Abstract
OBJECTIVE—Impaired glucose tolerance (IGT) represents a pre-diabetic state. Controversy continues in regards to its pathophysiology. The aim of this study was to investigate the differences in insulin sensitivity (IS) and secretion in obese adolescents with IGT compared with those with normal glucose tolerance (NGT) and type 2 diabetes.
RESEARCH DESIGN AND METHODS—A total of 12 obese adolescents with NGT, 19 with IGT, and 17 with type 2 diabetes underwent evaluation of insulin sensitivity (3-h hyperinsulinemic [80mu/m2/min]–euglycemic clamp), first-phase insulin and second-phase insulin secretion (2-h hyperglycemic clamp), body composition, and abdominal adiposity. Glucose disposition index (GDI) was calculated as the product of first-phase insulin × insulin sensitivity.
RESULTS—Insulin-stimulated glucose disposal was significantly lower in subjects with type 2 diabetes compared with subjects with NGT and IGT, with no difference between the latter two. However, compared with youth with NGT, youth with IGT have significantly lower first-phase insulin and C-peptide levels and GDI (P = 0.012), whereas youth with type 2 diabetes have an additional defect in second-phase insulin. Fasting and 2-h glucose correlated with GDI (r = −0.68, P < 0.001 and r = −0.73, P < 0.001, respectively) and first-phase insulin but not with insulin sensitivity.
CONCLUSIONS—Compared with youth with NGT, obese adolescents with IGT have evidence of a β-cell defect manifested in impaired first-phase insulin secretion, with a more profound defect in type 2 diabetes involving both first- and second-phase insulin. GDI shows a significantly declining pattern: it is highest in NGT, intermediate in IGT, and lowest in type 2 diabetes. Such data suggest that measures to prevent progression or conversion from pre-diabetes to type 2 diabetes should target improvement in β-cell function.
Impaired glucose tolerance (IGT) is a condition of altered glucose homeostasis associated with a high risk of progression to type 2 diabetes in adults (1) and children (2). The prevalence of IGT in children varies depending on the population studied, with rates varying from 4.1–4.5% in children recruited from the community (3,4) to up to 25% in youth from an obesity clinic (5). Also, 28% of high-risk Latino children with positive family history of type 2 diabetes have IGT (6). Therefore, against the backdrop of the obesity epidemic, IGT constitutes a significant problem in youth, especially those from ethnic minority populations and those with a family history of type 2 diabetes. However, the pathophysiology of IGT in children is not well understood.
In longitudinal studies of adult populations at high risk for type 2 diabetes, such as the Pima Indians (7), the progression from normal glucose tolerance (NGT) to IGT and type 2 diabetes was associated with an increase in body weight, worsening of insulin sensitivity, and decrease in biphasic insulin secretion (7,8). Longitudinal studies are not available in the pediatric age-group. Studies in pediatrics using different methodologies have shown conflicting results. Obese children and adolescents with IGT were reported to have higher BMI and worse fasting indexes of insulin resistance compared with those with NGT, but insulin secretion was estimated to be similar between the two groups (5). In overweight Latino children with a family history of type 2 diabetes, insulin sensitivity and acute insulin response were not different but glucose disposition index was lower in those with IGT (6). In our previous study of obese adolescent girls with polycystic ovary syndrome (PCOS), subjects with IGT and subjects with NGT of similar body composition and abdominal fat distribution had similar insulin sensitivity but lower first-phase insulin secretion and lower glucose disposition index (9). In the present study, we aimed to extend our previous observation and to investigate the differences in insulin sensitivity and insulin secretion not only between subjects with NGT and subjects with IGT but also between those with IGT and those with type 2 diabetes. We hypothesized that 1) insulin sensitivity is not significantly different between equally obese youth with IGT and those with NGT and 2) insulin secretion is impaired in IGT and type 2 diabetes compared with NGT, with a severity gradient from IGT to type 2 diabetes.
RESEARCH DESIGN AND METHODS
Study population
A total of 12 obese adolescents with NGT, 19 with IGT, and 17 with type 2 diabetes were studied; subjects were African-American and American Caucasian. All subjects had exogenous obesity and were not involved in any regular physical activity or weight-reduction programs. They were recruited through flyers posted in the community and the health center. The NGT and IGT adolescents had normal fasting glucose (<100 mg/dl), with a 2-h glucose value during an oral glucose tolerance test (OGTT) of <140 mg/dl in NGT and 140–199 mg/dl in IGT subjects. They were not on any medications that affect glucose metabolism. The adolescents with type 2 diabetes were clinically diagnosed according to American Diabetes Association and World Health Organization criteria (10), with a mean A1C of 10.1 ± 3.0% and glucose level of 277.2 ± 158.2 mg/dl at presentation and negative glutamic acid decarboxylase and islet cell autoantibodies. They all had adequate metabolic control, with an average A1C of 6.6 ± 0.2% (range 4.7–8.3%) and average duration of diabetes of 4.8 ± 5.7 months (0–18 months). They were on treatment consisting of lifestyle changes alone (n = 3), metformin (n = 6), metformin + insulin (n = 7), or insulin alone (n = 1). Metformin and long-acting insulin were discontinued 48 h before the clamp studies. All studies were approved by the institutional review board of the University of Pittsburgh. Informed consent was obtained. Characteristics of the study participants are summarized in Table 1.
Table 1.
Physical characteristics and fasting metabolic profile in adolescents with NGT, IGT, and type 2 diabetes
| NGT | IGT | Type 2 diabetes | |
|---|---|---|---|
| n | 12 | 19 | 17 |
| Age (years) | 14.2 ± 2.2 | 13.8 ± 1.5 | 14.7 ± 1.3 |
| Sex (male/female)* | 4/8 | 6/13 | 7/10 |
| Ethnicity* | |||
| African American | 5 | 5 | 7 |
| American Caucasian | 7 | 14 | 10 |
| Tanner Stage* | |||
| II–III | 4 | 3 | 2 |
| IV–V | 8 | 16 | 15 |
| Estradiol in females (pmol/l) | 232.7 ± 171.1 | 294.8 ± 211.8 | 222.8 ± 161.9 |
| DHEAS (nmol/l) | |||
| Females | 3,669.28 ± 759.2 | 4,108.8 ± 3,001.4 | 3,687.0 ± 1,902.0 |
| Males | 3,659.9 ± 1,366.0 | 5,415.0 ± 3,085.7 | 4,139.9 ± 2,332.0 |
| BMI (kg/m2) | 36.0 ± 5.2 | 35.0 ± 6.6 | 36.3 ± 5.3 |
| Waist circumference (cm) | 108.5 ± 18.9 | 104.3 ± 14.2 | 107.9 ± 11.8 |
| % Body fat | 45.4 ± 4.7 | 44.3 ± 4.3 | 41.0 ± 6.8 |
| Fat mass (kg) | 40.0 ± 6.9 | 40.7 ± 10.9 | 40.1 ± 10.5 |
| Subcutaneous abdominal fat (cm2) | 545.7 ± 168.6 | 501.6 ± 145.7 | 520.1 ± 152.4 |
| Visceral fat (cm2) | 75.8 ± 48.3 | 72.1 ± 25.1 | 78.7 ± 25.2 |
| A1C (%) | 5.2 ± 0.5a | 5.3 ± 0.4b | 6.8 ± 0.8 |
| Fasting glucose (mmol/l) | 5.1 ± 0.02a | 5.1 ± 0.2b | 6.6 ± 1.4 |
| Fasting insulin (pmol/l) | 252.6 ± 95.1 | 240.0 ± 130.7 | 274.2 ± 142.0 |
| Hepatic glucose production (μmol · kg−1 · min−1) | 10.5 ± 1.9 | 12.8 ± 3.5 | 13.3 ± 2.3 |
| Cholesterol (mmol/l) | 4.6 ± 0.9 | 4.3 ± 0.8 | 3.9 ± 0.8 |
| HDL cholesterol (mmol/l) | 1.1 ± 0.3 | 1.0 ± 0.3 | 0.9 ± 0.2 |
| LDL cholesterol (mmol/l) | 2.9 ± 0.9 | 2.6 ± 0.7 | 2.3 ± 0.6 |
| Triglycerides (mmol/l) | 1.5 ± 0.6 | 1.8 ± 1.0 | 1.4 ± 0.8 |
Data are means ± SD unless otherwise indicated. Four IGT subjects had VAT and subcutaneous abdominal adipose tissue evaluation by abdominal magnetic resonance imaging. Excluding these subjects from the analysis does not change the data. Superscripts are ANOVA P values for post-hoc analysis:
, P < 0.05 in NGT vs. type 2 diabetes;
, P < 0.05 in IGT vs. type 2 diabetes.
The χ2 analysis revealed no significant differences among groups with respect to ethnicity, sex, and Tanner stage. DHEAS, dehydroepiandrosterone sulfate.
Clamp studies
Participants were admitted twice within a 1–3 week period to the Pediatric Clinical and Translational Research Center on the days before the clamp studies, and a hyperinsulinemic-euglycemic clamp and a hyperglycemic clamp study were performed in random order. Each participant underwent a 2-h OGTT (1.75 g/kg of glucola [max 75 g]) the day before the first clamp.
In vivo insulin sensitivity
A fasting blood sample was obtained for determination of total cholesterol, LDL cholesterol, HDL cholesterol, VLDL cholesterol, triglycerides, and A1C. Fasting endogenous glucose production was measured with a primed constant rate infusion of [6, 6-2H2] glucose (0.306 ± 0.009 μmol · kg−1 · min−1) (Isotech, Miamisburg, OH) (11,12). Blood was sampled at the start of the 2-h stable isotope infusion and every 10 min from −30 to 0 min (basal period) for determination of plasma glucose, insulin, and isotopic enrichment of glucose. Following this basal period, insulin-mediated glucose metabolism and substrate utilization were evaluated during a 3-h hyperinsulinemic-euglycemic clamp (11,12). Intravenous crystalline insulin (Humulin; Lilly Indianapolis, IN) was infused at a constant rate of 80 mU/m2 per min, and plasma glucose was clamped at 5.6 mmol/l with a variable rate infusion of 20% dextrose as before (11). Continuous indirect calorimetry (Deltatrac Metabolic Monitor; Sensormedics, Anaheim, CA) was used to measure CO2 production, O2 consumption, and respiratory quotient for 30 min at baseline and at the end of the euglycemic clamp (12).
In vivo insulin secretion
First and second-phase insulin secretion was evaluated during a 2-h hyperglycemic clamp (12.5 mmol/l) as previously described (11).
Body composition
Body composition was determined by a dual-energy X-ray absorptiometry scan. Subcutaneous abdominal adipose tissue and visceral adipose tissue (VAT) were determined by a single-slice computed tomography (CT) scan at L4-L5, as before (11).
Biochemical measurements
Plasma glucose was measured with a glucose analyzer (Yellow Springs Instrument, Yellow Springs, OH), and insulin, C-peptide, and adiponectin were measured by radioimmunoassay, as before (11). A1C was measured by high-performance liquid chromatography (Tosoh Medics), and lipids were measured using the standards of the Centers for Disease Control and Prevention (11). Deuterium enrichment of glucose in the plasma was determined on a Hewlett-Packard Co. 5973 mass spectrometer (Palo Alto, CA) coupled to a 6,890 gas chromatograph (11,12).
Calculations
Fasting hepatic glucose production (HGP) was calculated during the last 30 min of the 2-h isotope infusion according to steady-state tracer dilution equations (11). Insulin-stimulated glucose disposal rate (Rd) was calculated during the last 30 min of the euglycemic clamp to be equal to the rate of exogenous glucose infusion. Peripheral insulin sensitivity was calculated by dividing the Rd by the steady-state clamp insulin level (11). Insulin-stimulated carbohydrate oxidation rates were calculated according to the formulas of Frayn (12) from the indirect calorimetry data. Nonoxidative glucose disposal was estimated by subtracting the rate of glucose oxidation from the total Rd. During the hyperglycemic clamp, the first- and second-phase insulin and C-peptide concentrations were calculated as described previously (11,12). Glucose disposition index (GDI) was calculated as the product of insulin sensitivity × first-phase insulin.
Statistics
Statistical analyses were performed using three-way ANOVA followed by post hoc Bonferroni or Dunnett's correction. Kruskal-Wallis test was used for multiple group comparison of nonparametric variables. Spearman's correlation and multiple regression analyses were used to evaluate bivariate and multivariate relationships, respectively, and χ2 was used to evaluate nonparametric variables. Data are presented as means ± SD. Two-tailed P < 0.05 was considered statistically significant.
RESULTS
Study subjects
Table 1 depicts the physical characteristics and fasting metabolic profile of the participants (NGT vs. IGT vs. type 2 diabetes). There were no significant differences in age, pubertal stage, or ethnic distribution among the three groups. There were no significant differences in body composition or abdominal fat distribution among the three groups.
Fasting metabolic profile
There were no significant differences in fasting lipids and insulin levels among the three groups. Fasting glucose and A1C were significantly higher in type 2 diabetic subjects compared with the two other groups. HGP was 20% higher in subjects with type 2 diabetes compared with the NGT group (P = 0.078). A1C did not differ among the four groups of type 2 diabetic subjects on different treatments (A1C 6.5 ± 0.5% with lifestyle alone, 7.0 ± 0.7% with metformin alone, 6.7 ± 1.1% with metformin + insulin, and 6.9% with insulin alone).
Clamp data
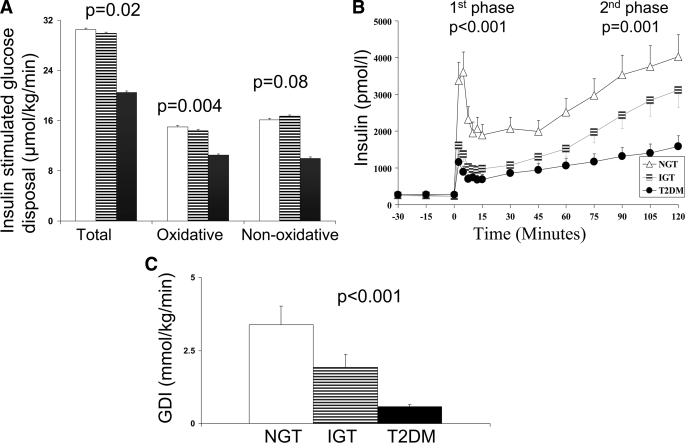
During the hyperinsulinemic-euglycemic clamp, steady-state glucose and insulin levels were not different among the three groups (NGT, IGT, type 2 diabetes) (glucose: 5.6 ± 0.08, 5.6 ± 0.10, and 5.6 ± 0.13 mmol/l, respectively; insulin: 2,020.8 ± 587.2, 1,737.6 ± 508.9, and 1,791.6 ± 674.2 pmol/l, respectively). Insulin-stimulated total and oxidative glucose disposal were not different between NGT and IGT but were significantly lower in type 2 diabetes (Fig. 1-A). Nonoxidative glucose disposal tended to be lower in those with type 2 diabetes than in those with NGT and IGT (P = 0.08) (Fig. 1-A). The data remained consistent when Rd was expressed per kg of fat-free mass (μmol · min−1 · kg−1) (P = 0.006).
Figure 1.
A: Insulin-stimulated total, oxidative, and nonoxidative glucose disposal in NGT (□), IGT ( ), and type 2 diabetes (▪). B: First and second-phase insulin levels in NGT (▵), IGT (
), and type 2 diabetes (▪). B: First and second-phase insulin levels in NGT (▵), IGT ( ), and type 2 diabetes (•). C: Glucose disposition index in NGT, IGT, and type 2 diabetes. Error bars reflect SEs.
), and type 2 diabetes (•). C: Glucose disposition index in NGT, IGT, and type 2 diabetes. Error bars reflect SEs.
During the hyperglycemic clamp, the IGT adolescents had lower first-phase insulin and C-peptide levels than subjects with NGT, with no difference in second-phase insulin (Table 2, Fig. 1B). Type 2 diabetic adolescents had lower first- and second-phase insulin and C-peptide levels compared with adolescents with NGT and a tendency for lower second-phase insulin (P = 0.07) and lower second-phase C-peptide (P = 0.012) compared with adolescents with IGT (Table 2, Fig. 1-B). GDI was significantly lower in IGT compared with NGT and lowest in type 2 diabetes (Fig. 1-C). GDI did not differ in the type 2 diabetic subjects in the four treatment groups (0.5 ± 0.3 mmol · kg−1 · min−1 in the lifestyle, 0.6 ± 0.4 mmol · kg−1 · min−1 in the metformin alone, 0.6 ± 0.3 mmol · kg−1 · min−1 in the metformin + insulin, and 0.6 mmol · kg−1 · min−1 in the insulin alone groups).
Table 2.
Hyperglycemic clamp data in the three groups
| NGT | IGT | Type 2 diabetes | ANOVA | |
|---|---|---|---|---|
| n | 12 | 19 | 17 | P |
| First-phase insulin (pmol/l) | 2,376.0 ± 1,729.9a,b | 1,182.0 ± 625.2 | 708.0 ± 938.4 | 0.001 |
| First-phase C-peptide (nmol/l) | 4.3 ± 2.0b | 3.0 ± 1.0 | 2.2 ± 1.4 | 0.001 |
| Second-phase insulin (pmol/l) | 2,563.2 ± 1,363.2b | 1,902.6 ± 1,336.2 | 982.2 ± 797.0 | 0.003 |
| Second-phase C-peptide (nmol/l) | 5.0 ± 1.5b | 4.7 ± 1.9c | 3.0 ± 1.1 | 0.002 |
Data are means ± SD unless otherwise indicated. C-peptide levels were not available in 3 subjects with IGT. Superscripts are ANOVA P values for post-hoc analysis (Bonferroni correction):
, P < 0.05 in NGT vs. IGT;
, P < 0.05 in NGT vs. type 2 diabetes;
: P < 0.05 in IGT vs. type 2 diabetes.
Relationship between OGTT indexes of glucose tolerance and clamp data
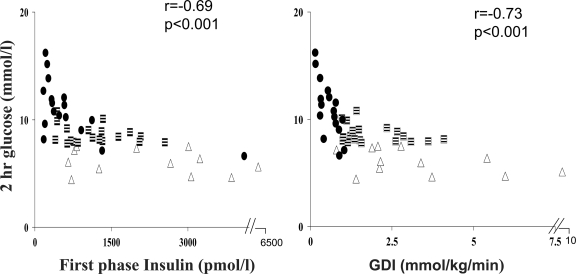
The 2-h glucose level during the OGTT correlated with GDI (r = −0.73, P < 0.001) and first-phase (r = −0.69, P < 0.001) and second-phase (r = −0.59, P < 0.001) insulin, but it did not correlate with insulin sensitivity (r = −0.13, P = 0.4) (Fig. 2). In multiple regression analysis with 2-h glucose post-OGTT as the dependent variable and VAT, GDI, and second-phase insulin as the independent variables, GDI (β = −0.54, P = 0.001) but not second-phase insulin nor VAT contributed significantly to the variance in the-2 h glucose (R2 = 0.44, P < 0.001). Similarly, fasting glucose correlated with GDI (r = −0.68, P < 0.001) and first-phase (r = −0.61, P < 0.001) and second-phase (r = −0.53, P < 0.001) insulin levels but not with insulin sensitivity (r = −0.2, P = 0.3).
Figure 2.
Relation of first-phase insulin and GDI to the 2-h glucose during the OGTT in NGT (▵), IGT ( ), and type 2 diabetes (•).
), and type 2 diabetes (•).
CONCLUSIONS
The present investigation demonstrates that IGT in youth is characterized by impaired insulin secretion relative to insulin sensitivity. The GDI is lowest in youth with type 2 diabetes, intermediate in those with IGT, and highest in those with NGT. Compared with NGT, glucose disposition index is ∼40% lower in those with pre-diabetes and 80% lower in those with type 2 diabetes. While insulin secretion is impaired in IGT, insulin-stimulated glucose disposal is not different from that in NGT. In youth with type 2 diabetes, the impairment in β-cell function is of greater magnitude and involves second-phase insulin secretion as well. The current study adds to the limited existing literature by 1) providing a comparison between three groups of equally obese adolescents (those with NGT, IGT, and type 2 diabetes) of similar BMI, pubertal stage, body composition, and abdominal fat distribution and 2) providing information on in vivo insulin sensitivity and secretion measured simultaneously by the clamp methodology.
Impaired glucose tolerance is a well- known pre-diabetic state with a linear relationship between the 2-h postchallenge glucose levels and subsequent risk for type 2 diabetes in adult prospective studies (1), with observed rates of progression from IGT to type 2 diabetes from 20% (13) to 60% (14,15) over an average duration of 2–8 years (1). Impaired glucose tolerance seems amenable to intervention with prevention of type 2 diabetes reported in many randomized control trials, with lifestyle intervention being at least as effective if not more effective than pharmacotherapy (16). Therefore, it is important to identify and characterize the pathopysiological mechanism(s) underlying IGT in youth in an effort to provide targeted intervention and prevention of progression to type 2 diabetes.
Our current findings are consistent with data in adults with IGT, where longitudinal studies point to the deterioration in insulin secretion relative to insulin sensitivity in the transition from NGT to IGT to type 2 diabetes (8). Pima Indians with isolated IGT had a modest decrease in acute insulin response (AIR), as measured by an intravenous glucose tolerance test, which was significant given increased insulin resistance in subjects with IGT compared with those with NGT (17). However, in these adult studies, the decrease in insulin sensitivity in subjects with IGT was attributed to aging over the 5 years of the study (8) or to higher BMI in the subjects with IGT (17). In our study, NGT and IGT groups had comparable obesity and body fat distribution. Furthermore, in Pima Indians, a greater defect in AIR was found in those who subsequently developed type 2 diabetes (7). Similarly, in Mexican Americans, the 7-year risk of progression to type 2 diabetes was significantly higher in subjects with IGT than in those with NGT (OR = 9.4), and both decreased insulin secretion (determined by Δ I30/Δ G30) and insulin resistance independently predicted the progression to type 2 diabetes (18). These adult longitudinal studies support the role of impaired β-cell function in the risk of progression from NGT to IGT to type 2 diabetes. Studies in pediatrics examining IGT have been few and somewhat contradictory.
Higher insulin resistance, as measured by the hyperinsulinemic-euglycemic clamp method, was reported in adolescents with IGT compared with control subjects with NGT, with no significant differences in insulin secretion (19). However, the group with IGT had a significantly higher ratio of visceral to subcutaneous abdominal fat (P = 0.002). We previously demonstrated that higher visceral fat is associated with lower insulin sensitivity in obese insulin-resistant youth (12). Thus, the lower insulin sensitivity in IGT in the former study could be related to the higher level of visceral fat. In another study, the same group reported that IGT is characterized by a decline in AIR, based on OGTT data (20). In that study, the children with IGT were heavier and had significantly higher BMI z scores than children with NGT, but abdominal adiposity was not evaluated (20). However, when researchers evaluated subjects with NGT, IGT, and of similar BMI and % body fat using mathematical modeling of the hyperglycemic clamp data, the glucose sensitivity of first-phase insulin secretion declined from NGT to IGT and from IGT to type 2 diabetes (although absolute insulin levels did not) (21). Also, recently, they reported decreased glucose sensitivity of first-phase insulin secretion in subjects with IGT compared with those with NGT, which was consistent with our findings (22). The different findings in these studies could be attributed to different methodologies used and the differences in BMI and body composition between the NGT and IGT groups. Data in high-risk overweight Latino children were consistent with our present observations. Subjects with NGT and IGT of similar body composition and abdominal fat distribution had similar insulin sensitivity, but subjects with IGT had relative insulin deficiency with significantly lower GDI than those with NGT (6). Lastly, the current findings confirm our previous observations in girls with PCOS (9).
With regards to type 2 diabetes, the impairment in first-phase insulin secretion is of greater magnitude than that in IGT, with the added dysfunction in second-phase insulin. In type 2 diabetes, first-phase insulin is ∼70% lower than that in NGT and ∼40% lower than that in IGT. Second-phase insulin is ∼60% lower in type 2 diabetes than in NGT, but it is preserved in IGT. This is consistent with our previous report of decreased second-phase insulin levels in type 2 diabetic subjects vs. obese control subjects (23) and with the findings of Weiss et al. (20) of decreased glucose sensitivity of second-phase insulin in type 2 diabetic subjects.
Hepatic glucose production was higher in type 2 diabetes than in NGT. This is consistent with our previous report of increased HGP in type 2 diabetic youth than in obese control subjects (23) and with adult data suggesting that increased endogenous glucose production contributes to fasting hyperglycemia (17). Finally, our data demonstrate that first-phase insulin and GDI are significant determinants of measures of glycemic regulation, including fasting glucose and 2-h glucose during the OGTT. These findings are in agreement with our findings in girls with PCOS (9) and with the adult literature in terms of the determinants of the glycemic status in subjects with IGT, although all the variables were not measured simultaneously in these subjects (24,25).
A potential limitation of the current study is that the IGT subjects were compared with type 2 diabetic children with different treatment modalities and diabetes duration. However, data analysis performed separately in the type 2 diabetic subjects did not show any significant differences in A1C or GDI in the four treatment groups. Also, the majority of these adolescents (12 out of 17) were studied within 6 months of diagnosis of type 2 diabetes, with no significant difference in GDI or A1C when evaluated according to duration of diabetes and no relationship between duration of diabetes and A1C, which was consistent with our previous findings (23). The other limitation of the relatively smaller sample size (NGT = 12) is offset by the use of the clamp methodology, which allowed us to demonstrate significant differences among the three groups.
In summary, our findings demonstrate that pre-diabetes, or IGT, is an intermediate stage in the impairment of β-cell function relative to insulin resistance, with type 2 diabetes having a more pronounced defect in first- and second-phase insulin secretion. Such data suggest that measures to prevent conversion/progression of IGT to type 2 diabetes should target recovery of β-cell function in addition to improvement in obesity and insulin resistance. The ultimate objective is restoration of glucose homeostasis through improved balance between insulin sensitivity and secretion.
Acknowledgments
This work was supported by U.S. Public Health Service Grants RO1 HD27503 and K24 HD01357 (S.A.A.), the Thrasher Research Fund (F.B., N.G.), the Pittsburgh Foundation (N.G.), Children's Hospital of Pittsburgh General Clinical Research Center Grant MO1 RR00084 Clinical and Translational Science Award, and (F.B., N.G., and S.A.A.) UL1 RR024153 (E.B. and S.A.A.).
No potential conflicts of interest relevant to this article were reported.
These studies would not have been possible without the nurses and staff of the Pediatric Clinical and Translational Research Center, the efforts of the research team, the laboratory expertise of Resa Stauffer, and the commitment of the study volunteers and their parents.
Published ahead of print at http://care.diabetesjournals.org on 3 October 2008.
N.G. is currently affiliated with the Children's Hospital at Scott and White, Texas A&M Health Science Center, Pediatric Endocrinology, Temple, Texas.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, Haffner SM, Pettitt DJ, Sorkin JD, Muller DC, Collins VR, Hamman RF: Predictors of progression from impaired glucose tolerance to NIDDM: An analysis of six prospective studies. Diabetes 46:701–710, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss R, Taksali S, Tamborlane W, Burgert T, Savoye M, Caprio S: Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 28:902–909, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Uwaifo GI, Elberg J, Yanovski JA: Impaired glucose tolerance in obese children and adolescents. N Engl J Med 347:290–292, 2002 [PubMed] [Google Scholar]
- 4.Invitti C, Gilardini L, Viberti G: Impaired glucose tolerance in obese children and adolescents. N Engl J Med 347:290–292, 2002 [PubMed] [Google Scholar]
- 5.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, Savoye M, Rieger V, Taksali S, Barbetta G, Sherwin RS, Capro S: Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 346:802–810, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Goran M, Bergman R, Avila Q, Watkins M, Ball G, Shaibi G, Weigensberg M, Cruz M: Impaired glucose tolerance and reduced beta cell function in overweight Latino children with a positive family history for Type 2 diabetes. J Clin Endocrinol Metab 89:207–212, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Festa A, Williams K, D'Agostino R Jr, Wagenknecht L, Haffner S: The natural course of B-cell function in nondiabetic and diabetic individuals: The Insulin Resistance Atherosclerosis Study. Diabetes 55:1114–1120, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Arslanian SA, Lewy VD, Danadian K: Glucose intolerance in obese adolescents with polycystic ovary syndrome: Roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab 86:66–71, 2001 [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26:3160–7, 2003. 14578255 [Google Scholar]
- 11.Bacha F, Saad R, Gungor N, Arslanina S: Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care 27:547–52, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Bacha F, Saad R, Gungor N, Arslanina SA: Are obesity-related metabolic risk factors modulated by the degree of insulin resistance in adolescents. Diabetes Care 29:1599–1604, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hamman RF, Marshall JA, Baxter J, Kahn LB, Mayer EJ, Orleans M, Murphy JR, Lezotte DC: Methods and prevalence of non-insulin-dependent diabetes mellitus in a biethnic Colorado population: the San Luis Valley Diabetes Study. Am J Epidemiol 129:295–311, 1989 [DOI] [PubMed] [Google Scholar]
- 14.Knowler WC, Pettit DJ, Saad MF, Bennett PH: Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabete Metab Rev 6:1–27, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH: The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med 319:1500–1506, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K: Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 334:299–308, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weyer C, Bogardus C, Pratley RE: Metabolic characteristics of individuals with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 48:2197–2203, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Haffner SM, Miettinen H, Gaskill SP, Stern MP: Decreased insulin secretion and increased insulin resistance are independently related to the 7-year risk of NIDDM in Mexican-Americans. Diabetes 44:1386–1391, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, Boselli L, Barbetta G, Allen K, Rife F, Savoye M, Dziura J, Sherwin R, Shulman GI, Caprio S: Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 362:951–957, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S: Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia 49:571–579, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R: β-cell function across the spectrum of glucose tolerance in obese youth. Diabetes 54:1735–1743, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Cali A, Bonadonna R, Trombetta M, Weiss R, Caprio S: Metabolic abnormalities underlying the different prediabetic phenotypes in obese adolescents. J Clin Endocrinol Metab 93:1767–1773, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gungor N, Bacha F, Saad R, Janosky J, Arslanian S: Youth type 2 diabetes: Insulin resistance, β-cell failure or both? Diabetes Care 28:638–644, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Festa A, D'Agostino R Jr, Hanley AJ, Karter AJ, Saad MF, Haffner SM: Differences in insulin resistance in nondiabetic subjects with isolated impaired glucose tolerance or isolated impaired fasting glucose. Diabetes 53:1549–1555, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA: beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 90:493–500, 2005 [DOI] [PubMed] [Google Scholar]