Abstract
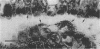
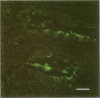
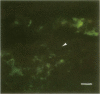
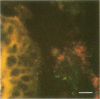
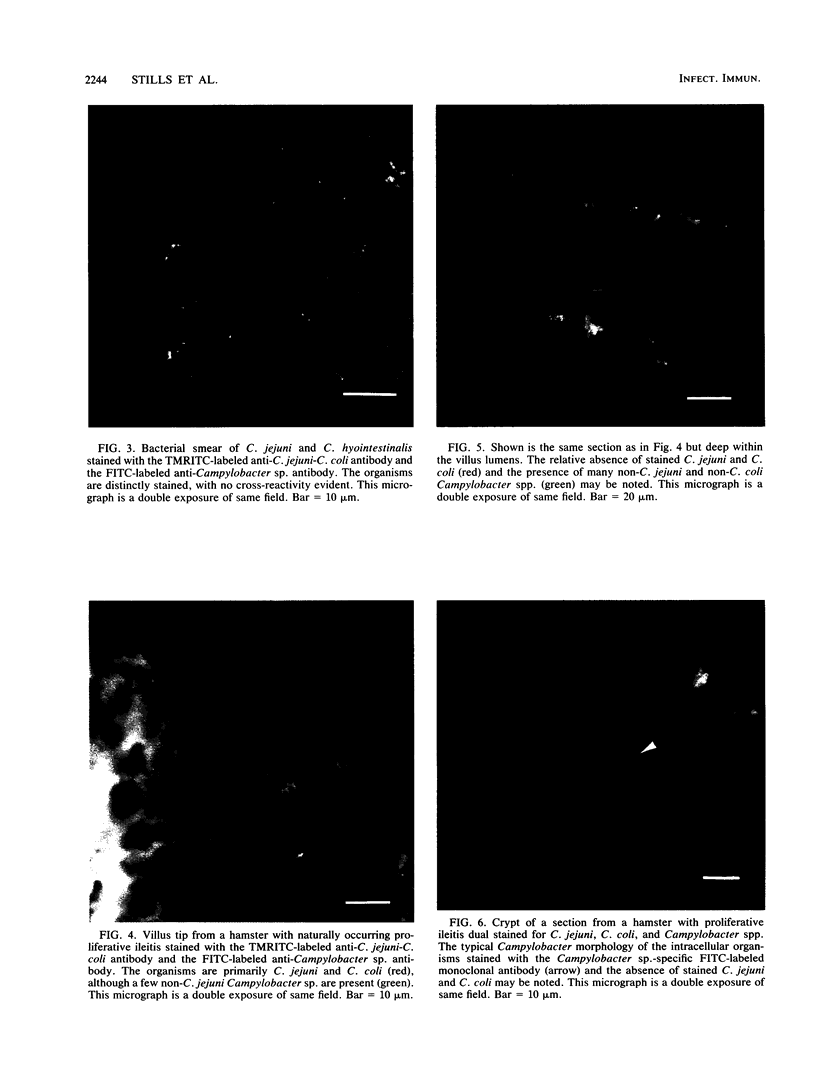
The role of Campylobacter jejuni in the pathogenesis of proliferative ileitis in Syrian hamsters was evaluated with monoclonal antibodies of different specificities. Monoclonal antibodies were produced with two different specificities: one for all members of the genus Campylobacter tested (antibody 8322-2E6) and one for C. jejuni and Campylobacter coli (antibodies 841-2A11, 841-4C6, and 841-5B1). Heal sections from healthy hamsters, from hamsters with naturally occurring proliferative ileitis, and from hamsters with experimentally induced proliferative ileitis were examined by using fluorescein isothiocyanate-labeled, Campylobacter sp.-specific 8322-2E6 and tetramethylrhodamine isothiocyanate-labeled C. jejuni-C. coli-specific 841-2A11 for direct dual-labeling immunofluorescence. Organisms which stained with the C. jejuni-C. coli-specific monoclonal antibody were observed in the ileal lumens and along the distal tips of the villi of hamsters with either experimentally induced or naturally occurring proliferative ileitis. In contrast, organisms identified by the Campylobacter sp.-specific monoclonal antibody were present deep within the villus lumens and crypts and intracellularly within the apical portions of the epithelial cells. No organisms stained with the C. jejuni-C. coli-specific monoclonal antibody were observed in ileal sections from control hamsters; an occasional intracellular organism stained with the Campylobacter sp.-specific monoclonal antibody was observed in 2 of 10 control hamsters. Thus, at least two immunologically distinct patterns were identified in ileal sections from hamsters with proliferative ileitis. On the basis of these results, we conclude that the organism seen intracellularly in ileal sections from hamsters with proliferative ileitis is a member of the genus Campylobacter but that it probably is not C. jejuni or C. coli.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amend N. K., Loeffler D. G., Ward B. C., Van Hoosier G. L., Jr Transmission of enteritis in the Syrian hamster. Lab Anim Sci. 1976 Aug;26(4):566–572. [PubMed] [Google Scholar]
- Boothe A. D., Cheville N. F. The pathology of proliferative ileitis of the golden syrian hamster. Pathol Vet. 1967;4(1):31–44. doi: 10.1177/030098586700400104. [DOI] [PubMed] [Google Scholar]
- Bryant J. L., Stills H. F., Lentsch R. H., Middleton C. C. Campylobacter jejuni isolated from patas monkeys with diarrhea. Lab Anim Sci. 1983 Jun;33(3):303–305. [PubMed] [Google Scholar]
- Cebra J. J., Goldstein G. Chromatographic purification of tetramethylrhodamine-immune globulin conjugates and their use in the cellular localization of rabbit gamma-globulin polypeptide chains. J Immunol. 1965 Aug;95(2):230–245. [PubMed] [Google Scholar]
- Chesterman F. C. Background pathology in a colony of golden hamsters. Prog Exp Tumor Res. 1972;16:51–68. [PubMed] [Google Scholar]
- Fox J. G., Zanotti S., Jordan H. V. The hamster as a reservoir of Campylobacter fetus subspecies jejuni. J Infect Dis. 1981 Jun;143(6):856–856. doi: 10.1093/infdis/143.6.856. [DOI] [PubMed] [Google Scholar]
- Frisk C. S., Wagner J. E. Experimental hamster enteritis: an electron microscopic study. Am J Vet Res. 1977 Nov;38(11):1861–1868. [PubMed] [Google Scholar]
- Frisk C. S., Wagner J. E., Owens D. R. Hamster (Mesocricetus auratus) enteritis caused by epithelial cell-invasive Escherichia coli. Infect Immun. 1981 Mar;31(3):1232–1238. doi: 10.1128/iai.31.3.1232-1238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhart C. J., Ward G. E., Chang K., Kurtz H. J. Campylobacter hyointestinalis (new species) isolated from swine with lesions of proliferative ileitis. Am J Vet Res. 1983 Mar;44(3):361–367. [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Goldman P. M., Andrews E. J., Lang C. M. A preliminary evaluation of Clostridium sp. in the etiology of hamster enteritis. Lab Anim Sci. 1972 Oct;22(5):721–724. [PubMed] [Google Scholar]
- Humphrey C. D., Montag D. M., Pittman F. E. Experimental infection of hamsters with Campylobacter jejuni. J Infect Dis. 1985 Mar;151(3):485–493. doi: 10.1093/infdis/151.3.485. [DOI] [PubMed] [Google Scholar]
- Jacoby R. O., Johnson E. A. Transmissible ileal hyperplasia. Adv Exp Med Biol. 1981;134:267–289. doi: 10.1007/978-1-4757-0495-2_25. [DOI] [PubMed] [Google Scholar]
- Jacoby R. O., Osbaldiston G. W., Jonas A. M. Experimental transmission of atypical ileal hyperplasia of hamsters. Lab Anim Sci. 1975 Aug;25(4):465–473. [PubMed] [Google Scholar]
- Jacoby R. O. Transmissible ileal hyperplasia of hamsters. I. Histogenesis and immunocytochemistry. Am J Pathol. 1978 Jun;91(3):433–450. [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Jacoby R. O. Transmissible ileal hyperplasia of hamsters. II. Ultrastructure. Am J Pathol. 1978 Jun;91(3):451–468. [PMC free article] [PubMed] [Google Scholar]
- Jonas A. M., Tomita Y., Wyand D. S. Enzootic intestinal adenocarcinoma in hamsters. J Am Vet Med Assoc. 1965 Nov 15;147(10):1102–1108. [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Herlyn D., Herlyn M. Study of antibodies against human melanoma produced by somatic cell hybrids. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3405–3409. doi: 10.1073/pnas.75.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Regina M., Lonigro J. Isolation of Campylobacter fetus subspecies jejuni from hamsters with proliferative ileitis. Lab Anim Sci. 1982 Dec;32(6):660–662. [PubMed] [Google Scholar]
- Lawson G. H., Rowland A. C., MacIntyre N. Demonstration of a new intracellular antigen in porcine intestinal adenomatosis and hamster proliferative ileitis. Vet Microbiol. 1985 Jun;10(4):303–313. doi: 10.1016/0378-1135(85)90001-x. [DOI] [PubMed] [Google Scholar]
- Lentsch R. H., McLaughlin R. M., Wagner J. E., Day T. J. Campylobacter fetus subspecies jejuni isolated from Syrian hamsters with proliferative ileitis. Lab Anim Sci. 1982 Oct;32(5):511–514. [PubMed] [Google Scholar]
- Lussier G., Pavilanis V. Presence of intranuclear inclusion bodies in proliferative ileitis of the hamster (Mesocricetus auratus). A preliminary report. Lab Anim Care. 1969 Jun;19(3):387–390. [PubMed] [Google Scholar]
- Nachamkin I., Hart A. M. Common and specific epitopes of Campylobacter flagellin recognized by monoclonal antibodies. Infect Immun. 1986 Aug;53(2):438–440. doi: 10.1128/iai.53.2.438-440.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G. Monoclonal antibodies directed against the flagella of Campylobacter jejuni: cross-reacting and serotypic specificity and potential use in diagnosis. J Hyg (Lond) 1986 Jun;96(3):377–384. doi: 10.1017/s0022172400066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEFFIELD F. W., BEVERIDGE E. Prophylaxis of "wettail" in hamsters. Nature. 1962 Oct 20;196:294–295. doi: 10.1038/196294b0. [DOI] [PubMed] [Google Scholar]
- Wagner J. E., Owens D. R., Troutt H. F. Proliferative ileitis of hamsters: electron microscopy of bacteria in cells. Am J Vet Res. 1973 Feb;34(2):249–252. [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]