Abstract
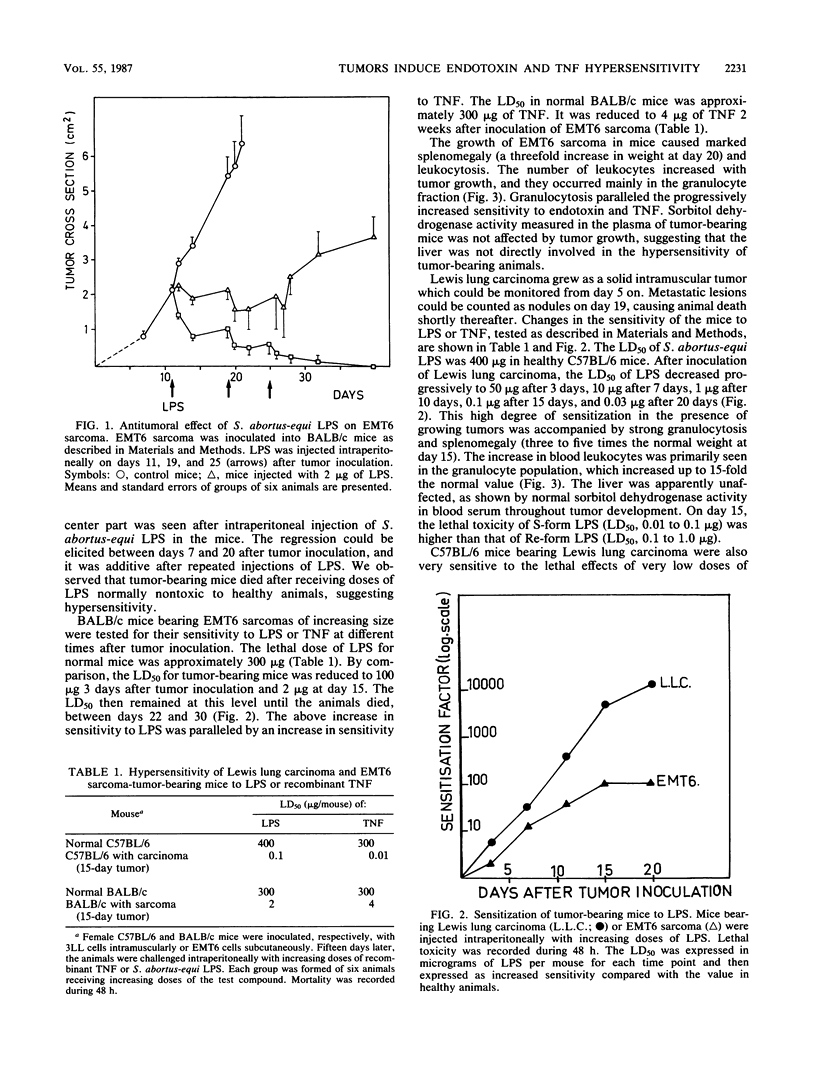
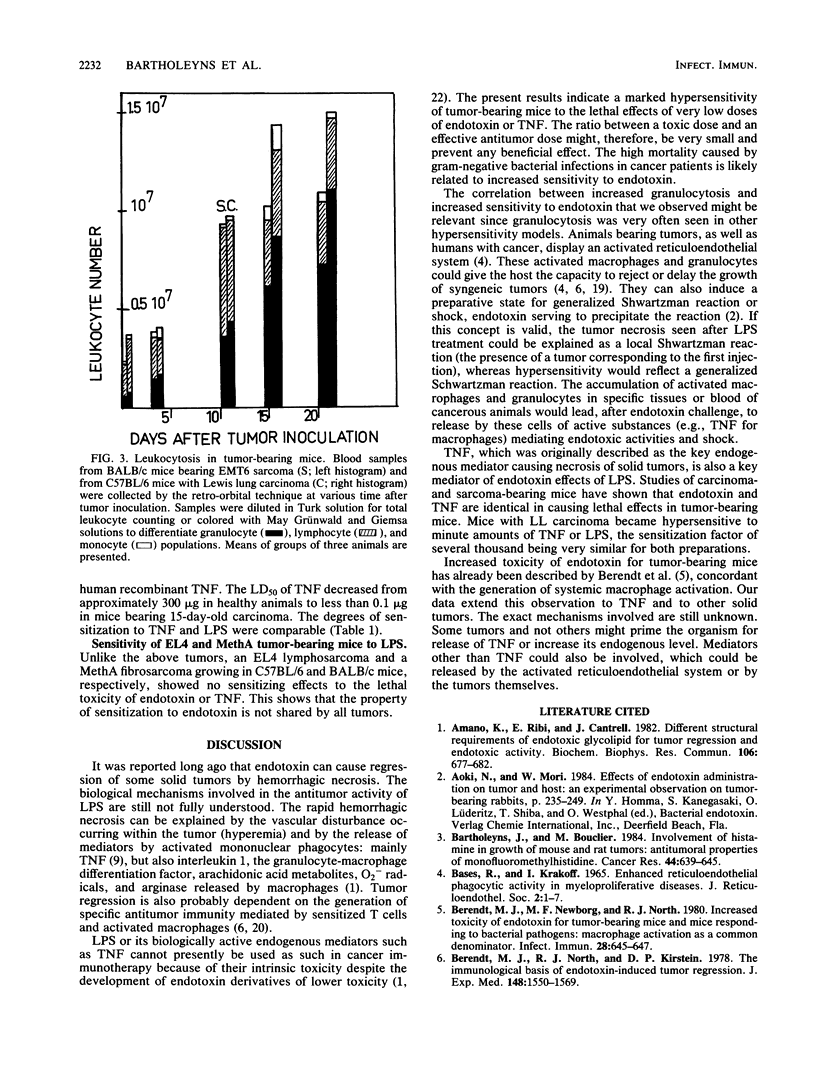
Lewis lung carcinoma and EMT6 sarcoma growing as solid tumors in mice caused a gradual increase in the susceptibility of the animals to lethal toxicity of endotoxins (lipopolysaccharides [LPS]). By day 15 following inoculation of the tumors, the 50% lethal dose of LPS, which in normal mice was approximately 400 micrograms, decreased to 2 micrograms for the sarcoma-bearing mice and 0.1 microgram for the carcinoma-bearing mice. This sensitization to endotoxin was paralleled by a high sensitization to tumor necrosis factor (TNF). Human recombinant TNF given to normal mice was lethal at about 500 micrograms. It was lethal for 50% of the animals bearing EMT6 or Lewis lung carcinoma tumors in amounts of 4 and 0.01 micrograms, respectively, on day 15 following tumor inoculation. The sensitization of tumor-bearing animals to LPS and TNF was paralleled by marked granulocytosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano K., Ribi E., Cantrell J. L. Different structural requirements of endotoxic glycolipid for tumor regression and endotoxic activity. Biochem Biophys Res Commun. 1982 Jun 15;106(3):677–682. doi: 10.1016/0006-291x(82)91764-8. [DOI] [PubMed] [Google Scholar]
- BASES R. E., KRAKOFF I. H. ENHANCED RETICULOENDOTHELIAL PHAGOCYTIC ACTIVITY IN MYELOPROLIFERATIVE DISEASES. J Reticuloendothel Soc. 1965 May;2:1–7. [PubMed] [Google Scholar]
- BERRY L. J., SMYTHE D. S. EFFECTS OF BACTERIAL ENDOTOXINS ON METABOLISM. VII. ENZYME INDUCTION AND CORTISONE PROTECTION. J Exp Med. 1964 Nov 1;120:721–732. doi: 10.1084/jem.120.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholeyns J., Bouclier M. Involvement of histamine in growth of mouse and rat tumors: antitumoral properties of monofluoromethylhistidine, an enzyme-activated irreversible inhibitor of histidine decarboxylase. Cancer Res. 1984 Feb;44(2):639–645. [PubMed] [Google Scholar]
- Berendt M. J., Newborg M. F., North R. J. Increased toxicity of endotoxin for tumor-bearing mice and mice responding to bacterial pathogens: macrophage activation as a common denominator. Infect Immun. 1980 May;28(2):645–647. doi: 10.1128/iai.28.2.645-647.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt M. J., North R. J., Kirstein D. P. The immunological basis of endotoxin-induced tumor regression. Requirement for T-cell-mediated immunity. J Exp Med. 1978 Dec 1;148(6):1550–1559. doi: 10.1084/jem.148.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Lehmann V., Freudenberg M. A., Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987 Mar 1;165(3):657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmenout A., Fransen L., Tavernier J., Van der Heyden J., Tizard R., Kawashima E., Shaw A., Johnson M. J., Semon D., Müller R. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem. 1985 Nov 4;152(3):515–522. doi: 10.1111/j.1432-1033.1985.tb09226.x. [DOI] [PubMed] [Google Scholar]
- Männel D. N. Biological aspects of tumor necrosis factor. Immunobiology. 1986 Sep;172(3-5):283–290. doi: 10.1016/S0171-2985(86)80110-3. [DOI] [PubMed] [Google Scholar]
- Männel D. N., Moore R. N., Mergenhagen S. E. Macrophages as a source of tumoricidal activity (tumor-necrotizing factor). Infect Immun. 1980 Nov;30(2):523–530. doi: 10.1128/iai.30.2.523-530.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J., Kirstein D. P. T-cell-mediated concomitant immunity to syngeneic tumors. I. Activated macrophages as the expressors of nonspecific immunity to unrelated tumors and bacterial parasites. J Exp Med. 1977 Feb 1;145(2):275–292. doi: 10.1084/jem.145.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr I., Wheeler E., Alexander P. Similarities of the anti-tumour actions of endotoxin, lipid A and double-stranded RNA. Br J Cancer. 1973 May;27(5):370–389. doi: 10.1038/bjc.1973.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Ribi E. E., Cantrell J. L., Von Eschen K. B., Schwartzman S. M. Enhancement of endotoxic shock by N-acetylmuramyl-L-alanyl-(L-seryl)-D-isoglutamine (muramyl dipeptide). Cancer Res. 1979 Nov;39(11):4756–4759. [PubMed] [Google Scholar]
- SKIPPER H. E., SCHMIDT L. H. A manual on quantitative drug evaluation in experimental tumor systems. I. Background, description of criteria, and presentation of quantitative therapeutic data on various classes of drugs obtained in diverse experimental tumor systems. Cancer Chemother Rep. 1962 Apr;17:1–143. [PubMed] [Google Scholar]
- SUTER E., ULLMAN G. E., HOFFMAN R. G. Sensitivity of mice to endotoxin after vaccination with BCG (Bacillus Calmette-Guérin). Proc Soc Exp Biol Med. 1958 Oct;99(1):167–169. doi: 10.3181/00379727-99-24282. [DOI] [PubMed] [Google Scholar]
- Schramek S., Kazar J., Sekeyova Z., Freudenberg M. A., Galanos C. Induction of hyperreactivity to endotoxin in mice by Coxiella burnetii. Infect Immun. 1984 Sep;45(3):713–717. doi: 10.1128/iai.45.3.713-717.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H., Tuchweber B., Bertók L. Effect of lead acetate on the susceptibility of rats to bacterial endotoxins. J Bacteriol. 1966 Feb;91(2):884–890. doi: 10.1128/jb.91.2.884-890.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



