Abstract
OBJECTIVE—All-trans retinoic acid (ATRA), a potent derivative of vitamin A, can regulate immune responses. However, its role in inducing immune tolerance associated with the prevention of islet inflammation and inhibition of type 1 diabetes remains unclear.
RESEARCH DESIGN AND METHODS—We investigated the mechanisms underlying the potential immunoregulatory effect of ATRA on type 1 diabetes using an adoptive transfer animal model of the disease.
RESULTS—Our data demonstrated that ATRA treatment inhibited diabetes in NOD mice with established insulitis. In addition, it suppressed interferon (IFN)-γ–producing CD4+ and CD8+ T effector (Teff) cells and expanded T regulatory (Treg) cells in recipient mice transferred with diabetic NOD splenocytes, without affecting either interleukin (IL)-17 –or IL-4–producing cells. Consistent with these results, ATRA reduced T-bet and STAT4 expression in T-cells and decreased islet-infiltrating CD8+ T-cells, suppressing their activation and IFN-γ/granzyme B expression. Depletion of CD4+CD25+ Treg cells impaired the inhibitory effect of ATRA on islet-infiltrating T-cells and blocked its protective effect on diabetes. Therefore, ATRA treatment induced Treg cell–dependent immune tolerance by suppressing both CD4+ and CD8+ Teff cells while promoting Treg cell expansion.
CONCLUSIONS—These results demonstrate that ATRA treatment promoted in vivo expansion of Treg cells and induced Treg cell–dependent immune tolerance by suppressing IFN-γ–producing T-cells, without affecting Th17 cells. Our study also provides novel insights into how ATRA induces immune tolerance in vivo via its effects on Teff and Treg cells.
Disorders associated with the inability to maintain the balance between different subsets of T-cells may result in T-cell–mediated destructive autoimmunity (1). For example, activation and expansion of CD4+ or CD8+ T effector (Teff) cells that produce proinflammatory cytokines, such as interferon (IFN)-γ, and/or attenuation of the number or function of CD4+ T regulatory (Treg) cells, can lead to target tissue destruction and induce autoimmune diseases (1–5). Recent studies demonstrated that, in addition to IFN-γ–producing Teff cells, interleukin (IL)-17–producing CD4+ Th17 cells represent a new population of Teff cells that induce potent inflammatory responses leading to autoimmune diseases (6). Additional studies showed that the differentiation of CD4+ T-cells directed either toward Foxp3+ Treg cells or Th17 cells was tightly regulated by cytokine and transcriptional control (6,7). These results suggest that the establishment of effective in vivo immune tolerance to treat autoimmune diseases such as type 1 diabetes requires simultaneous targeting of more than one T-cell population subset. Therefore, clinically relevant agents or methods that induce immune tolerance by affecting different T-cell subsets may represent an effective approach to treating human autoimmune diseases. Studies to understand how such an approach might affect various T-cell subsets will help to elucidate the mechanisms underlying the induction of immune tolerance and inhibition of inflammation in autoimmune diseases.
All-trans retinoic acid (ATRA) is a potent derivative of vitamin A that has been clinically used for effective treatment of acute promyelocytic leukemia and skin disease (8,9). Both vitamin A and ATRA have important immune modulatory functions. For example, vitamin A deficiency can lead to exacerbation of experimental autoimmune colitis (10), as well as an excess of Th1 and a reduction of Th2 cell responses in animals with parasite infection (11). Supplementation of vitamin A to animals results in a decrease in serum pro-inflammatory cytokines, including tumor necrosis factor-α and IFN-γ, and an increase in the immunosuppressive cytokine IL-10 (12,13). However, the mechanisms underlying the role of vitamin A or ATRA treatment in tolerance induction regulating autoimmune disease development have not been fully elucidated (13–17). Additionally, it is not clear whether ATRA treatment can inhibit type 1 diabetes. Because ATRA is a potent derivative of vitamin A and has been used in patient treatment, it is important to evaluate its role in regulating type 1 diabetes and to determine the mechanisms by which ATRA may inhibit the disease, so as to further elucidate the potential benefits of its clinical use in the treatment of type 1 diabetes.
Recent in vitro studies showed that ATRA may lead to expansion of Foxp3+ Treg cells and downregulation of Th1 and Th17 cell differentiation (18–20). ATRA-induced Treg cells showed gut-homing tendency and were more potent in inhibiting experimental autoimmune colitis than Treg cells induced by transforming growth factor (TGF)-β alone (20–22). In addition, dendritic cells isolated from gut and gut-associated lymph nodes, which are able to produce endogenous ATRA, induced greater Foxp3 expression when cultured in vitro with TGF-β than did dendritic cells isolated from other tissues (23). These in vitro results suggested that ATRA had distinctive effects on Teff cells compared with Treg cell subsets.
While these data demonstrated that ATRA had varied effects on different T-cell subsets, they did not clarify whether ATRA inhibited type 1 diabetes, nor did they elucidate the mechanisms underlying its in vivo induction of tolerance, which ultimately inhibits autoimmunity and leads to disease. This study addresses these important questions.
RESEARCH DESIGN AND METHODS
Mice.
NOD and NOD/scid mice, purchased from the Jackson Laboratory (Bar Harbor, ME), were bred and housed in a specific pathogen-free environment in the animal facility at the Beckman Research Institute (City of Hope, Duarte, CA). Around 70–80% of female NOD mice developed diabetes by the age of 6 months. NOD/scid mice at 6–8 weeks old were used as recipients for experiments.
Experimental model and ATRA treatment.
Splenocytes used for adoptive transfer studies were isolated from newly diabetic NOD mice (within 5 days of positive urine glucose tests of ≥2% for at least 2 consecutive days). Nondepleted splenocytes (1 × 107/mouse) were intravenously transferred into NOD/scid mice. From day 1 of cell transfer, mice were treated intraperitoneally with either ATRA (0.5 mg/mouse; Sigma, St. Louis, MO) or vehicle (corn oil) every other day (13). In some transfer studies, CD4+CD25+ cells were depleted from splenocytes using magnetic beads (Miltenyi Biotec, Auburn, CA). NOD mice (10 weeks old) were used for testing the effect of ATRA on spontaneous diabetes development.
Isolation of tissue cells and intracellular staining.
For isolation of islet-infiltrating lymphocytes, pancreata were inflated with RPMI medium containing collagenase P (1 mg/ml) and islets were isolated using a histopaque gradient. Lymph nodes were digested with collagenase D (2 mg/ml) for isolation of lymph node cells. To isolate splenocytes, spleens were mechanically disrupted.
Cells were stimulated (3 h) with phorbol myristate acetate (5 ng/ml) and ionomycin (500 ng/ml) in the presence of monensin (3 μmol/l), stained with antibodies, fixed with paraformaldehyde (4%), permeabilized in saponin (4%) buffer (24), and then used for intracellular staining.
In vivo IFN-γ secretion assay.
Mice were intravenously injected with anti-CD3 (145-2C11) (1.33 μg/mouse) ∼4 weeks after cell transfer and within 2 days of control recipient mice becoming diabetic. Splenocytes were isolated 60 min after injection and incubated (106 cells/ml, 2 or 4 h) in 96-well plates with RPMI medium plus 5% FBS. The cell culture supernatant was harvested for IFN-γ enzyme-linked immunosorbent assay (ELISA).
CD4+ cell in vitro differentiation assay.
CD4+ T-cells (106 cells/ml) from newly diabetic mice were activated in 96-well plates with anti-CD3 and anti-CD28 (5 μg/ml) with or without ATRA (1 μmol/l). Th1-conditioned cultures were supplemented with IL-12 (10 ng/ml) and anti–IL-4 (10 μg/ml) (19). Th17-conditioned cultures contained IL-6 (10 ng/ml), TGF-β1 (5 ng/ml), anti–IL-4 (10 μg/ml), and anti–IFN-γ (10 μg/ml) (19). Cells were cultured for 3 days and then stained with antibodies against IFN-γ and IL-17.
Detection of T-bet and Stat-4 expression in T-cells.
Four weeks after splenocyte transfer, splenic T-cells from diabetic control and nondiabetic ATRA-treated mice were stimulated (3 h) using phorbol myristate acetate and ionomycin, lysed with 1% Triton X-100; cell lysates were used for Western blot analyses.
Statistical analysis.
Kaplan-Meier survival analysis was used to compare cumulative diabetes incidence. The Student's t test was used for data analyses of all other studies. Significance was set at P ≤ 0.05.
RESULTS
ATRA treatment inhibits type 1 diabetes.
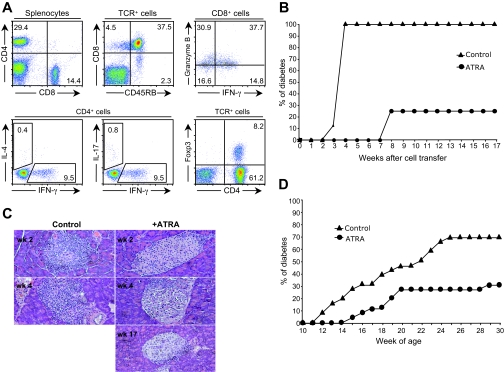
We used an adoptive transfer animal model of the disease to elucidate the mechanisms underlying the potential immunoregulatory effect of ATRA on type 1 diabetes. Splenocytes isolated from newly diabetic NOD mice were adoptively transferred to NOD/scid recipient mice. Donor T-cell phenotype, including CD4+ versus CD8+ T-cells, percentage of CD4+ Foxp3-expressing Treg cells, CD8+ cells expressing different markers, and their cytokine secretion profile are shown in Fig. 1A. The data showed that control recipient mice developed diabetes within 3 weeks, and all mice became diabetic within 4 weeks of cell transfer (Fig. 1B). In comparison, ATRA treatment (every other day, as of day 1 of cell transfer) not only significantly delayed the onset of diabetes for 5 weeks in recipient mice but also significantly reduced diabetes incidence compared with controls (25% vs. 100%, respectively) (Fig. 1B). Histological studies of pancreases from recipient mice showed that control mice had already developed severe destructive insulitis 2 weeks after cell transfer, whereas either intact islets or stationary peri-insulitis was observed in ATRA-treated mice as late as week 17 after cell transfer (Fig. 1C).
FIG. 1.
ATRA treatment inhibits type 1 diabetes. A: Phenotype, cytokine secretion profile, and Foxp3 expression of T-cells present in donor splenocytes used for adoptive transfer. Representative example of splenocytes isolated from more than five new-onset diabetic NOD mice were surface-stained with anti-CD4, -CD8, or -TCR β-chain and intracellular-stained using anti–IFN-γ, –granzyme B, –IL-4, –IL-17, or -Foxp3. B: Cumulative incidence of diabetes in recipient mice adoptively transferred with splenocytes from new-onset diabetic NOD mice with ATRA treatment was significantly different (P = 0.0001) from those without treatment (eight mice per group). Splenocytes were intravenously transferred into NOD/scid mice, treated with ATRA or vehicle on the day of cell transfer. C: Varied degrees of insulitis detected in recipient mice at different time points after cell transfer. H/E staining of pancreatic sections isolated from control or ATRA-treated recipient mice after cell transfer (n = 4 mice/group). There are no control mice available for staining on week 17 after cell transfer, since all control mice developed diabetes by week 4. D: Cumulative incidence of spontaneous diabetes in NOD mice with or without ATRA treatment. NOD mice (10 weeks old) were treated with ATRA or vehicle every other day from day 1 of cell transfer until 30 weeks old. ATRA treatment significantly inhibited spontaneous disease progress in NOD mice (26 mice/group; P = 0.0005). (Please see http://dx.doi.org/10.2337/db08-1154 for a high-quality digital representation of this figure.)
The effects of ATRA treatment on spontaneous diabetes development in NOD mice was also analyzed in studies where treatment was only initiated at 10 weeks of age. At this age, 2 weeks before the onset of overt diabetes, it is known that destructive insulitis has developed for several weeks (25–27). Our data showed that ATRA treatment of pre-diabetic NOD mice with established insulitis significantly reduced diabetes incidence to 30% compared with 70% in control untreated NOD mice (Fig. 1D), demonstrating that ATRA treatment was able to inhibit diabetes progress, although severe insulitis had already occurred in these animals.
ATRA treatment decreases the number of islet-infiltrating CD8+ cells and suppresses their activation and IFN-γ/granzyme B production.
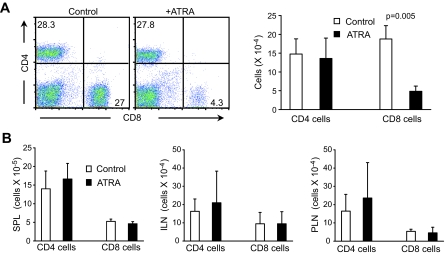
Several possibilities exist that may explain the inhibition of diabetes development by ATRA, including 1) reduction of Teff cell number, 2) suppression of activation and/or function of Teff cells, and 3) expansion of Treg cell number and/or enhancement of their function. To address these possibilities, we first determined whether ATRA treatment affected the number of CD4+ and CD8+ T-cells present in recipient mice transferred with splenocytes isolated from newly diabetic NOD mice. Our results showed that compared with controls, the percentage and number of islet-infiltrating CD8+ T-cells, but not CD4+ T-cells, was significantly reduced in ATRA-treated nondiabetic mice 4 weeks after cell transfer (Fig. 2A). At this time, all control mice had developed diabetes. ATRA treatment did not affect the number of CD4+ or CD8+ T-cells present in spleen and lymph nodes (Fig. 2B).
FIG. 2.
ATRA treatment decreases the percentage and number of islet-infiltrating CD8+ T-cells in recipient mice. A: FACS staining of islet-infiltrating cells (left); number of islet-infiltrating CD4+ or CD8+ T-cells isolated from recipient mice (right) at 4 weeks after cell transfer. B: Number of CD4+ and CD8+ T-cells in spleens (SPL), inguinal lymph nodes (ILN), and pancreatic lymph nodes (PLN). NOD/scid mice adoptively transferred with splenocytes isolated from newly diabetic NOD mice treated with vehicle or ATRA as of the day of cell transfer; cells were isolated from recipient mice 4 weeks after cell transfer. Control mice developed diabetes, and ATRA-treated recipient mice remained diabetes free. Numbers of islet-infiltrating CD8+ cells from ATRA-treated mice were significantly lower compared with controls. No difference was noted between control and ATRA-treated mice in islet-infiltrating CD4+ cells and CD4+ and CD8+ T-cells in other tissues (n = 3 experiments). (Please see http://dx.doi.org/10.2337/db08-1154 for a high-quality digital representation of this figure.)
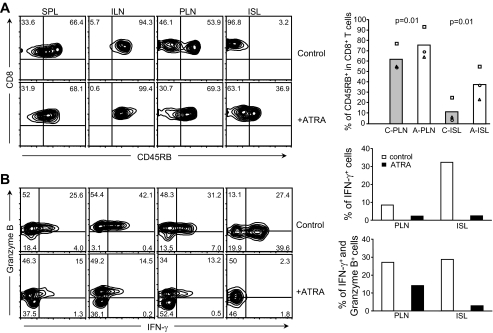
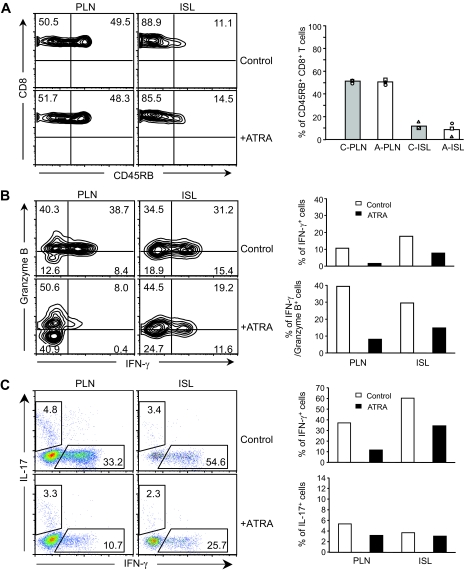
To better understand the effect of ATRA treatment on CD8+ T-cells, we analyzed the phenotype of CD8+ T-cells and their activation status in recipient mice 4 weeks after cell transfer, by quantifying the expression of CD45RB, CD44, CD62L, and CD69. Fluorescence-activated cell sorter (FACS) data revealed that the percentage of CD8+ T-cells expressing a higher level of CD45RB in pancreatic lymph nodes and islets was significantly greater in ATRA-treated mice than in control recipient mice (Fig. 3A). No differences were noted in CD44, CD62L, and CD69 expression (data not shown). Similarly, ATRA treatment did not alter the percentage of CD45RB-expressing CD8+ T-cells present in inguinal lymph nodes or spleens. These results showed that ATRA treatment blocked the downregulation of CD45RB on CD8+ T-cells and helped retain the expression of a higher level of CD45RB on CD8+ T-cells in pancreatic lymph nodes and, in particular, islets. It is known that CD45RB is downregulated upon activation of T-cells through their TCRs, indicating T-cell activation status (28). Therefore, compared with CD8+ T-cells present in pancreatic lymph nodes and islets of control mice, expression of a higher level of CD45RB on CD8+ T-cells in these two tissues suggested that these CD8+ T-cells were kept in a relatively less activated or inactivated status after ATRA treatment.
FIG. 3.
Effects of ATRA treatment on the functional and activation status of CD8+ T-cells. A: FACS analyses of CD45RB expression. ATRA treatment blocked CD45RB downregulation on CD8+ T-cells in pancreatic lymph nodes (PLN) and islets (ISL) compared with control recipient mice (left). Percentage of CD45RB+ cells in CD8+ T-cells in pancreatic lymph nodes and islets with or without ATRA treatment (right). A, ATRA-treated; C, control; ILN, inguinal lymph nodes; SPL, spleen. B: Intracellular FACS analyses of granzyme B and IFN-γ expression in CD8+ T-cells (left). ATRA treatment resulted in decreased granzyme B and IFN-γ expression in CD8+ T-cells in ISL, PLN, SPL, and ILN of NOD/scid recipient mice. Average percentage of CD8+ cells that expressed IFN-γ or IFN-γ and granzyme B in PLN and ISL with or without ATRA treatment (right) (n = 2) is shown. Control mice developed diabetes, whereas ATRA-treated recipient mice remained diabetes-free at 4 weeks after cell transfer.
To further evaluate the effect of ATRA treatment on the functional status of CD8+ T-cells in recipient mice, we examined the expression of granzyme B and IFN-γ expression in CD8+ T-cells. Previous studies showed that expression of granzyme B or IFN-γ by CD8+ T-cells either directly or indirectly contributed to CD8+ cell–mediated β-cell death (3,5,29). Our results indicated that in ATRA-treated recipient mice, the percentage of CD8+ T-cells expressing IFN-γ alone or both granzyme B and IFN-γ was reduced not only in pancreatic lymph nodes and islets but also in spleen and inguinal lymph nodes, compared with controls (Fig. 3B).
Our results suggested that ATRA treatment negatively regulated the activation and function of CD8+ Teff cells, in particular those infiltrating the islets, and maintained them in a relatively inactive status upon exposure to β-cell antigens.
ATRA treatment suppresses IFN-γ–but not IL-17–producing CD4+ T-cells in vivo.
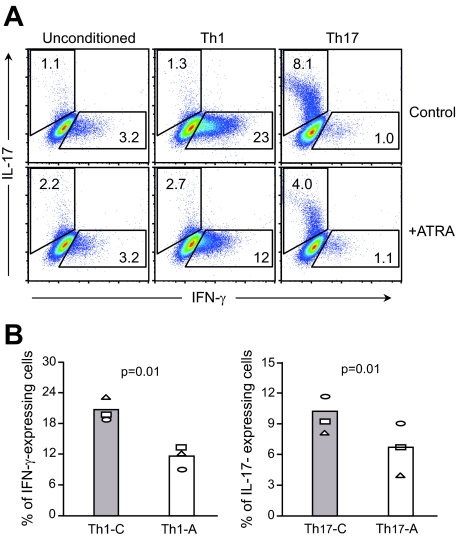
Although the number of islet-infiltrating CD4+ cells was not altered (Fig. 2A), ATRA treatment could potentially affect the functional status of CD4+ Teff cells and the differentiation of Th1 or Th17 cell lineages in recipient mice. While previous research in vitro has demonstrated ATRA regulation of Th1 and Th17 cell differentiation from naive CD4+ T-cells isolated from normal mice (18–20), its effects on the in vitro differentiation of CD4+ T-cells isolated from diabetic NOD mice are not known. We addressed this question by maintaining CD4+ splenic T-cells, purified from diabetic NOD mice, under culture conditions biased toward either Th1 or Th17 cell differentiation. The results from intracellular cytokine staining showed that, when compared with controls, addition of ATRA to cell cultures significantly reduced the percentage of IFN-γ–and IL-17–producing cells cultured under Th1 and Th17 conditions, respectively (Fig. 4).
FIG. 4.
ATRA suppresses in vitro differentiation of CD4+ T-cells from diabetic NOD mice into either IFN-γ–or IL-17–producing cells. A: Intracellular FACS staining of CD4+ T-cells cultured under conditions favoring either Th1 or Th17 cell differentiation. Addition of ATRA to CD4+ T-cells cultured under conditions toward either Th1 or Th17 cell differentiation suppressed IFN-γ–and IL-17–producing cells, respectively. B: Effects of ATRA treatment, in vitro, on the percentage of IFN-γ–or IL-17–producing cells cultured under either Th1- or Th17-conditioned medium. Th1-C/Th17-C: control CD4+ cells cultured in the absence of ATRA with either Th1- or Th17-conditioned medium; Th1-A/Th17-A: CD4+ cells cultured in the presence of ATRA with Th1- or Th17-conditioned medium (n = 3). (Please see http://dx.doi.org/10.2337/db08-1154 for a high-quality digital representation of this figure.)
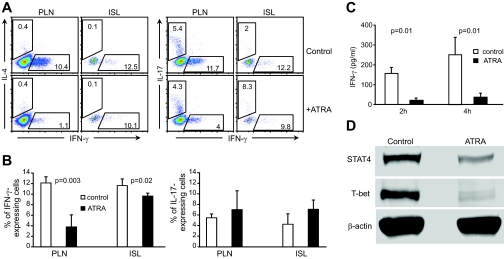
We next investigated whether ATRA treatment had similar negative effects on Th1 and Th17 cells in vivo; in particular, we examined the percentage of IFN-γ–and IL-17–producing cells in recipient mice. Cells isolated from pancreatic lymph nodes and islets of recipient mice were analyzed by intracellular cytokine staining 4 weeks after cell transfer. The results showed that the percentage of IFN-γ–producing CD4+ cells was significantly reduced in both pancreatic lymph nodes and islets of ATRA-treated recipient mice compared with controls (Fig. 5A and B). However, unexpectedly, the percentage of IL-17–producing CD4+ cells was not significantly changed in these tissues, nor was there an effect on IL-4–producing CD4+ cells (Fig. 5A).
FIG. 5.
In vivo effects of ATRA treatment on IFN-γ and IL-17 production in T-cells of recipient mice. A: Intracellular staining of CD4+ T-cells with antibodies against IFN-γ, IL-17, or IL-4. Cells were isolated from pancreatic lymph nodes (PLN) or islets (ISL) of diabetic control or diabetes-free ATRA-treated recipient mice 4 weeks after cell transfer and surface-stained with anti-CD4 and then intracellularly stained with antibody for cytokines. Cells were electronically gated on CD4+ T-cells. B: Percentage of cells producing either IFN-γ (left) or IL-17 (right). n = 5. C: ATRA treatment suppresses in vivo production of IFN-γ by T-cells in response to anti-CD3 stimulation, detected by ELISA. Four weeks after adoptive transfer of diabetic NOD mouse splenocytes, diabetic control recipient mice and nondiabetic ATRA-treated recipient mice were intravenously injected with anti-CD3 (n = 3). D: ATRA treatment suppresses T-bet and STAT4 expression in T-cells. T-cells were isolated from the spleen of control or ATRA-treated recipient mice at 4 weeks post–cell transfer. Isolated T-cells were then stimulated with phorbol myristate acetate plus ionomycin. Western blot of cell lysates for T-bet and STAT4 expression (n = 3). (Please see http://dx.doi.org/10.2337/db08-1154 for a high-quality digital representation of this figure.)
Given that ATRA treatment had negative in vivo effects on both CD4+ and CD8+ IFN-γ–producing T-cells, we further evaluated the in vivo effects on IFN-γ production in response to anti-CD3 stimulation in ATRA-treated recipient mice. Control and ATRA-treated recipient mice were injected intravenously with an anti-CD3 antibody, and splenocytes were isolated after antibody injection and cultured (2 and 4 h) in nonconditioned medium. ELISA results demonstrated a significant reduction in IFN-γ production by splenocytes from ATRA-treated animals, at both time points, compared with controls (Fig. 5C).
Biochemical studies were used to further confirm the effects of ATRA treatment on IFN-γ–producing T-cells. Western blot data showed that expression of STAT4 and T-bet transcription factors, which are critical to IFN-γ production and Th1 cell differentiation (30–32), were significantly reduced (48.9% in STAT4 and 83.3% in T-bet expression) in T-cells from ATRA-treated mice compared with untreated controls (Fig. 5D).
ATRA treatment leads to the expansion of Foxp3+ Treg cells in vivo.
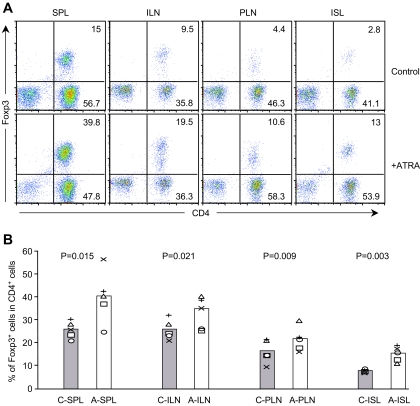
We next examined whether ATRA treatment also resulted in the expansion of Foxp3+ CD4+ Treg cells in vivo. Cells from various tissues of control and ATRA-treated recipient mice were isolated 4 weeks after cell transfer. Isolated cells were first surface stained with ani-CD4 and anti-TCR β antibody and then intracellularly stained with anti-Foxp3 antibody. FACS analysis data showed that the percentage of Foxp3+ CD4+ Treg cells present in the spleen, inguinal lymph nodes, pancreatic lymph nodes, and islets was significantly increased in ATRA-treated recipient mice compared with controls (Fig. 6A and B). Our in vivo study results demonstrated that ATRA treatment not only suppressed IFN-γ–producing Teff cells but also promoted the expansion of Foxp3+ CD4+ Treg cells without affecting Th17 cells.
FIG. 6.
ATRA treatment significantly increases Foxp3+ Treg cell expansion in animals. A: Intracellular staining of Foxp3 in CD4+ T-cells. Cells were isolated from various tissues of diabetic control or diabetes-free ATRA-treated recipient mice 4 weeks after cell transfer. Cells were electronically gated on TCR+ cells. B: Comparison of the percentage of Foxp3-expressing cells present in various tissues. A, ATRA-treated mice; C, control mice; ILN, inguinal lymph nodes; ISL, islets; PLN, pancreatic lymph nodes; SPL, spleen (n = 5). (Please see http://dx.doi.org/10.2337/db08-1154 for a high-quality digital representation of this figure.)
Depletion of CD4+CD25+ T-cells impairs the protective effect of ATRA treatment on diabetes.
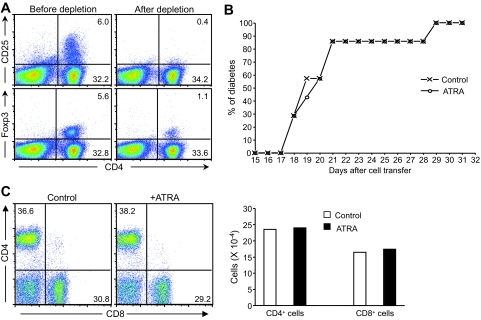
We further examined whether the inhibitory effects of ATRA treatment on diabetes were dependent upon Foxp3+ CD4+ Treg cells. CD4+CD25+ cells were depleted from donor splenocytes isolated from newly diabetic NOD mice, and CD4+CD25− splenocytes (Fig. 7A) were transferred into NOD/scid recipient mice. Our results demonstrated that control and ATRA-treated recipient mice developed accelerated diabetes and the kinetics of disease progression was nearly identical in both (Fig. 7B). Therefore, depletion of CD4+CD25+ T-cells from donor cells impaired the protective effect of ATRA treatment on diabetes development.
FIG. 7.
Depletion of CD4+CD25+ T-cells impairs the ability of ATRA to inhibit diabetes. A: Expression of CD25 or Foxp3 in CD4+ T-cells before and after depletion of CD4+CD25+ cells from donor splenocytes of newly diabetic NOD mice. Around 95% of CD4+CD25+ cells were depleted before they were adoptively transferred into NOD/scid recipient mice. About 1% of donor splenocytes still expressed Foxp3, a fivefold reduction compared with splenocytes before depletion. B: Cumulative diabetes incidence of ATRA-treated and control mice transferred with CD4+CD25+-depleted splenocytes (seven mice per group, n = 2). C: Depletion of CD4+CD25+ T-cells blocked the effect of ATRA in reducing the percentage and number of islet-infiltrating CD8+ T-cells. Islet-infiltrating cells isolated from control or ATRA-treated mice adoptively transferred with CD4+CD25+-depleted splenocytes are shown. Islet-infiltrating cells were analyzed by FACS staining using anti-CD4 and anti-CD8 antibodies (n = 2). (Please see http://dx.doi.org/10.2337/db08-1154 for a high-quality digital representation of this figure.)
Further analysis of islet-infiltrating lymphocytes isolated from recipient mice transferred with CD4+CD25+-depleted splenocytes showed that, unlike that observed in recipient mice transferred with nondepleted splenocytes (Fig. 2A), there were no differences in the percentage and number of CD8+ T-cells in control versus ATRA-treated mice (Fig. 7C). In addition, unlike the differences previously observed in CD45RB (Fig. 3A), no differences were noted in CD45RB expression on CD8+ T-cells in pancreatic lymph nodes and islets isolated from control and ATRA-treated recipient mice transferred with CD4+CD25+-depleted splenocytes (Fig. 8A). The results suggested that in the absence of a sufficient number of Treg, ATRA treatment failed to maintain islet-infiltrating CD8+ T-cells at a relatively less activated state.
FIG. 8.
Depletion of CD4+CD25+ T-cell impairs the ability of ATRA to suppress Teff cells. A: FACS staining of CD8+ T-cells (left). Depletion of CD4+CD25+ T-cells blocked the negative effect of ATRA treatment on CD45RB expression on CD8+ T-cells in pancreatic lymph nodes (PLN) and in islets (ISL). Percentage of CD45RB+ cells in CD8+ T-cells isolated from PLN and ISL of recipient mice with or without ATRA treatment (right) (A, ATRA; C, control) (n = 3). B: FACS analysis of CD8+ T-cells intracellularly stained for granzyme B and IFN-γ (left). Cells were isolated from pancreatic lymph nodes and islets of control and ATRA-treated recipient mice 4 weeks after being transferred with CD4+CD25+-depleted splenocytes. Cells were electronically gated on CD8+ T-cells. Average percentage of CD8+ cells expressing either IFN-γ alone or IFN-γ and granzyme B in PLN and ISL with or without ATRA treatment (right) (n = 2). C, left panel: FACS analysis of CD4+ T-cells intracellularly stained for IL-17 and IFN-γ. Cells were isolated from PLN and ISL of control and ATRA-treated recipient mice 4 weeks after being transferred with CD4+CD25+-depleted splenocytes. Cells were electronically gated on CD4+ T-cells. Average percentage of CD4+ cells expressing either IFN-γ or IL-17 in PLN and ISL with or without ATRA treatment (right) is shown. (Please see http://dx.doi.org/10.2337/db08-1154 for a high-quality digital representation of this figure.)
The results also showed that the percentage of CD8+ T-cells expressing either IFN-γ alone or IFN-γ and granzyme B was reduced in pancreatic lymph nodes and islets of ATRA-treated recipient mice transferred with CD4+CD25+-depleted splenocytes (Fig. 8B). However, despite this decrease, the percentage of islet-infiltrating CD8+ T-cells expressing either IFN-γ alone or IFN-γ and granzyme B in ATRA-treated mice transferred with depleted splenocytes was still higher than that found in ATRA-treated recipient mice transferred with nondepleted splenocytes (Fig. 8B; Fig. 3B). Similarly, while ATRA treatment also reduced the percentage of IFN-γ-expressing CD4+ T-cells in both pancreatic lymph nodes and islets of mice transferred with CD4+CD25+-depleted splenocytes, their percentage was still higher than that detected in mice receiving nondepleted splenocytes (Fig. 8C and Fig. 5A and B). In comparison, ATRA treatment did not affect IL-17 expression in CD4+ T-cells in pancreatic lymph nodes or islets from mice transferred with CD4+CD25+ cell-depleted splenocytes (Fig. 8C). Taken together, these results demonstrated that in the absence of a sufficient number of Foxp3+ CD4+ Treg cells, ATRA treatment could not efficiently suppress the activation and function of both CD8+ and CD4+ Teff cells and consequently failed to inhibit diabetes.
DISCUSSION
The findings in this report demonstrated that ATRA, a drug currently used to treat leukemia, effectively induced immune tolerance that inhibited islet inflammation and progression to overt diabetes. These novel findings extend the in vivo effects of ATRA on immune tolerance induction beyond its in vitro effects on CD4+ T-cells. Our results also implicated CD4+ and CD8+ Teff cells in ATRA-induced immune tolerance in vivo. ATRA treatment inhibited both CD4+ and CD8+ IFN-γ–producing cells without a detectable effect on CD4+ IL-17–producing cells. In addition, ATRA treatment not only suppressed the number but also the activation and functional status of CD8+ T-cells. Histological studies further showed that ATRA-treated nondiabetic animals were free of destructive insulitis, suggesting that the effects of ATRA on T-cells might have also prevented their trafficking to and infiltration into islets, thus inhibiting diabetes. In addition to its effect on Teff cells, our results showed that ATRA treatment promoted the in vivo expansion of Foxp3+ CD4+ Treg cells. Furthermore, the protective effects were impaired in the absence of a sufficient number of donor Foxp3+ CD4+ Treg cells. Altogether, these findings support a model in which ATRA exerted its in vivo disease-protective effect, at least in part, by suppressing both CD4+ and CD8+ IFN-γ–producing Teff cells but not IL-17–producing Teff cells, while promoting the expansion of Treg cells. In addition, this suppressive effect on Teff cells was dependent on the presence of Foxp3+ CD4+ Treg cells.
Our data provide novel insights as to how ATRA treatment induces immune tolerance, by suppressing both CD4+ and CD8+ Teff cells. IFN-γ–producing CD4+ Th1 cells and CD8+ Tc1 cells have been implicated in the pathogenesis of autoimmune diseases (3–5). Previous studies have demonstrated that both CD4+ and CD8+ Teff cells were involved in the onset and further development of type 1 diabetes (33–35). Our results suggested ATRA treatment significantly downregulated both the percentage of IFN-γ–producing CD4+ and CD8+ T-cells and the amount of IFN-γ produced by T-cells under in vitro and in vivo conditions. An unexpected finding of our study was that ATRA treatment did not alter the Th17 cell population, despite the fact that ATRA inhibited the in vitro differentiation of Th17 cells (Fig. 4) (18–20,23). Although Th17 cells may play an important role in pathogenesis in inflammatory bowel disease, experimental autoimmune encephalitis, and autoimmune arthritis (36–38), the role of Th17 cells in type 1 diabetes is not clear. Our data demonstrated that ATRA inhibited diabetes by suppressing both Th1 and Tc1 cells, without affecting Th17 cells. These results suggested that under these conditions, a presumably normal Th17 cell population alone was not able to induce type 1 diabetes and further demonstrated that one of the mechanisms by which ATRA effectively induced immune tolerance and inhibited diabetes was through the suppression of CD4+ Th1 and CD8+ Tc1 cells.
Our results further showed that ATRA treatment was able to suppress both the activation and functional status of islet-infiltrating CD8+ Teff cells in recipient mice. In control recipient mice, islet-infiltrating CD8+ T-cells downregulated the expression of CD45RB compared with that observed in other peripheral lymphoid tissues, including inguinal lymph nodes and the spleen. Downregulation of CD45RB in these islet-infiltrating CD8+ T-cells indicated they had been activated in the islets, which might have occurred after the encounter of their TCRs with their islet autoantigens. In comparison, CD45RB expression was not downregulated on islet-infiltrating CD8+ T-cells in ATRA-treated recipient mice, suggesting that ATRA suppressed the activation of these T-cells. Moreover, expression of granzyme B plays an important role in CD8+ Teff cell function (39). ATRA treatment suppressed granzyme B expression, especially coexpression with IFN-γ, in islet-infiltrating CD8+ T-cells, effectively suppressing the functional activation of islet-infiltrating CD8+ T-cells in an environment where they could easily encounter and be activated by their islet autoantigens. ATRA treatment also effectively prevented infiltration of T-cells into islets, thus precluding the development of destructive insulitis and diabetes. Histological analyses data showed that, although control recipient mice had already developed severe destructive insulitis at 2 weeks after cell transfer, only intact islets or peri-insulitis was detected in ATRA-treated recipient mice, even at a later time point (17 weeks after cell transfer). Therefore, by preventing recruitment of pathogenic T-cells to the islets, ATRA treatment might also prevent diabetes.
Additional questions regarding the relative role of ATRA treatment on various T-cell subsets and its subsequent protection against diabetes remain to be formally addressed. For example, it is currently unknown whether the increase in Treg cells occurred concomitantly with a decrease in Teff cells or whether these were separate or sequential events. One possibility is that ATRA induces an early expansion of Treg cells, which results in the suppression of both CD4+ and CD8+ Teff cells. However, our results that only islet-infiltrating CD8+ cell numbers were reduced by ATRA treatment suggest that a mechanism other than a simple expansion of Treg cells is responsible for the loss of CD8+ T-cells. Another possibility is that Treg cell expansion after ATRA treatment might affect a third cell population, which would lead to the selective suppression of different Teff cell subsets. Previous studies have suggested that Treg cells do not interact directly with Teff cells; instead, they may suppress target Teff cells through the interaction with antigen-presenting cells (40,41). Our results do not exclude the possible involvement of, for example, dendritic natural killer T-cells and natural killer cells, which have been suggested to play a role during islet inflammation and diabetes onset (27,42–46). Further studies are needed to determine the relative contribution of these different possibilities.
A novel finding in our study was that the disease-protection effect of ATRA was blocked when CD4+CD25+ T-cells, thus a majority of Foxp3+ Treg, were depleted from donor splenocytes. While the mechanisms responsible for the Treg dependency in ATRA's disease protection effects remain largely unclear, we found that ATRA treatment did not reduce the number of islet-infiltrating CD8+ T-cells in recipient mice transferred with CD4+CD25− splenocytes. Although the percentage of CD4+ or CD8+ T-cells producing IFN-γ alone or CD8+ cells producing both IFN-γ and granzyme B were reduced in recipient mice, the reduction level was less than that detected in recipient mice transferred with nondepleted splenocytes. These results indicated that after depletion of Treg cells from donor cells, ATRA treatment failed to inhibit diabetes, at least due in part to its inefficiency in suppressing these Teff cells. Future studies will further elucidate the molecular and cellular mechanisms underlying this Treg dependency.
In this article, we have focused on ATRA's effects in vivo on various populations of T-cells. Based on our findings, the dual in vivo effects of ATRA treatment, namely, the suppression of Teff cells and the promotion of Treg cell expansion, may distinguish the use of ATRA from other interventions that affect either Treg or Teff cells, as a therapeutic method for type 1 diabetes. Although ATRA treatment was able to inhibit spontaneous type 1 diabetes in pre-diabetic NOD mice with established insulitis, to further validate and establish the potential of using ATRA for therapy, not just prevention, it will be important for future studies to examine whether, other than using the adoptive transfer mouse model, ATRA treatment can inhibit ongoing spontaneously developed type 1 diabetes in nonmanipulated NOD mice. If so, it will also be important to uncover whether the same cellular and molecular mechanisms underlie the tolerance-inducing effect of ATRA in these mice. Furthermore, because ATRA is FDA-approved for the treatment of leukemia and skin disease, its well-studied toxicity and metabolic kinetics in humans suggest a clear advantage for its clinical use over other methods that might still require extensive human trials. Overall, our data support the use of ATRA treatment to induce immune tolerance may provide an effective method to inhibit type 1 diabetes.
Acknowledgments
This study was supported in part by funding from the American Diabetes Association, the Juvenile Diabetes Research Foundation, the American Heart Association, the National Institutes of Health, and the H.L. Snyder Medical Foundation.
No potential conflicts of interest relevant to this article were reported.
Published ahead of print at http://diabetes.diabetesjournals.org on 4 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 24.
REFERENCES
- 1.Asano M, Toda M, Sakaguchi N, et al.: Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med 184: 387–396, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amrani A, Verdaguer J, Thiessen S, et al.: IL-1alpha, IL-1beta, and IFN-gamma mark beta cells for Fas-dependent destruction by diabetogenic CD4(+) T lymphocytes. J Clin Invest 105: 459–468, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savinov AY, Wong FS, Chervonsky AV: IFN-gamma affects homing of diabetogenic T cells. J Immunol 167: 6637–6643, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Wang B, Andre I, Gonzalez A, et al.: Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci U S A 94: 13844–13849, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suarez-Pinzon W, Rajotte RV, Mosmann TR, et al.: Both CD4+ and CD8+ T-cells in syngeneic islet grafts in NOD mice produce interferon-γ during β-cell destruction. Diabetes 45: 1350–1357, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Weaver CT, Hatton RD, Mangan PR, et al.: IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25: 821–852, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Ziegler SF: FOXP3: of mice and men. Annu Rev Immunol 24: 209–226, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Castaigne S, Chomienne C, Daniel MT, Ballerini P, Berger R, Fenaux P, Degos L: All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood 76: 1704–1709, 1990 [PubMed] [Google Scholar]
- 9.Leyden JJ: Therapy for acne vulgaris. N Engl J Med 336: 1156–1162, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Reifen R, Nur T, Ghebermeskel K, et al.: Vitamin A deficiency exacerbates inflammation in a rat model of colitis through activation of nuclear factor-kappaB and collagen formation. J Nutr 132: 2743–2747, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Cantorna MT, Nashold FE, Hayes CE: In vitamin A deficiency multiple mechanisms establish a regulatory T helper cell imbalance with excess Th1 and insufficient Th2 function. J Immunol 152: 1515–1522, 1994 [PubMed] [Google Scholar]
- 12.Aukrust P, Muller F, Ueland T, et al.: Decreased vitamin A levels in common variable immunodeficiency: vitamin A supplementation in vivo enhances immunoglobulin production and downregulates inflammatory responses. Eur J Clin Invest 30: 252–259, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita K, Yoo BS, Nozaki Y, et al.: Retinoic acid reduces autoimmune renal injury and increases survival in NZB/W F1 mice. J Immunol 170: 5793–5798, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Miyagawa N, Homma T, Kagechika H, et al.: Effect of synthetic retinoid, TAC-101, on experimental autoimmune disease. Pharmacology 67: 21–31, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Osanai M, Nishikiori N, Murata M, et al.: Cellular retinoic acid bioavailability determines epithelial integrity: role of retinoic acid receptor alpha agonists in colitis. Mol Pharmacol 71: 250–258, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Zunino SJ, Storms DH, Stephensen CB: Diets rich in polyphenols and vitamin A inhibit the development of type I autoimmune diabetes in nonobese diabetic mice. J Nutr 137: 1216–1221, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Driscoll HK, Chertow BS, Jelic TM, et al.: Vitamin A status affects the development of diabetes and insulitis in BB rats. Metabolism 45: 248–253, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Schambach F, Schupp M, Lazar MA, et al.: Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol 37: 2396–2399, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Elias KM, Laurence A, Davidson TS, et al.: Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood 111: 1013–1020, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mucida D, Park Y, Kim G, et al.: Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317: 256–260, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Benson MJ, Pino-Lagos K, Rosemblatt M, et al.: All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med 204: 1765–1774, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang SG, Lim HW, Andrisani OM, et al.: Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol 179: 3724–3733, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Sun CM, Hall JA, Blank RB, et al.: Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 204: 1775–1785, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krymskaya L, Lee WH, Zhong L, et al.: Polarized development of memory cell-like IFN-gamma-producing cells in the absence of TCR zeta-chain. J Immunol 174: 1188–1195, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Andre I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D: Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci U S A 93: 2260–2263, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Q, Salomon B, Chen M, et al.: Reversal of spontaneous autoimmune insulitis in nonobese diabetic mice by soluble lymphotoxin receptor. J Exp Med 193: 1327–1332, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi FD, Flodstrom M, Balasa B, et al.: Germ line deletion of the CD1 locus exacerbates diabetes in the NOD mouse. Proc Natl Acad Sci U S A 98: 6777–6782, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birkeland ML, Johnson P, Trowbridge IS, et al.: Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc Natl Acad Sci U S A 86: 6734–6738, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estella E, McKenzie MD, Catterall T, et al.: Granzyme B-mediated death of pancreatic beta-cells requires the proapoptotic BH3-only molecule bid. Diabetes 55: 2212–2219, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Juedes AE, Rodrigo E, Togher L, et al.: T-bet controls autoaggressive CD8 lymphocyte responses in type 1 diabetes. J Exp Med 199: 1153–1162, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabo SJ, Kim ST, Costa GL, et al.: A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100: 655–669, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Nishikomori R, Usui T, Wu CY, et al.: Activated STAT4 has an essential role in Th1 differentiation and proliferation that is independent of its role in the maintenance of IL-12R beta 2 chain expression and signaling. J Immunol 169: 4388–4398, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Haskins K, McDuffie M: Acceleration of diabetes in young NOD mice with a CD4+ islet-specific T cell clone. Science 249: 1433–1436, 1990 [DOI] [PubMed] [Google Scholar]
- 34.Wicker LS, Leiter EH, Todd JA, et al.: β-2-Microglobulin–deficient NOD mice do not develop insulitis or diabetes. Diabetes 43: 500–504, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Santamaria P: Effector lymphocytes in islet cell autoimmunity. Rev Endocr Metab Disord 4: 271–280, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Fujino S, Andoh A, Bamba S, et al.: Increased expression of interleukin 17 in inflammatory bowel disease. Gut 52: 65–70, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batten M, Li J, Yi S, et al.: Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol 7: 929–936, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Koenders MI, Lubberts E, Oppers-Walgreen B, et al.: Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol 167: 141–149, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chowdhury D, Lieberman J: Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol 26: 389–420, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadokoro CE, Shakhar G, Shen S, et al.: Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med 203: 505–511, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Q, Adams JY, Tooley AJ, et al.: Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol 7: 83–92, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griseri T, Beaudoin L, Novak J, et al.: Invariant NKT cells exacerbate type 1 diabetes induced by CD8 T cells. J Immunol 175: 2091–2101, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Clare-Salzler MJ, Brooks J, Chai A, et al.: Prevention of diabetes in nonobese diabetic mice by dendritic cell transfer. J Clin Invest 90: 741–748, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludewig B, Odermatt B, Landmann S, et al.: Dendritic cells induce autoimmune diabetes and maintain disease via de novo formation of local lymphoid tissue. J Exp Med 188: 1493–1501, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carnaud C, Gombert J, Donnars O, et al.: Protection against diabetes and improved NK/NKT cell performance in NOD.NK1.1 mice congenic at the NK complex. J Immunol 166: 2404–2411, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Todd DJ, Forsberg EM, Greiner DL, et al.: Deficiencies in gut NK cell number and function precede diabetes onset in BB rats. J Immunol 172: 5356–5362, 2004 [DOI] [PubMed] [Google Scholar]