Abstract
OBJECTIVE—To investigate the relationships of serum adipocyte fatty acid–binding protein (A-FABP) and epidermal fatty acid–binding protein (E-FABP) with renal dysfunction and macrovascular complications in type 2 diabetic patients.
RESEARCH DESIGN AND METHODS—The associations of serum A-FABP and E-FABP with markers of renal function, nephropathy staging, and macrovascular complications were examined in 237 type 2 diabetic patients.
RESULTS—Serum A-FABP and E-FABP correlated significantly with serum creatinine, mean albumin excretion rate, and glomerular filtration rate (all P < 0.001) and were independently associated with diabetic nephropathy staging (P = 0.001 and P < 0.05, respectively). Circulating levels of both types of FABP were increased (P < 0.01) in subjects with macrovascular complications. Serum A-FABP was independently associated with macrovascular complications (odds ratio 2.92 [95% CI 1.42–6.01]; P = 0.004).
CONCLUSIONS—Serum A-FABP and E-FABP might be novel serum biomarkers for evaluating the progression of nephropathy and its cardiovascular risk in type 2 diabetic patients.
Adipocyte fatty acid–binding protein (A-FABP) and epidermal fatty acid–binding protein (E-FABP), highly expressed in adipocytes and macrophages, have been shown to mediate obesity-related metabolic disorders and atherosclerosis in animal studies (1). We have recently demonstrated that both types of FABP circulate in the human bloodstream (2,3), with levels closely associated with parameters of adiposity, insulin resistance, the metabolic syndrome, and carotid atherosclerosis (2–4). This study examined the relationships of circulating A-FABP and E-FABP with diabetic nephropathy and its associated macrovascular complications in 237 type 2 diabetic patients in various stages of nephropathy.
RESEARCH DESIGN AND METHODS
This cohort included 237 Chinese type 2 diabetic patients (mean ± SD age 55.8 ± 14.2 years, BMI 25.5 ± 4.3 kg/m2, 110 male and 127 female) from the Diabetes Clinic, Queen Mary Hospital (Hong Kong, China), with complete sets of renal function assessment data collected as part of two previously published studies (3,5). None of the patients were on thiazolidinediones. Informed consent was obtained, and the protocol was approved by the local ethics committee.
All subjects were assessed after at least 10 h of overnight fasting. Details on the assessment for hypertension, various anthropometric and biochemical parameters, serum high-sensitivity C-reactive protein, A-FABP (intra-assay coefficients of variation [CVs] 3.9–6.6% and interassay CVs 2.6–5.1%) and E-FABP (intra-assay CVs 4.5–4.9% and interassay CVs 5.7–5.9%) (BioVendor Laboratory Medicine, Modřice, Czech Republic) were reported previously (2–6). Serum soluble tumor necrosis factor receptor II (sTNF RII) was measured with ELISA kits from R&D Systems (Minneapolis, MN).
Mean albumin excretion rate (MAER) was estimated from the albumin content of two consecutive 12-h overnight urine samples, which were assayed nephelometrically. Subjects were classified as normoalbuminuric (MAER <20 μg/min), microalbuminuric (MAER 20–200 μg/min), or macroalbuminuric (MAER >200 μg/min) (6). Glomerular filtration rate (GFR) was estimated using the formula from the Modification of Diet in Renal Disease (MDRD) study (7). Macrovascular complications included ischemic heart disease, stroke, or peripheral vascular disease, diagnosed clinically and documented on electrocardiogram, myocardial perfusion scan, or coronary angiogram; computed tomography brain scan; or Doppler studies, respectively.
All analyses were performed with SPSS (version 15; SPSS, Chicago, IL). Skewed data were logarithmically transformed. One-way ANOVA or a χ2 test was used for comparisons between groups, and correlations between variables were adjusted using partial correlation. Multiple testing was corrected using Bonferroni correction. Multinomial and binary logistic regressions were used to determine the variables with independent significant associations with nephropathy staging and macrovascular complications, respectively. P <0.05 was considered statistically significant.
RESULTS
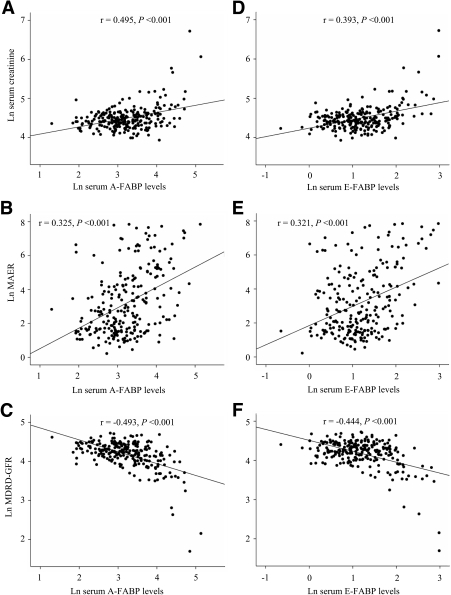
There were significant and progressive increases in serum A-FABP and E-FABP across the three stages of diabetic nephropathy (for both, with adjustment for trend, P < 0.001) (Table A1 in the online appendix, available at http://dx.doi.org/10.2337/dc08-1333). Serum A-FABP correlated positively with serum creatinine and MAER and negatively with MDRD-GFR (all P < 0.001, adjusted for sex, age, and waist circumference) (Fig. 1A–C). Age- and waist circumference–adjusted serum E-FABP also correlated positively with serum creatinine and MAER and negatively with MDRD-GFR (all P < 0.001) (Fig. 1D–F). The correlations of serum A-FABP or E-FABP with serum creatinine and MDRD-GFR remained significant even after adjusting for LDL cholesterol, hypertension, and sTNF RII (adjusted P = 0.015 to <0.001). There was no significant correlation of FABP with either A1C or statin use. Serum E-FABP, but not A-FABP, correlated with adiponectin (r = −0.213; P = 0.001).
Figure 1.
Correlations between serum levels of the two FABPs and indexes of renal function. Serum A-FABP levels correlated significantly with serum creatinine (A), MAER (B), and MDRD-GFR (C). Serum E-FABP also correlated significantly with serum creatinine (D), MAER (E), and MDRD-GFR (F). Ln, natural logarithm.
Multinomial regressions, including factors with biological relevance or significant associations (corrected P < 0.05) with nephropathy staging, showed that serum A-FABP was independently associated with nephropathy staging for microalbuminuria versus normoalbuminuria (odds ratio [OR] 3.04 [95% CI 1.61–5.74]; P = 0.001) and for macroalbuminuria versus normoalbuminuria (4.14 [1.83–9.33]; P = 0.001) together with LDL cholesterol (2.15 [1.23–3.77]; P = 0.007) (online appendix Table A2). Serum sTNF RII, A-FABP, and E-FABP were input one at a time because of their strong correlations (r > 0.4; P < 0.001). Repeated analysis yielded similar results for serum E-FABP (OR 1.83, P = 0.035 for microalbuminuria vs. normoalbuminuria, and OR 4.93, P < 0.001 for macroalbuminuria vs. normoalbuminuria).
Thirty-six micro- or macroalbuminuric subjects had macrovascular complications. They had higher serum A-FABP and E-FABP (both P < 0.01) than subjects with no macrovascular complications (online appendix Table A3). Binary logistic regression, which included parameters having significant associations or possible biological relevance with macrovascular complications, revealed that serum A-FABP (OR 2.92 [95% CI 1.42–6.01]; P = 0.004) and fasting glucose (1.15 [1.00–1.33]; P = 0.046) were independently associated with macrovascular complications (online appendix Table A4). Serum A-FABP, E-FABP, sTNF RII, and MDRD-GFR, being highly correlated, were analyzed one at a time. MDRD-GFR, but not serum E-FABP or sTNF RII, was significantly associated with macrovascular complications (OR 0.24; P = 0.006).
CONCLUSIONS
This study provides novel evidence that serum A-FABP and E-FABP in type 2 diabetic patients were independently associated with nephropathy staging and correlated positively with serum creatinine and negatively with MDRD-GFR, even after adjustment for age, adiposity, LDL cholesterol, hypertension, and sTNF RII. The elevated levels of these FABPs might have resulted from both impaired renal clearance and increased production by activated macrophages in diabetic nephropathy. Macrophage accumulation in the kidney, which increases with the progression of nephropathy in diabetes and renal injury (8), is the primary source of inflammation under this pathological condition. Both FABPs are highly expressed in macrophages (1), and several proinflammatory stimuli could induce A-FABP expressions in macrophages (9,10). On the other hand, both FABPs have been implicated as key mediators of inflammation (1). Taken together, macrophage accumulation in the kidney and the augmented expressions of these FABPs in macrophages would aggravate local inflammation and contribute to the progression of nephropathy in diabetes.
We have also demonstrated the significant increases of serum A-FABP and E-FABP in macrovascular complications and the independent association between serum A-FABP and macrovascular complications. These new findings would support a causative role of these FABPs in the pathogenesis of cardiovascular diseases, suggested by previous animal, genetic, and epidemiological studies (1,3,4,11). In summary, our findings raise the possibility that A-FABP and E-FABP may be used as serum biomarkers for stratifying nephropathy staging and cardiovascular risks in diabetic patients.
Supplementary Material
Acknowledgments
A.X. was supported by grant KKU 2/07C from the Collaborative Research Fund. K.L. was supported by grants HKU7637/05M and 7590/06M from the Hong Kong Research Grants Council.
No potential conflicts of interest relevant to this article were reported.
Published ahead of print at http://care.diabetesjournals.org on 17 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Furuhashi M, Hotamisligil GS: Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7:489–503, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang JL, Wat NM, Wong WK, Lam KS: Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem 52:405–413, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Yeung DC, Wang Y, Xu A, Cheung SC, Wat NM, Fong DY, Fong CH, Chau MT, Sham PC, Lam KS: Epidermal fatty-acid-binding protein: a new circulating biomarker associated with cardio-metabolic risk factors and carotid atherosclerosis. Eur Heart J. 4 July 2008 [Epub ahead of print] [DOI] [PubMed]
- 4.Yeung DC, Xu A, Cheung CW, Wat NM, Yau MH, Fong CH, Chau MT, Lam KS: Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol 27:1796–1802, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hoo RL, Chow WS, Yau MH, Xu A, Tso AW, Tse HF, Fong CH, Tam S, Chan L, Lam KS: Adiponectin mediates the suppressive effect of rosiglitazone on plasminogen activator inhibitor-1 production. Arterioscler Thromb Vasc Biol 27:2777–2782, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Liu YF, Wat NM, Chung SS, Ko BC, Lam KS: Diabetic nephropathy is associated with the 5′-end dinucleotide repeat polymorphism of the aldose reductase gene in Chinese subjects with type 2 diabetes. Diabet Med 19:113–118, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, the Modification of Diet in Renal Disease Study Group: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 140:461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Tesch GH: Intercellular adhesion molecule-1 deficiency is protective against nephropathy in type 2 diabetic db/db mice. J Am Soc Nephrol 16:1711–1722, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Kazemi MR, McDonald CM, Shigenaga JK, Grunfeld C, Feingold KR: Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol 25:1220–1224, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Fu Y, Luo N, Lopes-Virella MF: Oxidized LDL induces the expression of ALBP/aP2 mRNA and protein in human THP-1 macrophages. J Lipid Res 41:2017–2023, 2000 [PubMed] [Google Scholar]
- 11.Tuncman G, Erbay E, Hom X, De Vivo I, Campos H, Rimm EB, Hotamisligil GS: A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc Natl Acad Sci U S A 103:6970–6975, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.