Abstract
OBJECTIVE—Osteopontin (OPN) plays an important role in the development of insulin resistance and liver complications in dietary murine models. We aimed to determine the expression pattern of OPN and its receptor CD44 in obese patients and mice according to insulin resistance and liver steatosis.
RESEARCH DESIGN AND METHODS—OPN and CD44 expressions were studied in 52 morbidly obese patients and in mice. Cellular studies were performed in HepG2 cells.
RESULTS—Hepatic OPN and CD44 expressions were strongly correlated with liver steatosis and insulin resistance in obese patients and mice. This increased OPN expression could be due to the accumulation of triglycerides, since fat loading in HepG2 promotes OPN expression. In contrast, OPN expression in adipose tissue (AT) was enhanced independently of insulin resistance and hepatic steatosis in obese patients. The elevated OPN expression in AT was paralleled with the AT macrophage infiltration, and both phenomena were reversed after weight loss. The circulating OPN level was slightly elevated in obese patients and was not related to liver steatosis. Further, AT did not appear to secrete OPN. In contrast, bariatric surgery–induced weight loss induced a strong increase in circulating OPN.
CONCLUSIONS—The modestly elevated circulating OPN levels in morbidly obese patients were not related to liver steatosis and did not appear to result from adipose tissue secretion. In subcutaneous AT, expression of OPN was directly related to macrophage accumulation independently from liver complications. In contrast, hepatic OPN and CD44 expressions were related to insulin resistance and steatosis, suggesting their local implication in the progression of liver injury.
The incidence of overweight and obesity is rapidly increasing in many Western countries. This epidemic of obesity is associated with the development of type 2 diabetes, hypertension, and nonalcoholic fatty liver disease (NAFLD). These often-ignored hepatic abnormalities extend from simple steatosis to steatohepatitis (nonalcoholic steato-hepatitis [NASH]) and steatofibrosis leading, in some cases, to cirrhosis and hepatocellular carcinoma. NAFLD are frequently observed in the setting of visceral obesity, insulin resistance, and metabolic syndrome (1). Obesity is associated with a low-grade chronic inflammation, as evidenced by increased systemic concentrations of inflammatory markers and cytokines (2,3). The accumulation of macrophages in obese adipose tissue, a key source of inflammation (4,5), provides a causal link between the development of insulin resistance and liver complications (6). The combined elevation of plasma glucose and insulin levels promotes de novo lipid synthesis and impairs lipid oxidation within hepatocytes (6–8). Moreover, insulin resistance of adipose tissue leads to an enhanced delivery of free fatty acids to the liver, contributing to the excessive fatty acids accumulation (6,8). Recently, it has been proposed that osteopontin (OPN), a Th1 cytokine, could play an important role in the development of insulin resistance and NAFLD in dietary murine models (9–11).
OPN binds to multiple receptors including the integrin receptors and CD44 (12,13). This cytokine is involved in cell adhesion, chemo-attraction, and immunomodulation (13–15). In particular, OPN is highly secreted by macrophages at inflammation sites where it mediates monocyte adhesion, migration, and differentiation as well as phagocytosis (16–18). Recently, an elevated expression of OPN has been detected in human and mice adipose tissue (9,19,20). Elevated plasma levels of OPN have been associated with human and mice obesity (9,19), and weight loss after low-caloric diets was associated with a reduction of OPN plasma levels in obese patients (19). Furthermore, peroxisome proliferator–activated receptor (PPAR) ligands inhibited the OPN expression in macrophages (21,22), and treatment with bezafibrate in type 2 diabetic patients was correlated with reduced OPN levels (21). Recently, Nomiyama et al. (9) have identified OPN as a link between adipose tissue inflammation and insulin resistance in a murine model of diet-induced obesity. Similarly, OPN can also play an important role in the occurrence of liver complications, as suggested by Sahai et al. (10) in OPN null mice.
Based on the evidence that OPN can be considered as a potential actor in obesity-induced complications in mice (9,10), we first analyzed the expression pattern of OPN and its receptor CD44 in morbidly obese patients according to steatosis and insulin resistance. In addition, OPN and CD44 expressions were evaluated in human adipose tissue after a surgically induced weight loss associated with a marked reduction of inflammation and insulin resistance. We then looked for a direct effect of fat loading on OPN expression in a hepatocyte cell line.
RESEARCH DESIGN AND METHODS
Wild-type C57BL/6 male mice (7–10 weeks of age), obtained from Janvier (Le Genest-St-Isle, France), had free access to water and were fed a standard diet (n = 8) (TD2016, Harlan) or a high-fat diet (HFD, n = 10) containing 36% fat (TD99249, Harlan) for 15 weeks. At the time of death, white epididymal adipose tissue pads and liver samples were removed, immediately frozen in liquid nitrogen, and stored at −80°C until used. Serum OPN levels were determined by ELISA (R&D Systems, Lille, France).
Isolation of hepatocytes and nonparenchymal fraction from liver.
Mouse livers were perfused first with a HEPES buffer containing 8 g/l NaCl, 33 mg/l Na2HPO4, 200 mg/l KCl, and 2.38 g/l HEPES, pH 7.65, for 2 min at 5 ml/min and then with HEPES buffer supplemented with 1.5 g/l CaCl2 and 25 mg/l liberase (Roche Diagnostics) for 7 min at 5 ml/min. Livers were then carefully removed and minced, and the resultant cell suspension was then filtered (100 μm). Hepatocytes were collected by centrifugation for 5 min at 50g and then on a Percoll density gradient (20 min, 500g) (Sigma). Hepatocytes were then washed in NaCl 0.9%, isolated by centrifugation (5 min, 50g), and frozen at −80°C before RNA extraction, as described below. The nonparenchymal cells in the supernatant of first centrifugation were collected by centrifugation for 5 min at 450g and then incubated in NaCl 0.9% buffer supplemented with 0.02% pronase E (Sigma) for 20 min at 37°C under gentle agitation. Finally, cells were collected by centrifugation at 450g for 5 min and frozen at −80°C before RNA extraction as described below.
Patient population.
The patient cohort included 52 morbidly obese patients. These patients were recruited through the Department of Digestive Surgery and Liver Transplantation, where they underwent bariatric surgery for their morbid obesity (Nice and Paris hospitals). Bariatric surgery was indicated for these patients in accordance with the French Guidelines for obesity surgery. Briefly, they had a BMI ≥40 or ≥35 kg/m2 with at least one complication. Exclusion criteria were as follows: presence of hepatitis B or hepatitis C virus infection, excessive alcohol consumption (>20 g/day), or another cause of chronic liver diseases (primary biliary cirrhosis, autoimmune hepatitis, Wilson disease, genetic hemochromatosis, or biliary disease). The clinical and biological characteristics of the study groups are described in Table 1. Before surgery, fasting blood samples were obtained and used to measure alanine amino transferase (ALAT), glucose, insulin, HDL cholesterol, LDL cholesterol, triglycerides, and C-reactive protein (CRP) levels. Serum OPN levels were determined by ELISA (R&D Systems, Lille, France). The degree of insulin resistance was calculated by using the homeostatic model assessment–insulin resistance (HOMA-IR) index (23). Surgical liver biopsies were obtained at the time of bariatric surgery. Part of the biopsy was immediately frozen in liquid nitrogen and stored at −80°C until analyzed. The other part was fixed in Bouin solution, paraffin-embedded, sectioned, and stained with hematoxylin-eosin-safran and picro-sirius red. Steatosis was graded from 0–3 based on percent of hepatocytes in the biopsy affected: 0 for none (S0, n = 6); 1 for <30% (S1, mild steatosis, n = 13); 2 for 30–60% (S2, moderate steatosis, n = 9); and 3 for >60% (S3, severe steatosis, n = 24). Subcutaneous and visceral adipose tissue samples were also obtained from 39 patients and frozen until analyzed. The percentage of macrophages in subcutaneous adipose tissue of 19 patients has been evaluated by immunohistochemical analysis as previously described (24). In addition, abdominal subcutaneous adipose tissue was obtained from six women before (age 40.7 ± 3.2 years; BMI 42.7 ± 2.0 kg/m2) and 2 years after (BMI 26.0 ± 1.6 kg/m2) weight loss following bariatric surgery (mean weight loss 44.3 ± 11.4 kg). Serum was obtained from 25 patients before (BMI 43.9 ± 5.3 kg/m2) and 1 year after (BMI 27.9 ± 3.8 kg/m2) weight loss following bariatric surgery (mean weight loss 42.3 ± 12.3 kg). The second surgery was performed for cosmetic purpose.
TABLE 1.
Characteristics of the morbidly obese patients according to grade of hepatic steatosis
| S0 | S1 | S2 | S3 | |
|---|---|---|---|---|
| n | 6 | 13 | 9 | 24 |
| Sex (F/M) | 5/1 | 12/1 | 6/3 | 16/8 |
| BMI (kg/m2) | 42.1 ± 0.6 | 44.7 ± 1.5 | 45.0 ± 2.0 | 43.8 ± 1.1 |
| Age (years) | 41.8 ± 4.8 | 40.4 ± 2.3 | 43.2 ± 4.4 | 39.1 ± 1.9 |
| ALAT (IU/l) | 19.5 ± 4.4 | 19.8 ± 2.0 | 27.6 ± 3.6*† | 57.0 ± 10.9*†‡ |
| Blood glucose (mmol/l) | 5.1 ± 0.0 | 5.9 ± 0.9 | 7.5 ± 1.0*† | 6.6 ± 0.5*† |
| Insulin (μmol/l) | 8.0 ± 0.9 | 12.7 ± 3.9 | 14.4 ± 2.6 | 20.9 ± 2.7*† |
| HOMA-IR | 1.8 ± 0.2 | 3.3 ± 1.2 | 5.0 ± 1.3* | 6.1 ± 0.8*† |
| HDL cholesterol (mmol/l) | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| LDL cholesterol (mmol/l) | 3.2 ± 0.5 | 3.8 ± 0.3 | 3.2 ± 0.2 | 3.4 ± 0.2 |
| Triglycerides (mmol/l) | 1.2 ± 0.1 | 1.8 ± 0.2 | 1.4 ± 0.2 | 2.6 ± 0.4* |
| C-reactive protein (mg/l) | 8.8 ± 3.2 | 13.3 ± 1.6 | 9.1 ± 1.8 | 8.3 ± 1.1 |
Data are means ± SE and were compared by using the nonparametric Kruskal-Wallis test.
P < 0.05 compared with S0;
P < 0.05 compared with S1;
P < 0.05 compared with S2.
Control subjects.
Total RNA from six control livers was purchased from Stratagene (La Jolla, CA) (female, 34 years old, normal adjacent tissue to stromal sarcoma), Clontech (Mountain View, CA) (male, 51 years old, sudden death), and Biochain (Hayward, CA) (four males: 24, 26, 28, and 30 years old, sudden deaths). The Stratagene and Biochain companies confirmed that histological findings were completely normal with no evidence of fatty liver disease. No clinical or biological data were available for these individuals, but the absence of an inflammatory process was corroborated by low CRP mRNA expression levels, as previously reported (3,25). Control subcutaneous adipose tissue was obtained from seven lean subjects (five females and two males; age 36.7 ± 8.4 years; BMI 21.5 ± 1.9 kg/m2) undergoing lipectomy for cosmetic purposes. Finally, serum was obtained from eight lean healthy volunteers (six females and two males; age 31.6 ± 2.4 years; BMI 21.2 ± 0.3 kg/m2) after an overnight fast. All subjects gave their informed consent to participate in this research study according to French legislation regarding Ethic and Human Research (Huriet-Serusclat law, DGS 2003/0395).
Arteriovenous differences across the adipose tissue.
The protocol was approved by the Oxfordshire Clinical Research Ethics Committee, and all subjects gave written informed consent. Five overweight healthy subjects (four men, one woman, BMI 27 ± 1 kg/m2) were studied after an overnight fast. A 10 cm, a 22-gauge Hydrocath catheter (Becton Dickinson, U.K.) was introduced over a guide wire into a superficial vein on the anterior abdominal wall and threaded toward the groin so that its tip lay just superior to the inguinal ligament. This provided access to the venous drainage from the subcutaneous abdominal adipose tissue, uncontaminated by muscle drainage and with a relatively minor contribution from skin (26). A retrograde cannula was placed in a vein draining the hand, which was warmed in a hot-air box maintained at 60°C to obtain arterialized blood. The cannulae were kept patent by a slow infusion of 0.9% (wt/vol) saline. After a resting period of at least 30 min, blood samples were taken simultaneously from the arterialized and adipose tissue venous lines.
Real-time quantitative PCR analysis.
Total RNA was extracted from human and animal tissues using the RNeasy Mini Kit (Qiagen, Contraboeuf, France) and treated with Turbo DNA-free (Applied Biosystems, Contraboeuf, France) following the manufacturer's protocols. The quantity and quality of the isolated RNA were determined using the Agilent 2100 Bioanalyser with RNA 6000 Nano Kit (Agilent Technologies). One microgram of total RNA was reverse-transcribed with a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time quantitative PCR was performed in duplicate for each sample using the ABI PRISM 7500 Fast Real-Time PCR System and FAM dyes (Applied Biosystems, Contraboeuf, France) as previously described (3,25). The TaqMan gene expression assays were purchased from Applied Biosystems: OPN (Hs00167093_m1, Mm00436767_m1); CD44 (Hs00153304_m1; Mm01277163_m1), CD11b (Hs00355885_m1), CD68 (Hs00154355_m1), TNFα (Hs00174128_m1), F4/80 (Mm00802530_m1), RPLP0 (large P0 subunit of the acidic ribosomal phosphoprotein, Hs99999902_m1), and 36B4, (Mm99999223_gH).
OPN immunoblotting.
Proteins were extracted from patient frozen liver samples using the NucleoSpin RNA/Protein kit (Macherey-Nagel, France) according to the manufacturer's protocol. Proteins (50 μg) were separated by SDS-PAGE using a 10% resolving gel. The Western blotting was performed as previously described (27). The proteins were probed with anti-OPN (AKm2A1) or anti-GAPDH (FL-335) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) at 1 μg/ml.
Oleic acid–induced fat loading in HepG2 cells.
Human HepG2 cells were cultured in DMEM supplemented with 10% fetal bovine serum under 5% CO2 at 37°C. At 75% confluence, medium was exchanged for new medium with or without 1 mmol/l oleic acid for 24 h (28). Hepatic triglycerides were extracted with a methanol-chloroform mixture and measured using an enzymatic assay (Diagnostic Systems International, Germany). Results were normalized to protein content measured by BCA assay (Pierce, Perbio Science France, Brebiéres, France).
Statistical analysis.
Results are expressed as means ± SE. Statistical significance of differential gene expression between two study groups was determined using the nonparametric Kruskal-Wallis with the ΔCt of each group. Correlations were analyzed using Spearman's rank correlation test. Other data were statistically analyzed using the Student's t test. P < 0.05 was considered as significant.
RESULTS
OPN and CD44 gene expressions in livers from morbidly obese patients.
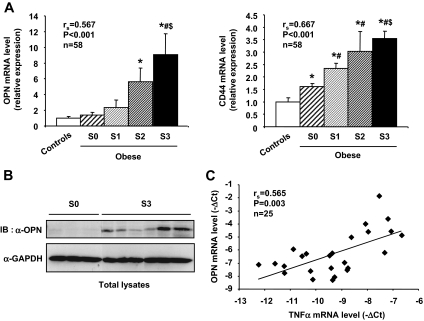
We studied the OPN and CD44 gene expression in the whole cohort of 52 morbidly obese patients included in the study (Table 1), who were stratified according to the degree of the fat infiltration in liver. We report for the first time that the hepatic OPN and CD44 gene expression was significantly correlated with the grade of hepatic steatosis (from S0 to S3) (Fig. 1A). Noteworthy, when compared with control values, OPN and CD44 gene expressions were markedly increased in S3 patients (9.2 ± 2.6-fold and 3.5 ± 0.3-fold, respectively; P < 0.05), whereas CD44 expression was barely increased in S0 patients (Fig. 1A). Furthermore, the hepatic OPN and CD44 gene expression was also significantly correlated with plasma ALAT levels (rs = 0.567, P < 0.001, and rs = 0.549, P < 0.001, respectively) and the insulin resistance as evaluated by the HOMA-IR (rs = 0.474, P = 0.001, and rs = 0.587, P < 0.001, respectively) (data not illustrated). Finally, the OPN protein expression was evaluated by Western blot in total liver lysates obtained from three morbidly obese patients without steatosis (S0) and six with severe steatosis (S3). In accordance with mRNA expression level, the OPN protein level was barely detectable in livers from S0 patients, whereas strong signals were observed in livers from S3 patients (Fig. 1B). Because tumor necrosis factor (TNF)-α can stimulate the OPN gene expression in hepatocytes (10), we evaluated the TNF-α gene expression of S3 patients (n = 15) compared with S0 patients (n = 6) and controls (n = 5). Interestingly, the gene expression of TNF-α was fourfold increased in S3 patients compared with S0 patients (P = 0.004) (data not shown). Moreover, the OPN gene expression was positively correlated with the TNF-α gene expression (Fig. 1C). These findings indicate that TNF-α and OPN expressions were closely related in vivo.
FIG. 1.
The hepatic OPN and CD44 expression is significantly correlated with the grade of steatosis in morbidly obese patients. A: Hepatic OPN and CD44 mRNA expression levels were analyzed by real-time quantitative PCR in lean patients (controls; n = 6), in morbidly obese patients without liver steatosis (S0; n = 6), and in morbidly obese patients with S1 (n = 13), S2 (n = 9), and S3 (n = 24) steatosis. The gene expression of OPN and CD44 was normalized to the mRNA levels of RPLP0. Results are expressed relative to the expression level in controls and expressed as means ± SE. *P < 0.05 compared with controls; #P < 0.05 compared with S0; $P < 0.05 compared with S1. B: Total lysates from liver biopsies obtained from three morbidly obese patients without steatosis (S0) and six with severe steatosis (S3) were separated by SDS-PAGE and immunoblotted with anti-OPN or anti-GAPDH antibodies. A representative immunoblot is shown. C: Correlation between OPN and TNF-α mRNA expression levels (ΔCt) in lean subjects (n = 5) and morbidly obese patients without (n = 6) or with severe steatosis (n = 14) was analyzed using Spearman's rank correlation test.
Induction of OPN expression in fatty HepG2 cells.
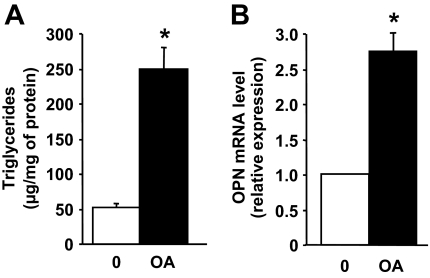
Because we show a correlation between OPN expression and steatosis, we wanted to determine if triglyceride accumulation was sufficient to induce OPN expression in cultured HepG2 cells. Triglyceride accumulation in HepG2 cells was induced by incubation with oleic acid for 24 h. Interestingly, this was associated with a marked increase in the OPN mRNA expression compared with cells cultured in the control medium (Fig. 2).
FIG. 2.
The OPN expression in human HepG2 cells is enhanced after the accumulation of triglycerides. HepG2 cells were incubated without (0) or with 1 mmol/l oleic acid (OA) for 24 h. Intracellular TG levels were determined and normalized to cell protein content. OPN mRNA expression was analyzed by real-time quantitative PCR. Data were expressed as means ± SE of three independent experiments. *P < 0.05.
OPN and CD44 gene expressions in adipose tissue from morbidly obese patients.
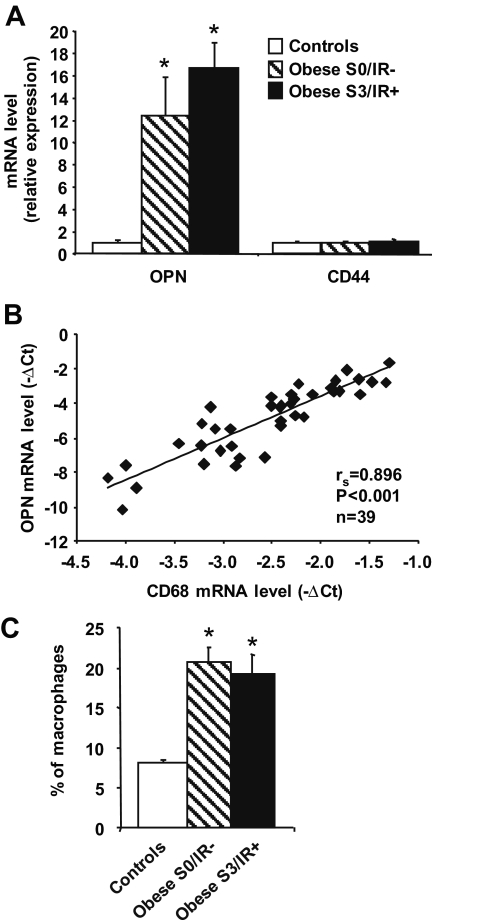
We next evaluated OPN and CD44 expressions in the adipose tissue that could be obtained in a set of patients without hepatic steatosis and insulin resistance (S0/IR−, n = 9) and in patients with severe hepatic steatosis and insulin resistance (S3/IR+, n = 23) compared with adipose tissue obtained in seven controls subjects. OPN mRNA expression level was significantly increased in the adipose tissue of morbidly obese patients (S0 and S3) compared with lean controls, but different from liver, independently from steatosis and insulin resistance (Fig. 3A). Although comparing mRNA expression from liver, subcutaneous adipose tissue (SCAT) and visceral adipose tissue (VAT) have to be cautiously taken; OPN mRNA expression was much higher in adipose tissues than in liver but did not significantly differ in both adipose tissues (VAT vs. liver: 2.96 ± 0.54, P < 0.05, n = 16; SCAT vs. liver: 5.02 ± 1.92, P < 0.05, n = 16; SCAT vs. VAT: 1.66 ± 0.40, NS, n = 16). There was no difference in CD44 mRNA expression levels in subcutaneous adipose tissue between lean controls and morbidly obese patients (S0 as well as S3) (Fig. 3A). Noteworthy, the CD44 gene expression was much higher in both adipose tissues than in liver (VAT vs. liver: 2.48 ± 0.37, P < 0.05, n = 16; SCAT vs. liver: 3.20 ± 0.55, P < 0.05, n = 16).
FIG. 3.
The OPN mRNA expression is increased in subcutaneous adipose tissue of morbidly obese patients and is significantly correlated with macrophage accumulation. A: OPN and CD44 mRNA expression levels were analyzed by real-time quantitative PCR in subcutaneous adipose tissue of lean patients (controls; n = 7), morbidly obese patients without liver steatosis and insulin resistance (S0/IR−; n = 9), and morbidly obese patients with severe steatosis and insulin resistance (S3/IR+; n = 23). Data are presented as relative mRNA normalized to RPLP0 mRNA and are expressed as means ± SE. *P < 0.05. B: Correlation between OPN and CD68 mRNA expression levels (ΔCt) was analyzed using Spearman's rank correlation test. C: The percentage of macrophages in subcutaneous adipose tissue has been evaluated in lean subjects (controls; n = 4), S0/IR− obese patients (n = 4), and S3/IR+ obese patients (n = 11).
Because invalidation of OPN prevents macrophages infiltration into mouse adipose tissue (9), we then compared the OPN gene expression in adipose tissue with that of a macrophages marker, CD68, in 39 patients from the cohort with a wide range of OPN expression. As shown in Fig. 3B, the OPN mRNA expression level was strongly and significantly correlated with the macrophage infiltration, as evaluated by CD68 expression. In accordance with this result, macrophage infiltration was increased in subcutaneous adipose tissue from obese patients (n = 19) versus lean subjects (n = 4) (Fig. 3C) and was correlated with the OPN gene expression (rs = 0.579, P = 0.009, n = 19) (data not shown).
Effect of weight loss on adipose gene expression.
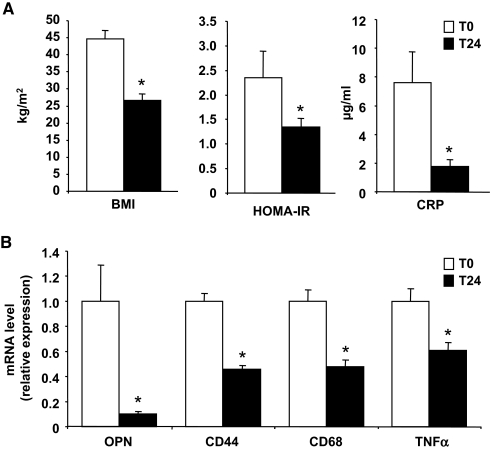
We then determined if weight loss could modify the gene expression of OPN, CD44, TNF-α, and the macrophage marker CD68 in abdominal subcutaneous adipose tissue. Six patients were reevaluated 2 years after their bariatric surgery. As expected, these patients had a marked weight loss (−44.3 ± 11.4 kg), leading to a notable improvement of systemic inflammation, as evaluated by the circulating levels of CRP and the insulin resistance as evaluated by the HOMA-IR (Fig. 4A). Interestingly, in their adipose tissue, the expression of both OPN and CD44 was drastically decreased and was associated with an important decrease in the macrophage infiltration, as evaluated by the CD68 gene expression (Fig. 4B). Furthermore, the OPN and CD44 mRNA expressions were significantly correlated with CD68 mRNA expression (rs = 0.769, P = 0.003; rs = 0.790, P = 0.002, respectively). In addition, while TNF-α expression was increased in obese patients compared with the lean patients (2.69 ± 0.26, P < 0.05) (data not shown), it was markedly decreased after weight loss (Fig. 4B) and was correlated with OPN mRNA expression (rs = 0.741, P = 0.006) (data not shown). These findings indicate that OPN and CD44 expressions are strongly related to macrophage accumulation in adipose tissue.
FIG. 4.
Weight loss is associated with a strong decrease in the gene expression of OPN, CD44, TNF-α, and the macrophage marker CD68 in subcutaneous adipose tissue. A: Six patients were studied before and 2 years after bariatric surgery for BMI, insulin resistance (as evaluated by the HOMA-IR), and systemic inflammation (as evaluated by the circulating levels of CRP). B: OPN, CD44, CD68, and TNF-α mRNA expressions were analyzed by real-time quantitative PCR in subcutaneous adipose tissue obtained from these patients before and after weight loss. Data are presented as relative mRNA normalized to RPLP0 mRNA and are expressed as means ± SE. *P < 0.05.
Circulating OPN levels in morbidly obese patients.
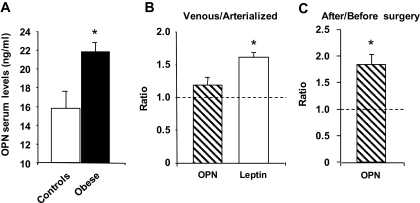
We then measured OPN serum levels in 52 morbidly obese patients without (S0, n = 6) or with liver steatosis (S1, n = 13; S2, n = 9; S3, n = 24) and control subjects (n = 8). As shown in Fig. 5A, circulating OPN concentrations were modestly but significantly elevated in morbidly obese patients compared with control subjects (n = 8), in agreement with Gomez-Ambrosi et al. (19). However, the circulating OPN levels were not correlated with the grade of liver steatosis (rs = 0.129; P = 0.268, n = 52) (data not shown). The relative contribution of liver and adipose tissue in circulating OPN levels is difficult to estimate. To determine whether circulating OPN could be of adipose tissue origin, we determined the OPN levels in the arterialized and adipose tissue venous plasma in five overweight patients. As shown in Fig. 5B, while adipose tissue enriched the circulating levels of leptin, no significant difference between arterialized and venous adipose tissue samples was observed for OPN. We then investigated if weight loss could modify the circulating levels of OPN in 25 patients. In accordance with recent reports (29–31), the circulating OPN level was 1.8-fold increased 1 year after bariatric surgery in our patients (Fig. 5C).
FIG. 5.
Circulating OPN levels are affected by obesity and weight loss but do not appear to be of adipose tissue origin. OPN levels were evaluated by ELISA (A) in serum of lean patients (controls, n = 8) and morbidly obese patients (obese, n = 52), in the arterial(ized) and adipose tissue venous plasma in five overweight patients (B), and in serum of 25 morbidly obese patients before and 1 year after bariatric surgery (C). Results are expressed as means ± SE. *P < 0.05.
OPN and CD44 expressions in obese mice.
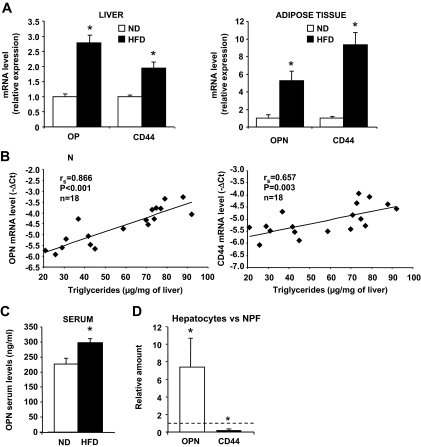
To corroborate the human findings and to further explore the potential role of OPN in liver complications of morbid obesity, a series of experiments were performed in various animal models. As expected, mice exposed to HFD exhibited increased weight (45.1 ± 3.2 vs. 28.2 ± 1.6 g in low-fat diet–fed controls), increased hepatic triglyceride content (73 ± 14 vs. 37 ± 12 μg/mg liver), hyperglycemia (208 ± 90 vs. 100 ± 16 mg/dl), and hyperinsulinemia (2.31 ± 0.63 vs. 0.95 ± 0.89 μg/l). HFD-induced obesity resulted in increased OPN and CD44 gene expressions in both fatty liver and epididymal WAT (Fig. 6A). Interestingly, the hepatic expressions of OPN and CD44 were also positively correlated with steatosis, as evaluated by hepatic triglyceride content (Fig. 6B). Similar findings were observed in genetic leptin-deficient (ob/ob) mice (n = 5), since OPN and CD44 gene expressions were found to be significantly enhanced in both liver (3.3 ± 0.3, P < 0.05, and 1.6 ± 0.1-fold, P < 0.05, respectively) and adipose tissue (23.4 ± 2.5, P < 0.05, and 5.2 ± 0.8-fold, P < 0.05, respectively) compared with lean littermates (n = 5). In addition, the adipose tissue expressions of OPN and CD44 were correlated with expression of the macrophage marker F4/80 in HFD-induced obese mice (rs = 0.820, P < 0.01, and rs = 0.843, P < 0.01, respectively). Furthermore, serum OPN levels were also significantly increased, but this increase was moderate, as occurred in liver and adipose tissue (Fig. 6C).
FIG. 6.
OPN and CD44 mRNA expression is increased in liver and adipose tissue of obese mice. A: OPN and CD44 mRNA expressions were analyzed by real-time quantitative PCR in liver and epididymal white adipose tissue of mice fed a normal diet (ND; n = 8) or HFD (n = 10) for 15 weeks. Data are presented as relative mRNA normalized to 36B4 mRNA and are expressed as means ± SE. B: Correlations between the expression levels of OPN or CD44 mRNA (−ΔCt) and the hepatic triglyceride content were analyzed using Spearman's rank correlation test. *P < 0.05. C: OPN levels were evaluated by ELISA in serum of HFD-induced obese mice (HFD, n = 6) and serum of lean littermates (ND, n = 6). D: Relative expression of OPN and CD44 was analyzed by real-time quantitative PCR in the isolated hepatocytes versus nonparenchymal fraction in the liver of ob/+ mice. Results are expressed as means ± SE of three independent experiments. P values were obtained using the nonparametric Kruskal-Wallis test. *P < 0.05.
We then investigated which liver cell population could be responsible for OPN and CD44 expression. Cells from livers of ob/+ mice were separated into hepatocytes and nonparenchymal fractions. As shown in Fig. 6D, real-time PCR analysis revealed that OPN expression was seven times higher in hepatocytes than in the nonparenchymal fraction. In contrast, CD44 was predominantly expressed in the nonparenchymal fraction. To secure the quality of the separation procedure, albumin and CD11b expression was used as markers of hepatocyte and inflammatory cells, respectively. Albumin was predominantly expressed in the hepatocyte fraction (45 ± 11, P < 0.05) as well as OPN, whereas CD11b was predominantly expressed in the nonparenchymal fraction (34 ± 27, P < 0.05) (data not shown) as well as CD44.
DISCUSSION
We report here that the increased expression of OPN in adipose tissue was associated with the accumulation of macrophages in the adipose tissue. We also describe for the first time that, in patients with severe steatosis and insulin resistance, the hepatic OPN gene expression, as well as the expression of its receptor CD44, were markedly increased and related to the severity of hepatic steatosis. This progressive upregulation of the hepatic OPN gene was significantly associated with liver injury (as evaluated by plasma ALT levels) and hepatic insulin resistance (as evaluated by the HOMA-IR). Local concentrations of OPN in liver and adipose tissue do not appear to affect its systemic levels. Indeed, the modest elevation of systemic OPN in obese patients is not related to liver steatosis or to its marked increase in adipose tissue. Furthermore, weight loss after bariatric surgery was indeed associated with a strong decrease in OPN expression in the adipose tissue, whereas an opposite increase was observed in its systemic level.
OPN plays an important role in infiltration and accumulation of macrophages in adipose tissue in the early stages of obesity. In vitro chemotaxis assays have revealed that OPN amplified macrophage migration (9). In addition, endogenous OPN in macrophage is required to maintain macrophage function including chemotaxis, an effect mediated by the interaction with its receptor CD44 (9,17,32,33). The increase in the production of such a pro-inflammatory adipokine could activate macrophages already present in the stromal vascular fraction of adipose tissue resulting in an increased production of migratory signal including OPN itself. This could contribute to the over-enrichment of adipose tissue in macrophages. However, the recruitment into adipose tissue of macrophages derived from the circulation in the early stage of obesity is a complex mechanism (5). Other chemokines may play an important role, such as monocyte chemoattractant protein-1 (MCP-1) (produced primarily by adipocytes) with elevated plasma levels in obese patients (34). Indeed, MCP-1 exerts additive effects on chemotaxis in the presence of OPN and amplifies macrophage migration to the same extent than OPN (9).
It has been proposed that the accumulation of macrophages in adipose tissue mediated by OPN, per se, as well as by MCP-1, resulting in an increased macrophage-related inflammatory activity, could be a link between inflammation and insulin resistance (9,35–37). Indeed, the respective invalidation of OPN, MCP-1, or its receptor CCR2 prevents the macrophage accumulation in adipose tissue, inflammation, and insulin resistance mediated by HFD-induced obesity (9,35–37). This appealing hypothesis was further supported by our studies confirming a marked increase in OPN as well as CD44 gene expressions in both liver and adipose tissue in two murine models of obesity. Of particular interest, in the HFD model, OPN and CD44 gene expressions were well correlated with the accumulation of macrophages in adipose tissue.
Because there is a direct link between OPN-induced macrophage accumulation and insulin resistance, the beneficial effect of weight loss or treatment with anti-inflammatory drugs (PPAR agonists) on inflammation and glucose homeostasis should be associated with a decrease in OPN expression. Indeed, we have shown that weight loss after bariatric surgery resulted in a strong reduction in OPN expression in adipose tissue, together with the marked diminution in the accumulation of macrophages also reported in previous studies (38,39). In these patients, weight loss was also associated with a decreased peripheral inflammation (as evaluated by plasma CRP levels) and an improvement in insulin sensitivity. On the other hand, PPAR agonists can suppress the OPN expression in macrophages (21,22) and decrease plasma levels in treated patients (21).
However, the role of circulating OPN in the macrophage infiltration into adipose tissue has not yet been established. We show here that in obese patients and mice, OPN expression was modestly elevated in serum but markedly increased in adipose tissue compared with lean subjects or mice. Whereas weight loss after bariatric surgery resulted in a strong reduction in OPN and CD44 expression and macrophage accumulation in adipose tissue, the circulating levels of OPN were enhanced. In accordance with this result, recent reports have shown that elevated OPN plasma concentration 1 year after bariatric surgery was correlated with markers of bone turnover but not with insulin resistance and inflammation (29,30). On the contrary, diet-induced weight loss could decrease circulating OPN levels (19). Local concentrations of OPN might be different from systemic levels. Furthermore, the tissues or organs contributing to the modestly elevated circulating OPN levels in obese patients were very difficult to estimate. Whereas we show for the first time that systemic OPN levels were not correlated with the grade of liver steatosis and that adipose tissue from overweight patients did not appear to secrete OPN, additional studies are required to know the organ origin of the circulating OPN.
It is currently accepted that insulin resistance can contribute to the development of hepatic steatosis (35,36) through the increased release of free fatty acids secondary to unsuppressed lipolysis (6,8). In addition, the combined elevation of plasma glucose and insulin levels promotes de novo lipid synthesis and impairs lipid oxidation within hepatocytes, further promoting the excessive fatty acids accumulation (6–8). The present data clearly show that in obese patients and mice, the expression of OPN in adipose tissue was positively correlated with macrophage markers. In addition, the hepatic OPN and CD44 gene expressions were significantly increased in the presence of severe steatosis, and this increase was correlated with the severity of steatosis. In parallel, the hepatic OPN protein itself was strongly elevated in obese patients with severe steatosis. Although direct cause-effect relationships cannot be inferred from our data, modification of all parameters could be linked to the severity of steatosis. While the role of OPN in the development of liver complications has not been fully elucidated, invalidation of the OPN gene reduces the plasma ALAT elevation as well as the hepatic inflammation and fibrosis in mice fed a choline-methionine–deficient diet (10).
The cellular origin of the increased hepatic OPN expression has not been yet determined. Although an increased hepatic OPN gene expression has been reported in macrophages, Küpffer cells, and stellate cells in response to carbon tetrachloride (40), we show here that hepatocytes versus the nonhepatocyte cells are the main source of OPN in normal mouse liver. Most interestingly, our in vitro steatosis studies clearly demonstrate that human HepG2 cells cultured in the presence of fatty acids rapidly accumulate triglycerides and, concomitantly, the OPN gene expression is rapidly induced.
In summary, we have shown that OPN expression was increased in the adipose tissue early in the course of obesity-induced insulin resistance and before the occurrence of liver steatosis. In morbidly obese patients, OPN could enhance macrophage infiltration and accumulation in the adipose tissue, thus contributing to inflammation and insulin resistance. In the presence of insulin resistance, the accumulation of triglycerides in liver was then associated with an additional upregulation of hepatic OPN and CD44 expression, well related to the severity of steatosis. In addition the hepatic gene expressions of OPN and of its receptor, CD44, were related to the liver injury and hepatic insulin resistance. Our original findings concerning the effects of triglyceride accumulation on hepatoma cells on OPN expression offer some interesting mechanistic explanations for the role of OPN in the development of NAFLD in humans and rodent models. Studies focusing on the behavior of hepatic OPN and CD44 signaling in the progression from normal liver toward steatosis, steatohepatitis, and fibrosis are attractive approaches to acquire more insight into the pathogenesis of human NAFLD.
Acknowledgments
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale (France), ANR-05-PCOD-025-02 PNRHGE to P.G., the University of Nice, the Programme Hospitalier de Recherche Clinique (CHU of Nice and Paris [AOR02076]) the Comité Doyen Jean Lépine (Nice, France), and the French Research Ministery (ACI JC5327 to P.G.). This work is part of the project “Hepatic and adipose tissue and functions in the metabolic syndrome” (HEPADIP, see http://www.hepadip.org), which is supported by the European Commission as an Integrated Project under the 6th Framework Programme (Contract LSHM-CT-2005-018734). A.B. was successively supported by the Programme Hospitalier de Recherche Clinique (CHU of Nice) and the Association pour la Recherche sur le Cancer (France). S.B. was supported by ANR-05-PCOD-025-02. Y.L.M.-B. and P.G. are the recipients of an Interface grant from CHU of Nice. S.L. is the recipient of an Interface grant from AP-HP.
This work was also supported by charitable donations from ALFEDIAM and AFEF/Schering-Plough (to P.G. and K.C.). No other potential conflicts of interest relevant to this article were reported.
We thank B. Bailly-Maitre for critical reading of the manuscript.
Published ahead of print at http://diabetes.diabetesjournals.org on 24 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Neuschwander-Tetri BA, Caldwell SH: Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 37: 1202–1219, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Shoelson SE, Herrero L, Naaz A: Obesity, inflammation, and insulin resistance. Gastroenterology 132: 2169–2180, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Anty R, Bekri S, Luciani N, Saint-Paul MC, Dahman M, Iannelli A, Ben Amor I, Staccini-Myx A, Huet PM, Gugenheim J, Sadoul JL, Le Marchand-Brustel Y, Tran A, Gual P: The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, type 2 diabetes, and NASH. Am J Gastroenterol 101: 1824–1833, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H: Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr: Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ: Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N: Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 107: 450–455, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN: Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Nomiyama T, Perez-Tilve D, Ogawa D, Gizard F, Zhao Y, Heywood EB, Jones KL, Kawamori R, Cassis LA, Tschop MH, Bruemmer D: Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest 117: 2877–2888, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahai A, Malladi P, Melin-Aldana H, Green RM, Whitington PF: Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. Am J Physiol Gastrointest Liver Physiol 287: G264–G273, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Sahai A, Malladi P, Pan X, Paul R, Melin-Aldana H, Green RM, Whitington PF: Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol 287: G1035–G1043, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Weber GF, Ashkar S, Glimcher MJ, Cantor H: Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 271: 509–512, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H: Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287: 860–864, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D, Segawa T, Maeda M, Hamuro J, Nakayama T, Taniguchi M, Yagita H, Van Kaer L, Onoe K, Denhardt D, Rittling S, Uede T: Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity 21: 539–550, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Mimura S, Mochida S, Inao M, Matsui A, Nagoshi S, Yoshimoto T, Fujiwara K: Massive liver necrosis after provocation of imbalance between Th1 and Th2 immune reactions in osteopontin transgenic mice. J Gastroenterol 39: 867–872, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M: Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol 152: 353–358, 1998 [PMC free article] [PubMed] [Google Scholar]
- 17.Nystrom T, Duner P, Hultgardh-Nilsson A: A constitutive endogenous osteopontin production is important for macrophage function and differentiation. Exp Cell Res 313: 1149–1160, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Murry CE, Giachelli CM, Schwartz SM, Vracko R: Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol 145: 1450–1462, 1994 [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Ambrosi J, Catalan V, Ramirez B, Rodriguez A, Colina I, Silva C, Rotellar F, Mugueta C, Gil MJ, Cienfuegos JA, Salvador J, Fruhbeck G: Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J Clin Endocrinol Metab 92: 3719–3727, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T, Aszmann O, Ludvik B, Silberhumer GR, Prager G, Stulnig TM: Osteopontin expression in human and murine obesity: extensive local upregulation in adipose tissue but minimal systemic alterations. Endocrinology 149: 1350–1357, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Nakamachi T, Nomiyama T, Gizard F, Heywood EB, Jones KL, Zhao Y, Fuentes L, Takebayashi K, Aso Y, Staels B, Inukai T, Bruemmer D: PPARalpha agonists suppress osteopontin expression in macrophages and decrease plasma levels in patients with type 2 diabetes. Diabetes 56: 1662–1670, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Oyama Y, Akuzawa N, Nagai R, Kurabayashi M: PPARgamma ligand inhibits osteopontin gene expression through interference with binding of nuclear factors to A/T-rich sequence in THP-1 cells. Circ Res 90: 348–355, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Wallace TM, Levy JC, Matthews DR: Use and abuse of HOMA modeling. Diabetes Care 27: 1487–1495, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clement K: Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 55: 1554–1561, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I, Saint-Paul MC, Huet PM, Sadoul JL, Gugenheim J, Srai SK, Tran A, Le Marchand-Brustel Y: Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology 131: 788–796, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Frayn KN, Coppack SW: Assessment of white adipose tissue metabolism by measurement of arteriovenous differences. Methods Mol Biol 155: 269–279, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Bertola A, Bonnafous S, Cormont M, Anty R, Tanti JF, Tran A, Le Marchand-Brustel Y, Gual P: Hepatocyte growth factor induces glucose uptake in 3T3–L1 adipocytes through A Gab1/phosphatidylinositol 3-kinase/Glut4 pathway. J Biol Chem 282: 10325–10332, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Lechon MJ, Donato MT, Martinez-Romero A, Jimenez N, Castell JV, O'Connor JE: A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact 165: 106–116, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Riedl M, Vila G, Maier C, Handisurya A, Shakeri-Manesch S, Prager G, Wagner O, Kautzky-Willer A, Ludvik B, Clodi M, Luger A: Plasma osteopontin increases after bariatric surgery and correlates with markers of bone turnover but not with insulin resistance. J Clin Endocrinol Metab 93: 2307–2312, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Schaller G, Aso Y, Schernthaner GH, Kopp HP, Inukai T, Kriwanek S, Schernthaner G: Increase of osteopontin plasma concentrations after bariatric surgery independent from inflammation and insulin resistance. Obes Surg 2008. [Epub ahead of print] [DOI] [PubMed]
- 31.Wucher H, Ciangura C, Poitou C, Czernichow S: Effects of weight loss on bone status after bariatric surgery: association between adipokines and bone markers. Obes Surg 18: 58–65, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J: Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. J Cell Physiol 184: 118–130, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Zhu B, Suzuki K, Goldberg HA, Rittling SR, Denhardt DT, McCulloch CA, Sodek J: Osteopontin modulates CD44-dependent chemotaxis of peritoneal macrophages through G-protein-coupled receptors: evidence of a role for an intracellular form of osteopontin. J Cell Physiol 198: 155–167, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Sartipy P, Loskutoff DJ: Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci U S A 100: 7265–7270, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M: MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 116: 1494–1505, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW Jr: CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 116: 115–124, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T: Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 281: 26602–26614, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K: Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54: 2277–2286, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Clement K, Langin D: Regulation of inflammation-related genes in human adipose tissue. J Intern Med 262: 422–430, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kawashima R, Mochida S, Matsui A, YouLuTu ZY, Ishikawa K, Toshima K, Yamanobe F, Inao M, Ikeda H, Ohno A, Nagoshi S, Uede T, Fujiwara K: Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem Biophys Res Commun 256: 527–531, 1999 [DOI] [PubMed] [Google Scholar]