Biogeochem. Cycles. 2004;18:GB4031. doi:10.1029/2004GB002247 [Google Scholar]
- Kok B. On the inhibition of photosynthesis by intense light. Biochim. Biophys. Acta. 1956;21:234–244. doi: 10.1016/0006-3002(56)90003-8. doi:10.1016/0006-3002(56)90003-8 [DOI] [PubMed] [Google Scholar]
- Korb R.E, Saville P.J, Johnston A.M, Raven J.A. Sources of inorganic carbon for photosynthesis by three species of marine diatom. J. Phycol. 1997;33:433–440. doi:10.1111/j.0022-3646.1997.00433.x [Google Scholar]
- Leverenz J.W, Falk S, Pilström C.-M, Samuelsson G. The effects of photoinhibition on the photosynthetic light-response curve of green plant cells (Chlamydomonas reinhardtii) Planta. 1990;182:161–168. doi: 10.1007/BF00197105. doi:10.1007/BF00197105 [DOI] [PubMed] [Google Scholar]
- Lindeman R.L. The trophic–dynamic aspect of ecology. Ecology. 1942;23:399–418. doi:10.2307/1930126 [Google Scholar]
- MacIntyre H.L, Geider R.J. Regulation of rubisco activity and its potential effect on photosynthesis during mixing in a turbid estuary. Mar. Ecol. Prog. Ser. 1996;144:247–264. doi:10.3354/meps144247 [Google Scholar]
- Mackey, K. R. M., Paytan, A., Grossman, A. R. & Bailey, S. In press. A photosynthetic strategy for coping in a high-light, low-nutrient environment. Limnol. Oceanogr
- Malkin R, Niyogi K. Photosynthesis. In: Buchannan B, Gruissem W, Jones R, editors. Biochemistry and molecular biology of plants. Wiley; Somerset, NJ: 2000. pp. 568–628. [Google Scholar]
- Maritorena S, Siegel D.A, Peterson A.R. Optimization of a semianalytical ocean color model for global-scale applications. Appl. Opt. 2002;41:2705–2714. doi: 10.1364/ao.41.002705. doi:10.1364/AO.41.002705 [DOI] [PubMed] [Google Scholar]
- Martin J.H, Fitzwater S.E. Iron deficiency limits phytoplankton growth in the north-east Pacific subarctic. Nature. 1988;331:341–343. doi:10.1038/331341a0 [Google Scholar]
- Merret M.J. Inorganic carbon transport in some marine microalgal species. Can. J. Bot. 1990;69:1032–1039. doi:10.1139/b91-133 [Google Scholar]
- Michel K.P, Pistorius E.K. Adaptation of the photosynthetic electron transport chain in cyanobacteria to iron deficiency: the function of IdiA and IsiA. Physiol. Plant. 2004;120:36–50. doi: 10.1111/j.0031-9317.2004.0229.x. doi:10.1111/j.0031-9317.2004.0229.x [DOI] [PubMed] [Google Scholar]
- Miller A.G, Espie G.S, Canvin D.T. Active transport of inorganic carbon increases the rate of O2 photoreduction by the cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988;88:6–9. doi: 10.1104/pp.88.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C, Asada K. The water–water cycle in algae. In: Larkum A.W.D, Douglas S.E, Raven J.A, editors. Advances in photosynthesis and respiration. Photosynthesis in algae. vol. 14. Kluwer Academic; Dordrecht, The Netherlands: 2003. pp. 183–204. [Google Scholar]
- Moore J.K, Braucher O. Sedimentary and mineral dust sources of iron to the world ocean. Biogeosciences. 2007;4:1279–1327. [Google Scholar]
- Moore C.M, Suggett D.J, Hickman A.E, Kim Y.N, Tweddle J.F, Sharples J, Geider R.J, Holligan P.M. Phytoplankton photoacclimation and photoadaptation in response to environmental gradients in a shelf sea. Limnol. Oceanogr. 2006;51:936–949. [Google Scholar]
- Morales F, Moise N, Quilez R, Abadia A, Abadia J, Moya I. Iron deficiency interrupts energy transfer from a disconnected part of the antenna to the rest of photosystem II. Photosyn. Res. 2001;70:207–220. doi: 10.1023/A:1017965229788. doi:10.1023/A:1017965229788 [DOI] [PubMed] [Google Scholar]
- Morel F.M.M, Reinfelder J.R, Roberts S.B, Chamberlain C.P, Lee J.G, Yee D. Zinc and carbon co-limitation of marine phytoplankton. Nature. 1994;369:740–742. doi:10.1038/369740a0 [Google Scholar]
- Nimer N.A, Warren M, Merrett M.J. The regulation of photosynthetic rate and activation of extracellular carbonic anhydrase under CO2-limiting conditions in the marine diatom Skeletonema costatum. Plant Cell Environ. 1998;21:805–812. doi:10.1046/j.1365-3040.1998.00321.x [Google Scholar]
- Park Y.I, Sandstrom S, Gustafsson P, Oquist G. Expression of the isiA gene is essential for the survival of the cyanobacterium Synechococcus sp. PCC7942 by protecting photosystem II from excess light under iron limitation. Mol. Microbiol. 1999;32:123–129. doi: 10.1046/j.1365-2958.1999.01332.x. doi:10.1046/j.1365-2958.1999.01332.x [DOI] [PubMed] [Google Scholar]
- Peltier G, Cournac L. Chlororespiration. Annu. Rev. Plant Biol. 2002;53:523–550. doi: 10.1146/annurev.arplant.53.100301.135242. doi:10.1146/annurev.arplant.53.100301.135242 [DOI] [PubMed] [Google Scholar]
- Pils D, Schmetterer G. Characterization of three bioenergetically active respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC 6803. FEMS Microbiol. Lett. 2001;203:217–222. doi: 10.1111/j.1574-6968.2001.tb10844.x. doi:10.1111/j.1574-6968.2001.tb10844.x [DOI] [PubMed] [Google Scholar]
- Platt T, Sathyendranath S, Ulloa O, Harrison W.G, Hoepffner N, Goes J. Nutrient control of phytoplankton photosynthesis in the western North Atlantic. Nature. 1992;356:229–231. doi:10.1038/356229a0 [Google Scholar]
- Price N.M, Morel F.M.M. Colimitation of phytoplankton growth by nickel and nitrogen. Limnol. Oceanogr. 1991;36:1071–1077. [Google Scholar]
- Raghavendra A.S, Padmasree K, Saradadevi K. Interdependence of photosynthesis and respiration in plant cells—interactions between chloroplasts and mitochondria. Plant Sci. 1994;97:1–14. doi:10.1016/0168-9452(94)90101-5 [Google Scholar]
- Raven J.A. The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytol. 1988;109:279–287. doi:10.1111/j.1469-8137.1988.tb04196.x [Google Scholar]
- Raven J.A. Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway. New Phytol. 1990;116:1–18. doi:10.1111/j.1469-8137.1990.tb00505.x [Google Scholar]
- Reinfelder J.R, Milligan A.J, Morel F.M.M. The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol. 2004;135:2106–2111. doi: 10.1104/pp.104.041319. doi:10.1104/pp.104.041319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithman H.C, Sherman L.A. Purification and characterization of an iron stress-induced chlorophyll–protein from the cyanobacterium Anacystis nidulans R2. Biochim. Biophys. Acta. 1988;935:141–151. doi: 10.1016/0005-2728(88)90211-3. doi:10.1016/0005-2728(88)90211-3 [DOI] [PubMed] [Google Scholar]
- Ritchie R.J, Nadolny C, Larkum A.W.D. Driving forces for bicarbonate transport in the cyanobacterium Synechococcus R-2 (PCC 7942) Plant Physiol. 1996;112:1573–1584. doi: 10.1104/pp.112.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotatore C, Colman B, Kuzma M. The active uptake of carbon dioxide by the marine diatoms Phaeodactylum ticornutum and Cyclotella sp. Plant Cell Environ. 1995;18:913–918. doi:10.1111/j.1365-3040.1995.tb00600.x [Google Scholar]
- Saito M.A, Goepfert T.J, Ritt J.T. Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnol. Oceanogr. 2008;53:276–290. [Google Scholar]
- Sandmann G. Consequences of iron deficiency on photosynthetic and respiratory electron transport in blue-green algae. Photosyn. Res. 1985;6:261–271. doi: 10.1007/BF00049282. doi:10.1007/BF00049282 [DOI] [PubMed] [Google Scholar]
- Sandström S, Ivanov A.G, Park Y.I, Oquist G, Gustafsson P. Iron stress responses in the cyanobacterium Synechococcus sp. PCC7942. Physiol. Plant. 2002;116:255–263. doi: 10.1034/j.1399-3054.2002.1160216.x. doi:10.1034/j.1399-3054.2002.1160216.x [DOI] [PubMed] [Google Scholar]
- Shuter B. A model of physiological adaptation in unicellular algae. J. Theor. Biol. 1979;78:519–552. doi: 10.1016/0022-5193(79)90189-9. doi:10.1016/0022-5193(79)90189-9 [DOI] [PubMed] [Google Scholar]
- Sicko-Goad L.M, Schelske C.L, Stoermer E.F. Estimation of intracellular carbon and silica content of diatoms from natural assemblages using morphometric techniques. Limnol. Oceanogr. 1984;29:1170–1178. [Google Scholar]
- Siegel D.A, Maritorena S, Nelson N.B, Hansell D.A, Lorenzi-Kayser M. Global distribution and dynamics of colored dissolved and detrital organic materials. J. Geophys. Res. Oceans. 2002;107:3228. doi:10.1029/2001JC000965 [Google Scholar]
- Steemann Nielsen E, Jørgensen E.G. The adaptation of plankton algae: I. General part. Physiol. Plant. 1968;21:401–413. doi:10.1111/j.1399-3054.1968.tb07264.x [Google Scholar]
- Steglich C, Behrenfeld M, Koblizek M, Claustre H, Penno S, Prasil O, Partensky F, Hess W.R. Nitrogen deprivation strongly affects photosystem II but not phycoerythrin level in the divinyl-chlorophyll b-containing cyanobacterium Prochlorococcus marinus. Biochim. Biophys. Acta. 2001;1503:341–349. doi: 10.1016/s0005-2728(00)00211-5. doi:10.1016/S0005-2728(00)00211-5 [DOI] [PubMed] [Google Scholar]
- Sterner R.W, Elser J.J. Princeton University Press; Princeton, NJ: 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. [Google Scholar]
- Straus N.A. Iron deprivation: physiology and gene regulation. In: Bryant D.A, editor. The molecular biology of cyanobacteria. Kluwer Academic; Dordrecht, The Netherlands: 1994. pp. 731–750. [Google Scholar]
- Sukenik A, Bennett J, Falkowski P. Light-saturated photosynthesis—limitation by electron transport or carbon fixation? Biochim. Biophys. Acta. 1987;891:205–215. doi:10.1016/0005-2728(87)90216-7 [Google Scholar]
- Sültemeyer D, Biehler K, Fock H.P. Evidence for the contribution of pseudocyclic photophosphorylation to the energy requirement of the mechanism for concentrating inorganic carbon in Chlamydomonas. Planta. 1993;189:235–242. doi:10.1007/BF00195082 [Google Scholar]
- Tortell P.D, Reinfelder J.R, Morel F.M.M. Active uptake of bicarbonate by diatoms. Nature. 1997;390:243–244. doi: 10.1038/36765. doi:10.1038/36765 [DOI] [PubMed] [Google Scholar]
- Tortell P.D, Rau G.H, Morel F.M.M. Inorganic carbon acquisition in coastal Pacific phytoplankton communities. Limnol. Oceanogr. 2000;45:1485–1500. [Google Scholar]
- Tsuda A, et al. A mesoscale iron enrichment in the western Subarctic Pacific induces a large centric diatom bloom. Science. 2003;300:958–961. doi: 10.1126/science.1082000. doi:10.1126/science.1082000 [DOI] [PubMed] [Google Scholar]
- Vassiliev I.R, Kolber Z, Wyman K.D, Mauzerall D, Shukla V.K, Falkowski P.G. Effects of iron limitation on photosystem II composition and light utilization in Dunaliella tertiolecta. Plant Physiol. 1995;109:963–972. doi: 10.1104/pp.109.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaulot D. The cell cycle of phytoplankton: coupling cell growth to population growth. In: Joint I, editor. Molecular ecology of aquatic microbes. NATO ASI Series. vol. G. 38. Springer; Berlin, Germany: 1995. pp. 303–322. [Google Scholar]
- von Caemmerer S, Furbank R.T. Modeling of C4 photosynthesis. In: Sage R.F, editor. The biology of C4 photosynthesis. Academic Press; San Diego, CA: 1999. pp. 169–207. [Google Scholar]
- Weinbaum S.A, Gressel J, Reisfeld A, Edelman M. Characterization of the 32,000 Dalton chloroplast membrane protein: probing its biological function in Spirodela. Plant Physiol. 1979;64:828–832. doi: 10.1104/pp.64.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberry, T., Behrenfeld, M. J., Siegel, D. A. & Boss, E. In press. Carbon-based primary productivity modeling with vertically resolved photoacclimation. Global Biogeochem. Cycles
- Wolf-Gladrow D.A, Riebesell U, Burkhardt S, Bijma J. Direct effects of CO2 concentration on growth and isotopic composition of marine plankton. Tellus B. 1999;51:461–476. doi:10.1034/j.1600-0889.1999.00023.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text and figures for SI parts I through IV