Abstract
OBJECTIVE—Adiponectin is an adipocyte-derived protein that acts to reduce insulin resistance in the liver and muscle and also inhibits atherosclerosis. Although adiponectin reportedly enhances AMP-activated protein kinase and inhibits tumor necrosis factor-α action downstream from the adiponectin signal, the precise physiological mechanisms by which adiponectin acts on skeletal muscles remain unknown.
RESEARCH DESIGN AND METHODS—We treated murine primary skeletal muscle cells with recombinant full-length human adiponectin for 12 h and searched, using two-dimensional electrophoresis, for proteins upregulated more than threefold by adiponectin compared with untreated cells.
RESULTS—We found one protein that was increased 6.3-fold with adiponectin incubation. MALDI-TOF (matrix-assisted laser desorption/ionization−top of flight) mass spectrometric analysis identified this protein as ferritin heavy chain (FHC). When murine primary skeletal muscle cells were treated with adiponectin, IκB-α phosphorylation was observed, suggesting that adiponectin stimulates nuclear factor (NF)-κB activity. In addition, FHC upregulation by adiponectin was inhibited by NF-κB inhibitors. These results suggest NF-κB activation to be involved in FHC upregulation by adiponectin. Other NF-κB target genes, manganese superoxide dismutase (MnSOD) and inducible nitric oxide synthase (iNOS), were also increased by adiponectin treatment. We performed a reactive oxygen species (ROS) assay using CM-H2DCFDA fluorescence and found that ROS-reducing effects of adiponectin were abrogated by FHC or MnSOD small-interfering RNA induction.
CONCLUSIONS—We have demonstrated that adiponectin upregulates FHC in murine skeletal muscle tissues, suggesting that FHC elevation might partially explain how adiponectin protects against oxidative stress in skeletal muscles.
Adipocytes have been recognized to secrete a variety of proteins, such as tumor necrosis factor (ΤΝF)-α, adipsin, plasminogen activator inhibitor-1, leptin, resistin, and adiponectin. These proteins are termed adipokines and are likely to physiologically exert a variety of hormonal actions (1). Among these proteins, adiponectin is exclusively expressed in adipose tissue and consists of an NH2-terminal collagenous domain and a COOH-terminal globular domain (2). Adiponectin belongs to the soluble collagen superfamily and has structural homology with collagens VIII and X, complement factor C1q (3), and the TNF family (2,4). Circulating adiponectin is extremely abundant (∼15 μg/ml), and adiponectin forms various oligomeric complexes, including low (LMW), medium (MMW), and high (HMW) molecular weight species. Adiponectin exerts antidiabetes effects on muscles and the liver through AMP-activated protein kinase activation (5) and antiath-erosclerotic effects by inhibiting monocyte adhesion to endothelial cells and lipid accumulation into macrophages (6,7). Thus, adiponectin increases glucose uptake and fatty acid oxidation in muscles via the type 1 adiponectin receptor (8), and decreases hepatic gluconeogenesis via the type 2 adiponectin receptor (8,9). On the other hand, nuclear factor (NF)-κB but not AMP-activated protein kinase activity was demonstrated to be enhanced by MMW or HMW adiponectin in muscles (10). According to recent studies (9–14), HMW adiponectin appears to be more important for the antidiabetes and antiatherosclerotic effects than the other two oligomeric complexes. Though the physiological role of HMW adiponectin in improving insulin resistance or reducing oxidative stress is clearly significant, the precise mechanisms by which adiponectin acts on skeletal muscles remain unknown.
Therefore, in the present study, we investigated adiponectin function in primary cultured skeletal muscle cells by comparing protein expressions in untreated cells using two-dimentional electrophoresis. A marked increase in FHC protein was observed with adiponectin incubation. FHC is one of two subunits of ferritin, the other being ferritin light chain (FLC) (15), and has ferroxidase activity, which is required for iron sequestration (16). FHC was reported to suppress reactive oxygen species (ROS) production (17), which may explain the ROS-reducing effects of adiponectin. FHC upregulation followed by an enhanced ROS-reducing effect is suggested to be a novel mechanism by which adiponectin acts directly against oxidative stress.
RESEARCH DESIGN AND METHODS
Cell culture and chemicals.
Murine primary cultured skeletal muscle cells were purchased from Cell Garage (Tokyo, Japan) in cultured flasks, and maintained in DMEM containing 10% fetal bovine serum (FBS). We switched the medium to DMEM containing 2% horse serum, which promotes differentiation of myocytes into myotubes, and continued the incubation for 5 days before the experiments. Human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex (Baltimore, MD) as cryopreserved cells. After thawing, the cells were plated in collagen-coated culture flasks and cultured to confluence in EBM-2 medium (Lonza, Walkersville, MD) containing the indicated ligands, 2% FBS, and antibiotics (BulletKit EGM-2, cat. no. CC-3162). C2C12 myoblasts were maintained in DMEM containing 10% FBS at 37°C in 5% CO2. After the C2C12 cells reached subconfluence, differentiation was induced by treatment with DMEM containing 5% horse serum for 7 days, at which time formation of myotubes was maximal. The following chemicals were purchased: H89 from Seikagaku (Tokyo, Japan); NF-κB inhibitor, NF-κB SN50, and BAY11-7082 from Biomol Research Laboratories (Plymouth Meeting, PA); forskolin, TNF-α, palmitate, and iron (II) sulfate heptahydrate from Sigma-Aldrich (St. Louis, MO); human recombinant adiponectin from R&D Systems (Minneapolis, MN); human recombinant globular adiponectin from BioVendor Laboratory Medicine (Modrice, Czech Republic); and 4-hydroxynonenal from Calbiochem (San Diego, CA). Fatty acid solution was prepared by a method described previously (18).
Two-dimensional electrophoresis.
A total of 3.0 × 106 murine primary cultured skeletal muscle cells were dissolved in lysis solution (7 mol/l urea, 2 mol/l thiourea, 4% CHAPS, 0.5% IPG buffer, 18 mmol/l dithiothreitol, and 2 mmol/l phenylmethanesulphonylfluoride, pH 8.5). Impurities such as salts, lipids, detergent, and nucleic acids were then removed from samples using a two-dimensional clean-up kit (Amersham Pharmacia Biotech, Amersham, U.K.). Samples were redissolved in rehydration solution and centrifuged at 24,000 rpm for 20 min at 10°C, and insoluble substances were removed. Using 450 μl of solution corresponding to 400 μg of murine primary cultured skeletal muscle cell protein, two-dimensional gel electrophoresis was performed according to the manufacturer's instructions (Amersham Pharmacia Biotech). The gels were Coomassie brilliant blue stained using PhastGel Blue R-350. Colloidal Coomassie blue–stained gels were scanned using a GS-800 calibrated densitometer (Bio-Rad Laboratories, Hercules, CA), and gel images were analyzed using PDQuest 2D-Image-Analysis software (version 7.3; Bio-Rad Laboratories). For this analysis, three independent sets consisting of a control sample gel and an adiponectin-treated sample gel were prepared. For a between-gel comparison, a set of spot-generation conditions was used. To analyze the proteins, we first chose one protein signal to assure that the number of proteins, with signals more intense than that initially chosen, would be ∼1,500. Then, we analyzed only these 1,500 protein signals. The computer allowed automatic detection and quantification of protein spots, as well as matching between the control and adiponectin-treated gels. Routine statistical analysis available within the software package was used to identify up- or down-expressed spots. The differentially expressed protein spots were identified by quantitative comparisons with control gels.
Identification of proteins upregulated by adiponectin.
Protein spots of interest were excised from the gels and subjected to matrix-assisted laser desorption/ionization−top of flight (MALDI-TOF) mass spectrometry. In-gel digestion of the individual protein spots was done by the following method. Pieces of gel were destained using 200 μl of 50 mmol/l ammonium bicarbonate in 50% acetonitrile, dehydrated in 200 μl of acetonitrile, and then completely dried by vacuuming and centrifuging. The samples were then allowed to expand in digestion buffer containing 100 mmol/l ammonium bicarbonate, 20 μg/ml of trypsin (Promega, Madison, WI), and 0.1% octyl β-d-glucopyranoside (Sigma-Aldrich) at 4°C. After a 30-min incubation, the samples were incubated overnight at 37°C. Peptides were then extracted twice using 0.1% trifluoroacetic acid in 30% acetonitrile with sonication. The peptide solution was vacuum concentrated until it had decreased to 10 μl and desalted according to the manufacturer's protocol. An AXIMA-CFR model MALDI-TOF mass spectrometer (Shimadzu, Kyoto, Japan) was used for mass analysis of tryptic peptide mixtures. Peptides were identified with the Mascot search program (Matrix Science, London, U.K.).
Western blotting and quantitative PCR.
Western blotting was performed as previously described (19). Briefly, after incubation with the indicated chemicals, primary cultured skeletal muscle cells were washed with ice-cold PBS, lysed in ice-cold lysis buffer, and then centrifuged at 14,000g for 10 min at 4°C. Supernatants including tissue protein extracts were resolved on 10% SDS-PAGE, followed by electrophoretic transfer to a nitrocellulose membrane. Membranes were incubated for 1 h at room temperature with the appropriate primary antibody. Commercial antibodies against phosphor–inhibitor of κB-α (IκB-α), intercellular adhesion molecule (ICAM)-1 FHC FLC, p65 NF-κB (Santa Cruz Biotechnology, Santa Cruz, CA), and IκB-α (Cell Signaling Technology, Palo Alto, CA) were purchased. After blotting with the indicated secondary antibody, detection was performed using an ECL chemiluminescent kit (Amersham Pharmacia Biotech), according to the manufacturer's instructions. Quantitations were performed using a Molecular Imager (Bio-Rad Laboratories). cDNA was synthesized from the purified total RNA using a reverse transcriptase kit (Amersham Pharmacia Biotech), according to the manufacturer's instructions. For quantitative analysis of FHC, manganese superoxide dismutase (MnSOD), and inductible nitric oxide synthase (iNOS), we conducted real-time PCR using an ABI PRISM model 7000 (Applied Biosystems, Foster City, CA), according to the manufacturer's instructions. The primer sets and probes for murine FHC (assay ID: Mm00850707_g1), murine MnSOD (assay ID: Mm00449726_m1), and murine iNOS (assay ID: Mm00440485_m1) were purchased. Nuclear protein extracts were prepared by separating the cell pellet into two compartments (i.e., the nucleus and the cytosol), as previously described (18).
Small-interfering RNA reagents and transfection.
C2C12 myotubes were transfected with small-interfering (siRNA) against FHC (ID 158606, 66945), MnSOD (ID 152022, 71294), and iNOS (ID 156550, 68442) (Applied Biosystems) using the transfection reagent (AM4510; Applied Biosystems), following the manufacturer's protocol. As the control, we utilized the commercially available siRNA control nonsilencing sequence (4611G; Applied Biosystems). The cells were used for experiments 48 h after siRNA transfection.
Generation and transfection of recombinant adenoviruses expressing FHC and adiponectin.
A full-length mouse FHC cDNA was isolated from mouse hepatic RNA by reverse-transcriptase PCR. The oligonucleotide sequences used for PCR were as follows: coding strand, 5′-ACCATGACCACCGCGTCTCCCTCGCAAGTG-3′; noncoding strand, 5′-AGCTTAGCTCTCATCACCGTGTCCCAGGGT-3′. The cDNA was subcloned into TA vectors, pCRII (Invitrogen Life Technologies, CA), sequenced to confirm their identities, and were observed to have no unexpected mutations. Adenovirus-expressing recombinant FHC was prepared by homologous recombination of the expression cosmid cassettes containing the corresponding cDNAs and the parental adenovirus genome, as described previously (20). Adenovirus-expressing recombinant adiponectin was prepared as reported previously (21). For adenovirus-mediated transfection, cultured cells were incubated for 2 h in 37°C with DMEM containing the adenovirus-expressing LacZ or FHC, and the growth media were then added. Experiments were performed 3 days after transfection. Mice were treated with recombinant adenovirus, expressing LacZ or adiponectin, by systemic injection into the tail vein.
Assay of intracellular cAMP contents.
cAMP was measured in murine primary cultured skeletal muscle cells using a direct enzyme immunoassay kit according to the instructions provided by the manufacturer (Amersham Pharmacia Biotech). Briefly, cell lysates (100 μl) were transferred to a new 96-well microplate coated with donkey anti-rabbit IgG. After addition of 100 μl of rabbit anti-cAMP serum to each well, the microplate contents were gently mixed and incubated at 4°C for 2 h. Then, after addition of 50 μl of cAMP peroxidase conjugate to each well, the microplates were gently agitated and incubated at 4°C for 60 min. We aspirated and washed each well four times with 400 μl of washing buffer and blotted the plate on tissue paper to remove any residual liquid. Next, we immediately dispensed 150 μl of enzyme substrate into each well, followed by mixing on a microplate shaker for exactly 60 min at room temperature. To halt the reaction, we added 100 μl of 1.0 mol/l sulfuric acid to each well. The optical density was determined in a plate reader at 450 nm.
Detection of intracellular ROS production.
Intracellular ROS production was monitored by flow cytometry (Becton Dickinson, Franklin Lakes, NJ) using 5-(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA). Cells were stimulated with the indicated reagents in culture dishes and incubated for 24 h. Then, these cells were washed twice in PBS, followed by addition of 10 μmol/l CM-H2DCFDA in PBS, and finally placed in the dark at 37°C for 1 h. The cells were washed once, harvested, and suspended in 500 μl PBS. Dead cells were excluded by adding 10 μmol/l propidium iodide, a nuclear stain to which viable cells are impermeable. ROS levels were measured by flow cytometrically by determining the mean fluorescent intensity relative to that of the control group. Using this method, we were able to measure not only H2O2 but also hydroxy radical (·OH) or peroxynitrite (ONOO−). As it was important to measure hydroxy radicals (OH), generated by the Fenton reaction, we adopted this method.
Animals.
Nine-week-old male mice (C57BL/KsJ, n = 14) were purchased from Clea Japan (Osaka, Japan). After a 2- to 3-day acclimatization period, all mice were maintained on a 12:12-h light-dark cycle, fed a standard rodent diet ad libitum, and given unlimited access to water. The mice were divided into a LacZ-transferred group (control construct) and an adiponectin-transferred group (adiponectin construct), and adenovirus-mediated gene transfer was performed. Before they were killed, the animals were fasted for 8 h. Three days after virus infection, increased serum adiponectin levels were confirmed using both a mouse/rat adiponectin ELISA kit (Otsuka, Tokushima, Japan) and immunoblot analysis with anti-murine adiponectin antibody (Chemicon International, Temecula, CA). Then, total hind limbs were removed and immediately homogenized with a Polytron homogenizer in six volumes of solubilization buffer. Extracts were centrifuged at 15,000g for 30 min at 4°C, and the supernatants were used as samples for immunoblotting with anti-FHC antibody.
Statistical analysis.
All data were expressed as means ± SE. The statistical significance of differences between groups was assessed with the unpaired Student's t test using Stat View software (version 5.01; SAS Institute, Cary, NC). A P value <0.05 was considered statistically significant.
RESULTS
Identification of proteins upregulated by adiponectin.
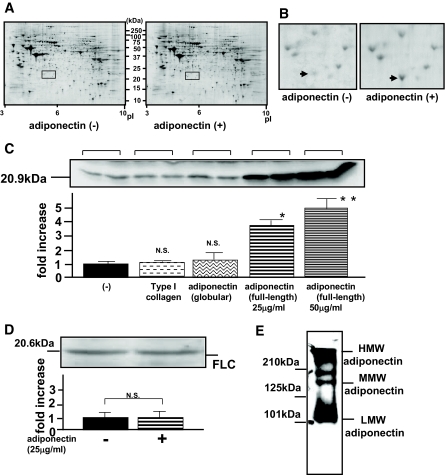
We treated murine primary cultured skeletal muscle cells with recombinant full-length human adiponectin, which was expressed in the mouse myeloma cell line NS0 and purified, and then we searched, using two-dimensional electrophoresis, for proteins upregulated more than threefold by adiponectin as compared with untreated cells. As confirmed by immunoblotting under nonreducing conditions (Fig. 1E), the adiponectin species used in this experiment may be atypical because these were essentially mixtures of the HMW and LMW isoforms of adiponectin, with little of the MMW form. The gels were stained with Coomassie brilliant blue (Fig. 1A) and scanned using a GS-800 calibrated densitometer, and gel images were analyzed. We selected 1,500 protein signals. Among these, only one protein was increased (6.3-fold) with adiponectin incubation. The protein spots in the absence or presence of adiponectin are indicated by the arrows in the magnified figure (Fig. 1B). We excised the protein spot from the gel (Fig. 1B, right panel) and identified four peptides, which were matched to FHC sequences (a.a.54–63, a.a.109–143, a.a.147–156, and a.a.158–172) using MALDI-TOF-MS analysis. The Score and Expect of the Mascot Search were 76 and 0.0022, respectively, both of which are highly definitive for FHC. As shown in Fig. 1C, FHC protein expressions were significantly increased in an adiponectin concentration–dependent manner but were unaffected by incubation with the same concentration of type I collagen or recombinant globular adiponectin, suggesting FHC upregulation to be specific to the multimer formation of adiponectin. Next, we assessed whether the expression of FLC is also upregulated by adiponectin. However, FLC expression was not altered (Fig. 1D).
FIG. 1.
Effects of incubation with HMW adiponectin on primary cultured skeletal muscles. Primary cultured murine skeletal muscles were incubated for 12 h in the presence (+) or absence (−) of adiponectin (25 μg/ml). A: Whole images of Coomassie brilliant blue–stained gels for two-dimensional electrophoresis in the absence (left) or presence (right) of adiponectin. B: Magnified images of two-dimensional electrophoresis revealing a spot apparently altered by adiponectin treatment (arrows). Three two-dimensional electrophoresis sets yielded similar results. C–E: After incubation with the indicated ligands, primary cultured skeletal muscle cells were lysed in ice-cold lysis buffer and centrifuged at 14,000g for 10 min at 4°C. Supernatants including tissue protein extracts were resolved on 10% SDS-PAGE, followed by electrophoretic transfer to a nitrocellulose membrane. Membranes were incubated for 1 h at room temperature with antibody against mouse FHC (C) or FLC (D). E: The recombinant full-length human adiponectin, which was expressed in a mouse myeloma cell line NS0, was resolved on 7.5% SDS-PAGE under nonreducing conditions and investigated by immunoblotting with anti-adiponectin antibody. After blotting with the indicated secondary antibody, detection was performed using an electrochemiluminescence chemiluminescent kit according to the manufacturer's instructions. Representative data from four independent experiments are presented. *Significant difference (P < 0.05) relative to FHC expression in control cells. **Significant difference (P < 0.05) relative to FHC expression with 25 μg/ml of adiponectin. N.S., not significant relative to control cells in the absence of adiponectin.
NF-κB activation is involved in FHC upregulation by adiponectin.
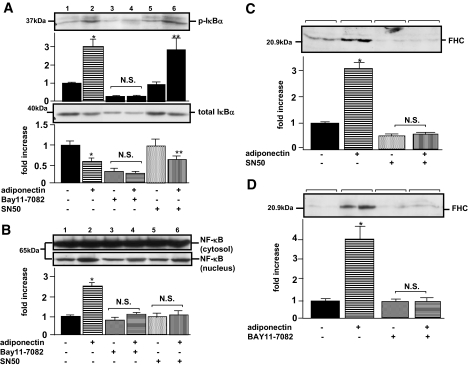
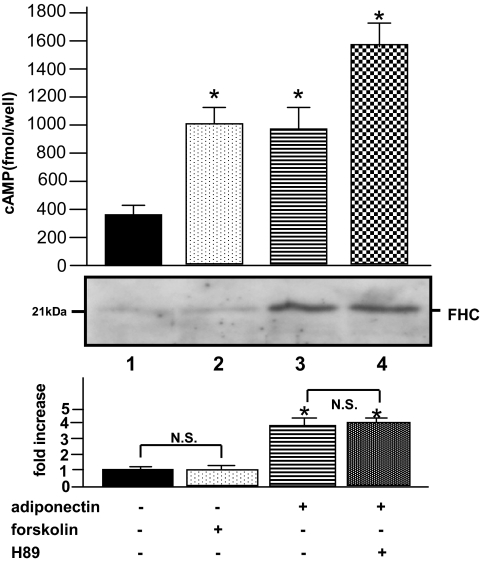
As FHC was reported to be transcriptionally upregulated in response to NF-κB activation (22), we next investigated whether adiponectin enhances NF-κB activity in primary skeletal muscle cells. The NF-κB bound to IκB-α is generally located in the cytosol before activation. In response to stimuli, IκB-α proteins are degraded, a process controlled by IκB-α phosphorylation, resulting in nuclear translocation of NF-κB and subsequent activation of NF-κB target gene transcription. When the cells were incubated with adiponectin, phosphorylation of IκB-α (Fig. 2A, upper panel, lanes 1 and 2) and a decrease in total IκB-α (Fig. 2A, lower panel, lanes 1 and 2) were observed, revealing that adiponectin actually stimulates NF-κB activation. The IκB-α phosphorylation required at least 3 h of incubation, suggesting that secondary effects might be involved in adiponectin-induced IκB-α phosphorylation. NF-κB SN50, an inhibitor of NF-κB translocation into the nucleus, did not affect the Iκ-B phosphorylation by adiponectin (Fig. 2A, lanes 5 and 6), whereas it was abolished by incubation with BAY11-7082, an inhibitor of IκB-α phosphorylation (Fig. 2A, lanes 3 and 4). To confirm that SN50 inhibits NF-κB translocation to the nucleus, we separated the myocyte-lysates into two compartments (i.e., nuclear extracts and cytosol) and performed immunoblotting using the anti-p65 NF-κB antibody. As shown in Fig. 2B, increases in nuclear NF-κB proteins were observed with adiponectin incubation (Fig. 2B, lane 2), indicating translocation of the activated NF-κB into the nucleus, whereas no significant translocation of NF-κB proteins was observed when both adiponectin and SN50 were present (Fig. 2B, lane 6). In addition, FHC upregulation by adiponectin was completely abolished by NF-κB SN50 (Fig. 2C) or BAY11-7082 (Fig. 2D), indicating that FHC was upregulated by adiponectin via an NF-κB–dependent pathway. Taking into consideration the reported upregulation of FHC by cAMP via a proximal cis-acting element containing the CCAAT motif (23), we further examined whether FHC is regulated by a cAMP–protein kinase A (PKA)-dependent pathway. When incubated with adiponectin, cAMP levels inside primary cultured muscle cells were increased by 2.8-fold with FHC upregulation (Fig. 3, lane 2). Unexpectedly, H89, a PKA inhibitor, failed to block this FHC upregulation by adiponectin (Fig. 3, lane 4). Furthermore, forskolin increased cAMP levels in these cells without FHC upregulation (Fig. 3, lane 2). These results suggest that cAMP elevation in response to adiponectin treatment was not associated with FHC upregulation.
FIG. 2.
Effects of IκB/NF-κB inhibitor on FHC in murine primary cultured skeletal muscle cells. Primary cultured skeletal muscle cells were pretreated with 100 μmol/l BAY11-7082 or 50 μg/ml NF-κB SN50 at 1 h before the cells were incubated with 40 μg/ml of adiponectin for 12 h. Cells were lysed in ice-cold lysis buffer and centrifuged at 14,000g for 10 min at 4°C. Supernatants including tissue protein extracts were subjected to SDS-PAGE. Transferred membranes were incubated for 1 h at room temperature with antibody against phosphor–IκB-α (A, upper panel), IκB-α (A, lower panel), p65 NF-κB (B), and FHC (C and D). After blotting with the indicated secondary antibody, detection was performed using an electrochemiluminescence chemiluminescent kit. Representative data (one sample each for A and B; two each for C and D) from four independent experiments (two samples for each experiment) are presented. Values are means ± SE. *Significant difference (P < 0.05) relative to IκB-α phosphorylation (A, upper panel), total IκB-α (A, lower panel), p65 NF-κB (B), or FHC expression (C and D) of control cells in the absence of adiponectin. **Significant difference (P < 0.05) relative to IκB-α phosphorylation (A, upper panel) or total IκB-α (A, lower panel) of paired control cells in the absence of adiponectin (lane 5). N.S., not significant relative to IκB-α phosphorylation (A, upper panel), total IκB-α (A, lower panel), p65 NF-κB (B), or FHC expression (C and D) of paired control cells in the absence of adiponectin.
FIG. 3.
Effects of recombinant human adiponectin on cAMP content of murine primary skeletal muscle cells. Primary skeletal muscle cells, cultured in 96-well plates, were exposed to 50 μg/ml of adiponectin and 5 μmol/l forskolin for 12 h. Adiponectin-treated cells were also pretreated with 1 μmol/l H89, a PKA inhibitor, for 1 h before the addition of adiponectin. The cAMP assay was performed according to the manufacturer's instructions. Cell lysates from primary cultured skeletal muscle cells were subjected to SDS-PAGE, followed by electrophoretic transfer to a nitrocellulose membrane. Membranes were incubated for 1 h at room temperature with antibody against mouse FHC. Detection was performed using an electrochemiluminescence chemiluminescent kit according to the manufacturer's instructions. Representative results from four independent experiments are presented. Values are means ± SE. *Significant difference (P < 0.05) relative to control cells in the absence of ligands (lane 1). N.S.; not significant relative to paired control cells in the absence of forskolin (lane 1) or H89 (lane 3).
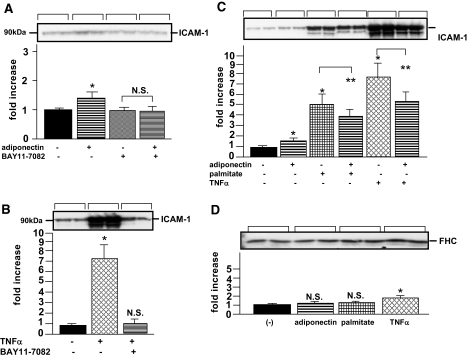
The effects of adiponectin, TNF-α, and free fatty acids on NF-κB target gene in HUVECs.
To investigate whether adiponectin increases FHC in other types of cultured cells, we further examined the NF-κB activation of HUVECs. When HUVECs were treated with adiponectin, ICAM-1, which is primarily regulated by the NF-κB transcription factor, was slightly, but significantly, increased (Fig. 4A). In contrast, TNF-α markedly increased ICAM-1 expression (Fig. 4B). These ICAM-1 inductions by adiponectin or TNF-α were both inhibited by the addition of BAY 11-7082. These results suggested that TNF-α upregulated ICAM-I via an NF-κB–dependent pathway in HUVECs to a far greater extent than adiponectin. In addition, we examined the synergistic effects of adiponectin and TNF-α on ICAM-1 expression. Unexpectedly, the induction of ICAM-1 by TNF-α was inhibited by further addition of adiponectin (Fig. 4C), indicating that TNF-α and adiponectin antagonized each other via the signaling pathways of these agents. This phenomenon, which was reported previously (7,24), was also observed in the actions of adiponectin and palmitate on ICAM-1 expression. Next, we examined the effects of these NF-κB activators on FHC expression in HUVECs. Although FHC expression was slightly increased by TNF-α, neither adiponectin nor palmitate treatment produced significant increases (Fig. 4D). Taken together, these observations indicated that induction of FHC by adiponectin does not occur in HUVECs despite the NF-κB activation, presumably due to the minor effect of adiponectin on NF-κB activation.
FIG. 4.
Effects of recombinant human adiponectin, TNF-α, and free fatty acids on NF-κB–regulated gene expressions in HUVECs. After pretreating HUVECs with or without 100 μmol/l BAY11-7082 for 1 h, 25 μg/ml of adiponectin (A) or 10 ng/ml of TNF-α (B) were added, followed by incubation for 12 h. After HUVECs had been pretreated with or without 25 μg/ml of adiponectin for 1 h, 10 ng/ml of TNF-α or 0.4 mmol/l palmitate was added, followed by incubation for 12 h (C and D). Cell lysates from HUVECs were subjected to SDS-PAGE, followed by electrophoretic transfer to a nitrocellulose membrane. Membranes were incubated for 1 h at room temperature with antibody against ICAM-1 (A, B, and C) or FHC (D). Detection was performed using an electrochemiluminescence chemiluminescent kit according to the manufacturer's instructions. Representative data (each bracket) from four independent experiments (two samples for each experiment) are presented. Values are means ± SE. *Significant difference (P < 0.05) relative to ICAM-1 (A–C) and FHC (D) expressions in control cells in the absence of ligands. **Significant difference (P < 0.05) relative to ICAM-1 expression (C) in paired control cells in the absence of adiponectin but in the presence of palmitate (lane 3) or TNF-α (lane 5). N.S., not significant relative to FHC expression in control cells in the absence of adiponectin (A), TNF-α (B), and each ligand (D).
Recombinant FHC reduces ROS production induced by oxidative stress.
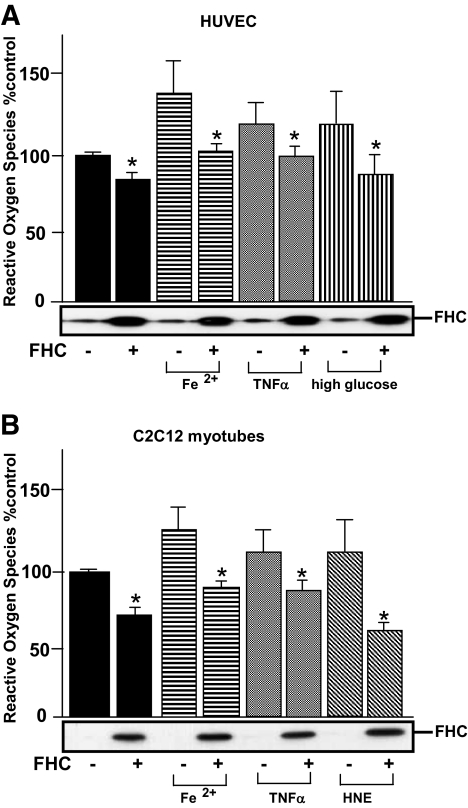
To investigate the cytoprotective effects of FHC against forms of damage mediated by oxidative stresses or inflammatory cytokines, we transfected adenovirus expressing recombinant FHC into HUVECs and C2C12 myocytes. As the murine primary cells exhibited susceptibility to adenovirus infection, we performed this experiment with C2C12 myotubes. Before the ROS assay, we confirmed recombinant FHC to be overexpressed by immunoblotting using anti-murine FHC antibodies. With overexpression of recombinant FHC, 13 and 28% reductions in relative ROS accumulations were observed in HUVECs and C2C12 myotubes, respectively (Fig. 5). In addition, FHC had a major effect on reducing ROS accumulation induced by various forms of oxidative stress (i.e., 26% [Fe2+], 18% [TNF-α], and 25% [high glucose] in HUVECs; and 26% [(Fe2+], 20% [TNF-α], and 49% [4-hydroxynonenal] in C2C12 myotubes), indicating that FHC exerts cytoprotective effects by reducing the ROS accumulation induced by various oxidative stresses. When these cells were treated with adiponectin, ROS-reducing effects, which were similar to those obtained with FHC overexpression, were also observed (data not shown). Taken together, these findings indicated FHC upregulation by adiponectin in skeletal muscle cells to at least partially explain the ROS-reducing effects of adiponectin.
FIG. 5.
Effects of recombinantly overexpressed FHC on ROS generation in C2C12 myotubes and HUVECs exposed to oxidative stresses. A: HUVECs, transfected with adenovirus overexpressing LacZ or recombinant FHC, were treated with 5.4 × 10−9 mol/l iron (II) sulfate heptahydrate and 25 mmol/l glucose. B: C2C12 myotubes, transfected with adenovirus overexpressing LacZ or recombinant FHC, were treated with 20 ng/ml of TNF-α and 100 μmol/l 4-hydroxynonenal (HNE). After a 24-h incubation, ROS generations were assayed by CM-H2DCFDA oxidation-based fluorescence. Representative results from three independent experiments are presented. Values are means ± SE. *Significant difference (P < 0.05) relative to ROS production by paired control cells in the absence of FHC overexpression.
Increased expression of NF-κB target genes with adiponectin incubation and their contributions to the ROS-reducing effects of adiponectin.
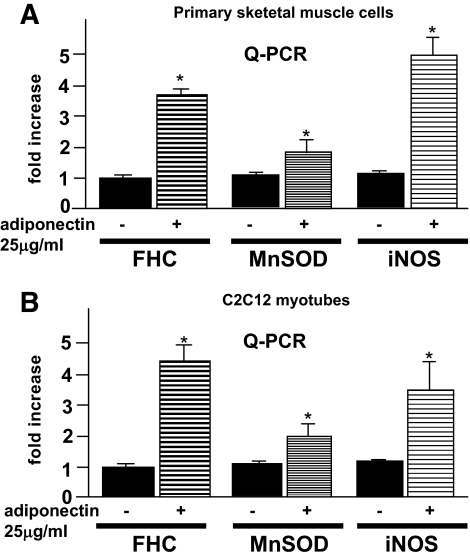
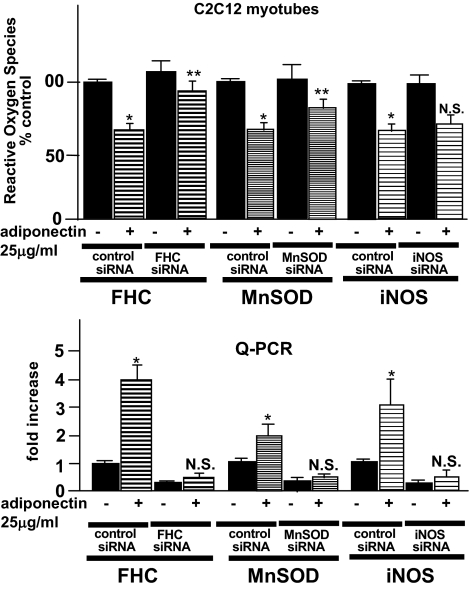
We further investigated NF-κB target gene expressions (e.g., MnSOD and iNOS) by quantitative PCR. As shown in Fig. 6, these proteins (FHC, MnSOD, and iNOS) were similarly upregulated by adiponectin incubation (3.6-, 1.6-, and 5.1-fold, respectively, in skeletal muscle cells and 4.3-, 1.8-, and 3.5-fold, respectively, in C2C12 myotubes). No MnSOD protein was identified on our two-dimensional electrophoresis search for proteins upregulated more than threefold by adiponectin. Though the reason for our inability to identify iNOS in two-dimensional electrophoresis was not entirely clear, the minute amounts of iNOS proteins in skeletal muscles made this form of analysis impractical (25). To clarify the relevance of the observed increase in all three proteins to the changes in ROS, we investigated the ROS levels in C2C12 myotubes, in which the expressions of these three proteins were inhibited by induction of siRNAs. As shown in Fig. 7, under the condition in which no significant increases in the three gene products were observed with adiponectin incubation, we investigated adiponectin-induced changes in ROS accumulation. When FHC siRNAs were induced, we observed a marked decrease in the ROS-reducing effects of adiponectin incubation. On the other hand, there was no significant change in ROS accumulation with iNOS siRNA induction, while a slight but significant decrease was observed with MnSOD siRNA induction. These results suggest that increased FHC expression has a major impact on ROS accumulation; however, this increase does not explain the entire ROS-reducing effect of adiponectin.
FIG. 6.
FHC, MnSOD, and iNOS mRNA levels in primary skeletal muscle cells (A) and C2C12 myotubes (B) were determined by quantitative PCR using an ABI PRISM model 7000 according to the manufacturer's instructions. Each column shows the mean ± SE obtained from four samples in the presence or absence of adiponectin. *Significant difference (P < 0.05) relative to control cells in the absence of adiponectin.
FIG. 7.
C2C12 myotubes were transfected with the control nonsilencing siRNA and siRNA against FHC, MnSOD, and iNOS using the transfection reagent. The cells were used for experiments 48 h after siRNA transfection and incubated with or without recombinant adiponectin at 12 h before the experiments. ROS generations and FHC, MnSOD, and iNOS mRNA in each of the cells were determined. Each column shows the mean ± SE obtained from four samples in the presence or absence of adiponectin. *Significant difference (P < 0.05) relative to the paired control cells in the absence of adiponectin. **Significant difference (P < 0.05) relative to control siRNA-transfected cells in the presence of adiponectin. N.S., not significant relative to paired control cells in the absence of adiponectin.
Increased serum adiponectin upregulates FHC in skeletal muscles in vivo.
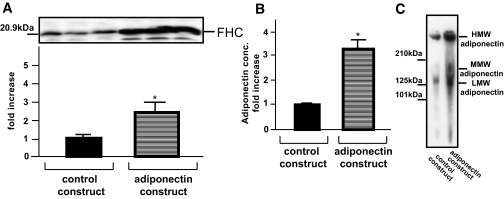
To further confirm the FHC upregulation by adiponectin in in vivo experiments, we prepared mice expressing recombinant adiponectin by systemic adenovirus injection into the tail vein. Adenovirus gene transfer revealed ectopic overexpression of adiponectin in the liver to markedly upregulate serum adiponectin (control construct: 14.4 ± 0.6 μg/ml and adiponectin construct: 44.5 ± 4.9 μg/ml) (Fig. 8B). In particular, mainly the HMW and LMW forms of adiponectin were increased (Fig. 8C). As shown by immunoblotting of skeletal muscles, FHC expression in these muscles was increased 2.5-fold in adiponectin-transferred mice (Fig. 8A) (i.e., in vitro experiments confirmed FHC upregulation under physiological conditions).
FIG. 8.
Effects of adiponectin overexpression on FHC regulation in skeletal muscles. Mice were systemically injected with recombinant adenovirus expressing LacZ (L group) or adiponectin (A group) via the tail vein. Three days after virus infection, we confirmed increased serum adiponectin levels, using a mouse/rat adiponectin enzyme-linked immunosorbent assay kit (B) and by immunoblot analysis (C) and quantitatively analyzed FHC expression in total hindlimbs (A), as previously described (19). Representative data of three mice (A) or one mouse (C) from each group are presented. Each column shows the means ± SE obtained from seven animals in each group. *Significant difference (P < 0.05) relative to L group.
DISCUSSION
Intensive previous studies (5,8) revealed adiponectin to improve insulin sensitivity and increase fatty acid oxidation in skeletal muscles. In fact, MMW or HMW adiponectin exerts these effects on skeletal muscles by activating AMP-activated protein kinase and peroxisome proliferator–activated receptor-α (26). On the other hand, HMW adiponectin was previously reported to activate NF-κB (10), the master coordinator of immunity, inflammation, differentiation, and cell survival (17,27–29). However, the physiological role of NF-κB activation in skeletal muscle cells has yet to be elucidated. In the present study, we treated murine primary cultured skeletal muscle cells with recombinant adiponectin and found FHC to be significantly increased. The two-dimensional gel electrophoresis–based proteomic approach used herein is generally acknowledged to be relatively insensitive in that it measures only a limited subset of tissue proteins and systematically excludes several classes. Adiponectin-induced FHC upregulation was seen only in cultured skeletal muscle cells, not endothelial cells (i.e., HUVECs). Judging from the small increase in ICAM-1 expression with adiponectin incubation, the NF-κB–activating effect of adiponectin was likely to be so small that we were unable to demonstrate FHC upregulation in HUVECs. These response differences between skeletal muscle and endothelial cells may be explained by variations in adiponectin species or different tissue localizations of molecules involved in adiponectin signaling, such as adiponectin receptors.
Our results demonstrate NF-κB activation to be involved in FHC upregulation by adiponectin. This mechanism of FHC upregulation is supported by the following data. First, in agreement with prior studies (10), we demonstrated that adiponectin does, in fact, really phosphorylate and degrade IκB-α in cultured skeletal muscle cells, thereby enhancing NF-κB activation. Second, FHC is regulated by NF-κB activation in response to enhanced oxidative stress (30). Third, FHC induction in response to adiponectin incubation is completely inhibited by BAY11-7082, an inhibitor of IκB-α phosphorylation, or NF-κB SN50, an inhibitor of NF-κB translocation into the nucleus. Though cAMP-dependent induction of FHC was previously demonstrated in human HeLa cells (31), our results show clearly that the cAMP/PKA pathway is not involved in FHC upregulation by adiponectin. Further study is needed to clarify the precise mechanisms by which FHC is regulated.
Ferritin is a major intracellular iron-storage protein that sequesters excess free iron molecules to minimize the generation of iron-catalyzed ROS (30,32). Ferritin consists of two subunits, FHC and FLC (15), and there are functional differences between these subunits. FHC has ferroxidase activity (i.e., the oxidation of Fe2+ to Fe3+), which is involved in rapid iron uptake and release and is required for iron sequestration (16). On the other hand, FLC has no ferroxidase activity but is likely to contribute to stabilization of assembled ferritin proteins for long-term iron storage (33). AP-1 motifs or NF-κB–responsive elements have been found in the promoter region of FHC (22,34) but not in that of FLC. Indeed, our results revealed only the expression of FHC (i.e., not that of FLC) to be increased in response to adiponectin exposure. In addition to this iron-mediated regulation, ferritin is regulated by immune/inflammatory cytokines. For example, similar changes in FHC-to-FLC ratios (i.e., increased FHC with no significant change in FLC expression) were observed in myoblasts following cytokine stimulation (35). Serum levels of ferritin were previously reported to be increased in patients with nonalcoholic steatohepatitis (36) or type 2 diabetes with obesity (37), disorders characterized by inflammation in the liver or adipose tissues. In these conditions, NF-κB plays a pivotal role in cytokine-induced FHC upregulation (17).
In recent decades, studies of NF-κB have concentrated mainly on discovering the molecules and biochemical processes essential to the signaling cascades controlling NF-κB activity, since NF-κB is known to be one of the critical transcription factors mediating inflammatory cellular responses, such as the production of cytokines and adhesion molecules (36,38). However, recent studies have focused on elucidating the novel mechanism whereby NF-κB exerts a protective effect against cytotoxicity. Notably, Pham et al. (17) discovered how NF-κB antagonizes TNF-α−induced apoptosis. They identified FHC as a critical mediator of NF-κB protective activity against TNF-α−induced cytotoxicity and concluded that FHC mediates suppression of ROS accumulation, which in turn prevents persistent activation of the Jun NH2-terminal kinase pathway, thereby inhibiting apoptosis. Thus, in adiponectin-sensitive tissues, such as skeletal muscle cells, FHC upregulation induced by adiponectin plays a pivotal role in the antagonistic cross-talk between the NF-κB and ROS/Jun NH2-terminal kinase pathways.
The physiological roles of NF-κB in skeletal muscles are currently unknown despite the well-recognized increase in NF-κB activity with acute exercise and muscle contraction (39). However, given the array of NF-κB target gene products in skeletal muscles, NF-κB is speculated to serve as a scavenger in states of oxidative stress because stress factors accumulate with exercise and muscle contraction (40). Along with FHC, MnSOD and iNOS were demonstrated to be increased and to be targets of the NF-κB gene product. Thus, our results suggest adiponectin to support NF-κB activation and thereby produce beneficial effects in skeletal muscle by reducing ROS via FHC upregulation.
In conclusion, we have clarified that NF-κB–targeted genes were upregulated by adiponectin in skeletal muscle cells, making this report, to our knowledge, the first ever demonstration of this property of adiponectin. Taking into consideration that ROS activity is subject to negative feedback regulation by NF-κB, adiponectin plays key roles in reducing oxidative stress and in cytoprotection against ROS in skeletal muscles. In fact, previous studies (41,42) have demonstrated a close relation between oxidative stress and insulin resistance. Thus, ROS production, following the accumulation of excessive fat, may account for the link between obesity and insulin resistance. Considering the beneficial effects of adiponectin on obesity-linked insulin resistance, FHC upregulation by adiponectin may play an important role in the mechanism by which adiponectin improves insulin resistance.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research no. 18591001 (to K.I.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
No potential conflicts of interest relevant to this article were reported.
We thank Y. Asami for help with some of the experiments and are grateful to S. Ohshima and N. Hirosawa for their excellent technical assistance with the flow cytometry and the two-dimensional electrophoresis, respectively.
Published ahead of print at http://diabetes.diabetesjournals.org on 17 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
REFERENCES
- 1.Guerre-Millo M: Adipose tissue and adipokines: for better or worse. Diabetes Metab 30: 13–19, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Shapiro L, Scherer PE: The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr Biol 8: 335–338, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara H, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T: The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K: Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 221: 286–289, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T: Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Ishigami M, Kuriyama H, Kishida K, Nishizawa H, Hotta K, Muraguchi M, Ohmoto Y, Yamashita S, Funahashi T, Matsuzawa Y: Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103: 1057–1063, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y: Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100: 2473–2476, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T: Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T: Impaired multimerization of human adiponectin mutants associated with diabetes: molecular structure and multimer formation of adiponectin. J Biol Chem 278: 40352–40363, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF: Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity: different oligomers activate different signal transduction pathways. J Biol Chem 278: 50810–50817, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE: Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem 279: 12152–12162, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y: Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 94: 27–31, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT: Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes 55: 249–259, 2006 [PubMed] [Google Scholar]
- 14.Nakashima R, Kamei N, Yamane K, Nakanishi S, Nakashima A, Kohno N: Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab 91: 3873–3877, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Arosio P, Levi S: Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med 33: 457–463, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Torti FM, Torti SV: Regulation of ferritin genes and protein. Blood 99: 3505–3516, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G: Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 119: 529–542, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Sinha S, Perdomo G, Brown NF, O'Doherty RM: Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem 279: 41294–41301, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Inukai K, Shewan AM, Pascow WS, Katayama S, James DE, Oka Y: Carboxy terminus of glucose transporter 3 contains an apical membrane targeting domain. Mol Endocrinol 18: 339–349, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I: Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci U S A 93: 1320–1324, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inukai K, Nakashima Y, Watanabe M, Takata N, Sawa T, Kurihara S, Awata T, Katayama S: Regulation of adiponectin receptor gene expression in diabetic mice. Am J Physiol Endocrinol Metab 288: E876–E882, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Kwak EL, Larochelle DA, Beaumont C, Torti SV, Torti FM: Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. J Biol Chem 270: 15285–15293, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Bevilacqua MA, Faniello MC, Quaresima B, Tiano MT, Giuliano P, Feliciello A, Avvedimento VE, Cimino F, Costanzo F: A common mechanism underlying the E1A repression and the cAMP stimulation of the H ferritin transcription. J Biol Chem 272: 20736–20741, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Hattori Y, Hattori S, Kasai K: Globular adiponectin activates nuclear factor-kappa B in vascular endothelial cells, which in turn induces expression of proinflammatory and adhesion molecule genes. Diabetes Care 29: 139–141, 2006 [DOI] [PubMed] [Google Scholar]
- 25.McConell GK, Bradley SJ, Stephens TJ, Canny BJ, Kingwell BA, Lee-Young RS: Skeletal muscle nNOSμ protein content is increased by exercise training in humans. Am J Physiol Regul Integr Comp Physiol 293: R831–R828, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB: Adiponectin increased fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor α. Diabetes 55: 2562–2570, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Barnes PJ, Karin M: Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066–1071, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Makarov SS: NF-kappaB as a therapeutic target in chronic inflammation: recent advances. Mol Med Today 6: 441–448, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Hayden MS, West AP, Ghosh S: NF-kappaB and the immune response. Oncogene 25: 6758–6780, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM: Ferritin and the response to oxidative stress. Biochem J 357: 241–247, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bevilacqua MA, Faniello MC, Russo T, Cimino F, Costanzo F: Transcriptional regulation of the human H ferritin-encoding gene (FERH) in G418-treated cells: role of the B-box-binding factor. Gene 141: 287–291, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Cozzi A, Corsi B, Levi S, Santambrogio P, Albertini A, Arosio P: Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J Biol Chem 275: 25122–25129, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Levi S, Luzzago A, Cesareni G Cozze A, Franceschinelli F, Albertini A, Arosio P: Mechanism of ferritin iron uptake: activity of the H-chain and deletion mapping of the ferro-oxidase site: a study of iron uptake and ferro-oxidase activity of human liver, recombinant H-chain ferritins, and of two H-chain deletion mutants. J Biol Chem 263: 18086–18092, 1988 [PubMed] [Google Scholar]
- 34.Tsuji Y, Akebi N, Lam TK, Nakabeppu Y, Torti SV, Torti FM: FER-1, an enhancer of the ferritin H gene and a target of E1A-mediated transcriptional repression. Mol Cell Biol 15: 5152–5164, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller LL, Miller SC, Torti SV, Tsuji Y, Torti FM: Iron independent induction of ferritin H-chain by tumor necrosis factor. Proc Natl Acad Sci U S A 88: 4946–4950, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fargion S, Mattioli M, Fracanzani AL, Sampietro M, Tavazzi D, Fociani P, Taioli E, Valenti L, Fiorelli G: Hyperferritinemia, iron overload, and multiple metabolic alterations identify patients at risk for nonalcoholic steatohepatitis. Am J Gastroenterol 96: 2448–2455, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Forouhi NG, Harding AH, Allison M, Sandhu MS, Welch A, Luben R, Bingham S, Khaw KT, Wareham NJ: Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-Norfolk prospective study. Diabetologia 50: 949–956, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY: Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem 276: 7614–7620, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Lappas M, Permezel M, Georgiou HM, Rice GE: Nuclear factor kappa B regulation of pro-inflammatory cytokines in human gestational tissues in vitro. Biol Reprod 67: 668–673, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Ho RC, Hirshman MF, Li Y, Cai D, Farmer JR, Aschenbach WG, Witczak CA, Shoelson SE, Goodyear LJ: Regulation of I-κB kinase and NF-κB in contracting adult rat skeletal muscle. Am J Physiol Cell Physiol 289: C794–C801, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N: Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3–L1 adipocytes. Diabetes 47: 1562–1569, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Houstis N, Rosen ED, Lander ES: Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440: 944–948, 2006 [DOI] [PubMed] [Google Scholar]