Abstract
Dopamine D2 and D3 receptors are similar subtypes with distinct interactions with arrestins; the D3 receptor mediates less agonist-induced translocation of arrestins than the D2 receptor. The goals of this study were to compare nonphosphorylated arrestin-binding determinants in the second intracellular domain (IC2) of the D2 and D3 receptors to identify residues that contribute to the differential binding of arrestin to the subtypes. Arrestin3 bound to glutathione transferase (GST) fusion proteins of the D2 receptor IC2 more avidly than to the D3 receptor IC2. Mutagenesis of the fusion proteins identified a residue at the C terminus of IC2, Lys149, that was important for the preferential binding of arrestin3 to D2-IC2; arrestin binding to D2-IC2-K149C was greatly decreased compared with wild-type D2-IC2, whereas binding to the reciprocal mutant D3-IC2-C147K was enhanced compared with wild-type D3-IC2. Mutating this lysine in the full-length D2 receptor to cysteine decreased the ability of the D2 receptor to mediate agonist-induced arrestin3 translocation to the membrane and decreased agonist-induced receptor internalization in human embryonic kidney 293 cells. The reciprocal mutation in the D3 receptor increased receptor-mediated translocation of arrestin3 without affecting agonist-induced receptor internalization. G protein-coupled receptor crystal structures suggest that Lys149, at the junction of IC2 and the fourth membrane-spanning helix, has intramolecular interactions that contribute to maintaining an inactive receptor state. It is suggested that the preferential agonist-induced binding of arrestin3 to the D2 receptor over the D3 receptor is due in part to Lys149, which could be exposed as a result of receptor activation.
The dopamine D2-like receptors, consisting of D2, D3, and D4 receptors, are the major targets of antipsychotic drugs. Despite the close sequence homology and similar pharmacological profiles of D2 and D3 receptors (Neve and Neve, 1997), the two subtypes differ substantially in their signaling and regulatory properties. In particular, several studies have found that the D3 receptor undergoes less agonist-induced receptor phosphorylation and, as a result, is less able to stimulate the translocation of arrestin to the plasma membrane (Kim et al., 2001, 2005; Cho et al., 2006; Kim and Caron, 2008). These differences can be attributed primarily to the second and third intracellular domains (IC2 and IC3) of the two receptors (Kim et al., 2001).
The nonvisual arrestins arrestin2 and -3 (also termed β-arrestin-1 and −2) are cytosolic proteins involved in homologous desensitization and resensitization of G protein-coupled receptors (GPCRs). The binding of arrestin to GPCRs involves both phosphorylation-dependent and independent mechanisms. Phosphorylation of receptor intra-cellular domains by GPCR kinases may create a binding site for positively charged arrestin residues (Gurevich and Gurevich, 2006) and may also cause the intracellular domains to undergo conformational changes exposing nonphosphorylated residues that participate in forming the arrestin binding site (Kim et al., 2004). Receptor activation is accompanied by conformational changes that also expose arrestin-interacting residues. Some sites potentially involved in activation-dependent binding of arrestin are in IC2, including the highly conserved DRY motif (Huttenrauch et al., 2002) and the position 6 residues distal to the DRY motif that is most frequently a proline in rhodopsin-class GPCRs (Raman et al., 1999; Marion et al., 2006).
We and others have used fusion proteins composed of glutathione transferase (GST) and GPCR intracellular domains to analyze the binding of arrestins to GPCRs (Gelber et al., 1999; Cheng et al., 2000; Cen et al., 2001; DeGraff et al., 2002; Macey et al., 2004, 2005). Because the GST fusion proteins are typically not phosphorylated, this method identifies only determinants of binding that are revealed by activation- or phosphorylation-dependent receptor conformational changes; the determinants are presumably occluded in the inactive, nonphosphorylated full-length receptor but exposed when expressed as receptor fragments. Thus, this in vitro binding assay may identify both phosphorylation-independent and some phosphorylation-dependent determinants of the interaction between receptor and arrestin. In vitro studies using receptor fragments, as peptides or as GST fusion proteins, suggest that all receptor intracellular domains, including IC2, contribute to the binding of arrestin (Krupnick et al., 1994; Wu et al., 1997; Gelber et al., 1999; Cen et al., 2001; Macey et al., 2005).
Our studies using GST fusion proteins of D2 and D3 receptor IC3 demonstrated that nonphosphorylated determinants of arrestin3 binding in IC3 are similar between the D2 and D3 receptor subtypes (Lan et al., 2009). Here we describe preferential binding of arrestin3 to D2-IC2 over D3-IC2. Most of the preferential binding to D2-IC2 can be attributed to the residue Lys149, the first residue of transmembrane helix 4; the D3 receptor has a cysteine residue at this position (Cys147). Substitution of cysteine for Lys149 in the full-length mutant receptor D2-K149C reduced agonist-induced translocation of arrestin3 to the membrane and receptor internalization. The reciprocal mutant D3-C147K mediated significantly more agonist-induced translocation of arrestin3 than did the wild-type D3 receptor, although agonist-induced internalization of wild-type and mutant D3 receptors was similar. Analysis of β-adrenergic receptor crystal structures suggests that when the D2 receptor is in an inactive state, Lys149 has a hydrogen-bonding interaction with a backbone carbonyl oxygen in IC1.
Materials and Methods
Materials. [3H]Sulpiride (77.7 Ci/mmol) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA), and serum from Hyclone Laboratories (Logan, UT). Dopamine, haloperidol, and most reagents, including culture medium, were purchased from Sigma-Aldrich (St. Louis, MO). Antibodies used include: mouse anti-rat arrestin2 (BD Transduction Laboratories, Lexington, KY), mouse anti-human arrestin3 (from Santa Cruz Biotechnology, Santa Cruz, CA), and horseradish peroxidase-conjugated goat anti-mouse IgG (Pierce Biotechnology, Rockford, IL). Arrestin3-pCMV5 was a generous gift from Dr. Marc Caron (Duke University, Durham, NC).
Generation of GST Fusion Proteins and D2 Receptor Constructs. For construction of the GST fusion proteins, the IC2 of the rat dopamine D2L receptor (D2-IC2), amino acids 129 to 153, and IC2 of the rat dopamine D3 receptor (D3-IC2), amino acids 125 to 151, were amplified by polymerase chain reaction, subcloned into SpeI-XhoI sites in pET-41a(+) (Novagen, Madison, Wisconsin), transformed into NovaBlue competent cells, sequenced, and subsequently transformed into Rosetta 2(DE3) competent cells (Novagen, Madison, Wisconsin). For purification of GST fusion proteins, Rosetta 2(DE3) cells were grown in 2× yeast-extract tryptone medium containing kanamycin (50 μg/ml) at 37°C to A600 = 0.8 and induced with 1 mM isopropyl-β-d-thiogalactopyranoside for 2 h at 32°C. Cells were pelleted, resuspended in lysis buffer (50 mM Tris, 1 mM EDTA, and 0.5 mg/ml lysozyme, pH 8.0) containing Complete protease inhibitor tablet (Roche Diagnostics, Mannheim, Germany), and incubated for 20 min with gentle rotation at room temperature. The homogenates were clarified by centrifugation, and supernatants were applied to microcentrifuge tubes containing Glutathione Sepharose 4B beads (GE Healthcare), and purified as described by the manufacturer. The amount of fusion protein in each sample was estimated by comparison with BSA after SDS-polyacrylamide gel electrophoresis and staining with Gel Code Blue (Pierce, Rockford, IL).
The GST-IC2 chimeras and the full-length receptor mutants D2-K149C and D3-C147K were constructed through one or more mutagenesis steps using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA), with GST-D2-IC2, GST-D3-IC2, a rat myc-D2L receptor cDNA (Lan et al., 2008), or a rat D3 receptor cDNA (Cox et al., 1995) as template.
Purified Arrestin Binding to GST Fusion Proteins. To obtain purified arrestins, plasmids were expressed in BL21 cells and arrestins purified using heparin-Sepharose chromatography, followed by Q-Sepharose chromatography (Han et al., 2001). GST fusion proteins bound to glutathione Sepharose 4B beads were incubated with purified bovine arrestin2 or arrestin3 in arrestin binding buffer (25 mM Tris-HCl, 150 mM NaCl, pH 7.2, Complete protease inhibitor tablet, 0.1% Triton X-100) for 30 min at room temperature. Incubation mixtures were washed four times in wash buffer (arrestin binding buffer without protease inhibitors), and the proteins were released with SDS sample loading buffer. Proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes that were then blocked with 5% nonfatdry milk in Tris-buffered saline (TBS), and detected by immunoblotting using anti-arrestin2 (1:300 dilution in TBS) or anti-arrestin3 (1:400 dilution in TBS) antibody, with horseradish peroxidase-conjugated goat anti-mouse IgG (1:10,000 dilution in TBS) as secondary antibody. Visualization of the secondary antibody was performed using the SuperSignal West Pico Chemiluminescence kit (Pierce, Rockford, IL) and quantified by IPLab (BD Biosciences Bioimaging, Rockville, MD). The amount of bound arrestin2 or arrestin3 was calculated by linear regression of a standard curve generated using background optical density (i.e., no arrestin) and three to six concentrations of arrestin2 or arrestin3 ranging between 0.25 and 20 ng. Saturation analysis of the binding of arrestin was carried out by incubating various concentrations of arrestin2 or −3 with a fixed concentration of GST alone (175 ng) or GST-IC2 (200 ng) for 30 min at room temperature. The amount of arrestin bound to GST was subtracted from the amount bound to GST-IC2 to determine specific binding at each concentration of arrestin. The resulting concentration-response curves were analyzed by nonlinear regression using Prism 3.0 (Graphpad Software, San Diego, CA) and statistical comparisons of the curves were made using two-way analysis of variance followed by Bonferroni post-test analysis. In experiments where only one concentration of arrestin was used, statistical significance was evaluated using a paired t test.
Receptor Internalization. Internalization was measured using the intact cell [3H]sulpiride binding assay described by Itokowa et al. (1996). HEK 293 cells grown to 80% confluence were cotransfected with D2 wild type (1 μg), D2-K149C (3 μg), D3 wild type(1 μg), or D3-C147K receptor DNA (0.5 μg; the amount of each plasmid was adjusted to achieve similar cell-surface expression levels) and 3 μg of arrestin3-pCMV5 using Lipofectamine2000 (Invitrogen, Carlsbad, CA). Cells from each transfection were split into four plates after 12 h, two for the internalization assay and two for translocation (see Arrestin3 Translocation). After 2 days, plates for internalization were rinsed once with prewarmed, calcium- and magnesium-free phosphate-buffered saline (CMF-PBS; 138 mM NaCl, 4.1 mM KCl, 5.1 mM sodium phosphate, 5 mM potassium phosphate, and 0.2% glucose, pH 7.4) and preincubated for 15 min with prewarmed, CO2-saturated serum-free Dulbecco’s modified Eagle’s medium containing 20 mM HEPES, pH 7.4, at 37°C. Cells were stimulated with 10 μM dopamine in the same HEPES-buffered medium at 37°C for 20 min. Stimulation was terminated by quickly cooling the plates on ice and washing the cells three times with ice-cold CMF-PBS, after which cells were gently dislodged from the plate in 2 ml of ice-cold CMF-PBS assay buffer (CMF-PBS containing 2 mM EDTA and 0.001% bovine serum albumin). Cells were mixed, added to assay tubes in a final volume of 250 μl with [3H]sulpiride (final concentration, 5 nM), and incubated at 4°C for 150 min in the absence and presence of unlabeled haloperidol (final concentration, 10 μM). The assay was terminated by filtration through Whatman GF/C filters pre-soaked with 0.05% polyethylenimine using a 96-well cell harvester (Tomtec, Orange, CT) and ice-cold wash buffer (10 mM Tris-HCl, pH 7.4, and 0.9% NaCl). Filters were allowed to dry, and BetaPlate Scint scintillation fluid (50 μl) was added to each sample. Radioactivity on the filters was determined using a Wallac 1205 BetaPlate scintillation counter (PerkinElmer Life and Analytical Sciences). The expression of arrestin3 in an aliquot of the cells was confirmed by immunoblotting as described under Purified Arrestin Binding to GST Fusion Proteins.
Arrestin3 Translocation. Transient expression of wild-type and mutant D2 or D3 receptors with arrestin3 and dopamine stimulation of cells were performed as described under Purified Arrestin Binding to GST Fusion Proteins. Dopamine stimulation was terminated by quickly cooling the plates on ice and washing the cells once with ice-cold CMF-PBS. Cells were lysed with 1 ml of ice-cold lysis buffer (20 mM HEPES, 20 mM NaCl, 5 mM EDTA, and Complete protease inhibitor tablet), scraped, collected, homogenized with a glass-Teflon homogenizer, and sonicated for 8 to 10 s. Samples were centrifuged at 1000g for 10 min at 4°C. Supernatants were transferred to new centrifuge tubes and centrifuged at 100,000g for 30 min at 4°C. Supernatants were collected; pellets were rinsed carefully with ice-cold CMF-PBS and then resuspended with 100 μl of CMF-PBS. The abundance of arrestin3 in both pellet and supernatant fractions was quantified by immunoblotting as described above, except with a 1:100 dilution of the anti-arrestin3 antibody and the addition of 0.1% Tween 20 and 5% dry milk to the incubations with primary and secondary antibodies.
Results
Binding of Arrestin3 to IC2. GST-D2-IC2 and GST-D3-IC2 were constructed, and the binding of arrestin determined using an in vitro GST pull-down assay. Having determined that the binding of arrestin3 to GST-D2-IC3 approached equilibrium within 15 min (Lan et al., 2009), a rapid time course consistent with other work using intracellular loop fragments of GPCRs (Gurevich et al., 1995; Cen et al., 2001), GST binding assays were carried out for 30 min. Arrestin3 bound more avidly to GST-D2-IC2 than to GST-D3-IC2 (Fig. 1). Using binding to GST alone to define nonspecific binding, specific binding was analyzed by nonlinear regression, yielding a calculated Kd value for binding of arrestin3 to D2-IC2 of 242 ± 66 nM. Arrestin3 bound with approximately 4-fold lower affinity to D3-IC2 (Table 1). Both of these values were higher (lower affinity) than for binding to IC3 of the D2 and D3 receptors (Lan et al., 2009). Arrestin2 bound weakly to both fusion proteins (Fig. 1).
Fig. 1.
Binding of arrestins to GST-D2-IC2 and GST-D3-IC2 fusion proteins. GST alone (GST, 175 ng) or receptor second intracellular loop GST fusion proteins (GST-D2-IC2 and GST-D3-IC2, 200 ng) were incubated with the indicated amount of arrestin2 or arrestin3. The amount of arrestin that coeluted with GST or the GST fusion proteins was determined by immunoblotting with anti-arrestin antibodies. Results were quantified using standard curves constructed with known amounts of arrestin2 and arrestin3. A, immunoblots are shown from an experiment representative of 3 independent experiments. Arrestin standards are shown on the right of each blot. B, the protein-stained gel demonstrates that equal amounts of GST-D2-IC2 and GST-D3-IC2 were included in the reactions. C, mean ± S.E.M. are shown for the binding of arrestins to IC3 fusion proteins. The amount of arrestin bound is plotted against the amount of arrestin included in the pull-down assay. *, p < 0.05; **, p < 0.01 for D2-IC2 compared with D3-IC2 by Bonferroni post test
TABLE 1.
Saturation analysis of binding of arrestin3 to receptor fragments
GST alone (175 ng), GST-D2-IC2 (200 ng), or GST-D3-IC2 (200 ng) was incubated with various concentrations of arrestin3 for 30 min, and total binding quantified by determining the arrestin immunoreactivity that co-precipitated in a GST pull-down assay. Specific binding was calculated by subtracting the amount bound to GST alone. Using nonlinear regression analysis of the resulting saturation curves, Kd values were calculated based on the molecular mass of arrestin3 (∼54 kDa). The total number of binding sites per 200 ng of fusion protein (Bmax) was also calculated. Each value represents the mean ± S.E.M. of three or four independent experiments. For GST-D3-IC2, the highest concentration of arrestin used was lower than the calculated Kd, producing error in the affinity and Bmax estimates that is reflected in the large S.E.M.; nevertheless, the similarity between Bmax values for D2-IC2 and D3-IC2 suggests that the Kd estimate for D3-IC2 is also reliable.
| Fusion Protein | Arrestin3
|
|
|---|---|---|
| Kd | Bmax | |
| nM | ng | |
| GST-D2-IC2 | 242 ± 114 | 5.5 ± 1.1 |
| GST-D3-IC2 | 1100 ± 881 | 5.0 ± 3.0 |
Binding of Arrestin3 to D2 and D3 Receptor IC2 Chimeras. Chimeras of the two receptor fragments were used in GST pull-down assays to identify residues contributing to the higher affinity of D2-IC2 for arrestin3. Alignment of D2-IC2 and D3-IC2 reveals that the sequences are almost identical for the first nine residues after the highly conserved DRY sequence that is at the cytoplasmic end of the third trans-membrane domain (Fig. 2A), suggesting that determinants of selectivity probably reside in the less homologous C-terminal portion of the loop. Thus, a chimera composed of the conserved N-terminal portion of the D2-IC2 combined with the C-terminal portion of D3-IC2 had a reduced level of arrestin binding that was indistinguishable from that of D3-IC2, whereas the reciprocal chimera containing the C-terminal portion of D2-IC2 displayed robust binding that was indistinguishable from binding to D2-IC2 (Fig. 2B).
Fig. 2.
Binding of arrestin3 to D2 and D3 receptor IC2 chimeras. A, alignment of IC2 from the rat D2 and D3 dopamine receptors, listing the name of each construct on the left and its sequence on the right, with mutated residues in bold. Each IC2 is divided into amino-terminal and carboxyl-terminal halves (NT and CT). The position in the full-length receptor of the first and last residues of each IC2 is indicated for the wild-type sequences. B–D, purified GST or GST fusion proteins (400 ng in B, 500 ng in C, and 250 ng in D) were incubated with 300 ng of purified arrestin3, and the amount of arrestin bound was determined by quantitative immunoblotting. The results shown are the mean ± S.E.M. from four to six independent experiments (*, p < 0.05; **, p < 0.01;, p < 0.001 versus the respective wild-type fusion protein by paired t test).
Chimeras were constructed to define more precisely the determinants of selective arrestin binding to IC2, with the hypothesis that such a determinant would be revealed by reciprocal effects on the binding of arrestin resulting from reciprocal changes in sequence. The first segment of the C-terminal portion of IC2 consists of a nonhomologous stretch of four (D2) or six (D3) residues; several chimeras were constructed to manipulate both the length and the composition of this segment (Fig. 2A). A number of these mutations resulted in severely impaired binding of arrestin to D2-IC2. Arrestin binding was greatly decreased by insertion of two D3-IC2 residues (D2-IC2+GQ) at the end of this nonhomologous segment or by replacing the four nonhomologous residues with six residues from D3-IC2 (D2-IC2M6). We were surprised to find that replacing the first two residues of this divergent segment with corresponding residues from D3-IC2 reversed the effect of the insertion of residues GQ; the chimera that combined these mutations (D2-IC2M4) exhibited robust binding of arrestin3 (Fig. 2C). It is noteworthy that none of the reciprocal chimeras constructed on the D3-IC2 background manifested arrestin binding that was greater than binding to wild-type D3-IC2 (Fig. 2C).
Following the divergent four to six residues of IC2 is a seven-residue sequence in which there are two nonconservative changes between D2 and D3 receptors. A pair of chimeras was constructed in which both divergent residues were replaced with the corresponding residues from the other receptor subtype (Fig. 2A). Replacing Lys149 with C and Thr153 with A (D2-IC2-K149C/T153A) decreased binding to the level observed for D3-IC2. The reciprocal chimera D3-IC2C147K/A151T exhibited arrestin binding that was significantly greater than to wild type D3-IC2, albeit less than to D2-IC2.
To determine which of the two substitutions contributed to selective binding of arrestin3, additional chimeras with individual substitutions were tested. The binding of arrestin to D2-IC2 was unaffected by the mutation T153A but was greatly decreased by the mutation K149C (Fig. 2D). Likewise, the binding of arrestin to D3-IC2 was unaffected by the mutation A151T but was significantly enhanced by the mutation C147K. We conclude that much of the selectivity of arrestin3 for D2-IC2 can be attributed to Lys149. In some contexts, however, the distinct N-terminal portions of D2-IC2 and D3-IC2 can differentially affect the binding of arrestin, presumably as a result of interactions with residues in the C-terminal portion. This is demonstrated by the observation that the C-terminal portions of D3-IC2-C147K/A151T and D2-IC2M6 are identical (Fig. 2A), yet more arrestin bound to D3-IC2-C147K/A151T than to D2-IC2M6 (Fig. 2C).
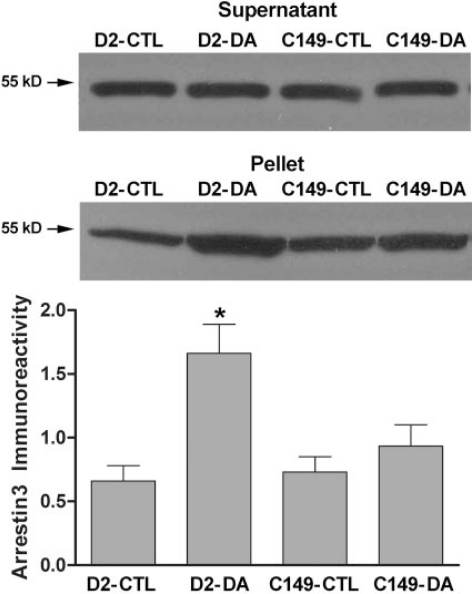
Arrestin Translocation Mediated by Wild-Type and Mutant D2 and D3 Receptors. To determine whether the presence of lysine or cysteine in IC2 at position 149 (D2)or 147 (D3), respectively, contributes to the differential ability of the full-length receptors to mediate agonist-induced trans-location of arrestin, we created the mutant receptors D2-K149C and D3-C147K. Wild-type and mutant receptors were transiently expressed in HEK 293 cells along with arrestin3, with the amount of D2 or D3 receptor plasmid DNA in the transfection mixture adjusted to yield 500 to 1000 fmol of cell-surface receptors/mg of protein, as determined by the binding of [3H]sulpiride. The abundance of arrestin3 in the plasma membrane was determined by quantitative immunoblotting after treatment of intact cells with dopamine (10 μM) or vehicle for 20 min. In cells expressing the wild-type D2 receptor, the amount of arrestin associated with the membrane fraction was more than doubled from 0.7 ± 0.1 ng of arrestin3 in untreated cells to 1.7 ± 0.2 ng after treatment with 10 μM dopamine for 20 min (Fig. 3). In contrast, dopamine treatment caused no significant translocation of arrestin3 to the membrane in cells expressing D2-K149C (vehicle = 0.7 ± 0.1 ng; dopamine = 0.9 ± 0.2 ng).
Fig. 3.
Agonist-induced translocation of arrestin3 in HEK 293 cells co-expressing arrestin3 and wild-type or mutant D2 receptors. Cells transiently transfected with arrestin3 and wild-type D2 receptor (WT) or D2-K149C (C149) were treated with 10 μM dopamine (DA) or vehicle (CTL) for 20 min, membranes were prepared, and levels of arrestin3 were determined by quantitative immunoblotting. Results were quantified using standard curves constructed with known amounts of purified arrestin3. A representative arrestin3 immunoblot is shown for the cytosolic fraction (supernatant) and the membrane fraction (pellet) from one of three independent experiments. The results from the supernatant demonstrate approximately equal expression of arrestin3 in all four conditions. The amount of arrestin in the membrane fractions from all three experiments is shown in the bar graph as the mean ± S.E.M. (*, p < 0.05 compared with vehicle-treated cells).
Consistent with other reports (Kim et al., 2001, 2005), the wild-type D3 receptor was unable to mediate dopamine-induced translocation of arrestin3 in HEK293 cells (vehicle = 1.1 ± 0.1 ng; dopamine = 1.2 ± 0.1 ng). Dopamine treatment of cells expressing the IC2 mutant D3-C147K, on the other hand, caused a modest but significant increase from 1.0 ± 0.1 to 1.4 ± 0.1 ng of arrestin3 in the membrane fraction (Fig. 4).
Fig. 4.
Agonist-induced translocation of arrestin3 in HEK 293 cells co-expressing arrestin3 and wild-type or mutant D3 receptors. Cells transiently transfected with arrestin3 and wild-type D3 receptor (WT) or D3-C147K (K147) were treated with 10 μM dopamine (DA) or vehicle (CTL) for 20 min, membranes were prepared, and levels of arrestin3 were determined by quantitative immunoblotting. Results were quantified using standard curves constructed with known amounts of purified arrestin3. A representative arrestin3 immunoblot is shown for the cytosolic fraction (supernatant) and the membrane fraction (pellet) from one of three independent experiments. The results from the supernatant demonstrate approximately equal expression of arrestin3 in all four conditions. The amount of arrestin in the membrane fractions from all three experiments is shown in the bar graph as the mean ± S.E.M. (*, p < 0.05 compared with vehicle-treated cells).
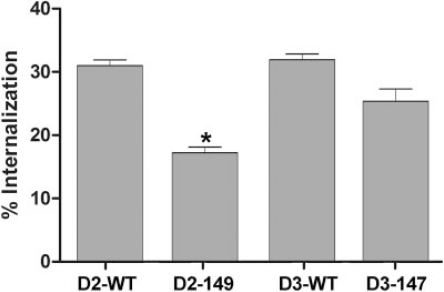
Agonist-Induced Internalization of Wild-Type and Mutant D2 and D3 Receptors. Agonist-induced internalization of D2 and D3 receptors is dependent on arrestin, and the lack of D3 receptor-mediated translocation of arrestin is typically reflected in poor internalization observed for that receptor subtype (Kim et al., 2001; Cho et al., 2006). In cells transiently expressing the D2 or D3 receptor and arrestin3, treatment with dopamine (10 μM, 20 min) caused a 31 ± 1% decrease in the abundance of cell-surface D2 receptors, measured using the hydrophilic ligand [3H]sulpiride (Fig. 5). We were surprised to find that the wild-type D3 receptor was internalized to a similar extent by dopamine treatment (33 ± 1%). The D2 receptor with the IC2 mutation K149C exhibited significantly decreased agonist-induced internalization (17 ± 1%), consistent with the reduced translocation of arrestin3, whereas the internalization of D3-C147K was not significantly different from the wild-type D3 receptor (Fig. 5).
Fig. 5.
Agonist-induced receptor internalization in HEK 293 cells coexpressing arrestin3 and wild-type or mutant D2 or D3 receptors. Cells were treated with 10 μM dopamine for 20 min and were subjected to the intact cell [3H]sulpiride binding assay. Results are expressed as the percentage by which the binding of [3H]sulpiride to cell surface receptors decreased after agonist stimulation and are shown as the mean ± S.E.M. from three to four independent experiments (*, p < 0.05 versus wild-type by paired t test).
Hydrogen-Bonding Interaction between Lys149 and IC1. Figure 6 depicts an alignment of D2-IC2 and D3-IC2 with three GPCRs for which crystal structures have been determined: rhodopsin (Palczewski et al., 2000), the β2-adrenergic receptor (Cherezov et al., 2007), and the β1-adrenergic receptor (Warne et al., 2008). Included in the alignment was a consensus IC2 sequence determined by analysis of 175 rhodopsin-class GPCRs (Marion et al., 2006). D2-Lys149 corresponds to the first residue in the TM4 α-helix of all three solved structures and to position 18 in the consensus IC2 sequence, a position that is most frequently proline (23%), a basic residue (23%) or one of the hydrophobic residues Ile/Leu/Val/Phe (23%)(Marion et al., 2006).
Fig. 6.
Alignment of IC2. Transmembrane (TM) helices 3 and 4 and IC2 from the D2 and D3 dopamine receptors and from the crystallized GPCRs bovine rhodopsin (Rh), the turkey β1-adrenergic receptor, and the human β2-adrenergic receptor were aligned, along with the consensus IC2 sequence (IC2) of Marion et al. (2006). This figure depicts the entire IC2 fragment characterized in this study, which included the cytoplasmic ends of the TM3 and TM4 α-helices (indicated with lines) and the intervening IC2. Residue numbering for the full-length receptor is indicated at the beginning and end of each sequence. D2 residue Lys149, the first residue of the TM4 α-helix, is in bold.
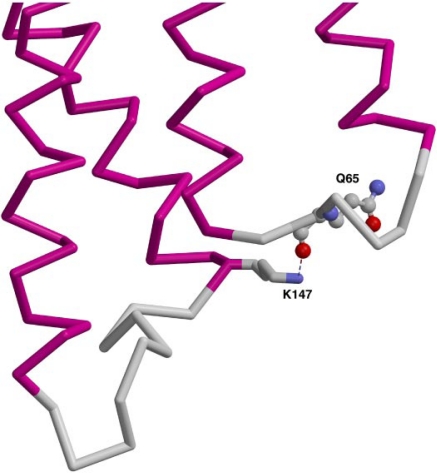
Examining the structures of the β1- and β2-adrenergic receptors, we observed that Lys147 (β2) and Arg155 (β1) bind to the backbone carbonyl oxygen of Gln in IC1 (Fig. 7), a residue that is conserved in the D2 and D3 receptors. In the higher resolution β2 structure, the bond distance from Lys147 to the carbonyl oxygen is approximately 2.9 Å. In the lower resolution β1 structure, the distance from Arg155 for three of the four independent molecules in the unit cell is 2.8 to 3.3 Å. The D2 receptor is likely to share this interaction between the base of TM4 and IC1, whereas the D3 receptor, with a cysteine at this position, would not be expected to have the same interaction with the carbonyl oxygen in the backbone.
Fig. 7.
Interaction of a basic residue near IC2 with IC1. In the human β2-adrenergic receptor (2RH1.pdb), the nitrogen in residue Lys147 hydrogen bonds to the backbone carbonyl of Q65 in IC1 (Cherezov et al., 2007). From left to right are transmembrane (TM) helices 3 and 4 (magenta), connected by IC2 (gray), and TM helices 2 and 1, connected by IC1.
Discussion
Studies of receptor-mediated translocation of GFP-tagged arrestin to the cell membrane have identified IC2 and IC3 as being particularly important for the preferential association of arrestin with the D2 over the D3 receptor (Kim et al., 2001). Having determined that nonphosphorylated determinants of binding in IC3 are similar in the two receptor subtypes (Lan et al., 2009), in the present study, we used GST fusion proteins to compare arrestin-binding determinants in D2-IC2 and D3-IC2. As observed for IC3, arrestin3 bound more avidly than arrestin2 to IC2. Arrestin bound with higher affinity to GST-D2-IC2 (240 nM) than to GST-D3-IC2 (1100 nM), although binding to IC2 from either subtype was with lower affinity than to GST-IC3 (70–80 nM; Lan et al., 2009). These affinity values for arrestin3, particularly at GST-D2-IC2, are similar to the estimated concentration of arrestins in neostriatal neurons (Gurevich et al., 2004), suggesting that these interactions are physiologically relevant.
Although both phosphorylated and nonphosphorylated residues contribute to the arrestin binding site (Gurevich and Gurevich, 2006), and differential phosphorylation of D2 and D3 receptors by GPCR kinases is likely to contribute to the differential affinity of the subtypes for arrestins (Kim et al., 2001; Cho et al., 2006), the finding that nonphosphorylated GST fusion proteins also display the preference of arrestin for the D2 receptor over the D3 receptor suggests that arrestin binding to phosphorylated residues does not entirely explain the preference for the D2 receptor, and that nonphosphorylated residues that are revealed by receptor activation or by phosphorylation of other sites on the receptor also contribute to subtype selectivity.
The D2-IC2 fragment evaluated in this study can be roughly divided into three segments: the proximal half that includes the cytoplasmic end of the third transmembrane helix and is highly conserved, with only two residues that are not identical or similar to the D3-IC2, as well as a shared proline residue that is an important determinant of arrestin binding in rhodopsin-class GPCRs (Raman et al., 1999; Marion et al., 2006), four residues with no homology to a stretch of six residues in the same location of the D3 receptor, and the distal segment that includes the beginning of the fourth transmembrane helix, with only two nonhomologous residues and a potential site of phosphorylation by protein kinase C. Using chimeric GST-IC2 constructs, we first determined that the two segments comprising the distal half of IC2 contain determinants of the selective binding of arrestin3 to D2-IC2. Although some substitutions within the first of these two segments dramatically decreased the binding of arrestin to D2-IC2, we did not identify any substitution that enhanced the binding of arrestin to D3-IC2. In the most distal segment of IC2, on the other hand, the presence of a lysine residue at position 149 of the D2 receptor seemed to be an important determinant of the binding of arrestin, because reciprocal substitutions between Lys149 and the cysteine at the corresponding position of the D3 receptor, Cys147, decreased arrestin3 binding to GST-D2-IC2 and increased binding to GSTD3-IC2.
Studies with full-length receptors have used direct binding of arrestin, arrestin translocation, and receptor internalization as assays to explore receptor determinants of arrestin binding, with results that collectively suggest that all intra-cellular domains participate in the binding site (Gurevich and Gurevich, 2006). We confirmed that the D3 has less capacity than the D2 receptor to mediate agonist-induced translocation of arrestin3 in HEK 293 cells. Under the conditions used in this study, however, D2 and D3 receptors internalized to a similar extent after dopamine treatment. The combination of deficient agonist-induced translocation of arrestin with robust receptor internalization could reflect the existence under basal conditions of a complex of the D3 receptor, arrestin3, and filamin (Kim et al., 2005) or the contribution of a non–arrestin-dependent pathway for internalization (Cho et al., 2007).
We made reciprocal substitutions of lysine and cysteine at this position in the full-length receptors, transiently coexpressed the receptors with arrestin3, and evaluated agonist-induced translocation of arrestin3 and receptor internalization. The D2 receptor mutant with a cysteine residue at position 149, D2-K149C, displayed reduced dopamine-induced arrestin3 translocation and receptor internalization compared with the wild-type D2 receptor. The reciprocal D3 receptor mutant D3-C147K exhibited increased dopamine-induced translocation of arrestin3, indicating that Lys149 contributes significantly to the higher affinity of the D2 receptor for arrestin3. It is noteworthy that a previous study identified D3-C147 as critical for rapid tolerance of the D3 receptor (Westrich and Kuzhikandathil, 2007).
The residue Lys149 aligns with the first residue of the TM4 α-helix in three GPCR crystal structures (Palczewski et al., 2000; Cherezov et al., 2007; Warne et al., 2008). In the two β-adrenergic receptors, the corresponding residues Lys147 (β2) and Arg155 (β1) have potential hydrogen-bonding interactions with a glutamine residue in IC1, a residue that is conserved in all of the D2-like receptors. The cysteine at this position in the D3 receptor would not be able to form the same hydrogen bond. Because the β-adrenergic receptor structures are inactive receptor conformations, we speculate that a similar hydrogen bond in the D2 receptor between Lys149 and Gln66 helps to constrain IC1 and IC2 and to maintain the receptor in an inactive conformation, also occluding Lys149 so that it is not accessible to arrestin. Agonist activation of the receptor could potentially break this interaction and make Lys149 available to contribute to the arrestin-binding site, as it presumably is in the GST-IC2 fusion protein.
ABBREVIATIONS:
- IC2
second intracellular loop of a GPCR
- IC3
third intracellular loop of a GPCR
- GPCR
G protein-coupled receptor
- GST
glutathione transferase
- IC3
third intracellular loop of a GPCR
- D2-IC2
second intracellular loop of the rat dopamine D2L receptor
- D3-IC2
second intracellular loop of the rat dopamine D3 receptor
- TBS
Tris-buffered saline
- CMF-PBS
calcium- and magnesium-free phosphate-buffered saline
Footnotes
This work was supported by United States Public Health Service grants MH045372 (to K.A.N.), GM077561 (to V.V.G.), and EY011500 (to V.V.G.), and the VA Merit Review and Career Scientist programs (to K.A.N.).
This article is a companion to “A Dopamine D2 Receptor Mutant Capable of G Protein-Mediated Signaling but Deficient Arrestin Binding,” by Lan H, Liu Y, Bell MI, Gurevich VV, and Neve KA on page 113 of this issue. That article should have been printed immediately preceding this article.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
References
- Cen B, Xiong Y, Ma L, Pei G. Direct and differential interaction of β-arrestins with the intracellular domains of different opioid receptors. Mol Pharmacol. 2001;59:758–764. doi: 10.1124/mol.59.4.758. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Zhao J, Sun Y, Hu W, Wu YL, Cen B, Wu GX, Pei G. β-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between β-arrestin and CXCR4. J Biol Chem. 2000;275:2479–2485. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DI, Beom S, Van Tol HH, Caron MG, Kim KM. Characterization of the desensitization properties of five dopamine receptor subtypes and alternatively spliced variants of dopamine D2 and D4 receptors. Biochem Biophys Res Commun. 2006;350:634–640. doi: 10.1016/j.bbrc.2006.09.090. [DOI] [PubMed] [Google Scholar]
- Cho EY, Cho DI, Park JH, Kurose H, Caron MG, Kim KM. Roles of protein kinase C and actin-binding protein 280 in the regulation of intracellular trafficking of dopamine D3 receptor. Mol Endocrinol. 2007;21:2242–2254. doi: 10.1210/me.2007-0202. [DOI] [PubMed] [Google Scholar]
- Cox BA, Rosser MP, Kozlowski MR, Duwe KM, Neve RL, Neve KA. Regulation and functional characterization of a rat recombinant dopamine D3 receptor. Synapse. 1995;21:1–9. doi: 10.1002/syn.890210102. [DOI] [PubMed] [Google Scholar]
- DeGraff JL, Gurevich VV, Benovic JL. The third intracellular loop of α2-adrenergic receptors determines subtype specificity of arrestin interaction. J Biol Chem. 2002;277:43247–43252. doi: 10.1074/jbc.M207495200. [DOI] [PubMed] [Google Scholar]
- Gelber EI, Kroeze WK, Willins DL, Gray JA, Sinar CA, Hyde EG, Gurevich V, Benovic J, Roth BL. Structure and function of the third intracellular loop of the 5-hydroxytryptamine2A receptor: the third intracellular loop is α-helical and binds purified arrestins. J Neurochem. 1999;72:2206–2214. doi: 10.1046/j.1471-4159.1999.0722206.x. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Benovic JL, Gurevich VV. Arrestin2 expression selectively increases during neural differentiation. J Neurochem. 2004;91:1404–1416. doi: 10.1111/j.1471-4159.2004.02830.x. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, Hosey MM, Benovic JL. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, β2-adrenergic, and m2 muscarinic cholinergic receptors. J Biol Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of β-arrestin at 1.9 Å: possible mechanism of receptor binding and membrane translocation. Struct Fold Des. 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- Huttenrauch F, Nitzki A, Lin FT, Höning S, Oppermann M. β-arrestin binding to CC chemokine receptor 5 requires multiple C-terminal receptor phosphorylation sites and involves a conserved Asp-Arg-Tyr sequence motif. J Biol Chem. 2002;277:30769–30777. doi: 10.1074/jbc.M204033200. [DOI] [PubMed] [Google Scholar]
- Itokawa M, Toru M, Ito K, Tsuga H, Kameyama K, Haga T, Arinami T, Hamaguchi H. Sequestration of the short and long isoforms of dopamine D2 receptors expressed in Chinese hamster ovary cells. Mol Pharmacol. 1996;49:560–566. [PubMed] [Google Scholar]
- Kim KM, Caron MG. Complementary roles of the DRY motif and C-terminus tail of GPCRS for G protein coupling and β-arrestin interaction. Biochem Biophys Res Commun. 2008;366:42–47. doi: 10.1016/j.bbrc.2007.11.055. [DOI] [PubMed] [Google Scholar]
- Kim KM, Gainetdinov RR, Laporte SA, Caron MG, Barak LS. G protein-coupled receptor kinase regulates dopamine D3 receptor signaling by modulating the stability of a receptor-filamin-β-arrestin complex. A case of autoreceptor regulation. J Biol Chem. 2005;280:12774–12780. doi: 10.1074/jbc.M408901200. [DOI] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and β-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Kim OJ, Gardner BR, Williams DB, Marinec PS, Cabrera DM, Peters JD, Mak CC, Kim KM, Sibley DR. The role of phosphorylation in D1 dopamine receptor desensitization: evidence for a novel mechanism of arrestin association. J Biol Chem. 2004;279:7999–8010. doi: 10.1074/jbc.M308281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Gurevich VV, Schepers T, Hamm HE, Benovic JL. Arrestinrhodopsin interaction. Multi-site binding delineated by peptide inhibition. J Biol Chem. 1994;269:3226–3232. [PubMed] [Google Scholar]
- Lan H, Liu Y, Bell MI, Gurevich VV, Neve KA. A dopamine D2 receptor mutant capable of G protein-mediated signaling but deficient in arrestin binding. Mol Pharmacol. 2009;75:113–123. doi: 10.1124/mol.108.050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey TA, Gurevich VV, Neve KA. Preferential interaction between the dopamine D2 receptor and arrestin2 in neostriatal neurons. Mol Pharmacol. 2004;66:1635–1642. doi: 10.1124/mol.104.001495. [DOI] [PubMed] [Google Scholar]
- Macey TA, Liu Y, Gurevich VV, Neve KA. Dopamine D1 receptor interaction with arrestin3 in neostriatal neurons. J Neurochem. 2005;93:128–134. doi: 10.1111/j.1471-4159.2004.02998.x. [DOI] [PubMed] [Google Scholar]
- Marion S, Oakley RH, Kim KM, Caron MG, Barak LS. A β-arrestin binding determinant common to the second intracellular loops of rhodopsin family G protein-coupled receptors. J Biol Chem. 2006;281:2932–2938. doi: 10.1074/jbc.M508074200. [DOI] [PubMed] [Google Scholar]
- Neve KA, Neve RL. Molecular biology of dopamine receptors. In: Neve KA, Neve RL, editors. The Dopamine Receptors. Humana Press; Totawa, NJ: 1997. pp. 27–76. [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- Raman D, Osawa S, Weiss ER. Binding of arrestin to cytoplasmic loop mutants of bovine rhodopsin. Biochemistry. 1999;38:5117–5123. doi: 10.1021/bi9824588. [DOI] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a β1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–491. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westrich L, Kuzhikandathil EV. The tolerance property of human D3 dopamine receptor is determined by specific amino acid residues in the second cytoplasmic loop. Biochim Biophys Acta. 2007;1773:1747–1758. doi: 10.1016/j.bbamcr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Wu GY, Krupnick JG, Benovic JL, Lanier SM. Interaction of arrestins with intracellular domains of muscarinic and α2-adrenergic receptors. J Biol Chem. 1997;272:17836–17842. doi: 10.1074/jbc.272.28.17836. [DOI] [PubMed] [Google Scholar]