Abstract
The atmosphere has apparently been oxygenated since the ‘Great Oxidation Event’ ca 2.4 Ga ago, but when the photosynthetic oxygen production began is debatable. However, geological and geochemical evidence from older sedimentary rocks indicates that oxygenic photosynthesis evolved well before this oxygenation event. Fluid-inclusion oils in ca 2.45 Ga sandstones contain hydrocarbon biomarkers evidently sourced from similarly ancient kerogen, preserved without subsequent contamination, and derived from organisms producing and requiring molecular oxygen. Mo and Re abundances and sulphur isotope systematics of slightly older (2.5 Ga) kerogenous shales record a transient pulse of atmospheric oxygen. As early as ca 2.7 Ga, stromatolites and biomarkers from evaporative lake sediments deficient in exogenous reducing power strongly imply that oxygen-producing cyanobacteria had already evolved. Even at ca 3.2 Ga, thick and widespread kerogenous shales are consistent with aerobic photoautrophic marine plankton, and U–Pb data from ca 3.8 Ga metasediments suggest that this metabolism could have arisen by the start of the geological record. Hence, the hypothesis that oxygenic photosynthesis evolved well before the atmosphere became permanently oxygenated seems well supported.
Keywords: oxygen, photosynthesis, biomarkers, fluid inclusions, trace metals, sulphur isotopes
1. Introduction
When did oxygenic photosynthesis evolve? The question is significant because photosynthetic oxygen production by cyanobacteria led to oxygenation of the atmosphere and oceans, in turn allowing aerobic respiration and the evolution of large, complex and ultimately intelligent organisms (Catling et al. 2005). However, the answer is not self-evident, because it is not clear that the first appearance of oxygenic photosynthesis necessarily coincided with the first signs of oxygen in the environment. Indeed, there are three schools of thought on the matter, which are as follows.
Oxygenic photosynthesis evolved hundreds of millions of years before the atmosphere became significantly oxygenated, because it took aeons to oxidize the continued production of reduced volcanic gases, hydrothermal fluids and crustal minerals (Catling & Claire 2005).
It arose at the ca 2.4 Ga (billion years ago) ‘Great Oxidation Event’, causing immediate environmental change (Kopp et al. 2005).
Biogenic oxygen production began very early in Earth's history, before the start of the geological record, leading to an Archaean (greater than 2.5 Ga) atmosphere that was highly oxygenated (Ohmoto 1997).
These various views are tenable because the traditional tools used to track the rise of oxygen—banded iron formations, uraniferous conglomerates and palaeosol geochemistry (Holland 1994)—are now considered by some to be rather uninformative. Banded iron formations could have been produced by photoferrotrophic bacteria (Widdel et al. 1993) or ultraviolet photochemistry (Braterman et al. 1986), without requiring environmental oxygen. Uraniferous conglomerates may have been produced by post-depositional hydrothermal activity rather than by detrital sedimentation in anoxic water bodies (Barnicoat et al. 1997). Few Archaean palaeosols show conclusive evidence of deposition under reducing conditions (Ohmoto 1996). Moreover, the Archaean microfossil record is very limited, without strong morphological evidence for oxygen-producing cyanobacteria (Buick 2001). Molecular phylogenetic trees based on extant organisms provide no compelling evidence for either the very early or the very late evolution of cyanobacteria. Hence, determining when oxygenic photosynthesis evolved requires the use of more subtle clues.
In recent years, the rise to ecological dominance of oxygenic photosynthesis has been tracked using several cryptic or scarce geological proxies such as hydrocarbon biomarkers, redox-sensitive metals, sulphur isotopes, non-marine stromatolites and marine U–Th–Pb isotopes. Here, I will review the records of these various palaeo-pO2 proxies in an attempt to test the various hypotheses for the evolutionary history of this metabolism. But first, it is necessary to establish when atmospheric oxygen levels became high enough to provide an uncontroversial evidence of the existence of oxygenic photosynthesis. This is provided by the Great Oxidation Event, the time after which there is a general consensus that photosynthetic oxygen production was well established.
2. The Great Oxidation Event
The most popular scenario for atmospheric oxygenation is that of a Great Oxidation Event in which the atmosphere changed from essentially anoxic, with perhaps approximately 10−14 present atmospheric level (PAL) O2 produced by UV photolysis of water (Catling & Zahnle 2002), to moderately oxygenated over a geologically short time interval before 2.32 Ga (Bekker et al. 2004). Only a small minority of Earth scientists support Model 3 of permanently high atmospheric O2 levels which, in the opinion of most workers, is contradicted by most available geological evidence. Although there are many indicators of the nearly anoxic atmospheric conditions in the Early Archaean, perhaps the most persuasive are geochemical proxies in rocks formed in close contact with the ancient atmosphere itself. Palaeosols, fossil soils that record the titration of bedrock by atmospheric rainout, are potentially the best, but they are rarely preserved because they form in terrestrial successions deposited above sea level and are thus subject to erosive destruction. Only one certainly Archaean example has been reported, from the ca 2.76 Ga Mt Roe Basalt in northwestern Australia (Rye & Holland 1998). The distribution of redox-sensitive metals, particularly iron, in this horizon shows that the more soluble reduced species were leached out of the more weathered upper portions of the profile, indicating anoxic conditions. Cerium is preserved in its more reduced state, further implying oxygen-deficient conditions. Redox-sensitive detrital minerals in fluvial sediments deposited by turbulent rivers where the water was well mixed with atmospheric gases, including pyrite, uraninite and especially siderite (Rasmussen & Buick 1999), constrain Archaean oxygen levels to less than roughly 0.1, 0.01 and 0.001 PAL, respectively. If these minerals were deposited by oxidized river waters, the uraninite would dissolve into soluble U6+ ions, pyrite would oxidize into sulphate ions and haematite, and siderite would rust to haematite. Lastly, non-mass-dependent fractionation of the rare sulphur isotopes 33S and 36S, a feature pervasively recorded in sedimentary sulphur minerals precipitated from Archaean surface waters, very strongly implies that volcanic sulphurous gases injected into the atmosphere were reprocessed by UV photolysis in the absence of atmospheric oxygen (Farquhar et al. 2000). The pO2 at which these non-mass-dependent isotopic signatures fail to develop or are homogenized is approximately 10−5 PAL (Pavlov & Kasting 2002).
The timing of the Great Oxidation Event is best constrained by the disappearance of large non-mass-dependent sulphur isotope fractionations. In the Huronian Supergroup of Canada and the Transvaal Supergroup of South Africa, where there are good sedimentary records during the critical time interval, this occurs between ca 2.4 and ca 2.3 Ga (Bekker et al. 2004; Papineau et al. 2007). Certainly by the time of the ca 2.32 Ga Makganyene glaciation and overlying bedded manganese deposits, atmospheric oxygen levels were manifestly greater than 10−3 PAL (Kopp et al. 2005). Thus, oxygenic photosynthesis was clearly well established by this time.
3. Hydrocaron biomarkers
Hydrocarbon biomarkers are the defunctionalized and fully saturated molecular skeletons of lipid and pigment biomolecules which are specific enough in their structure and biological distribution to be useful as taxonomic and environmental indicators (figure 1). They are preserved in ancient oil and kerogen (non-crystalline sedimentary organic matter), but are destroyed by metamorphism and oxidative weathering (Brocks & Pearson 2005). Thus, they can usually only be extracted from subsurface samples (drill-cores, mine workings) of low-grade sedimentary rocks.
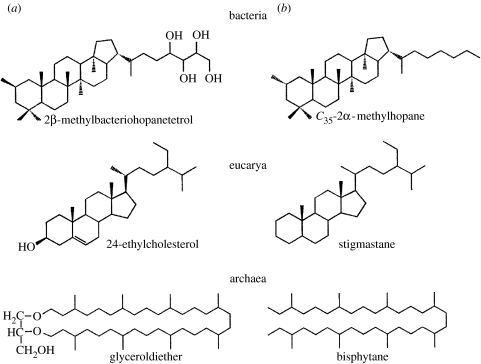
Figure 1.
(a) Biomolecules and their (b) geological biomarker derivatives for all three domains of life, including 2α-methylhopane and the sterane stignastane.
Two biomarker types are germane to the issue under discussion: hopanes and steranes. Hopanes are pentacyclic polyisoprenoid molecules with side chains of differing length and varying methyl-group substitution on the A-ring. They are derived from the bacteriohopanpolyols produced by many bacteria for the hypothesized purpose of stabilizing their cell membranes (Ourisson et al. 1987). In particular, >C31 2α-methylhopanes occur rarely outside cyanobacteria and have been suggested to be biomarkers for these oxygenic photosynthesizers (Summons et al. 1999). Steranes are tetracyclic isoprenoid molecules also with varying side chains and methyl-group substitution in the A-ring. They are usually derived from the sterols produced by Eukarya and have many physiological functions including modifying cell membrane properties to allow phagocytosis and thus endosymbiosis (reviewed by Summons et al. 2006). A few bacteria assimilate or synthesize a few simple sterols (lanosterol, parkeol, etc.), but only eukaryotes are known to synthesize broad suites of C26–C30 sterols (cholesterols, ergosterols, stigmasterols, etc.) with alkyl substituents at position C-24 (Brocks & Pearson 2005). The biosynthetic pathways leading to hopanols and sterols probably have a shared evolutionary history with one major distinction, biosynthesis of bacteriohopanpolyols can occur in the absence of molecular oxygen. However, all known sterol biosynthetic pathways require O2 at many steps, starting with squalene epoxidation (Summons et al. 2006).
(a) Archaean biomarkers
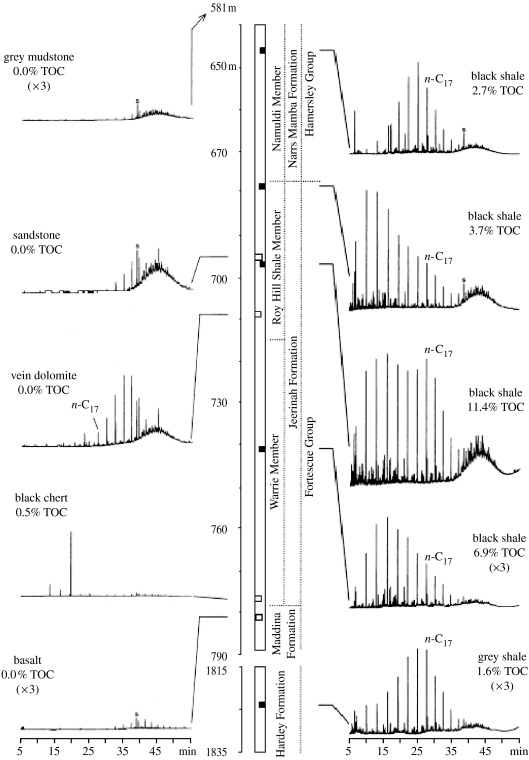
As most Archaean rocks have been subjected to metamorphism of greenschist facies or higher (greater than 350°C), it would seem that searching for biomarker molecules in kerogenous shales older than 2.5 Ga should be unsuccessful. However, biomarkers have been recovered from such rocks as old as ca 3.2 Ga, but in such low concentrations that the possibility of contamination cannot be excluded (Brocks 2001; Buick 2005). At ca 2.7 Ga, numerous shales yield diverse evidence suggesting that their biomarkers are indigenous, such as differing abundances and distributions in adjacent kerogen-rich versus kerogen-poor rocks (figure 2), stereoisomerism consistent with the metamorphic history of their host rocks, absence of obviously young molecules derived from plants, etc. (Brocks et al. 1999, 2003). However, the possibility of a post-Archaean origin for these particular biomarkers cannot be categorically excluded because their host rocks are an open system through which non-indigenous hydrocarbons could permeate, albeit with difficulty (Brocks 2001).
Figure 2.
Hydrocarbon distributions in organic-rich and organic-poor rocks from a drill-core through the Archaean upper Fortescue and lower Hamersley Groups of the Pilbara Craton, Australia (modified from Brocks et al. 1999).
Besides the problem of possible contamination, there are other difficulties with using biomarkers as indicators of Archaean photosynthesis. The very low yields obtained from metamorphosed Archaean rocks (i.e. detectability) and uncertainty about their biological sources (i.e. specificity) complicates their use. Specificity is currently insoluble, because very little of modern microbial diversity has been well characterized (Pace 1997), but the growing capacity to detect biosynthetic capabilities in uncultured microbes offers a possible long-term solution. Thus, at present, one can make only provisional statements about the source of biomarker molecules because a new microbe with differing metabolism but synthesizing the same molecule might be discovered at any time. However, the other problems can be overcome; to enhance detectability, very low-blank laboratory systems (Dutkiewicz et al. 2004; Volk et al. 2005) have been developed, and to avoid contamination, ultraclean drilling (Waldbauer et al. in press; Sherman et al. 2007) or oil-bearing fluid inclusions can be employed.
(b) Biomarkers in fluid-inclusion oils
Liquid oil occurs in fluid inclusions hosted by rocks up to 3.23 Ga old (Rasmussen & Buick 1999) and metamorphosed up to greenschist facies (greater than 300°C), in early diagenetic cements and syn-compactional fractures clearly Archaean in age (Dutkiewicz et al. 1998). This fluid-inclusion oil survives higher metamorphic grades than unconfined oil, due to its inert quartz host preventing catalytic degradation, closed-system confinement preventing escape of reaction products and high confining pressures slowing reaction rates (Dutkiewicz et al. 1998). It is present in very small volumes, particularly in Archaean inclusions which are generally less than 5 μm in diameter with a marginal smear of oil along with CO2, CH4 and H2O liquids and vapours. There may be several generations of oil-bearing fluid inclusions within one sedimentary rock, but the relative timing of entrapment can usually be determined by their petrographic relations (Dutkiewicz et al. 1998). Molecules derived from different inclusion generations cannot yet be differentiated, but if petrographic relationships show that all inclusions were trapped before peak metamorphism, then an average molecular spectrum of all pre-metamorphic oil can be obtained.
(c) Matinenda fluid inclusions
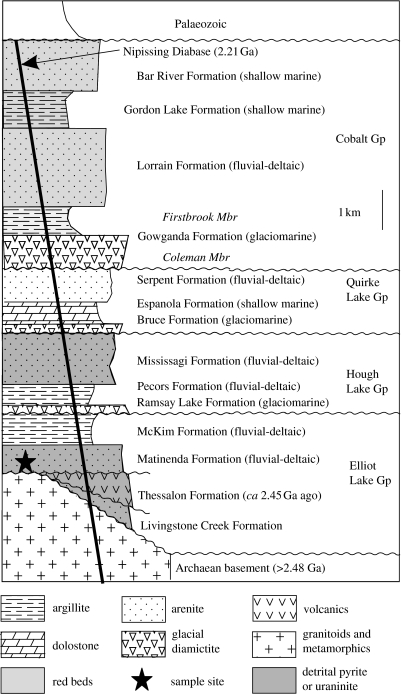
Dutkiewicz et al. (2006) and George et al. (2008) recently reported biomarkers from abundant oil-bearing fluid inclusions in sandstones and conglomerates from the Matinenda Formation in the Huronian Supergroup of Canada (figure 3). The oil source was most probably the immediately overlying McKim Formation, a kerogenous meta-shale that is the only such rock above or below the Matinenda Formation. Both the Matinenda and the McKim Formations were deposited shortly before the Great Oxidation Event and the first ‘Snowball Earth’ glaciation.
Figure 3.
Stratigraphy of the Huronian Supergroup, Canada, showing the position of the Matinenda Formation below the earliest Palaeoproterozoic glaciation and the first evidence of permanent atmospheric oxygenation in the form of red beds (modified from Dutkiewicz et al. 2006).
The Huronian rocks at Elliot Lake, from where the oil-bearing Matinenda samples were obtained, have been exposed to a maximum metamorphic grade of lower greenschist facies (approx. 350°C). These conditions were first attained during intrusion of the Nipissing dolerite dykes at 2.2 Ga (Mossman et al. 1993), and were repeated during the Penokean Orogeny at 1.9 Ga (Card 1978). The only significant post-Penokean event to affect the area was the deposition of a thin overlying veneer of Palaeozoic sediments, not thick enough to generate oil.
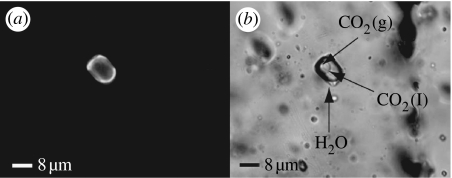
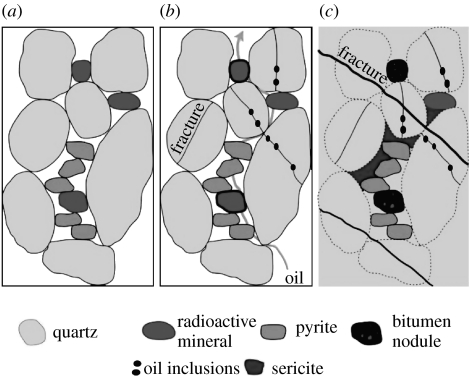
Many Matinenda fluid inclusions are aqueous only, but some contain thin films of fluorescent oil along with bubbles of CO2, CH4 and/or H2O vapour (figure 4). The oil inclusions occur mostly in intragranular microfractures within quartz and K-feldspar sand grains, indicating entrapment during compactional diagenesis (figure 5), although a few reside in pre-metamorphic but post-compactional transgranular fractures. Microthermometric analysis shows that early, low-temperature entrapment took place at 80–220°C and approximately 0.5–2.0 kbar, conditions typical of diagenesis (Dutkiewicz et al. 2003). Later, high-temperature entrapment occurred at 260–380°C and approximately 1–1.5 kbar, around lower greenschist facies conditions (Dutkiewicz et al. 2003). Because the fluid inclusions entrapped within intragranular fractures formed during compactional diagenesis, this oil was clearly generated, migrated and trapped before peak metamorphism ca 2.2 Ga. Inclusions in transgranular fractures also have properties congruent with trapping before peak metamorphism. However, because the inclusion oil was trapped and preserved under conditions different from those experienced by the kerogen in the source rock, which underwent low-pressure, open-system metamorphism in the presence of mineral catalysts, it is impossible to match biomarkers in the presumed source with the fluid-inclusion oils.
Figure 4.
Matinenda Formation fluid inclusions showing the distribution of volatile components, (a) UV epifluorescence showing fluorescent oil and (b) plane-polarized light showing gas bubbles (modified from Dutkiewicz et al. 2006).
Figure 5.
Entrapment model for Matinenda Formation fluid inclusions. (a) Deposition ca 2.45 Ga, (b) oil migration and (c) metamorphism ca 2.2 Ga (modified from Buick et al. 1998).
(d) Matinenda biomarkers
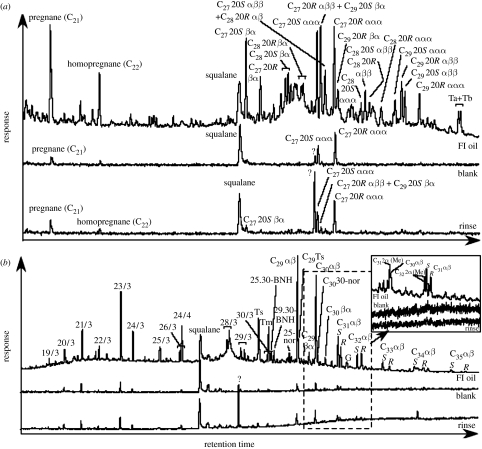
Organic geochemical studies of the Matinenda fluid-inclusion oils show that biomarkers apparently sourced from kerogens older than the Great Oxidation Event are preserved in abundance without subsequent contamination. These include abundant and diverse steranes (figure 6a), the sterol precursors of which are now biosynthesized exclusively by aerobic pathways requiring many O2 molecules (Summons et al. 2006). It has been argued that in the distant evolutionary past, sterols could have been synthesized in an anaerobic world by non-oxidative metabolic pathways that were subsequently supplanted by oxygen-dependent equivalents (Kopp et al. 2005), as has occurred in some other biosynthetic pathways (cf. Raymond & Blankenship 2004). However, there is no robust evidence to support this contention; there are no known organisms that manufacture sterols anaerobically (unlike other metabolic pathways in which anaerobic to aerobic replacement has occurred) and no known organisms make sterols by circumventing any of the oxidative steps in the pathway (Summons et al. 2006).Thus, the presence of such large quantities and diversity of steranes in the Matinenda fluid inclusions strongly suggests that at least some O2 was available in the Huronian water body.
Figure 6.
Gas chromatograph–mass spectrometry traces for Matinenda Formation fluid-inclusion hydrocarbons. (a) Steranes and (b) hopanes—inset shows methylhopanes (modified from George et al. 2008).
The Matinenda fluid-inclusion oils also contain high levels of >C31 2α-methylhopanes (figure 6b). The >C31 2α-methylbacteriohopanepolyol precursors of these molecules are almost exclusively produced by cyanobacteria (Summons et al. 1999). However, certain other microbes are also known to synthesize these molecules. Some soil-dwelling actinobacteria generate small amounts, but these cannot account for the large quantities found in many Precambrian marine kerogens (Summons et al. 2006). Recently, Rashby et al. (2007) reported that one strain (TIE-1) of Rhodopseudomonas palustris also produces abundant >C31 2-methylbacteriohopanepolyols in levels nearly equivalent to those found in cyanobacteria. Though R. palustris has a large metabolic plasticity and a broad habitat range (Larimer et al. 2004), the particular TIE-1 strain is the only one known to produce any methylated bacteriohopanepolyols and has only been found in non-marine settings (Jiao et al. 2005). Moreover, along with these molecules, it also produces other abundant and distinctive biomarker precursors such as tetrahymanols that convert in geological settings to gammaceranes (Rashby et al. 2007), molecules that are not found in the Matinenda fluid-inclusions or in other Archaean samples.
Thus, though all cyanobacteria perform oxygenic photosynthesis, not all cyanobacteria produce 2α-methylhopanols. Some organisms other than cyanobacteria do produce methylated bacteriohopanepolyols, which emphasizes that these molecules are not part of the oxygenic photosynthetic apparatus, and are therefore not specific for this metabolism. However, according to current knowledge, non-cyanobacterial methylated bacteriohopanepolyols are produced in low abundances, non-marine settings and/or with diagnostic partner molecules. Thus, the high relative abundances of >C31 2α-methylhopanes in Archaean and Proterozoic marine sedimentary rocks are still best attributed to, but not absolutely specific for, oxygenic photoautotrophic cyanobacteria. In particular, the abundant >C31 2α-methylhopanes in the Matinenda Formation fluid-inclusion oils provide very strong, but not infallible, evidence for the evolution of oxygenic photosynthesis prior to the Great Oxidation Event.
4. Other evidence for Archaean oxygen
If biomarkers cannot absolutely prove Archaean oxygen production, is there any other evidence? Though there are no other tools for directly demonstrating the existence of cyanobacteria or their metabolism (Archaean microfossils are not diagnostic, molecular phylogenies do not constrain time), there are geochemical indicators of moderate levels of atmospheric and marine oxygen, above those that could conceivably be produced by photolytic dissociation of water. Moreover, there are some Archaean stromatolites (laminated sediment mounds produced by particle accretion or precipitation by microbes) that can be attributed to cyanobacteria metabolizing by oxygenic photosynthesis owing to their unusual environmental setting. These will be discussed in the following sections.
(a) Redox-sensitive metals
Many metals vary in solubility and reactivity as they change their valence state. The major-element metals Fe and Mn have traditionally been used to examine environmental oxygenation (Holland 1994; Kopp et al. 2005), as their more oxidized species are relatively insoluble in seawater and precipitate out in the presence of moderate levels of dissolved oxygen, producing metal-enriched sediments such as ironstones and manganese ores. Trace metals, among them Mo, Re and U, can reveal differing aspects of the geochemical environment because they weather from different minerals and adsorb to different phases. For instance, dissolved marine Mo is derived only from oxic weathering of sulphide minerals and is proportionately removed by adsorption to organic matter. Hence, the presence of excess Mo in shales out of the proportion to kerogen indicates the presence of atmospheric or marine oxygen.
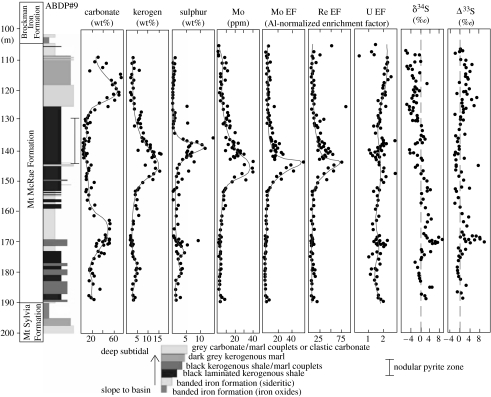
Anbar et al. (2007) reported a Mo spike in drill-core from the 2.5 Ga Mt McRae Shale (figure 7) in northwestern Australia. The Mo spike is still obvious even when corrected for organic adsorption (figure 7). Re shows the same behaviour, but U behaves differently, displaying constant levels throughout the section (figure 7). Anbar et al. (2007) argued that the Mo and Re enrichments resulted from weathering of terrestrial, not marine, sulphides because quite high oxygen levels are required to liberate such metal concentrations, not congruent with the relatively low levels of dissolved oxygen available even under a highly aerobic atmosphere. U, they argued, behaved differently because it largely resides in silicate species, not sulphides, which are not amenable to oxidative weathering. Thus, they concluded that a transient O2 ‘whiff’ occurred in the atmosphere over a hundred million years prior to the Great Oxidation Event.
Figure 7.
Redox-sensitive trace metal data (modified from Anbar et al. 2007) and sulphur isotopic values (modified from Kaufman et al. 2007) from the ca 2.5 Ga Mt McRae Formation, Hamersley Group, Pilbara Craton, Australia.
The Mo spike in the Mt McRae Shale coincided with a marked change in the sulphur isotope systematics of the sediment (Kaufman et al. 2007). At the peak of the Mo spike, δ34Ssulphide values switch from dominantly slightly positive to generally moderately negative (figure 7). Δ33Ssulphide shows a persistent non-mass-dependent fractionation throughout. These patterns reinforce the interpretation of and quantify the degree of the transient atmospheric oxygen pulse. The shift to negative δ34Ssulphide values records the onset of microbial sulphate reduction, indicating elevated sulphate concentrations in the marine water body (Kaufman et al. 2007). This implies an increase in oxic weathering of terrestrial sulphides, presumably by the oxygen pulse. Coupled with the Mo and Re data, this suggests that atmospheric O2 levels exceeded 10−6 PAL. The persistent non-mass-dependent fractionation of Δ33Ssulphide, however, constrains the magnitude of the atmospheric O2 whiff to less than 10−5 PAL (Pavlov & Kasting 2002), above which the non-mass-dependent fractionation of sulphur disappears due to the development of an ozone shield inhibiting short-wavelength UV photolysis of sulphur gases (Farquhar et al. 2001).
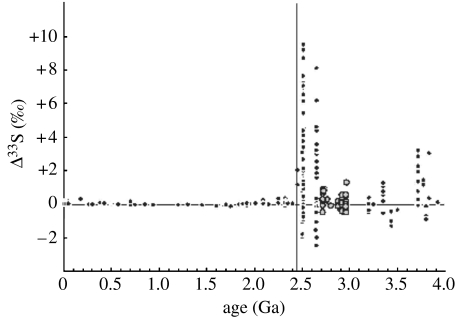
It is not yet known whether other O2 pulses occurred prior to the ca 2.4 Ga Great Oxidation Event. Photochemical modelling (Claire et al. 2006) predicted that at least one early oxygenation pulse would occur. It has been suggested (Ohmoto et al. 2006) that intervals of low Δ33Ssulphide between 2.8 and 3.0 Ga represented other transient oxygenation events of sufficient magnitude to suppress the non-mass-dependent fractionation, but Farquhar et al. (2007) recently demonstrated, using a larger and Δ36S-supplemented dataset, that small but significant Δ33S anomalies still occurred then (figure 8). Further coordinated trace-metal and sulphur-isotope studies are needed to reveal whether other oxygenation pulses occurred. Regardless, transient atmospheric O2 levels between 10−6 and 10−5 PAL at 2.5 Ga demonstrate that oxygenic photosynthesis, and hence cyanobacteria, had certainly evolved by this time.
Figure 8.
Δ33Ssulphide values through time (modified from Farquhar et al. 2007); note values of less than ±0.25 ppt after ca 2.3 Ga and greater than ±0.25 ppt between 2.8 and 3.0 Ga.
(b) Lacustrine stromatolites
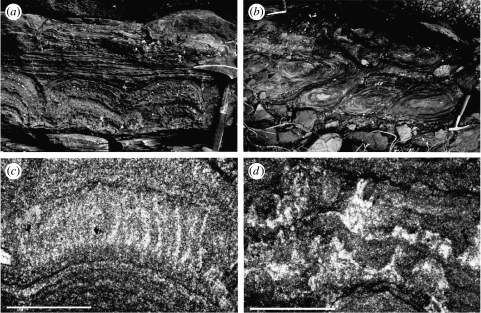
Somewhat older than the 2.5 Ga oxygen pulse, stromatolites (figure 9) that grew in 2.72 Ga lakes in the Tumbiana Formation, northwestern Australia, also provide information about the evolution of oxygenic photosynthesis. These structures, which are clearly biogenic as shown by their palimpsest textures of palisade and tufted microbial filaments (figure 9c,d), are large (up to 3 m across and high), diverse (at least six different morphotypes) and abundant (outcrops cover many hectares). Their microbial constructors were evidently phototropic, as their palisade filaments were concentrated on topographic highs and their tufted palimpsests show signs of phototaxy towards flexure crests (figure 9c,d). The stromatolites accreted in evaporative lakes formed on flood basalts. Significantly, there appears to have been no exogenous sources of reducing power within the lakes, precluding all metabolisms except for photosynthesis as the basis for the food chain (Buick 1992).
Figure 9.
(a–d) Lacustrine stromatolites from the ca 2.72 Ga Tumbiana Formation, Fortescue Group, Pilbara Craton, Australia (modified from Buick 1992).
Inflowing rivers deposited sediments lacking in organic matter, so photoheterotrophy of exogenous biogenic or abiogenic organic matter was evidently negligible. The basaltic substrate consists of olivine-poor tholeiites, severely restricting the amount of hydrogen or hydrocarbons that could be supplied from serpentinization. Though methanogenesis was locally important as shown by highly depleted δ13Corg values in these lakes (Hayes 1994; Eigenbrode & Freeman 2006), in the absence of an abiogenic H2 source this must have been a heterotrophic process recycling photoautrophic biomass through fermentative hydrogen production. Iron- and sulphur-rich sediments are absent from the lakes, except for minor late diagenetic pyrite, hence anoxygenic photosynthesis using Fe2+ or H2S as electron donors cannot have been significant. C/N ratios are low (Strauss & Moore 1992), hence the newly discovered nitrifying variety of anoxygenic photosynthesis (Griffin et al. 2007) was probably not significant. Hence, by a process of elimination, oxygenic photosynthesis was the only metabolism available to power the prolific lacustrine biota (Buick 2007). If so, this is congruent with the high >C31 2α-methylhopane indices of these rocks (Brocks et al. 2003) and suggests that these Archaean biomarkers are indeed indigenous and indicative of cyanobacteria. Therefore, converging lines of evidence provided by the unusual conditions prevailing in these restricted lacustrine basins are collectively and strongly indicative of oxygenic photosynthetic activity, at least locally, by 2.72 Ga.
(c) Older stromatolites, microfossils and kerogenous shales
All older stromatolites are marine with fabrics and textures not diagnostic of any particular metabolism. Their microstructure, with laminae thickening over flexure crests, indicates phototropism but not necessarily photosynthesis. The oldest known stromatolites, from the ca 3.49 Ga Dresser Formation in northwestern Australia (Walter et al. 1980; Buick et al. 1981), contain abundant sulphate crystals, once gypsum but now barite (Buick & Dunlop 1990). Where unweathered in subsurface drill-core, these rocks also contain abundant pyrite (Philippot et al. 2007). Thus, the stromatolites could have been constructed by anaerobic photosynthesizers using H2S as their electron donor, generating abundant sulphate which subsequently precipitated during periodic evaporation (Buick & Dunlop 1990). Residual sulphate was metabolized by sulphate-reducing bacteria to produce the pyrite (Shen et al. 2001). Thus, these stromatolites could well represent a sulphuretum ecosystem (c.f. Nisbet & Fowler 1999), revealing nothing about the evolution of oxygenic photosynthesis.
Similarly, ancient microfossils from nearby have been described as probable cyanobacterial remains (Schopf & Packer 1987; Schopf 1992, 1993, 2000), in which case they would have presumably been capable of oxygenic photosynthesis, as are all modern cyanobacteria. However, these purported microfossils are controversial, both in origin and age. They come from two localities, at one of which it has been argued that they were, in fact, formed abiotically in a subterranean hydrothermal fracture (Brasier et al. 2002), not an environment conducive to the preservation of cyanobacteria. At the other locality, the objects originally interpreted as cyanobacteria show marked morphological similarities with scoriaceous volcanic ash grains in their silicified vitric tuff host rock. All kerogen present at this locality is secondary, occurring only in black chert sills. Thus, neither of these early Archaean occurrences of purported cyanobacterial microfossils provides robust evidence of oxygenic photosynthesis.
Kerogenous shales also occur in early Archaean terrains and can potentially be informative about oxygenic photosynthesis. The oldest with total organic carbon contents of greater than 10% come from the ca 3.2 Ga Gorge Creek Group of northwestern Australia. They were deposited in deep marine environments, their beds are thick (hundreds of metres) and widespread (hundreds of kilometres), and are notably deficient in sulphur and iron, with almost no pyrite relative to the amount of kerogen. This suggests that anoxygenic photosynthesis was unlikely to have generated the organic matter, otherwise sulphur or iron mineralization should occur along with organic productivity. Indeed, it is hard to imagine any metabolism other than oxygenic photosynthesis having the necessary substrates available over so wide an area for such lengths of time in such abundance. Thus, although not conclusive evidence, the occurrence of widespread and thick, organic-rich but pyrite-poor shales at ca 3.2 Ga is certainly consistent with an oxygenic photosynthetic source for the kerogen.
(d) C and U–Th–Pb isotopes
The oldest known metasedimentary rocks on Earth come from the ca 3.8 Ga Isua supracrustal belt in west Greenland. Although the rocks have been metamorphosed several times to amphibolite facies and are highly deformed, original sedimentary structures are still evident in places. It has long been known that isotopically fractionated graphite (approx. −20‰) and carbonate (approx. −2‰) occurs at Isua (Schidlowski 1988). At first glance, these isotopic data seem consistent not just with the early evolution of autotrophic life but, owing to their similarity with modern marine carbon isotopic ratios, also with oxygenic photosynthesis. However, it is now clear that all of the carbonate is metasomatic and not sedimentary (Rose et al. 1996) and that much of the graphite formed by metamorphic decarbonation of siderite (Van Zuilen et al. 2002). Thus, isotopic data from such sources record only post-depositional abiotic fractionations. But some graphite particles with δ13Corg values of approximately −19‰ (Rosing 1999) are not associated with decrepitating carbonates and appear to have a genuinely sedimentary origin in turbiditic metapelite. Though this isotopic fractionation could conceivably result from oxygenic photosynthesis, in the absence of data from sedimentary carbonates providing information on the isotopic ratios on both sides of the reaction system, it is impossible to be sure (Buick 2007). Moreover, various pathways of autotrophic carbon fixation induce similar isotopic fractionations, so carbon isotope data alone are rarely sufficient to identify a specific metabolic process. Lastly, metamorphism resets carbon isotope values, hence attributing isotopic fractionations to a particular source in such high-grade rocks is fraught with danger.
Another way to determine whether oxygenic photosynthesis had already arisen by such ancient times is to look for signs of environmental oxygenation. To this end, Rosing & Frei (2004) interpreted the U–Th–Pb isotopic system of the ca 3.8 Ga metasediments from Isua as showing signs of photic zone oxygenation. They argued from the highly radiogenic Pb isotopic values that the metapelites record original and not secondary U–Th–Pb values, that they had high initial U abundances relative to Th, and that this indicated that U was environmentally mobile relative to Th. As U is soluble in oxidizing fluids but immobile under reduced conditions, whereas Th is immobile in both circumstances, the greater mobility of U implies that at least part of the Isua water body was relatively oxidized. This is a plausible argument, but if it is correct, somewhat similar isotopic patterns should occur in lower grade Archaean sediments deposited after oxygenic photosynthesis evolved but before general oxygenation. However, the Pb isotopic compositions observed in the Isua metasediments are apparently unique (Rosing & Frei 2004), so their significance remains uncertain.
5. Conclusions
Fluid-inclusion oils in the ca 2.45 Ga Matinenda Formation contain biomarkers evidently sourced from kerogens in the overlying McKim Formation that is older than the Great Oxidation Event. They are preserved in detectable abundances, and petrography and microthermometry of the fluid inclusions indicates that they were trapped before peak metamorphism (ca 2.2 Ga) without subsequent contamination. These biomarkers apparently record the existence of organisms producing and requiring molecular oxygen before the atmosphere became oxygenated.
Before this time, redox-sensitive metals and sulphur isotope data from the 2.5 Ga Mt McRae Shale indicate that a transient pulse of oxygen temporarily raised atmospheric O2 levels to near 10−5 PAL. Lacustrine stromatolites in sulphur- and iron-depleted basins strongly suggest that oxygen-producing photoautotrophs had evolved by 2.72 Ga. Thus, these lines of evidence all imply that oxygenic photosynthesis evolved well before abundant molecular oxygen appeared in the environment. In this case, hypothesis (i) in which atmospheric oxygenation is retarded for many hundreds of millions of years after the advent of oxygenic photosynthesis due to the necessity of first filling all near-surface oxygen sinks, is most probably correct.
A timeline for the appearance of oxygenic photosynthesis, based on current knowledge, might go as follows:
2.3 Ga. Oxygenic photosynthesis had certainly evolved by the end of the Great Oxidation Event which raised atmospheric oxygen permanently above the levels produced by photolysis of water.
2.45 Ga. Abundant and diverse steranes and 2α-methylhopanes in contamination-proof fluid-inclusion oils show that oxygenic photosynthesis had most probably evolved.
2.5 Ga. The pulse of oxygen indicated by Mo and Re abundances and sulphur isotope systematics is best explained by the operation of oxygenic photosynthesis.
2.72 Ga. Stromatolites and shale-hosted biomarkers in lake sediments deposited in basins deficient in exogenous sources of reducing power, although having some uncertainties, provide a concatenation of evidence most probably indicative of oxygenic photosynthesis.
3.2 Ga. Widespread, thick, non-pyritic but kerogen-rich black shales possibly provide evidence for oxygenic photosynthesis.
3.8 Ga. U–Th–Pb isotopes in metasomatized and metamorphosed turbidites perhaps represent the earliest evidence of oxygenic photosynthesis.
If all of these lines of evidence turn out to be correct, then the history of oxygenic photosynthesis before oxygen started accumulating in the atmosphere was very long indeed, almost as long as the geological record itself. This would imply that the history of life goes much deeper in time, and that the most recent 85% of Earth's and life's evolution has merely been an adjustment to more oxygen. Near-surface reduced reservoirs progressively oxidized and steadily more environments become ‘oxygen oases’ until the largely oxic world that we know today developed.
Acknowledgments
I thank Adriana Dutkiewicz, Simon George, Herbert Volk, John Ridley, Jochen Brocks, Ariel Anbar, Jay Kaufman and Roger Summons for their permission to adapt figures and for their advice about Archaean geobiology, and the NASA Astrobiology Institute, National Science Foundation, Australian Research Council and Petroleum Research Fund for financial support.
Footnotes
One contribution of 15 to a Discussion Meeting Issue ‘Photosynthetic and atmospheric evolution’.
Supplementary Material
References
- Anbar A.D, et al. A whiff of oxygen before the Great Oxidation Event? Science. 2007;317:1903–1906. doi: 10.1126/science.1140325. doi:10.1126/science.1140325 [DOI] [PubMed] [Google Scholar]
- Barnicoat A.C, et al. Hydrothermal gold mineralization in the Witwatersrand basin. Nature. 1997;386:820–824. doi:10.1038/386820a0 [Google Scholar]
- Bekker A, Holland H.D, Wang P.L, Rumble D.R, Stein H.J, Hannah J.L, Coetzee L.L, Beukes N.J. Dating the rise of atmospheric oxygen. Nature. 2004;427:117–120. doi: 10.1038/nature02260. doi:10.1038/nature02260 [DOI] [PubMed] [Google Scholar]
- Brasier M.D, Green O.R, Jephcoat A.P, Kleppe A.K, van Kranendonk M, Lindsay J.F, Steele A, Grassineau N. Questioning the evidence for Earth's oldest fossils. Nature. 2002;416:76–81. doi: 10.1038/416076a. doi:10.1038/416076a [DOI] [PubMed] [Google Scholar]
- Braterman P.S, Cairns-Smith A.G, Sloper R.W. Photo-oxidation of hydrated Fe2+—significance for banded iron formations. Nature. 1983;303:163–164. doi:10.1038/303163a0 [Google Scholar]
- Brocks, J. J. 2001 Molecular fossils in archean rocks. PhD thesis, University of Sydney.
- Brocks J.J, Pearson A. Building the biomarker tree of life. Rev. Mineral. Geochem. 2005;59:233–258. doi:10.2138/rmg.2005.59.10 [Google Scholar]
- Brocks J.J, Logan G.A, Buick R, Summons R.E. Archean molecular fossils and the early rise of eukaryotes. Science. 1999;285:1033–1036. doi: 10.1126/science.285.5430.1033. doi:10.1126/science.285.5430.1033 [DOI] [PubMed] [Google Scholar]
- Brocks J.J, Buick R, Logan G.A, Summons R.E. Composition and syngeneity of molecular fossils from the 2.78 to 2.45 billion-year-old Mount Bruce Supergroup, Pilbara Craton, Western Australia. Geochim. Cosmochim. Acta. 2003;67:4289–4319. doi:10.1016/S0016-7037(03)00208-4 [Google Scholar]
- Buick R. The antiquity of oxygenic photosynthesis: evidence from stromatolites in sulfate-deficient Archaean lakes. Science. 1992;255:74–77. doi: 10.1126/science.11536492. doi:10.1126/science.11536492 [DOI] [PubMed] [Google Scholar]
- Buick R. Life in the Archaean. In: Briggs D.E.G, Crowther P.R, editors. Palaeobiology II. Blackwell; Oxford, UK: 2001. pp. 13–21. [Google Scholar]
- Buick, R. 2005 Paleontological and geochemical records of early life in Archean rocks from the Pilbara Craton, Australia. In 16th Joint Meeting for Earth and Planetary Science, Makuhari, Japan, Abstr. P074-036. Tokyo, Japan: Japan Geoscience Union.
- Buick R. The earliest records of life. In: Sullivan W.T III, Baross J.A, editors. Planets and life: the emerging science of astrobiology. Cambridge University Press; Cambridge, UK: 2007. pp. 237–264. [Google Scholar]
- Buick R, Dunlop J.S.R. Evaporitic sediments of Early Archaean age from the Warrawoona Group, North Pole, Western Australia. Sedimentology. 1990;37:241–277. doi:10.1111/j.1365-3091.1990.tb00958.x [Google Scholar]
- Buick R, Dunlop J.S.R, Groves D.I. Stromatolite recognition in ancient rocks: an appraisal of irregularly laminated structures in an early Archaean chert–barite unit from North Pole, Warrawoona Group, Western Australia. Alcheringa. 1981;5:161–181. [Google Scholar]
- Buick R, Rasmussen B, Krapez B. Archaean oil: evidence for extensive hydrocarbon generation and migration 2.5–3.5 billion years ago. AAPG Bull. 1998;82:50–69. [Google Scholar]
- Card, K. D. 1978 Metamorphism of the Middle Precambrian (Aphebian) rocks of the eastern Southern Province. In Metamorphism in the Canadian Shield (eds J. A. Fraser & W. W. Heywood). Geological Survey of Canada, paper no. 78-10, pp. 269–282.
- Catling D.C, Claire M.W. How Earth's atmosphere evolved to an oxic state: a status report. Earth Planet. Sci. Lett. 2005;237:1–20. doi:10.1016/j.epsl.2005.06.013 [Google Scholar]
- Catling D, Zahnle K. Evolution of atmospheric oxygen. In: Holton J, Pyle J, Curry J, editors. Encyclopedia of atmospheric sciences. Academic Press; Amsterdam, The Netherlands: 2002. pp. 754–761. [Google Scholar]
- Catling D.C, Glein C.R, Zahnle K.J, McKay C.P. Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology. 2005;5:415–438. doi: 10.1089/ast.2005.5.415. doi:10.1089/ast.2005.5.415 [DOI] [PubMed] [Google Scholar]
- Claire M.W, Catling D.C, Zahnle K.J. Biogeochemical modelling of the rise of atmospheric oxygen. Geobiology. 2006;4:236–269. doi:10.1111/j.1472-4669.2006.00084.x [Google Scholar]
- Dutkiewicz A, Rasmussen B, Buick R. Oil preserved in fluid inclusions in Archaean sandstones. Nature. 1998;395:885–888. doi:10.1038/27644 [Google Scholar]
- Dutkiewicz A, Ridley J, Buick R. Oil-bearing CO2–CH4–H2O fluid inclusions: oil survival since the Palaeoproterozoic after high temperature entrapment. Chem. Geol. 2003;194:51–79. doi:10.1016/S0009-2541(02)00271-1 [Google Scholar]
- Dutkiewicz A, Volk H, Ridley J, George S. Geochemistry of oil in fluid inclusions in a Middle Proterozoic igneous intrusion: implications for the source of hydrocarbons in crystalline rocks. Org. Geochem. 2004;35:937–957. doi:10.1016/j.orggeochem.2004.03.007 [Google Scholar]
- Dutkiewicz A, Volk H, George S.C, Ridley J, Buick R. Biomarkers from Huronian oil-bearing fluid inclusions: an uncontaminated record of life before the Great Oxidation Event. Geology. 2006;34:437–440. doi:10.1130/G22360.1 [Google Scholar]
- Eigenbrode J.L, Freeman K.H. Late Archean rise of aerobic microbial ecosystems. Proc. Natl Acad. Sci. USA. 2006;103:15 759–15 764. doi: 10.1073/pnas.0607540103. doi:10.1073/pnas.0607540103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar J, Bao H, Thiemens M. Atmospheric influence of Earth's earliest sulfur cycle. Science. 2000;289:756–758. doi: 10.1126/science.289.5480.756. doi:10.1126/science.289.5480.756 [DOI] [PubMed] [Google Scholar]
- Farquhar J, Savarino J, Airieau S, Thiemens M.H. Observation of wavelength-sensitive mass-independent sulfur isotope effects during SO2 photolysis: implications for the early atmosphere. J. Geophys. Res. E. 2001;106:32 829–32 839. doi:10.1029/2000JE001437 [Google Scholar]
- Farquhar J, Peters M, Johnston D.T, Strauss H, Masterson A, Wiechert U, Kaufman A.J. Isotopic evidence for Mesoarchaean anoxia and changing atmospheric sulphur chemistry. Nature. 2007;449:706–709. doi: 10.1038/nature06202. doi:10.1038/nature06202 [DOI] [PubMed] [Google Scholar]
- George S.C, Volk H, Dutkiewicz A, Ridley J, Buick R. Preservation of hydrocarbons and biomarkers in oil trapped inside fluid inclusions for >2 billion years. Geochim. Cosmochim. Acta. 2008;72:844–870. doi:10.1016/j.gca.2007.11.021 [Google Scholar]
- Griffin B.M, Schott J, Schink B. Nitrite, an electron donor for anoxygenic photosynthesis. Science. 2007;316:1870. doi: 10.1126/science.1139478. doi:10.1126/science.1139478 [DOI] [PubMed] [Google Scholar]
- Hayes J.M. Global methanotrophy at the Archean–Proterozoic transition. In: Bengtson S, editor. Early life on Earth. Columbia University Press; New York, NY: 1994. pp. 220–236. [Google Scholar]
- Holland H.D. Early Proterozoic atmospheric change. In: Bengtson S, editor. Early life on Earth. Columbia University Press; New York, NY: 1994. pp. 237–244. [Google Scholar]
- Kaufman A.J, et al. Astrobiological insights into global biospheric oxygenation and atmospheric evolution. Science. 2007;317:1900–1903. doi: 10.1126/science.1138700. doi:10.1126/science.1138700 [DOI] [PubMed] [Google Scholar]
- Kopp R.E, Kirschvink J.L, Hilburn I.A, Nash C.Z. The Paleoproterozoic snowball Earth: a climate disaster triggered by the evolution of oxygenic photosynthesis. Proc. Natl Acad. Sci. USA. 2005;102:11 131–11 136. doi: 10.1073/pnas.0504878102. doi:10.1073/pnas.0504878102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Kappler A, Croal L.R, Newman D.K. Isolation and characterization of a genetically tractable photoautotrophic Fe(II)-oxidizing bacterium, Rhodopseudomonas palustris strain TIE-1. Appl. Environ. Microbiol. 2005;71:4487–4496. doi: 10.1128/AEM.71.8.4487-4496.2005. doi:10.1128/AEM.71.8.4487-4496.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larimer F.W, et al. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat. Biotechnol. 2004;22:55–61. doi: 10.1038/nbt923. doi:10.1038/nbt923 [DOI] [PubMed] [Google Scholar]
- Mossman D.J, Nagy B, Davis D.W. Hydrothermal alteration of organic matter in uranium ores, Elliot Lake, Canada: implications for selected organic-rich deposits. Geochim. Cosmochim. Acta. 1993;57:3251–3259. doi:10.1016/0016-7037(93)90538-8 [Google Scholar]
- Nisbet E.G, Fowler C.M.R. Archaean metabolic evolution of microbial mats. Proc. R. Soc. B. 1999;266:2375–2382. doi:10.1098/rspb.1999.0934 [Google Scholar]
- Ohmoto H. Evidence in pre-2.2 Ga paleosols for the early evolution of atmospheric oxygen and terrestrial biota. Geology. 1996;24:1135–1138. doi: 10.1130/0091-7613(1996)024<1135:eipgpf>2.3.co;2. doi:10.1130/0091-7613(1996)024<1135:EIPGPF>2.3.CO;2 [DOI] [PubMed] [Google Scholar]
- Ohmoto H. When did the Earth's atmosphere become oxic? Geochem. News. 1997;93:26–27. [Google Scholar]
- Ohmoto H, Watanabe Y, Ikemi H, Poulson S.R, Taylor B.E. Sulphur isotope evidence for an oxic Archaean atmosphere. Nature. 2006;442:908–911. doi: 10.1038/nature05044. doi:10.1038/nature05044 [DOI] [PubMed] [Google Scholar]
- Ourisson G, Rohmer M, Poralla K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Annu. Rev. Microbiol. 1987;41:301–333. doi: 10.1146/annurev.mi.41.100187.001505. doi:10.1146/annurev.mi.41.100187.001505 [DOI] [PubMed] [Google Scholar]
- Pace N.R. A molecular view of microbial diversity and the biosphere. Science. 1997;276:734–740. doi: 10.1126/science.276.5313.734. doi:10.1126/science.276.5313.734 [DOI] [PubMed] [Google Scholar]
- Papineau D, Mojzsis S.J, Schmitt A.K. Multiple sulfur isotopes from Paleoproterozoic Huronian interglacial sediments and the rise of atmospheric oxygen. Earth Planet. Sci. Lett. 2007;255:188–212. doi:10.1016/j.epsl.2006.12.015 [Google Scholar]
- Pavlov A.A, Kasting J.F. Mass-independent fractionation of sulfur isotopes in Archean sediments: strong evidence for an anoxic Archean atmosphere. Astrobiology. 2002;2:27–41. doi: 10.1089/153110702753621321. doi:10.1089/153110702753621321 [DOI] [PubMed] [Google Scholar]
- Philippot P, van Zuilen M, Lepot K, Thomazo C, Farquhar J, van Kranendonk M. Early Archaean organisms preferred elemental sulfur, not sulfate. Science. 2007;317:1534–1537. doi: 10.1126/science.1145861. doi:10.1126/science.1145861 [DOI] [PubMed] [Google Scholar]
- Rashby S.E, Sessions A.L, Summons R.E, Newman D.K. Biosynthesis of 2-methylbacteriohopanepolyols by an anoxygenic phototroph. Proc. Natl Acad. Sci. USA. 2007;104:15 099–15 104. doi: 10.1073/pnas.0704912104. doi:10.1073/pnas.0704912104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen B, Buick R. Redox state of the Archean atmosphere: evidence from detrital heavy minerals in ca. 3250–2750 Ma sandstones from the Pilbara Craton, Australia. Geology. 1999;27:115–118. doi:10.1130/0091-7613(1999)027<0115:RSOTAA>2.3.CO;2 [Google Scholar]
- Raymond J, Blankenship R.E. Biosynthetic pathways, gene replacement and the antiquity of life. Geobiology. 2004;2:199–203. doi:10.1111/j.1472-4677.2004.00037.x [Google Scholar]
- Rose N.M, Rosing M.T, Bridgwater D. The origin of metacarbonate rocks in the Archaean Isua supracrustal belt, west Greenland. Am. J. Sci. 1996;296:1004–1044. [Google Scholar]
- Rosing M.T. 13C-depleted carbon microparticles in >3700-Ma sea-floor sedimentary rocks from west Greenland. Science. 1999;283:674–676. doi: 10.1126/science.283.5402.674. doi:10.1126/science.283.5402.674 [DOI] [PubMed] [Google Scholar]
- Rosing M.T, Frei R. U-rich Archaean sea-floor sediments from Greenland: indications of >3700 Ma oxygenic photosynthesis. Earth Planet. Sci. Lett. 2004;217:237–244. doi:10.1016/S0012-821X(03)00609-5 [Google Scholar]
- Rye R, Holland H.D. Paleosols and the evolution of atmospheric oxygen: a critical review. Am. J. Sci. 1998;298:621–672. doi: 10.2475/ajs.298.8.621. [DOI] [PubMed] [Google Scholar]
- Schidlowski M. A 3,800-million-year isotopic record of life from carbon in sedimentary rocks. Nature. 1988;333:313–318. doi:10.1038/333313a0 [Google Scholar]
- Schopf J.W. Paleobiology of the Archean. In: Schopf J.W, Klein C, editors. The Proterozoic biosphere, a multidisciplinary study. Cambridge University Press; New York, NY: 1992. pp. 25–39. [Google Scholar]
- Schopf J.W. Microfossils of the early Archean Apex chert: new evidence of the antiquity of life. Science. 1993;260:640–646. doi: 10.1126/science.260.5108.640. doi:10.1126/science.260.5108.640 [DOI] [PubMed] [Google Scholar]
- Schopf J.W. The fossil record: tracing the roots of the cyanobacterial lineage. In: Whitton B.A, Potts M, editors. The ecology of cyanobacteria: their diversity in time and space. Kluwer Academic; Dordrecht, The Netherlands: 2000. pp. 13–35. [Google Scholar]
- Schopf J.W, Packer B.M. Early Archean (3.3-billion to 3.5-billion-year-old) micorofossils from Warrawoona Group, Australia. Science. 1987;237:70–73. doi: 10.1126/science.11539686. doi:10.1126/science.11539686 [DOI] [PubMed] [Google Scholar]
- Shen Y, Buick R, Canfield D.E. Isotopic evidence for microbial sulphate reduction in the early Archaean era. Nature. 2001;410:77–81. doi: 10.1038/35065071. doi:10.1038/35065071 [DOI] [PubMed] [Google Scholar]
- Sherman L.S, Waldbauer J.R, Summons R.E. Improved methods for isolating and validating indigenous biomarkers in Precambrian rocks. Org. Geochem. 2007;38:1987–2000. doi:10.1016/j.orggeochem.2007.08.012 [Google Scholar]
- Strauss H, Moore T.B. Abundances and isotopic compositions of carbon and sulfur species in whole rock and kerogen samples. In: Schopf J.W, Klein C, editors. The Proterozoic biosphere, a multidisciplinary study. Cambridge University Press; New York, NY: 1992. pp. 711–798. [Google Scholar]
- Summons R.E, Jahnke L.L, Logan G.A, Hope J.M. 2-Methylhopanoids as biomarkers for cyanobacterial oxygenic photosynthesis. Nature. 1999;400:554–557. doi: 10.1038/23005. doi:10.1038/23005 [DOI] [PubMed] [Google Scholar]
- Summons R.E, Bradley A.S, Jahnke L.L, Waldbauer J.R. Steroids, triterpenoids and molecular oxygen. Phil. Trans. R. Soc. B. 2006;361:951–968. doi: 10.1098/rstb.2006.1837. doi:10.1098/rstb.2006.1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zuilen M.A, Lepland A, Arrhenius G. Reassessing the evidence for the earliest traces of life. Nature. 2002;418:627–630. doi: 10.1038/nature00934. doi:10.1038/nature00934 [DOI] [PubMed] [Google Scholar]
- Volk H, George S.C, Dutkiewicz A, Ridley J. Characterisation of fluid inclusion oil in a Mid-Proterozoic sandstone and dolerite (Roper Superbasin Australia) Chem. Geol. 2005;223:109–135. doi:10.1016/j.chemgeo.2004.12.024 [Google Scholar]
- Waldbauer, J. R., Sherman, L. S., Sumner, D. Y. & Summons, R. E. In press. Late Archean molecular fossils from the Transvaal Supergroup record the antiquity of microbial diversity and aerobiosis. Precamb. Res
- Walter M.R, Buick R, Dunlop J.S.R. Stromatolites 3,400–3,500 Myr old from the North Pole area, Western Australia. Nature. 1980;284:443–445. doi:10.1038/284443a0 [Google Scholar]
- Widdel F, Schnell S, Heising S, Ehrenreich A, Assmus B, Schink B. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature. 1993;362:834–835. doi:10.1038/362834a0 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.