Abstract
OBJECTIVE—This study investigated the acute effects of treatment with vildagliptin on dipeptidyl peptidase-4 (DPP-4) activity, glucagon-like peptide 1 (GLP-1) concentration, pancreatic hormone levels, and glucose metabolism. The primary aims were to determine the effects of DPP-4 inhibition on GLP-1 clearance and on hepatic glucose uptake.
RESEARCH DESIGN AND METHODS—Fasted conscious dogs were studied in the presence (n = 6) or absence (control, n = 6) of oral vildagliptin (1 mg/kg). In both groups, GLP-1 was infused into the portal vein (1 pmol · kg−1 · min−1) for 240 min. During the same time, glucose was delivered into the portal vein at 4 mg · kg−1 · min−1 and into a peripheral vein at a variable rate to maintain the arterial plasma glucose level at 160 mg/dl.
RESULTS—Vildagliptin fully inhibited DPP-4 over the 4-h experimental period. GLP-1 concentrations were increased in the vildagliptin-treated group (50 ± 3 vs. 85 ± 7 pmol/l in the portal vein in control and vildagliptin-treated dogs, respectively; P < 0.05) as a result of a 40% decrease in GLP-1 clearance (38 ± 5 and 22 ± 2 ml · kg−1 · min−1, respectively; P < 0.05). Although hepatic insulin and glucagon levels were not significantly altered, there was a tendency for plasma insulin to be greater (hepatic levels were 73 ± 10 vs. 88 ± 15 μU/ml, respectively). During vildagliptin treatment, net hepatic glucose uptake was threefold greater than in the control group. This effect was greater than that predicted by the change in insulin.
CONCLUSIONS—Vildagliptin fully inhibited DPP-4 activity, reduced GLP-1 clearance by 40%, and increased hepatic glucose disposal by means beyond the effects of GLP-1 on insulin and glucagon secretion.
Glucagon-like peptide 1 (GLP-1) is a gut-derived hormone shown to enhance glucose-dependent insulin secretion, suppress inappropriately high glucagon secretion, slow gastric emptying, and reduce food intake (1). In some type 2 diabetic patients, GLP-1 levels are reduced, and elevation of GLP-1 by continuous infusion of the peptide leads to reductions in fasting glucose, postprandial glucose excursions, and A1C (2). The therapeutic potential of GLP-1 is limited, however, because it is rapidly inactivated by dipeptidyl peptidase-4 (DPP-4) (3,4).
Vildagliptin is an orally effective selective DPP-4 inhibitor. In diabetic patients, vildagliptin improved glycemic control, increased the plasma insulin–to–glucagon molar ratio, and reduced A1C levels (5,6). During a meal tolerance test, it augmented insulin secretion and decreased glucagon release, resulting in enhanced suppression of endogenous glucose production compared with placebo (7).
Ingested glucose and endogenously secreted GLP-1 are released from the gut into the hepatic portal vein, which then perfuses the liver. Typically, studies have investigated the effects of DPP-4 inhibition after a meal, when GLP-1 secretion is increased. In the present study, GLP-1 and glucose were infused directly into the hepatic portal vein in the presence or absence of DPP-4 inhibition. The first aim was to examine the effect of vildagliptin on GLP-1 clearance under these carefully controlled conditions. In addition, although GLP-1 can increase glucose disposal by stimulation of insulin secretion, the hormone has been suggested to affect glucose metabolism by actions over and above its effects on the pancreas. Therefore, the second aim of this study was to investigate the effect of DPP-4 inhibition on glucose disposal, in particular by the liver.
RESEARCH DESIGN AND METHODS
Experiments were conducted on 12 healthy, conscious, 18-h–fasted dogs (20–27 kg). Before the study, they were fed a standard chow diet once a day, and water was provided ad libitum. The surgical facility met the standards published by the American Association for the Accreditation of Laboratory Animal Care, and the protocols were approved by the Lovelace Respiratory Research Institute Institutional Animal Care and Use Committee before the start of the study. All dogs underwent a laparotomy 3 weeks before the experiment to implant infusion catheters into the jejunal and splenic veins. Sampling catheters were implanted into the hepatic portal vein, the left hepatic vein, and the left femoral artery. Ultrasonic flow probes (Transonic Systems, Ithaca, NY) were placed around the hepatic and right iliac arteries and the portal vein, as described previously (8). Intraportal catheters (splenic and jejunal) were used for the infusion of glucose (Baxter Healthcare, Deerfield, IL) and GLP-1 (Bachem, King of Prussia, PA). Each animal was used only once.
On the day of the study, intravenous catheters were placed into a leg vein for glucose delivery. Each experiment consisted of a basal period (−40 to 0 min) and an experimental period (0–240 min). Vildagliptin (1 mg/kg) or vehicle (sterile water; control) was administered via stomach gavage at −20 min. Vildagliptin was well tolerated by all animals. At 0 min, constant portal infusions of glucose (4 mg · kg−1 · min−1) and GLP-1 (1 pmol · kg−1 · min−1) were started, and glucose was infused into a peripheral vein to maintain arterial plasma glucose at 160 mg/dl.
Blood sampling and analytical procedures.
Blood samples were collected from the femoral artery and the hepatic portal and hepatic veins. Hematocrit; plasma glucose, glucagon, insulin, cortisol, and GLP-1; and blood alanine, lactate, and glycerol concentrations were determined as previously described (8). Hepatic blood flow was measured using ultrasonic flow probes and a Transit-time Perivascular Flow Meter (model T403; Transonic Systems, Ithaca, NY) as described elsewhere (8). DPP-4 activity was measured in plasma samples using 7-amino-4-methylcoumarin (AMC; cat. no. Q-1025; Bachem) as standard and H-Gly-Pro-AMC.HBr (cat. no. I-1225; Bachem) as substrate. Five microliters of plasma was incubated with 15 μl 100 μmol/l substrate for 20 min, and absorption was measured at excitation/emission wavelength of 360 nm/460 nm with a spectrophotometer. The DPP-4 activity is expressed as mU/ml where mU = nmol/min.
Data analysis.
Net hepatic substrate balance (NHB) was calculated with the arterial-venous difference method as NHB = loadout − loadin, where loadout = H × HF and loadin = (A × AF) + (P × PF), in which H, A, and P are the substrate concentrations in the hepatic vein, femoral artery, and hepatic portal vein blood or plasma, respectively, and HF, AF, and PF are the blood or plasma flows in the hepatic vein, hepatic artery, and portal vein, respectively, as determined by the ultrasonic flow probes. Using this calculation, a positive value represents net output by the liver, and a negative value represents net hepatic uptake. For glucose balance calculations, glucose concentrations were converted from plasma to blood values using previously published correction factors (9). Blood glucose concentrations were used for the calculation of net glucose balance because the use of whole-blood glucose ensures accurate balance measurements regardless of the characteristics of glucose entry into the erythrocyte. Nonhepatic glucose uptake was calculated as the glucose infusion rate (GIR) plus net hepatic glucose balance, with changes in the glucose mass accounted for when deviations from steady-state were present (10–12). The approximate substrate levels in plasma entering the liver sinusoids were determined using the formula [A × (AF/HF) + P × (PF/HF)], where hormone concentrations and flow are abbreviated as previously described. GLP-1 clearance was determined by dividing the hormone infusion rate by its arterial concentration after the basal GLP-1 level was subtracted.
Statistical analysis.
Data are presented as means ± SEM. Between-group differences were analyzed with two-way ANOVA, and univariate F tests were used for post hoc comparisons (SigmaStat; SPSS). One-way ANOVA was used for comparisons of mean data and area under the curve. Statistical significance was accepted at P < 0.05.
RESULTS
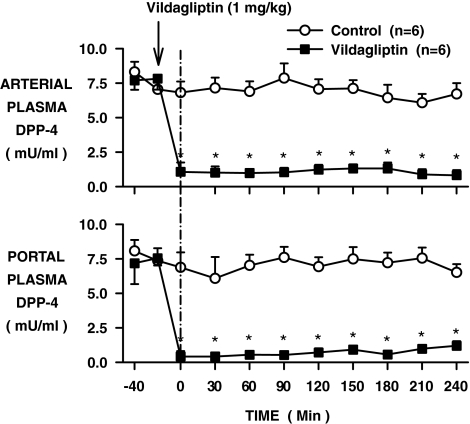
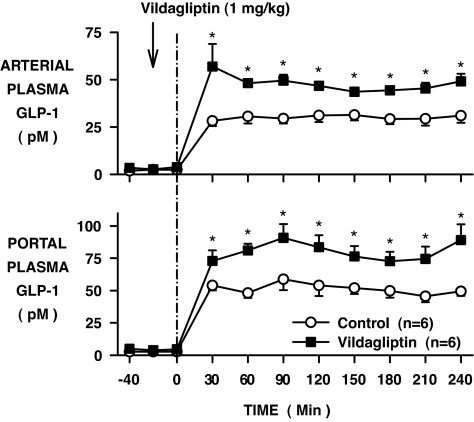
After oral administration of vildagliptin, the arterial and portal vein plasma DPP-4 activities remained fully suppressed over the 4-h experimental period (Fig. 1; P < 0.05). As a result of portal vein GLP-1 infusion, the plasma GLP-1 levels in the control group increased from 2 ± 1 to 30 ± 3 pmol/l in the artery and 3 ± 1 to 50 ± 3 pmol/l in the portal vein (basal period to average of the last 3 h of the experimental period; Fig. 2). The rise in GLP-1 was greater in the vildagliptin-treated group, increasing in the artery and portal vein, respectively, from 3 ± 1 to 51 ± 5 and 4 ± 2 to 85 ± 7 pmol/l (P < 0.05). Whole-body GLP-1 clearance rates were 38 ± 5 and 22 ± 2 ml · kg−1 · min−1 during the last 3 h in the control and vildagliptin-treated groups, respectively (P < 0.05).
FIG. 1.
Arterial and portal plasma DPP-4 activity in conscious dogs during the basal (−40 to 0 min) and experimental (0–240 min) periods treated with vehicle (○) or vildagliptin (▪) (means ± SE; n = 6 per group; *P < 0.05).
FIG. 2.
Arterial and portal plasma GLP-1 levels in conscious dogs during the basal (−40 to 0 min) and experimental (0–240 min) periods treated with vehicle (○) or vildagliptin (▪) (means ± SE; n = 6 per group; *P < 0.05).
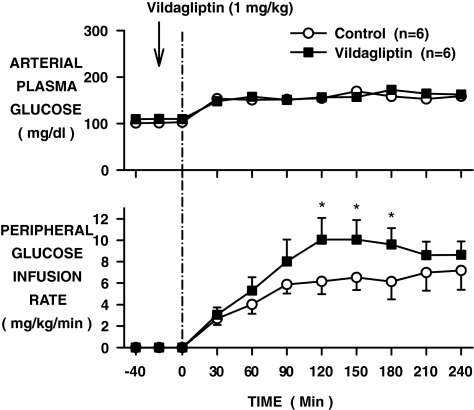
Glucose was infused to increase the fasting arterial plasma glucose level from ∼105 to ∼160 mg/dl in both groups (Fig. 3). The intraportal GIR was 4 mg · kg−1 · min−1 in both groups. To maintain the clamp, additional glucose had to be infused into a peripheral vein in both the control (6.1 ± 1.2 mg · kg−1 · min−1) and vildagliptin-treated (8.6 ± 1.5 mg · kg−1 · min−1; P < 0.05) groups (Fig. 3).
FIG. 3.
Arterial plasma glucose level and peripheral GIR in conscious dogs during the basal (−40 to 0 min) and experimental (0–240 min) periods treated with vehicle (○) or vildagliptin (▪) (means ± SE; n = 6 per group; *P < 0.05). Glucose was infused into the portal vein at 4 mg · kg−1 · min−1 in both groups during the experimental period.
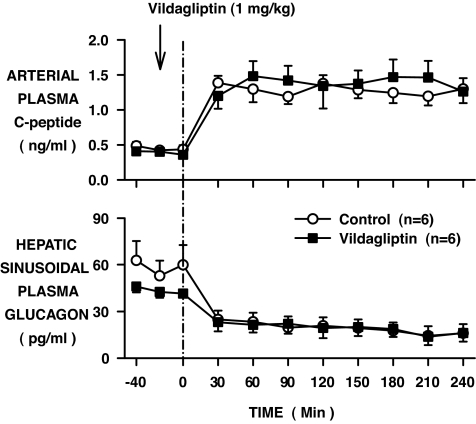
In response to the rise in the plasma glucose level, in the control group, the plasma insulin increased from 7 ± 1 to 26 ± 4 μU/ml in the artery and from 17 ± 3 to 73 ± 10 μU/ml in the hepatic sinusoids (increases of 4.1- and 4.3-fold, respectively, from the basal period to the last 3 h; Fig. 4). The rise in insulin in the vildagliptin-treated group tended to be larger (6 ± 1 to 36 ± 8 and 17 ± 3 to 88 ± 15 μU/ml; 5.5- and 5.4-fold), although they were not significantly different from the changes in the control group. The elevations in the arterial plasma C-peptide levels were not significantly different between groups, increasing in the control group from 0.45 ± 0.04 to 1.27 ± 0.09 ng/ml (2.9-fold) and in the vildagliptin-treated group from 0.39 ± 0.04 to 1.40 ± 0.20 ng/ml (3.6-fold) during the two periods, respectively (Fig. 5). Hepatic sinusoidal plasma glucagon levels decreased to similar values in the two groups; from 59 ± 11 to 23 ± 4 and 43 ± 3 to 23 ± 4 pg/ml in the control and vildagliptin-treated groups, respectively (Fig. 5). The hepatic plasma insulin–to–glucagon molar ratios were not significantly different (72 ± 10 and 86 ± 14 in the control and vildagliptin-treated groups, respectively) during the last 3 h of the study.
FIG. 4.
Arterial and hepatic sinusoidal plasma insulin levels in conscious dogs during the basal (−40 to 0 min) and experimental (0–240 min) periods treated with vehicle (○) or vildagliptin (▪) (means ± SE; n = 6 per group).
FIG. 5.
Arterial plasma C-peptide and hepatic sinusoidal plasma glucagon levels in conscious dogs during the basal (−40 to 0 min) and experimental (0–240 min) periods treated with vehicle (○) or vildagliptin (▪) (means ± SE; n = 6 per group).
After a meal, glucose uptake by the liver is stimulated by increased glucose and insulin levels in the blood. Therefore, in response to the increases in hepatic glucose load, portal vein insulin–to–glucagon molar ratio, and plasma GLP-1 level, there was a switch in net hepatic glucose balance in the control group from net output (2.4 ± 0.1 mg · kg−1 · min−1 during the basal period) to net uptake (−0.7 ± 0.1 mg · kg−1 · min−1 during the last 3 h of the experimental period; Fig. 6). In the vildagliptin-treated group, there was a greater response to these stimuli such that the liver switched from net hepatic glucose output of 2.1 ± 0.2 to net hepatic glucose uptake of −2.1 ± 0.5 mg · kg−1 · min−1 (P < 0.05). Nonhepatic glucose uptake (non-HGU; mg · kg−1 · min−1) increased from basal by 6.8 ± 1.2 in the control group (2.3 ± 0.2 during the basal period to 9.1 ± 1.2 during the last 3 h, respectively) and by 8.1 ± 1.4 in the vildagliptin-treated group (from 2.3 ± 0.3 to 10.4 ± 1.4, respectively; Fig. 6). Although the nonhepatic response was not significantly different between the two groups, the increases in the rate of glucose uptake by the liver and nonhepatic tissues were greater with vildagliptin treatment by about the same magnitude (∼1.2 mg · kg−1 · min−1). After vildagliptin treatment, net hepatic glucose fractional extraction was threefold greater (0.02 ± 0.01 vs. 0.06 ± 0.01 in the control and vildagliptin-treated groups, respectively; Fig. 7; P < 0.05), as was the ratio of net hepatic glucose uptake to the hepatic insulin level (0.01 ± 0.00 vs. 0.03 ± 0.01 in the two groups, respectively; Fig. 7; P < 0.05) during the last 3 h of the study. The ratios of non-HGU to arterial insulin, on the other hand, were similar in the two groups (0.38 ± 0.04 vs. 0.35 ± 0.05, respectively; Fig. 7).
FIG. 6.
Net hepatic glucose balance and nonhepatic glucose uptake in conscious dogs during the basal (−40 to 0 min) and experimental (0–240 min) periods treated with vehicle (○) or vildagliptin (▪) (means ± SE; n = 6 per group).
FIG. 7.
Net hepatic glucose fractional extraction (A), net hepatic glucose uptake–to–hepatic sinusoidal plasma insulin ratio (B), and nonhepatic glucose uptake–to–arterial plasma insulin ratio (C) during the last 3 h of the experimental period (60–240 min) in conscious dogs treated with vehicle (□) or vildagliptin (▪) (means ± SE; n = 6 per group; P < 0.05).
No differences in plasma cortisol (data not shown) were observed between groups. Free fatty acid and glycerol levels were ∼25% lower in the vildagliptin-treated group during the basal period and tended to remain lower during the study, but the levels were not significantly different between groups at the end of the experiment (Table 1). There was no treatment effect on the arterial level or net hepatic balance of blood lactate or alanine (Table 1).
TABLE 1.
Plasma free fatty acid levels; arterial blood lactate, alanine, and glycerol levels; and net hepatic balance in 18-h–fasted conscious dogs during the basal (−40 to 0 min) and experimental (0–240 min) periods
| Basal period
|
Experimental period (min)
|
|||||
|---|---|---|---|---|---|---|
| −40 to 0 | 60 | 120 | 180 | 210 | 240 | |
| Plasma free fatty acid level (μmol/l) | ||||||
| Control | 924 ± 69 | 245 ± 54 | 117 ± 23 | 115 ± 27 | 147 ± 48 | 124 ± 39 |
| Vildagliptin | 736 ± 118* | 111 ± 23 | 88 ± 26 | 71 ± 25 | 82 ± 31 | 66 ± 15 |
| Blood glycerol level (μmol/l) | ||||||
| Control | 97 ± 6 | 42 ± 9 | 28 ± 4 | 31 ± 5 | 35 ± 6 | 32 ± 6 |
| Vildagliptin | 70 ± 9* | 21 ± 4* | 24 ± 4 | 20 ± 5 | 19 ± 4 | 16 ± 2 |
| Net hepatic glycerol uptake (μmol · kg−1 · min−1) | ||||||
| Control | 1.7 ± 0.1 | 0.8 ± 0.2 | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 |
| Vildagliptin | 1.6 ± 0.3 | 0.5 ± 0.2 | 0.5 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.2 | 0.3 ± 0.1 |
| Blood lactate level (μmol/l) | ||||||
| Control | 482 ± 81 | 534 ± 71 | 536 ± 89 | 545 ± 66 | 593 ± 72 | 564 ± 59 |
| Vildagliptin | 433 ± 87 | 571 ± 82 | 535 ± 83 | 555 ± 63 | 543 ± 49 | 551 ± 68 |
| Net hepatic lactate balance (μmol · kg−1 · min−1) | ||||||
| Control | −4.5 ± 0.7 | 2.4 ± 1.7 | 1.1 ± 0.8 | 0.3 ± 1.2 | 0.7 ± 0.9 | 1.5 ± 1.1 |
| Vildagliptin | −2.3 ± 2.7 | 5.4 ± 2.4 | 2.1 ± 1.6 | 3.2 ± 1.7 | 2.5 ± 1.7 | 3.2 ± 1.9 |
| Blood alanine level (μmol/l) | ||||||
| Control | 289 ± 24 | 261 ± 23 | 239 ± 16 | 230 ± 14 | 235 ± 16 | 225 ± 15 |
| Vildagliptin | 300 ± 33 | 275 ± 24 | 245 ± 13 | 217 ± 18 | 221 ± 21 | 219 ± 24 |
| Net hepatic alanine uptake (μmol · kg−1 · min−1) | ||||||
| Control | 2.2 ± 0.2 | 1.5 ± 0.2 | 1.7 ± 0.1 | 2.1 ± 0.2 | 2.0 ± 0.1 | 2.2 ± 0.3 |
| Vildagliptin | 2.3 ± 0.4 | 1.8 ± 0.2 | 2.0 ± 0.5 | 2.2 ± 0.2 | 2.4 ± 0.2 | 2.5 ± 0.3 |
Data are means ± SE; n = 6 per group;
P < 0.05.
DISCUSSION
In this study, vildagliptin fully inhibited DPP-4 over a 4-h experimental period. As a result, GLP-1 clearance was reduced by 40%, and the levels of the hormone in plasma were increased. Although hepatic insulin and glucagon levels were not significantly altered by treatment with the DPP-4 inhibitor, there was a tendency for plasma insulin to be greater. Hepatic glucose disposal was nevertheless increased by treatment, over and above effects attributable to a rise in insulin. No such effect was seen on nonhepatic glucose uptake.
GLP-1 levels in the circulation are regulated primarily by NH2-terminal cleavage at the position 2 alanine by DPP-4 and by renal elimination, but the kidneys appear to only account for 10–20% of the degradation of intact GLP-1 (13). To our knowledge, an effect of vildagliptin on renal GLP-1 clearance is not known. In this study, DPP-4 inhibition resulted in a 70% increase in arterial and portal vein GLP-1 concentrations, which resulted from a 40% decrease in GLP-1 whole-body clearance. It should be noted that GLP-1 clearance was determined by dividing the rate of intraportal GLP-1 infusion by the change in plasma GLP-1. Although it is possible that a decrease in endogenous GLP-1 secretion may have occurred, the basal GLP-1 levels were low (2–3 pmol/l); therefore, any decrease during the clamp period would have had only a small effect on the estimate of clearance. In addition, these results are in line with previous findings in type 2 diabetic patients in which 4 weeks of treatment with vildagliptin resulted in a twofold or greater increase in the rise in active GLP-1 after meals (6,14).
GLP-1 has previously been shown to increase (1) or to have no effect on plasma insulin levels (15,16), and vildagliptin has been demonstrated to increase (17), to have no effect (6), or to decrease (14) the levels of insulin after a meal. Although the stimulatory effect of GLP-1 on insulin secretion is well established (1,18), in some studies, insulin levels increased with GLP-1 treatment as a result of reduced hepatic insulin clearance (19–22), whereas in others (23–26), clearance was not affected. In the present study, the increases in arterial C-peptide levels were greater in the vildagliptin group compared with the control group by the same relative magnitude as the increases in insulin levels that occurred, although none of the differences were statistically significant between groups. Thus, it appears that any elevation in plasma insulin levels during vildagliptin treatment was the result of a difference in insulin secretion, not a decrease in clearance.
The role of GLP-1 on canine insulin secretion is not well understood. An incretin effect has been clearly shown to be present in the dog (27), but the role of GLP-1 in this effect has not been clearly established. It is known that a pharmacological level of GLP-1 can induce insulin secretion in isolated canine pancreata (28) and islets (29). In addition, we have shown that a pharmacological dose of exendin-4 (a GLP-1R agonist) is able to induce insulin secretion (30), whereas an incretin effect has not been demonstrated in other studies in the dog when GLP-1 was infused intraportally in physiological amounts (16,27,31,32). In the present study, we once again did not observe a significant effect of elevated GLP-1 on insulin secretion, although it is possible that a greater difference in GLP-1 between groups would have revealed an incretin effect.
Patients with type 2 diabetes often exhibit inappropriately high postprandial glucagon levels, which can be suppressed by GLP-1 (33) and vildagliptin (7). In the present study, glucagon decreased to levels that were very similar in both groups. Because the animals studied were nondiabetic, pancreatic α-cell sensitivity to inhibition by hyperinsulinemia and hyperglycemia may have been maximal, even in the absence of drug. In addition, the elevated concentration of GLP-1 in the vehicle-treated group may have been sufficient to create the maximal effect of GLP-1 on α-cell suppression.
Although some studies in the human have not revealed extrapancreatic effects of GLP-1 (34–37), others have demonstrated insulin-independent effects, including inhibition of glucose production (38). Stimulation of liver glucose uptake by GLP-1 has been reported in the dog (39,40), whereas nonhepatic effects on glucose clearance have been reported in both dogs and humans (16,41–44). Recently, it was reported that during a meal tolerance test, patients with type 2 diabetes treated with vildagliptin showed greater suppression of endogenous glucose production (7). This effect was associated with increased plasma insulin and reduced plasma glucagon concentrations. No significant differences in the glucose disappearance or metabolic clearance rates were noted. In other recent studies, exenatide (an incretin mimetic) was shown to increase splanchnic (type 2 diabetes [45]) and hepatic glucose uptake (normal dog [30]). In both studies, the effect was associated with an increase in plasma insulin concentration. In another study (normal dog [31]), exenatide reduced postprandial glycemia independent of islet hormones and slowing of gastric emptying.
The present study extends these observations by demonstrating that DPP-4 inhibition, accompanied by increased GLP-1 levels, can increase hepatic glucose disposal. This occurred without a significant difference in the insulin-to-glucagon molar ratio, although the average hepatic insulin level in the vildagliptin-treated group was 15 μU/ml greater than in the control group. Even though this difference was not statistically significant, small differences in insulin can affect hepatic glucose production. Based on previous insulin dose response experiments (with intraportal glucose and insulin infusions), a 15 μU/ml difference would have increased net hepatic glucose uptake by ∼0.4 mg · kg−1 · min−1 (46). Thus, net hepatic glucose uptake was ∼1 mg · kg−1 · min−1 greater during vildagliptin treatment than would have been predicted from the difference in plasma insulin levels. Although hepatic glucose production was most likely fully inhibited in both groups because of the elevations in glucose and insulin, a difference in suppression of glucose production could also account for some of the difference in net hepatic glucose uptake between groups. Nevertheless, net hepatic glucose fractional extraction and the ratio of net hepatic glucose uptake to the insulin level at the liver were threefold greater during DPP-4 inhibition (Fig. 7). Conversely, the tendency for nonhepatic glucose uptake to be increased was solely attributable to the prevailing arterial insulin levels (Fig. 7).
DPP-4 inhibition also extends the half-life of gastric inhibitory polypeptide (GIP). However, because there was no stimulus for incretin secretion in the present study, GIP levels presumably remained close to basal. Additional experiments would have to be performed, however, to definitively rule out the possibility that the effects observed in this study were due to differences in GLP-1 levels and not to changes in the activity of another substrate of DPP-4. The likelihood that they were attributable to GLP-1 is supported by our earlier studies. In one experiment, which used a similar design as the present study (no pancreatic clamp and glucose clamped at 160 mg/dl), when saline or GLP-1 was infused into the liver via the portal vein or hepatic artery, plasma insulin and glucagon levels were not affected by GLP-1 infusion (16). Nevertheless, the change from basal net hepatic glucose uptake was greater with GLP-1 vs. saline infusion by 1–1.5 mg · kg−1 · min−1, although these differences were not statistically significant. In other studies in which hepatic insulin and glucagon levels were clamped at similar levels between groups, there was a linear relationship between net hepatic glucose uptake and the plasma concentration of GLP-1 (39). Thus, those studies support the present data, which suggest a dose-dependent increase in net hepatic glucose uptake.
The effect of vildagliptin treatment on liver glucose disposal was presumably mediated by the difference in GLP-1 concentrations between groups. The GLP-1 receptor has been identified in the hepatic portal vein (47) and the liver (48,49) and could exert its effect through binding at either site, although it appears that portal receptors may not be necessary for the effect (39). Glucose entering the liver may be directed into glycogen because GLP-1 has been shown to increase glycogen storage in rat hepatocytes (50). Although DPP-4 was maximally inhibited by the start of the experimental period and the maximal difference in GLP-1 was present by the first sampling point in that period, the maximal difference in response of the liver was not apparent until 90 min. This slow onset of action may reflect the time required for the synthesis of glucoregulatory proteins in the liver.
In summary, in the nondiabetic, overnight fasted dog, inhibition of DPP-4 by vildagliptin increased plasma GLP-1 levels by reducing its clearance by ∼40%. In addition, the rise in GLP-1 associated with DPP-4 inhibition produced an augmentation of hepatic glucose utilization that was not accounted for by the effects of the hormone on the pancreas.
Acknowledgments
This study was funded by Novartis Institutes for Biomedical Research (to C.H.H.). Lovelace Respiratory Research Institute received funding for these studies from Novartis Institutes for Biomedical Research. A.D. holds stock for Amylin, has served on a Scientific Advisory Board for Bristol Myers, and been a paid consultant for Merck. No other potential conflicts of interest relevant to this article were reported.
Published ahead of print at http://diabetes.diabetesjournals.org on 7 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Drucker DJ: The biology of incretin hormones. Cell Metab 3: 153–165, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Zander M, Madsbad S, Madsen JL, Holst JJ: Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359: 824–830, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ: Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44: 1126–1131, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Vilsboll T, Agerso H, Krarup T, Holst JJ: Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 88: 220–224, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Ahren B, Gomis R, Standl E, Mills D, Schweizer A: Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care 27: 2874–2880, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A: Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 89: 2078–2084, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Balas B, Baig MR, Watson C, Dunning BE, Ligueros-Saylan M, Wang Y, He YL, Darland C, Holst JJ, Deacon CF, Cusi K, Mari A, Foley JE, DeFronzo RA: The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J Clin Endocrinol Metab 92: 1249–1255, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Edgerton DS, Cardin S, Emshwiller M, Neal D, Chandramouli V, Schumann WC, Landau BR, Rossetti L, Cherrington AD: Small increases in insulin inhibit hepatic glucose production solely caused by an effect on glycogen metabolism. Diabetes 50: 1872–1882, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Edgerton DS, Neal DW, Scott M, Bowen L, Wilson W, Hobbs CH, Leach C, Sivakumaran S, Strack TR, Cherrington AD: Inhalation of insulin (Exubera) is associated with augmented disposal of portally infused glucose in dogs. Diabetes 54: 1164–1170, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Donmoyer CM, Chen SS, Hande SA, Lacy DB, Ejiofor J, McGuinness OP: Hyperinsulinemia compensates for infection-induced impairment in net hepatic glucose uptake during TPN. Am J Physiol Endocrinol Metab 279: E235–E243, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Galassetti P, Koyama Y, Coker RH, Lacy DB, Cherrington AD, Wasserman DH: Role of a negative arterial-portal venous glucose gradient in the postexercise state. Am J Physiol 277: E1038–E1045, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Moore MC, Hsieh PS, Neal DW, Cherrington AD: Nonhepatic response to portal glucose delivery in conscious dogs. Am J Physiol Endocrinol Metab 279: E1271–E1277, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Meier JJ, Nauck MA: The potential role of glucagon-like peptide 1 in diabetes. Curr Opin Investig Drugs 5: 402–410, 2004 [PubMed] [Google Scholar]
- 14.Mari A, Sallas WM, He YL, Watson C, Ligueros-Saylan M, Dunning BE, Deacon CF, Holst JJ, Foley JE: Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed beta-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab 90: 4888–4894, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Ionut V, Hucking K, Liberty IF, Bergman RN: Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia 48: 967–975, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Johnson KM, Edgerton DS, Rodewald T, Scott M, Farmer B, Neal D, Cherrington AD: Intraportal GLP-1 infusion increases nonhepatic glucose utilization without changing pancreatic hormone levels. Am J Physiol Endocrinol Metab 293: E1085–E1091, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Ahren B, Holst JJ, Martensson H, Balkan B: Improved glucose tolerance and insulin secretion by inhibition of dipeptidyl peptidase IV in mice. Eur J Pharmacol 404: 239–245, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Drucker DJ: Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 26: 2929–2940, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Ahren B, Thomaseth K, Pacini G: Reduced insulin clearance contributes to the increased insulin levels after administration of glucagon-like peptide 1 in mice. Diabetologia 48: 2140–2146, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Groop PH, Groop L, Totterman KJ, Fyhrquist F: Relationship between changes in GIP concentrations and changes in insulin and C-peptide concentrations after guar gum therapy. Scand J Clin Lab Invest 46: 505–510, 1986 [DOI] [PubMed] [Google Scholar]
- 21.Kindmark H, Pigon J, Efendic S: Glucose-dependent insulinotropic hormone potentiates the hypoglycemic effect of glibenclamide in healthy volunteers: evidence for an effect on insulin extraction. J Clin Endocrinol Metab 86: 2015–2019, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Rudovich NN, Rochlitz HJ, Pfeiffer AF: Reduced hepatic insulin extraction in response to gastric inhibitory polypeptide compensates for reduced insulin secretion in normal-weight and normal glucose tolerant first-degree relatives of type 2 diabetic patients. Diabetes 53: 2359–2365, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Brandt A, Katschinski M, Arnold R, Polonsky KS, Goke B, Byrne MM: GLP-1-induced alterations in the glucose-stimulated insulin secretory dose-response curve. Am J Physiol Endocrinol Metab 281: E242–E247, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hanks JB, Andersen DK, Wise JE, Putnam WS, Meyers WC, Jones RS: The hepatic extraction of gastric inhibitory polypeptide and insulin. Endocrinology 115: 1011–1018, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Meier JJ, Gallwitz B, Siepmann N, Holst JJ, Deacon CF, Schmidt WE, Nauck MA: The reduction in hepatic insulin clearance after oral glucose is not mediated by gastric inhibitory polypeptide (GIP). Regul Pept 113: 95–100, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Meier JJ, Holst JJ, Schmidt WE, Nauck MA: Reduction of hepatic insulin clearance after oral glucose ingestion is not mediated by glucagon-like peptide 1 or gastric inhibitory polypeptide in humans. Am J Physiol Endocrinol Metab 293: E849–E856, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Ionut V, Liberty IF, Hucking K, Lottati M, Stefanovski D, Zheng D, Bergman RN: Exogenously imposed postprandial-like rises in systemic glucose and GLP-1 do not produce an incretin effect, suggesting an indirect mechanism of GLP-1 action. Am J Physiol Endocrinol Metab 291: E779–E785, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kawai K, Suzuki S, Ohashi S, Mukai H, Ohmori H, Murayama Y, Yamashita K: Comparison of the effects of glucagon-like peptide-1-(1-37) and -(7-37) and glucagon on islet hormone release from isolated perfused canine and rat pancreases. Endocrinology 124: 1768–1773, 1989 [DOI] [PubMed] [Google Scholar]
- 29.van der Burg MP, Guicherit OR, Frolich M, Gooszen HG: Insulinotropic effects of cholecystokinin, gastric inhibitory polypeptide and glucagon-like peptide-1 during perifusion of short-term cultured canine isolated islets. Regul Pept 60: 61–67, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Edgerton DS, Stettler KM, Rodewald TD, Farmer B, Lautz M, Hastings J, Snead W, Scott M, Cherrington AD: Intraportal exendin delivery increases liver and muscle glucose utilization (Abstract). Diabetes 55: A28, 2006 [Google Scholar]
- 31.Ionut V, Zheng D, Stefanovski D, Bergman RN: Exenatide can reduce glucose independent of islet hormones or gastric emptying. Am J Physiol Endocrinol Metab 295: E269–E277, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson KM, Edgerton DS, Rodewald T, Scott M, Farmer B, Neal D, Cherrington AD: Intraportally delivered GLP-1, in the presence of hyperglycemia induced via peripheral glucose infusion, does not change whole body glucose utilization. Am J Physiol Endocrinol Metab 294: E380–E384, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hufner M, Schmiegel WH: Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 87: 1239–1246, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Ahren B, Larsson H, Holst JJ: Effects of glucagon-like peptide-1 on islet function and insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 82: 473–478, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Orskov L, Holst JJ, Moller J, Orskov C, Moller N, Alberti KG, Schmitz O: GLP-1 does not acutely affect insulin sensitivity in healthy man. Diabetologia 39: 1227–1232, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Ryan AS, Egan JM, Habener JF, Elahi D: Insulinotropic hormone glucagon-like peptide-1-(7-37) appears not to augment insulin-mediated glucose uptake in young men during euglycemia. J Clin Endocrinol Metab 83: 2399–2404, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Vella A, Shah P, Reed AS, Adkins AS, Basu R, Rizza RA: Lack of effect of exendin-4 and glucagon-like peptide-1-(7,36)-amide on insulin action in non-diabetic humans. Diabetologia 45: 1410–1415, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Prigeon RL, Quddusi S, Paty B, D'Alessio DA: Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285: E701–E707, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Dardevet D, Moore MC, DiCostanzo CA, Farmer B, Neal DW, Snead W, Lautz M, Cherrington AD: Insulin secretion-independent effects of GLP-1 on canine liver glucose metabolism do not involve portal vein GLP-1 receptors. Am J Physiol Gastrointest Liver Physiol 289: G806–G814, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dardevet D, Moore MC, Neal D, DiCostanzo CA, Snead W, Cherrington AD: Insulin-independent effects of GLP-1 on canine liver glucose metabolism: duration of infusion and involvement of hepatoportal region. Am J Physiol Endocrinol Metab 287: E75–E81, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Vella A, Shah P, Basu R, Basu A, Camilleri M, Schwenk FW, Holst JJ, Rizza RA: Effect of glucagon-like peptide-1(7-36)-amide on initial splanchnic glucose uptake and insulin action in humans with type 1 diabetes. Diabetes 50: 565–572, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Meneilly GS, McIntosh CH, Pederson RA, Habener JF, Gingerich R, Egan JM, Elahi D: Glucagon-like peptide-1 (7-37) augments insulin-mediated glucose uptake in elderly patients with diabetes. J Gerontol A Biol Sci Med Sci 56: M681–M685, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Meneilly GS, McIntosh CH, Pederson RA, Habener JF, Gingerich R, Egan JM, Finegood DT, Elahi D: Effect of glucagon-like peptide 1 on non-insulin-mediated glucose uptake in the elderly patient with diabetes. Diabetes Care 24: 1951–1956, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Egan JM, Montrose-Rafizadeh C, Wang Y, Bernier M, Roth J: Glucagon-like peptide-1(7-36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3–L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology 135: 2070–2075, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Cervera A, Wajcberg E, Sriwijitkamol A, Fernandez M, Zuo P, Triplitt C, Musi N, DeFronzo RA, Cersosimo E: Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am J Physiol Endocrinol Metab 294: E846–E852, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Myers SR, McGuinness OP, Neal DW, Cherrington AD: Intraportal glucose delivery alters the relationship between net hepatic glucose uptake and the insulin concentration. J Clin Invest 87: 930–939, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA: Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148: 4965–4973, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Villanueva-Penacarrillo ML, Delgado E, Trapote MA, Alcantara A, Clemente F, Luque MA, Perea A, Valverde I: Glucagon-like peptide-1 binding to rat hepatic membranes. J Endocrinol 146: 183–189, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Wheeler MB, Lu M, Dillon JS, Leng XH, Chen C, Boyd AE III: Functional expression of the rat glucagon-like peptide-I receptor, evidence for coupling to both adenylyl cyclase and phospholipase-C. Endocrinology 133: 57–62, 1993 [DOI] [PubMed] [Google Scholar]
- 50.Valverde I, Morales M, Clemente F, Lopez-Delgado MI, Delgado E, Perea A, Villanueva-Penacarrillo ML: Glucagon-like peptide 1: a potent glycogenic hormone. FEBS Lett 349: 313–316, 1994 [DOI] [PubMed] [Google Scholar]