Abstract
OBJECTIVE—Obesity is characterized by an overgrowth of adipose tissue that leads to the formation of hypoxic areas within this tissue. We investigated whether this phenomenon could be responsible for insulin resistance by studying the effect of hypoxia on the insulin signaling pathway in adipocytes.
RESEARCH DESIGN AND METHODS—The hypoxic signaling pathway was modulated in adipocytes from human and murine origins through incubation under hypoxic conditions (1% O2) or modulation of hypoxia-inducible factor (HIF) expression. Insulin signaling was monitored through the phosphorylation state of several key partners of the pathway and glucose transport.
RESULTS—In both human and murine adipocytes, hypoxia inhibits insulin signaling as revealed by a decrease in the phosphorylation of insulin receptor. In 3T3-L1 adipocytes, this inhibition of insulin receptor phosphorylation is followed by a decrease in the phosphorylation state of protein kinase B and AS160, as well as an inhibition of glucose transport in response to insulin. These processes were reversible under normoxic conditions. The mechanism of inhibition seems independent of protein tyrosine phosphatase activities. Overexpression of HIF-1α or -2α or activation of HIF transcription factor with CoCl2 mimicked the effect of hypoxia on insulin signaling, whereas downregulation of HIF-1α and -2α by small interfering RNA inhibited it.
CONCLUSIONS—We have demonstrated that hypoxia creates a state of insulin resistance in adipocytes that is dependent upon HIF transcription factor expression. Hypoxia could be envisioned as a new mechanism that participates in insulin resistance in adipose tissue of obese patients.
Obesity results from an imbalance between energy intake and energy expenditure. Abdominal obesity and adipose tissue dysfunction are major risk factors for chronic diseases, such as insulin resistance, type 2 diabetes, and cardiovascular diseases. Insulin resistance is associated with alterations in glucose and lipid homeostasis. At the molecular level, insulin resistance is triggered by a dysregulation of the insulin signaling cascade. Insulin stimulates the tyrosine kinase activity of its receptor, leading to tyrosine phosphorylation of its substrates, such as insulin receptor substrate (IRS)-1 and -2 or Shc. They are upstream of two major signaling pathways: the phosphatidylinositol 3-kinase/protein kinase B (PKB) pathway, responsible for most of the metabolic actions of insulin, and the Ras–extracellular signal–related kinase pathway, which regulates gene expression (1).
During the genesis of obesity, adipose tissue is one of the first tissues affected by insulin resistance. This phenomenon is closely associated with the development of a proinflammatory state within the adipose tissue. In addition to this proinflammatory state, obesity is associated with the formation of hypoxic areas within the tissue. This has been demonstrated in obese mice (ob/ob and dietary induced obesity) using various methods, such as immunohistochemistry with pimonidazole, use of O2 sensor probes, and lactate detection (2–4). Hypoxia, a deficiency in O2, is a major stimulus affecting a number of biological functions, such as angiogenesis, cell proliferation, apoptosis, and inflammation, and it switches cell metabolism from aerobic respiration to anaerobic glycolysis (5–7). Hypoxia mediates its effect through the activation of hypoxia-inducible factor (HIF), a basic helix-loop-helix transcription factor composed of two subunits, HIF-α and -β. Although HIF-β is constitutively expressed, HIF-α protein level is regulated. In the presence of O2, HIF-α is subjected to proline hydroxylation, leading to degradation by the proteasome. Hypoxia inactivates prolyl-hydroxylases, leading to HIF-α accumulation and formation of a functional heterodimeric transcription factor. Two α subunits, HIF-1α and -2α, show similarities in structure and regulation, but they regulate distinct sets of genes and are not redundant (5,7,8). HIF-1α and -2α expression are also regulated by O2-independent mechanisms because growth factors and cytokines stimulate HIF-1α and -2α protein synthesis via phosphatidylinositol 3-kinase or extracellular signal–related kinase pathways (9–12). Because hypoxia produces profound changes in cell metabolism, we investigated its effect on insulin signaling.
In the current study, we demonstrated that hypoxia creates an insulin-resistant state in adipocytes by inhibiting phosphorylation of the insulin receptor tyrosine, leading to a decrease in glucose transport. This phenomenon could contribute to the development of insulin resistance within adipose tissue.
RESEARCH DESIGN AND METHODS
Insulin was obtained from Lilly (Paris, France). Antibodies to HIF-1α (clone H1α67) and HIF-2α were purchased from Novus Biologicals (Littleton, CO). Antibodies to GLUT1 and HIF-2α were obtained from Abcam (Paris, France). Antibodies to phosphotyrosine, phospho-S6 kinase 1, phospho-Thr208 PKB, PKB, and GLUT-4 were purchased from Cell Signaling Technology (Beverly, MA). Antibody to phospho-S6 kinase 1, insulin receptor-β, and small interfering RNA (siRNA; control, HIF-1α, and -2α) were purchased from Santa Cruz Biotechnology (Tebu, France). Polyclonal insulin receptor substrate (IRS)-1 and -2 antibodies used in immunoprecipitation experiments were raised against a peptide corresponding to the last 14 amino acids of IRS-1 and a peptide corresponding to the last 16 amino acids of IRS-2 (Eurogentec, Seraing, Belgium). Polyclonal antibody directed against phospho-Ser632 IRS-1 has been described previously (13). Monoclonal anti–IRS-1 antibody used in immunoblotting experiments was purchased from BD Biosciences (PharMingen, San Diego, CA). Antibody to tubulin was purchased from Sigma-Aldrich. Culture media were from Invitrogen.
DNA plasmids.
Plasmid enhanced green fluorescent protein–C1-HIF-1α–green fluorescent protein (pEGFP-C1-HIF-1α-GFP) has been described previously (10). pcDNA3-HIF-2α cDNA was obtained from Steve McKnight and Richard Bruick (University of Texas Southwestern Medical Center) (14).
Cell culture.
Human embryonic kidney cells (HEK-293) were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 5% (vol/vol) FCS. 3T3-L1 fibroblasts were grown at 7% CO2 and 37°C in DMEM supplemented with 10% (vol/vol) calf serum and induced to differentiate as previously described (15). Human preadipocytes obtained from Biopredic (Rennes, France) were grown and induced to differentiate as previously described (16).
Transfection.
HEK-293 cells were transiently transfected using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN), according to the manufacturer's instructions. siRNAs were transfected using INTERFERin siRNA transfection reagent according to the manufacturer<5002>s instructions (Polyplus Transfection; Ozyme). Briefly, differentiated 3T3-L1 adipocytes were trypsinized and seeded in 12-well plates. After 24 h, siRNAs (50 nmol/l) directed against HIF-1α or -2α were transfected using INTERFERin and used 48 h after transfection.
Hypoxia treatment.
For hypoxic incubations, medium was replaced with DMEM containing 0.5% BSA (wt/vol), and adipocytes were incubated at 37°C in 95% air and 5% CO2 (normoxic conditions) or placed in a hypoxic chamber (Billups-Rothenberg, Dell Mar, CA) flushed for 10 min with gas mixture consisting of 1% O2, 5% CO2, and 94% N2 (hypoxic conditions). Cells were incubated for 16 h at 37°C. After hypoxic incubation, the chambers were opened in an anaerobic glove box (flushed with N2) to avoid reoxygenation. Cells were stimulated with ligands, and cell lysates were prepared. Absence of cytotoxicity was assessed by measuring lactate deshydrogenase activity according the manufacturer's instructions (Roche). For short-term hypoxia experiments, media were flushed with N2 within the anaerobic glove box to remove O2 from the medium prior to hypoxic incubation.
Western blot analysis.
Adipocytes were resuspended in lysis buffer (50 mmol/l HEPES [pH 7.4], 150 mmol/l NaCl, 10 mmol/l EDTA, 10 mmol/l Na4P2O7, 100 mmol/l NaF, 2 mmol/l vanadate, protease inhibitor cocktail [Complete; Roche], and 1% [vol/vol] Triton X-100) and immediately frozen in liquid nitrogen. Lysates were centrifuged (14,000 rpm) for 10 min at 4°C, and the protein concentration was determined using BCA protein assay reagent (Pierce). Cell lysates were either directly analyzed by Western blot or were subjected to immunoprecipitation. Immunoblottings were performed as previously described (10) and were revealed using a Fujifilm LAS-3000 imaging system. Quantifications were realized using MultiGauge software.
Measurement of reactive oxygen species.
3T3-L1 adipocytes were incubated for 30 min with 10 μmol/l 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA; Molecular Probes) in PBS. DCFH-DA was removed and adipocytes incubated in normoxia or hypoxia. After washes, adipocytes were sonicated in water. Fluorescence was determined at 485/520 nm and normalized to protein concentration.
Glucose transport.
3T3-L1 adipocytes were incubated for 16 h in normoxia or hypoxia and were stimulated with 100 nmol/l insulin for 20 min in normoxia or hypoxia. Glucose transport was determined by the addition of 2-[3H]deoxyglucose (0.1 mmol/l, 0.5 μCi/ml) as previously described (15).
Glycerol release and interleukin-1β and -6 measurement.
Glycerol was measured in cell culture media using free glycerol reagent (Sigma-Aldrich, Lyon, France), and interleukin (IL)-1β and -6 were measured in cell culture media using a RayBio enzyme-linked immunosorbent assay kit (Tebu, France) according to the manufacturer's instructions.
Protein tyrosine phosphatase activity.
Adipocytes were incubated in normoxia or hypoxia and were homogenized inside an anaerobic glove box (17). Insulin stimulation, cell homogenization, and protein tyrosine phosphatase (PTPase) assay were performed in anaerobic conditions to avoid any air oxidation. Cell were lysed in PTPase lysis buffer (150 mmol/l NaCl, 5 mmol/l EDTA, 5 mmol/l EGTA, and 50 mmol/l HEPES, pH 7.5, containing protease inhibitor cocktail [Complete; Roche] and 1% [vol/vol] Triton X-100) followed by centrifugation at 14,000 rpm for 15 min. PTPase activity was determined at 30°C for 30 min in a reaction buffer containing 20 mmol/l para-nitrophenyl phosphate in 50 mmol/l Tris (pH 7.5), 50 mmol/l NaCl, and 3 mmol/l dithiothreitol, and the absorption was determined at 410 nm (18). Positive control was performed using purified calf intestinal phosphatase.
Statistical analysis.
Statistical differences between groups were analyzed by Student's t test and were considered significant at P ≤ 0.05.
RESULTS
Hypoxia inhibits the insulin signaling pathway in human adipocytes and 3T3-L1 adipocytes.
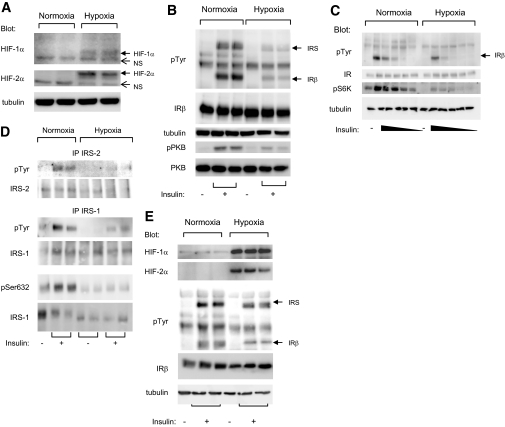
Because obesity is associated with the formation of hypoxic areas within the adipose tissue, we investigated the effect of hypoxia on the insulin signaling pathway in adipocytes. Murine 3T3-L1 adipocytes were incubated in normoxia or hypoxia (1%) for 16 h before being stimulated with insulin for 5 min. The condition of 1% O2 is similar to that found in the adipose tissue of obese mice (15.2 mmHg, 1–2% O2) (2,3). Hypoxia induced an increase in HIF-1α and -2α protein levels in 3T3-L1 adipocytes (Fig. 1A). In parallel, hypoxia inhibited the ability of insulin to stimulate the autophosphorylation of its receptor as well as the phosphorylation of PKB (Fig. 1B). 3T3-L1 adipocytes were incubated for 16 h in normoxia or in hypoxia before being stimulated with decreasing amounts of insulin (ranging from 100 to 0.01 nmol/l). The inhibition of insulin-induced insulin receptor phosphorylation by hypoxia was observed at maximal and submaximal insulin concentrations. Moreover, hypoxia inhibited S6 kinase (S6K) phosphorylation in response to insulin (Fig. 1C).
FIG. 1.
Hypoxia inhibited insulin-induced insulin receptor tyrosine phosphorylation in human and 3T3-L1 adipocytes. A: 3T3-L1 adipocytes were incubated for 16 h at 37°C in normoxia (21% O2) or hypoxia (1% O2). Cell lysates were analyzed by immunoblots with the indicated antibodies. B: 3T3-L1 adipocytes were incubated for 16 h at 37°C in normoxia (21% O2) or hypoxia (1% O2) before being stimulated with insulin (100 nmol/l) for 5 min. C: 3T3-L1 adipocytes were incubated for 16 h at 37°C in normoxia (21% O2) or hypoxia (1% O2) before being stimulated with decreasing concentrations of insulin ranging from 100 to 0.01 nmol/l. D: Cell lysates were subjected to immunoprecipitation using antibodies to IRS-1 or -2 followed by immunoblots using indicated antibodies. E: Human adipocytes were obtained after differentiation of preadipocytes and were incubated for 24 h at 37°C in normoxia (21% O2) or hypoxia (1% O2). After insulin stimulation (100 nmol/l) for 5 min, cell lysates were analyzed by immunoblots with indicated antibodies. Representative experiments of at least three independent experiments performed in duplicate or triplicate are shown. IP, immunoprecipitation; IR, insulin receptor; pTyr, phosphorylated tyrosine; pS6K, phospho-S6K 1; pSer632, phospho-Ser632.
Specific immunoprecipitation of IRS-1 or -2 revealed that hypoxia inhibited IRS-1 and -2 tyrosine phosphorylation (Fig. 1D). Because serine phosphorylation of IRS-1 is implicated in the inhibition of its tyrosine phosphorylation and in insulin resistance (19), we investigated the level of IRS-1 serine phosphorylation. Hypoxia inhibited the phosphorylation of Ser632 residue in response to insulin (Fig. 1D). No effect of hypoxia on the phosphorylation on Ser307 and Ser789 was detected (data not shown). It is to be noted that hypoxia did not induce cell toxicity, as measured by lactate deshydrogenase activity, or caspase 3 activity (data not shown). Because general tissue oxygenation is ∼50 mmHg (7% O2), the ability of 3T3-L1 adipocytes to respond to insulin treatment after incubation in 21%, 7%, or 1% O2 was examined. Adipocytes incubated in 21% or 7% O2 responded similarly to insulin stimulation (Online Appendix 1, available at http://dx.doi.org/10.2337/db08-0457).
Hypoxia also inhibited insulin signaling pathway in human adipocytes (Fig. 1E). Indeed, human adipocytes were incubated in normoxia (21% O2) or in hypoxia (1% O2) for 24 h and stimulated with insulin for 5 min. Hypoxia stimulated HIF-1α and -2α protein expression and inhibited insulin-induced insulin receptor and IRS tyrosine phosphorylation. In conclusion, in both human and murine adipocytes, hypoxia inhibited the ability of insulin to induce the phosphorylation of its receptor as well as IRS-1, IRS-2, PKB, and S6K.
Inhibition of insulin-induced insulin receptor tyrosine phosphorylation by hypoxia is rapid and reversible.
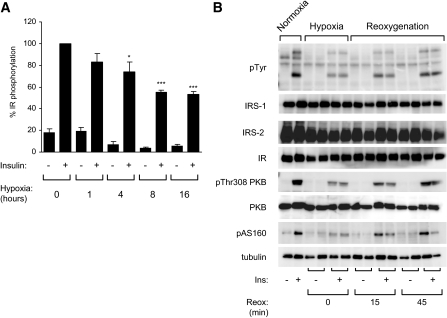
The time course of hypoxia-induced inhibition of insulin receptor phosphorylation was evaluated by incubating 3T3-L1 adipocytes in normoxia or hypoxia for 1, 4, 8, and 16 h before being stimulated with insulin for 5 min. As shown in Fig. 2A, the inhibitory effect of hypoxia on insulin receptor tyrosine phosphorylation was detected as soon as 1 h. These effects were more pronounced after 8 h of hypoxia. These observations demonstrate that hypoxia rapidly regulated the inhibition of the insulin signaling pathway.
FIG. 2.
The effect of hypoxia on the insulin signaling pathway is rapid and reversible. A: Prior to hypoxic incubation, the medium was flushed with N2 within an anaerobic glove chamber to remove O2 from the medium. 3T3-L1 adipocytes were incubated in normoxia (21% O2) or hypoxia (1% O2) for 1, 4, 8, and 16 h at 37°C. Adipocytes were stimulated with insulin (100 nmol/l) for 5 min, and cell lysates were analyzed by immunoblots with antiphosphotyrosine and insulin receptor antibodies. Quantification of insulin receptor tyrosine phosphorylation compared with the amount of insulin receptor of four to five independent experiments performed in duplicate is shown. B: 3T3-L1 adipocytes were incubated in normoxia or hypoxia (1% O2) for 16 h before being reoxygenated for 15 and 45 min at 37°C. Normoxic, hypoxic, and reoxygenated adipocytes were stimulated with insulin (100 nmol/l) for 5 min. Cell lysates were analyzed by immunoblots with indicated antibodies. A representative experiment of three independent experiments performed in duplicate is shown. Ins, insulin; IR, insulin receptor; pAS160, phospho-AS160; pThr308 PKB, phospho-Thr308 PKB; pTyr, phosphorylated tyrosine; Reox, reoxygenation. *P < 0.05; ***P < 0.001.
To determine whether the effect of hypoxia is reversible, 3T3-L1 adipocytes were incubated in hypoxia for 16 h. Hypoxic adipocytes were stimulated directly after hypoxia or were reoxygenated for 15 and 45 min before insulin stimulation (Fig. 2B). Control adipocytes were maintained in normoxia for 16 h and stimulated with insulin. Hypoxia impaired the ability of insulin to stimulate insulin receptor tyrosine phosphorylation as well as PKB and AS160 phosphorylation. During reoxygenation, the ability of insulin to stimulate phosphorylation of insulin receptor and signaling proteins was restored after 45 min.
Hypoxia inhibits glucose transport.
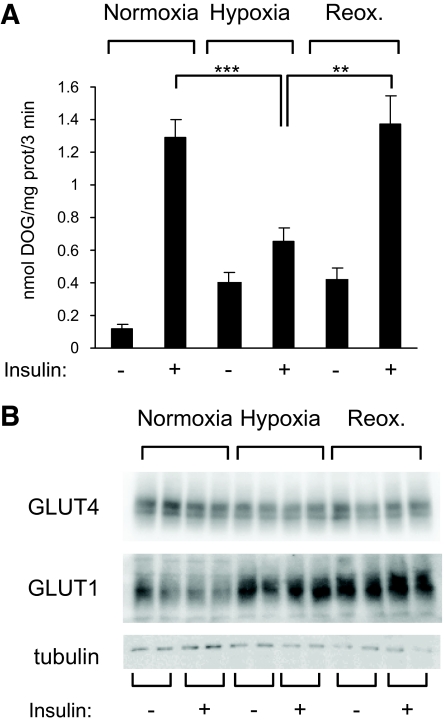
PKB and its substrate AS160 play an important role in insulin-induced GLUT4 translocation and glucose transport. We investigated whether hypoxia inhibited the downstream response of insulin, such as glucose transport in 3T3-L1 adipocytes. 3T3-L1 adipocytes were incubated in normoxia or hypoxia for 16 h or reoxygenated for 1 h. Glucose transport was determined by the increase in [3H]deoxyglucose (Fig. 3A). In normoxic adipocytes, insulin induced a 10-fold increase in glucose transport. Hypoxia upregulated basal glucose transport but inhibited insulin-induced glucose transport. Reoxygenation of adipocytes restored the ability of insulin to stimulate glucose entry to levels comparable to normoxic adipocytes. In reoxygenated adipocytes, basal glucose transport remained elevated. This increase in basal glucose uptake probably resulted from increased GLUT1 expression, which was maintained even after 1 h of reoxygenation, without modifying GLUT4 protein levels (Fig. 3B).
FIG. 3.
Hypoxia inhibits insulin-induced glucose transport. A: 3T3-L1 adipocytes were incubated for 16 h in normoxia or hypoxia before insulin-stimulated glucose transport was measured as described in research design and methods. Data are the means ± SE of three independent experiments performed in triplicate. B: Cell lysates were analyzed by immunoblots using the indicated antibodies. DOG, deoxyglucose; prot, protein; Reox, reoxygenation. **P < 0.01; ***P < 0.001.
Hypoxia induces reactive oxygen species generation in 3T3-L1 adipocytes.
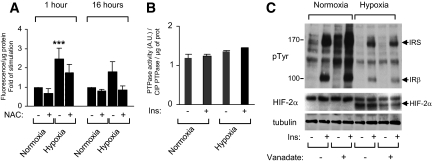
Insulin resistance has been associated with the generation of reactive oxygen species. We investigated whether hypoxia induced reactive oxygen species (ROS) production in 3T3-L1 adipocytes. To this end, we monitored the formation of ROS detected by the oxidation of the DCFH dye (20). Hypoxia stimulated the generation of ROS as soon as 1 h, which was maintained up to 16 h (Fig. 4A). Formation of ROS was in part inhibited by the antioxidant N-acetyl cysteine.
FIG. 4.
Hypoxia increased ROS generation but did not activate protein tyrosine phosphatases. A: 3T3-L1 adipocytes were incubated with DCFH-DA for 30 min before being incubated for 16 h in normoxia or hypoxia, in the absence or presence of N-acetyl cysteine (10 mmol/l). Adipocytes were lysed, and fluorescence was measured. Results are expressed as the fold of stimulation compared with controls. Data are the means ± SE of three independent experiments performed in triplicate. B: 3T3-L1 adipocytes were incubated in normoxia (21% O2) or hypoxia (1% O2) for 16 h before being stimulated for 5 min with insulin (100 nmol/l). Total phosphatase activity was determined by the dephosphorylation of the synthetic substrate pNPP (para-nitrophenyl phosphate) compared with the activity of CIP (calf intestine phosphatase). Data are the means ± SE of three independent experiments performed in triplicate. C: 3T3-L1 adipocytes were incubated in normoxia or hypoxia for 16 h in the absence or presence of 2 mmol/l vanadate. Adipocytes were stimulated with insulin (100 nmol/l) for 5 min, and cell lysates were analyzed by immunoblots with indicated antibodies. A representative experiment of three independent experiments is shown. Ins, insulin; NAC, N-acetyl cysteine; prot, protein; pTyr, phosphorylated tyrosine. ***P < 0.001.
Hypoxia does not inhibit insulin signaling through the activation of protein tyrosine phosphatases.
Attenuation of insulin signaling can occur through the dephosphorylation of the receptor by protein tyrosine phosphatase (PTPase). PTPase-1B and leukocyte antigen–related phosphatase have been implicated in insulin receptor dephosphorylation (21). We determined whether hypoxia modulated total phosphatase activity by measuring the dephosphorylation of para-nitrophenyl phosphate. Cell homogenization and measurement of phosphatase activities were performed in anaerobic conditions to avoid oxidation and their subsequent inhibition. No significant difference in total phosphatase activity was observed in hypoxia compared with normoxia (Fig. 4B).
To confirm the lack of implication of PTPases in hypoxia-mediated inhibition of the insulin pathway, we studied whether vanadate, a potent tyrosine phosphatase inhibitor, could reverse the effect of hypoxia. Adipocytes were incubated in normoxia or hypoxia in the absence or presence of vanadate for 16 h before insulin stimulation (Fig. 4C). Although vanadate increased the level of protein tyrosine phosphorylation in normoxia, because of the inhibition of cellular phosphatases, it did not restore the ability of insulin to stimulate the tyrosine phosphorylation of its receptor in hypoxia. Similar results were obtained with H2O2, which induced oxidation and subsequent inhibition of tyrosine phosphatases (data not shown). These results suggest that hypoxia did not mediate its action through the activation of tyrosine phosphatase activities.
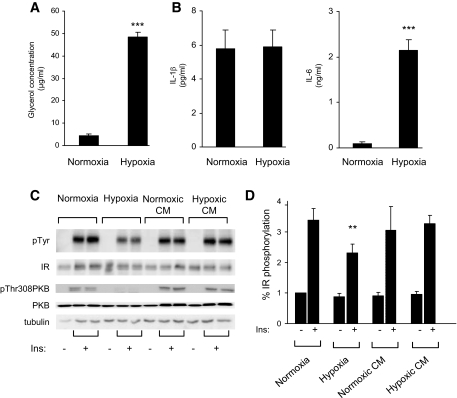
Hypoxia stimulates lipolysis and IL-6 secretion.
Free fatty acids and adipokines have been shown to be implicated in the development of insulin resistance. Thus, we investigated whether these factors could play a role in the inhibition of the insulin signaling pathway by hypoxia. First, we measured the release of glycerol and two adipokines, IL-1β and -6, in the conditioned media from normoxic or hypoxic adipocytes. 3T3-L1 adipocytes were subjected to normoxia or hypoxia for 16 h, conditioned media were collected, and glycerol (Fig. 5A) and IL-1β and -6 (Fig. 5B) were measured. Hypoxia induced a release of glycerol in the media (48 μg/ml ± 2 in hypoxia compared with 4 μg/ml ± 0.7 in normoxia). Moreover, IL-6 secretion was increased from 0.1 ± 0.02 ng/ml in normoxia up to 2.1 ± 0.28 ng/ml after 16 h of hypoxia. In contrast, the IL-1β protein level was not modified. Shorter incubations did not allow us to detect IL-6 or -1β expression (data not shown).
FIG. 5.
Effect of conditioned medium on insulin receptor phosphorylation. 3T3-L1 adipocytes were incubated for 16 h in normoxia or hypoxia. Conditioned media were collected, and secretion of glycerol (A) and IL-1β and -6 (B) was measured as described in research design and methods. Data are means ± SE of four independent experiments performed in triplicate. C: 3T3-L1 adipocytes incubated for 16 h in normoxia or hypoxia, or treated with conditioned medium from normoxic adipocytes (normoxic CM) or hypoxic adipocytes (hypoxic CM) were stimulated with 100 nmol/l insulin for 5 min. Cell lysates were analyzed with indicated antibodies. A representative experiment of four independent experiments performed in duplicate is shown. D: Quantification of tyrosine phosphorylation of insulin receptor compared with insulin receptor protein of four independent experiments is shown. Ins, insulin; IR, insulin receptor; pTyr, phosphorylated tyrosine. **P < 0.01; ***P < 0.001.
Then, we investigated whether the inhibition of insulin signaling by hypoxia could be mimicked by conditioned media. 3T3-L1 adipocytes were incubated under hypoxia or normoxia for 16 h, and the conditioned medium was collected and incubated with 3T3-L1 adipocytes for 16 h before stimulation with insulin (Fig. 5C). The effect of conditioned media was compared with the effect of hypoxia. As previously shown, hypoxia inhibited insulin-induced insulin receptor tyrosine phosphorylation and PKB phosphorylation compared with normoxic conditions. In the presence of conditioned media from hypoxic adipocytes, we did not observe a significant decrease in the insulin-induced phosphorylation of the insulin receptor or PKB. These results suggest that although secretion of adipokines is dysregulated during hypoxia, they cannot be responsible for the inhibition of insulin signaling induced by hypoxia.
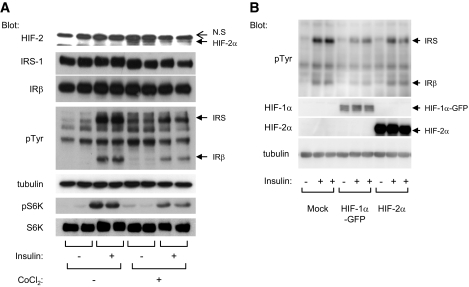
Effect of hypoxia on inhibition of the insulin signaling pathway is mimicked by CoCl2 and HIF-α expression.
Hypoxia allows the stabilization of HIF-α subunits, leading to the activation of the HIF transcription factor. CoCl2 is a hypoxia-mimicking agent that promotes HIF-α stabilization, HIF activation, and expression of hypoxia-induced genes (22). We investigated whether CoCl2 could mimic the effect of hypoxia on the insulin signaling pathway. 3T3-L1 adipocytes were treated with CoCl2 for 16 h followed by insulin stimulation. As shown in Fig. 6A, CoCl2 induced HIF-2α expression. Concomitantly, CoCl2 decreased the ability of insulin to stimulate insulin receptor and IRS tyrosine phosphorylation without affecting their respective protein levels. In parallel, CoCl2 inhibited the phosphorylation of S6K in response to insulin.
FIG. 6.
The effect of hypoxia on inhibition of the insulin signaling pathway is mimicked by CoCl2 and HIF-α expression. A: 3T3-L1 adipocytes were incubated for 16 h with CoCl2 (200 μmol/l) and stimulated with insulin (100 nmol/l) for 5 min. B: HEK-293 cells were transfected with indicated plasmids and stimulated with insulin for 5 min. Cell lysates were analyzed by immunoblots with indicated antibodies. A representative experiment of three independent experiments performed in duplicate is shown. GFP, green fluorescence protein; IR, insulin receptor; pS6K, phospho-S6K 1; pTyr, phosphorylated tyrosine.
Similar results were obtained when the expression of HIF-1α and -2α was induced by transfection of HIF-1α and -2α into HEK-293 cells before insulin stimulation. Ectopic expression of HIF-1α or -2α subunits led to a decrease in the ability of insulin to stimulate its receptor autophosphorylation compared with control cells (Fig. 6B). Together, these results show that enhanced HIF expression inhibits insulin signaling.
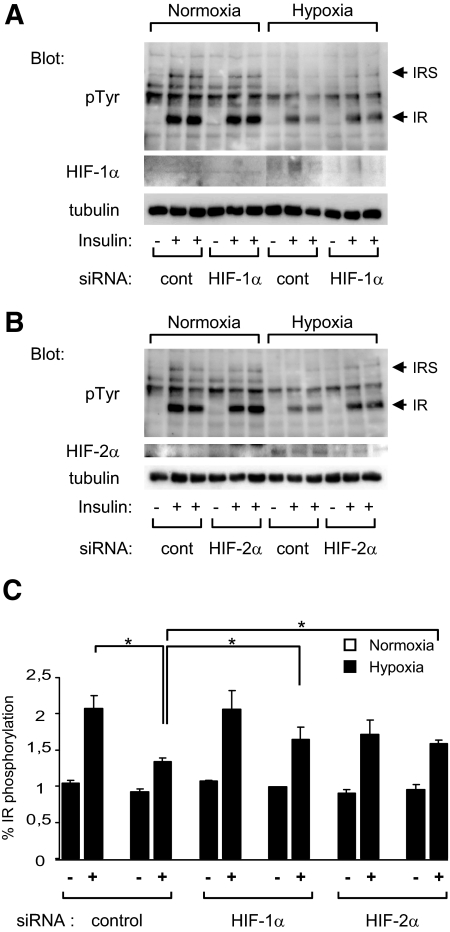
Inhibition of HIF activity restores insulin stimulation of the insulin receptor.
Because we showed that HIF expression was sufficient to induce insulin resistance, we investigated whether expression of these proteins was necessary for the inhibition of insulin signaling. HIF-1α or -2α expression was inhibited by siRNA transfection in 3T3-L1 adipocytes (Fig. 7). The siRNAs were able to partly inhibit (57 ± 3%) HIF-1α and -2α expression under hypoxia. This was accompanied by a partial restoration of the ability of insulin to stimulate the tyrosine phosphorylation of its receptor.
FIG. 7.
Inhibition of HIF-1α or -2α expression restores insulin stimulation of insulin receptors in hypoxia. 3T3-L1 adipocytes were transfected with siRNA for HIF-1α (A) or HIF-2α (B). At 48 h after transfection, adipocytes were incubated for 16 h in normoxia or in hypoxia for 16 h and stimulated with insulin (100 nmol/l) for 5 min. Cell lysates were analyzed by immunoblots with indicated antibodies. A representative experiment of three independent experiments performed in duplicate is shown. C: Quantification of insulin receptor tyrosine phosphorylation compared with insulin receptor amount. Data are the means ± SE of three independent experiments performed in duplicate. cont, control; IR, insulin receptor; pTyr, phosphorylated tyrosine. *P < 0.05.
The use of echinomycin, an inhibitor that has been characterized to inhibit HIF activity without modulating HIF-α subunit protein levels (23), led also to partial restoration of insulin-induced tyrosine phosphorylation of the insulin receptor in hypoxia (data not shown). Together, these results show that hypoxia inhibited insulin receptor and IRS tyrosine phosphorylation through mechanisms that are dependent of HIF transcription factors.
DISCUSSION
Obesity is associated with insulin resistance and type 2 diabetes. Massive development of the adipose tissue leads to the formation of hypoxic areas. As adipose tissue expands, some adipocytes become too distant from the vasculature to be correctly oxygenated. Indeed, development of hypoxia in the adipose tissue has been described in several genetic models of obesity in rodents. Partial pressure of O2 in the adipose tissue decreases from 47.9 mmHg in lean mice to 15.2 in ob/ob mice (2,3). In humans, the existence of hypoxia in the adipose tissue of obese patients is supported by the observation that although obese patients have more adipose tissue than lean patients, the cardiac output and blood flow directed to adipose tissue are not increased during obesity (24). Moreover, HIF-1 and HIF-1 target genes are overexpressed in the adipose tissue of obese individuals and decrease after weight loss (25). Finally, obesity is associated with hypertrophic adipocytes in which size prohibits a correct diffusion of O2 within the tissue.
In the current study, we propose that this hypoxic status within the adipose tissue could contribute to the development of insulin resistance directly through the inhibition of the insulin signaling pathway in adipocytes. In rodent adipocytes, hypoxia induces a rapid and robust inhibition of insulin signaling, as shown by a 50% inhibition of insulin receptor autophosphorylation and nearly complete inhibition of insulin-stimulated glucose transport. Because hypoxia affects the earliest intracellular step of insulin signaling, i.e., insulin receptor autophosphorylation, all subsequent steps, such as IRS and PKB phosphorylation and glucose transport, were affected. The mechanisms used by hypoxia to inhibit the insulin signaling pathway is not fully understood. Using experiments of gain and loss of function, we have shown that the inhibition of insulin signaling by hypoxia is dependent upon HIF-1 and -2 transcription factors. HIF-1 and -2 regulate >100 genes implicated in a broad range of cellular responses. HIF-1α and -2α are activated at different O2 concentrations, HIF-1α being more sensitive to O2 concentration. Although HIF-1 and -2 share common target genes, some specificity in gene expression has been revealed (5,7). Because downregulation of HIF-1α or -2α counteracts hypoxia-induced insulin resistance, it is likely that proteins involved are common target genes. These target genes are predicted to have a short half-life or to be rapidly inhibited. Indeed, hypoxia-induced insulin resistance is rapidly reversible. Reoxygenation restores insulin response after 45 min, as observed by an increase in insulin-stimulated insulin receptor phosphorylation and glucose transport. Interestingly, HIF proteins are not only induced during hypoxia but have also been reported to be induced by growth factors and cytokines. Because several of these circulating molecules have been shown to interact with insulin signaling, the HIF-mediated inhibition of insulin signaling described here could not be restricted to hypoxia. Moreover, we cannot exclude that HIF proteins mediate their effect independently of their action on transcription.
What could be the molecular mechanisms by which hypoxia mediates insulin resistance? PTPases, which could dephosphorylate the insulin receptor, seem not to be regulated by hypoxia. We show that hypoxia induces the production of ROS, which have been linked to impaired insulin signaling (26), through an increase in serine phosphorylation of IRS. We have not detected any increase in the phosphorylation status of IRS-1 serine phosphorylation, which has been associated with insulin resistance (Ser307, Ser789, and Ser632). Moreover, phosphorylation of these sites has not been reported to affect insulin receptor kinase activity. Hypoxia decreased IRS-1 serine phosphorylation on Ser632 residue, which is a target for mammalian target of rapamycin (mTOR) and S6K (19). Under hypoxia, energy expenditure is decreased, leading to the inhibition of high–energy-consuming events such as protein translation, leading to the inhibition of mTOR and S6K (27). However, one cannot exclude that ROS could affect insulin receptor tyrosine kinase activity directly or indirectly. Unfortunately, this is particularly difficult to investigate because ROS inhibitors by themselves directly affect insulin receptor tyrosine kinase activity (28) (data not shown).
In adipose tissue, hypoxia dysregulates the expression of some adipokines and proinflammatory cytokines (2–4,24,29,30), such as leptin, adiponectin, IL-1β, and IL-6, although tumor necrosis factor-α expression seems to be insensitive to hypoxia in human adipocytes (29). These cytokines are known to be involved in the downregulation of the insulin signaling pathway and to induce local and systemic insulin resistance (2,15). Analysis of conditioned media revealed that IL-6 protein secretion was increased by hypoxia but at concentrations inferior to that described to inhibit the insulin signaling pathway in adipocytes (2 vs. 20 ng/ml) (31). Thus, incubation of conditioned media from hypoxic adipocytes for 16 h did not mimic the effect of hypoxia on the inhibition of the insulin signaling pathway. This suggests that hypoxia-induced cytokine production by adipocytes is not the mechanism responsible for the inhibition of insulin signaling that we describe here. This is consistent with the observation that during hypoxia and obesity, cytokines are mainly produced by macrophages that invade the adipose tissue and not by adipocytes per se. Our in vitro system allowed us to show the direct effect of hypoxia on adipocytes.
The role of hypoxia in insulin resistance could be envisioned as a multistep process. In a first and early series of events, hypoxia induces local insulin resistance inside the adipose tissue by inhibiting the insulin signaling pathway. In a second and longer process, hypoxia attracts macrophages within the adipose tissue, leading to dysregulation of adipokine expression (3,32), which will be involved in the development of local, but also systemic, insulin resistance. Third, by creating a niche, hypoxia could increase mesenchymal stem cells’ self-renewal while inhibiting adipocyte differentiation in restricted parts of the tissue (6,33). Then, through angiogenesis, hypoxia could create new vessels that would lead to reoxygenation of these niches, leading to the development of new adipocytes. As obesity progresses, this mechanism could create a vicious circle aggravating the syndrome.
In conclusion, we show here that hypoxia inhibits insulin signaling in adipocytes through HIF proteins. This mechanism could be involved in the establishment of an insulin-resistant state during obesity.
Supplementary Material
Acknowledgments
This research was supported by funds from the Institut National de la Santé et de la Recherche Médicale (INSERM, France), the Association for Research on Diabetes (Paris, France), the University of Nice-Sophia Antipolis, Region Provence-Alpes-Cote-d'Azur, and Conseil Général des Alpes Maritimes. This work is part of the project “Hepatic and Adipose Tissue and Functions in the Metabolic Syndrome” (HEPADIP; http://www.hepadip.org/), which is supported by the European Commission as an integrated project under the 6th Framework Program (contract LSHM-CT-2005-018734).
This research was also supported by funding from the Association de Langue Française pour l'Etude du Diabète et des Maladies Métaboliques (ALFEDIAM)-Merck Lipha Santé and ALFEDIAM-Roche Diagnostics. No other potential conflicts of interest relevant to this article were reported.
We thank Teresa Gonzalez for her help in the culture and differentiation of human adipocytes.
Published ahead of print at http://diabetes.diabetesjournals.org on 4 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
See accompanying commentary, p. 26.
REFERENCES
- 1.Taniguchi CM, Emanuelli B, Kahn CR: Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Ye J, Gao Z, Yin J, He Q: Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293: E1118–E1128, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV: Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 32: 451–463, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I: Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56: 901–911, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Weidemann A, Johnson RS: Biology of HIF-1alpha. Cell Death Differ 15: 621–627, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Simon MC, Keith B: The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol 9: 285–296, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel SA, Simon MC: Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ 15: 628–634, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semenza GL: Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE 2007: cm8, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Catrina SB, Botusan IR, Rantanen A, Catrina AI, Pyakurel P, Savu O, Axelson M, Biberfeld P, Poellinger L, Brismar K: Hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha are expressed in kaposi sarcoma and modulated by insulin-like growth factor-I. Clin Cancer Res 12: 4506–4514, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouel-Kartmann MN, Van Obberghen E: Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor I. Mol Endocrinol 19: 1304–1317, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E: Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 277: 27975–27981, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL: HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8: S62–S67, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Gual P, Gremeaux T, Gonzalez T, Le Marchand-Brustel Y, Tanti JF: MAP kinases and mTOR mediate insulin-induced phosphorylation of insulin receptor substrate-1 on serine residues 307, 612 and 632. Diabetologia 46: 1532–1542, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Tian H, McKnight SL, Russell DW: Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11: 72–82, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF: Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 148: 241–251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aouadi M, Jager J, Laurent K, Gonzalez T, Cormont M, Binetruy B, Le Marchand-Brustel Y, Tanti JF, Bost F: p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett 581: 5591–5596, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Mahadev K, Zilbering A, Zhu L, Goldstein BJ: Insulin-stimulated hydrogen peroxide reversibly inhibits protein-tyrosine phosphatase 1b in vivo and enhances the early insulin action cascade. J Biol Chem 276: 21938–21942, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Picha KM, Patel SS, Mandiyan S, Koehn J, Wennogle LP: The role of the C-terminal domain of protein tyrosine phosphatase-1B in phosphatase activity and substrate binding. J Biol Chem 282: 2911–2917, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Gual P, Le Marchand-Brustel Y, Tanti JF: Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie 87: 99–109, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Emerling BM, Viollet B, Tormos KV, Chandel NS: Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett 581: 5727–5731, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng TC, Buckley DA, Galic S, Tiganis T, Tonks NK: Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J Biol Chem 279: 37716–37725, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Agani F, Semenza GL: Mersalyl is a novel inducer of vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol Pharmacol 54: 749–754, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G: Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res 65: 9047–9055, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Trayhurn P, Wang B, Wood IS: Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br J Nutr 100: 227–235, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K: Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54: 2277–2286, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Imoto K, Kukidome D, Nishikawa T, Matsuhisa T, Sonoda K, Fujisawa K, Yano M, Motoshima H, Taguchi T, Tsuruzoe K, Matsumura T, Ichijo H, Araki E: Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes 55: 1197–1204, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG Jr: Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev 18: 2893–2904, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmid E, El Benna J, Galter D, Klein G, Droge W: Redox priming of the insulin receptor beta-chain associated with altered tyrosine kinase activity and insulin responsiveness in the absence of tyrosine autophosphorylation. FASEB J 12: 863–870, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Wood IS, Trayhurn P: Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 455: 479–492, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang B, Wood IS, Trayhurn P: Hypoxia induces leptin gene expression and secretion in human preadipocytes: differential effects of hypoxia on adipokine expression by preadipocytes. J Endocrinol 198: 127–134, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Rotter V, Nagaev I, Smith U: Interleukin-6 (IL-6) induces insulin resistance in 3T3–L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 278: 45777–45784, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Trayhurn P, Wood IS: Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92: 347–355, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Yun Z, Maecker HL, Johnson RS, Giaccia AJ: Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell 2: 331–341, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.