Abstract
OBJECTIVE—To determine whether 1) hepatic ceramide and diacylglycerol concentrations, 2) SCD1 activity, and 3) hepatic lipogenic index are increased in the human nonalcoholic fatty liver.
RESEARCH DESIGN AND METHODS—We studied 16 subjects with (n = 8) and without (n = 8) histologically determined nonalcoholic fatty liver (NAFL+ and NAFL−) matched for age, sex, and BMI. Hepatic concentrations of lipids and fatty acids were quantitated using ultra-performance liquid chromatography coupled to mass spectrometry and gas chromatography.
RESULTS—The absolute (nmol/mg) hepatic concentrations of diacylglycerols but not ceramides were increased in the NAFL+ group compared with the NAFL− group. The livers of the NAFL+ group contained proportionally less long-chain polyunsaturated fatty acids as compared with the NAFL− group. Liver fat percent was positively related to hepatic stearoyl-CoA desaturase 1 (SCD1) activity index (r = 0.70, P = 0.003) and the hepatic lipogenic index (r = 0.54, P = 0.030). Hepatic SCD1 activity index was positively related to the concentrations of diacylglycerols (r = 0.71, P = 0.002) but not ceramides (r = 0.07, NS).
CONCLUSIONS—We conclude that diacylglycerols but not ceramides are increased in NAFL. The human fatty liver is also characterized by depletion of long polyunsaturated fatty acids in the liver and increases in hepatic SCD1 and lipogenic activities.
Nonalcoholic fatty liver disease (NAFLD) is characterized by lipid accumulation in the liver (≥10% of liver weight), which cannot be attributed to alcohol consumption or any other liver disease (1). NAFLD covers a range from simple nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) and fibrosis (1). The fatty liver is resistant to the action of insulin to inhibit hepatic glucose (2,3) and VLDL (4) production, resulting in hyperglycemia and hypertriglyceridemia. The mechanisms underlying insulin resistance in human NAFLD are unclear. While triacylglycerols themselves are inert, lipid intermediates may act as important regulators of both oxidative stress (5) and insulin signaling (6). In vitro studies as well as studies in animals suggest that diacylglycerols, which are immediate precursors of triacylglycerols (7), can induce insulin resistance by activating specific isoforms of protein kinase C (PKC) (8,9). The concentrations of diacylglycerols have recently been shown to be increased in human NAFLD compared with subjects with normal liver histology (10). Ceramides are another class of reactive lipids that mediate saturated fat–induced insulin resistance (6). There are no data comparing ceramide and diacylglycerol concentrations in the human liver or relating them to hepatic fat content.
Sources of hepatic lipids include dietary chylomicron remnants, free fatty acids released from either adipose tissue triacylglycerols or chylomicrons hydrolyzed at a rate in excess of what can be taken up by tissues (spillover), and de novo lipogenesis (11). Increased lipolysis is a major contributor to hepatic fat accumulation (12–14). In addition, when estimated using tracer techniques, de novo lipogenesis has been found to be significantly increased in subjects with NAFLD compared with normal subjects (12,15,16). De novo lipogenesis produces saturated fatty acids (17,18). Stearoyl-CoA desaturase 1 (SCD1) converts saturated fatty acids to monounsaturated fatty acids, which are major substrates for synthesis of triacylglycerols and other lipids (19). SCD1 knockout mice are resistant to the development of obesity and hepatic steatosis (20,21), whereas the activity of SCD1 is significantly increased in the fatty livers of ob/ob mice (20,22). These data thus suggest that hepatic SCD1 activity may contribute to lipid accumulation in NAFLD. There are, however, no data on hepatic SCD1 activity in human NAFLD.
To address the above questions, we quantified the full range of lipids and fatty acids using ultra-performance liquid chromatography (UPLC) coupled to mass spectrometry (MS) and gas chromatography in the human liver. These analyses were performed in two groups of subjects matched for age, sex, and BMI but with either a normal liver fat content (≤10% macrovesicular steatosis) or a nonalcoholic fatty liver (NAFL) (≥20% macrovesicular steatosis [1]).
RESEARCH DESIGN AND METHODS
The subjects (all Caucasians and Finns) were recruited from patients undergoing laparoscopic gastric bypass surgery based on the following inclusion criteria: 1) age 18–65 years; 2) no known acute or chronic disease except for obesity or type 2 diabetes based on history, physical examination, and standard laboratory tests (blood counts, serum creatinine, thyroid-stimulating hormone, and electrolyte concentrations), and electrocardiogram; and 3) alcohol consumption <20 g/day. The nature and potential risks of the study were explained to all subjects before obtaining their written informed consent. The patients had not lost weight before surgery (mean weight change over 10 months was −1.1 kg). The study protocol was approved by the ethics committee of the Helsinki University Central Hospital.
On the morning before surgery, blood samples were taken after an overnight fast for measurement of A1C, fasting serum insulin and C-peptide, liver enzymes, serum triglyceride, and LDL and HDL cholesterol concentrations. Body weight was recorded to the nearest kilogram using a calibrated weighting scale with subjects barefoot and wearing light indoor clothing. Wedge biopsies of the liver were taken at surgery. Approximately one-half of the liver sample was sent to the pathologist for routine histopathological assessment, while the rest was immediately frozen and stored in liquid nitrogen. The fat content of the liver biopsy specimens (percent of hepatocytes with macrovesicular and microvesicular steatosis) was determined by an experienced liver pathologist (J.A.) in a blinded fashion (23). The percent of macrovesicular steatosis was used as the liver fat percent. One of the patients had mild (grade 1) necroinflammatory and fibrotic changes, and two of the patients had mild (grades 1 and 2) fibrotic changes.
Lipidomic analysis.
Before analyzing the lipid composition of the liver, frozen samples (5 mg) were mixed with an IS mixture containing 0.5–1 μg/sample of phosphatidylcholine (PC) (17:0/0:0), ceramide (Cer) (d18:1/17:0), PC (17:0/17:0), phosphatidylethanolamine (PE) (17:0/17:0), triglycerides (TGs) (17:0/17:0/17:0), and 200 μl chloroform:methanol (2:1). The tissues were homogenized with grinding balls in a mixer mill at 25 Hz for 2 min, and 50 μl of 0.9% NaCl was added. The samples were vortexed for 2 min, and after 30 min standing, they were centrifuged at 10,000 rpm for 3 min. The labeled lipid standard mixture was added into the separated lipid extracts (1 μg/sample) before UPLC-MS analysis.
Lipid extracts were analyzed on a Waters Q-Tof Premier mass spectrometer combined with an Acquity UPLC. The column (at 50°C) was an Acquity UPLC bridged ethyl hybrid C18 1 × 50 mm with 1.7-μm particles. The solvent system included 1) water (1% 1 mol/l NH4Ac, 0.1% HCOOH) and 2) acetonitrile/isopropanol (5:2, 1% 1 mol/l NH4Ac, 0.1% HCOOH). The gradient started from 65% A/35% B, reached 100% B in 6 min, and remained there for the next 7 min. There was a 5-min reequilibration step before the next run. The flow rate was 0.200 ml/min, and the injected amount was 1.0 μl. Reserpine was used as the lock spray reference compound. Lipid profiling was carried out using ESI+ mode, and the data were collected at a mass range of 300–1,200 m/z, with a scan duration of 0.2 s. The data were processed by using MZmine software (version 6.0 [24]) and the lipid identification was based on an internal spectral library (25). The relative amounts of all the identified lipids were quantified by calibrating with corresponding class-specific internal standards if available. Sphingomyelins and diacylglycerols were calibrated with PC (17:0/17:0) as an internal standard, while all lysophospholipids were normalized using lysoPC (17:0). Characteristics of the analytical method have been described in detail in the supplement of our previous study (26). The applied platform affords broad screening of multiple lipid classes, including triacylglycerols, cholesterol esters, and major phospholipids, from total lipid extracts within a single sample run. Due to use of ESI+ mode in MS analysis, the platform is not optimal for detection of some negatively charged phospholipids such as phosphatidylserines and phosphatidylinositols. The method does not cover glycosphingolipids or the low–molecular weight reactive lipids such as eicosanoids and free fatty acids.
Fatty acid analysis.
Frozen liver tissues (5 mg) were spiked with 40–60 μg standards and homogenized in capped 2-ml microtubes (Sarstedt) at −20°C with chloroform:methanol (2:1; 400–600 μl) by using zirconium oxide balls in a mixer mill (25 Hz for 5 min). A total of 100–150 μl NaCl (0.9%) was added by vortexing, and after 1 h extraction time, the lower layer was separated by centrifuging at 10,000 rpm for 5 min. A total of 100–200 μl aliquots from the extracts were evaporated into dryness and used for fatty acid analyses.
The evaporation residues were redissolved into 700 μl petroleum ether (boiling point 40–60°C) by vortexing. Sodium methoxide (250 μl; 0.5 mol/l NaOMe in MeOH) and a couple of boiling stones were added, and the mixture was boiled at 45°C for 5 min (27). The samples were acidified with 500 μl of 15% NaHSO4, 100 μl petroleum ether was added, and the samples were centrifuged in 2 ml microtubes at 10,000 rpm for 5 min. The petroleum ether layers were separated into gas chromatography vials, evaporated, and redissolved into 100 μl hexane.
Gas chromatographic analysis of fatty acids was performed using the Agilent 5890 series II gas chromatography, equipped with a 25-m FFAP column (0.32 mm inner diameter). The injection volume was 2 μl and split ratio was 1:23. Helium was used as carrier gas and the oven temperature program was from 70°C to 240°C at 7°C/min. The injector and detector (frame ionization detector) temperatures were 260°C and 300°C, respectively.
Analytical procedures, measurements, and calculations.
Plasma glucose concentrations were measured in duplicate with the glucose oxidase method using Beckman Glucose Analyzer II (Beckman Instruments, Fullerton, CA) (28). Serum free insulin concentrations were measured with the Auto-DELFIA kit (Wallac, Turku, Finland) and C-peptide concentrations by radioimmunoassay (29). Serum HDL cholesterol and triacylglycerol concentrations were measured with the enzymatic kits from Roche Diagnostics using an autoanalyzer (Roche Diagnostics Hitachi, Hitachi, Tokyo, Japan). The concentration of LDL cholesterol was calculated using the Friedewald formula (30). Serum aspartate transaminase (AST), alanine transaminase (ALT), and γ glutamyltransferase (γGT) activities were determined, as recommended by the European Committee for Clinical Laboratory Standards.
Desaturase and elongase activities were estimated from product-to-precursor ratios of the percentages of individual fatty acids according to the following equations: SCD1 = (18:1/18:0), Δ6 desaturase = (18:3n − 6/18:2n − 6), Δ5 desaturase = (20:4/20:3), and elongase = (18:0/16:0) (31). Since SCD1 preferentially converts 18:0 to 18:1 rather than 16:0 to 16:1 (19), the 18:1/18:0 index was used to estimate SCD1 activity. The lipogenic index was determined from the 16:0/18:2n-6 ratio (32).
Statistical analysis.
Data are shown as means ± SE or, for non–normally distributed data, as median followed by the 25th and 75th percentiles. Correlation analyses were performed using Spearman's nonparametric rank correlation coefficient. The unpaired Student's t test was used to compare the NAFL− and NAFL+ groups. Calculations were made using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA), SysStat Statistical Package (SysStat version 10; SysStat, Evanston, IL), and SPSS 16.0 for Windows (SPSS, Chicago, IL). P < 0.05 was considered statistically significant.
RESULTS
Subject characteristics.
NAFL+ and NAFL− groups were comparable with respect to age, sex, body weight, BMI, fasting serum insulin, fasting serum C-peptide, A1C, fasting serum triacylglycerols, fasting serum LDL cholesterol, and serum γGT concentrations. Fasting serum HDL cholesterol concentrations were lower and serum ALT and serum AST concentrations were higher in the NAFL+ than in the NAFL− group (Table 1).
TABLE 1.
Clinical characteristics of the NAFL− and NAFL+ groups
| NAFL− | NAFL+ | |
|---|---|---|
| n (women) | 8 (4) | 8 (5) |
| Age (years) | 45 ± 2 | 41 ± 4 |
| Weight (kg) | 153 ± 10 | 145 ± 7 |
| BMI (kg/m2) | 51.8 ± 2.4 | 49.0 ± 1.1 |
| Fasting serum insulin (mU/l) | 10 (8–18) | 14 (10–25) |
| Fasting serum C-peptide (nmol/l) | 0.86 ± 0.17 | 1.32 ± 0.19 |
| A1C (%) | 5.4 (5.2–6.2) | 5.7 (5.5–6.5) |
| Fasting serum TGs (mmol/l) | 1.80 ± 0.26 | 2.08 ± 0.31 |
| Fasting serum HDL cholesterol (mmol/l) | 1.27 (1.13–1.29) | 0.95 (0.88–1.10)* |
| Fasting serum LDL cholesterol (mmol/l) | 2.3 ± 0.3 | 2.4 ± 0.3 |
| Serum ALT (U/l) | 25 ± 4 | 54 ± 7† |
| Serum AST (U/l) | 25 ± 2 | 39 ± 4† |
| Serum γGT (U/l) | 28 (17–47) | 30 (19–48) |
| Macrovesicular steatosis (%) | 0 (0–2.5) | 25 (30–50)‡ |
| Microvesicular steatosis (%) | 15 ± 6 | 44 ± 5† |
| Alcohol consumption (doses/day) | 0.09 ± 0.04 | 0.09 ± 0.05 |
Data are means ± SE or, for non–normally distributed data, medians (25th and 75th percentiles).
P < 0.05,
P < 0.01,
P < 0.001.
Lipids, fatty acids, and free fatty acids in the NAFL+ versus the NAFL− group.
The concentrations (nmol/mg liver tissue) of glycerophosphatidic acid, ether-linked phosphatidylcholines, lysophosphatidylethanolamine, ether-linked lysophosphatidylethanolamine, and di- and triacylglycerols were higher the NAFL+ than the NAFL− group (Table 2). The concentrations of ceramides, sphingomyelins, phosphatidylcholines, phosphatidylethanolamines, and ether-linked phosphatidylethanolamines were comparable between the groups. The results remained unchanged if the lipid data were normalized to total phospholipid concentrations (data not shown). Total hepatic lipid concentration was positively related to liver fat content (r = 0.53, P = 0.036). The livers of the NAFL+ group contained proportionally more esterified and free oleate (18:1n-9) and less esterified and free stearate (18:0) and long polyunsaturated fatty acids (Table 3) than the NAFL− group. The concentration of oleic (18:1n-9) fatty acid was higher in the NAFL+ group than in the NAFL− group (0.32 ± 0.04 vs. 0.18 ± 0.03 nmol/mg tissue, P = 0.007).
TABLE 2.
Absolute (nmol/mg tissue) lipid concentrations of the livers of the NAFL− and NAFL+ groups
| NAFL− | NAFL+ | |
|---|---|---|
| Ceramides | 0.089 ± 0.007 | 0.104 ± 0.009 |
| SM | 0.725 ± 0.043 | 0.889 ± 0.091 |
| GPA | 0.158 ± 0.020 | 0.297 ± 0.037* |
| PC | 11.47 ± 1.14 | 14.57 ± 1.77 |
| PC(e) | 0.087 ± 0.011 | 0.163 ± 0.032† |
| PE | 2.439 ± 0.227 | 2.543 ± 0.259 |
| PE(e) | 0.766 ± 0.049 | 1.046 ± 0.211 |
| lyso(tot) | 0.069 ± 0.005 | 0.189 ± 0.046† |
| lysoPC | 0.032 ± 0.003 | 0.034 ± 0.004 |
| lysoPE | 0.033 ± 0.004 | 0.139 ± 0.041† |
| lysoPE(e) | 0.004 ± 0.0006 | 0.016 ± 0.004† |
| Diacylglycerol | 0.014 ± 0.003 | 0.055 ± 0.015† |
| TGs | 49.07 ± 8.57 | 92.65 ± 14.99† |
Data are means ± SE.
P < 0.01,
P < 0.05. GPA, glycerophosphatidic acid; lysoPE(e), ether-linked lysoPE; lyso(tot), lysoPC and lysoPE; PC(e), ether-linked PC; PE(e), ether-linked PE; SM, sphingomyelin.
TABLE 3.
Proportional hepatic fatty acid composition of the NAFL− and NAFL+ groups
| NAFL− | NAFL+ | |
|---|---|---|
| 14:0 | 1.54 ± 0.19 | 1.87 ± 0.26 |
| 14:1n-9 | 0.28 ± 0.04 | 0.21 ± 0.02 |
| 16:0 | 27.6 ± 1.08 | 29.6 ± 1.11 |
| 16:1n-7 | 3.57 ± 0.32 | 3.89 ± 0.25 |
| 18:0 | 8.91 ± 0.50 | 6.86 ± 0.37* |
| 18:1n-9 | 31.7 ± 1.53 | 38.5 ± 1.20* |
| 18:1n-7 | 2.68 ± 0.14 | 2.771 ± 0.18 |
| 18:2n-6 | 12.9 ± 0.85 | 10.8 ± 0.94 |
| 18:3n-6 | 0.15 ± 0.03 | 0.12 ± 0.02 |
| 18:3n-3 | 0.90 ± 0.07 | 1.00 ± 0.07 |
| 20:3n-6 | 0.95 ± 0.10 | 0.45 ± 0.08* |
| 20:4n-6 | 4.56 ± 0.47 | 2.15 ± 0.35† |
| 20:5n-3 | 0.56 ± 0.07 | 0.20 ± 0.03† |
| 22:5n-3 | 0.66 ± 0.10 | 0.28 ± 0.05* |
| 22:6n-3 | 3.19 ± 0.44 | 1.37 ± 0.25* |
| 16:0 FFA | 41.3 ± 1.22 | 39.5 ± 0.63 |
| 18:0 FFA | 37.9 ± 1.96 | 30.7 ± 1.79‡ |
| 18:1 FFA | 14.1 ± 1.88 | 22.1 ± 1.66* |
| 18:2 FFA | 6.73 ± 0.94 | 7.78 ± 0.27 |
| 16:0 FFA (μg/mg) | 0.55 ± 0.08 | 0.58 ± 0.05 |
| 18:0 FFA (μg/mg) | 0.49 ± 0.07 | 0.45 ± 0.05 |
| 18:1 FFA (μg/mg) | 0.18 ± 0.03 | 0.32 ± 0.04* |
| 18:2 FFA (μg/mg) | 0.08 ± 0.01 | 0.11 ± 0.01‡ |
Data are means ± SE.
P < 0.01,
P < 0.05,
P < 0.001.
Fatty acid composition of hepatic TGs in relation to liver fat content.
Correlation coefficients between each of the 136 individual TGs (expressed relative to total hepatic TG) and histological liver fat content were calculated. These correlation coefficients were then plotted against the number of double bonds in the respective TGs. An inverse relationship was observed (r = −0.57, P < 0.0001, Fig. 1), implying that the TGs that were positively associated with liver fat content had only a few double bonds, whereas those TGs negatively related to liver fat content had many double bonds.
FIG. 1.
Correlation coefficients between each of the 136 individual TG (expressed relative to total hepatic TG) and histological liver fat contents were calculated. These correlation coefficients (Spearman's ρ) were then plotted against the number of double bonds in the respective TGs. An inverse relationship was observed, implying that the TGs that were positively associated with liver fat content had only a few double bonds, whereas those TGs negatively related to liver fat content had many double bonds. r = −0.57; P < 0.0001.
Hepatic desaturase and elongase activities in relation to liver fat content.
The SCD1 activity index, as estimated from the 18:1n-9–to–18:0 fatty acid ratio in the liver was 1.6-fold higher in the NAFL+ (5.7 ± 0.4) than in the NAFL− (3.7 ± 0.4, P = 0.004) group. Liver fat percent was positively related to hepatic SCD1 activity index (r = 0.70, P = 0.003, Fig. 2). The activities of Δ5 and Δ6 desaturases in the liver were comparable between the groups (data not shown). The elongase activity index (18:0/16:0) was lower in the NAFL+ (0.23 ± 0.02) than in the NAFL− (0.33 ± 0.02, P = 0.005) group and was inversely related to the percent liver fat (r = −0.78, P = 0.0004, Fig. 2). The hepatic lipogenic index (16:0/18:2n-6) was positively related to liver fat content (r = 0.54, P = 0.030, Fig. 2) but unrelated to hepatic SCD1 activity index (r = 0.28, NS).
FIG. 2.
The relationships between liver fat content and hepatic SCD1 activity index (A), hepatic elongase activity index (B), and hepatic lipogenic index (C).
The proportional amounts of long polyunsaturated fatty acids, such as 20:3n-6 and 22:6n-3, were strongly inversely related to SCD1 (r = −0.94, P < 0.0001, and r = −0.82, P < 0.0001) and positively to elongase (r = 0.83, P < 0.0001, and r = 0.86, P < 0.0001) activity indexes in the liver.
The relationships between hepatic concentrations of diacylglycerols, TGs, ceramides, and SCD1 activity index.
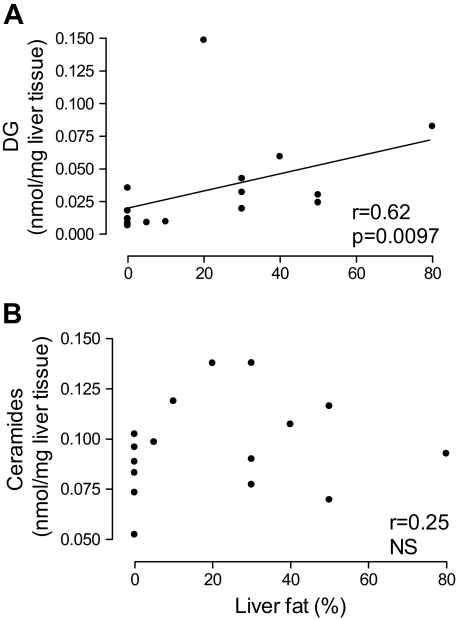
The hepatic concentrations of diacylglycerols were positively related to those of TGs (r = 0.58, P = 0.018) and to liver fat percent (r = 0.62, P = 0.0097, Fig. 3). Hepatic SCD1 activity index was positively related to the concentration of diacylglycerols (r = 0.71, P = 0.002) and TGs (r = 0.66, P = 0.005) but unrelated to those of ceramides (r = 0.07, NS) in the liver. The subjects (n = 3) with mild inflammatory or fibrotic changes fell on the same regression lines as others (data not shown).
FIG. 3.
The relationships between liver fat content and the hepatic concentrations of diacylglycerols (DG) (A) and ceramides (B).
DISCUSSION
Studies characterizing the human fatty liver are few because of methodological and ethical limitations to sample human liver tissue. In the present study, we analyzed the lipidome of the human liver of subjects with either normal or increased liver fat content due to nonalcoholic causes. We found that hepatic concentrations of diacylglycerols but not ceramides increase with increasing liver fat content. In addition, SCD1 activity index, as estimated from the product-to-precursor ratio, and lipogenic activities were increased, whereas long polyunsaturated fatty acids were depleted in fatty livers.
The final step in triacylglycerol synthesis is catalyzed by diacylglycerol acyltransferases (DGATs), which produce triacylglycerols from diacylglycerols (7). By definition, the concentrations of triacylglycerols in the human fatty liver are increased (1,10,33,34), which may explain the increase in their direct precursors, diacylglycerols (35). Consistent with this, the hepatic concentrations of diacylglycerols were positively related to those of triacylglycerols. In addition, we found hepatic diacylglycerol concentrations to be directly related to liver fat content, as determined by histology. Diacylglycerols are well-known allosteric activators of PKC, an enzyme which has been linked to insulin resistance in a variety of rodent models (36,37) as well as in the human liver (36). In mice, liver-specific overexpression of DGATs results in accumulation of triacylglycerols in the liver, whereas the concentrations of diacylglycerols and the activity of PKC remain unchanged (38). These mice do not exhibit any signs of hepatic insulin resistance (38). On the other hand, suppression of DGAT2 in mice decreases diacylglycerol concentrations and PKC activation and increases hepatic insulin sensitivity (39). These data together with the present findings would imply that diacylglycerols may contribute to hepatic insulin resistance, which is tightly related to liver fat content in humans (2,3,14).
Ceramides are sphingolipids that appear to mediate saturated fat–induced insulin resistance (6). Lipidomic analyses of livers of ob/ob mice have shown a strong association between hepatic ceramide content and the degree of steatosis (25). The relationships between hepatic ceramide concentrations and inflammatory changes were not analyzed in the latter study (25). In humans, we have previously found adipose tissue to be inflamed and contain more ceramides in subjects with high liver fat content compared with weight-matched subjects with low liver fat content without inflammation in adipose tissue (40). In the present study, we did not find hepatic ceramide concentrations to be related to liver fat content in human livers lacking significant inflammatory and fibrotic changes. These data do not exclude the possibility that ceramides contribute to hepatic insulin resistance in nonalcoholic steatohepatitis. Also, the present negative findings regarding ceramides need to be confirmed in larger groups of patients.
The concentrations of both diacylglycerols and ceramides in myocytes have been shown to increase as a consequence of silencing of SCD1 by siRNA (41). This raises the possibility that metabolism of these bioactive lipids may be regulated by SCD1. In the present study, we found the SCD1 activity index to be positively related to diacylglycerol but not ceramide concentrations. This is consistent with monounsaturated fatty acids, products of SCD1, being necessary for normal rates of synthesis of triacylglycerols (42,43). Hepatic RNA levels and activity of SCD1 are increased in obese (22) and lipoatrophic (44) mice with hepatic steatosis. In humans, SCD1 activity is considerable higher in skeletal muscle of obese compared with lean subjects (45). The present data suggest that, in the human liver, SCD1 activity increases with increasing liver fat content. Direct measurements of SCD1 activity would be helpful in this respect but could not be performed because of limited sample size.
It has recently been shown that short-term high-carbohydrate compared with high-fat feeding activates hepatic SCD1, as estimated from the composition of VLDL-TG fatty acids and de novo lipogenesis in healthy subjects with normal serum ALT concentrations (46). De novo lipogenesis is significantly increased in subjects with NAFLD compared with healthy controls (12,15,16), possibly as a consequence of an increase in sterol regulatory element–binding protein 1c (47–49), a key transcriptional activator of lipogenic genes, including SCD1 (50). In the present study, hepatic lipogenic index, estimated from the 16:0/18:2n-6 ratio (32), was positively related to liver fat content but not to SCD1 activity index. In contrast, the proportional amounts of long polyunsaturated fatty acids in the liver were strongly inversely related to hepatic SCD1 activity index and liver fat content. This is consistent with the ability of polyunsaturated fatty acids to suppress expression of both sterol regulatory element–binding protein 1c (51,52) and SCD1 (53) and activate genes involved in hepatic fatty acid oxidation (31). Subjects with NAFLD have been shown to consume less polyunsaturated fat than normal control subjects in some (54,55) but not all (56) cross-sectional studies.
Taken together, these data would suggest that depletion of long polyunsaturated fatty acids derived from essential fatty acids characterizes hepatic fat accumulation. These changes are associated with increases in both the hepatic lipogenic and SCD1 activity indexes. A recent pilot study suggested that prolonged supplementation of n-3 polyunsaturated fatty acids reduces liver fat in subjects with NAFLD (57), suggesting that polyunsaturated fatty acids could be beneficial in treating NAFLD.
Acknowledgments
This study was supported by research grants from the Academy of Finland (to H.Y.-J.), the Sigrid Juselius Foundation (to H.Y.-J.), the Finnish Diabetes Research Foundation (to A.K.), and the Biomedicum Helsinki Foundation (to A.K.). This work is part of the project “Hepatic and adipose tissue and functions in the metabolic syndrome” (www.hepadip.org), which is supported by the European Commission as an Integrated Project under the 6th Framework Programme (contract LSHM-CT-2005-018734) (to H.Y.-J. and M.O.).
This study also received grant support from Novo Nordisk Foundation (to H.Y.-J.). No other potential conflicts of interest relevant to this article were reported.
We gratefully acknowledge Mia Urjansson, Katja Tuominen, and Laxman Yetukuri for excellent technical assistance and the volunteers for their help.
Published ahead of print at http://diabetes.diabetesjournals.org on 24 October 2008.
M.O. and H.Y.-J. share senior authorship of this study.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Neuschwander-Tetri BA, Caldwell SH: Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 37: 1202–1219, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Ryysy L, Hakkinen AM, Goto T, Vehkavaara S, Westerbacka J, Halavaara J, Yki-Jarvinen H: Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 49: 749–758, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H: Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87: 3023–3028, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Hakkinen A, Olofsson SO, Yki-Jarvinen H, Boren J: Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia 49: 755–765, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Zoeller RA, Grazia TJ, LaCamera P, Park J, Gaposchkin DP, Farber HW: Increasing plasmalogen levels protects human endothelial cells during hypoxia. Am J Physiol Heart Circ Physiol 283: H671–H679, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA: Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167–179, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Lehner R, Kuksis A: Biosynthesis of triacylglycerols. Prog Lipid Res 35: 169–201, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Shmueli E, Alberti KG, Record CO: Diacylglycerol/protein kinase C signalling: a mechanism for insulin resistance? J Intern Med 234: 397–400, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O'Brien WR, Littman DR, Shulman GI: PKC-theta knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114: 823–827, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ: A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 46: 1081–1090, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Parks EJ, Hellerstein MK: Thematic review series: patient-oriented research. Recent advances in liver triacylglycerol and fatty acid metabolism using stable isotope labeling techniques. J Lipid Res 47: 1651–1660, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ: Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115: 1343–1351, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korenblat KM, Fabbrini E, Mohammed BS, Klein S: Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 134: 1369–1375, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotronen A, Vehkavaara S, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H: Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab 293: E1709–E1715, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Diraison F, Moulin P, Beylot M: Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab 29: 478–485, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Timlin MT, Parks EJ: Temporal pattern of de novo lipogenesis in the postprandial state in healthy men. Am J Clin Nutr 81: 35–42, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Aarsland A, Wolfe RR: Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res 39: 1280–1286, 1998 [PubMed] [Google Scholar]
- 18.Korchak HM: Regulation of hepatic lipogenesis. Tufts Folia Med 8: 134–143, 1962 [PubMed] [Google Scholar]
- 19.Ntambi JM, Miyazaki M: Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res 43: 91–104, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD: Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A 99: 11482–11486, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyazaki M, Dobrzyn A, Sampath H, Lee SH, Man WC, Chu K, Peters JM, Gonzalez FJ, Ntambi JM: Reduced adiposity and liver steatosis by stearoyl-CoA desaturase deficiency are independent of peroxisome proliferator-activated receptor-alpha. J Biol Chem 279: 35017–35024, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM: Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 297: 240–243, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR: Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467–2474, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Katajamaa M, Miettinen J, Oresic M: MZmine: Toolbox for processing and visualization of mass spectrometry based molecular profile data. Bioinformatics 22: 634–636, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Yetukuri L, Katajamaa M, Medina-Gomez G, Seppanen-Laakso T, Vidal-Puig A, Oresic M: Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst Biol 1: 12, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laaksonen R, Katajamaa M, Paiva H, Sysi-Aho M, Saarinen L, Junni P, Lutjohann D, Smet J, Van Coster R, Seppanen-Laakso T, Lehtimaki T, Soini J, Oresic M: A systems biology strategy reveals biological pathways and plasma biomarker candidates for potentially toxic statin-induced changes in muscle. PLoS ONE 1: e97, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seppanen-Laakso T, Laakso I, Hiltunen R: Analysis of fatty acids by gas chromatography, and its relevance to research on health and nutrition. Anal Chim Acta 465: 39–62, 2002 [Google Scholar]
- 28.Kadish AH, Little RL, Sternberg JC: A new and rapid method for the determination of glucose by measurement of rate of oxygen consumption. Clin Chem 14: 116–131, 1968 [Google Scholar]
- 29.Kuzuya H, Blix PM, Horwitz DL, Steiner DF, Rubenstein AH: Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes 26: 22–29, 1977 [DOI] [PubMed] [Google Scholar]
- 30.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 31.Nakamura MT, Nara TY: Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr 24: 345–376, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J: Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest 97: 2081–2091, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reunanen A, Miettinen TA, Nikkila EA: Quantitative lipid analysis of human liver needle biopsy specimens. Acta Med Scand 186: 149–150, 1969 [DOI] [PubMed] [Google Scholar]
- 34.Laurell S, Lundquist A: Lipid composition of human liver biopsy specimens. Acta Med Scand 189: 65–68, 1971 [DOI] [PubMed] [Google Scholar]
- 35.Schmitz-Peiffer C, Browne CL, Oakes ND, Watkinson A, Chisholm DJ, Kraegen EW, Biden TJ: Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat–fed rat. Diabetes 46: 169–178, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Considine RV, Nyce MR, Allen LE, Morales LM, Triester S, Serrano J, Colberg J, Lanza-Jacoby S, Caro JF: Protein kinase C is increased in the liver of humans and rats with non-insulin-dependent diabetes mellitus: an alteration not due to hyperglycemia. J Clin Invest 95: 2938–2944, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morino K, Petersen KF, Shulman GI: Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55 (Suppl. 2): S9–S15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RVS, Hevener AL, Farese RV Jr: Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6: 69–78, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, Zhang D, Wang A, Zhang XM, Kahn M, Cline GW, Pandey SK, Geisler JG, Bhanot S, Monia BP, Shulman GI: Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 282: 22678–22688, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Jarvinen H: Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 56: 1960–1968, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Pinnamaneni SK, Southgate RJ, Febbraio MA, Watt MJ: Stearoyl CoA desaturase 1 is elevated in obesity but protects against fatty acid-induced skeletal muscle insulin resistance in vitro. Diabetologia 49: 3027–3037, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM: The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem 275: 30132–30138, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Miyazaki M, Kim YC, Ntambi JM: A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res 42: 1018–1024, 2001 [PubMed] [Google Scholar]
- 44.Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND, Friedman JM: Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest 113: 414–424, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hulver MW, Berggren JR, Carper MJ, Miyazaki M, Ntambi JM, Hoffman EP, Thyfault JP, Stevens R, Dohm GL, Houmard JA, Muoio DM: Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab 2: 251–261, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA: Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr 87: 817–823, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Nakamura MT, Cheon Y, Li Y, Nara TY: Mechanisms of regulation of gene expression by fatty acids. Lipids 39: 1077–1083, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Luong A, Hannah VC, Brown MS, Goldstein JL: Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem 275: 26458–26466, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Magana MM, Lin SS, Dooley KA, Osborne TF: Sterol regulation of acetyl coenzyme A carboxylase promoter requires two interdependent binding sites for sterol regulatory element binding proteins. J Lipid Res 38: 1630–1638, 1997 [PubMed] [Google Scholar]
- 50.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM: Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem 279: 25164–25171, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Hannah VC, Ou J, Luong A, Goldstein JL, Brown MS: Unsaturated fatty acids down-regulate srebp isoforms 1a and 1c by two mechanisms in HEK-293 cells. J Biol Chem 276: 4365–4372, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Xu J, Nakamura MT, Cho HP, Clarke SD: Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids: a mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J Biol Chem 274: 23577–23583, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Ntambi JM, Sessler AM, Takova T: A model cell line to study regulation of stearoyl-CoA desaturase gene 1 expression by insulin and polyunsaturated fatty acids. Biochem Biophys Res Commun 220: 990–995, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Cortez-Pinto H, Jesus L, Barros H, Lopes C, Moura MC, Camilo ME: How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin Nutr 25: 816–823, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Faga E, Silli B, Pagano G: Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 37: 909–916, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, Oren R: Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 47: 711–717, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, Svegliati-Baroni G, Sofi F, Milani S, Abbate R, Surrenti C, Casini A: Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther 23: 1143–1151, 2006 [DOI] [PubMed] [Google Scholar]