Abstract
OBJECTIVE—In a prospective birth cohort study, we followed infants who had a first-degree relative with type 1 diabetes to investigate the relationship between early growth and infant feeding and the risk of islet autoimmunity.
RESEARCH DESIGN AND METHODS—Infants with a first-degree relative with type 1 diabetes were identified during their mother's pregnancy. Dietary intake was recorded prospectively to determine duration of breast-feeding and age at introduction of cow's milk protein, cereals, meat, fruit, and vegetables. At 6-month reviews, length (or height) and weight, antibodies to insulin, GAD65, the tyrosine phosphatase-like insulinoma antigen, and tissue transglutaminase were measured. Islet autoimmunity was defined as persistent elevation of one or more islet antibodies at consecutive 6-month intervals, including the most recent measure, and was the primary outcome measure.
RESULTS—Follow-up of 548 subjects for 5.7 ± 3.2 years identified 46 children with islet autoimmunity. Weight z score and BMI z score were continuous predictors of risk of islet autoimmunity (adjusted hazard ratios 1.43 [95% CI 1.10–1.84], P = 0.007, and 1.29 [1.01–1.67], P = 0.04, respectively). The risk of islet autoimmunity was greater in subjects with weight z score >0 than in those with weight z score ≤0 over time (2.61 [1.26–5.44], P = 0.01). Weight z score and BMI z score at 2 years and change in weight z score between birth and 2 years, but not dietary intake, also predicted risk of islet autoimmunity.
CONCLUSIONS—Weight gain in early life predicts risk of islet autoimmunity in children with a first-degree relative with type 1 diabetes.
Identical twin studies and geographic and temporal variations in incidence argue for a critical role of the environment in the development of type 1 diabetes (1). Environmental influences potentially initiate or accelerate the autoimmune destruction of the pancreatic islets. The incidence of type 1 diabetes is increasing in populations worldwide with an earlier age of onset described in European and Oceania populations (1). This rise in childhood incidence parallels in time the overweight/obesity epidemic in Western childhood populations.
The accelerator hypothesis proposes that weight and associated insulin resistance accelerate loss of β-cells in both type 1 and type 2 diabetes, such that they are distinguished only by their rate of progression (2). Proposed accelerators include genes, insulin resistance, and autoimmunity. Although the full implications of this hypothesis are being debated, there is increasing evidence of the importance of weight, BMI, and relative insulin insensitivity in the development of type 1 diabetes. Younger age of onset of type 1 diabetes is associated with higher BMI at diagnosis in large cohorts (3), and in one study, the association was seen only in children with lower fasting C-peptide levels (4). We and others have shown that surrogate markers of insulin resistance and BMI predict progression to type 1 diabetes in subjects with islet autoimmunity (5,6). These findings support weight and relative insulin insensitivity as accelerating β-cell loss after the development of islet autoimmunity when insulin secretion is falling rather than before. However, only prospective studies from birth can resolve this question.
Retrospective case-control studies in Europe link increased linear growth and weight gain in childhood, particularly in the first 2 years of life, with later onset of type 1 diabetes (7–9). In one study, infant growth was related to detection of tyrosine phosphatase-like insulinoma antigen (insulinoma-associated protein 2 [IA2]) antibodies at diagnosis (9). Reports from Scandinavia, Germany, and Colorado in the U.S. and our own data have provided prospective data from birth (10–17), but there are no data examining the effect of weight gain on the development of islet autoimmunity or type 1 diabetes in birth cohort studies. Diet, in terms of introduction and intake of cow's milk protein, cereals, ω-3 fatty acids, fruits, and root vegetables, is a putative influence on the development of islet autoimmunity (12–17).
The Australian Baby Diab Study has prospectively followed from birth infants who have a first-degree relative with type 1 diabetes and live in Victoria or South Australia (10,11). We aimed to investigate the relationship between early growth (weight, length, and height gain) and infant feeding and the risk of development of islet autoimmunity.
RESEARCH DESIGN AND METHODS
All subjects were participants in the Australian Baby Diab Study. Probands included mothers, fathers, and siblings with type 1 diabetes. In 17 of 548 (3.1%) families, there was more than one first-degree relative with type 1 diabetes. Subjects were identified through local diabetes associations and clinics, recruited during their pregnancy, and followed prospectively from birth with 6-month reviews. All infants had normal growth and development apart from four with congenital anomalies who were excluded from the study. At each 6-month review, length (if <2 years of age) or height and weight were measured in the majority of infants using a clinic Harpenden stadiometer (Holtain, Crymych, Wales) to the nearest 0.1 cm and balance scales. Measurements were converted to weight z score, height z score, or length z score to account for normal growth in childhood. After 2 years of age, BMI (weight in kilograms divided by the square of height in meters) and BMI z score were also calculated.
Dietary intake was documented in a home diary by parents and recorded during face-to-face interviews at 6-month reviews. No systematic infant feeding advice was given. Six-month venous specimens were collected for measurement of insulin autoantibodies and antibodies to GAD65, IA2, and tissue transglutaminase. HLA typing was performed on cord blood.
The study was approved by the Women's and Children's Hospital's Human Research and Ethics Committee and the Royal Melbourne Hospital's Human Research and Ethics Committee. Parents gave written informed consent for their child to enter the study.
Dietary records
The following were recorded by the child's parent or guardian in the home diary: 1) duration of exclusive breast-feeding, 2) total duration of breast-feeding, 3) dairy products consumed by the mother while breast-feeding, 4) age at introduction of cow's milk–based infant formulae, 5) commercial brand of infant formulae, 6) age at introduction of cow's milk as cow's milk or other dairy products, 7) age at introduction of nongluten (rice)-containing cereals, 8) age at introduction of gluten-containing (wheat, barley, rye, and oats) cereals, and 9) age at introduction of meat, meat products, fruit, and vegetable solids.
Autoantibodies
Insulin autoantibodies (IAAs) were assayed by a modification of the fluid phase radiobinding assay as described previously (10,11). The interassay coefficient of variation (CV) was 16%. GAD and IA2 antibodies were assayed by immunoprecipitation of [35S]methionine-labeled recombinant human proteins as described previously (10,11). The interassay CVs for GAD and IA2 were 12 and 19%, respectively. In the 2005 Diabetes Autoantibody Standardization Program, workshop sensitivity and specificity for the GAD antibody assay were scored as 86 and 99%, for the IA2 antibody assay 64 and 100%, and for the IAA antibody assay 22 and 99%, respectively.
Elevation of islet antibodies was defined as IAAs >5.5%, GAD antibodies >5 units, or IA2 antibodies >3 units. Islet autoimmunity was defined as persistent elevation of one or more islet antibodies on consecutive 6-month tests, including the most recent measure, and was the primary outcome measure.
Tissue transglutaminase antibodies were measured using a Quanta Lite h-tTG IgA kit (code 708760; INOVA Diagnostics, San Diego, CA). Elevation of tissue transglutaminase antibodies was defined as >20 units. The interassay CV for the negative control (3.6 units/ml) was 12.2%, and the interassay CV for the positive control (105.0 units/ml) was 3.3%.
HLA typing
HLA-A and -B (class I) typing was performed by a standard serologically based microlymphocytotoxicity assay using T lymphocytes separated from whole blood using magnetic beads coated with a T-cell monoclonal antibody. Class II (DRB1) typing was performed by hybridization of PCR-amplified DRB1 exon 2 DNA with sequence-specific oligonucleotide probes according to the 11th International Histocompatibility Workshop protocol but with minor modifications as described previously (10,11).
Statistical analysis
Time-to-event analyses were used to explore the effect of weight and diet on the risk of islet autoimmunity. Parametric survival models were used to compare the age when islet autoimmunity developed across categories of exposure to the risk factors of interest. Time to development of islet autoimmunity was assumed to follow a Gompertz distribution. Unadjusted and adjusted hazard ratios (HRs) were calculated by parametric survival models, which accounted for inconsistent length of follow-up and right-censored data. A total of 4,598 weight and length or height measurements were obtained from 548 subjects. These were converted into weight z score and BMI z score (from 2 years) by reference to the Centers for Disease Control and Prevention 2000 standardized reference growth charts (http://www.cdc.gov/nchs/). Subjects with missing data for HLA or dietary variables >5% were allocated to the “unknown” category, which was included in the analysis (Tables 1–3). The maximum number of subjects with missing data for a risk factor was 30% (Table 1), and these subjects included those who had fewer missing data for other variables. Of the 548 subjects, 44 (8.0%) subjects had a missing weight at 2 or 4 years, and 18 of 548 (3%) subjects had a missing birth weight; these subjects were excluded from analysis of weight z score and BMI z score at these time points.
Table 1.
Characteristics of subjects categorized according to the development of islet autoimmunity
| Islet autoimmune | Not islet autoimmune | P | |
|---|---|---|---|
| n | 46 | 502 | |
| Age in years* | 2.2 ± 1.7 | 5.8 ± 3.2 | |
| Caucasian | 45 | 491 | 0.92 |
| Male sex | 21 (46) | 275 (54) | 0.28 |
| HLA DR-3,4 | 16 (35) | 50 (10) | 0.001 |
| HLA DR-3x or HLA DR-4x | 20 (43) | 266 (53) | |
| Other | 5 (11) | 65 (13) | |
| Unknown | 5 (11) | 118 (24) | |
| Birth weight z score | 0.28 ± 1.28 | 0.25 ± 1.18 | 0.86 |
| Gestational age | 38.6 ± 2.2 | 38.1 ± 2.7 | 0.25 |
| Weight z score | |||
| At 2 years | 0.79 ± 1.12 | 0.34 ± 1.17 | 0.02 |
| At 4 years | 0.77 ± 0.91 | 0.46 ± 1.10 | 0.08 |
| BMI z score | |||
| At 2 years | 0.90 ± 0.90 | 0.39 ± 1.21 | 0.02 |
| At 4 years | 0.76 ± 0.96 | 0.44 ± 1.18 | 0.11 |
| Duration of exclusive breast-feeding | |||
| 0 months | 8 (17) | 98 (19) | 0.98 |
| >0–3 months | 10 (22) | 99 (20) | |
| >3 months | 16 (35) | 176 (35) | |
| Unknown | 12 (26) | 129 (26) | |
| Total duration of breast-feeding | |||
| 0 months | 4 (9) | 49 (10) | 0.74 |
| >0–3 months | 7 (15) | 100 (20) | |
| >3 months | 23 (50) | 252 (50) | |
| Unknown | 12 (26) | 101 (20) | |
| Age of introduction of cow's milk protein† | |||
| 0–3 months | 15 (33) | 182 (36) | 0.21 |
| >3 months | 28 (61) | 248 (49) | |
| Unknown | 3 (7) | 72 (14) | |
| Age of introduction of nongluten cereal | |||
| >0–4 months | 18 (39) | 176 (35) | 0.80 |
| >4 months | 14 (31) | 174 (34) | |
| Unknown | 14 (30) | 152 (30) | |
| Age of introduction of gluten | |||
| >0–4 months | 5 (11) | 59 (12) | 0.86 |
| >4 months | 30 (66) | 340 (68) | |
| Unknown | 11 (24) | 103 (21) | |
| Breast-fed at introduction of cereals | |||
| Yes | 21 (46) | 228 (45) | 0.83 |
| No | 13 (28) | 160 (32) | |
| Unknown | 12 (26) | 114 (23) | |
| Breast-fed at introduction of cow's milk | |||
| Yes | 12 (26) | 134 (26) | 0.78 |
| No | 21 (46) | 249 (50) | |
| Unknown | 13 (28) | 119 (24) |
Data are means ± SD or n (%).
For islet autoimmune, age at visit when first tested positive for autoantibodies. For not islet autoimmune, age at last visit.
Cow's milk protein introduced as infant formulae, dairy products, or cow's milk.
Table 3.
Association of weight z score at 2 years and 4 years with islet autoimmunity
| Adjusted HR (95% CI)* | P | |
|---|---|---|
| Model 1 | ||
| Weight z score at 2 years | 1.43 (1.07–1.93) | 0.02 |
| Birth weight z score | 0.86 (0.66–1.14) | 0.3 |
| HLA-DR-3,4 | 1 | 0.002 |
| HLA-DR-3x or DR-4x | 0.31 (0.15–0.62) | |
| Other | 0.30 (0.11–0.85) | |
| Unknown | 0.16 (0.05–0.57) | |
| Model 2 | ||
| Weight z score at 2 years | 1.35 (0.99–1.84) | 0.06 |
| Birth weight z score | 0.88 (0.67–1.16) | 0.36 |
| HLA-DR-3,4 | 1 | 0.002 |
| HLA-DR-3x or DR-4x | 0.31 (0.15–0.62) | |
| Other | 0.30 (0.11–0.85) | |
| Unknown | 0.16 (0.05–0.57) |
Adjusted HRs for weight z score at 2 years (model 1) and 4 years (model 2) corrected for relationship with birth weight and HLA type.
Weight z scores and BMI z scores over time (continuous and categories) were entered into their respective parametric survival model as time-dependent covariates. Adjusted HRs were used to analyze the development of islet autoimmunity with weight z score and BMI z score over time while controlling for HLA type and with weight z score and BMI z score at 2 and 4 years while controlling for birth weight z score and HLA type. For each variable, subjects with incomplete data did not differ significantly from subjects with complete data with respect to mean follow-up time and frequency of islet autoimmunity. Statistical analyses were performed in Stata version 9 and SPSS 14.0. The level of significance was 0.05.
RESULTS
Of the 548 subjects, 46 (8.4%; 21 male) who were followed from birth for a mean ± SD of 5.7 ± 3.2 years developed islet autoimmunity as defined above, of whom 12 progressed to type 1 diabetes at 4.2 ± 2.1 years. Median age of onset of islet autoimmunity was 1.7 years (range 0.2–8.6 years). Characteristics of the study cohort are shown in Table 1. The most common raised islet antibody was IAA in 39 of 46 (85%) subjects. In the islet autoimmunity group, 14 subjects (30%) were positive for one islet antibody, 18 (39%) for two islet antibodies, and 14 (30%) for all three islet antibodies. In 2 of 20 subjects with transplacental transfer of an islet antibody from their mother with type 1 diabetes, this result was not transient, and they were categorized as having islet autoimmunity. Twenty-four of 421 (5.7%) subjects (12 male) developed raised tissue transglutaminase antibodies at 6.4 ± 2.6 years.
Weight z score
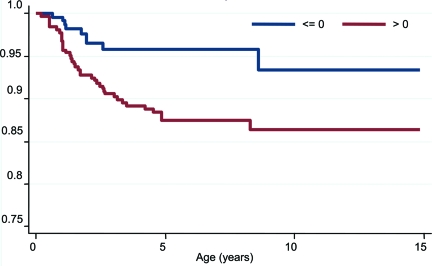
Weight z score over time was a continuous predictor of risk of islet autoimmunity (Table 2) and remained a continuous predictor of risk of islet autoimmunity after controlling for HLA type (adjusted HR 1.43 [95% CI 1.10–1.84] per unit increase in z, P = 0.007). Weight z score and BMI z score at 2 and 4 years were normally distributed. Weight z score at 2 years and change in weight z score between birth and 2 years predicted risk of islet autoimmunity (Tables 2 and 3). Weight z score at 4 years and change in weight z score between birth and 4 years showed a trend toward predicting risk of islet autoimmunity (Table 2). Birth weight z score was associated with weight z score at 2 years (r = 0.27, P < 0.001) and 4 years (r = 0.28, P < 0.001). Weight z score at 2 years remained a significant predictor of islet autoimmunity after controlling for birth weight z score and HLA type (Table 3). When weight z score over time was recorded in four categories (−1 or less, more than −1 to 0 or less, >0 to ≤1, and >1), there was a significant difference in the risk of islet autoimmunity between the four categories [χ2(3) = 8.1, P = 0.04]. When weight z score over time was dichotomized as ≤0 or >0, there was a significant difference in the risk of islet autoimmunity between the two categories [χ2(1) = 7.5, P = 0.006] (Fig. 1), and this remained after controlling for the effect of HLA type (adjusted HR 1 for weight z score ≤0; HR 2.61 [95% CI 1.26–5.44] for weight z score >0, P = 0.01). Length z score or height z score did not predict risk of islet autoimmunity development over time, and length z score at 2 years or height z score at 4 years did not either.
Table 2.
Parametric survival model on risk of islet autoimmunity
| Risk factor | n | Unadjusted HR (95% CI) | P |
|---|---|---|---|
| Weight z score over time | 1.44 (1.12–1.87) | 0.004 | |
| Weight z score | |||
| ≤0 | 1,932 | 1.0 | 0.006 |
| >0 | 2,664 | 2.77 (1.33–5.75) | |
| 2 years | 1.38 (1.05–1.83) | 0.02 | |
| 4 years | 1.29 (0.96–1.73) | 0.08 | |
| Change in weight z score | |||
| Between birth and 2 years | 1.28 (1.04–1.59) | 0.02 | |
| Between birth and 4 years | 1.23 (0.98–1.53) | 0.07 | |
| BMI z score over time | 1.31 (1.02–1.68) | 0.03 | |
| BMI z score | |||
| ≤0 | 1,902 | 1.0 | 0.09 |
| >0 | 2,431 | 1.81 (0.93–3.52) | |
| 2 years | 1.42 (1.06–1.89) | 0.02 | |
| 4 years | 1.27 (0.95–1.72) | 0.11 | |
| Age of introduction of gluten | |||
| ≤4 months | 0.90 (0.35–2.33) | 0.62 | |
| >4 months | 1 | ||
| Unknown | 1.39 (0.70–2.78) | ||
| Age of introduction of nongluten cereals | |||
| ≤4 months | 1.21 (0.60–2.42) | 0.70 | |
| >4 months | 1 | ||
| Unknown | 1.33 (0.63–2.79) | ||
| Duration of exclusive breast-feeding | |||
| 0 months | 0.95 (0.40–2.21) | 0.94 | |
| >0–3 months | 1.12 (0.51–2.46) | ||
| >3 months | 1 | ||
| Unknown | 1.21 (0.60–2.56) | ||
| Total duration of breast-feeding | |||
| 0 months | 0 | 0.93 (0.32–2.70) | 0.49 |
| >0–3 months | 0.82 (0.35–1.91) | ||
| >3 months | 1 | ||
| Unknown | 1.57 (0.78–3.15) | ||
| Age of introduction of cow's milk protein* | |||
| 0–3 months | 0.78 (0.42–1.46) | 0.47 | |
| >3 months | 1 | ||
| Unknown | 0.28 (0.52–1.71) | ||
| Breast fed at introduction of cow's milk* | |||
| Yes | 1.01 (0.50–2.05) | 0.49 | |
| No | 1 | ||
| Unknown | 1.49 (075–2.97) | ||
| Breast fed at introduction of cereals | |||
| Yes | 1.08 (0.54–2.15) | 0.61 | |
| No | 1 | ||
| Unknown | 1.45 (0.66–3.18) | ||
| Parity | 1.02 (0.77–1.37) | 0.85 | |
| Sex | |||
| Male | 1.0 | 0.24 | |
| Female | 1.42 (0.78–2.53) | ||
| Birth weight z score | 1.04 (0.82–1.33) | 0.74 | |
| Gestational age | 1.10 (0.95–1.28) | 0.20 | |
| HLA-DR | |||
| 3, 4 | 1 | <0.001 | |
| 3x or 4x | 0.27 (0.14–0.51) | ||
| Unknown | 0.21 (0.08–0.57) |
Cow's milk protein introduced as infant formulae, dairy products, or cow's milk.
Figure 1.
Kaplan-Meier curve of the development of islet autoimmunity over time in samples from subjects with weight z score >0 (n = 2,664 [58%]) and samples from subjects with weight z score ≤0 (n = 1,932 [42%]). Numbers of subjects at 0, 1, 2, 3, 4, and 5 years of follow-up were 548 (100%), 512 (93%), 449 (82%), 401 (73%), 349 (64%), and 286 (52%), respectively. In the parametric survival model, there was a significant difference between the HRs for the risk of islet autoimmunity in the two weight z score categories: HR 1 for weight z score ≤0, HR 2.77 (95% CI 1.33–5.75) for weight z score >0, χ2(1) = 7.5; P = 0.006.
BMI z score
BMI z score over time was a continuous predictor of risk of islet autoimmunity (Table 2) and remained a continuous predictor of risk of islet autoimmunity after controlling for HLA type (adjusted HR 1.29 [95% CI 1.01–1.67] per unit increase in z, P = 0.04). BMI z score predicted risk of islet autoimmunity at 2 years but not 4 years (Table 2). When BMI z score over time was dichotomized as ≤0 or >0, there was a borderline significant difference in risk of islet autoimmunity between the two categories [χ2(1) = 2.7, P = 0.09] (Table 2). There was no significant difference in risk of islet autoimmunity between the two BMI z score categories after controlling for HLA type (adjusted HR 1 for BMI z score ≤0, HR 1.69 [95% CI 0.90–3.30] for BMI z score >0, P = 0.12). Weight z score or BMI z score did not relate to the number of islet antibodies detected in subjects with islet autoimmunity.
Dietary intake
A food diary was completed for 505 of 548 (92.1%) subjects. Duration of breast- feeding and introduction of cow's milk protein and cereals are shown in Table 1. No subject who developed islet autoimmunity received cereals before 3 months of age including 16 subjects exposed to gluten; therefore, introduction of cereal was categorized before and after 4 months. Risk of development of islet autoimmunity did not relate to the timing of introduction of gluten-containing or gluten-free cereals (rice cereals), cow's milk–based infant formulae (containing intact cow's milk protein), cow's milk, dairy products (Table 2), soy milk, meat, fruit, or vegetables. Risk of development of islet autoimmunity did not relate to duration of exclusive breast-feeding or total duration of breast-feeding (Table 2). Risk of development of islet autoimmunity was not altered by whether a subject was breast-fed at the time of introduction of cereals or cow's milk (Table 2).
Pregnancy and birth
Parity, sex of the child, gestational age, and birth weight z score did not relate to risk of islet autoimmunity (Table 2).
HLA genotypes
HLA genotypes were categorized as HLA-DR-3,4; HLA-DR-4,x; HLA-DR-3,x; HLA-DR-2,x; and HLA-DR-x,x. There was no difference between the frequency of HLA-DR-3,4; HLA-DR-4,x; and HLA-DR-3,x between those with weight z score ≤0 (81%) and those with weight z score >0 (84%) in the islet autoimmunity group (P = 0.7). HLA-DR-3,x was present in 13 of 21 subjects who developed transglutaminase antibodies.
CONCLUSIONS
This prospective study from birth shows for the first time that early weight gain in childhood independently predicts the risk of early islet autoimmunity in children with an increased genetic risk of type 1 diabetes. The effect of weight gain both as weight z score and BMI z score was seen continuously over the 6 years of follow-up. Weight gain during the first 2 years of life was more predictive than weight gain during the first 4 years. This finding was expected because islet autoimmunity developed on average by 2 years and emphasizes the contribution of weight gain on risk of islet autoimmunity from birth. Similarly, weight z score was expected to be a stronger predictor than BMI z score, as BMI is not calculated until 2 years of age.
Rapid weight gain, as occurs in early life, may increase β-cell destruction if metabolically active β-cells are more vulnerable to damage than resting β-cells. Increased insulin secretion and high glucose upregulate GAD and Fas to Fas ligand, respectively, in β-cells in vitro (18,19). Metabolically active β-cells may therefore be destroyed by autoimmune (18) and nonautoimmune mechanisms (19); this destruction could occur independently or together according to the individual's genetic makeup.
We found no relationship between infant feeding and risk of islet autoimmunity, including detailed analysis of breast-feeding duration (total and exclusive), introduction of cow's milk protein (in infant formulae and milk solids), and introduction of gluten-free and gluten-containing cereals. There is an interaction between diet and weight gain in early childhood in the normal population. Formula-fed infants have accelerated weight gain from 3 months in comparison with breast-fed infants and an increased risk of overweight during childhood (20,21). Infant feeding practices themselves are interrelated in most populations in that breast-fed infants receive cereals in their diet later than bottle-fed infants. However, it is not possible to reconcile variable findings of the effect of infant diet on the development of islet autoimmunity (11–17), including reported harmful effects of early introduction of cow's milk or cereals (12–14), by an overriding risk of weight gain. To our knowledge no previous prospective birth cohort study of the development of islet autoimmunity has analyzed the contribution of weight and height data, in addition to infant diet, to risk of islet autoimmunity.
A limitation of this study is that the outcome measure was development of islet autoimmunity rather than type 1 diabetes. However, a number of studies have shown that persistent islet autoimmunity confers a high risk of later development of type 1 diabetes in first-degree relatives of individuals with type 1 diabetes (22). In addition, there were significant missing data in prospective diet records for breast-feeding and introduction of cereals, restricting the power of these risk analyses such that an effect could have been missed. These limitations are common to other birth cohort studies also and, in the case of prospective diet data, similar rates of data collection are described (12). We had robust power to relate weight and BMI to risk of islet autoimmunity.
Prior observations support weight gain driving β-cell loss after the development of islet autoimmunity as children progress to type 1 diabetes (4–6). We have extended this argument to show, in the first birth cohort study, that being above average in weight in early life increases the risk of islet autoimmunity in children with increased genetic risk. Over the next decade, the cohort can be followed to determine whether weight gain in the first years of life also predicts speed of progression to type 1 diabetes. Our findings have major implications for interventions to curb the epidemic of type 1 diabetes in early childhood and to target nutrition and weight gain in the first and second years of life.
Acknowledgments
This work was supported by grants from the Women's and Children's Hospital Research Foundation, South Australia, and Victorian Health Promotion Foundation, Victoria, Australia.
No potential conflicts of interest relevant to this article were reported.
We thank Cheryl Steele, Tania Kelly, and Fiona Williams for expert research nurse assistance and Shane Gellert and Natalie Stone for expert laboratory assistance.
Published ahead of print at http://care.diabetesjournals.org on 3 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.International Diabetes Federation: Incidence [article online], 2005. Available from http://www.eatlas.idf.org/Incidence. Accessed 11 November 2008
- 2.Wilkin TJ: The accelerator hypothesis: weight gain as the missing link between type I and type II diabetes. Diabetologia 44:914–922, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Knerr I, Wolf J, Reinehr T, Stachow R, Grabert M, Schober E, Rascher W, Holl RW, DPV Scientific Initiative of Germany and Austria: The accelerator hypothesis: relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9,248 German and Austrian children with type 1 diabetes. Diabetologia 48:2501–2504, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D, D'Agostino RB, Mayer-Davis EJ, Pettitt DJ, Imperatore G, Dolan LM, Pihoker C, Hillier TA, Marcovina SM, Linder B, Ruggiero AM, Hamman RF, Search for Diabetes in Youth Study Group: Testing the accelerator hypothesis: body size, β-cell function, and age at onset of type 1 (autoimmune) diabetes. Diabetes Care 29:290–294, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC: Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 47:1661–1667, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Sosenko JM, Krischer JP, Palmer JP, Mahon J, Cowie C, Greenbaum CJ, Cutherbertson D, Lachin JM, Skyler JS, Diabetes Prevention Trial–Type 1 Study Group: A risk score for type 1 diabetes derived from autoantibody-positive participants in the Diabetes Prevention Trial–Type 1. Diabetes Care 31:528–533, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Hypponen E, Kenward MG, Virtanen SM, Plitulainen A, Virta-Autio P, Tuomilehto J, Knip M, Akerblom HK: Infant feeding, early weight gain and risk of type 1 diabetes. Diabetes Care 22:1961–1965, 1999 [DOI] [PubMed] [Google Scholar]
- 8.EURODIAB Substudy 2 Study Group: Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care 25:1755–1760, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Bruining GJ, Netherlands Kolbrie Study Group of Childhood Diabetes: Association between infant growth before onset of juvenile type 1 diabetes and autoantibodies to IA2. Lancet 356; 655–656, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Colman PG, Steele C, Couper JJ, Beresford SJ, Powell T, Kewming K, Pollard A, Gellert S, Tait B, Honeyman M, Harrison LC: Islet autoimmunity in infants with a type 1 diabetic relative is common but is frequently restricted to one autoantibody. Diabetologia 43:203–209, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Couper JJ, Steele C, Beresford S, Powell T, McCaul K, Pollard A, Gellert S, Tait B, Harrison LC, Colman PG: Lack of association between breast feeding and cow's milk introduction and the development of islet autoimmunity in infants at risk of type 1 diabetes. Diabetes 48:2145–2149, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E: Early infant feeding and risk of developing type 1 diabetes associated autoantibodies. JAMA 290:1721–1728, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Kimpimaki T, Erkkola M, Korhonen S, Kupila A, Virtanen SM, Ilonen J, Simell O, Knip M: Short-term exclusive breastfeeding predisposes young children with increased genetic risk of type I diabetes to progressive β-cell autoimmunity. Diabetologia 44:63–69, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, Rewers M: Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 290:1713–1720, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Oroton HD, Baron AE, Clare-Salzer M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M: Omega 3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk of type 1 diabetes. JAMA 298:1420–1428, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Fronczak CM, Baron AE, Chase HP, Ross C, Brady HL, Hoffman M, Eisenbarth GS, Rewers M: In utero dietary exposures and risk of islet autoimmunity in children. Diabetes Care 26:3237–3242, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Virtanen SM, Kenward MG, Erkkola M, Kautiainen S, Kronberg-Kippila C, Hakulinen T, Ahonen S, Uusitalo L, Niinisto S, Veijola R, Simell O, Ilonen J, Knip M: Age at introduction of new foods and advanced β cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia 49:1512–1521, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Björk E, Kampe O, Andersson A, Karlsson FA: Expression of the 64 kDa/glutamic acid decarboxylase rat islet cell antigen is influenced by the rate of insulin secretion. Diabetologia 32:490–493, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Maedler K, Spinas GA, Lehmann R, Sergeev, Weber M, Fonatana A, Kaiser N, Donath MY: Glucose induces β-cell apoptosis via upregulation of the Fas receptor in human islets. Diabetes 50:1683–1690, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Stettler N: Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life. Int J Obes 31:1035–1043, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Moreno LA, Rodriguez G: Dietary risk factors for development of childhood obesity. Curr Opin Clin Nutr Metab Care 10:336–341, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS: Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes 45:926–933, 1996 [DOI] [PubMed] [Google Scholar]