Abstract
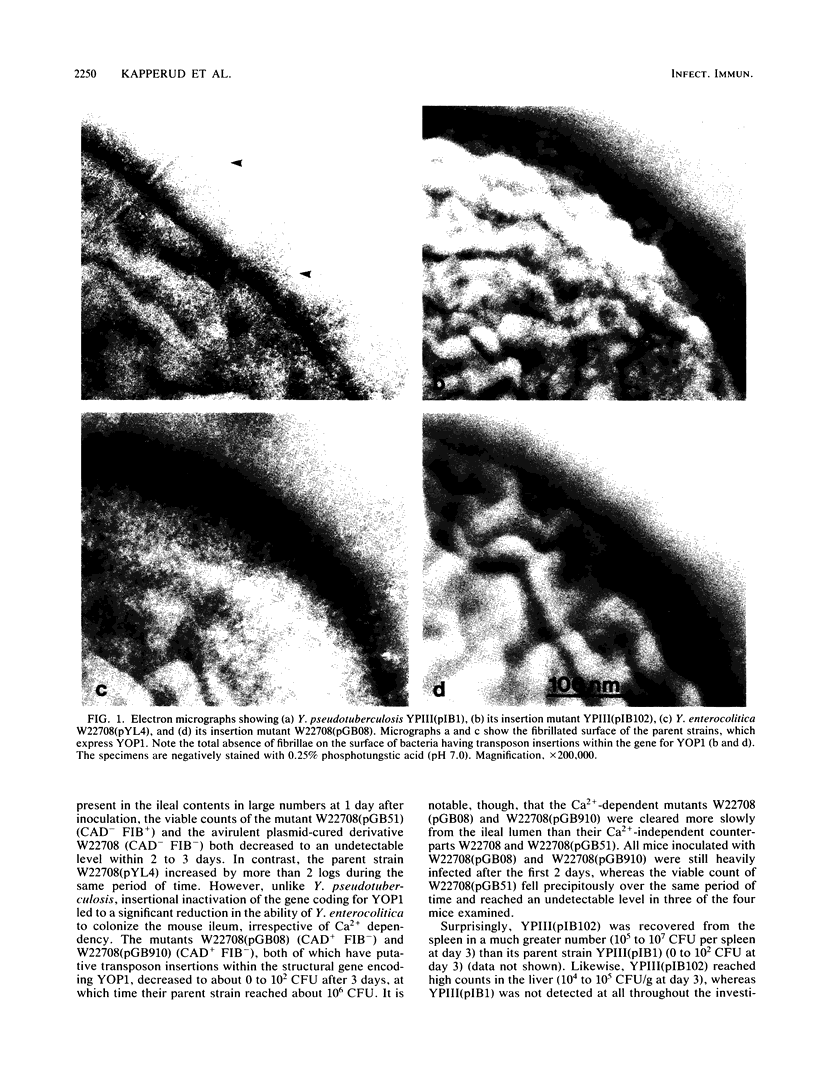
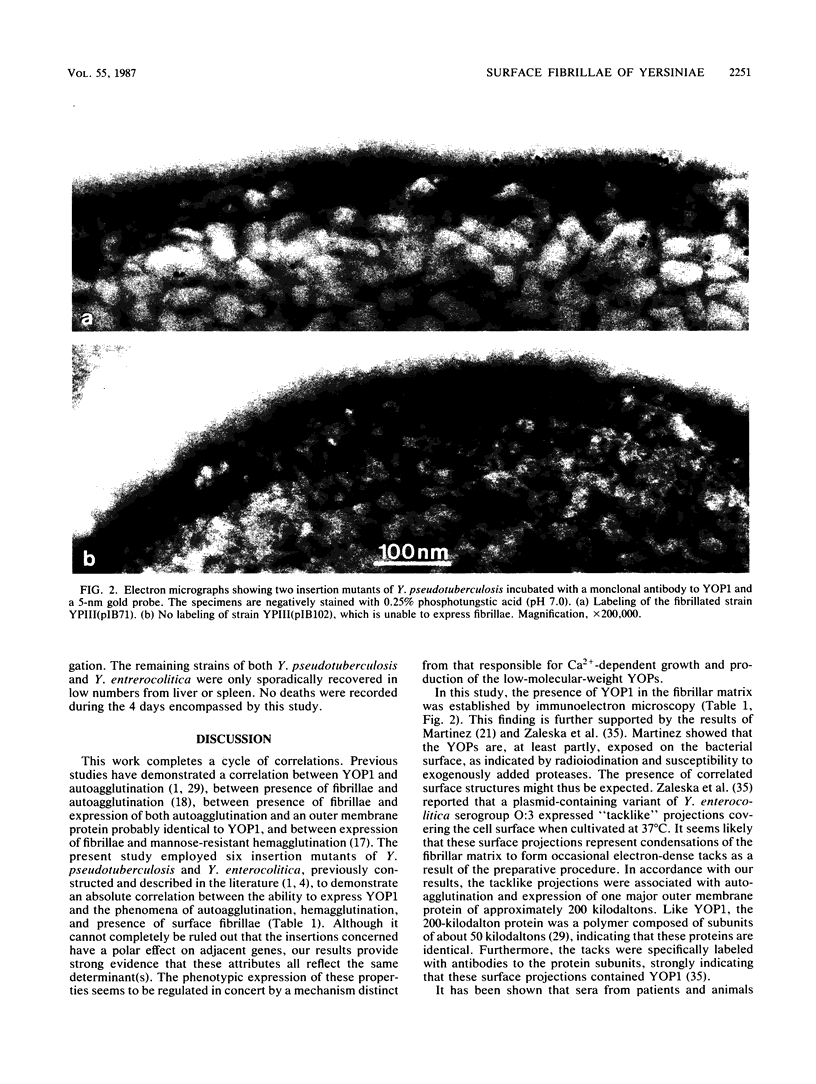
The cell surface properties of Yersinia pseudotuberculosis and Yersinia enterocolitica mutants, constructed by insertional inactivation of genes located on the 40- to 50-megadalton virulence plasmid, were examined. Electron microscopy revealed an absolute correlation between expression of four plasmid-dependent, temperature-inducible properties related to the bacterial surface: (i) a fibrillar matrix covering the outer membrane, (ii) outer membrane protein YOP1, (iii) spontaneous autoagglutination, and (iv) mannose-resistant hemagglutination of guinea pig erythrocytes. Immunoelectron microscopy indicated that YOP1 is a structural component of the fibrillae. Experiments demonstrating inhibition of hemagglutination by anti-YOP1 monoclonal antibody suggested a potential role for YOP1 in adhesion. Insertional inactivation of the gene coding for YOP1, with resultant loss of the ability to express fibrillae, led to a significant reduction in the capacity of Y. enterocolitica, but not Y. pseudotuberculosis, to colonize the ileum of orogastrically infected mice. In both Y. enterocolitica and Y. pseudotuberculosis, inactivation of the genes coding for Ca2+ dependency reduced the ability to maintain intestinal colonization, regardless of the ability to express fibrillae. Both surface fibrillae and Ca2+ dependency seem to reflect pathogenic determinants which are required for the establishment of Y. enterocolitica infection. In Y. pseudotuberculosis, however, no clinical significance of the fibrillae has so far been defined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. THE EFFECT OF CA++ AND MG++ ON LYSIS, GROWTH, AND PRODUCTION OF VIRULENCE ANTIGENS BY PASTEURELLA PESTIS. J Infect Dis. 1964 Feb;114:13–25. doi: 10.1093/infdis/114.1.13. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. The effects of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br J Exp Pathol. 1958 Jun;39(3):278–291. [PMC free article] [PubMed] [Google Scholar]
- Balligand G., Laroche Y., Cornelis G. Genetic analysis of virulence plasmid from a serogroup 9 Yersinia enterocolitica strain: role of outer membrane protein P1 in resistance to human serum and autoagglutination. Infect Immun. 1985 Jun;48(3):782–786. doi: 10.1128/iai.48.3.782-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölin I., Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984 Jan;43(1):72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis G., Sory M. P., Laroche Y., Derclaye I. Genetic analysis of the plasmid region controlling virulence in Yersinia enterocolitica 0:9 by Mini-Mu insertions and lac gene fusions. Microb Pathog. 1986 Aug;1(4):349–359. doi: 10.1016/0882-4010(86)90067-7. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Pennington J. E., Kubler A. M., Conscience J. F. A simple, single-step technique for selecting and cloning hybridomas for the production of monoclonal antibodies. J Immunol Methods. 1982;50(2):161–171. doi: 10.1016/0022-1759(82)90222-8. [DOI] [PubMed] [Google Scholar]
- Ferber D. M., Brubaker R. R. Plasmids in Yersinia pestis. Infect Immun. 1981 Feb;31(2):839–841. doi: 10.1128/iai.31.2.839-841.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T., Wohlhieter J. A. Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun. 1980 Jun;28(3):1044–1047. doi: 10.1128/iai.28.3.1044-1047.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguen J. D., Yother J., Straley S. C. Genetic analysis of the low calcium response in Yersinia pestis mu d1(Ap lac) insertion mutants. J Bacteriol. 1984 Dec;160(3):842–848. doi: 10.1128/jb.160.3.842-848.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Lassen J. Relationship of virulence-associated autoagglutination to hemagglutinin production in Yersinia enterocolitica and Yersinia enterocolitica-like bacteria. Infect Immun. 1983 Oct;42(1):163–169. doi: 10.1128/iai.42.1.163-169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skarpeid H. J. Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect Immun. 1985 Feb;47(2):561–566. doi: 10.1128/iai.47.2.561-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L., Ferris W. R. Association of fibril structure formation with cell surface properties of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):272–275. doi: 10.1128/iai.46.1.272-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird W. J., Cavanaugh D. C. Correlation of autoagglutination and virulence of yersiniae. J Clin Microbiol. 1980 Apr;11(4):430–432. doi: 10.1128/jcm.11.4.430-432.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclagan R. M., Old D. C. Haemagglutinins and fimbriae in different serotypes and biotypes of Yersinia enterocolitica. J Appl Bacteriol. 1980 Oct;49(2):353–360. doi: 10.1111/j.1365-2672.1980.tb05135.x. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983 Sep;41(3):921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namork E., Johansen B. V. Surface activation of carbon film supports for biological electron microscopy. Ultramicroscopy. 1982;7(4):321–330. doi: 10.1016/0304-3991(82)90257-1. [DOI] [PubMed] [Google Scholar]
- Perry R. D., Brubaker R. R. Vwa+ phenotype of Yersinia enterocolitica. Infect Immun. 1983 Apr;40(1):166–171. doi: 10.1128/iai.40.1.166-171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Blank H. F., Kingsbury D. T., Falkow S. Genetic analysis of essential plasmid determinants of pathogenicity in Yersinia pestis. J Infect Dis. 1983 Aug;148(2):297–304. doi: 10.1093/infdis/148.2.297. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981 Dec;148(3):877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Martinez R. J. Role of a plasmid in the pathogenicity of Yersinia species. Curr Top Microbiol Immunol. 1985;118:29–51. doi: 10.1007/978-3-642-70586-1_3. [DOI] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Bölin I., Heikkinen H., Piha S., Wolf-Watz H. Virulence plasmid-associated autoagglutination in Yersinia spp. J Bacteriol. 1984 Jun;158(3):1033–1036. doi: 10.1128/jb.158.3.1033-1036.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M. Expression of antigens encoded by the virulence plasmid of Yersinia enterocolitica under different growth conditions. Infect Immun. 1985 Jan;47(1):183–190. doi: 10.1128/iai.47.1.183-190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M., Poikonen K. Experimental intestinal infection of rats by Yersinia enterocolitica 0:3. A follow-up study with specific antibodies to the virulence plasmid specified antigens. Scand J Infect Dis. 1986;18(4):355–364. doi: 10.3109/00365548609032347. [DOI] [PubMed] [Google Scholar]
- Straley S. C., Bowmer W. S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986 Feb;51(2):445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Portnoy D. A., Bölin I., Falkow S. Transfer of the virulence plasmid of Yersinia pestis to Yersinia pseudotuberculosis. Infect Immun. 1985 Apr;48(1):241–243. doi: 10.1128/iai.48.1.241-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yother J., Goguen J. D. Isolation and characterization of Ca2+-blind mutants of Yersinia pestis. J Bacteriol. 1985 Nov;164(2):704–711. doi: 10.1128/jb.164.2.704-711.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleska M., Lounatmaa K., Nurminen M., Wahlström E., Mäkelä P. H. A novel virulence-associated cell surface structure composed of 47-kd protein subunits in Yersinia enterocolitica. EMBO J. 1985 Apr;4(4):1013–1018. doi: 10.1002/j.1460-2075.1985.tb03732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]