Abstract
The photonic structures of butterfly wings are among the most anatomically diverse of all those in nature, giving rise to an unrivalled display of structural colours. These have recently become the focus of research by workers in a variety of disciplines, stimulated by their potential applications to technology (‘biomimetics’). This interest, together with the discovery of unpublished electron micrographs taken by the late Dr John Huxley (Natural History Museum, London), prompted this review of butterfly photonics in general. The current work provides a synopsis of the literature to date, covering the diversity and evolution of these optical structures and incorporating Huxley's work, which represents an important biomimetic and evolutionary database on its own. This review deals with butterfly photonic devices according to the parts of the butterfly scales on which they occur. In this way, the information is ripe for evolutionary study.
Keywords: photonic, interference, structural colour, butterfly, diversity, evolution
1. Introduction
Structural colours are the result of the interaction of light with physical structures, which are in or on the surface of a substratum and are of a size comparable to the wavelength of light. Such colours usually cause bright directional effects as opposed to chemical pigments, which scatter light diffusely. We have been aware of structural colours for some time, Newton (1704) having surmised that they were responsible for the iridescence of male peacock tail feathers. Since then they have been found in a diverse range of taxa, including birds (Prum et al. 1999; Zi et al. 2003; Li et al. 2005; Vigneron et al. 2006), fishes (Denton & Nichol 1965, 1966), molluscs (Denton & Land 1971), annelids (Parker et al. 2001), cnidarians (Welch et al. 2005, 2006) and arthropods (Parker 1995; Parker et al. 1998, 2003; Parker & Hegedus 2003; Vigneron et al. 2005). Butterflies, however, reveal the most diverse range of reflectors in any taxon, probably because they possess scales that bear complex architectures at the sub-micron level—a very suitable platform for the evolution of structural colours. Some of the foremost studies were written in the late nineteenth century by Mayer (1896) and in the early twentieth century by Onslow (1921), Mason (1926, 1927a,b) and Gentil and Suffert (Suffert 1924; Gentil 1942). Much of this early work was verified later with the advent of the electron microscope in the mid-twentieth century, which enabled the nanostructure of the butterfly wing scales, causing the iridescence, to be observed for the first time (Anderson & Richards 1942; Suffert 1942; Lippert & Gentil 1951).
More recently, scientists from the fields of materials science, engineering (Tabata et al. 1996; Tada et al. 1998) and physics (Argyros et al. 2002; Vukusic et al. 2004; Kinoshita & Yoshioka 2005; Berthier et al. 2006; Prum et al. 2006; Wickham et al. 2006; Yoshioka & Kinoshita 2006) have become interested in butterfly structural colours. This work has, in some cases, provided new insights into our understanding of the physical and applied aspects of the photonic structures causing these colours, although it has also proved problematic in terms of scale terminology, which remains unstandardized.1
The most recent review of butterfly scales and photonics was written by Ghiradella (1998) and dealt mainly with the biological aspects of the subject. However, since then, there have been a number of key papers published in a diverse range of periodicals, demonstrating the expansion of butterfly photonics research into new fields. In addition, previously unseen scale architectures have recently been discovered in the form of unpublished micrographs and accompanying laboratory books belonging to the late John Huxley at the Natural History Museum (NHM). Huxley's interests lay predominantly in butterfly coloration, on which he conducted a vast amount of work, yet published only two (often-cited) papers (Huxley 1975, 1976) due to his untimely death. He was also interested in the origin of scale reflectors in insects generally, leading to an examination of the caddis flies (Trichoptera; Huxley & Barnard 1988). During his 30 years (1961–1990) at the NHM, he studied one of the world's largest butterfly collections and took a considerable number of scanning electron micrographs (SEMs) of a diverse range of butterfly species, which represented the variety of optical effects in butterflies based on his specialist experience. Unfortunately, Huxley died suddenly, leaving almost all of these data unpublished. When we were later shown them, we, along with John Huxley's former colleagues and Rob Huxley (brother), felt that they should be published posthumously as they represent important results to the field. This provided the stimulus for the current review, which summarizes the varied butterfly photonics literature, covering the diversity and evolution of scale reflectors, into which we incorporate Huxley's work.
2. Scales: the source of wing colour
There are normally two layers of chitinous scales tiled distally across each of the dorsal and ventral wing surfaces: the basal scales, which lie directly above the wing lamina, and the cover scales, which overlay these (figure 1a). Previous studies have shown that it is usually the cover scales (on the dorsal wing surface since structural colours are typically, although not exclusively, restricted to this region), which are primarily responsible for producing reflected colour. There are, however, exceptions to this; for example, Morpho species (Vukusic et al. 1999; Yoshioka & Kinoshita 2004) in which dorsal wing colour results from the combined optical interactions within the basal and cover scales.
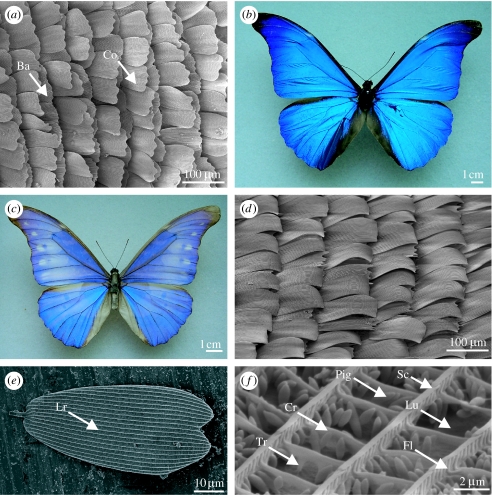
Figure 1.
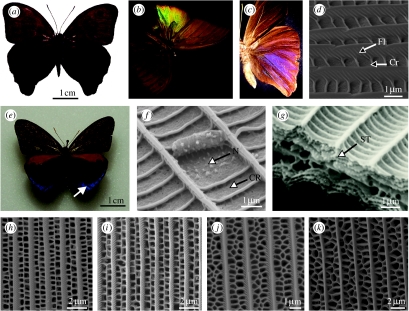
(a) SEM of the dorsal wing scales of Heliophorus saphir (Lycaeninae: Lycaenidae) showing cover scales (Co) overlaying basal scales (Ba). The figure is orientated left to right, towards the wing edge. (b) An unbleached Morpho rhetenor. (c) A bleached M. rhetenor showing paler blue appearance due to melanin degradation. (d) SEM of the dorsal wing scales of Eumaeus childrenae (Theclinae: Lycaenidae) showing scales curling away from the wing membrane beneath. (e,f) Pieris brassicae (Pierinae: Pieridae). (e) SEM of a single scale indicating the longitudinal ridges (Lr). (f) Nanostructure of a cover scale (Cr, cross rib; Fl, flutes; Lu, lumen; Pig, pigment granules; Sc, scutes; Tr, trabecula).
Melanin is typically contained in the basal scales, where it absorbs much or all of the transmitted light, thus preventing desaturation by wavelengths back-reflected by the cover scales, to enhance spectral purity (Ghiradella 1998). This is demonstrated by comparing an unbleached and bleached specimen (figure 1b,c, respectively), the latter of which appears paler due to the destruction of the melanin by exposure to UV light. Melanin is clearly integral to the visibility of structural wing colour (Ghiradella 1974; Yoshioka & Kinoshita 2004).
Previous studies have shown that often the scales do not lie flat against the wing membrane; for example, the cover scales may be curled up vertically from the wing (Allyn & Downey 1976; figure 1d). The angle at which the scale is tilted with respect to the wing membrane can be significant, and it has been shown that calculations of theoretical spectra are unreliable if this is not accounted for (Berthier et al. 2003; Yoshioka & Kinoshita 2007; Ingram et al. accepted, in press). There are also the potential effects of scale tilt on structural colours caused by components of the scale, which are themselves tilted. The angle of these components with respect to the dorsal wing surface may be exaggerated or suppressed if they and the scale are tilted in the same or opposite directions, respectively. It is therefore important to consider the contribution of the scale macrostructure in determining the overall visual effect.
Both the basal and cover scales are of the order of 100 μm long (figure 1e) and resemble dorsoventrally flattened sacs with an upper (obverse) and lower (reverse) lamina. The reverse lamina is generally smooth, whereas the obverse may possess an intricate architecture, typically composed of a series of longitudinal ridges (figure 1e). Dorsal outgrowths of these, termed scutes (figure 1f), overlap anteriorly and may vary in the angle to which they are oriented with respect to the obverse lamina. Further outgrowths or flutes (figure 1f) are often located laterally on the ridges and may extend between them, forming cross ribs (figure 1f). These structures are joined to the obverse lamina by vertical supports or trabeculae (figure 1f) and the region between the trabeculae is termed the lumen (figure 1f). Additional pigmentary granules may be present (figure 1f), although these have only been found in the Pieridae (Stavenga et al. 2004). More commonly, pigments, for example melanin (Ghiradella & Radigan 1976) or fluorescent pigments (Vukusic & Hooper 2005), are incorporated into the scale's structure.
These scale components can form the basis of a wide diversity of architectures, which, when compared with the wavelength of light, interact to produce structural colours. Many of Huxley's micrographs2 show variations on known architectures but in previously unstudied species. There are also those that are entirely new and are presented here for the first time: the role of these novel structures, if any, in wing colour has yet to be determined. However, their possible role is discussed here in the context of the current literature and in terms of the evolution of photonic structures in butterflies.
3. Diversity of photonic structures
To date, butterflies have been the taxon most studied for structural colour, revealing a wide diversity of photonic structures. Currently, three types have been identified: diffraction gratings; multilayer reflectors; and photonic crystals. The first two fall into the category of simple optical reflectors; that is, a light wavelength is reflected only once within the structure (Parker 2006; Parker & Townley 2007). Photonic crystals are comparatively more complex and light is reflected many more times from each structure (Parker 2006; Parker & Townley 2007).
(a) Diffraction gratings3
Diffraction gratings consist of periodic parallel slits or grooves, the spacing of which is of the order of magnitude of the wavelength of light (Jenkins & White 1981; figure 2a). Each slit behaves as a line scatterer of incident light, diffracting it into its component wavelengths. The combined effect of each scatterer is reinforced for each wavelength at a different viewing angle, causing different colours to be perceived at different angles of observation. Wavelengths are diffracted into orders (m=0, 1, 2, …) at each periodic structure; m=0 occurring at the specular (or mirror) angle, which is equal to the angle of incidence (figure 2a). A variation on this occurs when the periodic structures are blazed or asymmetric, giving a sawtooth profile. In this case, the incident light energy tends to be concentrated into one order (Jenkins & White 1981).
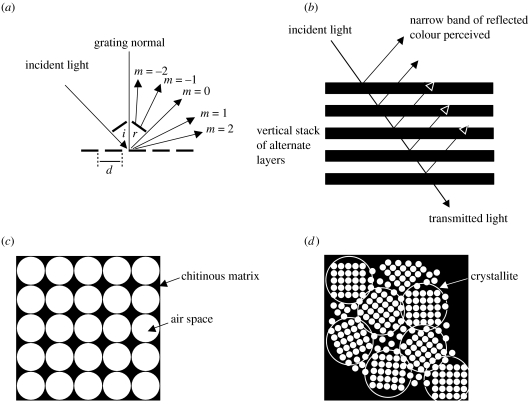
Figure 2.
Three types of photonic structure identified from butterflies. (a) Diffraction grating showing an incident light beam being diffracted into orders around the specular (mirror) angle at m=0. Note that the angle of the incident beam (i) is equal to the angle of the reflected beam (r) at m=0, d is the distance (period) between slits. (b) ‘Ideal’ multilayer reflector, in which alternate layers are of the same optical thickness, causing light to be reflected coherently. (c) Monocrystalline three-dimensional inverse opal structure composed of a matrix of chitin containing air spaces of approximately 250 nm in diameter. Light is reflected and diffracted from the air–chitin interfaces more than once. (d) Polycrystalline three-dimensional inverse opal, in which the structure is subdivided into domains or crystallites (indicated by circles).
Diffraction gratings can be formed by modifications of the scutes, longitudinal ridges, flutes or cross ribs.
(b) Multilayer reflectors4
In contrast to diffraction gratings, which are periodic parallel to the surface of the photonic structure, the periodicity of multilayer reflectors runs perpendicular to it (figure 2b). They are the most common type of photonic structures found in butterflies and may be formed by almost every component of a scale, including the scutes, flutes and cross ribs. They are also found within the lumen. In each case, the multilayer reflector is composed of alternating layers of chitin (the high refractive index material) and air (forming the low refractive index layers; Land 1972). At each interface, light is either reflected, transmitted or absorbed: the thickness of each layer dictates which wavelengths will be reflected and of these, which will constructively or destructively interfere at specific viewing angles. In the case of the former, the reflectance and therefore intensity of colour increases with increasing number of layers, to the point at which 100% reflection is effectively attained when there are approximately 10 or more layers present (Land 1972). This is found in ‘ideal’ reflectors in which the layers have the same optical thickness (actual layer thickness ‘d’×refractive index of material ‘n’), equal to a quarter the wavelength of the reflected light. In this case, layers give proportionally greater reflectance and wider bandwidth per layer than in the ‘non-ideal’ condition (Land 1972). However, the terms ideal and non-ideal refer only to the optical system and may not apply to evolution or animal behaviour (Parker et al. 1998).
(c) Three-dimensional photonic crystals
Three-dimensional photonic crystals are complex periodic lattices within which the propagation of light is controlled at the level of a single wave (Yablonovitch 1999). This is because light of a particular wavelength is reflected entirely, irrespective of incidence angle. In contrast, light is only reflected from an ideal multilayer reflector at specific incidence angles. Photonic crystals are characterized by the presence of band gaps, in which certain wavelengths cannot propagate as a result of the three-dimensional periodicity of the lattice (Yablonovitch 1987). These complex structures have been reported from the lumen of a number of butterflies, particularly the Lycaenidae (Allyn & Downey 1976; Tilley 1988; Biró et al. 2003; Vértesy et al. 2004). They are typically composed of a matrix of chitin (high refractive index material) containing regularly arranged spherical air spaces (low refractive index material) of approximately 250 nm in diameter (Bálint et al. 2005; Welch 2005; figure 2c). This is often termed ‘inverse opal’, since in the mineral opal, a low refractive index material forms the matrix in which spheres of the high refractive index material, silicon dioxide, are regularly arranged. In butterflies, the lattice may be either monocrystalline (figure 2c), in which the lattice is oriented in a single horizontal plane and is periodic across the entire volume (Kertész et al. 2006a), or polycrystalline (figure 2d), where it is subdivided into domains or crystallites that differ from one another in their horizontal orientation but display structural periodicity within each crystallite (Allyn & Downey 1976). The former arrangement is, to date, unique to butterflies (Welch & Vigneron 2007). The geometry of photonic crystals in butterflies is usually face-centred cubic: a central space is surrounded by others, one at each of the corners and another at the centre of each face (Ghiradella 1989). However, it can also rarely be hexagonally packed, in which a central space is surrounded by six others (Kertész et al. 2006b).
The appearance of scales containing photonic crystals may vary from being relatively matt and invariant with viewing angle in the case of a polycrystalline lattice in which colour averaging (‘pointillism’) occurs, or iridescent and variable in colour with angle in the case of a monocrystalline structure.
Each of these photonic structures, which interfere with light in a specific way, is dealt with individually in this review. However, as will become evident, they are not mutually exclusive in butterflies: we are discovering that individual scales, which had previously been thought only to cause colour by a single type of interference, can in some species produce colour by a complex system of, for example, diffraction and multilayer reflection from the same scale structure (Vukusic et al. 2002). Moreover, recently, Ingram et al. (accepted, in press) identified two separate scale components interspersed on the same scale, each of which functions independently as a diffraction grating, producing two entirely independent iridescence signals visible from different directions. Such discoveries have made the task of understanding butterfly photonics ever more challenging.
4. Scale components as photonic structures
The photonic structures described in §3 are produced by variation within the existing basic structural components that characterize butterfly scale architecture. This variation will now be described in further detail.
(a) Scutes5
Scutes are among the most well-documented photonic structures in the Lepidoptera and have been shown to behave as multilayer reflecting and diffracting elements, individually on a single scale or more rarely, together on the same scale. When present, scutes can vary in three main respects, each of which has been shown to affect their reflective role. These are as follows: (i) total number of layers (including thickness and spacing), (ii) tilt with respect to the obverse scale lamina, and (iii) position either side of a longitudinal ridge (opposite or alternate).
(i) Total number of layers
Morpho species (Morphinae: Nymphalidae) are probably the most well-known species to produce colour by means of scutes—the group having been studied exhaustively since the last century (Gentil 1942; Suffert 1942; Lippert & Gentil 1959; Bingham et al. 1995; Vukusic et al. 1999; Gralak et al. 2001; Kinoshita et al. 2002; Plattner 2004; Saito et al. 2004; Yoshioka & Kinoshita 2004; Berthier et al. 2006). Morpho scutes show much of the variability in scute morphology and optical function, demonstrating how these structures can function as both multilayer reflectors and diffraction gratings. Typically, Morpho species possess two layers of scales, the unpigmented cover (or glass) scales (figure 3a) and the basal scales, which contain melanin and multiple scute layers (figure 3b; Ghiradella 1994). The scutes of the latter behave as multilayer reflectors but also as diffracting elements (figure 3b), producing the observed blue coloration, while the ridges of the cover scales (figure 3a) broaden the angle over which the blue colour is observed, by means of diffraction (Vukusic et al. 1999). Studies of the Morpho species have shown the effect of variation in the total number of scute layers in the basal scales on the intensity of the reflected colour: increasing layers producing brighter colours (as predicted by thin film interference theory). One such study compared the peak reflectance of the glossy blue wings of Morpho rhetenor Cramer 1775, the scales of which possess approximately ten layers, with those of the whitish-blue wings of Morpho didius Hopffer 1874, which possess approximately four to six layers (Vukusic et al. 1999). The peak reflectance for M. rhetenor was 70%—almost double that of M. didius (40%). The larger number of layers was concluded to be responsible for the blue intensity. This in turn has been shown, in a separate study, to affect the appearance of the wings: greater intensity (and angular anisotropy of the reflected beam) producing a more glossy appearance (Yoshioka & Kinoshita 2004).
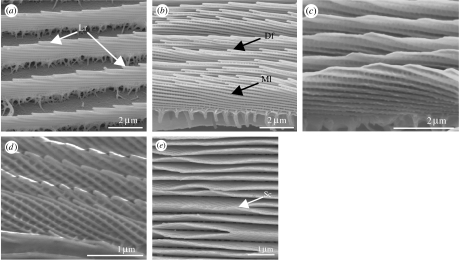
Figure 3.
(a) Scanning electron micrograph (SEM) of an unpigmented cover scale from a male Morpho sp. showing the diffracting longitudinal ridges (Lr). (b) SEM of a pigmented basal scale from the same specimen as in (a), showing the multilayered (Ml) and diffractive (Df) scutes. (c,d) SEMs showing the lateral views of the cover scales from the iridescent blue regions. (c) Doxocopa cherubina (Apaturinae: Nymphalidae). (d) Ptychandra lorquini (Satyrinae: Nymphalidae). (e) Sithon nedymond chitra (Theclinae: Lycaenidae). SEM of a plan view of scutes showing lateral contact between adjacent scutes (Sc).
There are two examples in Huxley's collection of previously unstudied species that are a similarly intense, broad blue colour, thought also to be as a result of multilayered scutes (A. L. Ingram 2007, unpublished results): Doxocopa cherubina C&R Felder 1876 (Apaturinae : Nymphalidae) (figure 3c) and Ptychandra lorquini C&R Felder 1861 (both Satyrinae: Nymphalidae; figure 3d), both of which possess up to 10 layers in the dorsal wing cover scales. Interestingly, a survey of all of the species with multilayered scutes among Huxley's collection and considering those studied elsewhere revealed that none possessed more than 12 layers. This could be because at frequencies above this, there is no significant benefit in terms of increased reflectance (approx. 10 ideal layers will give maximum reflectance; Land 1972), and thus diminishing returns do not favour the evolution of higher numbers of multilayer scute reflectors. Structural stability may be another limiting factor. For example, it is noteworthy that in a number of the species Huxley examined, including Sithon nedymond chitra (figure 3e) and also M. rhetenor, the dorsal regions of adjacent scutes often touch, which could suggest some instability. Lateral contact could also prevent incident light from hitting the scutes in these areas, leading to reduced reflectance, which, if undesirable, would also not favour the evolution of high numbers of scutes.
(ii) Tilt with respect to the obverse scale lamina
The tilt of scutes with respect to the obverse scale lamina affects the angle over which the resultant colour is visible and the spectrum of colours observed, both of which decrease with increasing tilt angle from the horizontal plane of the scale. Where present, tilted scutes are typically angled towards the proximal end of the scale.
When characterizing a structural colour caused by tilted scutes (or any other tilted scale structure), it is important to note whether the scale itself lies flat against the wing or if it is at some angle towards the body, since this can cause an additive effect on the total angle of the scute with respect to the wing surface and therefore the resultant visual effect.
Tilted scutes have been identified from a range of species, which possess scutes angled from approximately 10° to 30° with respect to the obverse scale lamina. For example, in M. rhetenor, the basal scale scutes are tilted at approximately 10°, contributing to the broad blue wing coloration (Vukusic et al. 1999). The males of Colias eurytheme Boisduval 1852 (Coliadinae: Pieridae; see Ghiradella 1974) and Eurema lisa Boisduval & Leconte 1829 (Coliadinae: Pieridae; see Ghiradella et al. 1972) possess scutes that are further tilted at approximately 20°. These form a multilayer reflector with a broad UV signal that has been shown to be employed in intrasexual communication (Silberglied & Taylor 1973; Rutowski 1981). The scutes of Troides magellanus Felder & Felder 1862 (Papilioninae: Papilionidae; figure 4a) and Ancyluris meliboeus Fabricius 1777 (Riodininae: Lycaenidae) are tilted to an even larger degree (30°), in each case resulting in limited-view iridescence, although effected by different means. In A. meliboeus, the structural colour is located on the ventral wings, where the scutes of the hindwing cover scales form both a multilayer reflector vertically and a diffraction grating horizontally (Vukusic et al. 2002). The grating results in a broad range of iridescent coloration, while the tilted multilayer reflector restricts the angle over which the colour is visible. This enables the butterfly to flash colour with minimal wing movement, which is advantageous in terms of energy expenditure. By contrast, the scutes of the yellow dorsal hindwing (DHW) regions of T. magellanus form a bi-grating, in which the exposed distal tips and the vertical interface of each scute form diffracting elements in the horizontal and vertical planes, respectively (figure 4a; Lawrence et al. 2002). This two-dimensional structure causes scattering at the blue end of the visible spectrum (figure 4b), which is only observable in the same plane as that of the incident light (backscatter) owing to the tilting of the scutes. Huxley also noted this blue reflection, stimulating the examination of another related species, Troides miranda Butler 1868 (figure 4c), in which the scutes are tilted to a similarly large degree in the yellow dorsal regions (figure 4d). The flutes have been completely suppressed in T. miranda as they have in T. magellanus, lying instead beneath the scutes close to the obverse scale lamina (figure 4d). However, unlike those of T. magellanus, the scutes of T. miranda extend vertically beyond the longitudinal ridge, causing the edge to appear ragged. On first inspection, there is no iridescence observable from T. miranda as there is in T. magellanus, suggesting that the scutes do not play a diffractive or multilayer reflective role. There is, however, broadly visible fluorescence—shared by both the species, though not mentioned by Lawrence et al. (2002). The scutes may therefore serve to scatter incident light onto the fluorescent pigment contained within the scale nanostructure of both species.
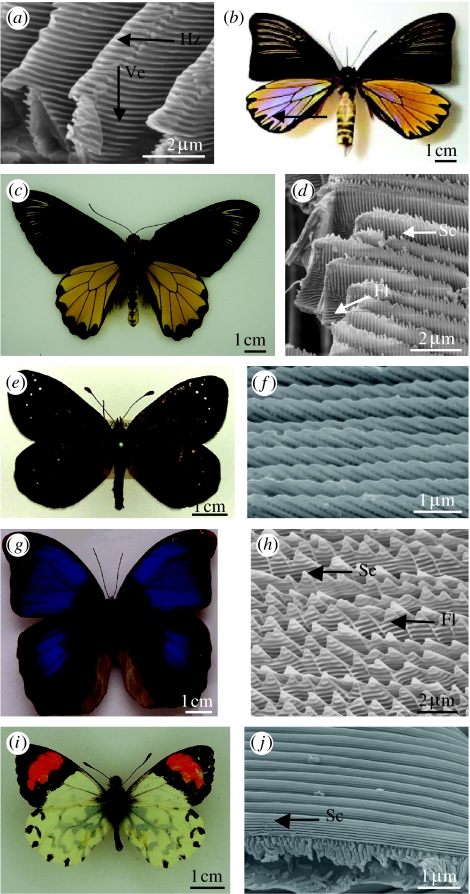
Figure 4.
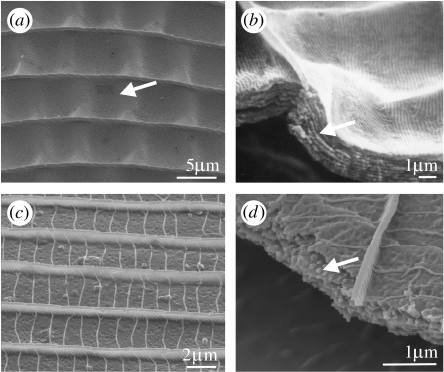
The variation in scute morphology. Dorsal views of males and SEMs of the lateral views of the cover scales from the iridescent regions. (a,b) Troides magellanus (Papilioninae: Papilionidae). (a) SEM showing horizontal (Hz) and vertical (Ve) diffracting scute tips from the iridescent blue dorsal regions. (b) Blue iridescence visible in backscatter. (c,d) Troides miranda (Papilioninae: Papilionidae). (d) SEM showing positions of scutes (Sc) and flutes (Fl) from scales in yellow regions. (e,f)Percnodaimon pluto (Satyrinae: Nymphalidae). (f) SEM showing tilted scutes from iridescent region on dorsal wings. (g,h) Eryphanis aesacus (Morphinae: Nymphalidae). (h) SEM showing flutes (Fl) and scutes (Sc). (i,j) Eroessa chiliensis (Pierinae: Pieridae). (j) Parallel continuous scutes in the orange regions.
Like T. magellanus, Percnodaimon pluto Butler 1876 (Satyrinae: Nymphalidae; figure 4e), from Huxley's collection, is also thought to employ the distal tips of the scutes, tilted at 30° (figure 4f), in structural wing colour (A. L. Ingram, unpublished data). Initial observations of this species showed that when white light is shone from directly above onto the dorsal wings, it appears dark brown. However, when illuminated laterally across the wings, bright iridescence from green through to violet is observable with increasing angle of incidence with respect to the wing surface. This suggests the presence of a diffraction grating, which is likely to be formed by the scute tips, since they are appropriately sized (period=440 nm) and angled to give the observed iridescence.
The scutes are approaching 45° to the obverse scale lamina in Caligo uranus Herrich-Scäffer 1850 (Morphinae: Nymphalidae; Ghiradella 1984), causing violet to be reflected close to the horizontal surface of the wing. Beyond this angle of tilt, as seen in Eryphanis aesacus Herrich-Scäffer 1850 (Morphinae: Nymphalidae; figure 4g,h), the flutes are thought to form the primary photonic structure (Ghiradella 1998).
So far, all of the scutes we have discussed have been tilted with respect to the dorsal scale lamina. However, this is not a requirement for their optical function as demonstrated by those of Eroessa chiliensis Guérin-Méneville 1830 (Pierinae: Pieridae; figure 4i,j) in Huxley's collection. The scutes of this species lie parallel to the dorsal lamina and run uninterrupted for the entire length of the scale. The scutes are located in the cover scales of the dorsal orange regions and are thought to be responsible for the UV reflectance measured over a broad range of angles (J. Huxley, unpublished results6).
(iii) Position either side of a longitudinal ridge (opposite or alternate)
The effect of the position of the scutes with respect to one another on either side of the longitudinal ridges has, until recently, been unclear. Alternate scutes, in which the structures do not lie opposite one another, have only previously been identified from Morpho species (Berthier et al. 2006). However, they are also present in P. lorquini (figure 5a) and Doxocopa laurentia Godart 1824 (both Satyrinae: Nymphalidae) from Huxley's collection. Alternate scutes should not, theoretically, cause differences in the colour or intensity of the reflection, provided the thickness and frequency of all the scutes remains the same. However, differences in polarization have been revealed recently in a study of 14 Morpho species, which suggested that alternate pairs of scutes form a blazed diffraction grating perpendicular to the plane of the multilayer reflector made by the scutes horizontally (Berthier et al. 2006). These reflectors were found to (plane) polarize incident light. The function of the polarized signal is unknown. However, like other dorsal structural wing colours, it may be employed in intraspecific recognition (Wiklund 2003).
Figure 5.
SEMs of the cover scales from males. (a) Ptychandra lorquini (Satyrinae: Nymphalidae), showing alternate scutes. (b) Troides miranda (Papilioninae: Papilionidae), showing opposite scutes, each opposed pair merging and terminating in a point beyond the dorsal region of the longitudinal ridge.
Opposite scutes are, as the name suggests, opposed to one another, either side of the longitudinal ridges. Troides miranda (figure 5b), from Huxley's collection, displays this arrangement of scutes, each opposed pair merging and terminating in a point beyond the dorsal region of the longitudinal ridge. The combined effect of the opposed scutes, which are of the same thickness and frequency, will be to reinforce the multilayer reflection, enhancing the intensity of the resultant colour. It is not known whether there are any additional polarization effects caused.
(b) Flutes and cross ribs
Flutes refer to the lateral projections located either side of the longitudinal ridges. These may extend between adjacent ridges and connect ventrally to the obverse scale surface via trabeculae, forming cross ribs. In both cross ribs and flutes, there is much variation in the morphology, tilt and spacing—the latter of which determines the presence of windows into the lumen in the case of the cross ribs.
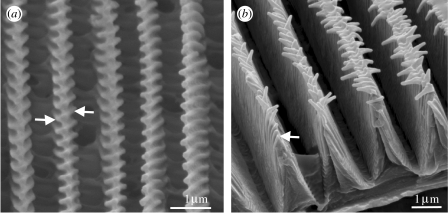
The cross ribs are typically discrete structures perpendicular to the dorsal scale lamina. They are perhaps one of the most morphologically diverse scale structures as demonstrated by Huxley's collection. For example, those of Lamprolenis nitida Godman & Salvin 1881 (Satyrinae: Nymphalidae; figure 6a), which are plate-like and tilted at approximately 40° to the dorsal scale surface. Together with the flutes, which are also tilted, the cross ribs cause the normally matt brown wings of the male (figure 6a) to appear brightly iridescent (Ingram et al. accepted, in press). Under certain conditions of illumination, the hindwings emit two entirely independent iridescent signals: when illuminated and observed anteroposteriorly, bright green to red iridescence is observed (figure 6b). If illumination and observation positions are then rotated approximately 180°, further iridescence from blue to violet is visible posteroanteriorly, from the same location on the wing (figure 6c). It was revealed that the two signals were produced by two separate blazed diffraction gratings formed by the cross ribs and flutes present on the same scale (figure 6d). This type of grating is asymmetric and produces correspondingly asymmetric diffraction patterns, driving most of the incident light into one diffraction order, visible in one direction only. The sloping grooves of the two gratings are opposed, giving rise to two diffraction patterns visible in different directions from the butterfly wing. Lamprolenis nitida is so far unique among the Lepidoptera and also in nature, in emitting such a complex optical signature, since typically a single photonic structure provides a single optical signal: multiple signals from independent photonic structures within the same sub-micron device are currently unknown in animals.
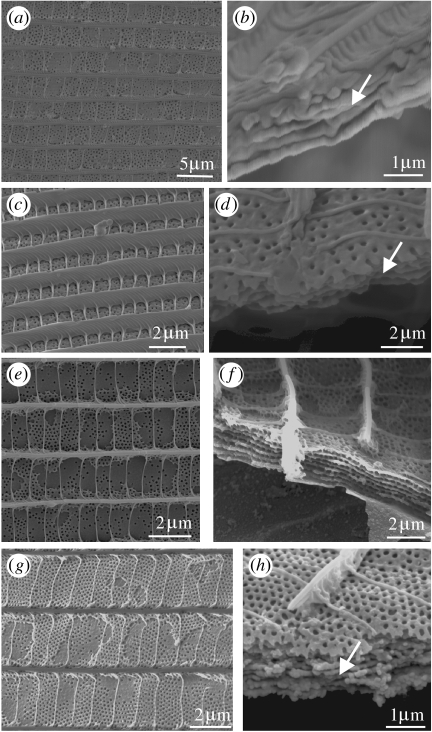
Figure 6.
Variation in cross rib morphology. (a–d) Male L. nitida (Satyrinae: Nymphalidae). (a) Dorsal view. (b) Effect of illuminating and observing the DHW anteroposteriorly. (c) The same region of the hindwing displaying violet structural colour when illuminated and observed posteroanteriorly. (d) SEM of the cover scale from the DHW, indicating the position of the flutes (Fl) and cross ribs (Cr). (e–g) Male E. tricolor (Satyrinae: Nymphalidae). (e) Dorsal view. (f) SEM of a scale from the region indicated by arrow in (e), showing the cross rib plates (CR) connected by nodules (N). (g) Transverse SEM of the same scale, showing a stack (ST) formed by adjacent cross ribs. (h–k) SEMs of the cover scales from the DHWs of papilionids (Papilioninae: Papilionidae; regions in brackets). (h) Graphium kirbyi (brown). (i) Graphium illyris (brown). (j) Lamproptera meges ennuis (black). (k) Papilio phorcas (brown).
The cross ribs are similarly tilted in Euptychia tricolor Hewitson 1851 (Satyrinae: Nymphalidae; figure 6e). However, in this case, adjacent cross ribs are no longer separate but are joined by nodules as shown in Huxley's micrographs (figure 6f), such that they resemble a stack of thin layers in transverse section (figure 6g). Initial observations suggest that these layers are behaving as a multilayer reflector, since there are two regions of blue structural colour visible on the DHW at 180° to one another: SEM revealed the presence of layered cross ribs tilted in correspondingly opposite directions in scales from each area (A. L. Ingram, unpublished results).
In many species examined by Huxley, adjacent cross ribs have become fused to varying degrees, forming a network of windows into the lumen. This is common among the papilionids (Papilioninae: Papilionidae), which demonstrate regular pairs of opposite windows, for example, in Graphium kirbyi or Graphium illyris Hewitson 1872 (figure 6h,i) to more numerous irregular arrangements, for example, in Lamproptera meges ennuis Zinken 1831 (figure 6j) or Papilio phorcas Cramer 1775 (figure 6k). The cross ribs can in some cases extend vertically to the obverse scale lamina, forming channels, termed alveoli (Huxley 1975). For example, in the blue dorsal regions of Papilio zalmoxis Hewitson 1864 (see Huxley 1976; figure 7a), Papilio ophidicephalus Oberthur 1878 (figure 7b) and Papilio bromius Doubleday 1845 (figure 7c). In Huxley's (1976) study of P. zalmoxis, he postulated that the alveoli caused Tyndall scattering, resulting in the observed blue coloration on the dorsal wings. Tyndall or Rayleigh blues are the result of the incoherent or random scattering of white light by heterogeneous particles of less than 575 nm in diameter (Mason 1923). All wavelengths are scattered but those which are shorter (blue to violet) are scattered more strongly, giving an overall perception of blue. Recent research employing Fourier analysis now, however, casts some doubt on Huxley's conclusions, since it was shown that the alveoli are inappropriately spaced to incoherently scatter light (Prum et al. 2006). The blue was in fact suggested to originate from a fluorescent pigment contained within the alveoli and the function of the nanostructure was to coherently scatter incident light onto the pigment, enhancing the fluorescence (Vukusic & Hooper 2005; Prum et al. 2006). It is probable that this is the same mechanism causing the blue in the other Papilio species mentioned above, which also possess alveoli.
Figure 7.
SEMs of the cover scales from the DHW unless otherwise stated. (a) Papilio zalmoxis (Papilioninae: Papilionidae; basal scale from the blue region). (b) Papilio ophidicephalus (Papilioninae; blue). (c) Papilio bromius (Papilioninae; blue). (d) Papilio mackinoni (Papilioninae; brown). (e) Heliconius doris (Heliconiinae: Nymphalidae; blue). (f) Parnassius hardwicki (Parnassiinae; violet). (g) Archon apollinus bellargus (Parnassiinae; violet).
Alveoli have not only been identified from scales which appear blue but they are also found in those appearing brown and black, for example, in the papilionids, Papilio mackinoni Sharpe 1891 (Papilioninae; figure 7d) from Huxley's collection, Papilio ulysses Linnaeus 1758 (Papilioninae) and Archaeoprepona meander Cramer 1775 (Charaxinae). In each case, the alveoli perform the same scattering function to enhance pigmentary absorption within the alveolar walls. However, this time, the alveoli are infused with melanin and are thought to increase both the intensity of the blackness (Vukusic et al. 2004) and solar radiation absorption (Berthier 2005).
Huxley thought that the cross ribs may also be involved in scattering to produce the blue observed on the DHWs of Heliconius doris Linnaeus 1771 (Heliconiinae: Nymphalidae; figure 7e). The blue appears iridescent and turns violet when illuminated and viewed at angles close to parallel to the wing surface. Since other structures are too widely spaced or altogether absent, the cross ribs, which are discrete structures in the cover scales, are perhaps the source of the colour. However, this requires confirmation.
In some of the species examined by Huxley, the cross ribs and flutes are entirely absent, for example, in the dorsal iridescent violet regions of the papilionids Parnassius hardwickei Gray 1831 (Parnassiinae) and Archon apollinus bellargus Herbst 1789 (Parnassiinae; figure 7f,g, respectively). In these species, the cause of the structural violet is not immediately obvious.
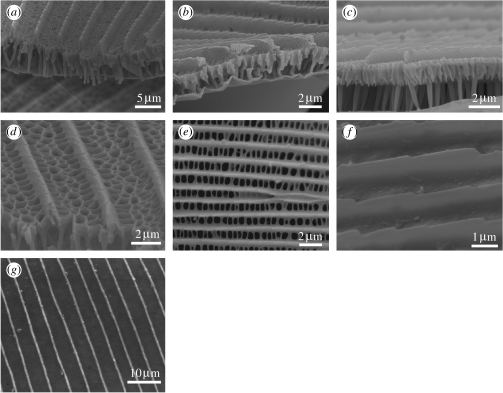
The cross ribs can conversely be so proliferate as to cover the region between longitudinal ridges entirely, leading to the absence of windows to the lumen. This arrangement is demonstrated in Huxley's collection by Philaethria dido Herbst 1789 (Parnassiinae: Papilionidae; figure 8a), Graphium sarpedon Linnaeus 1758 and Graphium weiskei Ribbe 1900 (Papilioninae: Papilionidae; figure 8b,c, respectively) and Eumaeus childrenae Gray 1834 (Theclinae: Lycaenidae; figure 8d). It is not clear whether the structure of these cross ribs contributes to the optical function in the first three species, since the regions from which the scales originated do not exhibit any iridescent coloration. However, in E. childrenae, the cross ribs are located in iridescent green–blue spots and are thought to be behaving as a diffraction grating. Evidence for this originates from a study of similar cross ribs found in the gold patches of the moth Thysanoplusia orichalcea (Fabricius, 1775), which were shown to diffract incident light (Brink et al. 1995; Brink & Lee 1996).
Figure 8.
SEMs of the cover scales from the DHW regions. (a) Philaethria dido (Parnassiinae: Papilionidae; green transparent). (b) Graphium sarpedon (Papilioninae; turquoise). (c) Graphium weiskei (Papilioninae; blue scale from the ventral forewing). (d) Eumaeus childrenae (Theclinae: Lycaenidae). SEM of an iridescent blue/green scale. (e) Pierella rhea (Satyrinae: Nymphalidae). SEM of the violet hindwing region.
The role of the flutes in photonic wing coloration has, by contrast, largely been ignored. A recent study (Ingram et al. accepted, in press), however, suggests that additional, although weak, violet iridescence is caused by these structures in some species. The flutes are thought to be producing violet in the same way in Pierella rhea Fabricius 1775 (figure 8e) and Pierella ceryce Hewitson 1874 (Satyrinae: Nymphalidae; J. Huxley, unpublished results). Since the variation in flute morphology and position, between species that possess them, is small (A. L. Ingram, unpublished results), it is probable that when these structures occur, they will play some secondary part in the optical signature of a butterfly. Their presence should therefore be considered when studying the overall structural wing colour.
(c) Structures within the lumen of the scale
A number of different types of photonic structure have been identified in the lumen of the scale, some of which involve the most complex scale architectures known in the Lepidoptera. These include thin film layers and mono- or polycrystalline structures, referred to as inverse opal.
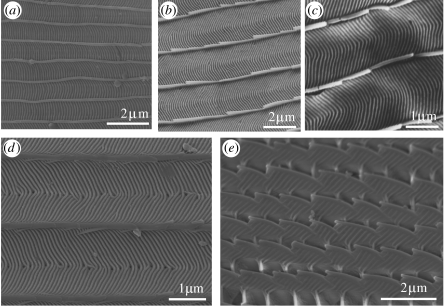
Layers within the lumen function optically as multilayer reflectors. They can vary morphologically in a number of respects, including the presence of undulations and the presence and frequency of perforations, all of which can alter the resultant visual effect. Undulating layers were first revealed by Huxley (1975) in the iridescent green DHWs of Papilio karna C&R Felder 1864 and Papilio palinurus Fabricius 1787 (Papilioninae: Papilionidae). In each species, the layers are entire and when viewed from above, the uppermost is covered in concavities (figure 9a). In his study, Huxley (1975) noted that the scales of both the species contained approximately 10 layers (figure 9b), which he concluded were functioning as multilayer reflectors for green. This work was later developed by Vukusic et al. (2000) in an analysis of P. palinurus. It was shown that the green resulted from the combination of structural yellow and blue: the yellow, emerging from the central region of each undulation, is the result of incident light being reflected at the surface normal. The blue resulted from the double reflection of incident light from orthogonal multilayer reflectors forming the sides of each undulation, a process which causes polarization.
Figure 9.
SEMs showing variation in layers located within the lumen: all of the cover scales from the male DHW. Plan and transverse views are shown, respectively. (a,b) Green regions of P. karna (Papilioninae: Papilionidae). (a) Concavity in the dorsal-most layer indicated. (b) Transverse section showing multilayers. (c,d) Bronze regions of H. brahma (Lycaeninae: Lycaenidae). (c) Plan view of the entire layers. (d) Transverse section indicating nodules separating each layer.
Since Huxley's work, similar undulating multilayer reflectors have been found in other papilionids, again in the dorsal green cover scales, including Papilio blumei Boisduval 1836 (Tada et al. 1998), Papilio lorquinianus C&R Felder 1865 and Troides priamus Linnaeus 1758 (Ghiradella 1985), suggesting that this type of layer may be restricted to this family.
While undulating layers are relatively rare, those which are flat are more common among the Lepidoptera and can vary in their integrity, depending on the presence of air holes. This is demonstrated by a number of specimens in Huxley's collection. Unperforated layers are found, for example, in the bronze dorsal regions of Heliophorus brahma Moore 1857 (Lycaeninae; figure 9c), in which five layers are each separated by nodular spacers (figure 9d). In other species, layers are to varying degrees perforated. For example, those of the cover scales from the iridescent blue DHWs of the lycaenids Udara blackburnii Zimmermann 1958 (Polyommatinae; figure 10a,b), Aphnaeus orcas hollandi Drury 1782 (figure 10c,d) and Arhopala meander Boisduval 1832 (figure 10e,f) and the green regions of Arhopala chamaeleona Bethune-Baker 1903 (all Theclinae; figure 10g,h) contain layers, the uppermost of which is punctuated by regularly arranged air holes. Subsequent layers are again separated by nodules. Similar layers have also been noted in the blue Celastrina ladon Cramer 1780 (Polyommatinae: Lycaenidae; Ghiradella 1984, 1998) and the violet dorsal cover scales of Quercusia quercus Linnaeus 1758 and Laeosopis roboris Esper 1793 (Theclinae: Lycaenidae; Jones & Tilley 1999). In all of these species, the layers are likely to behave as multilayer reflectors.
Figure 10.
(a–h) SEMs showing the plan and transverse views of multilayered structures within the lumen. Dorsal-most layer is perforated: subsequent layers are separated by nodules. (a,b) Violet region of Vaga blackburnii (Polyommatinae). (c,d) Blue region of A. o. hollandi (Theclinae). (e,f) Blue region of A. meander (Theclinae). (g,h) Green/blue region of A. chamaeleona (Theclinae).
Inverse opal, consisting of regularly arranged spherical air spaces in a matrix of chitin, has also been identified in butterflies, most commonly in the Lycaenidae. Polycrystalline photonic crystals, which cause the wings to appear matt, have to date been found exclusively on the ventral wing surfaces where they reflect green. For example, in Callophrys species (Theclinae: Lycaenidae), including Callophrys rubi Linnaeus 1758 (Morris 1975; Tilley 2000; figure 11a) and Callophrys gryneus (Allyn & Downey 1976; Ghiradella 1984). They also appear in Cyanophrys remus Hewitson 1877 (Bálint et al. 2005) and V. blackburnii Zimmermann 1958 (Polyommatinae: Lycaenidae; Huxley's collection; figure 11b). In each case, the green is thought to be employed in antipredation, by camouflaging the stationary butterfly when the wings are brought together to display the ventral surface (Darwin 1880; Ingram in press).
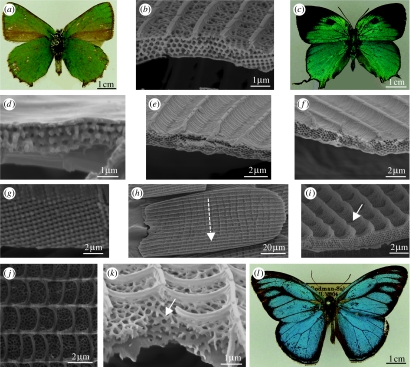
Figure 11.
(a) Green ventral wing surface of male Callophrys rubi (Theclinae: Lycaenidae). (b) SEM showing a transverse view of a green cover scale from the ventral wing of V. blackburnii (Polyommatinae: Lycaenidae). (c) Turquoise dorsal wing surface of T. imperialis (Theclinae: Lycaenidae). (d) Lysandra coridon (Polyommatinae: Lycaenidae). SEM showing a transverse view of the cover scale from the blue region of the DHW. (e) Thecla coronata (Theclinae: Lycaenidae). SEM of the cover scale from the blue region of the DHW. (f) Thecla imperialis (Theclinae: Lycaenidae). SEM of the cover scale from the blue region of the DHW. (g) Transverse section of the cover scale from the green dorsal wing of T. imperialis (Papilioninae: Papilionidae). (h,i) Hypochrysops polycletus (Theclinae: Lycaenidae). Cover scales from the blue region of the DHW. (h) SEM showing a plan view of a scale with ‘stripe’. (i) Transverse section, arrow indicates a concavity. (j,l) Euptychia cephus (Satyrinae: Nymphalidae). Cover scales from the blue region of the DHW. (j) Plan view of perforations. (k) Transverse view indicating perforated layers. (l) Dorsal view of a matt blue male.
Monocrystalline structures have conversely been identified exclusively from the dorsal wing surfaces, where they produce brightly iridescent blues and greens for the purpose of intraspecific communication (Wiklund 2003; Ingram in press; figure 11c). For example, in C. remus (Kertész et al. 2006b), Polyommatus bellargus (Vértesy et al. 2004), Polyommatus icarus (Moss & Gibbs 1997; Vértesy et al. 2004), Lysandra coridon Poda 1761 (Polyommatinae; figure 11d), Thecla coronata Draudt 1919 (figure 11e) and Thecla imperialis Draudt 1919 (Theclinae: Lycaenidae; Huxley's collection; Tilley 1988; figure 11f). The green dorsal cover scales of Teinopalpus imperialis (Papilioninae: Papilionidae) also possess a monocrystalline face-centred cubic structure (Argyros et al. 2002; figure 11g). This species is so far unique in containing the largest volume of crystalline structure we have encountered in a butterfly scale: the effect is to produce a diffuse reflection appearing matt green.
Huxley's collection contains two previously unstudied species, the dorsal blue wings of which contain a monocrystalline photonic structure (Ingram et al. submitted). A plan view of the cover scales of Hypochrysops polycletus Linnaeus 1758 (Theclinae: Lycaenidae) reveals novel transverse ‘stripes’ over the surface (figure 11h). The transverse sections of these scales showed that the stripes are the result of regular undulations in the surface of the crystalline structure (figure 11i). The effects of these undulations on the resultant reflection are currently under investigation (Ingram et al. submitted). The crystalline structure of a second species, the nymphalid, Euptychia cephus Fabricius 1775 (Satyrinae: Nymphalidae), appears different from that of H. polycletus, in that there are a number of perforated layers, rather than a lattice (figure 11j,k). The structure is reminiscent of the ‘pepper pot’ photonic crystal identified in the dorsal blue regions of male Polyommatus daphnis (Denis & Schifferemller 1775; Biró et al. 2003). In E. cephus, there may be increasing disorder in the crystal, however, leading to relatively less coherent scattering, since this species appears matt blue (figure 11l) compared with P. daphnis, which is strongly iridescent.
5. The evolution of photonic scale structures
There has been little research into the evolution of butterfly photonic structures. However, recently two studies were published which had conflicting outcomes. The reason for this lay with different assumptions about the relative contributions of genetic material to the expression, and therefore evolution, of photonic scale structures. The first study by Wickham et al. (2006) provided weak evidence7 to suggest that as simple optical structures, multilayer reflectors give rise to increasingly complex nano-architectures. This is through gradual optical refinement over time due to incremental changes in the genome. This theory presupposes that the evolution of simple reflectors is fully controlled by the random mutations of genes, which code for individual photonic structures. Wickham et al. (2006) based their conclusions on an examination of multilayer reflectors in 10 unrelated species from the Nymphalidae and Papilionidae. Three types of reflector were recognized: untilted scute; untilted flute; and tilted scute (see previously figure 1f for the first two types and figure 3(b–e) for the third type). Simple and Dollo parsimony analyses showed that the untilted scute reflector (equivalent to a basic multilayer) was always at the base of the tree, suggesting that this represented the least-derived character state. The flute- and tilted scute multilayer structures evolved later, suggesting that they represent a more refined version of the untilted scute structure with a greater angle dependence and exaggeration of colour. These examples represent optical refinement for a specific optical function (assuming that this is the primary purpose of these nanostructures).
Wickham et al. (2006) had followed a model where optical reflectors had been demonstrated to evolve by increasing complexity and optical efficiency in ostracods (Crustacea; Parker 1995). However, in this case, the reflectors are formed by multiple cells contributing to the same optical structure, and optical improvements probably arose due to incremental changes in the genome, which led directly to incremental changes in chitin deposition. In butterflies, however, developmental studies have shown that individual scales (optical devices) originate from single cells (Overton 1966).
Gradual increases in complexity over time are in line with traditional evolutionary theory and are based on the supposition that the genotype is proportional to the phenotype in the case of structural colours—multiple genes producing the complex scale architectures composed of multiple photonic structures, which give rise to different colours and effects. Indeed, something that emerges from Huxley's collection of electron micrographs is that the morphological plasticity of nanostructure is matched by that of the hues and intensities of colours and the visual effects produced by these simple photonic structures. However, there is an alternative explanation for how such architectural variation on the nanoscale may evolve.
In contrast to the conclusions of Wickham et al.'s (2006) study, evidence from developmental observations suggests that control over the expression and evolution of simple and also complex photonic scale structures is not entirely genetic but may also involve self-assembly—the formation of a structure from components without the aid of enzymes, independent of the structure—and mechanical processes such as buckling, cracking or splitting (Ghiradella 1974, 1989, 1991, 1994; Ghiradella & Radigan 1976; Parker 2006). Here, further formation of the structure's architecture takes place after protein biosynthesis from the ribonucleic acid.
Developmental studies of lepidopteran scales were first reported in the 1960s (e.g. Paweletz & Schlote 1964; Overton 1966; Greenstein 1972). From this work, we know that basic (unspecialized) scales develop from scale cells, which extrude the cell membrane to form a sac—the rudimentary scale. Bundles of microfilaments assemble longitudinally around the inner surface of the cell membrane, intermittently forming regions of close contact with the membrane. Epicuticle is then extracellularly secreted onto the cell membrane. The longitudinal ridges subsequently develop between the microfilament bundles, which appear to behave as spacers. Procuticle is then deposited inside the epicuticle onto the ridges, leaving the regions between without the cuticle, which will later form windows into the lumen. Finally, the cell membrane withdraws and eventually dies back completely to leave the scale. Specialized scale photonic structures are then elaborations on this basic framework.
Ghiradella and her colleagues were the first to realize the potential importance of mechanical processes in photonic structural development, in studies of the simple multilayered UV-reflective scales of C. eurytheme (Pieridae) males (Ghiradella 1974, 1989; Ghiradella & Radigan 1976). They suggested that the scutes were formed by a combination of elastic buckling of the ridge cuticle and tension exerted through contraction of the microfilaments (part of the cytoskeleton of the cell).
Later, self-assembly was also suggested by Ghiradella (1989) to be involved in the formation of basic multilayer reflectors. Studies of C. ladon (Lycaenidae) revealed that the contents of scale cells were arranged into pockets, between which ran fibrils and tubules of nascent cuticle, which appeared to be aligning themselves into stacks upon the ventral scale lamina. The fibrils and tubules eventually assembled to form the layers and spacers, separating adjacent layers.
Self-assembly has also been observed to occur with the addition of intracellular components of individual scale cells acting as templates (Ghiradella 1989). This was reported during the development of complex three-dimensional polycrystalline structures (Ghiradella 1989) in Mitoura grynea (Lycaenidae). Tubular units were observed within the cytosol, which consisted of a membranous sleeve surrounding the nascent cuticle, termed membrane cuticle (MC) units. The sleeves appeared to be invaginations of the plasma membrane and therefore continuous extracellularly. These MC units self-assembled into clumps, packed in a face-centred cubic configuration, forming crystallites of the final lattice. Within each crystallite, the MC units enclosed tubules of smooth endoplasmic reticulum (SER), which was acting as a cuticular template. After approximately 2 days, the SER and the rest of the cell died back, leaving the completed lattice. Ghiradella (1989) concluded from these observations that the three-dimensional lattice development was achieved by a combination of self-assembly of the MC units and SER templates. Although some aspects of the self-assembly templating and cracking and splitting processes will be under genetic control, the genotype does not directly determine the phenotype and hence we should not expect to find evolution via gradualism (Parker 2006).
Parker (2006) recently highlighted the similarities between photonic crystals (‘complex’ optical devices) across taxa and even kingdoms, showing that there are just four basic types (two-dimensional periodic stacks of (i) solid rods or (ii) hollow tubes (Parker et al. 2001; Zi et al. 2003) and three-dimensional (iii) opal or (iv) inverse opal (see Parker et al. 2003; Biró et al. 2003). All are morphologically very similar, consisting of components that are circular in cross section, though each is of variable dimensions. This suggests that intracellular structures, common to the general eukaryotic cell, may be employed in the manufacturing process (Parker 2006), supporting the hypothesis proposed by Ghiradella (1989). It also suggests that the highly complex inverse opal-type structures could appear ‘suddenly’ in evolutionary time (without having to evolve stepwise). Developmental studies will prove crucial to this work in the future, having already shown that intracellular components of individual scale cells act as templates for some photonic structures (Overton 1966; Ghiradella 1974).
Huxley's collection reinforces Parker's (2006) hypothesis, in that the inverse opal-type three-dimensional photonic crystals can be found in species of the butterfly orders Lycaenidae and the Papilionidae (Papilionidae) and also in the moths (Microlepidoptera). In comparing the closest living relatives of these species, no traces of potential ‘intermediate stages’, or phenotypic steps towards the inverse opal structure, were evident. Although this subject requires more attention, the evidence for intracellular-structure-assisted evolution of butterfly scale reflectors is strong.
6. Conclusions
Photonic structures in nature have attracted much attention recently due to their potential for applications in technology—none more so than those of butterflies, since this group displays the widest diversity of structures, resultant colours and visual effects of any living organism. The literature describing butterfly photonics is equally diverse and it is hoped that this review will provide a useful reference tool, bringing together research that has emerged over the last 40 years with ongoing efforts in the field. Also included here for the first time is John Huxley's unpublished work on butterfly photonic structures, which forms the largest known collection of data on this subject. By incorporating this work, we have been able to extend our current understanding of the diversity of these optical structures. Furthermore, a number of questions have been raised, for example: why do butterflies predominantly reflect UV to green, when their visual systems are capable of perceiving a greater range of colours (Arikawa et al. 1987, 1999a,b; Arikawa & Stavenga 1997)? Are some photonic structures trade-off with aerodynamic and/or thermal devices? What is the effect of motion/time on the visual effect, and has motion been a selection pressure? In order to answer these and other questions, there is still a need for basic research to characterize butterfly photonic structures. However, instead of studying ‘random’ species of interest, it may now be more informative to concentrate our efforts on examining specific individuals, which are, for example, closely related or live in close proximity, to answer some of the overarching questions above.
Acknowledgments
Funding was provided by the European BioPhot (NEST) project (no. 12915). A.R.P. was supported by the Royal Society and the Australian Research Council. We thank P. Ackery and J. Chainey (NHM, London) for butterfly specimens. R. Huxley and D. Vane-Wright are thanked for historical information regarding John Huxley's work at the museum. R. H. Telling and R. Graves are also thanked for their helpful discussions of the manuscript. A. Ball is thanked for his assistance with scanning electron microscope imaging.
Endnotes
This review conforms to the terminology of Downey & Allyn (1975).
Where Huxley's original electron micrographs were of poor quality, the authors have retaken them to improve clarity.
Sometimes referred to as two-dimensional photonic crystals (Berthier 2007).
Multilayer reflectors may also be referred to as one-dimensional photonic crystals (Berthier et al. 2006).
Also known as lamellae (see Ghiradella 1998).
A summary of the results relating to a study of this species was given by Huxley (undated) in his notebooks held at the museum. The original data (spectra, etc.) did not, however, accompany them.
A significant and fundamental problem associated with the study was that the species examined are so distantly related as to render the analysis almost meaningless due to inherent variability.
References
- Allyn A.C, Downey J.C. Diffraction structures in the wing scales of Callophrys (Mitoura) siva siva (Lycaenidae) Bull. Allyn Museum. 1976;40:1–6. [Google Scholar]
- Anderson T.F, Richards A.G. An electron microscope study of some structural colours of insects. J. Appl. Phys. 1942;13:748–758. doi:10.1063/1.1714827 [Google Scholar]
- Argyros A, Manos S, Large M.C.J, McKenzie D.R, Cox G.C, Dwarte D.M. Electron tomography and computer visualisation of a three-dimensional ’photonic’ crystal in a butterfly wing-scale. Micron. 2002;33:483–487. doi: 10.1016/s0968-4328(01)00044-0. doi:10.1016/S0968-4328(01)00044-0 [DOI] [PubMed] [Google Scholar]
- Arikawa K, Stavenga D.G. Random array of colour filters in the eyes of butterflies. J. Exp. Biol. 1997;200:2501–2506. doi: 10.1242/jeb.200.19.2501. [DOI] [PubMed] [Google Scholar]
- Arikawa K, Inokuma K, Eguchi E. Pentachromatic visual system in a butterfly. Naturwissenschaften. 1987;74:297–298. doi:10.1007/BF00366422 [Google Scholar]
- Arikawa K, Mizuno S, Scholten D.G.W, Kinoshita M, Seki T, Kitamoto J, Stavenga D.G. An ultraviolet-absorbing pigment causes a narrow-band violet receptor and a single-peaked green receptor in the eye of the butterfly Papilio. Vis. Res. 1999a;39:1–8. doi: 10.1016/s0042-6989(98)00070-4. doi:10.1016/S0042-6989(98)00070-4 [DOI] [PubMed] [Google Scholar]
- Arikawa K, Scholten D.G.W, Kinoshita M, Stavenga D.G. Tuning of photoreceptor spectral sensitivities by red and yellow pigments in the butterfly Papilio xuthus. Zool. Sci. 1999b;16:17–24. doi:10.2108/zsj.16.17 [Google Scholar]
- Bálint Z, Vértesy Z, Biró L.P. Microstructures and nanostructures of high Andean Penaincisalia lycaenid butterfly scales (Lepidoptera: Lycaenidae): descriptions and interpretations. J. Nat. Hist. 2005;39:2935–2952. doi:10.1080/00222930500140629 [Google Scholar]
- Berthier S. Thermoregulation and spectral selectivity of the tropical butterfly Prepona meander: a remarkable example of temperature auto-regulation. Appl. Phys. A. 2005;80:1397–1400. doi:10.1007/s00339-004-3185-x [Google Scholar]
- Berthier S.Iridescences: The Physical Colors of Insects2007Springer; New York [Google Scholar]
- Berthier S, Charron E, Da Silva A. Determination of the cuticle index of the scales of the iridescent butterfly Morpho menelaus. Optics Commun. 2003;228:349–356. doi:10.1016/j.optcom.2003.10.032 [Google Scholar]
- Berthier S, Charron E, Boulenguez J. Morphological structure and optical properties of the wings of Morphidae. Insect Sci. 2006;13:145–157. doi:10.1111/j.1744-7917.2006.00077.x [Google Scholar]
- Bingham L, Bingham I, Geary S, Tanner J, Driscoll C, Cluff B, Gardener J.S. SEM comparison of Morpho butterfly dorsal and ventral scales. Microsc. Res. Tech. 1995;31:93–94. doi: 10.1002/jemt.1070310108. doi:10.1002/jemt.1070310108 [DOI] [PubMed] [Google Scholar]
- Biró L.P, et al. Role of photonic crystal-type structures in the thermal regulation of a lycaenid butterfly sister species pair. Phys. Rev. E. 2003;67:0 219 071–0 219 077. doi: 10.1103/PhysRevE.67.021907. doi:10.1103/PhysRevE.67.021907 [DOI] [PubMed] [Google Scholar]
- Brink D.J, Lee M.E. Ellipsometry of diffractive insect reflectors. Appl. Opt. 1996;35:1950–1955. doi: 10.1364/AO.35.001950. [DOI] [PubMed] [Google Scholar]
- Brink D.J, Smit J.E, Lee M.E, Moller A. Optical diffraction by the microstructure of the wing of a moth. Appl. Opt. 1995;34:6049–6057. doi: 10.1364/AO.34.006049. [DOI] [PubMed] [Google Scholar]
- Darwin C. The sexual colours of certain butterflies. Nature. 1880;8:237. [Google Scholar]
- Denton E.J, Land M.F. Mechanism of reflexion in silvery layers of fish and cephalopods. Proc. R. Soc. A. 1971;178:43–61. doi: 10.1098/rspb.1971.0051. [DOI] [PubMed] [Google Scholar]
- Denton E.J, Nichol J.A.C. Studies on reflexion of light from silvery surfaces of fishes, with special reference to the bleak, Alburnus alburnus. J. Mar. Biol. Assoc. UK. 1965;45:683–702. [Google Scholar]
- Denton E.J, Nichol J.A.C. A survey of reflectivity in silvery teleosts. J. Mar. Biol. Assoc. UK. 1966;46:685–722. [Google Scholar]
- Downey J.C, Allyn A.C. Wing-scale morphology and nomenclature. Bull. Allyn Museum. 1975;31:1–32. [Google Scholar]
- Gentil K. Elektronenmikrosckopische Unetersuchung des Feinbaues schillernder Leisten von Morpho-Schuppen. Z. Morphol. Okol. Tiere. 1942;38:344. doi:10.1007/BF01434120 [Google Scholar]
- Ghiradella H. Development of ultraviolet-reflecting butterfly scales: how to make an interference filter. J. Morphol. 1974;142:395–410. doi: 10.1002/jmor.1051420404. doi:10.1002/jmor.1051420404 [DOI] [PubMed] [Google Scholar]
- Ghiradella H. Structure of iridescent lepidopteran scales—variations on several themes. Ann. Entomol. Soc. Am. 1984;77:637–645. [Google Scholar]
- Ghiradella H. Structure and development of iridescent Lepidopteran scales—the Papilionidae as a showcase family. Ann. Entomol. Soc. Am. 1985;78:252–264. [Google Scholar]
- Ghiradella H. Structure and development of iridescent butterfly scales—lattices and laminae. J. Morphol. 1989;202:69–88. doi: 10.1002/jmor.1052020106. doi:10.1002/jmor.1052020106 [DOI] [PubMed] [Google Scholar]
- Ghiradella H. Light and color on the wing—structural colors in butterflies and moths. Appl. Opt. 1991;30:3492–3500. doi: 10.1364/AO.30.003492. [DOI] [PubMed] [Google Scholar]
- Ghiradella H. Structure of butterfly scales—patterning in an insect cuticle. Microsc. Res. Tech. 1994;27:429–438. doi: 10.1002/jemt.1070270509. doi:10.1002/jemt.1070270509 [DOI] [PubMed] [Google Scholar]
- Ghiradella H. Hairs, bristles and scales. In: Locke M, editor. Microscopic anatomy of invertebrates. Wiley–Liss Publishers; New York, NY: 1998. pp. 257–287. [Google Scholar]
- Ghiradella H, Radigan W. Development of butterfly scales. II. Struts, lattices and surface tension. J. Morphol. 1976;150:279–298. doi: 10.1002/jmor.1051500202. doi:10.1002/jmor.1051500202 [DOI] [PubMed] [Google Scholar]
- Ghiradella H, Aneshansley D, Eisner T, Silberglied R.E, Hinton H.E. Ultraviolet reflection of a male butterfly : interference color caused by thin-layer elaboration of wing scales. Science. 1972;178:1214–1217. doi: 10.1126/science.178.4066.1214. doi:10.1126/science.178.4066.1214 [DOI] [PubMed] [Google Scholar]
- Gralak B, Tayeb G, Enoch S. Morpho butterflies wings color modeled with lamellar grating theory. Opt. Express. 2001;9:567–578. doi: 10.1364/oe.9.000567. [DOI] [PubMed] [Google Scholar]
- Greenstein M.E. The ultrastructure of developing wings in the giant silkmoth, Hyalophora cecropia. I. Generalized epidermal cells. J. Morphol. 1972;136:1–22. doi: 10.1002/jmor.1051360102. doi:10.1002/jmor.1051360102 [DOI] [PubMed] [Google Scholar]
- Huxley J. The basis of structural colour variation in two species of Papilio. J. Entomol. (A) 1975;50:9–22. [Google Scholar]
- Huxley J. The colouration of Papilio zalmoxis and P. antimachus and the discovery of Tyndall blue in butterflies. Proc. R. Soc. B. 1976;193:441–453. doi:10.1098/rspb.1976.0056 [Google Scholar]
- Huxley J, Barnard P.C. Wing scales of Pseudoleptocerus chirindensis Kimmins (Trichoptera, Leptoceridae) Zool. J. Linn. Soc. 1988;92:285–312. [Google Scholar]
- Ingram, A. L. In press. Butterfly photonics. In Functional surfaces in biology (ed. S. Gorb). New York, NY: Springer.
- Ingram, A. L., Vigneron, J. P., Parker, A. R. & Lousse, V. Accepted. Dual gratings interspersed on a single butterfly scale. J. R. Soc. Interface [DOI] [PMC free article] [PubMed]
- Ingram, A. L., Vigneron, J. P., Parker, A. R. & Lousse, V. In press. Blazed gratings on a butterfly scale produce novel iridescence patterns. Phys. Rev. E
- Ingram, A. L., Lousse, V. Vigneron, J. P. & Parker, A. R. Submitted. Structural origin of the blue iridescence on the wings of Hypochrysops polycletus
- Jenkins F.A, White H.E. Fundamentals of optics. McGraw-Hill International Editions; Singapore: 1981. [Google Scholar]
- Jones R.W, Tilley R.J.D. Colour, ultraviolet reflectivity and iridescent scale structure in Quercusia quercus (1.,1758) and Laeosopis roboris (Esper, 1793) (Lepidoptera: Lycaenidae) Entomol. Gaz. 1999;50:181–187. [Google Scholar]
- Kertész K, Bálint Z, Vértesy Z, Mark G.I, Lousse V, Vigneron J.P, Biró L.P. Photonic crystal type structures of biological origin: structural and spectral characterization. Curr. Appl. Phys. 2006a;6:252–258. doi:10.1016/j.cap.2005.07.051 [Google Scholar]
- Kertész K, Bálint Z, Vértesy Z, Mark G.I, Lousse V, Vigneron J.P, Rassart M, Biró L.P. Gleaming and dull surface textures from photonic crystal type nanostructures in the butterfly Cyanophrys remus. Phys. Rev. E. 2006b;74:15. doi: 10.1103/PhysRevE.74.021922. doi:10.1103/PhysRevE.74.021922 [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Yoshioka S. Structural colors in nature: the role of regularity and irregularity in the structure. ChemPhysChem. 2005;6:1442–1459. doi: 10.1002/cphc.200500007. doi:10.1002/cphc.200500007 [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Yoshioka S, Kawagoe K. Mechanisms of structural colour in the Morpho butterfly: cooperation of regularity and irregularity in an iridescent scale. Proc. R. Soc. B. 2002;269:1417–1421. doi: 10.1098/rspb.2002.2019. doi:10.1098/rspb.2002.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M.F. The physics and biology of animal reflectors. Prog. Biophys. Mol. Biol. 1972;24:75–106. doi: 10.1016/0079-6107(72)90004-1. doi:10.1016/0079-6107(72)90004-1 [DOI] [PubMed] [Google Scholar]
- Lawrence C, Vukusic P, Sambles R. Grazing-incidence iridescence from a butterfly wing. Appl. Opt. 2002;41:437–441. doi: 10.1364/ao.41.000437. doi:10.1364/AO.41.000437 [DOI] [PubMed] [Google Scholar]
- Li Y.H, Lu Z.H, Yin H.W, Yu X.D, Liu X.H, Zi J. Structural origin of the brown color of barbules in male peacock tail feathers. Phys. Rev. E. 2005;72:010902. doi: 10.1103/PhysRevE.72.010902. doi:10.1103/PhysRevE.72.010902 [DOI] [PubMed] [Google Scholar]
- Lippert W, Gentil K. Elektronenmikroskopische studien uber micellare strukturen bei schmetterlingsschuppen vom Morpho-typ. Z. wiss. Mikr. 1951;61:95–100. [Google Scholar]
- Lippert W, Gentil K. Uber lamellare feinstrukturen bei den schillerschuppen der schmetterlinge vom Urania und Morpho-typ. Z. Morphol. Okol. Tiere. 1959;48:115–122. doi:10.1007/BF00407836 [Google Scholar]
- Mason C.W. Structural colors of feathers. Int. J. Phys. Chem. 1923;27:201–251. doi:10.1021/j150228a001 [Google Scholar]
- Mason C.W. Structural colors in insects. I. Int. J. Phys. Chem. 1926;30:383–395. doi:10.1021/j150261a009 [Google Scholar]
- Mason C.W. Structural colors in insects. II. Int. J. Phys. Chem. 1927a;31:321–354. doi:10.1021/j150273a001 [Google Scholar]
- Mason C.W. Structural colors in insects. III. Int. J. Phys. Chem. 1927b;31:1856–1872. doi:10.1021/j150282a008 [Google Scholar]
- Mayer A.G. On the color and color-patterns of moths and butterflies. Bull. Museum Comp. Zool. 1896;30:169–256. [Google Scholar]
- Morris R.B. Iridescence from diffraction structures in the wing scales of Callophrys rubi, the Green Hairstreak. J. Entomol. 1975;49:149–154. [Google Scholar]
- Moss M.O, Gibbs G. The wing scales of the common blue butterfly. Quekett J. Microsc. 1997;38:49–55. [Google Scholar]
- Newton, I. 1704 Opticks London, UK: The Royal Society.
- Onslow H. On a periodic structure in many insect scales, and the cause of their iridescent colours. Phil. Trans. R. Soc. B. 1921;211:1–74. doi:10.1098/rstb.1923.0001 [Google Scholar]
- Overton J. Microtubules and microfibrils in morphogenesis of the scale cells of Ephestia kuhniella. J. Cell Biol. 1966;29:293–305. doi: 10.1083/jcb.29.2.293. doi:10.1083/jcb.29.2.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A.R. Discovery of functional iridescence and its coevolution with eyes in the phylogeny of Ostracoda (Crustacea) Proc. R. Soc. B. 1995;262:349–355. doi:10.1098/rspb.1995.0216 [Google Scholar]
- Parker A.R. Conservative photonic crystals imply indirect transcription from genotype to phenotype. Recent Res. Dev. Entomol. 2006;5:1–10. [Google Scholar]
- Parker A.R, Hegedus Z. Diffractive optics in spiders. J. Opt. A: Pure Appl. Opt. 2003;5:S111–S116. doi:10.1088/1464-4258/5/4/364 [Google Scholar]
- Parker A.R, Townley H.E. Biomimetics of photonic nanostructures. Nat. Nanotechnol. 2007;2:347–353. doi: 10.1038/nnano.2007.152. doi:10.1038/nnano.2007.152 [DOI] [PubMed] [Google Scholar]
- Parker A.R, McKenzie D.R, Ahyong S.T. A unique form of light reflector and the evolution of signalling in Ovalipes (Crustacea: Decapoda: Portunidae) Proc. R. Soc. B. 1998;265:861–867. doi:10.1098/rspb.1998.0371 [Google Scholar]
- Parker A.R, McPhedran R.C, McKenzie D.R, Botten L.C, Nicorovici N.A.P. Photonic engineering—Aphrodite's iridescence. Nature. 2001;409:36–37. doi: 10.1038/35051168. doi:10.1038/35051168 [DOI] [PubMed] [Google Scholar]
- Parker A.R, Welch V.L, Driver D, Martini N. Structural colour—opal analogue discovered in a weevil. Nature. 2003;426:786–787. doi: 10.1038/426786a. doi:10.1038/426786a [DOI] [PubMed] [Google Scholar]
- Paweletz N, Schlote F.-W. Die entwicklung der schmetterlingsschuppe bei Ephestia kuhniella zeller. Zeitschrift fur Zellforschung. 1964;63:840–870. doi:10.1007/BF00336225 [PubMed] [Google Scholar]
- Plattner L. Optical properties of the scales of Morpho rhetenor butterflies: theoretical and experimental investigation of the back-scattering of light in the visible spectrum. J. R. Soc. Interface. 2004;1:49–59. doi: 10.1098/rsif.2004.0006. doi:10.1098/rsif.2004.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prum R.O, Torres R, Williamson S, Dyck J. Two-dimensional Fourier analysis of the spongy medullary keratin of structurally coloured feather barbs. Proc. R. Soc. B. 1999;266:13–22. doi:10.1098/rspb.1999.0598 [Google Scholar]
- Prum R.O, Quinn T, Torres R.H. Anatomically diverse butterfly scales all produce structural colours by coherent scattering. J. Exp. Biol. 2006;209:748–765. doi: 10.1242/jeb.02051. doi:10.1242/jeb.02051 [DOI] [PubMed] [Google Scholar]
- Rutowski R.L. Sexual discrimination using visual cues in the checkered white butterfly (Pieris protidice) Z. Tierpsychol. 1981;55:325–334. [Google Scholar]
- Saito A, Yoshioka S, Kinoshita S. Reproduction of the Morpho butterfly's blue: arbitration of contradicting factors. In: Chen P.T.C, Fleming J.C, Dittman M.G, editors. Optical systems degradation, contamination and stray light: effects, measurements and control. SPIE; Bellingham, WA: 2004. pp. 188–194. [Google Scholar]
- Silberglied R.E, Taylor O.R. Ultraviolet differences between the sulphur butterflies, Colias eurytheme and C. philodice, and a possible isolating mechanism. Nature. 1973;241:406–408. doi: 10.1038/241406a0. doi:10.1038/241406a0 [DOI] [PubMed] [Google Scholar]
- Stavenga D.G, Stowe S, Siebke K, Zeil J, Arikawa K. Butterfly wing colours: scale beads make white pierid wings brighter. Proc. R. Soc. B. 2004;271:1577–1584. doi: 10.1098/rspb.2004.2781. doi:10.1098/rspb.2004.2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffert F. Morphologie und optik der schmetterlingsschuppen. Z. Morphol. Okol. Tierre. 1924;1:171–308. doi:10.1007/BF00403572 [Google Scholar]
- Suffert F. Elektronenmikroskopische untersuchung des feinbaues schillernder leisten von Morpho-schuppen. Zeitschr. Morphol. Okol. Tierre. 1942;38:344–355. doi:10.1007/BF01434120 [Google Scholar]
- Tabata H, Kumazawa K, Funakawa M, Takimoto J, Akimoto M. Microstructures and optical properties of scales of butterfly wings. Opt. Rev. 1996;3:139–145. doi:10.1007/s10043-996-0139-x [Google Scholar]
- Tada H, Mann S.E, Miaoulis I.N, Wong P.Y. Effects of a butterfly scale microstructure on the iridescent color observed at different angles. Appl. Opt. 1998;37:1579–1584. doi: 10.1364/ao.37.001579. [DOI] [PubMed] [Google Scholar]
- Tilley R.J.D. Scale structure and blue colour in the chalkhill blue butterfly, Lysandra coridon (Poda): (Lepidoptera: Lycaenidae) Entomologist. 1988;107:82–89. [Google Scholar]
- Tilley R.J.D. Further considerations of the colour of the green scales on the underside of the wings of the butterflies Callophrys rubi (Linnaeus, 1758) and C. avis (Chapman, 1909) Entomol. Gazette. 2000;51:191–193. [Google Scholar]
- Vértesy Z, Bálint Z, Kertesz K, Mehn D, Kiricsi I, Lousse V, Vigneron J.-P, Biró L.P. Modifications to wing scale microstructures in lycaenid butterflies. Microsc. Anal. 2004;18:25–27. [Google Scholar]
- Vigneron J.P, Colomer J.F, Vigneron N, Lousse V. Natural layer-by-layer photonic structure in the squamae of Hoplia coerulea (Coleoptera) Phys. Rev. E. 2005;72:061 904. doi: 10.1103/PhysRevE.72.061904. doi:10.1103/PhysRevE.72.061904 [DOI] [PubMed] [Google Scholar]
- Vigneron J.P, Colomer J.F, Rassart M, Ingram A.L, Lousse V. Structural origin of the colored reflections from the black-billed magpie feathers. Phys. Rev. E. 2006;73:021 914. doi: 10.1103/PhysRevE.73.021914. doi:10.1103/PhysRevE.73.021914 [DOI] [PubMed] [Google Scholar]
- Vukusic P, Hooper I. Directionally controlled fluorescence emission in butterflies. Science. 2005;310:1151. doi: 10.1126/science.1116612. doi:10.1126/science.1116612 [DOI] [PubMed] [Google Scholar]
- Vukusic P, Sambles J.R, Lawrence C.R, Wootton R.J. Quantified interference and diffraction in single Morpho butterfly scales. Proc. R. Soc. B. 1999;266:1403–1411. doi:10.1098/rspb.1999.0794 [Google Scholar]
- Vukusic P, Sambles J.R, Lawrence C.R. Structural colour—colour mixing in wing scales of a butterfly. Nature. 2000;404:457. doi: 10.1038/35006561. doi:10.1038/35006561 [DOI] [PubMed] [Google Scholar]
- Vukusic P, Sambles J.R, Lawrence C.R, Wootton R.J. Limited-view iridescence in the butterfly Ancyluris meliboeus. Proc. R. Soc. B. 2002;269:7–14. doi: 10.1098/rspb.2001.1836. doi:10.1098/rspb.2001.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukusic P, Sambles J.R, Lawrence C.R. Structurally assisted blackness in butterfly scales. Proc. R. Soc. B. 2004;271:S237–S239. doi: 10.1098/rsbl.2003.0150. doi:10.1098/rsbl.2003.0150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch V.L. Photonic crystals in biology. In: Kinoshita S, Yoshioka S, editors. Structural colors in biological systems. Osaka University Press; Osaka, Japan: 2005. pp. 53–71. [Google Scholar]
- Welch V.L, Vigneron J.P. Beyond butterflies-the diversity of biological photonic crystals. Opt. Quant. Electron. 2007;39:295–303. doi:10.1007/s11082-007-9094-4 [Google Scholar]
- Welch V.L, Vigneron J.P, Parker A.R. The cause of colouration in the ctenophore Beroe cucumis. Curr. Biol. 2005;15:R985–R986. doi: 10.1016/j.cub.2005.11.060. doi:10.1016/j.cub.2005.11.060 [DOI] [PubMed] [Google Scholar]
- Welch V, Vigneron J.P, Lousse V, Parker A. Optical properties of the iridescent organ of the comb-jellyfish Beroe cucumis (Ctenophora) Phys. Rev. E. 2006;73:0 419 161–0 419 167. doi: 10.1103/PhysRevE.73.041916. doi:10.1103/PhysRevE.73.041916 [DOI] [PubMed] [Google Scholar]
- Wickham S, Large M.C.J, Poladian L, Jermiin L. Exaggeration and suppression of iridescence: the evolution of two-dimensional butterfly structural colours. J. R. Soc. Interface. 2006;3:99–109. doi: 10.1098/rsif.2005.0071. doi:10.1098/rsif.2005.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund C. Sexual selection and the evolution of butterfly mating systems. In: Boggs C.L, Watt W.B, Ehrlich P.R, editors. Butterflies: ecology and evolution taking flight. The University of Chicago Press; Chicago, IL: 2003. pp. 67–90. [Google Scholar]
- Yablonovitch E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. E. 1987;58:2059–2062. doi: 10.1103/PhysRevLett.58.2059. doi:10.1103/PhysRevLett.58.2059 [DOI] [PubMed] [Google Scholar]
- Yablonovitch E. Liquid versus photonic crystals. Nature. 1999;401:539–541. doi:10.1038/44038 [Google Scholar]
- Yoshioka S, Kinoshita S. Wavelength-selective and anisotropic light-diffusing scale on the wing of the Morpho butterfly. Proc. R. Soc. B. 2004;271:581–587. doi: 10.1098/rspb.2003.2618. doi:10.1098/rspb.2003.2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka S, Kinoshita S. Single-scale spectroscopy of structurally colored butterflies: measurements of quantified reflectance and transmittance. J. Opt. Soc. Am. A: Opt. Image Sci. Vis. 2006;23:134–141. doi: 10.1364/josaa.23.000134. doi:10.1364/JOSAA.23.000134 [DOI] [PubMed] [Google Scholar]
- Yoshioka S, Kinoshita S. Polarization-sensitive color mixing in the wing of the Madagascan sunset moth. Opt. Express. 2007;15:2691–2701. doi: 10.1364/oe.15.002691. doi:10.1364/OE.15.002691 [DOI] [PubMed] [Google Scholar]
- Zi J, Yu X, Li Y, Hu X, Xu C, Wang X, Lui X, Fu M. Colouration strategies in peacock feathers. Proc. Natl Acad. Sci. USA. 2003;100:12 576–12 578. doi: 10.1073/pnas.2133313100. doi:10.1073/pnas.2133313100 [DOI] [PMC free article] [PubMed] [Google Scholar]