Abstract
OBJECTIVE—The aim of this study was to examine the association of physical activity with glucose tolerance and resting energy expenditure (REE) among adolescents.
RESEARCH DESIGN AND METHODS—Subjects were 32 male and female adolescents aged 12–18 years. Intravenous glucose tolerance (Kg) and REE were assessed under inpatient conditions after an overnight fast. Kg was determined as the inverse slope of time versus (ln) glucose over minutes 8–19 of an intravenous glucose tolerance test. Physical activity was assessed over 8 days using accelerometry (counts per minute).
RESULTS—In multiple linear regression analysis, Kg was positively associated with total physical activity (TPA), moderate physical activity (MPA), and 5-min bouts of MPA. Similarly, REE was positively associated with TPA, MPA, and 5-min bouts of MPA.
CONCLUSIONS—In this population, physical activity was positively related to both glucose tolerance and REE. These results suggest that moderate activity may be beneficial in the prevention of diabetes in adolescent populations both through promoting efficient glucose disposal and through increasing energy expenditure.
Traditionally, type 2 diabetes has been considered a disease that primarily affects adults; however, in the last decade, there has been an increasing and alarming incidence of type 2 diabetes in adolescent populations. Although type 1 diabetes remains the prevailing form in teens in the U.S., the prevalence of type 2 diabetes is expected to be predominant in many ethnic groups within 10 years (1). The first large-scale, population-based study of diabetes in American youth, the SEARCH for Diabetes in Youth Study (SEARCH) found that in 2001, 3.5% of the 10- to 19-year-old study population had type 2 diabetes (2). Furthermore, the American Diabetes Association states that one in six overweight adolescents has pre-diabetes (3). The epidemic is imminent; escalating rates of diabetes are paralleling that of the epidemic of childhood obesity (4).
There is, however, convincing evidence in adults that increased physical activity can prevent or delay the development of type 2 diabetes. Large adult prevention trials such as the Diabetes Prevention Program (DPP) (5) and the Finnish Diabetes Prevention Group (6) showed that intensive lifestyle interventions, including exercise, were 58% more effective in retarding progression from impaired glucose tolerance (IGT) to diabetes than the control. Remarkably, lifestyle intervention in the DPP study also resulted in 39% less incidence of diabetes than pharmacological intervention (metformin) (5). The Da Qing IGT and Diabetes Study (7) showed that exercise alone reduced risk of disease progression by 46%. There have been no trials in pediatric populations to evaluate progression of IGT to diabetes. Whether the results of prevention trials in adults can be extrapolated to adolescents is not clear.
Randomized, controlled clinical trials reiterate the conclusions drawn from prevention trials with physiological evidence. Exercise has been shown to enhance insulin signaling and, consequently, increase the rate of insulin-stimulated glucose uptake by GLUT 4 glucose transporter proteins (8). Independent of insulin signaling, muscle contraction also results in increased abundance and redistribution of GLUT 4 (9), the promotion of muscle mass, capillary recruitment (10), and capillary proliferation (11) in muscles and a higher proportion of insulin-sensitive muscle fiber types (12), thereby increasing overall insulin sensitivity (13). Current research suggests that exercise promotes partitioning of excessive fatty acid uptake within the muscle to triglycerides as opposed to fatty acid intermediates known to ultimately induce insulin resistance (8).
In addition to acute promotion of glucose uptake, chronic exercise may decrease the risk for type 2 diabetes via increasing energy expenditure, thereby limiting gains in fat mass. Exercise can increase energy expenditure directly related to the exercise bout, leisure-time energy expenditure (14), and resting energy expenditure (REE). The increase in REE can occur because of both an increase in skeletal muscle mass (15) and, with vigorous exercise, excess postexercise oxygen consumption for 24–48 h (16).
The purpose of this study was to examine the association of physical activity, as assessed by accelerometry, with both glucose tolerance and REE in an adolescent population. Specifically, we hypothesized that time and intensity of physical activity would be positively associated with both glucose tolerance (Kg) and REE.
RESEARCH DESIGN AND METHODS
This study was part of the longitudinal parent study, “Intra-Abdominal Fat and Risk of Disease in Adolescents,” conducted at the University of Alabama at Birmingham (UAB). Subjects were recruited through informational fliers, notices posted in pediatric office waiting rooms, and newspaper advertisements. The parent study excluded adolescents taking medications known to affect body composition or physical activity (e.g., prednisone, Ritalin, or growth hormone) and diagnoses of syndromes known to affect body composition/fat distribution (e.g., Cushing's syndrome, Down's syndrome, type 1 and 2 diabetes, or hypothyroidism). Children with any confounding medical conditions, acute or chronic, were also excluded from participation. All current subjects of the parent study were given the opportunity to participate in this substudy.
Participants in the substudy were 32 African American and Caucasian adolescents between the ages of 12 and 18 years (56% female and 47% African American). Further, there were 10 Caucasian male, 8 Caucasian female, 4 African American male, and 10 African American female subjects. This study was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham. Each subject signed an assent form, and the guardian signed a consent form before enrollment.
Protocol
All data were collected during one inpatient visit to the General Clinical Research Center (GCRC) at the UAB Hospital and Clinics and one follow-up visit 8 days later. On the afternoon of admission to the GCRC, body composition was assessed using dual-energy X-ray absorptiometry in the Energy Metabolism Laboratory at the UAB Department of Nutrition Sciences. The morning after admission consisted of measurement of REE by indirect calorimetry and Kg by an intravenous glucose tolerance test. At midday, teens were fitted with accelerometers and discharged. At the follow-up visit, accelerometers were turned in to investigators for data analysis.
Accelerometry
Free-living physical activity was assessed with Computer Science and Applications ActiGraph monitors (model 7164, version 2.2; MTI Health Services, Fort Walton Beach, FL). The monitor is a small, lightweight, unidirectional accelerometer that measures vertical acceleration and deceleration. “Counts” are the summation of the accelerations measured in 1 minute, and acceleration is measured 10 times/s. Therefore, 600 measurements are summed and recorded at the end of 1 minute.
Parent and teen were given verbal and written instructions for wearing the ActiGraph, and an investigator secured the monitor above the iliac crest of the right hip with an elastic band before discharge from the GCRC. The monitor was programmed to begin collecting data at 12:00 p.m. on the day of discharge. The subject wore the activity monitor 24 h/day for 8.5 days, except when swimming and bathing. The first 12 h of data were not analyzed but regarded as a period of adaptation. The outcome variables were total body movement (counts per day), which is an indicator of the total volume of physical activity, and time (minutes per day) spent at different activity intensity categories. At the end of the collection period, counts were categorized to the following groups: 1) <1,952 counts = <2.99 METS (walking >24 min/mile) = light activity; 2) 1,953–5,724 counts = 3.0–5.99 METS (walking 15–24 min/mile) = moderate activity; 3) 5,725–9,498 counts = 6.0–8.99 METS (jogging 8–15 min/mile) = hard activity; and 4) >9,499 counts = >9.0 METS (running <8 min/mile) = very hard activity (17). Mean numbers of 5-, 10-, and 20-min bouts per day of moderate physical activity were also calculated.
Body composition
Total fat mass and fat-free mass (FFM) were determined via dual-energy X-ray absorptiometry using a Lunar Prodigy densitometer (with software version 6.10.029; GE-Lunar, Madison, WI). Subjects were scanned in the supine position with their hands placed at their sides. For the purposes of this article, we will refer to measures of fat-free soft tissue as FFM. At the GCRC, height and weight were recorded to the nearest 0.1 cm and kg, respectively. A standardized stadiometer and a Scale-Tronix digital scale were used for measurements.
REE
Each adolescent fasted for at least 8 hours after an evening admission. Each participant's resting metabolic rate was measured using a Delta Trac II Metabolic Cart (SensorMedics, Anaheim, CA) in the morning immediately upon awakening. After calibration using standard gases, the clear plastic canopy was placed over the subject's head. After a 5-min acclimation period, respiratory gas exchange was measured for 25 min, and the average REE was calculated.
An in-house, quality control, alcohol burn test was performed quarterly on the Delta Trac instrument or whenever questions or problems arose. At all times during the project period, the instrument generated respiratory quotient values between 0.64 and 0.69, which are reflective of accurate function, as indicated in the manufacturer's guidelines. In addition, the instrument was serviced annually by the manufacturer to assure accurate function and calibration.
Intravenous glucose tolerance test
At approximately 7:00 a.m., after subjects had fasted for 12 h, flexible intravenous catheters were placed in the antecubital spaces of both arms. At time “zero,” glucose (300 mg/kg) was administered intravenously. At minute 20 after glucose administration, subjects received a 5-min infusion of insulin (0.02 unit/kg). Blood samples were collected at −30, −15, 1, 2, 3, 4, 5, 6, 8, 10, 12, 15, 19, 20, 21, 22, 24, 25, 26, 28, 30, 35, 40, 50, 60, 70, and 240 min relative to glucose injection. Sera subsequently were analyzed for glucose and insulin, and values were entered into the MINMOD computer program (version 3.0, Richard N. Bergman) for determination of the insulin sensitivity index (SI) and the acute insulin response to glucose. Acute insulin response to glucose is the integrated incremental area under the curve for insulin during the first 10 min of the test. The average of the −30- and −15-min glucose and insulin values was used for determination of basal glucose and insulin concentrations. Intravenous glucose tolerance (Kg, percent per minute) was determined from the inverse slope of the regression line of time (minutes) versus ln glucose (milligrams per deciliter) from minute 8 through minute 19 of the test. A higher number implies higher (“better”) glucose tolerance. Intravenous glucose tolerance is the rate at which glucose declines after administration, which primarily reflects glucose uptake and utilization by skeletal muscle. Glucose tolerance was assessed because it captures several processes that affect glucose disposal, including insulin sensitivity, β-cell responsiveness, hepatic insulin extraction, insulin suppression of glucose production, vascularization of skeletal muscle, and skeletal muscle perfusion. All of these processes may be affected by aspects of physiology, metabolism, and the environment. Thus, glucose tolerance is an integrated measure of numerous processes that affect the ability to dispose of glucose.
Assay of glucose and insulin
Analyses were performed in the Core Laboratory of the GCRC and the Clinical Nutrition Research Center at UAB. Glucose was measured in 10 μl of sera using an Ektachem DT II System (Johnson & Johnson Clinical Diagnostics). Insulin was assayed in duplicate 100-μl aliquots with Linco Research Products (St. Charles, MO) reagents. In the Core Laboratory, this assay has a sensitivity of 3.35 μIU/ml, a mean intra-assay coefficient of variation (CV) of 3.49%, and a mean interassay CV of 5.57%. Commercial quality control sera of low, medium, and high insulin concentration are included in every assay to monitor variation over time.
Statistical methods
Descriptive statistics were computed for the physical activity variables, REE, metabolic variables, and demographic variables. Kg and SI values were log transformed (using a log10 scale) to follow an approximate normal distribution. Differences between ethnic groups and sex were examined (separately) using two-group t tests or the two-group t test for unequal variances as appropriate. Relationships between physical activity variables and Kg were examined using Pearson correlation analysis. Multiple linear regression models were developed for predicting Kg and REE. For the Kg model, the independent variables were total physical activity (TPA), very hard physical activity (VHPA), hard physical activity (HPA), moderate physical activity (MPA), and 5-min bouts of MPA, each used in a separate model; covariates in all models were race, sex, and total fat mass. Although race and fat mass are not significant in the model, both are known determinants of glucose metabolism and therefore Kg. We elected a priori to include both. For the REE model, independent variables were TPA, VHPA, HPA, MPA, and 5-min bouts of MPA, each used in a separate model; covariates used in all models were race and FFM. In preliminary regression models, sex was examined as a predictor of REE and was found not to be statistically significant (P > 0.05); thus, sex was not included in the final model. All statistical tests used a significance level of 5% and were two-tailed. Statistical analyses were performed using SAS (version 9.1; SAS Institute, Cary, NC).
RESULTS
Study population demographics are described in Table 1. Female subjects had significantly higher fat mass (P = 0.0124), higher percent fat (P = 0.0002), lower FFM (P = 0.0001), lower REE (P = 0.0173), and higher Kg (P = 0.0171) than male subjects. There were no significant differences in demographic or metabolic characteristics between groups of African American and Caucasian subjects.
Table 1.
Baseline demographic and metabolic characteristics: all subjects combined and by ethnic and sex groups
| Total population | Caucasian | African American | Male | Female | |
|---|---|---|---|---|---|
| n | 32 | 18 | 14 | 14 | 18 |
| Age (years) | 16.0 ± 1.6 | 16.1 ± 1.3 | 15.9 ± 2.0 | 15.9 ± 1.5 | 16.1 ± 1.8 |
| Tanner stage | 4.8 ± 0.5 | 4.8 ± 0.5 | 4.9 ± 0.3 | 4.9 ± 0.4 | 4.8 ± 0.5 |
| Weight-for-height percentile | 66.2 ± 32.1 | 57.4 ± 37.1 | 77.3 ± 20.4 | 60.1 ± 31.0 | 70.8 ± 33.0 |
| Total fat mass (kg) | 23.4 ± 15.5 | 22.2 ± 15.8 | 24.9 ± 15.6 | 15.8 ± 15.0 | 29.3 ± 13.5* |
| % Fat | 29.5 ± 14.2 | 30.0 ± 13.7 | 28.9 ± 15.4 | 19.7 ± 12.4 | 37.5 ± 10.1* |
| Total FFM (kg) | 45.7 ± 12.1 | 45.6 ± 10.5 | 45.8 ± 14.3 | 54.1 ± 8.7 | 39.2 ± 10.3* |
| REE (kcal/day) | 1,565 ± 253 | 1,602 ± 309 | 1,524 ± 171 | 1,688 ± 283 | 1,471 ± 185* |
| SI (×10−4 · min−1/μIU/ml) | 3.41 ± 1.87 | 3.46 ± 2.15 | 3.36 ± 1.49 | 3.77 ± 2.12 | 3.12 ± 1.65 |
| Kg (%/min) | 2.15 ± 1.21 | 1.83 ± 0.87 | 2.57 ± 1.47 | 1.63 ± 0.67 | 2.56 ± 1.38* |
Data are means ± SD.
Significantly different between male and female subjects (P < 0.05).
Male subjects engaged in significantly more TPA, MPA, and HPA (P = 0.0108, P = 0.0379, and P = 0.0329, respectively) than female subjects (Table 2). Race was a determinant of total activity counts and was significantly higher in African American teens than in Caucasian teens. Race was not a determinant of minutes spent in TPA, MPA, HPA, or VHPA.
Table 2.
Mean physical activity by accelerometry: all subjects combined and by ethnic and sex groups
| Total population | Caucasian | African American | Male | Female | |
|---|---|---|---|---|---|
| n | 32 | 18 | 14 | 14 | 18 |
| TAC (counts/day) | 382,863 ± 144,581 | 325,962 ± 133,681 | 456,021 ± 127,402* | 426,980 ± 146,056 | 348,550 ± 137,681 |
| TPA (min/day) | 41.5 ± 27.8 | 34.8 ± 27.8 | 50.2 ± 26.2 | 55.3 ± 28.7 | 30.8 ± 22.3† |
| MPA (min/day) | 37.7 ± 24.7 | 30.8 ± 24.7 | 46.6 ± 22.8 | 47.9 ± 26.2 | 29.8 ± 21.1† |
| HPA (min/day) | 3.0 ± 5.8 | 3.3 ± 7.5 | 2.8 ± 3.0 | 6.0 ± 8.0 | 0.8 ± 1.2† |
| VHPA (min/day) | 0.4 ± 1.2 | 0.3 ± 0.4 | 0.8 ± 1.9 | 0.9 ± 1.8 | 0.2 ± 0.4 |
Data are means ± SD.
Significantly different between African American and Caucasian subjects (P < 0.001).
Significantly different between male and female subjects (P < 0.05). TAC, total activity counts.
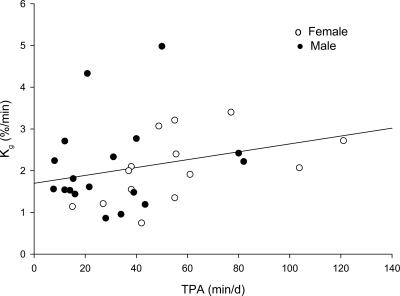
Multiple linear regression analysis indicated significant, independent associations between Kg and TPA (P = 0.026) (Fig. 1), MPA (P = 0.031), and 5-min bouts of MPA (P = 0.035). HPA and VHPA did not make significant contributions to Kg (P = 0.717 and P = 0.830, respectively) (data not shown).
Figure 1.
Glucose tolerance relative to total daily minutes spent in physical activity after adjustments for fat mass, sex, and race (significant at P = 0.026). d, day.
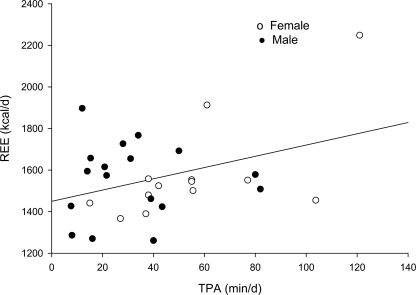
A positive, independent relationship was observed between REE and TPA (P = 0.016) (Fig. 2), MPA (P = 0.032), HPA (P = 0.040), and 5-min bouts of MPA (P = 0.011). VHPA was not independently associated with REE (P = 0.507) (data not shown).
Figure 2.
Relationship between REE and TPA after adjustments for race and FFM (significant at P = 0.016). d, day.
CONCLUSIONS
The aim of this study was to examine the association of physical activity with Kg and REE in an adolescent sample. In this study, physical activity was positively associated with both Kg and REE. The relationship between physical activity and carbohydrate metabolism is well established in adult populations; however, this research provides new information in teenaged populations and may be important as obesity-related diseases, such as diabetes, expand into this age-group. Further research is needed to determine whether moderate physical activity can decrease the risk for obesity and glucose intolerance in this population.
The significant differences in percent body fat, FFM, and REE between male and female subjects in this study verify findings of previous research in an adolescent population (18). We did not see REE differences between ethnicities in this study. Similarly, our previous research in young children indicated that REE was similar in African American and Caucasian children (19). Other literature showed that Caucasian teens as a group had higher REE than African American teens, but when analyzed for sex, Caucasian boys had lower REE than African American boys, whereas the opposite was true for girls (18). It is possible that the reported ethnic difference in REE is sex-specific and that it develops during adolescence.
Assessment of Kg in an adolescent population is a novel undertaking. In this study, Kg was higher in girls than in boys, independent of body composition, race, and physical activity. Sex differences in aspects of glucose metabolism have been attributed to an estrogenic hormonal environment. Estrogen stimulates skeletal muscle glucose uptake (20), which is reported to be greater in women than in men (21). This study suggests that sex differences in glucose metabolism are apparent in adolescence.
The study sample engaged in ∼40 min of physical activity per day, the majority spent in a moderate level of physical activity. Less than 5 min/day were spent in HPA and <1 min/day in VHPA. This cohort engaged in few bouts of HPA or VHPA, as shown by the mean bouts per day <1. Others have shown that boys and girls in grades 1–12 exhibited few bouts of vigorous physical activity (22). In our study sample, male subjects engaged in significantly more physical activity than female subjects, also consistent with other literature reports (23). When we compared the TPA of Caucasians and African Americans, there were no statistically significant differences. Other literature reports also suggested greater similarities in physical activity among ethnic groups than sex groups, although African American adolescent populations were less active than Caucasian (24).
We saw a significant association between Kg and TPA (Fig. 1), MPA, and 5-min bouts of MPA. Exercise is a major mediator of glucose transport activity in muscle, and this occurs through an increase in the maximal velocity of transport (13). Another major mechanism that increases glucose uptake through exercise is the translocation of glucose transporter proteins from an intracellular compartment to the surface of the cell. Because exercise affects glucose transport through several mechanisms, we do not know the exact mechanism responsible for the relationship observed in this study between physical activity and Kg. The absence of relationships in this study of Kg with HPA and VHPA may have been due to the minimal amounts of these activities performed by this study sample.
Our results indicate that, as TPA (Fig. 2), MPA, and HPA increased, an adolescent's mass-specific REE increased. Data from adults also indicate that exercise can increase REE, adjusted for FFM (16). Our observation of a positive association between physical activity and REE suggests that promotion of movement, such as leisure-time activity and planned exercise, among adolescents may be a particularly useful means of combating obesity within this age-group. However, further research is needed to determine whether an intervention to increase physical activity will likewise increase REE.
Strengths of the study include robust measures of Kg and time spent in moderate to vigorous physical activity. Limitations include a relatively small sample size (n = 32), which may have limited our ability to detect racial differences among outcomes of interest. In particular, the small number of African American male subjects may have constrained the outcomes further. A limitation of this and all cross-sectional studies is that causality cannot be inferred from statistical relationships. Further research is needed to determine whether a physical activity intervention is associated with changes in REE and Kg. In addition, the ActiGraph may underestimate sedentary and light intensity physical activity.
In summary, adolescents in this study engaged primarily in MPA, with very little HPA or VHPA. Physical activity was significantly associated with Kg and REE. Longitudinal studies with a larger population are needed to determine whether an increase in physical activity results in increases in REE and Kg, and, if so, to examine the relevant mechanisms involved. Likewise, further research is needed to determine whether consistent physical activity increases REE and limits obesity in the adolescent population.
Acknowledgments
This work was supported by grants M01-RR-00032 (GCRC) and P30-DK56336 (Clinical Nutrition Research Unit).
No potential conflicts of interest relevant to this article were reported.
The Metabolism Core laboratory of the GCRC/Clinical Nutrition Research Center is acknowledged for laboratory analyses.
Parts of this study were presented in abstract form at the Southeastern American College of Sports Medicine conference, Charlotte, North Carolina, 16 February 2007.
Published ahead of print at http://care.diabetesjournals.org on 7 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.The Consensus Workshop Group: Type 2 diabetes in the young: the evolving epidemic; the International Diabetes Federation Consensus Workshop. Diabetes Care 27:1798–1811, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Search for Diabetes Study Group: The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. J Pediatr 118:1510–1518, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Total prevalence of diabetes and pre-diabetes [article online], 2005. Available from http://diabetes.org/diabetes-statistics/prevalence.jsp. Accessed 28 November 2007
- 4.Zimmet P, Alberti KG, Shaw J: Global and societal implications of the diabetes epidemic. Nature 414:782–787, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnish Diabetes Prevention Study Group: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Ziao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard B: Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 20:537–544, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Horowitz JF: Exercise-induced alterations in muscle lipid metabolism improve insulin sensitivity. Exerc Sport Sci Rev 35:192–196, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Douen AG, Ramalal T, Klip A, Young DA, Cartee GD, Holloszy JO: Exercise-induced increase in glucose transporters in plasma membranes of rat skeletal muscle. Endocrinology 124:449–454, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Honig CR, Odoroff CL, Frierson JL: Capillary recruitment in exercise: rate, extent uniformity, and relation to blood flow. Am J Physiol Heart Circ Physiol 243:H196–H206, 1982 [DOI] [PubMed] [Google Scholar]
- 11.Injer F: Capillary supply and mitochondrial content of different skeletal muscle fiber types in untrained and endurance-trained men: a histochemical and ultra-structural study. Eur J Appl Physiol 40:197–209, 1979 [DOI] [PubMed] [Google Scholar]
- 12.Daugaard JR, Tichter EA: Relationship between muscle fibre composition, glucose transporter protein 4 and exercise training: possible consequences in non-insulin dependent diabetes mellitus. Acta Physiol Scand 171:267–276, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Goodyear LJ, Kahn BB: Exercise, glucose transport, and insulin sensitivity. Annu Rev Med 49:235–261, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM: Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol 89:977–984, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Byrne HK, Wilmore JH: The relationship of mode and intensity on resting metabolic rate in women. Int J Sport Nutr Exerc Metab 11:1–14, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Tremblay A, Nadeau A, Fournier G, Bouchard C: Effect of a three-day interruption of exercise-training on resting metabolic rate and glucose-induced thermogenesis in trained individuals. Int J Obes 12:163–168, 1988 [PubMed] [Google Scholar]
- 17.Freedson PS, Melanson E, Sirard J: Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30:777–781, 1998 [DOI] [PubMed] [Google Scholar]
- 18.DeLany JP, Bray GA, Harsha DW, Volaufova J: Energy expenditure in African American and white boys and girls in a 2-y follow-up of the Baton Rouge Children's Study. Am J Clin Nutr 79:268–273, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Sun M, Gower BA, Nagy TR, Trowbridge CA, Dezenberg C, Goran MI: Total, resting, and activity-related energy expenditures are similar in Caucasian and African-American children. Am J Physiol 274:E232–E237, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Kumagai S, Holmang A, Bjorntorp P: The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol 149:91–97, 1993 [DOI] [PubMed] [Google Scholar]
- 21.Donahue RP, Prineas RJ, Donahue RD, Bean JA, Skyler JS: The female “insulin advantage” in a biracial cohort: results from the Miami Community Health Study. Int J Obes 20:76–82, 1996 [PubMed] [Google Scholar]
- 22.Pate RR, Freedson PS, Sallis JF, Taylor WC, Sirard J, Trost SG, Dowda M: Compliance with physical activity guidelines: prevalence in a population of children and youth. Ann Epidemiol 12:303–308, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Trost SG, Pate RR, Sallis JF, Freedson PS, Taylor WC, Dowda M, Sirard J: Age and gender differences in objectively measured physical activity in youth. Med Sci Sports Exerc 34:350–355, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Gordon-Larson P, McMurray RG, Popkin BM: Adolescent physical activity and inactivity vary by ethnicity: the national longitudinal study of adolescent health. J Pediatr 135:301–306, 1999 [DOI] [PubMed] [Google Scholar]