Abstract
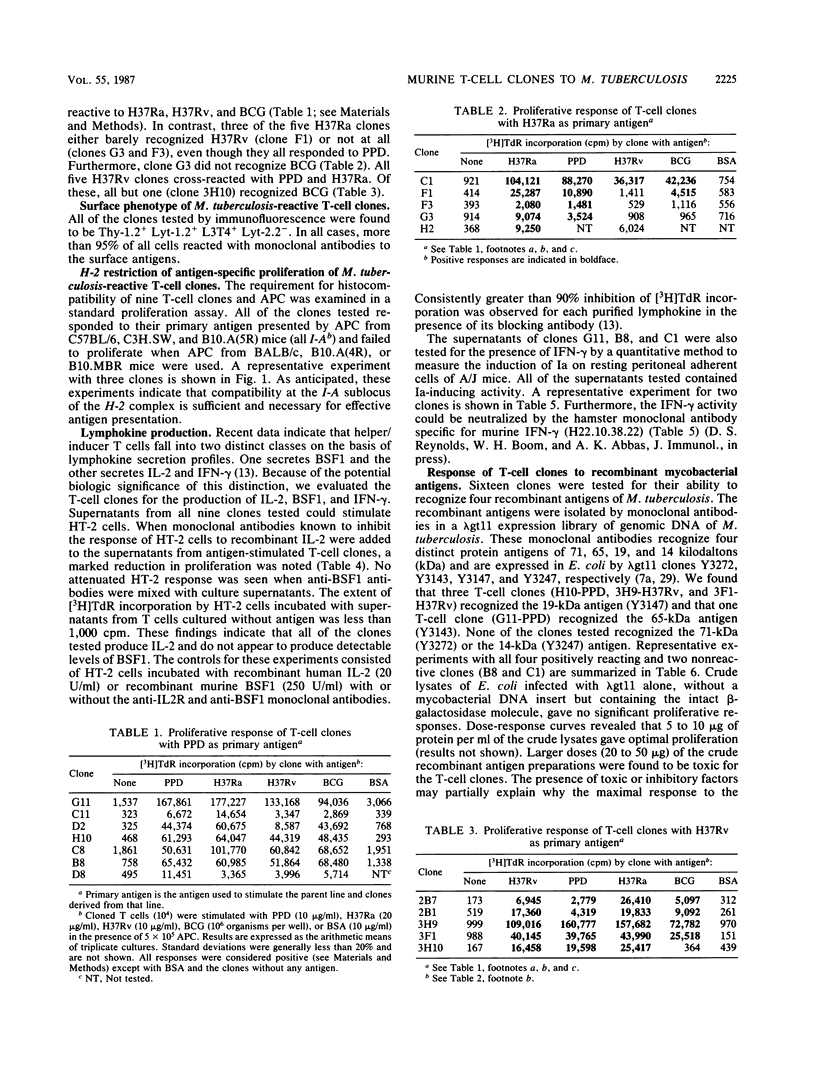
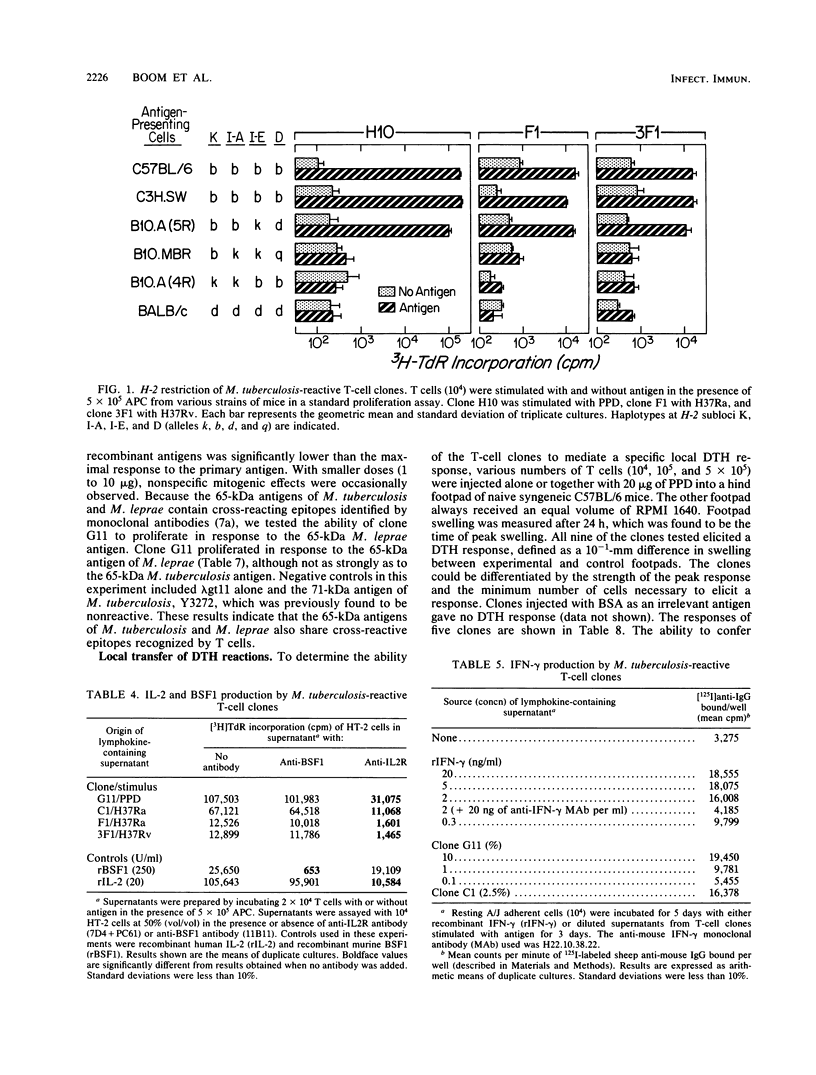
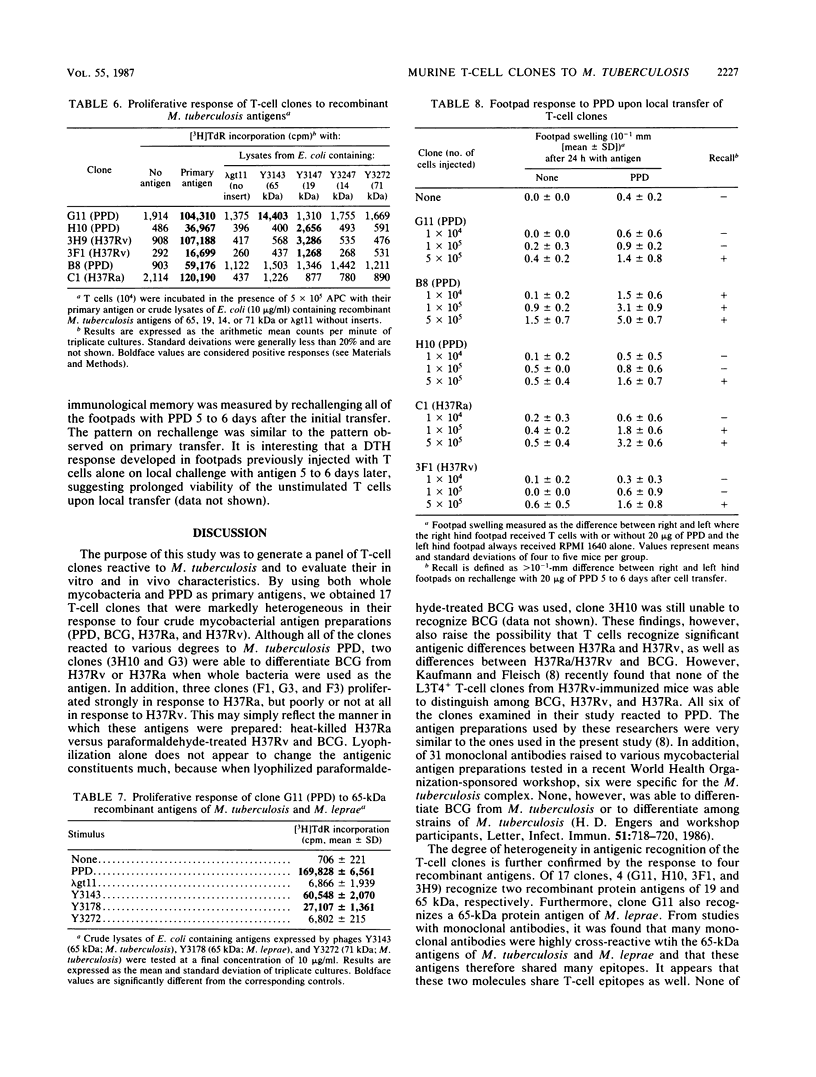
Seventeen helper T-cell clones were derived by stimulating lymph node cells from sensitized C57BL/6 mice with Mycobacterium tuberculosis H37Ra, M. tuberculosis H37Rv, or purified protein derivative. Most clones cross-reacted with Mycobacterium bovis BCG, H37Ra, H37Rv, and purified protein derivative. However, four clones were able to differentiate H37Rv from H37Ra, or BCG from H37Ra and H37Rv. In addition, four other T-cell clones recognized recombinant antigens of 19 and 65 kilodaltons isolated from a genomic expression library of M. tuberculosis by using monoclonal antibodies. All clones were Ia restricted and had the Thy-1.2+ Lyt-1+ L3T4+ Lyt-2- phenotype. On stimulation with antigen, all of the clones tested secreted interleukin-2 and gamma interferon but not B-cell stimulatory factor 1. All of the clones tested induced an antigen-specific delayed-type hypersensitivity response upon local cell transfer, although the magnitude of this response differed markedly among clones.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baily G. V. Tuberculosis prevention Trial, Madras. Indian J Med Res. 1980 Jul;72 (Suppl):1–74. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Drug-resistant tuberculosis among the homeless--Boston. MMWR Morb Mortal Wkly Rep. 1985 Jul 19;34(28):429–431. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Tuberculosis--United States, 1984. MMWR Morb Mortal Wkly Rep. 1985 May 31;34(21):299-302, 307. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Tuberculosis--United States, 1985--and the possible impact of human T-lymphotropic virus type III/lymphadenopathy-associated virus infection. MMWR Morb Mortal Wkly Rep. 1986 Feb 7;35(5):74–76. [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husson R. N., Young R. A. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1679–1683. doi: 10.1073/pnas.84.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Flesch I. Function and antigen recognition pattern of L3T4+ T-cell clones from Mycobacterium tuberculosis-immune mice. Infect Immun. 1986 Nov;54(2):291–296. doi: 10.1128/iai.54.2.291-296.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto M., Fathman C. G. Antigen-reactive T cell clones. I. Transcomplementing hybrid I-A-region gene products function effectively in antigen presentation. J Exp Med. 1980 Oct 1;152(4):759–770. doi: 10.1084/jem.152.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Immune response to Mycobacterium tuberculosis in rats. Infect Immun. 1973 Aug;8(2):182–189. doi: 10.1128/iai.8.2.182-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman A. H., Kurt-Jones E. A., Abbas A. K. B-cell stimulatory factor 1 and not interleukin 2 is the autocrine growth factor for some helper T lymphocytes. Proc Natl Acad Sci U S A. 1987 Feb;84(3):824–827. doi: 10.1073/pnas.84.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987 Jan 1;138(1):293–298. [PubMed] [Google Scholar]
- Pedrazzini T., Louis J. A. Functional analysis in vitro and in vivo of Mycobacterium bovis strain BCG-specific T cell clones. J Immunol. 1986 Mar 1;136(5):1828–1834. [PubMed] [Google Scholar]
- Pelletier M., Forget A., Bourassa D., Gros P., Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982 Nov;129(5):2179–2185. [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Failure of passive serum transfer of immunity against aerogenic tuberculosis in rabbits. Proc Soc Exp Biol Med. 1974 Jan;145(1):173–175. doi: 10.3181/00379727-145-37771. [DOI] [PubMed] [Google Scholar]
- Sheppard H. W., Scott P. A., Dwyer D. M. Recognition of Leishmania donovani antigens by murine T lymphocyte lines and clones. Species cross-reactivity, functional correlates of cell-mediated immunity, and antigen characterization. J Immunol. 1983 Sep;131(3):1496–1503. [PubMed] [Google Scholar]
- Stead W. W., Lofgren J. P., Warren E., Thomas C. Tuberculosis as an endemic and nosocomial infection among the elderly in nursing homes. N Engl J Med. 1985 Jun 6;312(23):1483–1487. doi: 10.1056/NEJM198506063122304. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Wittenberg G. F., Unanue E. R. Immune complex effects on murine macrophages. I. Immune complexes suppress interferon-gamma induction of Ia expression. J Immunol. 1985 Dec;135(6):3735–3743. [PubMed] [Google Scholar]
- Walker L., Lowrie D. B. Killing of Mycobacterium microti by immunologically activated macrophages. Nature. 1981 Sep 3;293(5827):69–71. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Mehra V., Sweetser D., Buchanan T., Clark-Curtiss J., Davis R. W., Bloom B. R. Genes for the major protein antigens of the leprosy parasite Mycobacterium leprae. Nature. 1985 Aug 1;316(6027):450–452. doi: 10.1038/316450a0. [DOI] [PubMed] [Google Scholar]



