Abstract
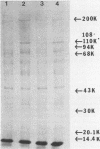
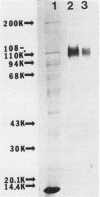
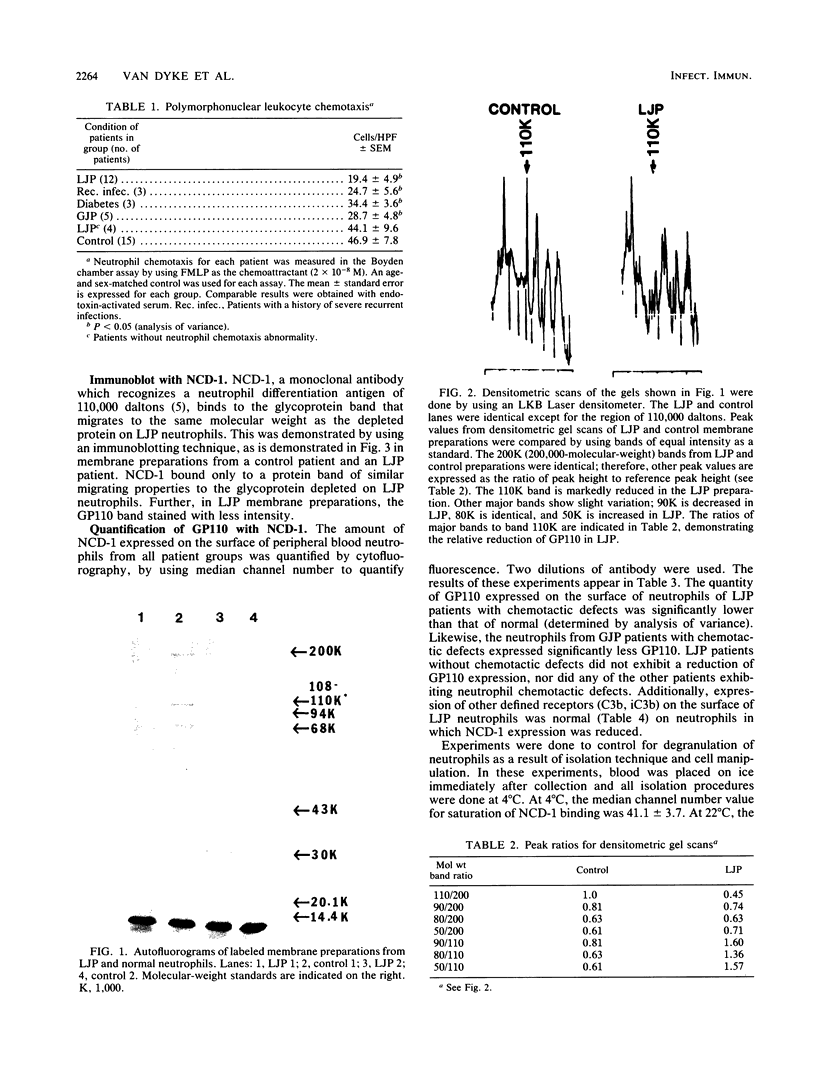
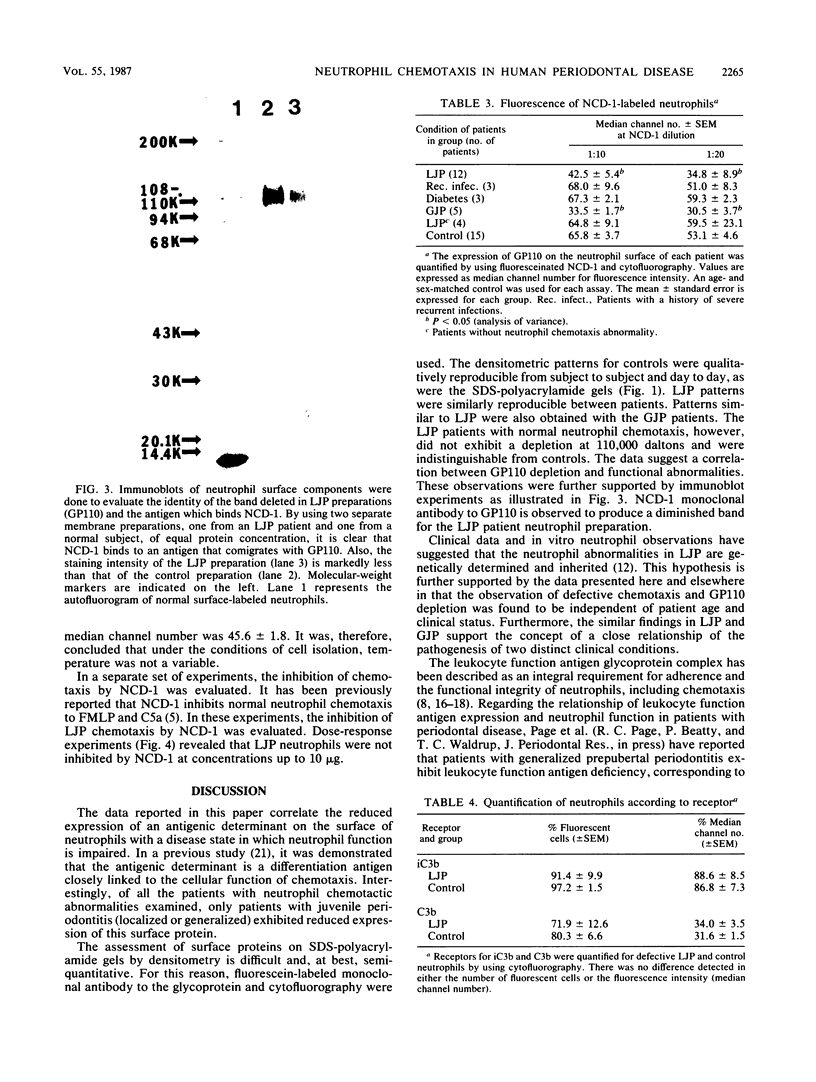
Localized juvenile periodontitis (LJP) is characterized by severe, early-onset, molar and incisor bone loss; neutrophil chemotaxis disorders; and a high prevalence of Actinobacillus actinomycetemcomitans infection. LJP is further characterized by significant familial aggregation of the disease. Recent work in our laboratory has demonstrated the selective depletion of a surface glycoprotein of 110,000 Mr (GP110) from LJP neutrophils by using surface labeling with [14C]formaldehyde and autofluorography. The function of GP110 is unknown; however, it does not appear to be a chemotactic factor receptor. Rather, it is bound by a monoclonal antibody (NCD-1) that recognizes a neutrophil differentiation antigen and which itself alters neutrophil chemotactic and secreting functions. To quantify GP110 on LJP and normal neutrophils, fluorescein-labeled NCD-1 was bound to neutrophils and the amount of fluorescence was evaluated by using cytofluorography. Our results indicate that there is a quantifiable reduction (40%) of GP110 on the surface of LJP and GJP neutrophils, compared with controls. Other patients with neutrophil defects express normal quantities of GP110, suggesting disease specificity. Our data suggest that GP110 may be a useful disease marker for LJP and may provide a useful probe for the study of neutrophil chemotactic function and dysfunction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein P. C., Stossel T. P. Prevention of degradation of human polymorphonuclear leukocyte proteins by diisopropylfluorophosphate. Blood. 1980 Sep;56(3):442–447. [PubMed] [Google Scholar]
- Baer P. N. The case for periodontosis as a clinical entity. J Periodontol. 1971 Aug;42(8):516–520. doi: 10.1902/jop.1971.42.8.516. [DOI] [PubMed] [Google Scholar]
- Cainciola L. J., Genco R. J., Patters M. R., McKenna J., van Oss C. J. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977 Feb 3;265(5593):445–447. doi: 10.1038/265445a0. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Page R. C., Wilde G. Defective neutrophil chemotaxis in juvenile periodontitis. Infect Immun. 1977 Dec;18(3):694–700. doi: 10.1128/iai.18.3.694-700.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter T. G., Henson P. M. Purification and characterization of an antigen involved in neutrophil chemotaxis and degranulation using a monoclonal antibody. Eur J Immunol. 1984 Jul;14(7):605–609. doi: 10.1002/eji.1830140705. [DOI] [PubMed] [Google Scholar]
- Cotter T. G., Spears P., Henson P. M. A monoclonal antibody inhibiting human neutrophil chemotaxis and degranulation. J Immunol. 1981 Oct;127(4):1355–1360. [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Fischer A., Seger R., Durandy A., Grospierre B., Virelizier J. L., Le Deist F., Griscelli C., Fischer E., Kazatchkine M., Bohler M. C. Deficiency of the adhesive protein complex lymphocyte function antigen 1, complement receptor type 3, glycoprotein p150,95 in a girl with recurrent bacterial infections. Effects on phagocytic cells and lymphocyte functions. J Clin Invest. 1985 Dec;76(6):2385–2392. doi: 10.1172/JCI112251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. P., Seligmann B. E. Monitoring human neutrophil granule secretion by flow cytometry: secretion and membrane potential changes assessed by light scatter and a fluorescent probe of membrane potential. J Leukoc Biol. 1985 Apr;37(4):431–447. doi: 10.1002/jlb.37.4.431. [DOI] [PubMed] [Google Scholar]
- Gallin J. I. Abnormal phagocyte chemotaxis: pathophysiology, clinical manifestations, and management of patients. Rev Infect Dis. 1981 Nov-Dec;3(6):1196–1220. doi: 10.1093/clinids/3.6.1196. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wolff S. M. Leucocyte chemotaxis: physiological considerations and abnormalities. Clin Haematol. 1975 Oct;4(3):567–607. [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavine W. S., Maderazo E. G., Stolman J., Ward P. A., Cogen R. B., Greenblatt I., Robertson P. B. Impaired neutrophil chemotaxis in patients with juvenile and rapidly progressing periodontitis. J Periodontal Res. 1979 Jan;14(1):10–19. doi: 10.1111/j.1600-0765.1979.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Miedema F., Tetteroo P. A., Terpstra F. G., Keizer G., Roos M., Weening R. S., Weemaes C. M., Roos D., Melief C. J. Immunologic studies with LFA-1- and Mo1-deficient lymphocytes from a patient with recurrent bacterial infections. J Immunol. 1985 May;134(5):3075–3081. [PubMed] [Google Scholar]
- Ross G. D. Clinical and laboratory features of patients with an inherited deficiency of neutrophil membrane complement receptor type 3 (CR3) and the related membrane antigens LFA-1 and p150,95. J Clin Immunol. 1986 Mar;6(2):107–113. doi: 10.1007/BF00918742. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Anderson D. C. The importance of the Mac-1, LFA-1 glycoprotein family in monocyte and granulocyte adherence, chemotaxis, and migration into inflammatory sites: insights from an experiment of nature. Ciba Found Symp. 1986;118:102–126. doi: 10.1002/9780470720998.ch8. [DOI] [PubMed] [Google Scholar]
- Suzuki J. B., Collison B. C., Falkler W. A., Jr, Nauman R. K. Immunologic profile of juvenile periodontitis. II. Neutrophil chemotaxis, phagocytosis and spore germination. J Periodontol. 1984 Aug;55(8):461–467. doi: 10.1902/jop.1984.55.8.461. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Peralta J. M., Simons A. R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Horoszewicz H. U., Cianciola L. J., Genco R. J. Neutrophil chemotaxis dysfunction in human periodontitis. Infect Immun. 1980 Jan;27(1):124–132. doi: 10.1128/iai.27.1.124-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. E., Levine M. J., Tabak L. A., Genco R. J. Juvenile periodontitis as a model for neutrophil function: reduced binding of the complement chemotactic fragment, C5a. J Dent Res. 1983 Aug;62(8):870–872. doi: 10.1177/00220345830620080301. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Levine M. J., Tabak L. A., Genco R. J. Reduced chemotactic peptide binding in juvenile periodontitis: a model for neutrophil function. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1278–1284. doi: 10.1016/0006-291x(81)91962-8. [DOI] [PubMed] [Google Scholar]
- Van Dyke T. E., Reilly A. A., Horoszewicz H., Gagliardi N., Genco R. J. A rapid, semi-automated procedure for the evaluation of leukocyte locomotion in the micropore filter assay. J Immunol Methods. 1979;31(3-4):271–282. doi: 10.1016/0022-1759(79)90140-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wright J. T., Elmer W. A., Dunlop A. T. A simple method for plasma membrane isolation from embryonic tissue using positively charged microcarriers. Anal Biochem. 1982 Sep 1;125(1):100–104. doi: 10.1016/0003-2697(82)90388-8. [DOI] [PubMed] [Google Scholar]