Abstract
We tested the hypothesis that a nitric oxide donor, DETA-NONOate upregulates Stromal cell-Derived Factor-1 (SDF1) and Angiopoietin 1 (Ang1) in the ischemic brain and their, respective, receptors chemokine CXC motif receptor 4 (CXCR4) and Tie2 in the subventricular zone (SVZ) and thereby promote SVZ neuroblast cell migration after stroke. C57BL/6J mice were subjected to middle cerebral artery occlusion (MCAo) and 24 hours later DETA-NONOate (0.4 mg/kg) or phosphate buffered solution were intravenously administered. Mice were sacrificed at 14 days for histological assessment or sacrificed at 3 days for analysis real-time polymerase chain reaction and migration after MCAo. To elucidate whether SDF1/CXCR4 and Ang1/Tie2 pathways mediate DETA-NONOate induced SVZ migration after stroke, SDF1α, Ang1 peptide and a specific antagonist of CXCR4 (AMD3100) and a neutralizing antibody of Tie2 (anti-Tie2) were used in vitro. DETA-NONOate significantly increased the percent area of doublecortin (a marker of migrating neuroblasts) immunoreactive-cells in the SVZ and ischemic boundary zone. DETA-NONOate significantly increased the expression of SDF1 and Ang1 in the ischemic border and upregulated CXCR4 and Tie2 in the SVZ compared with MCAo control. DCX-positive cell migration from SVZ explants was significantly increased in the DETA-NONOate treatment group compared with MCAo alone animals. In vitro, SDF1α and Ang1 significantly increased SVZ explants cell migration. In addition, inhibition of CXCR4 or Tie2 significantly attenuated DETA-NONOate induced SVZ cell migration. Our data indicated that treatment of stroke with a nitric oxide donor upregulates SDF1/CXCR4 and Ang1/Tie2 pathways and thereby likely increases SVZ neuroblast cell migration.
Keywords: nitric oxide donor, neural progenitor cells, stromal cell-derived factor-1/ chemokine (CXC motif) receptor 4, Angiopoietin-1/Tie2, stroke
INTRODUCTION
Ischemic stroke induces the proliferation of endogenous neural progenitor cells (NPCs) and increases the number of immature neurons in the subventricular zone (SVZ) of the adult rodent brain (Darsalia et al. 2005; Sun et al. 2003; Zhang et al. 2001b). The expanded neuroblasts in the SVZ migrate to the ischemic boundary zone (IBZ) and have the potential to differentiate into neurons and thereby replace neurons lost after stroke (Zhang et al. 2004; Zhang et al. 2007a; Zhang et al. 2003). In addition, neuroblasts may act synergistically with microvasculature to stimulate the local synaptic microenvironment. Increased neuroblast migration in SVZ improves neurological recovery from stroke (Bernal and Peterson 2004; Ohab et al. 2006; Yamashita et al. 2006).
Our previous studies show that treatment of stroke with a nitric oxide (NO) donor, DETA-NONOate [(Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) aminio] diazen-1-ium-1, 2-diolate], promotes neurogenesis (i.e. increases SVZ cell proliferation and migration), angiogenesis and improves functional outcome after stroke in young adult rats (Chen et al. 2006b; Zacharek et al. 2006; Zhang et al. 2001a). These data suggests that DETA-NONOate treatment improves functional outcome after stroke via upregulation of the endothelial-growth factor Angiopoietin-1 (Ang1) and its receptor tyrosine kinase Tie2 pathway, which enhances angiogenesis in the ischemic brain (Zacharek et al. 2006). Neurogenesis includes NPCs proliferation, migration and differentiation (Chen et al. 2005a; Parent et al. 2002; Zhang et al. 2001b; Zhang et al. 2003; Zhang et al. 2004; Zhang et al. 2007b). In our previous study, we investigated the effect of DETA-NONOate treatment on regulation of NPCs proliferation (Chen et al. 2004; Chen et al. 2006b; Zhang et al. 2001a). However, the mechanism underlying DETA-NONOate enhancement of migration is not fully understood.
Chemokines are important factors controlling cellular migration. Stromal cell-Derived Factor 1 (SDF1) has the chemokine (CXC motif) receptor 4 (CXCR4) as its only receptor (Bagri et al. 2002). SDF1 is a master regulator of tracking of various types of CXCR4 positive cells (Bhakta et al. 2006; Ceradini et al. 2004; Kucia et al. 2005). Ang1 / Tie2 not only promotes angiogenesis and vascular maturation, but also regulates mesenchymal cell and neuroblast migration (Metheny-Barlow et al. 2004; Ohab et al. 2006). Our previous study demonstrated that DETA-NONOate increased endogenous ischemic brain SDF1 and transplanted bone marrow stromal cells (BMSCs) CXCR4 expression and thereby enhanced exogenous BMSC migration into the ischemic brain after stroke (Cui et al. 2007). In this study, we seek to investigate whether DETA-NONOate enhances endogenous SVZ neuroblast migration in the ischemic brain, and whether SDF1/CXCR4 and Ang1/Tie2 pathways contribute to SVZ neuroblast cell migration induced by DETA-NONOate treatment after stroke in mice.
MATERIALS AND METHODS
MCAo Model and Experimental Groups
Adult male C57BL/6J mice weighing 22-25 g (n=62, Charles River, Wilmington, MA) were used in all experiments. All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and the Institutional Animal Care and Use Committee of Henry Ford Health System. Thirty mice were anesthetized with halothane and subjected to transient (2.5 hours) monofilament right middle cerebral artery occlusion (MCAo) (Chen et al. 2005b). Sham-operated mice underwent the same surgical procedure without suture insertion. Twenty four hours after surgery, MCAo and sham-operated mice were intravenously administered via the tail vein with: 1) sham + 0.2 ml phosphate buffered solution (PBS) (n = 15); 2) sham + DETA-NONOate treatment (0.4 mg/kg in 0.2 ml of PBS; Alexis Biochemical, San Diego, CA, n = 12); 3) MCAo + PBS (n = 20); 4) MCAo + DETA-NONOate treatment (0.4 mg/kg, n = 15). One set of mice were sacrificed at 14 days after MCAo for immunostaining (n = 9 / group); the other set of mice were sacrificed at 3 days after MCAo for real-time PCR (n = 3 / group) and to isolate the SVZ for measurement of SVZ explant cell migration (n = 3, 6 or 9 / group).
Histological Assessment
Mice were sacrificed at 14 days after MCAo (n = 9 / group). The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin (Chen et al. 2005b). A standard paraffin block was obtained from the center of the lesion (bregma -1 mm to +1 mm). A series of 6 μm thick sections were cut from the block. For lesion volume evaluation, the seven coronal brain sections were traced using a Global Lab Image analysis system (Data Translation, Marlboro, MA, USA). The indirect lesion area, in which the intact area of the ipsilateral hemisphere was subtracted from the area of the contralateral hemisphere, was calculated (Swanson et al. 1990). Lesion volume is presented as a volume percentage of the lesion compared with the contralateral hemisphere.
Immunohistochemistry
Every 10th coronal section for a total 5 sections was used for immunohistochemical staining. Antibody against doublecortin (DCX, 1:100; C-18, Santa Cruz Biotechnology Inc., Santa Cruz, CA), a protein expressed in immature neurons and specifically in a migrating subpopulation (Englund et al. 2002; Gleeson et al. 1999; Hwang et al. 2008; Nygren et al. 2006), SDF1 (1:250; Santa Cruz Biotechnology Inc.), CXCR4 (1:400; AB1846, Chemicon, Temecula, CA), and Ang1 (1:2000; Abcam, Cambridge, MA) immunohistochemical staining were performed to detect migrating neuroblasts, and the expression of SDF1 and Ang1 in the ischemic brain and CXCR4 in SVZ. Antibody against Tie2 (1:80, Santa Cruz Biotechnology Inc.) with Cy3 (1:200, Jackson Immunoresearch Laboratories, West Grove, PA) immunofluorescence staining was used to measure the expression of Tie2. Control experiments consisted of staining brain coronal tissue sections as outlined above, but the primary antibodies were omitted, as previously described (Li et al. 1998).
Double Immunofluorescence Staining
To specifically identify whether DCX-reactive cells co-localized with CXCR4 or Tie2 positive cells in the SVZ, double immunofluorescence staining for DCX/CXCR4 and DCX/Tie2 were employed. Antibodies against DCX (1:200, Santa Cruz) with a monoclonal antibody against CXCR4 (1:250, Chemicon) or Tie2 (1:80, Santa Cruz Biotechnology Inc.) were used, respectively. Each coronal section was first treated with the primary anti-DCX antibody with fluorescein isothiocyanate (FITC, 1:200, Calbiochem), and were then followed by anti-CXCR4 or anti-Tie2 conjugated with Cy3 (1:200, Jackson Immunoresearch) staining. Control experiments consisted of staining brain coronal tissue sections as outlined above, but omitted the primary antibodies.
Quantification
For quantitative measurement of DCX, SDF1, Ang1 and CXCR4 expression, five slides from each block of an immunostained coronal section were digitized using a 40× objective (BX40; Olympus Optical, Tokyo, Japan) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with a Micro Computer Imaging Device (MCID) software (Imaging Research, Saint Catharines, ON, Canada). For quantitative detection Tie2, the images of five slides from each block of a immunofluorescent coronal section were acquired using fluorescent microscopy (Axiophot2, HB0100 W/2, Carl Zeissi, New York, NY) with a digital camera (C4742-95, Hamamatsu, Japan). Each slide contains 8 fields consisting of cortex and striatum from the IBZ for measurement of SDF1 and Ang1, and all fields of SVZ for measurement of CXCR4 and Tie2. For measurement of DCX, 4 fields of striatum from the IBZ and SVZ were selected (a schematic image as show in Fig.1). The percentages of DCX, SDF1, Ang1, CXCR4 and Tie2 positive area were quantified by a blinded investigator using MCID software, respectively (Chen et al. 2003). Data are presented as the percentage of positive immunoreactive area to the total scan area.
Fig.1.
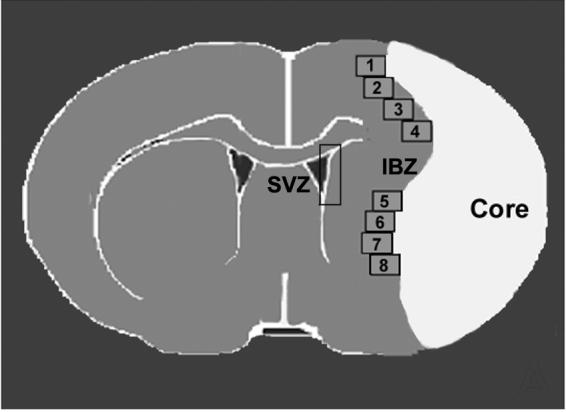
Schematic image of coronal brain section shows 8 fields selected along the ischemic boundary zone (IBZ) and the subventricular zone (SVZ) for quantitative measurement of scan areas.
Real-Time PCR
Three days after stroke, the ischemic hemisphere brain tissue from the MCAo control and MCAo + DETA-NONate treatment group (n = 3 / group) were collected, and the total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA) following a standard protocol (Zacharek et al. 2007). Quantitative polymerase chain reaction (PCR) was performed using the SYBR Green real-time PCR method on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA) using three-stage program parameters provided by the manufacturer. Each sample was tested in triplicate, and relative gene expression data were analyzed using the 2-ΔΔCT method. The following primers for real-time PCR were designed using Primer Express software (Applied Biosystems): GAPDH (forward, AGA ACA TCA TCC CTG CAT CC; reverse, CAC ATT GGG GGT AGG AAC AC), SDF1 (forward, CCC GGA TCC ATG AAC GCC AAG GTC GTG; reverse, AGA GCT GGG CTC CTA CTG TGC GGC CGC GGG), Ang1 (forward, TAT TTT GTG ATT CTG GTG ATT; reverse, GTT TCG CTT TAT TTT TGT AATG).
SVZ Explant Cell Migration
To investigate whether DETA-NONOate increases endogenous SVZ cell migration and the mechanisms underlying SDF1 and Ang1 pathway induced migration, the SVZ derived from both sham and MCAo mice treated with or without DETA-NONOate (n = 6) were dissociated and cultured in vitro for 3 days: 1) non-treatment for control; 2) DETA-NONOate treatment (0.4 mg/kg); 3) Control + SDF1α 200 μg/L (Sigma, Saint Louis, MO); 4) Control + Ang1 200 μg/L (MS Angiopoietin-1, Chemicon).
To further investigate whether DETA-NONOate enhanced SVZ cell migration after stroke is mediated by the SDF1/CXCR4 and Ang1/Tie2 pathways, the following SVZ explants derived from stroke mice (n = 5) were cultured: 1) MCAo control; 2) + CXCR4 specific antagonist (AMD3100, 20 μmol/L, AnorMed, Groton, CT, Canada); 3) + neutralizing antibody of Tie2 (anti-Tie2, recombinant mouse Tie2/FC, 2 mg/L, Chimera, R&D System, Cambridge, MA); 4) + DETA-NONOate 0.1 μmol/L; 5) + DETA-NONOate 0.1 μmol/L + AMD3100 20 μmol/L; 6) + DETA-NONOate 0.1 μmol/L + anti-Tie2 2 mg/L.
The SVZ explants were cut to 1 mm3 and plated in BD Matrigel™ Matrix (BD Biosciences, Bedford, MA) in 24 wells (6 wells each group) with 1 ml of Neuralbasal-A medium (Invitrogen, Carlsbad, CA) containing 2% of B27 supplement (Invitrogen) (Chen et al. 2005a). The SVZ cell migration length was measured at 3 days after culture. For specific measurement of SVZ neuroblast cell migration, DCX-immunofluorescent conjugated with Cy3 staining was performed at 3 days after SVZ culture. The 4× objective with 1.5× electronic zoom of an Olympus IX71 microscope with a CCD camera (CoolSNAP, Proper Scientific Photometrics) and Meta View software (Universal Imaging, West Chester, PA) was used for acquiring images. The averages of distances of DCX-positive cell migration from the explants culture edge were measured by a blinded investigator in each explanted culture using the Meta View software.
Statistical Analysis
Independent Samples T-Test was used for testing the infarct lesion volume, SDF1 and Ang1 mRNA level , and the migration distance of DCX-positive neuroblasts between the two groups. One-way ANOVA and Least Significant Difference (LSD) analysis after Post Hoc Test were used for testing the expression of DCX, SDF1, Ang1, CXCR4 and Tie2-immunoreactive positive areas in the IBZ or in the SVZ, and the SVZ explant cell migration in vitro. Statistical analysis was blinded performed. The data are presented as mean ± SE; P < 0.05 is considered significant.
RESULTS
Lesion Volume
The infarction volume was not significantly decreased in the MCAo + DETA-NONOate treatment group mice (17.27% ± 4.61%) compared with the MCAo alone group (20.87% ± 6.44%), which indicated that the lesion severity was not affected by injection of DETA-NONOate after 14 days of stroke.
DETA-NONOate Treatment of Stroke Increases Neuroblast Migration in the Ischemic Brain
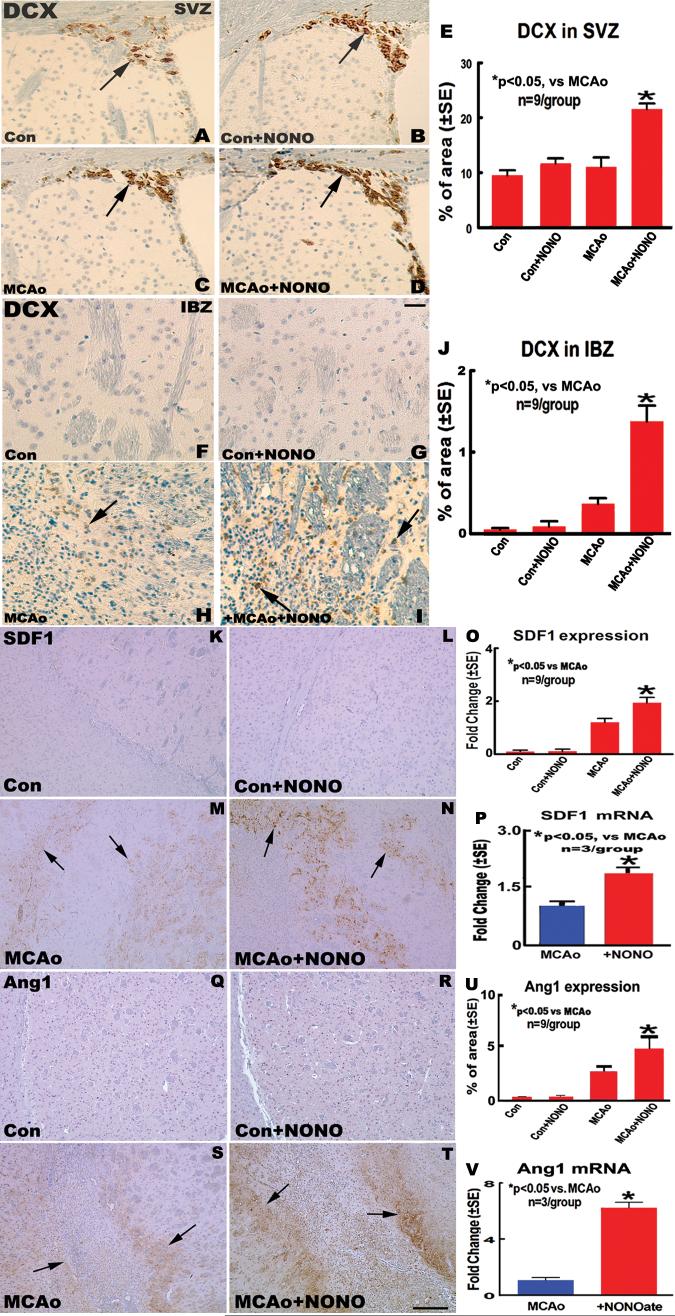
To determine whether DETA-NONOate treatment of stroke increases neuroblasts migration in the SVZ and in the IBZ of the striatum, DCX-immunohistostaining, a marker of migrating neuroblasts, was performed. The percentage of DCX-immunoreactive positive area was measured both in the SVZ and in the IBZ. The data show that the percentage of DCX-immunoreactive positive area present in the SVZ (Fig. 2C-E) and in the IBZ (Fig. 2H-J) significantly increased in the MCAo + DETA-NONOate treatment group compared with MCAo alone group 14 days after stroke (P <0.05). However, no significant differences in the percentage of DCX-immunoreactive positive area both in the SVZ (Fig. 2A, B and E) and in the striatum (Fig. 2F, G and J) were observed between the sham mice treated with or without DETA-NONOate. Our data indicated that DETA-NONOate treatment increases neuroblast migration in the ischemic brain.
Fig.2.
DETA-NONOate treatment upregulates Stromal cell-Derived Factor 1 (SDF1) and Angiopoietin 1 (Ang1) in the IBZ and increases neuroblast migration in the ischemic brain after middle cerebral artery occlusion (MCAo) in mice. A-D and F-I: doublecortin (DCX) immunostaining in sham control (A and F); sham + DETA-NONOate treatment (B and G); MCAo control (C and H); and MCAo + DETA-NONOate treatment (D and I) in the SVZ and IBZ, respectively. E and J: Quantitative data of DCX-immunoreactive neuroblasts in the SVZ (E) and IBZ (J). K-N and Q-T: SDF1 and Ang1 immunohistochemical expression in the IBZ in sham control (K and Q); sham + DETA-NONOate (L and R); MCAo control (M and S); and MCAo + DETA-NONOate treatment (N and T). O and U: Quantitative SDF1 (O) and Ang1 (U) immunostaining data. P and V: SDF1 (P) and Ang1 (V) gene expression measured by real time polymerase chain reaction (PCR). Bar in G = 50 μm; Bar in T = 200 μm.
DETA-NONOate Promotes SDF1 and Ang1 Gene and Protein Expression in the IBZ and Upregulates CXCR4 and Tie2 in the SVZ After Stroke
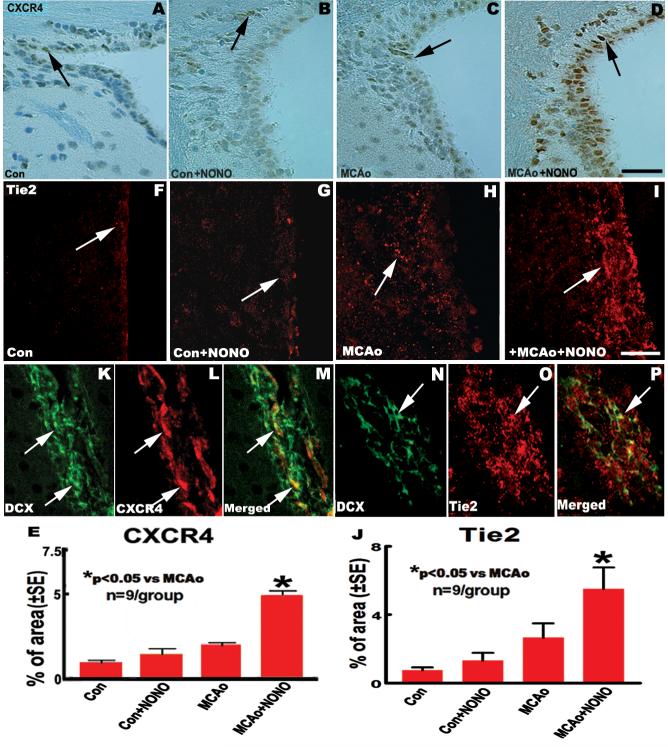
We hypothesized that DETA-NONOate enhancement of the directed migration of new neurons towards the ischemic injury is dependent on SDF1/CXCR4 and Ang1/Tie2 chemotaxis. We therefore tested whether DETA-NONOate treatment increases SDF1 and Ang1 immunoreactivity and SDF1 and Ang1 mRNA level in the ischemic hemisphere. The data show that the percentage of SDF1 (Fig. 2M-O) and Ang1 (Fig. 2S-U) immunoreactive positive area and the fold of SDF1 (Fig. 2P) and Ang1 (Fig. 2V) mRNA changes in the IBZ in MCAo + DETA-NONOate treatment group significantly increased compared with the MCAo alone group (P < 0.05). We then analyzed whether DETA-NONOate treatment upregulates CXCR4 and Tie2 in the SVZ using CXCR4 and Tie2 immunostaining method. The data show that MCAo + DETA-NONOate treatment significantly upregulates CXCR4 (Fig. 3C-E) and Tie2 expression (Fig. 3H-J) in the SVZ compared with MCAo control group (P <0.05). However, significant differences in the expression of SDF1 (Fig. 2K, L and O) and Ang1 (Fig. 2Q, R and U) in the striatum or cortex, CXCR4 (Fig. 3A, B and E) and Tie2 (Fig. 3F, G and J) in the SVZ were not observed between the sham control mice treated with or without DETA-NONOate.
Fig.3.
DETA-NONOate treatment upregulates chemokine (CXC motif) receptor 4 (CXCR4) and Tie2 in the SVZ after stroke in mice. A-D and F-I: CXCR4 and Tie2 immunostaining in the SVZ in sham control (A and F); sham + DETA-NONOate (B and G); MCAo control (C and H) and MCAo + DETA-NONOate treatment (D and I). E and J: Quantitative data of CXCR4 (E) and Tie2 (J) immunoreactive positive area. K-P: CXCR4 (K-M) and Tie2 (N-P) double-immunofluorecence staining with DCX in the SVZ. Bar in D = 50 μm; Bar in I = 25 μm.
DCX-positive Cells are Colocalized with CXCR4- and Tie2-positive Cells in the SVZ
To test whether DETA-NONOate treatment mediated increase of SDF1/CXCR4 and Ang1/Tie2 expression is related to SVZ neuroblast cell migration, DCX/CXCR4 and DCX/Tie2 double immunostaining was performed. Double-immunohistofluorescent staining of brain sections revealed that DCX labeling was partially co-localized with CXCR4-positive cells (Fig. 3K-M), and was also partially co-localized with Tie2-positive cells (Fig. 3N-P) in SVZ. These data suggest that SDF1/CXCR4 and Ang1/Tie2 signals are associated with SVZ neuroblast cell migration.
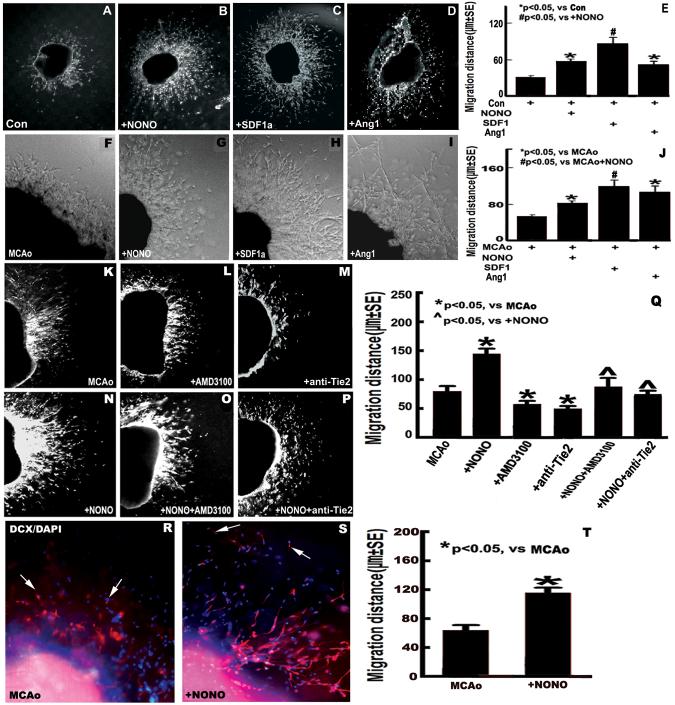
DETA-NONOate, SDF1α and Ang1 Promote SVZ Explant Cell Migration
To investigate whether DETA-NONOate treatment enhances SVZ explant cell migration, the migration of SVZ explants derived from both sham mice and MCAo mice were measured. Fig. 4A, B, E, F, G and J show that the length (μm) of SVZ explant cell migration in DETA-NONOate treatment group (B and G) significantly increased compared with sham (A) and MCAo alone group (F) (P <0.05), respectively. Fig. 4C-E, H-J show that incubation of SVZ explants derived from both sham and MCAo groups with SDF1α (200 μg/L) or Ang1 (200 μg/L) significantly increases SVZ explant cell migration compared with non-treatment control (P <0.05), respectively.
Fig.4.
DETA-NONOate increases SVZ explant cell migration by upregulating SDF1/CXCR4 and Ang1/Tie2 pathways. A-D and F-I: images of SVZ explant cell migration derived from sham and MCAo mice (A and F: control; B and G: + DETA-NONOate 0.4 mg/kg; C and H: + SDF1α 200 μg/L; D and I: + Ang1 200 μg/L). E and J: Quantitative data of SVZ explant cell migration derived from sham (E) and MCAo (J) mice. K-P: images of SVZ explant cell migration derived from MCAo mice (K: MCAo alone; L: + AMD3100 20 μmol/L; M: + anti-Tie2 2 mg/L; N: + DETA-NONOate 0.1 μmol/L; O: + DETA-NONOate 0.1 μmol/L + AMD3100 20 μmol/L; P: + DETA-NONOate 0.1 μmol/L + anti-Tie2 2 mg/L). Q: Quantitative evaluation of SVZ explant cell migration derived from MCAo mice. R and S: DCX-immunofluorecence staining in the cultured SVZ explants derived from MCAo alone (R) and DETA-NONOate treatment (0.4 mg/kg) (S). T: Quantitative data of DCX-positive cell migration. n = 6 well / group.
DETA-NONOate Induced SVZ Explant Cell Migration is Regulated by SDF1α/CXCR4 and Ang1/Tie2 Signaling
For verification that DETA-NONOate stimulates endogenous SVZ explant cell migration after stroke, and to further test the hypothesis that SDF1/CXCR4 and Ang1/Tie2 pathways contribute to DETA-NONOate promotion of SVZ cell migration, measurement of SVZ explant cell migration derived from stroke mice were performed. Fig. 4K-Q show that the migration distance of SVZ explants cell significantly increased in the DETA-NONOate treatment group compared with MCAo alone (P <0.05). Inhibition of CXCR4 or Tie2 both in the MCAo alone and MCAo + DETA-NONOate treatment group significantly decreased SVZ explant cell migration compared with the non-inhibition group, respectively (P <0.05). Our findings indicate an important role for SDF1/CXCR4 and Ang1/Tie2 signaling in the SVZ cell migration by DETA-NONOate treatment after stroke.
DETA-NONOate Promotes SVZ Explant Neuroblast Migration
To test whether DETA-NONOate regulates SVZ explant neuroblast migration after stroke, DCX, a marker of migrating neuroblasts, immunostaining was performed. DCX-positive cell migration was measured. Fig. 4R-T shows the DCX-positive cell migration significantly increased in the DETA-NONOate treatment group compared to the MCAo alone group (p<0.05).
DISCUSSION
In this study, we found that DETA-NONOate significantly enhances endogenous brain neuroblast cell migration in the ischemic brain after stroke compared to PBS treatment mice. In addition, DETA-NONOate failed to increase neuroblast cell migration in sham control mice compared to PBS treated sham control mice. DETA-NONOate increases SDF1 and Ang1 expression in the ischemic brain and it also upregulates CXCR4 and Tie2 expression in the SVZ. Inhibition of CXCR4 by using a CXCR4 specific antagonist (AMD3100) or inhibition of Tie2 using a neutralized Tie2 antibody (anti-Tie2) significantly decreased DETA-NONOate induced SVZ explant cell migration. Thus, it is likely that SDF1/CXCR4 along with Ang1/Tie2 axis mediate DETA-NONOate induced neuroblast migration after stroke.
Endogenous NPCs play an important role for the treatment of nervous system diseases (Goldman 2005). NPCs provide a cellular reservoir for replacement of cells lost during normal cell turnover and after brain injury (Ohab et al. 2006; Zhang et al. 2007a). As a pharmacologic neurorestorative approach, NO is a reactive molecule with numerous physiological and pathophysiological roles affecting the nervous and cardiovascular systems. NO regulates synaptic remodeling and neuronal differentiation in the adult mammal central nervous system (Bicker 2001; Ciani et al. 2004; Seidel and Bicker 2000; Sunico et al. 2005) and in insects (Truman et al. 1996). NO promotes neuronal differentiation (Cheng et al. 2003; Moreno-Lopez et al. 2004) and neurogenesis (Packer et al. 2003). An NO donor, DETA-NONOate regulates neuronal differentiation, and neurite outgrowth in both young and retired breeder neurospheres and improves functional outcome after stroke and brain injury (Chen et al. 2006b; Chen et al. 2005a; Zacharek et al. 2006; Zhang et al. 2001a). Administration of DETA-NONOate to young adult rats increases cell proliferation and migration in the SVZ. NO is involved in the regulation of progenitor cells and neurogenesis, and reduces functional deficits after stroke in the adult brain (Chen et al. 2004; Chen et al. 2006b; Zhang et al. 2001a). In this study, we demonstrate that DETA-NONOate treatment of stroke enhances endogenous neuroblast cell migration in the ischemic brain, which may improve therapeutic outcome after stroke.
Interaction between SDF1 and CXCR4 mediates migration of endogenous neuroblasts and exogenous transplanted bone marrow-derived cells to the sites of brain injury (Cui et al. 2007; Hill et al. 2004; Ji et al. 2004b; Ni et al. 2004) and migration of transplanted neural stem cells (NSCs) to the infarcted area after stroke (Imitola et al. 2004; Robin et al. 2006). The chemokine receptor CXCR4 is present both in the adult rat SVZ and in cultured adult rat neurospheres and regulates the migration of NPCs after stroke (Ji et al. 2004a; Robin et al. 2006; Tran et al. 2007; Tran et al. 2004). SDF1/CXCR4 signaling directs the migration of sensory neuron progenitors to the dorsal root ganglia (DRGs) in mice (Belmadani et al. 2005). SDF1/CXCR4 expression in the SVZ is the essential pathway of interaction between precursors of projection neurons and invading interneurons during corticogenesis (Tiveron et al. 2006). In mice lacking CXCR4 or SDF1, GABAergic neurons fail to complete their migration, and regulated expression of SDF1 in the intermediate neurons and SVZ influences lateromedial tangential migration of CXCR4-expressing GABAergic neurons (Stumm et al. 2007). NO donors significantly enhanced the SDF1α-induced hematopoietic progenitor cells migration, whereas various inhibitors of NO synthase markedly abrogated the chemotactic response in a concentration-dependent manner (Cherla and Ganju 2001). Thus, the interaction of SDF1/CXCR4 likely controls the trafficking of endogenous neuroblast in the SVZ and DETA-NONOate may enhance SDF1-induced chemotaxis. Our previous studies showed that SDF1 is primarily expressed in astrocytes and partially in endothelial cells (Cui et al. 2007). In the present study, our data show that DETA-NONOate treatment of stroke significantly increases endogenous brain SDF1 gene and protein expression in the ischemic brain. In vitro, SDF1α directly regulates SVZ explant cell migration both in the sham and in the MCAo groups, which is consistent with the previous studies (Robin et al. 2006). In addition, CXCR4 colocalizes with DCX-positive cells in the SVZ, which is also consistent with other studies (Robin et al. 2006; Tran et al. 2007). The specific CXCR4 blocker AMD3100 did not change the total number of DCX positive cells, but suppressed the migration of the newly born neurons (Thored et al. 2006; Watanabe M 2007). DETA-NONOate significantly increased SDF1 expression in the IBZ as well as upregulated CXCR4 in the SVZ, and inhibition of CXCR4 significantly decreased SVZ explant cell migration. These findings show that SDF1 generated in the stroke hemisphere may guide SVZ cell migration towards the ischemic boundary via binding to its receptor CXCR4 in the SVZ cells. Thus, our data indicate that DETA-NONOate-induced augmentation of the SDF1/CXCR4 axis is important for mediating SVZ cell migration after stroke. However, inhibition of CXCR4 only partially suppressed SVZ cell migration in vitro, which suggests that other mechanisms also direct SVZ cell migration.
Ang1 and Tie2 not only promote angiogenesis and vascular maturation, but also regulate cell migration (Kobayashi et al. 2006; Metheny-Barlow et al. 2004). Ang1/Tie2 promotes post-stroke neuroblast migration to the peri-infarct cortex and improves animal behavioral recovery (Ohab et al. 2006). DETA-NONOate increases expression of Ang1 and Tie2, which may regulate angiogenesis and vascular integrity after stroke in rats (Chen et al. 2006a; Zacharek et al. 2006). Ang1 is primarily expressed in the ischemic border around the blood vessels in astrocytes, endothelial cells and pericytes (Zacharek et al. 2007). Blood vessels in peri-infarct cortex express Ang1, and their neighboring neuroblasts express their receptors Tie2 (Ohab et al. 2006). The present study showed that Tie2 colocalizes with DCX-positive cells in the SVZ, which is consistent with previous studies (Ohab et al. 2006). These data indicated that DETA-NONOate increased the expression of Ang1 in the IBZ and upregulated their neighboring neuroblasts Tie2 in the SVZ after stroke. In addition, DETA-NONOate significantly enhanced DCX-positive cell migration in vivo and in vitro. Blocking Tie2 with a specific neutralizer significantly attenuated DETA-NONOate-induced SVZ cell migration. These data demonstrated that DETA-NONOate increases Ang1 in the ischemic boundary and the Tie2 receptor in neuroblasts, which in concert exert a trophic effect on migrating of neuroblasts.
In addition, our data show that DETA-NONOate treatment induces SDF1/CXCR4 and Ang1/Tie2 expression in the ischemic brain, as well as increases neuroblast cell migration in the ischemic brain after stroke but not in sham control mice. Thus, DETA-NONOate treatment induced neuroblast cell migration occurs under ischemic conditions. However, in vitro we found that DETA-NONOate induces SVZ cell migration derived from both sham and stroked mice. This apparent contradiction between in vitro and in vivo data to requires future investigation.
The present data provide insight into the molecular mechanisms underlying the migration of neuroblasts after stroke. Our study also demonstrates an important aspect of the restorative use of an NO donor for the treatment of stroke, amplification of neuroblast migration mediated by SDF1/CXCR4 and Ang1/Tie2.
ACKNOWLEDGEMENT
The authors wish to thank Qinge Lu and Supata Santra for technical assistance.
Grant sponsor: National Institute of Neurological Disorders and Stroke (P50 NS23393 and RO1 NS047682); American Heart Association (0750048Z).
REFERENCES
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129(18):4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25(16):3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal GM, Peterson DA. Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell. 2004;3(6):345–351. doi: 10.1111/j.1474-9728.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- Bhakta S, Hong P, Koc O. The surface adhesion molecule CXCR4 stimulates mesenchymal stem cell migration to stromal cell-derived factor-1 in vitro but does not decrease apoptosis under serum deprivation. Cardiovasc Revasc Med. 2006;7(1):19–24. doi: 10.1016/j.carrev.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Bicker G. Nitric oxide: an unconventional messenger in the nervous system of an orthopteroid insect. Arch Insect Biochem Physiol. 2001;48(2):100–110. doi: 10.1002/arch.1062. [DOI] [PubMed] [Google Scholar]
- Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature medicine. 2004;10(8):858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. Journal of neuroscience research. 2003;73(6):778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang R, Katakowski M, Gautam SC, Xu Y, Lu M, Zhang Z, Chopp M. Combination therapy of stroke in rats with a nitric oxide donor and human bone marrow stromal cells enhances angiogenesis and neurogenesis. Brain research. 2004;1005(1-2):21–28. doi: 10.1016/j.brainres.2003.11.080. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li A, Zhang C, Ding J, Roberts C, Lu M, Kapke A, Chopp M. Vascular endothelial growth factor mediates atorvastatin-induced mammalian achaetescute homologue-1 gene expression and neuronal differentiation after stroke in retired breeder rats. Neuroscience. 2006a;141(2):737–744. doi: 10.1016/j.neuroscience.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Li Y, Li A, Wang L, Katakowski M, Roberts C, Lu M, Chopp M. N-cadherin mediates nitric oxide-induced neurogenesis in young and retired breeder neurospheres. Neuroscience. 2006b;140(2):377–388. doi: 10.1016/j.neuroscience.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005a;25(9):2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005b;25(2):281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Developmental biology. 2003;258(2):319–333. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Cherla RP, Ganju RK. Stromal cell-derived factor 1 alpha-induced chemotaxis in T cells is mediated by nitric oxide signaling pathways. J Immunol. 2001;166(5):3067–3074. doi: 10.4049/jimmunol.166.5.3067. [DOI] [PubMed] [Google Scholar]
- Ciani E, Severi S, Contestabile A, Bartesaghi R. Nitric oxide negatively regulates proliferation and promotes neuronal differentiation through N-Myc downregulation. J Cell Sci. 2004;117(Pt 20):4727–4737. doi: 10.1242/jcs.01348. [DOI] [PubMed] [Google Scholar]
- Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, Savant-Bhonsale S, Chopp M. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem cells (Dayton, Ohio) 2007;25(11):2777–2785. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36(8):1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- Englund U, Bjorklund A, Wictorin K. Migration patterns and phenotypic differentiation of long-term expanded human neural progenitor cells after transplantation into the adult rat brain. Brain Res Dev Brain Res. 2002;134(1-2):123–141. doi: 10.1016/s0165-3806(01)00330-3. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23(2):257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nature biotechnology. 2005;23(7):862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, Maeda M, Fagan SC, Carroll JE, Conway SJ. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63(1):84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoon YS, Choi JH, Yoo KY, Yi SS, Chung DW, Kim HJ, Kim CS, Do SG, Seong JK, Lee IS, Won MH. Doublecortin-immunoreactive neuronal precursors in the dentate gyrus of spontaneously hypertensive rats at various age stages: comparison with sprague-dawley rats. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 2008;70(4):373–377. doi: 10.1292/jvms.70.373. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neuroscience letters. 2004a;355(3):236–240. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem cells (Dayton, Ohio) 2004b;22(3):415–427. doi: 10.1634/stemcells.22-3-415. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, DeBusk LM, Babichev YO, Dumont DJ, Lin PC. Hepatocyte growth factor mediates angiopoietin-induced smooth muscle cell recruitment. Blood. 2006;108(4):1260–1266. doi: 10.1182/blood-2005-09-012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, Ratajczak J, Ratajczak MZ. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem cells (Dayton, Ohio) 2005;23(7):879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29(9):1972–1980. doi: 10.1161/01.str.29.9.1972. [DOI] [PubMed] [Google Scholar]
- Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of VEGF. Microvasc Res. 2004;68(3):221–230. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Moreno-Lopez B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24(1):85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HT, Hu S, Sheng WS, Olson JM, Cheeran MC, Chan AS, Lokensgard JR, Peterson PK. High-level expression of functional chemokine receptor CXCR4 on human neural precursor cells. Brain Res Dev Brain Res. 2004;152(2):159–169. doi: 10.1016/j.devbrainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Nygren J, Kokaia M, Wieloch T. Decreased expression of brain-derived neurotrophic factor in BDNF(+/-) mice is associated with enhanced recovery of motor performance and increased neuroblast number following experimental stroke. Journal of neuroscience research. 2006;84(3):626–631. doi: 10.1002/jnr.20956. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26(50):13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, Goldman SA, Enikolopov G. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci U S A. 2003;100(16):9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52(6):802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(1):125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Seidel C, Bicker G. Nitric oxide and cGMP influence axonogenesis of antennal pioneer neurons. Development. 2000;127(21):4541–4549. doi: 10.1242/dev.127.21.4541. [DOI] [PubMed] [Google Scholar]
- Stumm R, Kolodziej A, Schulz S, Kohtz JD, Hollt V. Patterns of SDF-1alpha and SDF-1gamma mRNAs, migration pathways, and phenotypes of CXCR4-expressing neurons in the developing rat telencephalon. J Comp Neurol. 2007;502(3):382–399. doi: 10.1002/cne.21336. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111(12):1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunico CR, Portillo F, Gonzalez-Forero D, Moreno-Lopez B. Nitric oxide-directed synaptic remodeling in the adult mammal CNS. J Neurosci. 2005;25(6):1448–1458. doi: 10.1523/JNEUROSCI.4600-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10(2):290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem cells (Dayton, Ohio) 2006;24(3):739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Rossel M, Moepps B, Zhang YL, Seidenfaden R, Favor J, Konig N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26(51):13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500(6):1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. Journal of neuroscience research. 2004;76(1):20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Truman JW, De Vente J, Ball EE. Nitric oxide-sensitive guanylate cyclase activity is associated with the maturational phase of neuronal development in insects. Development. 1996;122(12):3949–3958. doi: 10.1242/dev.122.12.3949. [DOI] [PubMed] [Google Scholar]
- Watanabe M MW, Shirahama Y, Mitsuyama H, Oonakahara K, Noma S, Higashimoto I, Osame M, Arimura K. Dual effect of AMD3100, a CXCR4 antagonist, on bleomycin-induced lung inflammation. J Immunol. 2007;178(9):11. doi: 10.4049/jimmunol.178.9.5888. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26(24):6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27(10):1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Zhang C, Cui X, Roberts C, Jiang H, Teng H, Chopp M. Nitric oxide regulates Angiopoietin1/Tie2 expression after stroke. Neuroscience letters. 2006;404(1-2):28–32. doi: 10.1016/j.neulet.2006.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang L, Zhang Z, Wang Y, Lu M, Lapointe M, Chopp M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann Neurol. 2001a;50(5):602–611. doi: 10.1002/ana.1249. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24(4):441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007a;27(12):3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang L, Zhang ZG, Morris D, Jiang Q, Wang L, Zhang LJ, Chopp M. Migration and differentiation of adult rat subventricular zone progenitor cells transplanted into the adult rat striatum. Neuroscience. 2003;116(2):373–382. doi: 10.1016/s0306-4522(02)00696-6. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Chopp M. Gene profiles within the adult subventricular zone niche: proliferation, differentiation and migration of neural progenitor cells in the ischemic brain. Current molecular medicine. 2007b;7(5):459–462. doi: 10.2174/156652407781387136. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001b;105(1):33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]