Abstract
OBJECTIVE—Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome is caused by FOXP3 mutations. We aimed to determine the prevalence, genetics, and clinical phenotype of FOXP3 mutations in a large cohort with permanent neonatal diabetes (PNDM).
RESEARCH DESIGN AND METHODS—The 11 coding exons and the polyadenylation region of FOXP3 were sequenced in 26 male subjects with diabetes diagnosed before 6 months of age in whom common genetic causes of PNDM had been excluded. Ten subjects had at least one additional immune-related disorder, and the remaining 16 had isolated diabetes.
RESULTS—We identified four hemizygous FOXP3 mutations in 6 of 10 patients with associated immune-related disorders and in 0 of 16 patients with isolated diabetes (P = 0.002). Three patients with two novel mutations (R337Q and P339A) and the previously reported L76QfsX53 developed classic IPEX syndrome and died within the first 13 months. The novel mutation V408M was found in three patients from two unrelated families and had a mild phenotype with hypothyroidism and autoimmune enteropathy (n = 2) or nephrotic syndrome (n = 1) and survival to 12–15 years.
CONCLUSIONS—FOXP3 mutations result in ∼4% of cases of male patients with permanent diabetes diagnosed before 6 months. Patients not only have classic IPEX syndrome but, unexpectedly, may have a more benign phenotype. FOXP3 sequencing should be performed in any male patient with the diagnosis of diabetes in the first 6 months who develops other possible autoimmune-associated conditions, even in the absence of full IPEX syndrome.
Type 1 diabetes is the leading cause of diabetes among children except for those in whom diabetes is diagnosed before the age of 6 months. HLA studies have shown that patients in whom diabetes is diagnosed within the first 6 months of life (permanent neonatal diabetes [PNDM]) do not harbor high-risk HLA haplotypes and hence are very unlikely to have classic type 1 diabetes (1,2). These patients should be tested for monogenic causes of neonatal diabetes.
Mutations in FOXP3 have been associated with a severe, early-onset, male-limited autoimmunity syndrome known as IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked; OMIM [Online Mendelian Inheritance in Man] 304930) (3–5). The gene maps to chromosome Xp11.23 and encodes a 431–amino acid protein, also named scurfin, required for the generation and functioning of CD4+CD25+ regulatory T lymphocytes. FOXP3-expressing CD4+ T cells are potent suppressors of self-reactive T-cell activation and proliferation, presumably via direct cell-cell interaction. Thus, lack of these cells results in an uncontrolled autoimmune reactivity in male patients with hemizygous FOXP3 mutations (6). In keeping with an X-linked recessive mode of inheritance, heterozygous carrier females remain completely asymptomatic, but each son has a 50% risk of being affected with IPEX syndrome. One patient with IPEX syndrome due to recessive inheritance of CD25 mutations has recently been reported (7).
Most patients with IPEX syndrome described to date have developed symptoms shortly after birth or during the first 3–4 months of life. The most common findings have been enteropathy (nearly 100% of patients), diabetes (∼70%), skin disease (∼65%), failure to thrive (∼50%), thyroiditis (∼30%), and recurrent infections (∼20%). Less common additional features include autoimmune cytopenias, pneumonitis, nephritis, hepatitis, vasculitis, arthritis, myositis, and alopecia as well as lymphadenopathy and splenomegaly. These disorders often appear sequentially rather than simultaneously, and the affected organ spectrum varies substantially from patient to patient (8). The life expectancy of patients with IPEX syndrome rarely extends beyond infancy.
However, a milder phenotype has been reported in a number of patients, who can live longer, sometimes into adulthood. Enteropathy was present in virtually all of them, although diabetes was frequently absent (9–11).
To our knowledge, FOXP3 has not been systematically studied before in patients with early-onset diabetes. Hence, we aimed to explore the prevalence of FOXP3 mutations in the largest worldwide cohort of PNDM.
RESEARCH DESIGN AND METHODS
This study was conducted in accordance with the Declaration of Helsinki, as revised in 2000. Informed consent was obtained from all patients, with parental consent given on behalf of children.
The study population originated from the International Society of Pediatric and Adolescent Diabetes (ISPAD) Rare Diabetes Study. In total, 296 patients (154 boys) with diabetes diagnosed in the first 6 months of life who were receiving insulin treatment at the time of referral were studied. A genetic cause for the disease had previously been identified in 171 subjects: KCNJ11 in 85 patients, INS in 37 patients, ABCC8 in 30 patients, GCK in 8 patients, EIF2AK3 in 8 patients, PTF1A in 2 patients, and IPF1 in 1 patient (12–14; S.E., A.T.H., unpublished data). The most common causes of PNDM had been excluded by direct sequencing in the remaining 125 patients, 70 of whom were male. Specifically, mutations in KCNJ11 had been excluded in 68 patients, ABCC8 mutations had been excluded in 63 patients, INS mutations had been excluded in 63 patients, and GCK mutations had been excluded in 31 patients.
Among the 70 male patients without a molecular diagnosis, we sequenced FOXP3 in 10 subjects from 9 unrelated families on the basis of the presence of PNDM and any other immune-mediated disease. As a comparison, we also sequenced FOXP3 in 16 further male patients with PNDM but without any associated immune disease.
DNA samples were collected in Exeter. Clinical information was obtained from hospital records with assistance from the referring clinicians. Pancreatic autoantibodies were measured locally according to the standard clinical practice. Sequence variants were tested for their presence in family members whenever a DNA sample was available.
Molecular genetic analysis
Genomic DNA was extracted from peripheral leukocytes using standard procedures. The 11 coding exons, minimal promoter, and the 3′-untranslated region of the FOXP3 gene including the polyadenylation signal were amplified by PCR; primers and conditions are available upon request. Sequence-specific primers for each amplicon were tagged with 5′ M13 tails to allow sequencing to be performed with a “universal” M13 primer.
Single strand sequencing was carried out using standard methods on an ABI 3730 sequencer (Applied Biosystems, Warrington, U.K.). Sequences were compared with the published sequence (NM_014009.3) using Mutation Surveyor (version 3.10). Any changes in the sequence were checked against published polymorphisms and mutations and for conservation across species.
We used a panel of microsatellites for chromosome 20q to confirm family relationships in those families in whom the mutation seemed to have arisen de novo. Patients with PNDM and other autoimmune disease in whom no FOXP3 mutation was identified were also investigated for the presence of CD25 mutations by sequencing of the coding region.
Molecular models
Predicted three-dimensional structures for the forkhead DNA-binding domain (DBD) (residues 335–423) of normal and variant FOXP3 proteins were generated using SWISS-MODEL (http://swissmodel.expasy.org/), an Internet-based tool for automated comparative protein modeling (15). Structures were visualized and images were generated using either MDL Chime (Symyx Technologies) or DeepView (Swiss-PdbViewer) programs (16).
RESULTS
Molecular genetics
We identified four different mutations in FOXP3 in six male subjects with PNDM from five unrelated families, all of whom presented with an associated immune-mediated disease (see below). None of the 16 patients with isolated PNDM had a variant in the FOXP3 sequence (Fisher's exact test; P = 0.002). No CD25 mutations were identified in probands lacking FOXP3 mutations.
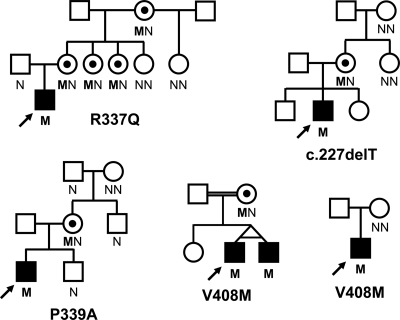
There were three novel FOXP3 missense mutations: R337Q (c.1010G>A; p.Arg337Gln), P339A (c.1015C>G; p.Pro339Ala), and V408M (c.1222G>A; p.Val408Met), the latter being present in three patients (including monozygotic twins) from two unrelated pedigrees (Fig. 1). The affected amino acid residues were conserved among species as well as in several other members of the forkhead transcription factor superfamily (data not shown). None of them was found in 510 European Caucasian or in 147 Turkish control X chromosomes. Only one of the mutations had previously been reported (c.227delT; p.Leu76GlnfsX53). It introduces a frameshift and generates a premature stop codon (17,18). The inheritance within families of the mutations is in keeping with an X-linked inheritance as shown in Fig. 1.
Figure 1.
Pedigrees for families with individuals harboring a FOXP3 mutation. Filled symbols represent patients with early-onset diabetes; black dots represent asymptomatic female carriers. The genotype is shown underneath each symbol; M and N denote mutant and wild-type alleles, respectively. An arrow indicates the proband.
Clinical features
The clinical information from patients bearing a FOXP3 mutation is shown in Table 1. There were no significant clinical differences (including age at diabetes onset, birth weight, or type of associated autoimmune disease) between those who were positive and negative for FOXP3 mutations (data not shown). Classic IPEX syndrome was present in three of the six patients, all of whom died by 13 months. In contrast, all three patients with the V408M mutation had a milder phenotype as well as prolonged survival (still alive at age 12–15 years).
Table 1.
Clinical characteristics of the patients with a FOXP3 mutation
| Subject | Country | Gestational age (weeks) | Birth weight (g) | Age at onset of diabetes | Glucose at diagnosis (mmol/l) | Ketosis at diagnosis | FOXP3 mutation | Current status | Associated clinical features |
|---|---|---|---|---|---|---|---|---|---|
| I | Czech Republic | 36 | 2,400 (−0.74 SDS) | 2 days | 15 | Negative | V408M | Alive at 15 years | Mildly elevated TSH at 3 years (thyroid antibodies negative); nephrotic syndrome at 6 years (good response to steroids); transient ischemic attack at 13 years (normal brain MRI scan) GAD and IA2 antibodies negative at 14 years; No diarrhea or malabsorption (anti-endomysial and anti-transglutaminase IgA negative); current A1C 10%; chronic diabetes complications (microalbuminuria and polyneuropathy) |
| IIa | Germany (Turkish) | 40 | 3,720 (+0.34 SDS) | 3 weeks | 48.5 | NA | V408M | Alive at 12 years | Autoimmune hypothyroidism at 1 year (TSH: 193 μU/ml; TPO Abs >3,000 units/l; TG Abs >2,000 units/l); enteropathy at 3 years (anti-enterocyte, anti-gliadin IgG, anti-endomysium and anti-transglutaminase IgA, parietal cell antibodies all positive; villous atrophy on jejunal biopsy); recurrent respiratory and gastrointestinal infections; mucocutaneous candidiasis; ICA and GAD Abs negative; current A1C 8.6%; no chronic diabetes complications; mild intellectual impairment (IQ 74) |
| IIb | Germany (Turkish) | 40 | 3,750 (+0.40 SDS) | 3.5 months | 29.7 | NA | V408M | Alive at 12 years | Autoimmune hypothyroidism at 1 year (TSH: 177 μU/ml; TPO Abs: >3,000 units/l; TG Abs >2,000 units/l); enteropathy since age 3 years (anti-enterocyte, anti-gliadin IgG, anti-intrinsic factor and parietal cell Abs positive; anti-endomysial and anti-transglutaminase IgA Abs negative; no villous atrophy on jejunal biopsy); recurrent respiratory ant gastrointestinal infections; mucocutaneous candidiasis; hypochromic microcytic anemia since age 10 years; ICA and GAD Abs negative; current A1C 8.5%; no chronic diabetes complications; mild intellectual impairment (IQ 80) |
| III | Argentina | 38 + 3 | 3,180 (+0.12 SDS) | 30 days | 40 | DKA | R337Q | Died at 13 months | ICA and GAD Abs negative at diagnosis, positive at 4 months (120 JDF units and 38 units/l, respectively); watery diarrhea at 3 months (severe villous atrophy, absent antienterocyte antibodies); serum IgE 2,266 units/ml at 3 months; normal thyroid function |
| IV | Germany | 38 + 3 | 1,590 (−3.67 SDS) | 1 week | 25.6 | NA | P339A | Died at 5.5 months | Maldigestion; cholestasis (attributed to parenteral nutrition); eczema; euthyroid thyroiditis at 6 weeks (TPO Abs 117 units/l; TG Abs 253 units/l) |
| V | England | 33 + 5 | 1,250 (−2.65 SDS) | 1 day | 23 | NA | L76QfsX53 | Died at 8 months | Absent GAD, ICA, and IA2 antibodies; watery diarrhea; low fecal elastase at 2.5 months, initially normal (small portion of pancreatic tissue in abdominal MRI scan); anemia; neutropenia; thrombocytopenia; increased IgE (132 units/ml); thyroid dysfunction (negative thyroid antibodies); recurrent respiratory tract infections and sepsis |
Ab, antibody; DKA, diabetic ketoacidosis; IA2, insulinoma-associated protein 2; ICA, islet cell antibody; JDF, Juvenile Diabetes Foundation; NA, not available; SDS, standard deviation score; TG, thyroglobulin; TPO, thyroperoxidase; TSH, thyroid-stimulating hormone.
Diabetes was the presenting feature in all subjects. Age at diagnosis varied from the first day of life to 3.5 months and did not correlate with the outcome of the disease. Birth weight was a feature of disease severity as it was markedly reduced only in the two patients who died early in infancy. Antibodies against β-cell antigens were reported to be unequivocally present in just one patient; in this patient results were negative at the time diabetes was diagnosed and became positive only during follow-up.
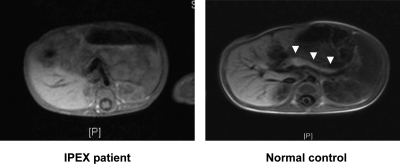
Gastrointestinal symptoms developed in five of six patients, although both the timing and the clinical severity varied widely. Interestingly, one of the most severely affected infants (Table 1, proband V) had diarrhea with low fecal elastase, and a magnetic resonance imaging (MRI) scan performed at 4 months showed only a tiny remnant of pancreatic tissue (Fig. 2). Thyroid disease was also found in five of six patients, but again it ranged between very mildly elevated thyroid-stimulating hormone levels without any detectable thyroid antibodies to severe autoimmune hypothyroidism. Proband I (hemizygous for V408M) did not have any gastrointestinal problem and only had a steroid-sensitive nephrotic syndrome that was considered to represent minimal change disease by the attending nephrologist.
Figure 2.
Abdominal MRI scan showing the absence of recognizable pancreatic tissue in one of the patients with classic IPEX syndrome (c.227delT, p.Leu74GlnfsX53 mutation). White arrowheads point to the pancreas in a control subject.
Molecular models
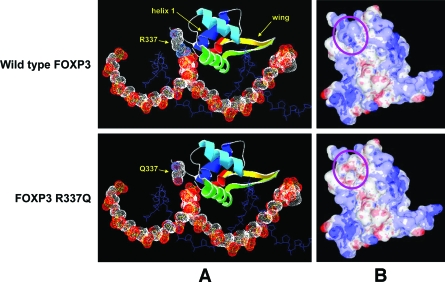
Inspection of the predicted structures of the DBD of FOXP3 suggested that all three novel missense FOXP3 mutations are likely to have deleterious effects on protein function and therefore be pathogenic, although to various degrees. In normal FOXP3, the side chain of arginine 337 is predicted to make close contact with the DNA backbone (Fig. 3A, top panel). The presence of a strong basic residue at this position is conserved in all forkhead transcription factors, and it contributes to DNA binding through both hydrogen bonding and electrostatic interactions. The R337Q substitution destroys this close contact with the DNA backbone (Fig. 3A, bottom panel) and is predicted to cause a significant loss of positive charge at the DNA-binding surface of FOXP3 (Fig. 3B). Taken together, the data strongly suggest that the R337Q mutation will result in a significant loss of DNA binding affinity.
Figure 3.
Predicted structures of the DBD of normal and R337Q FOXP3. A: Predicted interaction of FOXP3 with DNA. FOXP3 is represented in ribbon form, colored blue (NH2-terminal) to red (COOH-terminal), except for residue 337 (labeled) that shows the van der Waals radii of backbone and side chain atoms as dotted surfaces (white, carbon; blue, nitrogen; red, oxygen). Other features referred to in the text (helix 1 and the wing region) are labeled; the main DNA recognition helix (helix 3, green) lies in the major groove of the DNA. The DNA strand predicted to contact FOXP3 R337 is represented as a space-filling model, showing the van der Waals radii of the backbone atoms (white, carbon; red, oxygen; yellow, phosphorus). For clarity, each of the second DNA strands is shown only as a stick model of the backbone (blue). B: Molecular surface of FOXP3, showing areas of positive (blue) or negative (red) electrostatic potential. The structure shown in A has been rotated upward to look toward the DNA-binding surface; the position of residue 337 is indicated by the purple oval, and the DNA strands have been omitted for clarity. Structures were visualized and molecular surfaces were calculated using DeepView (A) or MDL Chime (B) programs.
Proline 339 lies in a γ-turn between Arg337 and helix 1 of the DBD. Substitution of this residue by alanine (as in the P339A mutation) is likely to affect the topology of Arg337 and/or helix 1 relative to the DNA and so would also be expected to have a deleterious effect on DNA binding affinity.
Valine 408 lies in a β-turn at the COOH-terminal of wing 1; in the predicted structure of FOXP3 its side chain points away from the DNA-binding surface and toward the side chain of arginine 347 in helix 1 (data not shown), although there is no apparent contact between the two side chains. Interestingly, replacing Val408 with the longer methionine is predicted to allow van der Waals contact between the two side chains (data not shown). This increased interaction between wing 1 and helix 1 is likely to have some effect on protein flexibility within the DBD, and it is reasonable to suspect that reduced flexibility in the DBD of FOXP3 will have an impact on DNA binding affinity. However, given the weak nature of van der Waals interactions, such effects are likely to be relatively modest, and the V408M substitution has no apparent effect either on the predicted hydrogen bond between the nearby residue Tyr406 and the DNA backbone or on surface charge distribution (data not shown). Therefore, the effects of the V408M substitution on protein structure are milder than the other mutations, which is consistent with the mild phenotype observed among subjects with this mutation.
CONCLUSIONS
Our systematic search for FOXP3 mutations in a large cohort of male probands with PNDM in whom the common causes had been excluded has shown that mutations are common in males who subsequently develop possible immune-mediated disorders (6 of 10 [60%]) but are absent or very rare in those who have isolated diabetes (0 of 16 [0%]). We show that there is clinical heterogeneity with three of the six mutation carriers having a severe phenotype and dying by 13 months, whereas the other three are still alive at 12–15 years.
We report on four different mutations, including three novel mutations. The previously reported single base pair deletion at position 227 generates a frameshift and introduces a premature stop codon. If the mutant mRNA evades nonsense-mediated decay, the resulting protein would lack the forkhead as well as the zinc finger and the leucine zipper domains and is predicted to be completely inactive. Consistent with this null mutation, the patient presented with classic IPEX syndrome and died within the first year of life. The likely pathogenicity of the three novel missense mutations was explored by using molecular modeling software. All of them are located within the forkhead domain of FOXP3; their predicted structural effects are likely to alter function of the protein and can be broadly correlated with the observed phenotype. The pathogenicity of R337Q is further supported as a different substitution at position 337 (R337P) as was previously described in a patient with IPEX syndrome (19). A substitution of asparagine for aspartic acid at position 409 of FOXP3 (adjacent to the site of the V408M mutation) also produces IPEX syndrome (20). Thus, we believe that the three novel mutations are likely to be responsible for the observed phenotypes.
Permanent diabetes was the presenting feature in the six patients with a FOXP3 mutation, although age at diagnosis varied. There was no correlation between the age at presentation and the clinical outcome of the patients; however, there was a suggestion that low birth weight, a reflection of intrauterine insulin secretion, was a prognostic marker: two of the three patients who died within the first 13 months of life as a result of severe IPEX syndrome were born with severe intrauterine growth retardation. Low birth weight might be a marker of poor prognosis among patients with FOXP3 mutations.
Autoantibodies against β-cell antigens were only persistently found in one of our six affected children, and in this child they were not present at diagnosis, suggesting that the main pathogenic mechanism leading to β-cell destruction is mediated by activated T lymphocytes. A systematic investigation of all possible β-cell autoantibodies was not performed in these children, and serum was not available for a retrospective analysis in a central laboratory. Therefore, the complete lack of antibodies cannot definitely be confirmed. However, our results show that antibodies against β-cell proteins as they are used in routine clinical practice may be absent in patients with proven FOXP3 mutations. Thus, the absence of pancreatic autoantibodies should not be taken as a screening test to exclude the possibility of a FOXP3 mutation.
One patient with classic IPEX syndrome only had a small remnant of pancreatic tissue seen on an abdominal MRI scan and had evidence of evolving exocrine pancreatic dysfunction, suggesting the development of pancreatic atrophy. Although both mild pancreatic exocrine insufficiency and a reduced pancreas volume are relatively common findings in long-standing classic type 1 diabetes, they are not as severe as in this patient. It is possible that a massive autoimmune response against the pancreas might be responsible for both PNDM and pancreatic atrophy in this patient as seen in maternal enteroviral infection (21).
There was considerable clinical heterogeneity among the patients with FOXP3 mutations. Three patients with classic IPEX syndrome died during the first 13 months of life despite the use of immunosuppressive therapy. However, the phenotypic spectrum is extended from that previously described in the three patients with the V408M mutation. PNDM was the only clinical feature in two twin brothers from one of the pedigrees with this mutation until the age of 1 year, when autoimmune hypothyroidism was diagnosed in both of them. Subsequently, they both developed recurrent diarrhea, and autoimmune enteropathy was diagnosed on the basis of positive antienterocyte antibodies in both twins. They had multiple antibodies indicating gastrointestinal autoimmunity (Table 1). Twin IIa had a jejunal intestinal biopsy showing villous atrophy, but the second twin (IIb) had a normal jejunal biopsy. Interestingly, they both developed very well on a gluten-free diet. These patients are now 12 years old, and no other significant problems have been detected except that both have intellectual impairment (IQ of 74 and 80, respectively). The other patient with a V408M mutation has the mildest phenotype associated with a FOXP3 mutation to date. Although diabetes was diagnosed 2 days after birth, no other evidence of immune dysfunction has been detected apart from an episode of nephrotic proteinuria at 6 years. Clinical remission was achieved with steroid treatment, and the disease has not relapsed. The patient is currently 15 years, and no other autoimmune problems have arisen since then. Nephrotic syndrome and classic type 1 diabetes can be associated, indicating a shared immunological basis for both disorders (22,23), and nephrotic syndrome has previously been described in a patient with classic IPEX syndrome (24). We therefore believe that there may be a causal link between both diabetes and nephrotic syndrome and the presence of the V408M mutation in our patient.
IPEX syndrome is thought to be an extremely rare condition with only approximately 50 cases reported in the literature. As with any other X-linked recessive disorder with reduced survival, IPEX syndrome frequently presents as a single case either caused by de novo or maternally inherited mutations. Patients with a mild presentation are likely to have been under-reported, so the true incidence of the disease might actually be higher than is currently perceived. A clinical diagnosis of IPEX syndrome was made in only one of our patients before the genetic analysis, whereas the others were referred for genetic testing because of their PNDM. The varied clinical spectrum resulting from mutations in FOXP3 emphasizes the need to be aware of this possibility in male patients with PNDM who develop any other immune-mediated disorder later in life.
Acknowledgments
This work was funded by the Wellcome Trust (grant 067463/Z/2/Z). O.R.C. is supported by an “Ayuda para contratos post-Formación Sanitaria Especializada” from the “Instituto de Salud Carlos III” (FIS CM06/00013). Z.S. is supported by the Czech Ministry of Education (grant 021620819) and by the Czech Ministry of Health (grant 64203). A.T.H. is a Wellcome Trust Research Leave Fellow.
No potential conflicts of interest relevant to this article were reported.
We thank Andrew Parrish for his technical assistance. We also thank Olga Kordonouri for her clinical input and help with the writing of the manuscript.
Published ahead of print at http://care.diabetesjournals.org on 17 October 2008.
O.R.-C. and J.A.L.M. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Edghill EL, Dix RJ, Flanagan SE, Bingley PJ, Hattersley AT, Ellard S, Gillespie KM: HLA genotyping supports a nonautoimmune etiology in patients diagnosed with diabetes under the age of 6 months. Diabetes 55:1895–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Iafusco D, Stazi MA, Cotichini R, Cotellessa M, Martinucci ME, Mazzella M, Cherubini V, Barbetti F, Martinetti M, Cerutti F, Prisco F; Early Onset Diabetes Study Group of the Italian Society of Paediatric Endocrinology and Diabetology: Permanent diabetes mellitus in the first year of life. Diabetologia 45:798–804, 2002. [erratum in Diabetologia46:140, 2003] [DOI] [PubMed] [Google Scholar]
- 3.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM: JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest 106:R75–R81, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME: X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet 27:18–20, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD: The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet 27:20–21, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Valencia X, Lipsky PE: CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol 3:619–626, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW: CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol 119:482–487, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Moraes-Vasconcelos D, Costa-Carvalho BT, Torgerson TR, Ochs HD: Primary immune deficiency disorders presenting as autoimmune diseases: IPEX and APECED. J Clin Immunol 28 (Suppl. 1):11–19, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Powell BR, Buist NR, Stenzel P: An X-linked syndrome of diarrhea, polyendocrinopathy, and fatal infection in infancy. J Pediatr 100:731–737, 1982 [DOI] [PubMed] [Google Scholar]
- 10.De Benedetti F, Insalaco A, Diamanti A, Cortis E, Muratori F, Lamioni A, Carsetti R, Cusano R, De Vito R, Perroni L, Gambarara M, Castro M, Bottazzo GF, Ugazio AG: Mechanistic associations of a mild phenotype of immunodysregulation, polyendocrinopathy, enteropathy, x-linked syndrome. Clin Gastroenterol Hepatol 4:653–659, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG: Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest 116:1713–1722, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flanagan SE, Patch AM, Mackay DJ, Edghill EL, Gloyn AL, Robinson D, Shield JP, Temple K, Ellard S, Hattersley AT: Mutations in ATP-sensitive K+ channel genes cause transient neonatal diabetes and permanent diabetes in childhood or adulthood. Diabetes 56:1930–1937, 2007. [erratum in Diabetes 57: 523, 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellard S, Flanagan SE, Girard CA, Patch AM, Harries LW, Parrish A, Edghill EL, Mackay DJ, Proks P, Shimomura K, Haberland H, Carson DJ, Shield JP, Hattersley AT, Ashcroft FM: Permanent neonatal diabetes caused by dominant, recessive, or compound heterozygous SUR1 mutations with opposite functional effects. Am J Hum Genet 81:375–382, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edghill EL, Flanagan SE, Patch AM, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Støy J, Steiner DF, Philipson LH, Bell GI, Neonatal Diabetes International Collaborative Group, Hattersley AT, Ellard S: Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes 57:1034–1042, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold K, Bordoli L, Kopp J, Schwede T: The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Guex N, Peitsch MC: SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modelling. Electrophoresis 18:2714–2723, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi I, Shiari R, Yamada M, Kawamura N, Okano M, Yara A, Iguchi A, Ishikawa N, Ariga T, Sakiyama Y, Ochs HD, Kobayashi K: Novel mutations of FOXP3 in two Japanese patients with immune dysregulation, polyendocrinopathy, enteropathy, X linked syndrome (IPEX). J Med Genet 38:874–876, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchizawa T, Adachi Y, Ito Y, Higashiyama H, Kanegane H, Futatani T, Kobayashi I, Kamachi Y, Sakamoto T, Tsuge I, Tanaka H, Banham AH, Ochs HD, Miyawaki T: Developmental changes of FOXP3-expressing CD4+CD25+ regulatory T cells and their impairment in patients with FOXP3 gene mutations. Clin Immunol 125:237–246, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lopes JE, Torgerson TR, Schubert LA, Anover SD, Ocheltree EL, Ochs HD, Ziegler SF: Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J Immunol 177:3133–3142, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Rao A, Kamani N, Filipovich A, Lee SM, Davies SM, Dalal J, Shenoy S: Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood 109:383–385, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Otonkoski T, Roivainen M, Vaarala O, Dinesen B, Leipälä JA, Hovi T, Knip M: Neonatal type I diabetes associated with maternal echovirus 6 infection: a case report. Diabetologia 43:1235–1238, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Agras PI, Kinik ST, Cengiz N, Baskin E: Type 1 diabetes mellitus associated with nephrotic syndrome. J Pediatr Endocrinol Metab 19:1045–1048, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Aaltonen P, Rinta-Valkama J, Pätäri A, Tossavainen P, Palmén T, Kulmala P, Knip M, Holthöfer H: Circulating antibodies to nephrin in patients with type 1 diabetes. Nephrol Dial Transplant 22:146–153, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Moudgil A, Perriello P, Loechelt B, Przygodzki R, Fitzerald W, Kamani N: Immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome: an unusual cause of proteinuria in infancy. Pediatr Nephrol 22:1799–1802, 2007 [DOI] [PubMed] [Google Scholar]