Abstract
Echidna and platypus brains were sectioned and stained by Nissl or myelin stains or immunocytochemically for calcium-binding proteins, gamma aminobutyric acid (GABA) or other antigens. Cyto- and myeloarchitecture revealed thalami that are fundamentally mammalian in organization, with the three principal divisions of the thalamus (epithalamus, dorsal thalamus and ventral thalamus) identifiable as in marsupials and eutherian mammals. The dorsal thalamus exhibits more nuclear parcellation than hitherto described, but lack of an internal medullary lamina, caused by splaying out of afferent fibre tracts that contribute to it in other mammals, makes identification of anterior, medial and intralaminar nuclear groups difficult. Differentiation of the ventral nuclei is evident with the ventral posterior nucleus of the platypus enormously expanded into the interior of the cerebral hemisphere, where it adopts a relationship to the striatum not seen in other mammals. Other nuclei such as the lateral dorsal become identifiable by expression of patterns of calcium-binding proteins identical to those found in other mammals. GABA cells are present in the ventral and dorsal thalamic nuclei, and in the ventral thalamus form a remarkable continuum with GABA cells of the two segments of the globus pallidus and pars reticulata of the substantia nigra.
Keywords: platypus, echidna, dorsal thalamus, ventral thalamus, epithalamus, GABA cells
1. Introduction
The thalamus has a fundamentally similar pattern of nuclear organization and connectivity in all mammals. The basic divisions of the thalamus into ventral thalamus, dorsal thalamus and epithalamus can be readily identified in all metatherian and eutherian species, even in those in which the component nuclei of these divisions are least differentiated (Jones 1985, 2007). In two of the three living monotremes, the platypus and short-beaked echidna, the cytoarchitecture of the thalamus has been described as being rather monotonous, the nuclear divisions being less evident than in marsupials and eutherian mammals. The monotreme thalamus as a consequence has come to be regarded as less differentiated than that of other mammals (Ziehen 1897; Hines 1929; Abbie 1934; Lende 1964; Campbell & Hayhow 1971, 1972; Welker & Lende 1980; Ulinski 1984). The thalamus of the third living monotreme species, the rare long-beaked echidna, has not been examined. The ancestors of the monotremes are thought to have split off from the line ancestral to metatherian and eutherian mammals ca 150 Myr ago, with the forerunners of marsupials and eutherian mammals only separating from a common stock much later at ca 100 Myr ago (e.g. Radinsky 1987; Musser 2003; Van Rheede et al. 2006). The supposedly undifferentiated thalamus of the living monotremes, therefore, could potentially provide clues to what may have been the ancestral state of the thalamus at a time when, in the course of evolution, the earliest reptile-like mammals had emerged.
Recent experimental work devoted to mapping the principal sensory and motor areas of the cerebral cortex of the platypus and echidna shows that despite some significant differences in the degree to which these areas can be delineated from one another, they are laid out in a manner very comparable to that found in other mammals (Rowe 1990; Krubitzer et al. 1995; Manger et al. 1996). All evidence points to the parcellation of the mammalian cerebral cortex into functional areas occurring in close conjunction with a similar differentiation of the thalamic nuclei that provide input to the cortical areas (Jones 1985, 2007). Hence, there may be a far greater degree of nuclear differentiation in the monotreme thalamus than hitherto assumed. There have been no comprehensive studies of the structure of the thalamus of the extant monotremes that would permit the necessary re-evaluation, and none carried out with the full armamentarium of neuro- and chemoanatomical labels that have proven so valuable in extending our knowledge of the structural and functional organization of the thalamus of the common experimental animals. Recent partial study of the thalamus of the echidna indicates that it is possible to apply some of the more common histo- and immunocytochemical staining techniques to the monotreme thalamus (Ashwell & Paxinos 2005) and implies that a more complete analysis with a broader range of techniques would be profitable.(table 1)
Table 1.
List of abbreviations.
| A | anterior nuclei of thalamus |
| AC | anterior commissure |
| Am | amygdala |
| CALB | calbindin |
| Ce | central nuclei |
| CG | central gray |
| CP | cerebral peduncle |
| F | fornix |
| FF | field of Forel |
| GPe | external division of globus pallidus |
| GPi | internal division of globus pallidus |
| HC | habenular commissure |
| Hl | lateral habenular nucleus |
| Hm | medial habenular nucleus |
| HPT | habenulopeduncular tract |
| Hy | hypothalamus |
| IC | internal capsule |
| IP | interpeduncular complex |
| IPC | interpallidal commissure |
| LD | lateral dorsal nucleus of thalamus |
| LGd | dorsal lateral geniculate nucleus |
| LGv | ventral lateral geniculate nucleus |
| LM | medial lemniscus |
| ?LP | possible lateral posterior nucleus of thalamus |
| M | medial nucleus of thalamus |
| ?MG | possible medial geniculate complex |
| MTT | mamillothalamic tract |
| MV | medioventral nucleus of thalamus |
| NST | nucleus of stria terminalis |
| OT | optic tract |
| Pa | paraventricular nucleus of epithalamus |
| PARV | parvalbumin |
| PC | posterior commissure |
| Pi | pineal body |
| Pf | parafascicular nucleus of thalamus |
| PT | pretectum |
| Pt | parataenial nucleus of thalamus |
| R | reticular nucleus of thalamus |
| S | striatum |
| Sb | subthalamic nucleus |
| SC | superior colliculus |
| SM | stria medullaris |
| SNc | pars compacta of substantia nigra |
| SNr | pars reticulata of substantia nigra |
| ST | stria terminalis |
| VL | ventral lateral nucleus of thalamus |
| VM | ventral medial nucleus of thalamus |
| VMb | basal ventral medial nucleus of thalamus |
| VP | ventral posterior nucleus of thalamus |
| VT | vena terminalis |
| VTA | ventral tegmental area |
| ZI | zona incerta |
| ? | unknown dorsal thalamic nucleus |
| ?? | second unknown thalamic nucleus |
2. Material and methods
This study was carried out on the brains of three platypuses (Ornithorhynchus anatinus) and four short-beaked echidnas (Tachyglossus aculeatus). The brains from two of the platypuses and two of the echidnas came from animals recently caught in the wild in Queensland, Australia, with the appropriate permits (see Siegel et al. 1996, 1999 for details). The remaining brains came from an archive of already sectioned material in the collection of one of the authors (E.G.J.).
The newly caught animals were anaesthetized with 30 mg kg−1 ketamine and 1.3 mg kg−1 xylazine injected intramuscularly, given a lethal dose of intraperitoneal sodium pentobarbital, and perfused through the heart with normal saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). The brains were removed, infiltrated with phosphate-buffered sucrose and later frozen in dry ice. All procedures on the animals prior to sectioning the brains were carried out at the University of Queensland following the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Staining. The new brains were sectioned serially at a thickness of 30 μm on a sliding microtome and the sections collected in groups of five in 0.1 M phosphate buffer. One platypus brain and one echidna brain were sectioned in the frontal plane, and one platypus brain and one echidna brain were sectioned in the parasagittal plane. From each brain, a one-in-five series of sections was stained with thionin, a one-in-ten series was stained with haematoxylin, and one-in-five or one-in-ten series were stained for acetyl cholinesterase or cytochrome oxidase activity or immunocytochemically for parvalbumin, 28 kDa calbindin, 29 kDa calretinin, tyrosine hydroxylase, serotonin, gamma aminobutyric acid (GABA), α-type II calcium/calmodulin-dependent protein kinase or with the monoclonal antibody, SMI-32. The following primary antibodies or antisera were used: parvalbumin (Sigma, 1 : 1000); calbindin (provided by Dr P. C. Emson, 1 : 2000); calretinin (Chemicon, 1 : 2000); tyrosine hydroxylase (Eugene Tech, 1 : 500); serotonin (Sigma, 1 : 4000); GABA (Sigma, 1 : 10 000); α-type II calcium/calmodulin-dependent protein kinase (Boehringer-Manheim, 1 : 2000); and SMI-32 (Sternberger Monoclonals, 1 : 1000). All antibodies or antisera were diluted in 0.1 M phosphate buffer containing 3% normal serum from the species in which the secondary antibody was raised, 1% bovine serum albumin and 0.25% Triton X-100. After incubation for 12–24 h in the primary antibody at 4°C, sections were washed in phosphate buffer and reincubated in a 1 : 200 solution of biotinylated secondary antibody for 1–2 h at room temperature. They were then washed again and reacted for 1 h in avidin–biotin–peroxidase complex (ABC kit, Vector laboratories), washed and reacted in phosphate buffer containing 0.5 mg ml−1 3,3′ diaminobenzidine 4HCl and 0.01% H2O2. Controls consisted of staining selected sections with the same procedure but replacing the primary antibody with preimmune serum. Sections, after staining, were dried, dehydrated, cleared and mounted in DPX. The brains from the archives had all been sectioned in the frontal plane and all sections had been stained with thionin.
Imaging. Stained sections mounted on glass microscope slides were scanned at 20× (0.46 μm per pixel) in a ScanScope T3 scanner (Aperio Technologies, Vista, CA) adapted to accommodate 3″×2″ slides. Virtual slides prepared in this way were saved as JPEG-compressed TIFF files and uploaded to a server at www.brainmaps.org (Mikula et al. 2007). The 100 000 pixel wide images were down sampled to approximately 10% original size to allow importing into Adobe Photoshop for preparation and labelling of figures.
Nuclei recognized as having similar cytoarchitectonic features and relationships to those found in other mammals have been given standardized names (e.g. Jones 2007). Where a nuclear identity has been conjectured, this is indicated by ? before the abbreviation. Divisions of the thalamus that could not be recognized as the equivalents of those in other mammals have been left unlabelled.
3. Results
(a) Cyto- and myeloarchitecture
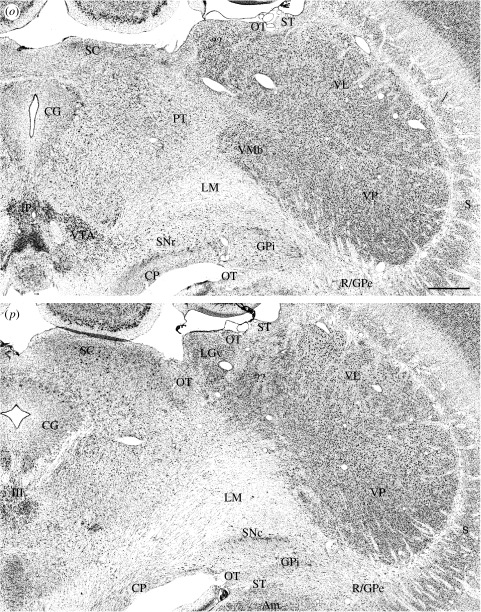
Nissl- and myelin-stained sections from the thalami of both the platypus and the echidna display a clear differentiation of epithalamus, dorsal thalamus and ventral thalamus (figures 1–5). The platypus thalamus is much larger than that of the echidna and distorted by a relatively enormous, lobe-like, posterolateral expansion of the ventral posterior part of the dorsal thalamus (figure 1l–t). Unlike in mammals, such as primates, in which posterior enlargement of the lateral posterior–pulvinar complex extends along the lateral surface of the midbrain, in the platypus the enlargement of the thalamus invades and becomes engulfed in the posterior part of the cerebral hemisphere. The echidna thalamus does not present this appearance and remains attached to the lateral aspect of the midbrain separate from the cerebral hemisphere (figure 2h–l).
Figure 1.
(a–t) Nissl-stained frontal sections in (a) anterior to (t) posterior order through the thalamus of a platypus. Scale bar, 1 mm. See table 1 for abbreviations. (Cont.)
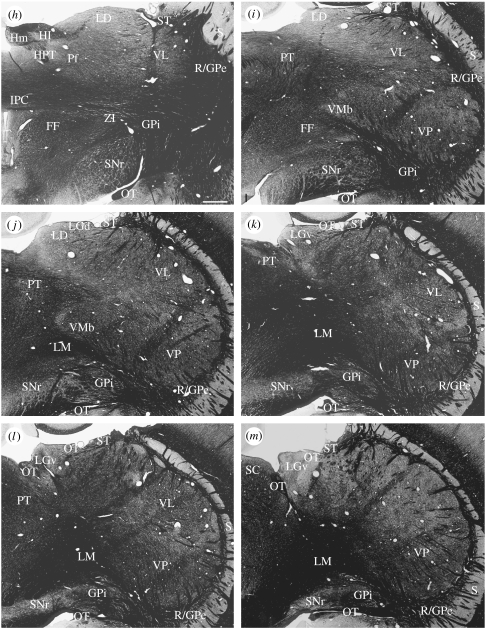
Figure 2.
(a–m) Myelin-stained frontal sections from the same brain as in figure 1, arranged in (a) anterior to (m) posterior order. Scale bar, 1 mm. See table 1 for abbreviations. (Cont.)
Figure 3.
(a–h) Nissl-stained parasagittal sections in (a) medial to (h) lateral order through the thalamus of a platypus. Scale bar, 1 mm. See table 1 for abbreviations. (Cont.)
Figure 4.
(a–l) Frontal sections in (a) anterior to (l) posterior order through the thalamus of an echidna. All sections are Nissl stained except (b), which is myelin stained. Scale bar, 1 mm. See table 1 for abbreviations. (Cont.)
Figure 5.
(a–l) Nissl-stained parasagittal sections in (a) medial to (l) lateral order through the thalamus of an echidna. Scale bar, 1 mm. See table 1 for abbreviations. (Cont.)
The three primary divisions of the thalamus (epithalamus, dorsal thalamus and ventral thalamus) can be readily identified in both species. The greater part of the epithalamus of both species is well defined and consists of clearly distinguishable medial and lateral habenular nuclei; they are relatively anteriorly placed, but have cytological characteristics identical to those of other mammals (figures 1h–l, 3a,b, 4e–i and 5a–c). The medial habenular nucleus is the larger of the two habenular nuclei; it is made up of closely packed small neurons that tend to form lobulated clumps with relatively acellular intervening spaces. Many of these neurons are immunoreactive for choline acetyl transferase (Manger et al. 2002a). The lateral habenular nucleus is relatively small; it contains both moderately large cells that tend to be concentrated dorsolaterally and smaller cells that tend to be concentrated ventromedially. There is a well-defined stria medullaris ascending the anterior aspect of the thalamus and entering the habenular nuclei, and a large habenulopeduncular tract emerges from their ventral aspects. Paraventricular nuclei are much less easy to define than in other mammals. Posteriorly, between the two medial habenular nuclei, there are two small nuclei of small densely packed cells that are merged in the midline, and thus resemble the posterior paraventricular nucleus of other mammals (figure 1g–k). Anteriorly, however, the ependyma lining the dorsal recess of the third ventricle is not underlain by the typically compacted, small-celled anterior paraventricular nucleus. Instead, there is a region of neuropil containing only a few scattered cells in that position in the platypus and a zone of compact cells extending up either side of the dorsal recess in the echidna (figures 1d–f and 4a).
In the ventral thalamus, which is clearly separated from the dorsal thalamus by an external medullary lamina, there is a distinct reticular nucleus and a zona incerta; these divisions of the ventral thalamus have cytoarchitectures comparable to those in other mammals, but they are anteriorly placed and the reticular nucleus does not encompass the whole lateral aspect of the dorsal thalamus (figures 1d–i and 4a–d). Throughout its extent, the reticular nucleus is closely apposed to the cells of the external segment of the globus pallidus and, over considerable distances, the cells of the two nuclei are essentially continuous with one another (figure 1i–t and see below). In anterior sections, the extended reticular nucleus/external segment of the globus pallidus comes to resemble the transient ‘perireticular nucleus’ of developing mammals (e.g. Hayes et al. 2003). The reticular nucleus comes to its end at about the middle of the anteroposterior extent of the dorsal thalamus, and from that point on, the cells of the external division of the globus pallidus are directly applied to the surface of the dorsal thalamus in such a manner that the posterior lobe-like expansion of the dorsal thalamus into the cerebral hemisphere in the platypus is surrounded by a thin line of pallidal cells that at first sight resemble the reticular nucleus (figures 1q–t and 3h). The posterior lobe-like expansion also causes the dorsal thalamus to override the internal segment of the globus pallidus and the substantia nigra; the internal segment of the globus pallidus becomes applied to the dorsal aspect of the internal capsule as it joins the cerebral peduncle so as to appear like the entopeduncular nucleus of many other mammals. The appearance is most marked in the platypus but evident also in the echidna (figures 1o–t and 2, and see below). In the platypus, the cerebral peduncle, substantia nigra and internal segment of the globus pallidus are also drawn up the medial side of the posterior expansion of the thalamus (figure 1p–t). In overriding the entopeduncular nucleus and the substantia nigra, the enlarged posterior lobe causes the reticular nucleus, external segment of the globus pallidus, entopeduncular nucleus and pars reticulata of the substantia nigra to merge into a continuous sequence of large, deeply staining cells without well-defined boundaries between the different nuclear components (figure 1q–t).
The zona incerta is relatively large, extending from a distinct subparafascicular nucleus and field of Forel medially across the ventral aspect of the dorsal thalamus, extending laterally up the side of the latter for a more considerable extent than occurs in other mammals. It too merges with the common reticular nucleus, globus pallidus and pars reticulata mass of cells, especially in the platypus (figure 1k–m). Running through the zona incerta towards the posterior aspect of the thalamus in the echidna, but more anteriorly located in the platypus on account of the giant posterior protrusion of the dorsal thalamus, is a commissure not evident in marsupials or eutherian mammals (figure 6). It does not obviously connect any parts of the thalamus; instead, it clearly emerges as a series of bundles from the two segments of the globus pallidus of one side and ends similarly on the other side. It has been named the interpallidal commissure (Jones 2007).
Figure 6.
Interpeduncular commissure of the platypus, as seen in Nissl-stained (a) frontal and (b) sagittal sections and in (c) a myelin-stained section adjacent to (a). Scale bars, (a) 500 μm and (b,c) 1 mm. See table 1 for abbreviations.
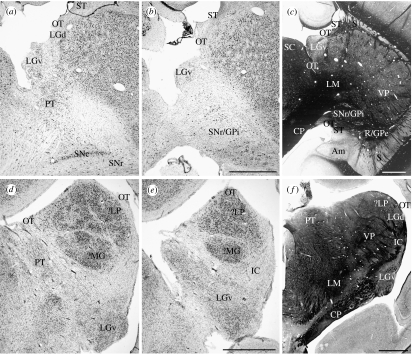
The remaining major division of the ventral thalamus, the ventral lateral geniculate nucleus, is differently located in the platypus and echidna and occupies positions quite dissimilar to its location in other mammals. A nucleus identical to that identified by the tracing of connections from the eye, and called LGNa by Campbell & Hayhow (1971), is relatively large and situated posteriorly beside the cerebral peduncle in the echidna; it contains large and small cells which can even be partially laminated, resembling the ventral lateral geniculate nucleus of other mammals in all except its pendulous shape and location at the posterior pole of the thalamus. In this posterior position, it extends up the medial side of the posterior pole of the dorsal thalamus to meet the lateral surface of the superior colliculus (figures 1p,q and 7). In the platypus, the whole ventral lateral geniculate nucleus, which is relatively large and partially laminated with alternating large- and small-celled regions as in the echidna, is displaced dorsomedially and lies adjacent to the anterior pole of the superior colliculus (figures 1p,q and 7). Like the dorsal lateral geniculate nucleus (see below), it can be more confidently regarded as the ventral lateral geniculate nucleus by tracing the optic tract to it (Campbell & Hayhow 1971). The optic tract of the echidna can be traced to the ventral lateral geniculate nucleus and then continues as a medially directed arm that follows the dorsally extending ventral lateral geniculate nucleus to the brachium of the superior colliculus, and as a lateral arm, located on the dorsolateral aspect of the dorsal thalamus and ending in the dorsal lateral geniculate nucleus (figures 1m–t, 2h–m, 3f, 4g–l, 5h–j, 7 and 8). In the platypus, the study of Campbell & Hayhow (1972) on the retinal projections in the platypus is key to identifying the optic tract and the ventral and dorsal lateral geniculate nuclei, for the enormous posterior outgrowth of the dorsal thalamus has occurred in such a manner as to displace the whole optic tract medially in relation to the dorsal thalamus. The large ventral lateral geniculate nucleus thus lies in an uncharacteristic position dorsolaterally adjacent to the superior colliculus (figures 1m, 7 and 8). The fibres of the optic tract ascend to the brachium of the superior colliculus along a trajectory similar to that of the echidna, but this bundle of fibres also contains fibres destined for a tiny dorsal lateral geniculate nucleus located lateral to the dorsally displaced ventral lateral geniculate nucleus (figures 1m, 7a, 8 and see below).
Figure 7.
(a,b) Nissl- or (c) myelin-stained frontal sections through the ventral lateral geniculate nucleus and the adjacent regions of the platypus thalamus. Similar sections (d,e,f) through comparable regions of the echidna thalamus. Scale bar, 1 mm. See table 1 for abbreviations.
Figure 8.
Parts of adjacent frontal sections showing the dorsal aspect of the thalamus in a platypus, (a) stained by the Nissl stain or (b) immunocytochemically for parvalbumin, showing the optic tract (OT), its separation from the stria terminalis (SM) by the vena terminalis (VT), and the small dorsal lateral geniculate nucleus (LGd). Scale bar, 500 μm. See table 1 for abbreviations.
In both the platypus and the echidna, the dorsal thalamus presents an initial impression of a lack of nuclear differentiation even in comparison with the least differentiated thalami of marsupials or eutherian mammals. On close inspection, however, the cellular architecture of the dorsal thalamus is by no means homogeneous in either species.
In the dorsal thalamus of both species, an internal medullary lamina is not present so anterior, medial and ventral/lateral divisions of the dorsal thalamic nuclei cannot be delineated by their relationships to an internal medullary lamina, and no groups of cells that could be regarded as the equivalents of the intralaminar nuclei can be identified with certainty. The fibre tracts that normally contribute to the lamina in other mammals become markedly dispersed on entering the thalamus. The mamillothalamic tract, although large in the monotremes, begins to splay out in small bundles immediately on entry into the thalamus (figure 2b–e). These small bundles radiate through the anterior region of the dorsal thalamus (figures 2c,d and 5b) but, in failing to consolidate as an internal medullary lamina, do not isolate a population of cells that can be immediately identified as the anterior group of dorsal thalamic nuclei. Scattered among the bundles of the mamillothalamic tract are cells whose architecture resembles that of the anteroventral nucleus of other mammals and we identify this region as the anterior nuclear complex, but there is no differentiation into anteroventral, anteromedial or anterodorsal nuclei typical of therian mammals. At the anterior pole of the thalamus and closely applied to the undersurface of the stria medullaris (taenia thalami) is a population of cells whose architecture resembles that of the parataenial nucleus of rodents and marsupials (figure 1c–e). Fibre bundles entering the dorsal thalamus from the internal capsule dorsally and posteroventrally also tend to splay out immediately on entry (figures 2, 6 and 7f) and these, too, fail to contribute to an internal medullary lamina.
Ventromedially in the anterior part of the dorsal thalamus, located on either side of the third ventricle, are paired masses of cells that are slightly more densely packed than elsewhere. These merge in the midline, to form a bilobed structure resembling a nucleus reuniens of other mammals (figures 1d–g, 3a–d, 4a,b and 5a). Hence, we call these nuclei individually the medioventral nucleus and the fused nuclei the nucleus reuniens, following the practice introduced by Berman & Jones (1982).
As the dorsal thalamus expands posteriorly, there is a large region of dorsally located, relatively dispersed cells that are not well delineated from adjacent cell populations but, as described below, are all calbindin immunoreactive; this is a feature of the lateral dorsal nucleus of other mammals and we accordingly call the region by this name. Medially, there is a rounded region of slightly more densely stained cells that might be considered a mediodorsal nucleus, but it is not surrounded by intralaminar or midline nuclei and thus its borders are ill-defined. We have labelled it M for medial nuclear complex in the figures (figures 1–6). Posteriorly, this putative mediodorsal nucleus is penetrated by the habenulopeduncular tract, around which the cells become a little more densely stained and, on the basis of its relationship to the tract and the density of cell staining, this region can be provisionally identified as a parafascicular nucleus (figures 1k,l, 3a,b, 4f,g, 5d,e and 6a).
At the region at which the interpallidal commissure crosses the midline, the medioventral nucleus gives way to a quite distinct region of slightly larger cells, which is provisionally identified as a ventral medial nucleus (figures 1h–j, 3d, 4c–f, 5b–d and 6). Extending laterally from this and along the commissure is a thin zone of larger, more deeply staining cells, which expands posterolaterally (figures 1k–n and 4d–g). In the platypus, with the downward growth of the ventral posterior nucleus, this line of cells is drawn up medially along the anterior pretectal nucleus and it comes to occupy a small triangular region at the boundary between dorsal thalamus and pretectum that might represent a limitans–suprageniculate nucleus. In the echidna, it is more rounded in shape.
Cytoarchitectonic differentiation is far greater in the posterior regions of the dorsal thalamus of both monotremes than in the anterior regions. The ventral posterior nucleus is the most distinct (figures 1l–t, 3f–h, 4d–i and 5h–k). In both species, it lies along the interpallidal commissure anteriorly and extends posteriorly as a large, multilobular, ovoid structure in the echidna and as an enormous, laterally extended, pear-shaped structure in the platypus. The ventral posterior nucleus of the echidna extends back towards the midbrain in a manner in which anatomical relationships between the thalamus and midbrain can be recognized as distinctly similar to those found in other mammals. In the platypus, however, the extreme enlargement of the ventral posterior nucleus causes the dorsal thalamus to invade the cerebral hemispheres and to continue within them to a level well posterior to that at which the hemispheres separate from the diencephalon (figures 1t and 3g,h).
The architecture of the ventral posterior nucleus is similar in both monotremes. There are three incompletely separated divisions (figures 1l–t and 4d–i). Medially is a region of relatively small, densely packed cells, which resembles the basal ventral medial nucleus of other mammals. Anteriorly, it lies along the interpallidal commissure, but posteriorly it expands up the lateral side and over the posterior pole of the ventral posterior nucleus. In the platypus, the region is streaked by three or more myelinated fibre bundles running across the nucleus with a dorsolateral to ventromedial orientation (figure 2i–m). These streaked bundles are not associated with any associated differences in cell packing density or cell type within the nucleus. In the echidna, the small-celled part of the ventral posterior nucleus is very highly lobulated without the fibre streaking (figures 4g–i and 5h–j). Ventromedially, the medial lemniscus is large and multifasciculated, with fascicles radiating into the ventral posterior nucleus; a large medial bundle within the lemniscus of the platypus suggests an ipsilateral trigeminothalamic tract (figures 1n–q, 2j–m, 3f,g, 4g–k and 5f–h).
The other two divisions of the ventral posterior nucleus are more evident in the platypus than in the echidna. There is a central region of somewhat less densely packed cells and a dorsal region of larger, somewhat more dispersed cells. In neither animal can an arcuate lamella be discerned and no divisions resembling ventral posterior medial and ventral posterior lateral nuclei can be identified.
Anterior to the ventral posterior nucleus is a region of relatively large, less densely packed cells that possibly represents a ventral lateral nucleus (figures 1f–p, 3e–h, 4c–e and 5h–k). It has been labelled accordingly. Dorsal to it, the lateral dorsal nucleus gradually tapers to an end and is replaced by a larger region of slightly more densely packed cells; by exclusion, we will call this the lateral posterior nucleus. It is quite large in both species of monotreme but it progressively narrows and disappears as the ventral posterior nucleus expands to fill the whole posterior aspect of the thalamus in the platypus, but it extends over the posterior pole of the dorsal thalamus in the echidna.
In neither species can a medial geniculate complex be identified with any certainty. Fibres forming a brachium of the inferior colliculus can be seen entering the diencephalon from the midbrain, but they tend to disappear into the posterior pole of the ventral posterior nucleus in the echidna and into the ventromedial aspect of the same nucleus in the platypus. It is possible that some of the cell lobules in the ventral aspect of the echidna ventral posterior nucleus (figures 4h–k and 5i–l) could represent a medial geniculate complex (identified by ?MG in the figures; Campbell & Hayhow 1971). No similar groups can be identified in the platypus.
A dorsal lateral geniculate nucleus (figure 1m) can be identified on the dorsal aspect of the platypus thalamus as described above. It is very small and only recognizable by following the optic tract to it (figures 1m and 7a; Campbell & Hayhow 1972). Hines (1929) mistook it for the bed nucleus of the stria terminalis which lies immediately lateral to it but separated from it by the vena terminalis (figure 8). In the echidna, the dorsal lateral geniculate nucleus is dorsolaterally located as in many other mammals; it is much larger than in the platypus, and its cells tend to form lobular masses that posteriorly merge with those of the more distinct putative lateral posterior nucleus (figures 4i–j and 5j–l); it descends over the posterior pole of the echidna thalamus towards the ventral lateral geniculate nucleus, so that the relative positions of the two geniculate nuclei there become the same as in marsupials and non-anthropoid mammals. The massive growth of the ventral posterior nucleus in the platypus prevents a comparable union and the ventral lateral geniculate nucleus, which is relatively large and partially laminated with alternating large- and small-celled regions, is displaced to the lateral edge of the superior colliculus (figures 1p,q and 7a–c).
(b) Chemical anatomy
Acetyl cholinesterase staining does not provide evidence of an incipient internal medullary lamina in either species. Staining is absent throughout all parts of the thalamus with the exception of the habenular nuclei, habenulopeduncular tract and in a thin lamina within the ventral lateral geniculate nuclei (figure 9a–d).
Figure 9.
Acetyl cholinesterase staining of the (a) habenular nuclei, of the merged substantia nigra, zona incerta, internal and external segments of the globus pallidus and (b) reticular nucleus and (c,d) of a lamina within the ventral lateral geniculate nucleus of a platypus. Scale bar, 500 μm. See table 1 for abbreviations.
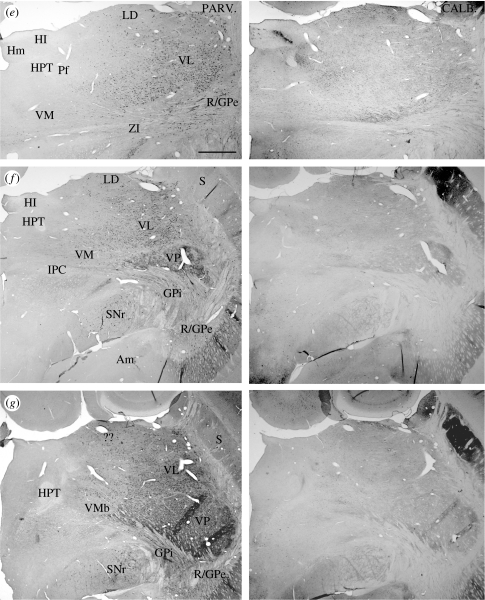
Parvalbumin and calbindin are expressed in thalamic neurons of both the platypus and the echidna; as in other mammals, they are expressed in different populations of neurons (figure 10). Regions containing large populations of parvalbumin immunoreactive cells are associated with enhanced cytochrome oxidase staining (not shown). Regions containing large populations of calbindin immunoreactive cells are associated with weak cytochrome oxidase staining as in other mammals (Rausell & Jones 1991a,b; Rausell et al. 1992; Jones 1998). Parvalbumin immunoreactivity is characteristic of all cells in the reticular nucleus of the two monotremes and is found in many cells of the zona incerta in both, as well as in the cells of the substantia nigra pars reticulata and the two divisions of the globus pallidus (figures 10–12). In the dorsal thalamus, there are no parvalbumin immunoreactive cells anteriorly, except for a small number of well-stained cells in the putative anterior nuclear complex (figure 11a). These look remarkably similar to a group identified as invaders from the reticular nucleus in other mammals (Jones 2007). The number of parvalbumin immunoreactive cells increases posteriorly, and a large population fills the ventral lateral and ventral posterior nuclei and the overlying putative lateral posterior nucleus (figure 11c). Throughout the length of the dorsal thalamus, however, there are no parvalbumin immunoreactive cells in medial regions, including in the putative parafascicular nucleus, and few or none in the lateral dorsal nucleus (figures 10, 11b,c and 12d).
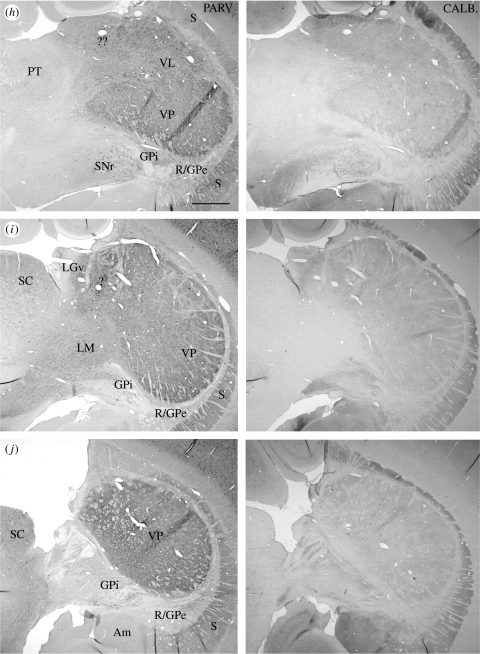
Figure 10.
(a–j) Pairs of adjacent frontal sections through the thalamus of a platypus, stained immunocytochemically for parvalbumin (left member of each pair) or calbindin (right member of each pair), showing the complementary patterns of distribution of cells immunoreactive for the calcium-binding proteins. Scale bar, 1 mm. See table 1 for abbreviations. (Cont.)
Figure 11.
Immunocytochemical staining for parvalbumin in the thalamus of a platypus. (a) Anteriorly, immunostained cells are largely confined to the reticular nucleus. (b) At middle levels, parvalbumin cells are found in the region formed by merging of the reticular nucleus and external segment of the globus pallidus on the lateral surface of the dorsal thalamus. (c) Posteriorly, the number of parvalbumin immunoreactive cells increases considerably in ventral and lateral parts of the dorsal thalamus but few or none are found dorsally or medially. Scale bars, (a,c) 1 mm and (b) 500 μm. See table 1 for abbreviations.
Figure 12.
Immunocytochemical staining for (a–c) calbindin or (d) parvalbumin in the thalamus of a platypus. Calbindin immunoreactive cells are concentrated anteriorly, medially and dorsally, in a manner complementary to the distribution of parvalbumin immunoreactive cells. Scale bars, 500 μm. See table 1 for abbreviations.
In the ventral posterior nucleus, there are many densely parvalbumin immunoreactive smaller cells embedded in a dense parvalbumin immunoreactive neuropil. Extending across the ventral posterior nucleus from dorsolateral to ventromedial, there are four bands of enhanced parvalbumin neuropil immunoreactivity (figure 11c). Two are located anteriorly in the nucleus and two posteriorly. These are the same bands that display enhanced staining for myelin (see above), and also display enhanced staining for cytochrome oxidase, calbindin and GABA. The fibres of the medial lemniscus are immunostained for parvalbumin, and this reveals its very large size and extent in relation to the ventromedial aspect of the dorsal thalamus.
In the epithalamus, the medial habenular nucleus contains only a few parvalbumin immunoreactive cells. In the ventral thalamus, the reticular nucleus and zona incerta possess many parvalbumin cells that are continuous with similarly immunoreactive cells in the two segments of the globus pallidus and pars reticulata of the substantia nigra (figure 11c); the ventral lateral geniculate nucleus, however, has few or none.
Cell populations exhibiting calbindin immunoreactivity in the monotreme thalamus are largely complementary to those displaying parvalbumin immunoreactivity (figures 10–12). The region identified as the anterior nuclear complex contains many densely packed calbindin immunoreactive cells, and the region identified as the lateral dorsal nucleus is particularly densely packed with them (figure 12a–c). This high density of calbindin cells serves to outline a large lateral dorsal nucleus that extends across most of the mediolateral extent of the dorsal thalamus and well back towards the posterior pole before being replaced by the putative lateral posterior nucleus, in which there are smaller numbers of more weakly calbindin immunoreactive cells and an equal population of parvalbumin immunoreactive cells as well (figure 10h). The putative medial, medioventral and ventral medial nuclei contain many calbindin immunoreactive cells (figures 10 and 12a–c), but the number in the ventral posterior nucleus is small and neuropil staining is weak, apart from that in the oblique bands described above. Outside the dorsal thalamus, calbindin immunoreactive cells are found in the zona incerta, posterior paraventricular nucleus, as a band in the dorsolateral aspect of the medial habenular nucleus, and as a small group in the dorsomedial corner of the ventral lateral geniculate nucleus (figure 10c–e,i).
Immunoreactivity for GABA is widespread in cells of the monotreme thalamus. All cells of the reticular nucleus and some in the zona incerta and ventral lateral geniculate nucleus are GABA immunoreactive (figure 13). Those of the external segment of the globus pallidus merge with and replace those of the reticular nucleus on the posterodorsal aspect of the thalamus, and in the platypus encircle the huge posterior extension of the dorsal thalamus into the cerebral hemisphere. These GABAergic cells also form the continuum extending from reticular nucleus through the external segment of the globus pallidus and entopeduncular nucleus into the pars reticulata of the substantia nigra described above. In the dorsal thalamus, the pattern of cellular immunoreactivity for GABA follows closely that of parvalbumin; there are a few well-stained GABA cells in the putative anterior nuclei, and their numbers increase on going posteriorly in the lateral parts of the thalamus. There are no GABA immunoreactive cells in the medial regions of the dorsal thalamus.
Figure 13.
Immunocytochemical staining of (a–c) GABA neurons in the (a,b) reticular nucleus and (c) dorsal thalamus of a platypus and of (d) tyrosine hydroxylase fibres in medial regions of the dorsal thalamus of an echidna. Scale bars, 500 μm. See table 1 for abbreviations.
Immunoreactivity for tyrosine hydroxylase, serotonin and choline acetyl transferase reveals permeation of the dorsal thalamus by fibres immunoreactive for these epitopes, and there are higher densities of immunoreactive fibres in the reticular nucleus and zona incerta and in the (posterior) paraventricular nucleus (not shown). No accumulation of immunoreactive fibres that could be construed as a nascent internal medullary lamina could be detected, but there was a strong tendency for tyrosine hydroxylase-immunoreactive fibres to form a plexus in the medial regions of the dorsal thalamus (figure 13d). The only choline acetyl transferase-immunoreactive cells are located in the medial habenular nucleus (Manger et al. 2002a). Neurons immunoreactive for choline acetyl transferase, serotonin or tyrosine hydroxylase, from which the afferents to the monotreme thalamus presumably arise, are located in brainstem nuclei comparable to those of other mammals (Manger et al. 2002a–c).
4. Discussion
The cyto- and chemoarchitecture of the monotreme thalamus, when examined in depth, reveals far more similarities to the thalami of marsupial and placental mammals than a cursory glance at a Nissl-stained preparation suggests. The homologues of ventral, dorsal and epithalamus are clearly evident and organized in a manner fundamentally similar to that of metatherian and eutherian mammals, and these divisions have similar immunocytochemical characteristics. The foreshortening of the reticular nucleus, its continuity with the globus pallidus and its replacement by the GABA cells of the external segment of the globus pallidus posteriorly, and the obvious continuity between the GABA cells of the ventral thalamus, globus pallidus and substantia nigra are unique features of the monotreme diencephalon. In the epithalamus, the difficulty in identifying an anterior paraventricular nucleus, especially in the platypus, is puzzling but otherwise the divisions of the epithalamus are very similar to those of other mammals. Lobulation of the medial habenular nucleus is found in many other mammals and large- and small-celled populations can also usually be identified in the lateral habenular nucleus of other mammals, becoming so well defined in the primates that they reach the status of separate subnuclei (Jones 2007).
The dorsal thalamus of the monotremes shows the greatest variation from other mammals until examined in depth and with the benefit of complementary immunocytochemical staining for the calcium-binding proteins. The lack of an internal medullary lamina, which seems to be determined by an unusual splaying out of fibres entering the thalamus from major fibre tracts such as the mamillothalamic, is a cause of uncertainty in making nuclear definitions in the anterior and medial regions of the dorsal thalamus. However, immunocytochemical staining patterns and the enclosing of the anterior polar region by the splayed out fascicles of the mamillothalamic tract suggest the presence of an anterior group or complex of nuclei. The lack of an enfolding arm of the internal medullary lamina may have influenced Ashwell & Paxinos (2005) in referring to the region we call the anterior nuclei as the parataenial nucleus in the echidna. The lack of identifiable anterior nuclei, coupled with the relatively enormous size of the frontal cortex in the echidna, influenced Regidor & Divac (1987) in identifying the anterior region of the echidna thalamus as all belonging to the mediodorsal nucleus. This whole anterior region undergoes retrograde degeneration after destruction of the frontal cortex in the echidna (Welker & Lende 1980), but the lesions could have encompassed the target cortex of anterior nuclei projections as well as those of a mediodorsal nucleus equivalent, the one equivalent to cingulate cortex and the other to prefrontal cortex.
The calbindin immunostaining pattern makes identification of a lateral dorsal nucleus, even in the absence of an unmistakable cytoarchitecture, almost incontrovertible owing to the consistency with which this nucleus expresses calbindin in all mammals that have been studied (Jones 2007). Medioventral and ventral medial nuclei are distinguishable on cytoarchitectonic grounds and the former on the basis of calbindin immunoreactivity as well.
The lack of cell groups that might be regarded as the equivalents of the intralaminar system, other than a somewhat indistinct parafascicular nucleus, is puzzling. Given the presence of a striatum of significant size, one would anticipate the existence of a substantial thalamostriatal projection, which in other mammals arises predominantly from cells located in intralaminar nuclei and their midline extensions. Conceivably, the tyrosine hydroxylase immunoreactive plexus illustrated in figure 13d identifies a region of striatally projecting cells, since the internal medullary lamina of other mammals usually contains a high concentration of such fibres.
In the posterior and lateral parts of the dorsal thalamus, the ventral posterior nucleus stands out very clearly in both monotremes and over it can be seen a nucleus different in immunocytochemical characteristics from the lateral dorsal nucleus and, therefore, likely to be a lateral posterior nucleus. The enormous size of the trigeminal nuclei and the medial lemniscus in both monotremes, especially the platypus, suggests that a substantial part of the ventral posterior nucleus should be devoted to the representation of the bill of the platypus and the beak of the echidna. However, no division of the ventral posterior nucleus into subnuclei comparable to the ventral posterior medial (trigeminal) and ventral posterior lateral (rest of the body) representations could be discerned. The densely packed small-celled medial part of the ventral posterior nucleus suggests a basal ventral medial (taste and visceral) nucleus (Jones 1985, 2007).
In the echidna, regions that include the ventral posterior nucleus described here project to a somatosensory responsive region in the cerebral cortex (Lende 1964; Bohringer & Rowe 1977). This cortical region appears to consist of two architectonic fields the anterior of which receives inputs from dorsal parts of the lateral nuclear mass and the posterior from ventral parts (Ulinski 1984) comparable to the ventral lateral and ventral posterior nuclei identified in the present study. Anterior to the somatosensory area, a motor area that partially overlapped the somatosensory representation and posterior to it visual and auditory cortical fields were also defined in early studies (Lende 1964; Allison & Goff 1972; Bohringer & Rowe 1977). The sensory areas are situated near the posterior pole of the cerebral hemisphere in the echidna, which therefore possesses a relatively enormous frontal cortex. In a recent mapping study, Krubitzer et al. (1995) identified a large cortical region in which multiunit responses to somaesthetic stimuli could be elicited. Within it, a first somatosensory area (SI) could be recognized on the basis of myelo- and chemoarchitecture and contained a systematic representation of the contralateral body surface like that found in other mammals. Anterior to SI was a second representation based on responses to deep stimuli, and anterior to that again a putative motor representation. Posterior to SI and partially separated from it by a small auditory representation was a further contralateral body representation thought to be homologous to the parietal ventral field (PV) of other mammals.
In the platypus, the somatosensory-responsive cortex is also large but less dramatically displaced posteriorly as in the echidna. The same fields (SI, deep, motor and PV) as in the echidna were described by Krubitzer et al. (1995). In addition, however, the platypus SI exhibits a remarkable anteroventral expansion in which the contralateral side of the bill is represented and in which some neurons may respond to low-amplitude electrical stimulation, probably reflecting the presence of electroreceptors in the bill of the platypus (Manger et al. 1995, 1996; Manger & Pettigrew 1996; Proske et al. 1998). The bill representation field is distinguished by patches of enhanced cytochrome oxidase staining separated by less densely stained zones (Krubitzer et al. 1995). Manger et al. (1996) found that the cytochrome oxidase-dense patches receive inputs from mechanoreceptors and the intervening less well-stained regions from a combination of mechanoreceptors and electroreceptors. This region most probably receives its thalamic input from the equally enlarged posterior, lobe-like expansion of the ventral posterior nucleus which is also characteristically lobulated.
It is impossible conclusively to identify a medial geniculate complex in either species. The brachium of the inferior colliculus offers no assistance here for it loses its identity as a compact tract before it enters the thalamus, and thus does not define a region of termination detectable in Nissl or myelin staining. A medial geniculate complex may be incorporated into regions identified overall as the ventral posterior nucleus. The appearance of an overhanging lateral posterior nucleus in the echidna gives some validity to this idea for that appearance is typical of many mammals, but there is no similar configuration in the platypus.
Identification of the dorsal lateral geniculate nucleus owing to its small size, especially in the platypus, is not easy but it can be identified by tracing the optic tract to it in either Nissl- or myelin-stained preparations and by correlation with the sites of termination of the optic tracts as revealed in axonal tracing studies by Campbell & Hayhow (1971, 1972). The enormous posterior growth of the ventral posterior nucleus in the platypus has tended to obscure the expected topographical relationships between the dorsal and ventral lateral geniculate nuclei, the latter being displaced to a dorsal position adjacent to the superior colliculus. Here, apart from its input from the optic tract, it can be recognized by a cellular architecture and chemical anatomy quite the same as that of other mammals (Jones 2007). The ventral lateral geniculate nucleus of the echidna is not only far less significantly displaced, but also reaches up the medial side of the dorsal thalamus to the superior colliculus. Campbell & Hayhow (1971, 1972) called the dorsal and ventral lateral geniculate nuclei of the echidna and platypus LGNb and LGNa, respectively. Since LGNb underwent retrograde degeneration after ablation of the visual cortex whereas LGNa did not (Welker & Lende 1980), LGNb was regarded as equivalent to the dorsal and LGNa to the ventral lateral geniculate nucleus. The similarities in cyto- and chemoarchitecture between LGNa and the ventral lateral geniculate nucleus of other mammals confirm this. LGNb is the same nucleus that we have identified as the dorsal lateral geniculate nucleus.
GABA cells are typical of the reticular nucleus in the monotremes and they show colocalization of parvalbumin as in all mammals, presumably two long-conserved traits. The presence of GABA cells within the dorsal thalamus is surprising in view of their absence from nuclei other than the dorsal lateral geniculate nucleus of many marsupials and smaller eutherian mammals such as rodents and bats (Houser et al. 1980; Ohara et al. 1983; Spreafico et al. 1983; reviewed in Jones 2007). They are still restricted, however, to the lateral and ventral nuclei in the monotremes and are singularly absent from medial regions. Finally, the evidence from immunostaining for the transmitters of the non-specific cholinergic, serotoninergic and noradrenergic afferents from the brainstem (Manger et al. 2002a–c) indicates that the monotreme thalamus is permeated by these fibres in the same manner as the thalamus of other mammals.
At middle and posterior levels of the thalamus in the monotremes, a thin strip of GABA/parvalbumin cells interposed between the dorsal thalamus and the putamen represents a common reticular nucleus/external segment of the globus pallidus and forms part of a continuum of the GABA/parvalbumin cells running from the ventral thalamus through the external segment of the globus pallidus, internal segment of the globus pallidus (entopeduncular nucleus) and pars reticulata of the substantia nigra. Recent evidence has revealed that the GABA cells of these nuclei, along with those of the perireticular nucleus, also form part of a developmental continuum that becomes broken up as development proceeds in other mammals (Jones 2001; Hayes et al. 2003). In embryonic rodents, ferrets and monkeys, the GABAergic cells of the ventral thalamus arise from an anterior part of the third ventricle neuroepithelium that is continuous with the proliferative ganglionic eminence of the cerebral hemisphere. Postmitotic cells migrate back from this region through the precursor of the external medullary lamina (the zona limitans intrathalamica) to populate the ventral lateral geniculate nucleus, reticular nucleus, zona incerta and nucleus of the field of Forel. Many of these cells continue without interruption into the developing pars reticulata of the substantia nigra. A second stream of postmitotic GABAergic cells descending from the part of the ganglionic eminence lying adjacent to the thalamus continues to add GABAergic cells to the reticular nucleus and, via the perireticular nucleus, to the substantia nigra pars reticulata and the internal segment of the globus pallidus (entopeduncular nucleus) late into development. With time, and reduction of the perireticular nucleus, the continuity between these components of the ventral thalamus, upper midbrain and midbrain/forebrain junctional region is largely lost. The continuity between the entopeduncular nucleus and pars reticulata of the substantia nigra remains the least tenuous and there is good reason to believe, from connectional patterns, that these two should be regarded as part of a common internal pallidal division (Nauta 1979). The appearances in the monotremes suggest an even wider expansion of the idea to embrace a common pallidal/nigral/ventral thalamic GABA population and in this regard it is noteworthy that all the nuclei incorporated into this common group send GABAergic projections into the dorsal thalamus (reviewed by Jones 2007), indicating at least one common pattern of connectivity.
We can conclude that the thalami of the two monotreme species examined are distinctly mammalian in type and bear little or no resemblance to the thalami of reptiles. While sharing the same broad organization of the three divisions of the thalamus as living marsupials and eutherian mammals, the thalami of the monotremes differ from the basic organizational pattern in a number of important ways. The marsupial and eutherian thalami have a great deal in common with one another. In marsupials, the same nuclear groups of the dorsal, ventral and epithalamus as in eutherians can be distinguished, their organization usually resembling that of a rodent. Some exhibit elaborations of nuclei such as the ventral posterior or dorsal lateral geniculate that can be as highly developed and differentiated as in murine or sciurid rodents, respectively. While the opossum and many other polyprotodont marsupials possess thalami that are relatively undifferentiated, apart from the ventral posterior nucleus, the opossum thalamus is, on the whole, no less differentiated than that of a mouse. And the thalami of many diprotodont marsupials are far more elaborated than that of the mouse; some even possess barreloids in the ventral posterior nucleus (Haight & Neylon 1978; Jones 1985). The early divergence of the monotreme lineage in the course of mammalian evolution appears to have been associated with the development of a thalamus that is different in certain aspects of cyto-, myelo- and chemoarchitecture, particularly in its lack of an internal medullary lamina; but it is not so uniquely different that the basic mammalian organizational plan of three major divisions with constituent nuclei cannot be discerned. Moreover, the nuclei of the monotreme thalamus share many of the cyto-, myelo- and chemoarchitectonic characteristics of metatherian and eutherian mammals. These include the elaboration of nuclei associated with the inputs from the chief sensory receiving surfaces of the animals, the expression of GABA as the inhibitory transmitter, the presence of inputs from all three of the major non-specific brainstem modulatory systems and the distinction of relay cells by expression of a calbindin or parvalbumin phenotype.
Acknowledgments
This work was supported by research grants numbers NS21377 and NS39094 from the National Institutes of Health, United States Public Health Service, by grant number MH60975 jointly funded by the National Institutes of Health and the National Science Foundation, and by the W. M. Keck Foundation Program in Neuroscience Imaging. Digital images of many of the preparations illustrated here can be found at www.brainmaps.org. We thank Phong Nguyen for valuable technical assistance.
References
- Abbie A.A. The brain-stem and cerebellum of Echidna aculeata. Phil. Trans. R. Soc. B. 1934;224:1–74. doi:10.1098/rstb.1934.0015 [Google Scholar]
- Allison T, Goff W.R. Electrophysiological studies of the echidna, Tachyglossus aculeatus. III. Sensory and interhemispheric evoked responses. Arch. Ital. Biol. 1972;110:195–216. [PubMed] [Google Scholar]
- Ashwell K.W.S, Paxinos G. Cyto- and chemoarchitecture of the dorsal thalamus of the monotreme Tachyglossus aculeatus, the short beaked echidna. J. Chem. Neuroanat. 2005;30:161–183. doi: 10.1016/j.jchemneu.2005.07.002. doi:10.1016/j.jchemneu.2005.07.002 [DOI] [PubMed] [Google Scholar]
- Berman A.L, Jones E.G. University of Wisconsin Press; Madison, WI: 1982. The thalamus and basal telencephalon of the cat: a cytoarchitectonic atlas with stereotaxic coordinates. [Google Scholar]
- Bohringer R.C, Rowe M.J. The organization of the sensory and motor areas of cerebral cortex in the platypus (Ornithorhynchus anatinus) J. Comp. Neurol. 1977;174:1–14. doi: 10.1002/cne.901740102. doi:10.1002/cne.901740102 [DOI] [PubMed] [Google Scholar]
- Campbell C.B.G, Hayhow W.R. Primary optic pathways in the echidna, Tachyglossus aculeatus: an experimental degeneration study. J. Comp. Neurol. 1971;143:119–136. doi: 10.1002/cne.901430108. doi:10.1002/cne.901430108 [DOI] [PubMed] [Google Scholar]
- Campbell C.B.G, Hayhow W.R. Primary optic pathways in the duckbill platypus Ornithorynchus anatinus: an experimental degeneration study. J. Comp. Neurol. 1972;145:195–208. doi: 10.1002/cne.901450206. doi:10.1002/cne.901450206 [DOI] [PubMed] [Google Scholar]
- Haight J.R, Neylon L. An atlas of the dorsal thalamus of the marsupial brush-tailed possum, Trichosurus vulpecula. J. Anat. 1978;126:225–245. [PMC free article] [PubMed] [Google Scholar]
- Hayes S.G, Murray K.D, Jones E.G. Two epochs in the development of GABA cells in the ferret thalamus. J. Comp. Neurol. 2003;463:45–65. doi: 10.1002/cne.10749. doi:10.1002/cne.10749 [DOI] [PubMed] [Google Scholar]
- Hines M. The brain of Ornithorhynchus anatinus. Phil. Trans. R. Soc. B. 1929;217:155–287. doi:10.1098/rstb.1929.0004 [Google Scholar]
- Houser C.R, Vaughn J.E, Barber R.P, Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980;200:341–354. doi: 10.1016/0006-8993(80)90925-7. doi:10.1016/0006-8993(80)90925-7 [DOI] [PubMed] [Google Scholar]
- Jones E.G. Plenum; New York, NY: 1985. The thalamus. [Google Scholar]
- Jones E.G. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 1998;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. doi:10.1016/S0306-4522(97)00581-2 [DOI] [PubMed] [Google Scholar]
- Jones E.G. Dichronous appearance and unusual origins of GABA neurons during development of the mammalian thalamus. Thalamus Related Syst. 2001;1:283–288. [Google Scholar]
- Jones E.G. 2nd edn. Cambridge University Press; Cambridge, UK: 2007. The thalamus. Two volumes. [Google Scholar]
- Krubitzer L.A, Manger P.R, Pettigrew J.D, Calford M.B. Organization of somatosensory cortex in monotremes: in search of the prototypical plan. J. Comp. Neurol. 1995;351:261–306. doi: 10.1002/cne.903510206. doi:10.1002/cne.903510206 [DOI] [PubMed] [Google Scholar]
- Lende R.A. Representation in the cerebral cortex of a primitive mammal: sensorimotor visual and auditory fields in the echidna (Tachyglossus aculeatus) J. Neurophysiol. 1964;27:37–48. doi: 10.1152/jn.1964.27.1.37. [DOI] [PubMed] [Google Scholar]
- Manger P.R, Pettigrew J.D. Ultrastructure, number, distribution and innervation of electroreceptors and mechanoreceptors in the bill skin of the platypus. Brain Behav. Evol. 1996;48:27–54. doi: 10.1159/000113185. [DOI] [PubMed] [Google Scholar]
- Manger P.R, Pettigrew J.D, Keast J, Bauer A. Nerve terminals of mucous gland electroreceptors in the platypus. Proc. R. Soc. B. 1995;260:13–19. doi: 10.1098/rspb.1995.0053. doi:10.1098/rspb.1995.0053 [DOI] [PubMed] [Google Scholar]
- Manger P.R, Calford M.B, Pettigrew J.D. Properties of electrosensory neurons in the cortex of the platypus (Ornithorhynchus anatinus): implications for processing of electrosensory stimuli. Proc. R. Soc. B. 1996;263:611–617. doi:10.1098/rspb.1996.0092 [Google Scholar]
- Manger P.R, Fahringer H.M, Pettigrew J.D, Siegel J.M. The distribution and morphological characteristics of cholinergic cells in the brain of monotremes as revealed by ChAT immunohistochemistry. Brain Behav. Evol. 2002a;60:275–297. doi: 10.1159/000067195. doi:10.1159/000067195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger P.R, Fahringer H.M, Pettigrew J.D, Siegel J.M. The distribution and morphological characteristics of catecholaminergic cells in the brain of monotremes as revealed by tyrosine hydroxylase immunohistochemistry. Brain Behav. Evol. 2002b;60:298–314. doi: 10.1159/000067193. doi:10.1159/000067193 [DOI] [PubMed] [Google Scholar]
- Manger P.R, Fahringer H.M, Pettigrew J.D, Siegel J.M. The distribution and morphological characteristics of serotonergic cells in the brain of monotremes. Brain Behav. Evol. 2002c;60:315–332. doi: 10.1159/000067194. doi:10.1159/000067194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikula S, Trotts I, Stone J.M, Jones E.G. Internet-enabled high resolution brain mapping and virtual microscopy. NeuroImage. 2007;35:9–15. doi: 10.1016/j.neuroimage.2006.11.053. doi:10.1016/j.neuroimage.2006.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser A.M. Review of the monotreme fossil record and comparison of paleontological and molecular data. Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2003;136:927–942. doi: 10.1016/s1095-6433(03)00275-7. doi:10.1016/S1095-6433(03)00275-7 [DOI] [PubMed] [Google Scholar]
- Nauta H.J.W. Projections of the pallidal complex: an autoradiographic study in the cat. Neuroscience. 1979;4:1853–1873. doi: 10.1016/0306-4522(79)90060-5. doi:10.1016/0306-4522(79)90060-5 [DOI] [PubMed] [Google Scholar]
- Ohara P.T, Lieberman A.R, Hunt S.P, Wu J.-Y. Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat; immunohistochemical studies by light and electron microscopy. Neuroscience. 1983;8:189–212. doi: 10.1016/0306-4522(83)90060-x. doi:10.1016/0306-4522(83)90060-X [DOI] [PubMed] [Google Scholar]
- Proske U, Gregory J.E, Iggo A. Sensory receptors in monotremes. Phil. Trans. R. Soc. B. 1998;353:1187–1198. doi: 10.1098/rstb.1998.0275. doi:10.1098/rstb.1998.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radinsky L.B. University of Chicago Press; Chicago, IL: 1987. The evolution of vertebrate design. [Google Scholar]
- Rausell E, Jones E.G. Chemically distinct compartments of the thalamic VPM nucleus in monkeys relay principal and spinal trigeminal pathways to different layers of the somatosensory cortex. J. Neurosci. 1991a;11:226–237. doi: 10.1523/JNEUROSCI.11-01-00226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Jones E.G. Histochemical and immunocytochemical compartments of the thalamic VPM nucleus in monkeys and their relationship to the representational map. J. Neurosci. 1991b;11:210–225. doi: 10.1523/JNEUROSCI.11-01-00210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausell E, Bae C.S, Viñuela A, Huntley G.W, Jones E.G. Calbindin and parvalbumin cells in monkey VPL thalamic nucleus: distribution, laminar cortical projections, and relations to spinothalamic terminations. J. Neurosci. 1992;12:4088–4111. doi: 10.1523/JNEUROSCI.12-10-04088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regidor J, Divac I. Architectonics of the thalamus in the echidna (Tachyglossus aculeatus): search for the mediodorsal nucleus. Brain Behav. Evol. 1987;30:328–341. doi: 10.1159/000118655. [DOI] [PubMed] [Google Scholar]
- Rowe M.J. Organization of the cerebral cortex in monotremes and marsupials. In: Jones E.G, Peters A, editors. Cerebral cortex. Comparative structure and evolution of cerebral cortex, Part II. vol. 8B. Plenum; New York, NY: 1990. pp. 263–334. [Google Scholar]
- Siegel J.M, Manger P.R, Nienhuis R, Fahringer H.M, Pettigrew J.D. The echidna Tachyglossus aculeatus combines REM and non-REM aspects in a single sleep state: implications for the evolution of sleep. J. Neurosci. 1996;16:3500–3506. doi: 10.1523/JNEUROSCI.16-10-03500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.M, Manger P.R, Nienhuis R, Fahringer H.M, Shalita T, Pettigrew J.D. Sleep in the platypus. Neuroscience. 1999;9:391–400. doi: 10.1016/s0306-4522(98)00588-0. doi:10.1016/S0306-4522(98)00588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreafico R, Schmechel D.E, Ellis I.C, Jr, Rustioni A. Cortical relay neurons and interneurons in the N. ventralis posterolateralis of cats: a horseradish peroxidase, electron-microscopic, Golgi and immunocytochemical study. Neuroscience. 1983;9:491–510. doi: 10.1016/0306-4522(83)90168-9. doi:10.1016/0306-4522(83)90168-9 [DOI] [PubMed] [Google Scholar]
- Ulinski P.S. Thalamic projections to the somatosensory cortex of the echidna, Tachyglossus aculeatus. J. Comp. Neurol. 1984;229:153–170. doi: 10.1002/cne.902290203. doi:10.1002/cne.902290203 [DOI] [PubMed] [Google Scholar]
- Van Rheede T, Bastianns T, Boone D.N, Hedges S.B, de Jong W.W, Madsen O. The platypus in its place: nuclear genes and indels confirm the sister group relation of monotremes and therians. Mol. Biol. Evol. 2006;23:587–597. doi: 10.1093/molbev/msj064. doi:10.1093/molbev/msj064 [DOI] [PubMed] [Google Scholar]
- Welker W.I, Lende R.A. Thalamocortical relationships in echidna (Tachglossus aculeatus) In: Ebbesson S.O.E, editor. Comparative neurology of the telencephalon. Plenum; New York, NY: 1980. pp. 449–481. [Google Scholar]
- Ziehen T. Semon's Zool. Forschung. vol. 3. Fischer; Jena, Germany: 1897. Das Centralnervensystem der Monotremen und Marsupialier. Part 2, pp. 789–921. [Google Scholar]