Abstract
OBJECTIVE—The aim of this study was to examine the effect of protein kinase Cβ inhibition with ruboxistaurin on renal hemodynamic function and urinary biomarkers (monocyte chemoattractant protein-1 [MCP-1] and epidermal growth factor) in renin angiotensin system blockade-treated type 1 diabetic subjects.
RESEARCH DESIGN AND METHODS—Albuminuric subjects were randomized (2:1) to ruboxistaurin (32 mg daily; n = 13) or placebo (n = 7) for 8 weeks. Renal hemodynamic function was measured during clamped euglycemia or hyperglycemia and before and after ruboxistaurin or placebo.
RESULTS—Ruboxistaurin was not associated with between-group differences during clamped euglycemia or hyperglycemia. In a post hoc analysis comparing hyperfilterers with normofilterers during euglycemia, glomerular filtration rate and MCP-1 decreased, whereas the epidermal growth factor–to–MCP-1 ratio increased in hyperfilterers versus normofilterers (all P < 0.05).
CONCLUSIONS—The effect of ruboxistaurin is modest and dependent, at least in part, on the level of ambient glycemia and baseline glomerular filtration rate.
Experimental studies of diabetes have suggested that the activation of the intracellular signaling molecule protein kinase Cβ (PKCβ) is associated with renal hyperfiltration and development of diabetes complications (1,2). PKCβ activation in diabetes is associated with loss of key protective trophic factors, such as epidermal growth factor (EGF) (3), and with expression of proinflammatory mediators such as monocyte chemoattractant protein-1 (MCP-1) (4,5). Much less is known about the role of PKCβ activation in the pathogenesis of renal hemodynamic and molecular abnormalities in human diabetes.
Accordingly, in this pilot study, we hypothesized that ruboxistaurin would reverse the hemodynamic effects of diabetes and blunt the response to clamped hyperglycemia. In a post hoc analysis, we analyzed subjects on the basis of the presence of renal hyperfiltration during clamped euglycemia (6,7). We also examined the effect of ruboxistaurin on the excretion of the urinary biomarkers MCP-1 and EGF (4,8,9).
RESEARCH DESIGN AND METHODS
After giving informed consent, subjects (Table A1, with inclusion/exclusion criteria, is available in an online appendix at http://dx.doi.org/10.2337/dc08-1609) adhered to a diet that was Na replete and moderate in protein for 7 days before each experiment (6,7). Euglycemic (blood glucose 4–6 mmol/l) and hyperglycemic (blood glucose 9–11 mmol/l) conditions were maintained on two consecutive days using a modified glucose clamp technique, and renal hemodynamic function was measured using inulin and para-aminohippurate (6,7). Urinary biomarkers were measured by ELISA (Quantikine; R&D Systems, Minneapolis, MN) before and after treatment with ruboxistaurin or placebo, normalized for urinary creatinine. Subjects were then randomized (2:1) to ruboxistaurin (32 mg daily for 8 weeks) or a placebo in a double-blind fashion. All subjects were taking an ACE inhibitor, an angiotensin receptor blocker (ARB), or a combination throughout the study. The University Health Network Research Ethics Board approved the protocol.
The primary analysis examined hemodynamic responses during clamped euglycemia and hyperglycemia before and after treatment with ruboxistaurin or the placebo. In a post hoc analysis, we analyzed subjects on the basis of filtration status (hyperfiltration, glomerular filtration rate [GFR] ≥135 ml/min per 1.73 m2; normofiltration, <135 ml/min per 1.73 m2) (6,7). Between-group comparisons of all parameters at baseline were made using parametric methods (unpaired Student's t test). Within-subject and between-group differences in the response to PKCβ inhibition were determined by repeated-measures ANOVA. All statistical analyses were performed using SPSS (version 14; SPSS, Chicago, IL).
RESULTS
Baseline clinical characteristics are shown in online appendix Table A2. At baseline, mean ± SEM arterial pressure was higher in the ruboxistaurin group (96 ± 1 mmHg) than in the placebo group (81 ± 4 mmHg) during clamped euglycemia. In the primary analysis, during clamped euglycemia, ruboxistaurin was associated with a reduction in mean arterial pressure (96 ± 1 to 91 ± 2 mmHg; P = 0.032) but did not influence renal hemodynamic function. During clamped hyperglycemia (online appendix Table A3), ruboxistaurin was associated with declines in effective renal plasma flow (ERPF) and renal blood flow and a rise in filtration fraction (all P < 0.05). Ruboxistaurin did not change MCP-1, EGF, or the EGF–to–MCP-1 ratio in urinary excretion.
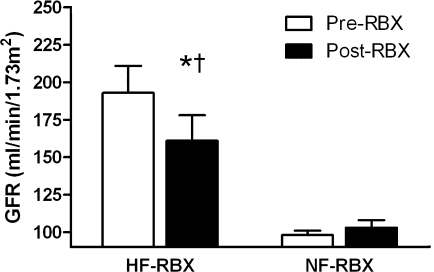
When analyzed on the basis of filtration status, hyperfiltration (n = 4) and normofiltration (n = 9) subjects were similar at baseline (data not shown). In hyperfiltration subjects, ruboxistaurin was associated with a decline in GFR that was significant compared with the response in normofiltration subjects (Fig. 1). When analyzed on the basis of filtration status, ruboxistaurin was associated with a decrease in MCP-1 (P = 0.041) and a rise in the EGF–to–MCP-1 ratio (P = 0.041) in hyperfiltration versus normofiltration subjects (online appendix Figures A1–A3).
Figure 1.
The effect of ruboxistaurin (RBX) on GFR during euglycemia in hyperfiltration and normofiltration subjects (mean ± SEM). HF, hyperfiltration; NF, normofiltration. *P = 0.009 vs. baseline in hyperfiltration subjects. †P = 0.003 vs. response in normofiltration subjects.
CONCLUSIONS
The aim of this study was to determine the role of PKCβ inhibition in humans with diabetes. Our major findings were that 1) during clamped hyperglycemia, ruboxistaurin lowered ERPF and renal blood flow, and 2) in a post hoc analysis based on filtration status, ruboxistaurin partially corrected hyperfiltration during clamped euglycemia, while MCP-1 decreased and the EGF–to–MCP-1 ratio increased in hyperfiltration versus normofiltration subjects.
Renal hemodynamic complications, including hyperfiltration, occur early in the natural history of diabetes and may in part be due to a hyperglycemia-mediated increase in PKCβ activity (1). Our first major finding was that during clamped euglycemia, ruboxistaurin did not significantly affect renal hemodynamic function. In contrast, during clamped hyperglycemia, ruboxistaurin lowered ERPF but there was no effect on GFR. Although from this experiment we could not determine why ruboxistaurin failed to lower GFR during hyperglycemia, the explanation may involve activation of redundant hemodynamic pathways, such as endothelin or cyclooxygenase-2, leading to the maintenance of GFR (10,11).
We have previously reported that baseline GFR during clamped euglycemia is a determinant of renal hemodynamic responsiveness (6). Our second major finding was that ruboxistaurin was associated with reductions in GFR and filtration fraction without affecting ERPF in hyperfiltration subjects but was not associated with such reductions in normofiltration subjects. Furthermore, the change in the urinary biomarkers in hyperfiltration subjects was consistent with protection against renal injury (a decline in MCP-1 and a rise in the EGF–to–MCP-1 ratio) (12). Although we cannot determine the precise pathway responsible for these hemodynamic and molecular effects, we favor the theory that ruboxistaurin may have inhibited the effects of angiotensin II, leading to vasodilatation and a fall in GFR. The responsible mechanisms for these observations require further investigation in animal studies because these parameters cannot be assessed in humans.
This study has important limitations. We attempted to minimize the effect of the small sample size with homogeneous study groups, careful prestudy dietary preparation, and a study design that allowed each subject to act as his or her own control. We were also prevented from studying the independent effect of ruboxistaurin because of ethical concerns about discontinuing ACE inhibitor or ARB therapy in this proteinuric cohort of subjects. Therefore, the effect of ruboxistaurin may have been blunted by a preexisting renin angiotensin system blockade.
In conclusion, PKCβ appears to play a role in the maintenance of hyperfiltration in patients with type 1 diabetes, and its inhibition results in a decrease in GFR and proinflammatory urinary biomarkers. The role of PKCβ may be enhanced in hyperfiltration patients, suggesting that the impact of ruboxistaurin depends on filtration status.
Supplementary Material
Acknowledgments
Operating funds for this study were provided by Heart and Stroke Foundation Grant NA-5296 (to J.A.M.). D.Z.C. and H.R. were supported by funding from the Kidney Research Scientist Core Education and National Training Program (sponsored by the Canadian Institutes of Health Research, the Kidney Foundation of Canada, the Canadian Society of Nephrology, and Ortho Biotech), and the Clinician Scientist Program at the University of Toronto. J.W.S. is the Canadian Institutes of Health Research/Amgen Canada Kidney Research Chair at the University Health Network, University of Toronto.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 41st annual meeting of the American Society of Nephrology, Philadelphia, Pennsylvania, 4–9 November 2008.
The authors thank Eli Lilly Canada for providing the study drug.
Published ahead of print at http://care.diabetesjournals.org on 22 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Ishii H, Koya D, King GL: Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J Mol Med 76:21–31, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL: Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science 272:728–731, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Gilbert RE, Cox A, McNally PG, Wu LL, Dziadek M, Cooper ME, Jerums G: Increased epidermal growth factor in experimental diabetes related kidney growth in rats. Diabetologia 40:778–785, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Tuttle KR: Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol 16:1537–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Giunti S, Tesch GH, Pinach S, Burt DJ, Cooper ME, Cavallo-Perin P, Camussi G, Gruden G: Monocyte chemoattractant protein-1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia 51:198–207, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Cherney DZ, Miller JA, Scholey JW, Bradley TJ, Slorach C, Curtis JR, Dekker MG, Nasrallah R, Hébert RL, Sochett EB: The effect of cyclooxygenase-2 inhibition on renal hemodynamic function in humans with type 1 diabetes. Diabetes 57:688–695, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Sochett EB, Cherney DZ, Curtis JR, Dekker MG, Scholey JW, Miller JA: Impact of renin angiotensin system modulation on the hyperfiltration state in type 1 diabetes. J Am Soc Nephrol 17:1703–1709, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Tesch GH, Lan HY, Nikolic-Paterson DJ: Treatment of tissue sections for in situ hybridization. Methods Mol Biol 326:1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR: Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med 190:1813–1824, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touyz RM, Schiffrin EL: Role of protein kinase C in the anti-aggregatory effects of endothelin-1 on human platelets. Clin Sci (Lond) 88:277–283, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H: Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol 14:S241–245, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Torres DD, Rossini M, Manno C, Mattace-Raso F, D'Altri C, Ranieri E, Pontrelli P, Grandaliano G, Gesualdo L, Schena FP: The ratio of epidermal growth factor to monocyte chemotactic peptide-1 in the urine predicts renal prognosis in IgA nephropathy. Kidney Int 73:327–333, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.