With the advent of more intensive glucose management, hypoglycemia has emerged as a primary limitation in the treatment of insulin-dependent diabetes. It is now recognized that the increased incidence of hypoglycemia derives not only from imperfect insulin replacement but also from impaired counterregulation and hypoglycemic unawareness (1). The latter two observations have led to a renewed interest in the mechanisms underlying hypoglycemic detection. As a result of intensive research over the past decade, the traditional hypothalamocentric model of glucose sensing has been replaced with one emphasizing a widespread neural network involving numerous aspects of the central nervous system, as well as peripheral sensory input. Thus, in addition to the ventromedial hypothalamus, the paraventricular hypothalamus, arcuate nucleus, area postrema, nucleus of the solitary tract, and dorsal motor nucleus all appear to play important roles (2,3). In the periphery, important glucose sensors have been identified in the carotid bodies (4), gastrointestinal tract (5), and portal-mesenteric vein (6). For hypoglycemic detection, the glucose sensors of the portal-mesenteric vein have garnered the most attention. Animal studies have repeatedly demonstrated that blocking portal glucose sensing via portal glucose infusion (7) or denervating the portal vein (8) substantially suppresses the sympathoadrenal response to hypoglycemia. More recently, it was shown that portal-mesenteric vein glucose sensing is particularly important when hypoglycemia develops slowly and, under these conditions, modulates over 90% of the sympathoadrenal response to hypoglycemia (9).
While portal vein glucose sensing appears to be conserved across several species (7,9,10), demonstration of consistent findings in humans has proven elusive. An obvious limitation for human studies is the lack of direct access to the portal vein, which severely constrains experimental interventions. To circumvent this problem, Rossetti et al. (11) employed an oral glucose load to elevate portal glucose concentration during a hyperinsulinemic-hypoglycemic clamp. Oral glucose was administered before the clamp to establish a portal-arterial gradient before the onset of hypoglycemia. Hypoglycemia was then allowed to develop slowly—an important aspect of this study considering the previously mentioned animal experiments and the clinical relevance. Despite these efforts, they observed no effect of the oral glucose load on counterregulatory or symptomatic responses to hypoglycemia. The authors conclude that the portal glucose sensor plays no significant role in hypoglycemic detection for humans. This is not the first time such an approach has been employed in an attempt to elucidate the potential role of portal glucose sensing in humans (12–14). While all previous reports demonstrated a significant impact of an oral glucose load on the hormonal responses to hypoglycemia, results have been anything but consistent.
In addition to the negative findings for Rossetti et al., oral glucose during a hyperinsulinimic-hypoglycemic clamp has been shown to suppress (14), augment (13), and initially suppress and then augment (12) the sympathoadrenal response to hypoglycemia in humans. As noted by Rossetti et al. (11), subtle differences in the respective protocols (e.g., rate of fall in glycemia, the timing and/or mass of the oral glucose load) may explain some of the observed differences. However, critical to the interpretation of these findings is the assumption that the oral glucose load actually elevates the portal vein glucose concentration above the glycemic threshold for the duration of the experiment. Because portal glucose concentration cannot be measured directly in humans, it must be based on estimated rates of glucose appearance and portal blood flow. A number of sophisticated modeling approaches employing multiple tracers have been developed for estimating the appearance of an oral glucose load (15,16) but, to date, have not been used in studies of portal glucose sensing in humans. Further confounding estimates of portal glucose concentration is the wide range of values reported for human portal blood flow, (10–18 ml · kg−1 · min−1 [17]), which may increase substantially in response to oral glucose ingestion.
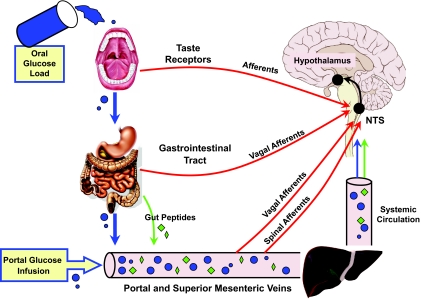
Alternatively, the disparate findings for these human studies may result from the complexity of introducing an oral glucose load, as opposed to simply infusing glucose in the portal vein (Fig. 1). As noted in one recent review (3), glucose sensing of an oral glucose load begins in the oral cavity and continues in the gut, the portal-mesenteric vein, and, finally, the systemic circulation. In particular, the gastrointestinal tract is now recognized as an important locus for glucose detection. The ability to sense glucose in the luminal contents of the gut not only allows for intrinsic control but also provides important sensory feedback to the central nervous system via extrinsic afferent nerves and blood-borne peptides (5). Many of the peptides secreted by the enteroendocrine cells of the gut (e.g., glucagon-like peptide 1 [GLP-1], glucose-dependent insulinotropic peptide, and peptide YY) are now well recognized for their impact on glucose and energy homeostasis. While considerable insight has been gained regarding their role in hyperglycemic and euglycemic states, their impact under hypoglycemic conditions is poorly understood and not always obvious. For example, the ability of GLP-1 to suppress glucagon secretion is apparently lost under hypoglycemic conditions (18). Also, the vagal glucose-sensitive afferents of the portal vein, which are inhibited by glucose, are activated by GLP-1 (19), a peptide released in response to oral glucose (5). While vagal afferents are apparently not involved in hypoglycemic detection at the portal vein (20), if the spinal glucose-sensitive afferents (8) demonstrate similar reciprocal responses to glucose and GLP-1, this might explain some of the observed disparity in these human studies. It is also important to recognize that all peripheral glucose sensory input, i.e., gut, portal-mesenteric, and gustatory, converges on the nucleus of the solitary tract, where local glycemic conditions are likely to impact on the eventual efferent response (3).
FIG. 1.
Glucose sensory input: For an oral glucose load, afferent inputs include the oral cavity, gastrointestinal tract, and portal-superior mesenteric veins (vagal and spinal), all of which converge on the nucleus of the solitary tract (NTS). In addition, gut peptides released by an oral glucose load can activate sensory neurons in the gastrointestinal tract and portal vein, as well as activate the central nervous system directly. For portal vein glucose infusion during a hyperinsulinimic-hypoglycemic clamp, input is restricted to glucose sensing afferents in the portal-mesenteric vein.
Given the marked disparity in findings for humans, it is perhaps premature to conclude that hypoglycemic detection at the portal vein is not important for humans as proposed by Rossetti et al. (11). Beyond the substantial technical obstacles faced by such studies, there is the fundamental question of whether glucose introduced to the portal circulation via the gut is equivalent to a direct glucose infusion. As our understanding of the neural network underlying glucose sensing improves, it is likely that at least some of the apparent differences in human and animal hypoglycemic detection will be resolved.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
See accompanying original article, p. 194.
REFERENCES
- 1.Cryer PE: Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 54: 3592–3601, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Levin BE, Routh VH, Kang L, Sanders NM, Dunn Meynell AA: Neuronal glucosensing: what do we know after 50 years? Diabetes 53: 2521–2528, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Marty N, Dallaporta M, Thorens B: Brain glucose sensing, counterregulation, and energy homeostasis. Physiology 22: 241–251, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Koyama Y, Coker RH, Stone EE, Lacy B, Jabbour K, Williams PE, Wasserman DH: Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49: 1434–1442, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Berthoud H-R: Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20: 64–72, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donovan CM: Portal vein glucose sensing. Diabetes Nutr Metab 15: 308–312, 2002 [PubMed] [Google Scholar]
- 7.Donovan C, Hamilton-Wessler M, Halter J, Bergman R: Primacy of liver glucosensors in the sympathetic response to progressive hypoglycemia. Proc Natl Acad Sci 91: 2863–2867, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita S, Bohland MA, Sanchez-Watts G, Watts AG, Donovan CM: Hypoglycemic detection at the portal vein is mediated by capsaicin-sensitive primary sensory neurons. Am J Physiol 293: E96–E101, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Saberi M, Bohland MA, Donovan CM: The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of the portal-superior mesenteric vein in glucose sensing. Diabetes 57: 1380–1386, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Burcelin R, Dolci W, Thorens B: Glucose sensing by the hepatoportal sensor is GLUT2-dependent. Diabetes 49: 1643–1648, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Rossetti P, Porcellati F, Lucidi P, Busciantella Ricci N, Candeloro P, Cioli P, Santeusanio F, Bolli GB, Fannelli CG: Portal vein glucose sensors do not play a major role in modulating physiological responses to insulin-induced hypoglycemia in humans. Diabetes 58: 194–202, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ertl AC, Mann S, Richardson A, Briscoe VJ, Tate HB, Davis SN: Effects of oral carbohydrate on autonomic nervous system counterregulatory responses during hyperinsulinemic hypoglycemica and euglycemia. Am J Physiol 295: E618–E625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heptulla RA, Tamborlane WV, Ma TY-Z, Rife F, Sherwin RS: Oral glucose augments the counterregulatory hormone response during insulin-induced hypoglycemia in humans. J Clin Endocrinol Metabol 86: 645–648, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Smith D, Pernet A, Reid H, Bingham E, Rosenthal J, Macdonald I, Umpleby A, Amiel SA: The role of hepatic portal glucose sensing in modulating responses to hypoglycemia in man. Diabetologia 45: 1416–1424, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Basu R, Di Camillo B, Toffolo G, Basu A, Shah P, Vella A, Rizza R, Cobelli C: Use of a novel triple-tracer approach to assess postprandial glucose metabolism. Am J Physiol 284: E55–E69, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Livesey G, Wilson PDG, Dainty JR, Brown JC, Faulks RM, Roe MA, Newman TA, Eagles J, Mellon FA, Greenwood RH: Simultaneous time-varying systemic appearance of oral and hepatic glucose in adults monitored with stable isotopes. Am J Physiol 275: E717–E728, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Tamada T, Moriyasu F, Ono S, Shimizu K, Kajimura K, Soh Y, Kawasaki T, Kimura T, Yamashita Y, Someda H: Portal blood flow: measurement with MR imaging. Radiology 173: 639–644, 1989 [DOI] [PubMed] [Google Scholar]
- 18.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hufner M, Schmiegel WH: Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 87: 1239–1246, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A: Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol 271: E803–E813, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Jackson P, Pagliassotti M, Shiota M, Neal D, Cardin S, Cherrington A: Effects of vagal blockade on the counterregulatory response to insulin-induced hypoglycemia in the dog. Am J Physiol 273: E1178–E1188, 1997 [DOI] [PubMed] [Google Scholar]