Abstract
In highly seasonal environments, offspring production by vertebrates is timed to coincide with the annual peak of resource availability. For herbivores, this resource peak is represented by the annual onset and progression of the plant growth season. As plant phenology advances in response to climatic warming, there is potential for development of a mismatch between the peak of resource demands by reproducing herbivores and the peak of resource availability. For migratory herbivores, such as caribou, development of a trophic mismatch is particularly likely because the timing of their seasonal migration to summer ranges, where calves are born, is cued by changes in day length, while onset of the plant-growing season on the same ranges is cued by local temperatures. Using data collected since 1993 on timing of calving by caribou and timing of plant growth in West Greenland, we document the consequences for reproductive success of a developing trophic mismatch between caribou and their forage plants. As mean spring temperatures at our study site have risen by more than 4°C, caribou have not kept pace with advancement of the plant-growing season on their calving range. As a consequence, offspring mortality has risen and offspring production has dropped fourfold.
Keywords: caribou, climate change, global warming, plant phenology
1. Introduction
Recent studies of the effects of climate change on timing of breeding and reproductive success in migratory birds have focused attention on the phenomenon of trophic mismatch between the timing of nesting and the timing of food availability (Visser et al. 1998), which may reduce breeding success (Stevenson & Bryant 2000). Such a mismatch occurs because increases in springtime temperatures lead to advances in the timing of spring events such as plant growth and insect emergence on breeding grounds (Visser & Holleman 2001), while timing of migration from winter areas, which may be cued by seasonal changes in length of daylight, remains constant (Visser et al. 1998). Alternatively, trophic mismatch may increase if organisms on different trophic levels display differential plasticity in their responses to similar changes in climate (Høye et al. 2007). The ultimate consequence of trophic mismatch is population decline, due to reproductive failure or reduced recruitment, as has been documented in some European populations of the pied flycatcher (Ficedula hypoleuca; Both et al. 2006). Such mechanistic insights into indirect consequences of climate change for population dynamics are possible only through simultaneous studies at multiple trophic levels (Gunn & Skogland 1997).
As in other seasonal environments, herbivores in the Arctic display seasonal reproduction which is timed to coincide with a highly pulsed peak in resource availability (Post 2003a). Caribou and wild reindeer (both Rangifer tarandus), for instance, exhibit highly synchronous parturition that coincides with the onset of the plant-growing season (Skogland 1989; Post & Klein 1999; Post et al. 2003). However, both the timing and duration of the plant-growing season are expected to respond to climate change in the Arctic (Molau 1997), with the onset beginning earlier and the duration possibly shortening as abiotic constraints on phenological progression are alleviated (Post et al. 2001, in press). Indeed, numerous studies have already documented shifts in the timing of plant growth at high latitudes associated with recent climate change (Walther et al. 2002; Post 2003b; Forchhammer et al. 2005). There is, therefore, potential for development of a trophic mismatch between the timing of caribou arrival on their calving ranges and the timing of peak resource availability on-site. Such a trophic mismatch could have negative consequences for offspring production and survival in mammalian herbivores such as caribou because the energetic demands of lactation, which would otherwise be met by intake of newly emergent plant tissue at peak nutritional value, are the highest resource demands of the annual reproductive cycle (Robbins 1983; Clutton-Brock 1991).
Numerous recent studies have focused on adverse effects of recent climate change on caribou populations throughout the Arctic. In the High Arctic of Canada, a population of the endangered Peary caribou (Rangifer tarandus pearyi) recently experienced a catastrophic and near-total population crash associated with increasing winter snow and ice crust formation consistent with climate change projections (Miller & Gunn 2003). As well, the dynamics of multiple populations of caribou in West Greenland have become synchronized towards the end of the twentieth century in response to rising winter temperatures (Post & Forchhammer 2004). Furthermore, dynamics of caribou and reindeer populations throughout the Northern Hemisphere respond to fluctuations in the North Atlantic Oscillation/Arctic Oscillation (Forchhammer et al. 2002; Post 2005), and in some cases are entrained to the point of highly synchronous dynamics across distances of thousands of kilometres (Post & Forchhammer 2006). Such climate-induced synchrony represents a potentially adverse consequence of climate change because synchronously fluctuating populations face a greater risk of global extinction than do independently fluctuating populations (Palmquist & Lundberg 1998).
While these examples suggest direct adverse effects of climate change on caribou populations, it is also possible that increasing temperatures and associated changes in precipitation may influence caribou populations indirectly through changes in the timing of plant growth (Gunn & Skogland 1997). Caribou migrate between seasonal ranges and time their migration to calving ranges to coincide with the timing of emergence of nutritious, highly digestible forage plants, which is crucial to the successful provisioning of newborn calves by female caribou (Gunn & Skogland 1997). To our knowledge, however, no study has yet demonstrated the consequences of trophic mismatch for offspring production and survival in caribou or wild reindeer. In fact, the only study to date that has explicitly addressed trophic mismatch in a mammalian herbivore focused on Soay sheep (Ovis aries) on the Scottish island of Hirta (Durant et al. 2005). That study found no evidence for an effect of trophic mismatch on offspring survival in Soay sheep, however, presumably because resource dynamics on Hirta are only weakly seasonal (Durant et al. 2005). Our focus was therefore to investigate the interaction between trophic mismatch and reproductive success in a system where the potential for its development was high: an Arctic plant–herbivore system.
2. Study site and methods
(a) Caribou calving
Since 1993, we have collected data during six summers (1993, 2002–2006) on the annual timing and progression of the calving season in the Kangerlussuaq population of caribou in West Greenland. Each year, we have visited our field site beginning in mid- to late May and recorded numbers of adult female caribou and calves on a daily to near-daily basis.
This population occupies low Arctic coastal and inland ranges in West Greenland, in the area bounded by the Davis Strait to the west, the Inland Ice to the east, Nordre Isortoq River to the north and Sukkertoppen Icecap to the south (66–67° N, 50–52° W; Bøving & Post 1997). During and following the calving season, the Kangerlussuaq population occupies the furthest eastern inland portion of its range, at the western edge of the Inland Ice (Thing 1984; Bøving & Post 1997). This area is characterized by a dry, continental climate with mean daily minimum and maximum temperatures during the calving and post-calving seasons (late May–late June) of 1.6 and 12.5°C, respectively (Thing 1984).
Annually, we have quantified the seasonal progression of births in this population according to Caughley's ‘Indirect Method A’ (Caughley 1977) and its modification for wild populations, for which capture is not necessary (Caughley & Caughley 1974). According to this method, we observe groups of pre-parturient and parturient female caribou on a daily or near-daily basis. Our efforts have focused on observing congregations of females in areas where they occur predictably. A minimum of 50 and an average of 100–200 adult females are observed each day. On each day of observation, using spotting scopes and binoculars from elevated vantage points, we record the numbers of adult females and calves observed. Animals under observation are not aware of our presence.
The onset and progression of the calving season is recorded as the daily proportions of calves (calves/(adult females+calves)) observed (Post et al. 2003). These observations display a clear sigmoidal pattern when plotted against ‘day of year’, indicating a gradual start to, rapid increase in, and levelling off of, numbers of calves produced per day. The proportion of calves observed invariably declines from the maximum observed at peak calving as early mortality occurs (Post et al. 2003). Our observations continue each year as long as caribou are present in the core calving area and extend several days after caribou have migrated out of the calving area. The last count of calves observed in late June to early July each year is considered the final proportion of calves produced for the season. It has been estimated that 85% of caribou calf mortality occurs within 8 days of birth (Adams et al. 1995), with up to 75% of calf mortality occurring within 48 hours of birth (Whitten et al. 1992).
Daily estimates of proportion calves are converted to daily estimates of per cent births in the population according to Caughley & Caughley (1974) and Caughley (1977). The date on which we observe the maximum proportion calves is considered to be the date of 100% births, i.e. the date on which all females that will give birth in a given year have given birth. The proportion of the annual total of the number of births observed on any given day is then quantified as
| (2.1) |
in which pi is proportion calves on date i and pmax is maximum proportion calves observed that year. This method does not require that sampling periods are contiguous or that observations are spaced evenly throughout the birth season, nor does it require that numbers (of adult females) per sample be equal (Caughley 1977). Moreover, the estimated per cent births to date are statistically independent of each other, and neither the beginning nor the ending of the birth season requires sampling for statistically accurate estimates of the tails of the birth season (Caughley & Caughley 1974; Caughley 1977).
(b) Plant phenology
In 2002, we established twelve (0.5 m2) permanently marked phenology plots in the study site, adjacent to the core caribou calving area. These plots replaced those originally established and monitored by us in 1993 (Post et al. 2003). Each year, we have monitored them with the same regularity and frequency that caribou are observed. Plots are distributed randomly among three meadows of comparable species composition, aspect and elevation within the study site; these meadows were chosen to be representative of, and adjacent to, the areas where parturient caribou are observed. Information on species composition has been published (Post et al. 2003). All species monitored on our plots occur in the diets of caribou in the Kangerlussuaq population during the calving season (Thing 1984; Post et al. 2003).
On each visit to the phenology plots, we record the names and numbers of plant species emergent in each plot. For analytical purposes, owing to differences in numbers of species present among plots, daily numbers of species emergent are transformed to daily proportions of the final number of species emergent in each plot. Using the final number of species emergent in each plot at the end of monitoring, we can back-calculate the percentage of this final number in an emergent state on each day of observation prior to the last day of monitoring. This is comparable to the method used for estimating daily per cent births for caribou (equation (2.1)).
(c) Quantifying trophic mismatch
Our index of the degree of trophic mismatch each year is based on the percentage of forage species emergent on the date at which 50% of caribou births have occurred. This index quantifies the temporal state of the forage resource midway through the season of caribou births. We considered using an index of trophic mismatch defined as the percentage of forage species emergent on the date at which 5% of births have occurred, which would quantify the temporal state of the forage resource at the onset of the season of caribou births. However, in some years, our observations of plant phenology were sparse at the beginning of the growing season, and we were concerned about the bias inherent to nonlinear-based estimation of values at the tails of a distribution of observed values (Rachlow & Bowyer 1991). Nonetheless, the index of trophic mismatch we used, that was based on the state of plant phenology on the date of 50% caribou births, correlated closely with the index of trophic mismatch we did not use, that was based on the state of plant phenology on the date of 5% caribou births (r=0.93).
In a simple sense, the more species that have already emerged by the midpoint of the birth season, the greater is the extent to which caribou calving has lagged behind plant phenology, and the greater the trophic mismatch. Conversely, the fewer species that have emerged by the midpoint of the caribou birth season, the more closely caribou calving ‘matches’ plant phenology, and the lower the trophic mismatch. This index is also closely and inversely related to the correlation between per cent caribou births and per cent of forage species emergent (r=−0.74). The correlation between daily per cent births and daily per cent of forage species emergent could also have been used as an index of how closely caribou calving tracks plant phenology each year, but it presents the disadvantage of not allowing us to determine whether a poor correlation indicates that caribou calving precedes emergence of most forage species or lags behind their emergence. Therefore, we used the index that quantifies the temporal state of forage resources midway through the season of births.
To use this approach, we first quantified the relationship between per cent births and per cent of forage species emergent for each year. Because this relationship is approximately sigmoidal, we used the following nonlinear regression model:
| (2.2) |
in which Y and X are per cent births and per cent species emergent, respectively (Thing 1984; Post & Klein 1999; Post et al. 2003). Percent births were estimated from proportions of calves observed each day (figure 1b) on the basis of Caughley's Indirect Method as described above (Caughley 1977). Coefficients (a and b) from equation (2.2) were then used to estimate the degree of trophic mismatch each year as per cent of births at the date of 50% emergence of forage species.
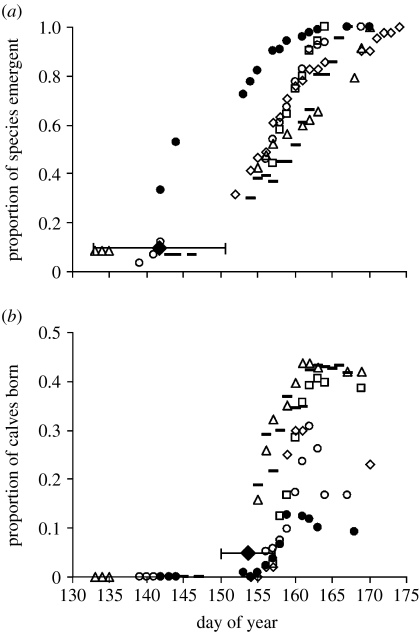
Figure 1.
Onset and progression of the annual seasons of (a) plant growth and (b) calving by caribou in the Kangerlussuaq population, West Greenland, 1993 and 2002–2006. In (a), the data are expressed as the mean daily proportion of the final number of species observed on each plot versus day of observation. In (b), the data are expressed as the proportion of calves (calves/(adult females+calves)) observed each day. See §2 for further details. In each panel, the filled diamond represents the mean (±1 s.d.) among years. In both panels, symbols are unique to each year of observation. Between panels, identical symbols represent observations of plant phenology (a) or calving (b) in the same year.
(d) Relating caribou calf production and survival to trophic mismatch
Offspring production by caribou was quantified in two ways: as the maximum proportion calves observed each year (PCmax); and as the final proportion calves observed each year (PCfinal), presumably after most early calf mortality has occurred (Adams et al. 1995). Annual early calf mortality (i.e. mortality occurring during the season of parturition) was then quantified as the difference between PCmax and PCfinal. In our analysis of the influence of trophic mismatch on caribou calf survival, however, we used an index of calf mortality scaled to calf production each year termed ‘relative calf mortality’, Mr, estimated as
| (2.3) |
(e) Relating trophic mismatch to abiotic conditions
To determine what abiotic conditions contribute to or ameliorate trophic mismatch between caribou calving and plant phenology, we used our nonlinear regression estimates of the onset and progression of the season of plant growth. We tested for relations between monthly mean temperatures and monthly total precipitation, as well as average spring temperature (the mean of temperature for the period March–May) and total spring precipitation (the total of precipitation for the period March–May). Weather data were obtained from the station maintained in Kangerlussuaq by the Danish Meteorological Institute. Although we recognize that temperature and precipitation probably interact to influence plant phenology and thereby trophic mismatch, the low number of years of data we have did not lend themselves to multiple regression analyses. Therefore, we report our results as simple linear correlations.
3. Results and discussion
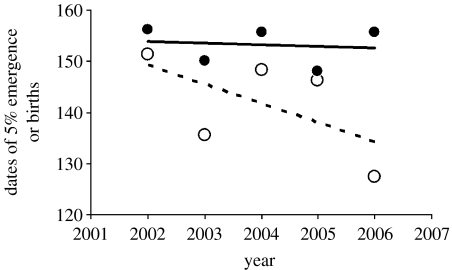
Over the course of the study, the mean (±1 s.d.) date of onset of the plant-growing season (the date of emergence of 5% of species) was 22 May (Julian day 142; ±8.9 days), whereas the mean date of onset of the caribou calving season (the date of 5% births) was 3 June (Julian day 154; ±3.6 days; figure 1). Between 1993 and 2006, the timing of onset of the plant-growing season (estimated as the date of emergence of 5% of plant species) advanced by 4.59 days; for the subset of those years for which data are continuous (2002–2006), onset of the plant-growing season advanced by 14.8 days (figure 2: open circles). By contrast, between 1993 and 2006, timing of onset of calving (date of 5% births) advanced by 3.82 days; whereas from 2002 to 2006, when advancement of the plant-growing season was most pronounced, onset of calving advanced by only 1.28 days (figure 2: solid circles). Moreover, interannual variability in onset of plant growth (CV=6.28) was approximately twice as great as that of caribou calving (CV=2.34), though this difference was not significant (F5,5=2.69, p>0.50). Taken together, these results suggest that caribou display less interannual variability in the timing of their reproductive cycle than do the forage plants upon which they depend for offspring provisioning at the period of peak resource demand. They furthermore indicate a rapidly developing mismatch between caribou reproduction and the timing of availability of their forage (figure 2).
Figure 2.
Dates (in day of year) of emergence of 5% of forage species (open circles, dashed line) and of 5% of caribou births (filled circles, solid line) at the study site in Kangerlussuaq, West Greenland, during the period of continuous annual data collection from 2002 to 2006. Fitted lines are linear regressions.
Among years, the timing of onset of the plant-growing season was most closely related to mean April temperature (r=−0.57; p=0.20). Timing of onset of caribou calving displayed its strongest correlation to mean spring (March–May) temperature (r=−0.71; p=0.12). Onset of calving was not, however, closely correlated with that of the plant-growing season (r=0.04; p>0.50), presumably because, as noted above, the onset of plant growth was considerably more variable among years than was the onset of calving (figure 1). Mean spring temperature, over the course of the study, increased by 4.63°C, though the correlation with ‘year’ is only marginally significant (r=0.52, p=0.058). We assume that the poor correlation of onset of the growing season with any of our abiotic predictors may be explained by either or both of the following factors. First, because our sampling did not begin early enough to record observations of 0 species emergent (figure 1a), our nonlinear regression estimates of the beginning of the growing season each year are less precise than those of the onset of calving (figure 1b). Second, as in other parts of the Arctic, onset of the plant-growing season may be determined by the combined influences of temperature and snow cover (Høye et al. 2007), and our sample was too small to justify multiple regression analysis.
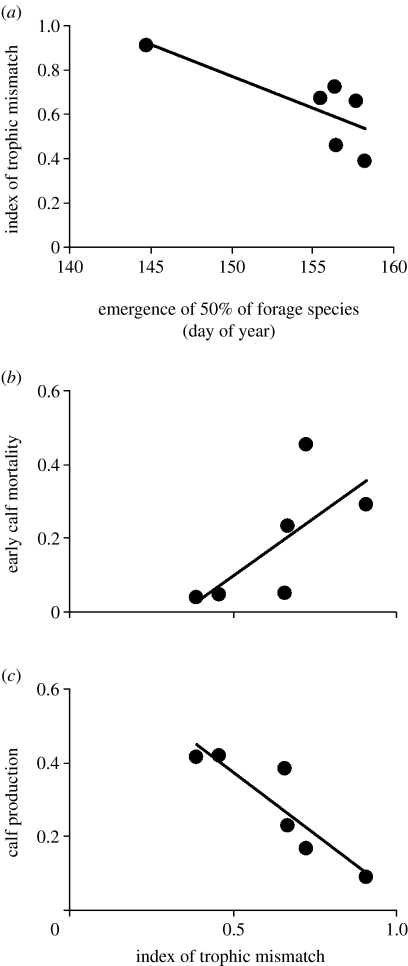
The progression of the plant-growing season was closely related to its onset, as the date of emergence of 50% of forage species was highly positively correlated with the date of emergence of 5% of species (r=0.84, p<0.05). Hence, warm springs were followed by early onset and rapid progression of the plant-growing season. In turn, a more rapid progression of the plant-growing season led to greater trophic mismatch between caribou calving and plant phenology (r=−0.77, p=0.07): the per cent of forage species emergent at the date of 50% births was nearly twice as great in the earliest and most rapid spring than in the latest and most gradual spring (figure 3a).
Figure 3.
(a) Relation between the midpoint of the plant-growing season and the index of trophic mismatch between caribou calving and plant phenology each year; an earlier occurrence of the midpoint of the plant-growing season leads to greater trophic mismatch. (b) Relation between the magnitude of trophic mismatch between caribou calving and plant phenology and early calf mortality. Calf mortality is calculated according to equation (2.3) in §2. (c) Relation between the magnitude of trophic mismatch between caribou calving and plant phenology and calf production. Calf production is estimated as the final proportion of calves observed each year according to the approach described in §2.
Early caribou calf mortality was closely related to the degree of trophic mismatch around the time of calving (r=0.70, p=0.12). Calf mortality varied sevenfold between the lowest and highest degrees of trophic mismatch observed (figure 3b). Accordingly, calf production declined with increasing trophic mismatch (r=−0.89, p<0.02), varying fourfold between the lowest and highest levels of trophic mismatch observed (figure 3c).
For animals inhabiting seasonal environments, successful reproduction depends on synchronizing offspring production with the time of year when resources are most abundant or of highest quality. In the far north, nutritional content and digestibility of plants reach a peak soon after emergence and decline rapidly thereafter (Klein 1990; Albon & Langvatn 1992). Hence, timing of parturition by caribou and wild reindeer (also R. tarandus) is closely linked to the start of the plant-growing season (Post & Klein 1999; Post et al. 2003). The extent to which onset of parturition in caribou—or in any northern herbivore—can track shifts in plant phenology induced by climatic warming is therefore a key question. While gestation length is fixed at approximately 240 days for this species (Leader-Williams 1988) and the annual reproductive cycle is entrained by seasonal changes in day length (Lincoln & Short 1980), there is some indication that caribou might be able to adjust the timing of their annual reproductive cycle to match, to some extent, changes in plant phenology. For instance, geographical variation in onset and peak of calving among populations of caribou and wild reindeer correlates closely with geographical variation in the timing of the plant-growing season among the areas inhabited by those populations (Skogland 1989). As well, Norwegian reindeer introduced to the sub-Antarctic island of South Georgia completely reversed their annual reproductive cycle by six months within 2 years of introduction, although it would seem this reversal was ultimately driven by the seasonal reversal of day length variation from the Northern to Southern Hemisphere (Leader-Williams 1988).
Of key importance to caribou in this population, however, is the rate at which plant phenology will advance with further changes in spring temperature. Our results indicate that, whereas onset of plant growth is highly variable among years, onset of parturition by caribou is not (figures 1 and 2). This would suggest a ‘bet-hedging’ strategy in caribou of timing parturition to coincide with a long-term average onset of favourable conditions. Over the course of our study, an advance in the onset and progression of the plant-growing season by approximately two weeks precipitated an increase in calf mortality and fourfold decline in calf production (figure 3). This two-week advance in plant phenology corresponded to an increase in average spring (March–May) temperature of 4.63°C in our study site over the same period (figure 2). With a further 3–5°C increase in warming expected throughout the Arctic (Maxwell 1997), the extent to which plant phenology will further advance is critically important to the future reproductive success of caribou in this population. The results of a warming experiment we conducted at the same study site indicated that an increase of 4°C advanced phenology of key species, used by caribou at the time of calving, by up to 10 days (Post et al. in press, submitted).
To our knowledge, our results are the first such documentation of a developing trophic mismatch in an Arctic mammal and its consequences for offspring production. While the patterns are clear, our analyses are, in some cases, hampered by low sample sizes. Therefore, our results cannot be considered conclusive. Nonetheless, they corroborate results from better-studied systems with longer-term data documenting the implications of climate change for trophic mismatch in aquatic and marine systems (Edwards & Richardson 2004; Winder & Schindler 2004), and of consequences of trophic mismatch for reproductive success and population dynamics in migratory birds. By far, the best studied of such systems is that of insectivorous birds including great tits (Parus major) and pied flycatchers (F. hypoleuca) in The Netherlands. Insectivorous birds should be especially susceptible to trophic mismatch due to climate change because emergence of their forage species in spring habitats is cued by local temperatures, whereas spring migration by passerines is cued by changes in day length. Great tits, for example, have been shown to suffer mistimed reproduction as climatic warming has advanced the appearance of invertebrate prey but not the timing of their own offspring production (Visser et al. 1998). In pied flycatchers nesting in The Netherlands, the timing of spring arrival on nesting grounds has not advanced in association with warming over the past two decades, whereas timing of egg laying has advanced due to selection pressure on offspring provisioning (Both & Visser 2001). Nonetheless, the advance in laying date has not kept pace with the advance of emergence of key forage species of invertebrates, and the magnitude of decline in several Dutch populations matches the extent of the temporal mismatch between caterpillar emergence and nestling production (Both et al. 2006).
The example of population declines in pied flycatchers illustrates the consequences of mistimed reproduction in migratory species that are unable to fully compensate through adjustments in their reproductive phenology for climate-driven changes in the timing of availability of resources. We might expect intense selection for earlier reproduction in caribou and other Arctic herbivores if further climatic warming and greater trophic mismatch reduce reproductive success. Some of the female reindeer introduced to the island of South Georgia, for instance, were pregnant and produced offspring in their first May on the island; their calves, however, died because their birth coincided with the onset of winter on the island (Leader-Williams 1988). Nonetheless, those same females eventually adjusted their reproductive cycles to coincide with the sub-Antarctic seasons, and reindeer persist there today (Leader-Williams 1988). We suggest, however, that the role of trophic mismatch in reproductive success and population dynamics of this and other Arctic species warrants urgent attention.
Acknowledgements
Procedures for observation of animals in this study were approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University
This research was conceived of by E.P. and supported by grants from the University of Alaska, Penn State Institutes of Energy and the Environment, and the National Geographic Society Committee for Research and Exploration. We are grateful for field assistance from Pernille Bøving, Toke Høye, Megan MacArthur, Christian Pedersen, Taylor Rees, Chris Wilmers and Tyler Yenter. We also benefited from fruitful discussions with David R. Klein and Henning Thing who guided our efforts during the initiation of this study.
Footnotes
One contribution of 12 to a Theme Issue ‘The boreal forest and global change’.
References
- Adams L.G, Singer F.J, Dale B.W. Caribou calf mortality in Denali National Park. J. Wildl. Manage. 1995;59:584–594. [Google Scholar]
- Albon S.D, Langvatn R. Plant phenology and the benefits of migration in a temperate ungulate. Oikos. 1992;65:502–513. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Both C, Visser M. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature. 2001;411:296–298. doi: 10.2307/3545568. [DOI] [PubMed] [Google Scholar]
- Both C, Bouwhuis S, Lessells C.M, Visser M. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.2307/3545568. [DOI] [PubMed] [Google Scholar]
- Bøving P.S, Post E. Vigilance and foraging behaviour of female caribou in relation to predation risk. Rangifer. 1997;17:55–63. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Caughley G. Wiley; London, UK: 1977. Analysis of vertebrate populations. [Google Scholar]
- Caughley G, Caughley J. Estimating median date of birth. J. Wildl. Manage. 1974;38:552–556. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Clutton-Brock T.H. Princeton University Press; Princeton, NJ: 1991. The evolution of parental care. [Google Scholar]
- Durant J.M, Hjermann D.Ø, Anker-Nilssen T, Beaugrand G, Mysterud A, Pettorelli N, Stenseth N.C. Timing and abundance as key mechanisms affecting trophic interactions in variable environments. Ecol. Lett. 2005;8:952–958. doi: 10.2307/3545568. [DOI] [PubMed] [Google Scholar]
- Edwards M, Richardson A.J. Impact of climate change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–884. doi: 10.2307/3545568. [DOI] [PubMed] [Google Scholar]
- Forchhammer M.C, Post E, Stenseth N.C, Boertmann D. Long-term responses in arctic ungulate dynamics to variation in climate and trophic processes. Popul. Ecol. 2002;44:113–120. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Forchhammer M.C, Post E, Berg T.B.G, Høye T.T, Schmidt N.-M. Large-scale climatic fingerprint in local short-term plant and herbivore behaviour. Ecology. 2005;86:2644–2651. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Gunn A, Skogland T. Responses of caribou and reindeer to global warming. In: Oechel W.C, Callaghan T, Gilmanov T, Holten J.I, Maxwell B, Molau U, Sveinbjörnsson B, editors. Global change and Arctic terrestrial ecosystems. Springer; New York, NY: 1997. pp. 189–200. [Google Scholar]
- Høye T.T, Post E, Meltofte H, Schmidt N.M, Forchhammer M.C. Rapid advancement of spring in the High Arctic. Curr. Biol. 2007;17:R449–R451. doi: 10.1016/j.cub.2007.04.047. [DOI] [PubMed] [Google Scholar]
- Klein D.R. Variation in quality of caribou and reindeer forage plants associated with season, plant part, and phenology. Rangifer, Special Issue. 1990;3:123–130. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Leader-Williams N. Cambridge University Press; Cambridge, UK: 1988. Reindeer on South Georgia. [Google Scholar]
- Lincoln G.A, Short R.V. Seasonal breeding: nature's contraceptive. Rec. Prog. Horm. Res. 1980;36:1–52. doi: 10.2307/3545568. [DOI] [PubMed] [Google Scholar]
- Maxwell B. Recent climate patterns in the Arctic. In: Oechel W.C, Callaghan T, Gilmanov T, Holten J.I, Maxwell B, Molau U, Sveinbjörnsson B, editors. Global change and Arctic terrestrial ecosystems. Springer; New York, NY: 1997. pp. 21–46. [Google Scholar]
- Miller F.L, Gunn A. Catastrophic die-off of Peary caribou on the western Queen Elizabeth Islands, Canadian High Arctic. Arctic. 2003;56:381–390. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Molau U. Phenology and reproductive success in arctic plants: susceptibility to climate change. In: Oechel W.C, Callaghan T, Gilmanov T, Holten T, Maxwell J.I, Molau U, Sveinbjörnsson B, editors. Global change and Arctic terrestrial ecosystems. Springer; New York, NY: 1997. pp. 153–170. [Google Scholar]
- Palmquist E, Lundberg P. Population extinctions in correlated environments. Oikos. 1998;83:359–367. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Post E. Large-scale climate synchronizes timing of flowering by multiple species. Ecology. 2003a;84:277–281. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Post E. Timing of reproduction in large mammals. In: Schwartz M.D, editor. Phenology: an integrative environmental science. Kluwer; New York, NY: 2003b. pp. 437–450. [Google Scholar]
- Post E. Large-scale spatial gradients in herbivore population dynamics. Ecology. 2005;86:2320–2328. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Post E, Forchhammer M.C. Spatial synchrony of local populations has increased in association with the recent Northern Hemisphere climate trend. Proc. Natl Acad. Sci. USA. 2004;101:9286–9290. doi: 10.2307/3545568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E, Forchhammer M.C. Spatially synchronous population dynamics: an indicator of Pleistocene faunal response to large-scale environmental change in the Holocene. Quat. Int. 2006;151:99–105. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Post E, Klein D.R. Caribou calf production and seasonal range quality during a population decline. J. Wildl. Manage. 1999;63:335–345. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Post E, Forchhammer M.C, Stenseth N.C, Callaghan T.V. The timing of life-history events in a changing climate. Proc. R. Soc. B. 2001;268:15–23. doi: 10.2307/3545568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E, Bøving P.S, Pedersen C, MacArthur M.A. Synchrony between caribou calving and plant phenology in depredated and non-depredated populations. Can. J. Zool. 2003;81:1709–1714. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Post, E., Pedersen, C., Wilmers, C. C. & Forchhammer, M. C. Submitted. Spatial and temporal compression of resource availability by warming: implications for large herbivores.
- Post, E., Pedersen, C., Wilmers, C. C. & Forchhammer, M. C. In press. Phenological sequences reveal aggregate life history response to climatic warming. Ecology. [DOI] [PubMed]
- Rachlow J.L, Bowyer R.T. Interannual variation in timing and synchrony of parturition in Dall's sheep. J. Mamm. 1991;72:487–492. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Robbins C.T. Academic Press; New York, NY: 1983. Wildlife feeding and nutrition. [Google Scholar]
- Skogland T. Paul Parey; Berlin, Germany: 1989. Comparative social organization of wild reindeer in relation to food, mates, and predator avoidance. [Google Scholar]
- Stevenson I.R, Bryant D.M. Climate change and constraints on breeding. Nature. 2000;406:366–367. doi: 10.2307/3545568. [DOI] [PubMed] [Google Scholar]
- Thing H. Feeding ecology of the West Greenland caribou (Rangifer tarandus groenlandicus) in the Sisimiut–Kangerlussuaq region. Dan. Rev. Game Biol. 1984;12:1–53. doi: 10.2307/3545568. [DOI] [Google Scholar]
- Visser M.E, Holleman L.J.M. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. B. 2001;268:289–294. doi: 10.2307/3545568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E, Van Noordwijk A.J, Tinbergen J.M, Lessells C.M. Warmer springs lead to mistimed reproduction in great tits (Parus major) Proc. R. Soc. B. 1998;265:1867–1870. doi: 10.1098/rspb.1998.0514. [DOI] [Google Scholar]
- Walther G.-R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.-M, Guldberg O.H, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. [DOI] [PubMed] [Google Scholar]
- Whitten K.R, Garner G.W, Mauer F.J, Harris R.B. Productivity and early calf survival in the Porcupine Caribou Herd. J. Wildl. Manage. 1992;56:201–212. doi: 10.1038/416389a. [DOI] [Google Scholar]
- Winder M, Schindler D.E. Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology. 2004;85:2100–2106. doi: 10.1038/416389a. [DOI] [Google Scholar]