Abstract
OBJECTIVE—Immune-mediated destruction of β-cells resulting in type 1 diabetes involves activation of proinflammatory, islet autoreactive T-cells, a process under the control of dendritic cells of the innate immune system. We tested the hypothesis that type 1 diabetes development is associated with disturbance of blood dendritic cell subsets that could enhance islet-specific autoimmunity.
RESEARCH DESIGN AND METHODS—We examined blood dendritic cells (plasmacytoid and myeloid) in 40 patients with recent-onset diabetes (median duration 28 days) and matched control subjects. We also examined the relative ability of different dendritic cell subsets to process and present soluble or immune complexed islet cell autoantigen (the islet tyrosine phosphatase IA-2) to responder CD4 T-cells.
RESULTS—The balance of blood dendritic cells was profoundly disturbed at diabetes diagnosis, with a significantly elevated proportion of plasmacytoid and reduction of myeloid cells compared with control subjects. Dendritic cell subset distribution was normal in long-standing disease and in patients with type 2 diabetes. Both dendritic cell subsets processed and presented soluble IA-2 to CD4 T-cells after short-term culture, but only plasmacytoid dendritic cells enhanced (by as much as 100%) autoantigen presentation in the presence of IA-2+ autoantibody patient serum.
CONCLUSIONS—The plasmacytoid subset of dendritic cells is overrepresented in the blood close to diabetes onset and shows a distinctive ability to capture islet autoantigenic immune complexes and enhance autoantigen-driven CD4 T-cell activation. This suggests a synergistic proinflammatory role for plasmacytoid dendritic cells and islet cell autoantibodies in type 1 diabetes.
Type 1 diabetes is an autoimmune disease resulting from T-cell–mediated destruction of insulin-producing β-cells (1–3). Although the precise aetiopathogenesis of the disease is unknown, it is apparent that β-cell damage involves the generation of activated, proinflammatory, islet-autoreactive, effector CD4 and CD8 T-cells (3,4). The priming, differentiation, and expansion of effector T-cells is largely under the control of a heterogeneous group of immune cells that go under the collective term of dendritic cells, because of their distinctive morphology (5). Dendritic cells have numerous specialized forms present in peripheral tissues, lymph nodes, and the blood, and collectively, these cells are responsible for the sensing and ingestion of pathogens and activation of T-cells of relevant specificity. Because activated dendritic cells are a requirement for priming of naïve T-cells, it is likely that a similar process pertains during the development of islet autoreactivity, although the activating stimuli and islet autoantigens involved remain obscure. It is also likely that once this process is initiated, dendritic cell presentation of islet autoantigens remains a feature of the disease, because spreading of the autoimmune response to additional autoantigens and epitopes develops (6).
Given the pivotal role of dendritic cells in the activation of naïve T-cells, there is a strong justification for investigating their activity in type 1 diabetes. Until relatively recently, however, opportunities to study dendritic cells in a human disease setting were limited. In recent years, there has been an increasing recognition that two of the major dendritic cell subsets, the myeloid (myeloid dendritic cell) and plasmacytoid (plasmacytoid dendritic cell) forms are present at low levels in the circulation and can be identified by their expression of distinct lineage and functional markers (7,8). Plasmacytoid dendritic cells, also known as the type I interferon (IFN)-producing cells, are of particular interest, being specialized in the sensing of virus infection through selective expression of Toll-like receptors (TLRs) specific for viral single-stranded RNA (TLR7) and double-stranded DNA (TLR9) (9). Ligation of such viral receptors results in the rapid secretion of type I IFNs, such as IFN-α, at levels 100-1,000 times more than any other cell type. Serum IFN-α levels are elevated in children at diagnosis of type 1 diabetes (10); IFN mRNA subtypes are found in post mortem pancreas samples from type 1 diabetic patients (11); and critically, IFN-α treatment for diseases such as chronic viral hepatitis and cancer has precipitated the clinical manifestation of autoimmune disease, including type 1 diabetes, in a number of cases (12,13). Moreover, there is emerging evidence of a close relationship between plasmacytoid dendritic cells, excessive amounts of type I IFNs, and other autoimmune conditions (14).
We hypothesized that the existence of a relationship between type I IFNs and type 1 diabetes close to the onset of the disease might be reflected in a disturbance in blood dendritic cell subsets. Our study demonstrates a profound disturbance in the normal balance of plasmacytoid dendritic cells and myeloid dendritic cells in peripheral blood in the immediate period after diagnosis. Moreover, we show that plasmacytoid dendritic cells capture islet cell autoantigens via immune complexes and in so doing enhance CD4 T-cell activation, suggesting synergy between elements of the innate and adaptive immune systems, and islet cell autoantibodies in particular, in type 1 diabetes–related autoimmunity.
RESEARCH DESIGN AND METHODS
For dendritic cell enumeration studies, fresh heparinized blood was obtained from 40 patients with new-onset type 1 diabetes (mean age 27 ± 7.2 years; disease duration 30 ± 15 days) and 40 nondiabetic control subjects of similar age, sex, and HLA type with no family history of autoimmune disease (mean age 29 ± 5.2 years). Eighteen patients with long-standing type 1 diabetes (median disease duration 19.5 years, range 3–58 years; mean age 44 ± 13.8 years) were also studied along with an additional, appropriately matched nondiabetic control group (n = 18; mean age 34 ± 10 years). In addition, 16 patients with type 2 diabetes (median disease duration 7.5 years, range 2–16 years, mean age 59.8 ± 11.7 years) were also studied along with an appropriately matched nondiabetic control group (n = 16; mean age 51 ± 8.7 years). Blood was also obtained from a further group of patients with new-onset type 1 diabetes and from control subjects of similar age, sex, and HLA-type for detailed analysis of dendritic cell phenotype (n = 13 in each group) and dendritic cell cytokine production after in vitro stimulation (n = 9 in each group). All type 1 diabetic patients gave a history of acute onset of symptoms typical of diabetes and required insulin from diagnosis. Typically, blood from a patient was analyzed alongside that of an appropriate control taken at the same time of day to avoid any potential confounding effects of diurnal rhythm and season. Patients or control subjects reporting recent (up to 2 weeks) symptoms of “virus-like” illnesses were excluded from participation in the study. Metabolic control was assessed by measurement of A1C at the time of blood sampling for dendritic cell studies. The presence of autoantibodies to IA-2 and glutamic acid decarboxylase-65 (GAD65) was detected by radioimmunoassay (RSR, Cardiff, U.K.).
Dendritic cell enumeration.
Lysed whole blood direct immunofluoresence staining for the quantitation of dendritic cell subsets was performed using the Blood DC Enumeration Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Briefly, 300 μl whole blood was incubated with either the anti-BDCA cocktail, containing monoclonal antibodies (mAbs) specific for the dendritic cell markers BDCA-1 (CD1c for detecting myeloid dendritic cell 1), BDCA-2 (CD303 for detecting plasmacytoid dendritic cells), and BDCA-3 (CD141 for detecting myeloid dendritic cell 2); anti-CD14 (monocytes); and anti-CD19 (B-cells) or the control cocktail (containing appropriate isotype control mAbs). After 10-min photoincubation with these and a dead cell discriminator on ice, erythrocytes were lysed, and remaining cells were washed in PBS containing 1% BSA (Sigma-Aldrich, Poole, U.K.), 0.1% sodium azide, 2% FCS (Invitrogen, Paisley, U.K.), and 1% human AB serum (PAA, Linz, Austria) (flow buffer). Cells were fixed and analyzed by four-color flow cytometry using a FACSCalibur cytometer (BD Biosciences, San Jose, CA). CD14+, CD19+, and dead cells were excluded, and dendritic cell subsets were enumerated. A minimum of 3.5 × 105 events in the mononuclear (forward and side scatter) gate were collected, of which a mean of 0.8% are dendritic cells (∼2,800 events). Gates denoting positivity for markers of myeloid dendritic cell 1, myeloid dendritic cell 2, and plasmacytoid dendritic cell were set using the appropriate isotype control mAbs and the number of dendritic cells in a sample expressed as a percentage of the total number of gated mononuclear cells or the total number of dendritic cells.
Dendritic cell isolation, phenotypic analysis, and stimulation.
Peripheral blood mononuclear cells (PBMCs) were obtained from whole blood by density gradient centrifugation (Lymphoprep; Axis Shield, Dundee, U.K.). CD14+ and CD19+ cells were depleted from PBMCs using magnetic cell sorting technology (AutoMACS; Miltenyi Biotech). Myeloid dendritic cells were then positively isolated from the remaining PBMCs using BDCA-1+–conjugated magnetic microbeads and plasmacytoid dendritic cells then isolated from the remaining cells using BDCA-4+–conjugated magnetic microbeads. Averaging from 20 separations, mean ± SD purity was 78.8 ± 10.9% for plasmacytoid dendritic cells and 75.0 ± 19.5% for myeloid dendritic cells.
Markers of activation and function were analyzed by four-color flow cytometry using 2 × 104 dendritic cells in 100 μl fluorescence-activated cell sorting buffer along with the appropriate fluorochrome-labeled mAbs. Samples were incubated on ice in the dark for 30 min and then washed twice in ice-cold flow buffer before immediate analysis. The following mAbs were used: fluorescein isothiocyanate–labeled anti-CD19 (clone LT19), anti–HLA-ABC (clone W6/32), and anti-CD83 (clone HB15e) mAbs; phycoerythrin (PE)-labeled anti-CD62L (clone FMC46) and anti-CD80 (clone MEM-233) mAbs (all from Serotec, Oxford, U.K.); PE-labeled anti-CD4 (clone RPA-T4), peridinin chlorophyll protein–labeled anti-CD14 (clone Mφ P9), and anti–HLA-DR (clone TU36) mAbs; and allophycocyanin-labeled anti-CD3 (clone UCHT1), anti-CD86 (clone 2331FUN-1) (all BD Pharmingen, San Diego, CA), and anti-CD123 (clone AC145) mAbs (Miltenyi Biotec). Antibody concentrations used were based on data supplied by the manufacturers and in-house optimization studies. For these studies, the selected dendritic cell subset was always gated using a specific marker (BDCA1 for myeloid dendritic cell and CD123 for plasmacytoid dendritic cell). Gated cells were then assessed for the mean fluorescence intensity of the specific activation/maturation marker.
In studies on the cytokine potential of isolated blood dendritic cell subsets, myeloid dendritic cells and plasmacytoid dendritic cells were resuspended to 2 × 105 per ml in RPMI 1640 supplemented with penicillin, streptomycin, and fungizone (Invitrogen) and 10% human AB serum. Myeloid dendritic cells were stimulated with lipopolysaccharide (Sigma-Aldrich) at concentrations of 0 and 10 ng/ml in a total volume of 200 μl in duplicate samples. Plasmacytoid dendritic cells underwent similar treatment in cultures supplemented with 10 ng/ml recombinant human interleukin (IL)-3 (Strathmann Biotec, Hamburg, Germany) and were stimulated with unmethylated oligodeoxynucleotides (ODN 2216, a potent TLR-9 ligand and IFN-α secretagog; Autogen Bioclear, Calne, U.K.) at concentrations of 0, 1, 3, 10, and 30 μg/ml in a total volume of 200 μl in duplicate samples (15,16). After 16 h of stimulation, supernatants were collected and stored at −80°C for later analysis. IFN-α production in plasmacytoid dendritic cell culture supernatants was quantified by specific sandwich ELISA using the IFN-α module set (detection range 8–5,000 pg/ml; Bender MedSystems). There are 14 different IFN-α isoforms, of which the IFN-α module set detects the majority, with the exception of B and F. Supernatants from myeloid dendritic cell and plasmacytoid dendritic cell cultures were analyzed using the Beadlyte Human Cytokine Detection kit 3 (Upstate, Lake Placid, NY) according to the manufacturer's instructions. Cytokines IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, Il-12p70, tumor necrosis factor-α (TNF-α), and IFN-γ were measured (detection range 6.9–5,000 pg/ml). Data were analyzed on the Luminex 100 instrument system using STarstation software.
Presentation of autoantigens by dendritic cells.
cDNA encoding human IA2-intracellular portion (IA-2ic) was modified to include a coding sequence for the HLA-DR4 restricted influenza hemagglutinin CD4 T-cell epitope (HA307–319), inserted at the NH2-terminus of IA-2ic, and recombinant IA-2ic-HA307–319 protein was prepared as described previously (17,18). An HLA-DR4 restricted CD4 T-cell clone specific for HA307–319 (JNZ-1) was generated by peptide stimulation of PBMC cultures, followed by flow cytometric sorting of cells stained positive with an HLA-DR4 tetramer loaded with HA307–319 (provided by Dr. G. Nepom, Benaroya Research Institute, Seattle, WA). Plasmacytoid dendritic cells, myeloid dendritic cells, and CD14+ monocytes were isolated from HLA-DR4+ subjects and resuspended to 5 × 104/ml, and 100 μl was dispensed into 96-well flat-bottomed plates in serum-free X-VIVO 15 (Cambrex, Charles City, IA). Cultures were supplemented with serum from type 1 diabetic patients positive for either IA-2 or GAD65 autoantibodies, or pooled AB (1–30%) and IA-2ic-HA307–319 (10 μg/ml). In some experiments, the IgG fraction in IA-2+ autoantibody serum was depleted by incubation with protein A matrix (binding capacity 18–43 mg/ml; Repligen, Waltham, MA) at a 1:1 ratio for 90 min at room temperature according to the manufacturer's instructions. After centrifugation (15 s at 10,000g), unbound serum was carefully removed, and IgG depletion was confirmed by laser nephelometric analysis of IgG levels on samples before and after adsorption. After 24 h of dendritic cell culture with serum under these conditions, JNZ-1 clone cells (105/well) were added, and 48 h later, IFN-γ levels in supernatants were quantified by ELISA (detection range 2–500 pg/ml; Immunotools, Friesoythe, Germany).
Statistical analysis.
Dendritic cell subsets, expressed as a percentage of total dendritic cells, were normally distributed in all study groups according to Kolmogorov-Smirnov analysis. Mean levels of dendritic cell subsets were therefore compared between study groups using the Student's t test. Relationships between dendritic cell subset numbers and clinical parameters were compared by calculation of Pearson's correlation coefficient. Differences in distribution were compared by χ2 analysis. P values of <0.05 were considered significant. Differences in IFN-γ production were compared using Student's t tests.
RESULTS
Detection of dendritic cell subsets in whole blood.
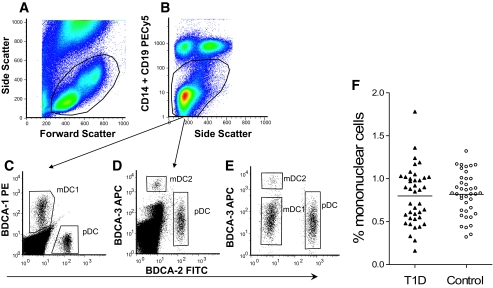
Dendritic cell subsets were identified in whole blood using specific mAbs among mononuclear cells identified by their physical side and forward scatter properties (Fig. 1A) after removal of CD14+, CD19+, and dead cells by exclusion gating (Fig. 1B). Dendritic cell numbers were expressed as a percentage of mononuclear cells and dendritic cell subsets as a proportion of total dendritic cells. Representative examples of BDCA-1+ (myeloid dendritic cells), BDCA-2+ (plasmacytoid dendritic cells), and BDCA-3+ (myeloid dendritic cell 2) cells are shown in Fig. 1C–E. The majority of events in the CD14−/CD19− gate in Fig. 1B are T-cells, with some natural killer and natural killer T-cells. The relative proportion of this population was not different between type 1 diabetic patients and control subjects (data not shown).
FIG. 1.
Example of flow cytometry analysis and gating strategy for identification and quantification of blood dendritic cell subsets. The mononuclear cell population in whole blood is identified by a combination of forward and side scatter properties (A) followed by exclusion gating of live CD14+ and CD19+ mononuclear cells and dead cells (B), before the identification and enumeration of plasmacytoid dendritic cell (pDC), myeloid dendritic cell 1 (mDC1), and myeloid dendritic cell 2 (mDC2) subsets, as shown in C–E with the relevant blood dendritic cell (BDC) marker. F: Total dendritic cell populations as a percentage of mononuclear cells in 40 patients with new-onset type 1 diabetes and matched control subjects. (Please see http://dx.doi.org/10.2337/db08-0964 for a high-quality digital representation of this figure.)
Dendritic cell subsets in type 1 diabetic patients.
The total number of dendritic cells (i.e., cells expressing BDCA-1, BDCA-2, or BDCA-3) when expressed as a percentage of the total mononuclear cell population was similar in new-onset type 1 diabetic patients and nondiabetic control subjects (Fig. 1F).
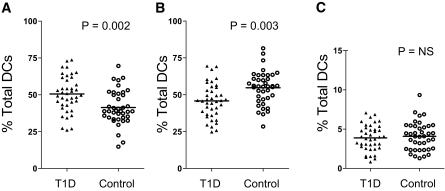
However, within the total dendritic cell population, dendritic cell subsets had a different proportional distribution in the two study groups. The mean percentage of dendritic cells belonging to the plasmacytoid dendritic cell subset was significantly higher in new-onset type 1 diabetic patients when compared with nondiabetic control subjects (P = 0.002; Fig. 2A). In contrast, the mean percentage of myeloid dendritic cells was significantly lower in new-onset type 1 diabetic patients compared with the nondiabetic control group (P = 0.003; Fig. 2B). These findings were similar regardless of whether dendritic cell subsets were expressed as a percentage of total dendritic cells or of mononuclear cells. There was no difference between the study groups in the proportion of dendritic cells belonging to the BDCA3+ myeloid dendritic cell 2 populations (Fig. 2C). Among the new-onset group, there was no correlation between percentages of dendritic cell subsets and age or duration of diabetes or A1C levels as a marker of metabolic control. The percentage levels of dendritic cell subsets were not different between patients with and without islet cell autoantibodies and did not differ between patient subgroups divided according to HLA class II genotype.
FIG. 2.
Dendritic cell subsets in new-onset type 1 diabetic patients and control subjects showing plasmacytoid dendritic cells (A), type 1 myeloid dendritic cells (B), and type 2 myeloid dendritic cells (C). Results are expressed as percentage of total dendritic cell population. Mean values for each group are indicated by a horizontal line, and P values are given where significant.
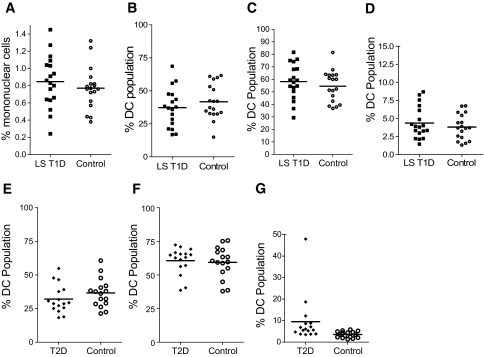
To assess whether the abnormalities in dendritic cell subsets observed in new-onset type 1 diabetic patients could be genetically determined, we also studied patients in whom type 1 diabetes had been diagnosed for a minimum of 3 years (median duration of disease 19.5 years, maximum 58 years). In these patients, mean levels of total dendritic cells as a percentage of mononuclear cells and the distribution of dendritic cell subsets were similar to those in age-matched nondiabetic control subjects (Fig. 3A–D). We also examined the potential influence of hyperglycemia per se on dendritic cell subsets by analysis of patients with type 2 diabetes with similar levels of A1C (mean ± SD 7.9 ± 1.9%) compared with the new-onset patients (8.73 ± 2.1%; NS). Chronic hyperglycemia alone could not account for the changes we observed, because proportions of dendritic cell subsets did not differ significantly in patients with type 2 diabetes compared with control subjects (Fig. 3E–G).
FIG. 3.
Dendritic cell subsets in long-standing type 1 diabetic patients (LS T1D) and age-matched control subjects showing total dendritic cell population as a percentage of mononuclear cells (A) and the plasmacytoid dendritic cell (B), type 1 myeloid dendritic cell (C), and type 2 myeloid dendritic cell (D) subsets as a percentage of total dendritic cells. Mean values for each group are indicated by a horizontal line and were similar between study groups. Similarly, E–G show plasmacytoid dendritic cell (E), type 1 myeloid dendritic cell (F), and type 2 myeloid dendritic cell (G) subsets as a percentage of total dendritic cells in patients with type 2 diabetes and an appropriately age-matched control group. Mean levels are similar in the two groups (NS).
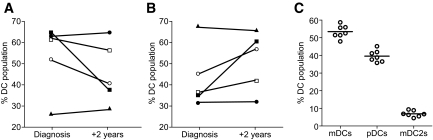
Although the populations of patients with type 1 diabetes (new-onset and long-standing) were not closely age-matched, we also compared the distribution of dendritic cell subsets between them; this comparison seemed justified in the absence of any relationship between age and dendritic cell subset number in the patient and control subject groups. Mean levels of plasmacytoid dendritic cells were higher, and mean levels of myeloid dendritic cells were lower in the new-onset patients (P = 0.0013 and 0.0014, respectively). These data suggested that dendritic cell subset balance is disturbed near to disease diagnosis but gradually normalizes. Further evidence for this was obtained by repeat analysis of blood samples on five patients tested both at diagnosis and 2 years later. Three of the five show evidence of declining plasmacytoid dendritic cell percentages, and four had increased/unchanged percentages of myeloid dendritic cells at year 2 (Fig. 4A and B), although with small numbers of subjects, these can only be considered indicative trends (NS). This contrasts with data from a control individual analyzed seven times over the same 2-year period, in whom plasmacytoid dendritic cell and myeloid dendritic cell subsets were remarkably stable (Fig. 4C). Taken together, these data indicate that type 1 diabetes is characterized by an abnormal distribution of dendritic cells, resulting in high levels of plasmacytoid dendritic cells and low levels of myeloid dendritic cells but that these changes are restricted to the peri-diagnosis period.
FIG. 4.
Plasmacytoid (A) and myeloid (B) dendritic cell subsets as a percentage of total dendritic cells in five patients with new-onset type 1 diabetes measured at diagnosis and 2 years later. There is a trend in three of the patients for plasmacytoid dendritic cell levels to decline and in three of the patients for myeloid dendritic cell levels to increase/remain unchanged. C: Dendritic cell subset levels (expressed as a percentage of dendritic cells) in a nondiabetic control individual studied on seven separate occasions over the same 2-year period.
Expression of differentiation and activation markers on plasmacytoid dendritic cells and myeloid dendritic cells in type 1 diabetic patients.
Because the plasmacytoid dendritic cell population appeared to be expanded at diagnosis of type 1 diabetes, we next wished to determine whether this expansion was associated with activation of this subset. Blood plasmacytoid dendritic cells expressed high basal levels of HLA molecules (both class I and class II), IL-3 receptor (CD123), and l-selectin (CD62L) but low levels of the dendritic cell activation marker CD83 and the costimulatory molecules CD80 and CD86, which are typically upregulated on activation. Myeloid dendritic cells were also found to express high levels of HLA class I and class II molecules. Myeloid dendritic cells also expressed detectable levels of CD83, CD80, and CD86 but without any sign of upregulation of these activation molecules in patients (supplementary Fig. 1, available in an online appendix at http://dx.doi.org/10.2337/db08-0964). Formal comparison of the levels of expression of these markers in new-onset type 1 diabetic patients and nondiabetic control subjects (n = 13 in each group) failed to show any significant differences.
Cytokine and chemokine production by plasmacytoid dendritic cells and myeloid dendritic cells.
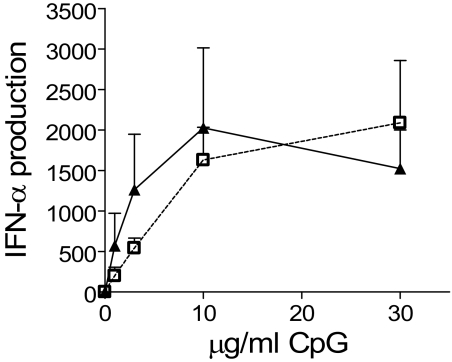
Plasmacytoid dendritic cells are capable of producing very large quantities of type I IFNs after stimulation; and to assess their IFN-α–producing capacity in type 1 diabetes, cells isolated from the study groups were cultured with a TLR9 ligand, and IFN-α secretion was measured. Plasmacytoid dendritic cells from both new-onset type 1 diabetic patients and nondiabetic control subjects (n = 9 in each group) did not produce IFN-α without stimulation. After stimulation with CpG oligonucleotide, plasmacytoid dendritic cells from patients and control subjects produced similar, large amounts of IFN-α in a dose-dependent manner (Fig. 5). Thus, per-plasmacytoid dendritic cell IFN-α secretion is not abnormal in patients, and there is no evidence of spontaneous ex vivo IFN-α secretion by these cells, making it unlikely that they are the source of the elevated serum levels of this cytokine that has been reported in type 1 diabetic patients (10). Using the same samples, we also examined basal and stimulated secretion of IL-1β, IL-6, IL-8, IL-10, IL-12p70, TNF-α, and IFN-γ by plasmacytoid dendritic cells and myeloid dendritic cells. No differences between patients and control subjects were observed.
FIG. 5.
IFN-α secretion by isolated plasmacytoid dendritic cells in type 1 diabetic patients and control subjects in response to stimulation with increasing concentrations of the unmethylated CpG oligonucleotide 2,216. Each point represents the mean value of IFN-α secretion from nine different patients (▴) and nine control subjects (□). Vertical bars represent SEs.
Islet cell autoantibody–positive serum enhances autoantigen presentation to plasmacytoid dendritic cells.
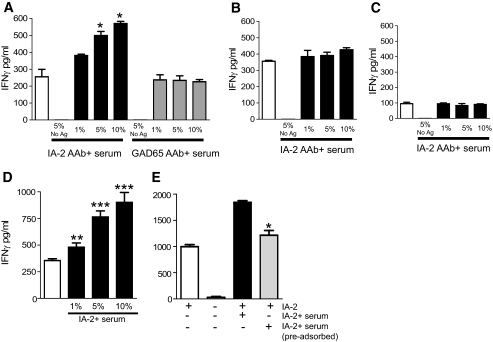
It has recently been reported that plasmacytoid dendritic cell presentation of antigen is enhanced in the presence of immune complexes containing the relevant antigen (19). We designed experiments in which plasmacytoid dendritic cells, myeloid dendritic cells, and monocytes presented a hybrid construct of IA-2ic containing the influenza hemagglutinin epitope HA307–319. Activation of an HA307–319-specific CD4 T-cell clone (JNZ-1) was used as the measure of efficiency of IA-2ic-HA307–319 presentation. Serum positive or negative for IA-2 autoantibodies was then added to examine whether presentation was enhanced in the presence of relevant autoantibody specificities. Presentation of IA-2ic-HA307–319 by plasmacytoid dendritic cells was enhanced in the presence of serum containing IA-2, but not GAD65, autoantibodies (Fig. 6A). IFN-γ production by the T-cell clone was more than doubled in the presence of IA-2 autoantibodies (P < 0.05 compared with control serum for 5 and 10% serum supplementation). In contrast, myeloid dendritic cell and monocyte presentation of IA-2ic-HA307–319 was largely unaffected by the presence of islet cell autoantibodies (Fig. 6B and C). In the case of myeloid dendritic cells, it is probable that their uptake and presentation of antigen through pinocytosis is highly efficient in the absence of immune complexes and therefore not enhanced to any great degree in their presence.
FIG. 6.
Effect of islet cell autoantibody–positive serum on presentation by plasmacytoid dendritic cells (A), myeloid dendritic cells (B), and CD14+ monocytes (C) of the islet cell autoantigen IA-2ic-HA307–319 to CD4 T-cells (JNZ-1 clone) specific for the influenza HA307–319 epitope. CD4 T-cell clone responses are indicated by levels of IFN-γ produced in the culture supernatants. A: In the presence of soluble 10 μg/ml IA-2ic-HA307–319 without serum supplementation (□), IA-2ic is processed and presented with comparable efficiency by plasmacytoid dendritic cells and myeloid dendritic cells, with CD14+ monocytes markedly less proficient. Addition of IA-2+ autoantibody (AAb) serum (3,500 World Health Organization [WHO] units) at 1, 5, and 10% (▪) considerably and significantly enhances the presentation of IA-2ic by plasmacytoid dendritic cells but has minimal effects on myeloid dendritic cells and CD14+ monocytes (*P < 0.05 vs. presence of antigen alone; □). GAD65+ autoantibody serum (1,700 WHO units;  ; 1–10%) has no effect on presentation. Likewise, pooled AB serum at these concentrations has no effect (data not shown). In the presence of 5% serum but no IA-2ic-HA307–319, there is no T-cell activation. Data represent one of four experiments using four different cell donors. D: Pooled data from a total of four donors (including the one used in A) for plasmacytoid dendritic cell enhancement of IA-2ic-HA307–319 (▪) presentation to JNZ-1 clone cells (*P < 0.05, **P < 0.01, and ***P < 0.001 vs. presence of antigen alone; □). The additional sera contained 3,875.1, 3,263.1, and 3,007.8 WHO units of IA-2 autoantibodies. Bars represent means ± SE from quadruplicate wells of individual experiments. E: IA-2+ serum–enhanced IA-2ic-HA307–319 presentation is dependent on the serum IgG fraction. Serum has been adsorbed using Protein A matrix. Serum IgG level before adsorption, 6.54 g/l; after adsorption, 0.22 g/l. Serum was used at 5% concentration in these experiments. Serum IA-2 autoantibody level before adsorption, 2,152 WHO units; after adsorption, IA-2 autoantibodies were not detectable. *Significant reduction in IFN-γ production by clone JNZ-1 in the presence of adsorbed compared with nonadsorbed serum (P < 0.05).
; 1–10%) has no effect on presentation. Likewise, pooled AB serum at these concentrations has no effect (data not shown). In the presence of 5% serum but no IA-2ic-HA307–319, there is no T-cell activation. Data represent one of four experiments using four different cell donors. D: Pooled data from a total of four donors (including the one used in A) for plasmacytoid dendritic cell enhancement of IA-2ic-HA307–319 (▪) presentation to JNZ-1 clone cells (*P < 0.05, **P < 0.01, and ***P < 0.001 vs. presence of antigen alone; □). The additional sera contained 3,875.1, 3,263.1, and 3,007.8 WHO units of IA-2 autoantibodies. Bars represent means ± SE from quadruplicate wells of individual experiments. E: IA-2+ serum–enhanced IA-2ic-HA307–319 presentation is dependent on the serum IgG fraction. Serum has been adsorbed using Protein A matrix. Serum IgG level before adsorption, 6.54 g/l; after adsorption, 0.22 g/l. Serum was used at 5% concentration in these experiments. Serum IA-2 autoantibody level before adsorption, 2,152 WHO units; after adsorption, IA-2 autoantibodies were not detectable. *Significant reduction in IFN-γ production by clone JNZ-1 in the presence of adsorbed compared with nonadsorbed serum (P < 0.05).
The effect of IA-2+ serum samples in enhancing antigen presentation of IA-2ic-HA307–319 was seen in samples from more than one patient with IA-2 autoantibodies and was highly significant (Fig. 6D and E). The enhancing effect was attributable to the IgG fraction in the serum samples, because it was significantly diminished after IgG depletion using a protein A-Sepharose column (Fig. 6F). Enhanced presentation of IA-2 by plasmacytoid dendritic cells was not associated with IFN-α production.
DISCUSSION
Our study shows an abnormal distribution of the main blood dendritic cell subsets in type 1 diabetes. IFN-producing plasmacytoid dendritic cells, rather than myeloid dendritic cells, predominate proportionally among dendritic cells in type 1 diabetic patients, and because these changes were only observed at diagnosis of disease, it seems unlikely that the alterations are genetically determined. The increased proportion of plasmacytoid dendritic cells and reduction in myeloid dendritic cells was not accompanied by changes in activation status of these cells, nor, in the case of plasmacytoid dendritic cells, by a change in their capacity for IFN-α secretion. There is no immediate explanation for this increased representation of plasmacytoid dendritic cells, but taken together with previous reports of abnormal levels of circulating and tissue-deposited type I IFNs in type 1 diabetes, these findings extend the concept of a relationship between type I IFNs, their major cellular source the plasmacytoid dendritic cell, and the onset of type 1 diabetes. Further weight is given to the potential importance of this relationship by our demonstration that plasmacytoid dendritic cells have enhanced ability to activate primed effector CD4 T-cells in the presence of islet cell autoantibodies, indicating a route through which autoantibodies could promote cell-mediated islet autoimmunity.
Reagents for the reliable detection of dendritic cell subsets in peripheral blood have only recently become available, and few investigators have yet had the opportunity to deploy this technology in autoimmune diseases. To the best of our knowledge, this is the first study to demonstrate an increase in plasmacytoid dendritic cells in new-onset type 1 diabetic patients using this approach. These findings are similar to those in the preliminary report of Peng et al. (20), who did not use the BDCA dendritic cell markers but rather detected plasmacytoid dendritic cells as lineage-negative/HLA-DR+/CD11c−/CD123+ and myeloid dendritic cells as lineage-negative/HLA-DR+/CD11c+/CD123− cells. This group also found a higher proportion of plasmacytoid dendritic cells in type 1 diabetes, although specific results are not reported, limiting the opportunity for direct comparison. A more recent report focuses on patients with type 1 diabetes of at least 5 years duration and finds no abnormality of blood dendritic cell balance, similar to our own analysis of long-standing patients (21). In contrast, Vuckovic et al. (22) have reported decreased dendritic cell numbers in children with new-onset type 1 diabetes. However, these authors did not use specific markers of dendritic cell subsets, and they report levels of blood dendritic cells (>100 cells/μl) that are vastly in excess of the known concentration of these cells (∼1% of PBMCs, equivalent to 10–20 cells/μl [7]).
Using an in vitro culture system, we show that in the presence of islet cell autoantibodies of the relevant specificity, the ability of plasmacytoid dendritic cells to process and present islet autoantigens to CD4 T-cells is significantly enhanced. This immune complex capture is known to operate through the Fcγ RII-receptor for IgG (CD32), probably through enhanced antigen uptake (19). Antibody-enhanced cross-presentation of an islet antigen has also been shown to break tolerance in a murine model of autoimmune diabetes (23). We consider it unlikely that such a pathway is involved in the initial priming of islet autoimmunity in man, but it could have an important role in the amplification and maintenance of T-cell responses and also in epitope spreading (24). The finding that immune complex capture by plasmacytoid dendritic cells enhances autoreactive T-cell activation highlights a potential route through which autoantibodies could participate in cell-mediated autoimmune responses, in addition to that offered by B-cell presentation of autoantigens.
It is unclear why dendritic cell balance should be perturbed near to diagnosis of type 1 diabetes, but our studies exclude any possible effects of glycemic control. One explanation is that selective migration of myeloid dendritic cells into tissues or lymph nodes results in a relative expansion of blood plasmacytoid dendritic cells, especially if accompanied by proliferation of the dendritic cell-precursor pool. The possible effect of dendritic cell migration on blood levels has been noted in diseases in which both inflamed tissues and the circulation have been examined, such as psoriasis (25), systemic lupus erythematosus (26), and respiratory syncytial virus infection (27), each of which are associated with a reduction in plasmacytoid dendritic cells in peripheral blood and infiltration of the tissues. An alternative hypothesis is that plasmacytoid dendritic cell expansion occurs in response to a virus infection that arises toward the end of the islet destructive process. Virus infection at this late stage of disease is known to be capable of accelerating disease onset in animal models (28), and it is tempting to speculate that this induces a shift in the ratio of plasmacytoid and myeloid dendritic cells in the periphery.
At this stage, it is only possible to speculate on the pathological relevance of our findings. Although the possibility of a proinflammatory role is attractive, studies in animal models show that an increase in plasmacytoid dendritic cell/myeloid dendritic cell ratio may prevent antigen-specific inflammatory responses, including diabetes (29–31), and a similar role in type 1 diabetes cannot be excluded. Future studies that examine whether dendritic cell subsets are disturbed in the pre-diabetic period and therefore reflect events involved in the chronic autoimmune process of islet destruction or whether plasmacytoid dendritic cell expansion is related solely to peri-diagnosis events may clarify this.
Supplementary Material
Acknowledgments
This work was supported by a grant from Diabetes UK and by the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St. Thomas’ NHS Foundation Trust in partnership with King's College London. B.L.-O. is in receipt of a Juvenile Diabetes Research Foundation post-doctoral fellowship (3-2007-643).
No potential conflicts of interest relevant to this article were reported.
We are grateful to Dr. G. Nepom for supply of the HA307–319-DR4 tetramer.
Published ahead of print at http://diabetes.diabetesjournals.org on 3 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 12.
REFERENCES
- 1.Tree TI, Peakman M: Autoreactive T cells in human type 1 diabetes. Endocrinol Metab Clin North Am 33: 113–133, ix–x, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS: Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358: 221–229, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Roep BO: The role of T-cells in the pathogenesis of type 1 diabetes: from cause to cure. Diabetologia 46: 305–321, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Staeva-Vieira T, Peakman M, von Herrath M: Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol 148: 17–31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinman RM, Hemmi H: Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol 311: 17–58, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Lehmann PV, Forsthuber T, Miller A, Sercarz EE: Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature 358: 155–157, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J: BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 165: 6037–6046, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J: BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med 194: 1823–1834, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YJ: IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 23: 275–306, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Chehadeh W, Weill J, Vantyghem MC, Alm G, Lefebvre J, Wattre P, Hober D: Increased level of interferon-alpha in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J Infect Dis 181: 1929–1939, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Yuang J, Goddard A, Foulis A, James RF, Lernmark A, Pujol-Borrell R, Rabinovitch A, Somoza N, Stewart TA: Interferon expression in the pancreases of patients with type I diabetes. Diabetes 44: 658–664, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Fabris P, Floreani A, Tositti G, Vergani D, De Lalla F, Betterle C: Type 1 diabetes mellitus in patients with chronic hepatitis C before and after interferon therapy. Aliment Pharmacol Ther 18: 549–558, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Guerci AP, Guerci B, Levy-Marchal C, Ongagna J, Ziegler O, Candiloros H, Guerci O, Drouin P: Onset of insulin-dependent diabetes mellitus after interferon-alfa therapy for hairy cell leukaemia. Lancet 343: 1167–1168, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Crow MK, Kirou KA: Interferon-alpha in systemic lupus erythematosus. Curr Opin Rheumatol 16: 541–547, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kawamura K, Kadowaki N, Kitawaki T, Uchiyama T: Virus-stimulated plasmacytoid dendritic cells induce CD4+ cytotoxic regulatory T cells. Blood 107: 1031–1038, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, Hartmann G: Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol 31: 2154–2163, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M: Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest 113: 451–463, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peakman M, Stevens EJ, Lohmann T, Narendran P, Dromey J, Alexander A, Tomlinson AJ, Trucco M, Gorga JC, Chicz RM: Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J Clin Invest 104: 1449–1457, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benitez-Ribas D, Adema GJ, Winkels G, Klasen IS, Punt CJ, Figdor CG, de Vries IJ: Plasmacytoid dendritic cells of melanoma patients present exogenous proteins to CD4+ T cells after Fc gamma RII-mediated uptake. J Exp Med 203: 1629–1635, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng R, Li Y, Brezner K, Litherland S, Clare-Salzler MJ: Abnormal peripheral blood dendritic cell populations in type 1 diabetes. Ann N Y Acad Sci 1005: 222–225, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Summers KL, Marleau AM, Mahon JL, McManus R, Hramiak I, Singh B: Reduced IFN-alpha secretion by blood dendritic cells in human diabetes. Clin Immunol 121: 81–89, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Vuckovic S, Withers G, Harris M, Khalil D, Gardiner D, Flesch I, Tepes S, Greer R, Cowley D, Cotterill A, Hart DN: Decreased blood dendritic cell counts in type 1 diabetic children. Clin Immunol 123: 281–288, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Harbers SO, Crocker A, Catalano G, D'Agati V, Jung S, Desai DD, Clynes R: Antibody-enhanced cross-presentation of self antigen breaks T cell tolerance. J Clin Invest 117: 1361–1369, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G: Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today 14: 203–208, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, Burg G, Liu YJ, Gilliet M: Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med 202: 135–143, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blomberg S, Eloranta ML, Magnusson M, Alm GV, Ronnblom L: Expression of the markers BDCA-2 and BDCA-4 and production of interferon-alpha by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum 48: 2524–2532, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Gill MA, Palucka AK, Barton T, Ghaffar F, Jafri H, Banchereau J, Ramilo O: Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J Infect Dis 191: 1105–1115, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Tracy S, Drescher KM: Coxsackievirus infections and NOD mice: relevant models of protection from, and induction of, type 1 diabetes. Ann N Y Acad Sci 1103: 143–151, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Chilton PM, Rezzoug F, Fugier-Vivier I, Weeter LA, Xu H, Huang Y, Ray MB, Ildstad ST: Flt3-ligand treatment prevents diabetes in NOD mice. Diabetes 53: 1995–2002, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Kared H, Masson A, Adle-Biassette H, Bach JF, Chatenoud L, Zavala F: Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4+CD25+ regulatory T-cells. Diabetes 54: 78–84, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD: The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol 179: 5041–5053, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.