Abstract
In recent years the ventral pallidum has become a focus of great research interest as a mechanism of reward and incentive motivation. As a major output for limbic signals, the ventral pallidum was once associated primarily with motor functions rather than regarded as a reward structure in its own right. However, ample evidence now suggests that ventral pallidum function is a major mechanism of reward in the brain. We review data indicating that 1) an intact ventral pallidum is necessary for normal reward and motivation, 2) stimulated activation of ventral pallidum is sufficient to cause reward and motivation enhancements, and 3) activation patterns in ventral pallidum neurons specifically encode reward and motivation signals via phasic bursts of excitation to incentive and hedonic stimuli. We conclude that the ventral pallidum may serve as an important ‘limbic final common pathway’ for mesocorticolimbic processing of many rewards.
Author Keywords: Substantia Innominata, Pleasure, Limbic, Addiction, Obesity, Liking, Wanting, Learning
Ventral Pallidum Function: Moving Beyond Movement
The ventral pallidum was recognized as a distinct anatomical structure only a few decades ago. Heimer and Wilson first identified the ventral pallidum in 1975 as the primary output for the ventral striatum (nucleus accumbens), and suggested it served a role similar to globus pallidus in the striatal-pallidal circuitry for dorsal striatum (caudate-putamen) [1]. Previously the ventral pallidum often had been lumped with adjacent areas including the globus pallidus, substantia innominata, extended amygdala system, lateral preoptic area of hypothalamus (far rostral and lateral hypothalamus), or the polymorph layer of the olfactory tubercle. Today, however, its distinctive limbic-thalamocortical anatomical connectivity, histochemical and neuronal makeup (e.g. high levels of substance P, enkephalins, and iron; heterogeneous cell types including cholinergic and GABAergic projection neurons; basal firing rates that are generally slower than dorsal pallidal but faster than striatal projection neurons) are recognized to distinguish ventral pallidum from other surrounding structures [1–16].
Notions of the ventral pallidum as a striatal output for movement, comparable to globus pallidus, contributed originally to a view that it functioned as a motor expression site [17,18]. For example, based on a series of behavioral studies, Mogenson and colleagues proposed that nucleus accumbens projections to the ventral pallidum translated limbic motivation signals into motor output [18,19]. This account attributed “limbic-motor integration” [19] to accumbens-pallidal systems, and specifically identified ventral pallidal projections to brainstem (e.g. pedunculopontine tegmentum) as a primary motor output for limbic motivation signals. However, transferring input from accumbens to brainstem motor-related targets is only one feature of ventral pallidum connectivity. The ventral pallidum is also a central convergent point for input from orbitofrontal, prefrontal and infralimbic cortex, the amygdala, lateral hypothalamus, ventral tegmental area, parabrachial nucleus, subthalamic nucleus, and other structures related to reward [20–35]. Conversely, the ventral pallidum projects back to nearly all of its input sources including the nucleus accumbens for reciprocal information exchange [8,13,36–41]. Further, ventral pallidum outputs re-enter corticolimbic loops via direct projections to medial prefrontal cortex, and dense projections to mediodorsal nucleus of thalamus, which relays in turn to prefrontal cortex [6,10,11,13,36,38,42,43]. Such limbic-related anatomical connectivity sets the stage for the ventral pallidum to mediate reward and motivation functions at many levels in the brain, beyond merely aiding translation to movement [35,40,44–54].
The most crucial evidence that ventral pallidum mediates reward, however, must come from actual functional demonstrations that ventral pallidum manipulations have consequences for reward. That is, do manipulations of ventral pallidum actually alter reward-related measures of neural activation and reward-directed behavior? Many such studies have now been conducted, which we review below. Together they provide strong evidence that the ventral pallidum is needed for normal reward, that it can add new reward value to stimuli, and that its neurons can encode reward and incentive motivation to gain external rewards.
The Ventral Pallidum is Necessary for Reward
Necessary for motivation to eat and hedonic impact
Perhaps the earliest experiments to implicate ventral pallidum in reward and motivation functions were a set of studies by Morgane that pushed the boundaries of food reward functions beyond the lateral hypothalamus to include the ventral pallidum and globus pallidus [55]. Morgane reported that electrolytic lesions to the globus pallidus (which now can be recognized to have damaged ventral pallidum), caused aphagia (failure to voluntarily eat) and adipsia (failure to drink) in rats, similar to lesions of the lateral hypothalamus [55–61], despite not damaging the lateral hypothalamus (the pallidal lesions being anterior, further lateral or dorsal to the hypothalamus). This early lesion study did not distinguish between globus pallidus and ventral pallidum, but rather damaged both, and used the name of globus pallidus for the entire damaged region. However, our own inspection of Morgane’s lesions, as well as early lateral hypothalamic lesions, in published histological figures indicates the aphagia-inducing lesions damaged ventral pallidum as well as their intended target structure. These data, together with a later study that found that aphagia can be produced by lesions of the posterior ventral pallidum that do not invade globus pallidus or lateral hypothalamus [60], confirmed a role for the ventral pallidum as a key component of the neural system for eating and food ‘wanting’ [62].
The ventral pallidum may play an even more unique role in mediating reward beyond being necessary for motivated eating: it is the only structure known to us in which local lesions also eliminate normal ‘liking’ for sucrose, and replace it with ‘disliking’ [60]. ‘Liking’ is a second core component of reward, in addition to ‘wanting,’ and for many the most crucial. For natural food rewards, ‘liking’ has objective consequences in affective reactions patterns, such as orofacial reactivity patterns in response to tastes that are homologous across species [63–66].
Lesion studies of ventral pallidum and lateral hypothalamus have attempted to map the locus for a particular affective change in reward that often accompanied aphagia-producing lesions: the loss of acceptance or positive hedonic reactions to the taste of palatable food (such as tongue protrusions and lip licking), and replacement by active aversion reactions (such as gapes or headshakes). In the late 1970s, studies by Schallert and Whishaw [59] and Stellar, Brooks, and Mills [61] found that active avoidance of food (e.g. withdrawal from a food- or chocolate-containing spoon) and aversion to intraorally infused food (e.g. ejection of the reward followed by aversion reactions like face washing) was produced by what was described as damage to the anterior portion of lateral hypothalamus [59,61]. That damage encroached on what is now known to be ventral pallidum. Active avoidance-aversion was not produced by damage to more posterior subregions in the lateral hypothalamus (which caused aphagia without active aversion) [59]. A later mapping study actually contrasted ventral pallidum, globus pallidus or lateral hypothalamic excitotoxin lesions, and found that active aversion to sucrose was caused only if a lesion damaged the ventral pallidum (specifically its posterior end, overlapping with part of the adjacent substantia innominata) [60] (Figure 1) 1. Of note, the posterior and medial edge of ventral pallidum abuts the anterior and lateral edge of the lateral hypothalamus, and in fact, many classical electrolytic lesions of the lateral hypothalamus that produced aphagia with aversion also damaged the ventral pallidum as well as lateral hypothalamus [59,60]. This positioning may be important, given evidence for a ‘hedonic hotspot’ in the posterior ventral pallidum that we will describe below where ‘liking’ can actually be enhanced by neurochemical activity.
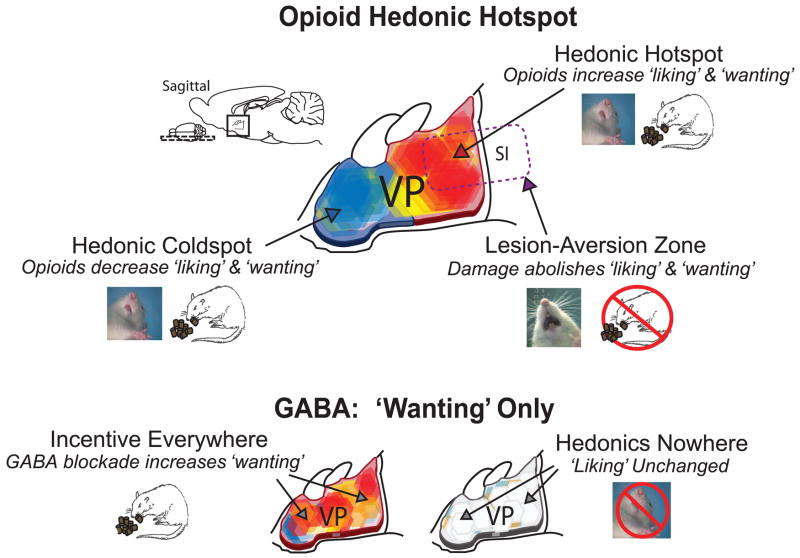
Figure 1.
Neurochemical maps of hedonic ‘liking’ and motivational ‘wanting’ in the ventral pallidum. Microinjections of DAMGO to stimulate opioid transmission in the posterior ventral pallidum hotspot enhance reward ‘liking’ (increased hedonic orofacial reactions to sweet taste such as tongue protrusion shown in image insert) and also enhance ‘wanting’ (increased motivated eating behavior) (top; red hexagons). The same DAMGO microinjections in an anterior coldspot decrease both ‘liking’ and ‘wanting’ measures below normal (blue). The posterior hedonic hotspot overlaps with the crucial zone where ventral forebrain lesions produce aversion to palatable tastes and aphagia (purple outline). By contrast, blockade of ventral pallidal GABA with bicuculline microinjections increase food ‘wanting’ and eating behavior virtually throughout the ventral pallidum (bottom left; red), but fail to change normal hedonic ‘liking’ reactions at the same sites (bottom right; white). Maps depict data reconstructed from [60,123]. VP ventral pallidum; SI substantia innominata.
This elimination of normal food reward (suppressed ‘liking’ and ‘wanting’, with enhanced aversion) that follows posterior ventral pallidum lesions may also be achieved with temporary neurochemical inactivation of ventral pallidal activity by excessive GABAergic inhibition. Microinjection of the GABAA agonist muscimol in the ventral pallidum was recently reported to attenuate intake of saccharine-flavored water or of bitter quinine-water, and to replace positive hedonic taste reactions to saccharine taste with aversive reactions [78]. These GABA microinjections that enhanced aversion did not explicitly distinguish subregions of the ventral pallidum, and reported effects were averaged for the entire ventral pallidum (though placements fell within the posterior lesion-aversion zone and extended anteriorly too).
Some evidence from humans also supports the idea that the ventral pallidum may be needed for normal motivation and hedonics. A recent clinical report describes a drug-addicted human patient with partial lesions to the ventral pallidum (overlapping with globus pallidus) who, after the lesions, “reported the disappearance of all drug cravings and remained abstinent from all recreational drugs other than an occasional glass of wine with dinner,” and “reported that he no longer experienced pleasure from drinking alcohol” (p. 786) [79]. The patient also “endorsed a depressed mood” and doctors noted a general “anhedonia” (p. 786) [79]. However, the patient also gained weight throughout this period, contrary to the lesion-induced aphagic effects in rodents described above, which may perhaps reflect spared parts of the ventral pallidum in the patient (the extent of damage is not clear from the published report of the still-living patient). In a second recent case [80], a patient with bilateral damage to the globus pallidus (the lesion is described by the authors as perhaps extending into the ventral pallidum) reported an “inability to feel emotions”, and was noted by analysts to have a flat affect and “a profound lack of motivation” (p. 413). In a reward task, the patient worked much less than normal to increase viewing time of pleasant pictures like food (by pressing a keyboard key), and reported less arousal from the pictures. His ratings of picture pleasantness were normal, and he worked normally to decrease viewing time of unpleasant pictures. The lesions thus appear to have impaired his ability to motivate behavior towards positive visual stimuli, though the extent of ventral pallidum damage is again not clear.
Necessary for reward learning and performance
Lesion or inactivation studies have further shown the ventral pallidum to be crucial for learning or performing learned responses related to rewards, in addition to generating the impact of unconditioned rewards. In operant and place preference conditioning studies, for example, ventral pallidum excitotoxin lesions or temporary inactivation (by microinjection of GABA or glutamate drugs, or lidocaine to block sodium channels) reduce baseline or primed lever pressing for alcohol, i.v. cocaine, and electrical stimulation to the medial forebrain bundle [81–88]. Rats with ventral pallidum GABAergic inactivation also have diminished willingness to work hard on an instrumental task to obtain sucrose reward, and instead shift their choice toward normal chow that can be obtained more easily, an effort shift similar to that produced by depletion of mesolimbic dopamine [89]. Ventral pallidum inactivation also reduces Pavlovian incentive learning about rewards, such as acquiring and expressing learned preferences for environments paired with sucrose, amphetamine, and morphine reward [90–92], and additionally impair performance in a variety of discrimination or matching tasks [93–104].
Thus, in total, features of normal reward learning and memory, motivational ‘wanting’, and hedonic ‘liking’ all appear to depend critically on the ventral pallidum. Among these reward components, normal hedonics may quite specifically require the ventral pallidum in a relatively unique way, whereas the other components can also be impaired by damage to other brain structures.
Ventral Pallidal Mechanisms for Enhancing Reward Impact
Beyond being necessary for normal reward, as reflected by impairments induced by lesion or inactivation, specific neurobiological activations in the ventral pallidum may also be sufficient to cause increases in the hedonic or motivational impact of stimuli. That is, it may be possible to enhance reward ‘liking’ or ‘wanting’ by chemical or other stimulation of the ventral pallidum. Necessary and sufficient causations are distinct forms of reward mediation, and do not always converge on the same reward substrates [54,105], but they may do so in the ventral pallidum.
Perhaps the first evidence suggesting that ventral pallidum activations can enhance the rewarding impact of stimuli and actions came from brain stimulation studies in the 1990s, which demonstrated that animals would repeatedly press a lever to self-stimulate through electrodes implanted in ventral pallidum [53,106,107], similarly to electrodes in the lateral hypothalamus and medial forebrain bundle [106,108–110]. This finding indicated that circuit activation through ventral pallidal stimulation was sufficient to activate a reward for instrumental pursuit, and placed the ventral pallidum as part of a larger network of limbic sites where reward might actually be generated in neural activity. More recent studies have begun to characterize the ventral pallidal neurotransmitters systems that play a role in enhancing reward ‘liking’ versus ‘wanting’, with some intriguing chemical and anatomical compartmentalizations.
Disinhibition of ventral pallidum from GABA suppression: stimulation of food ‘wanting’ (but not ‘liking’)
The use of intracranial drug microinjection to manipulate neurotransmission in limbic circuits has been particularly important for evaluating the natural chemical signals that mediate reward enhancement. This work has indicated the possibility that release of ventral pallidum neurons from tonic inhibitory GABA inputs from nucleus accumbens, central amygdala, and other areas (i.e. disinhibition), is a chief ‘downstream’ mechanism by which hyperpolarizations in nucleus accumbens stimulate motivation and reward [44,111–114]. Microinjections in nucleus accumbens of GABA agonists, glutamate AMPA antagonists, or opioid or cannabinoid agonists all stimulate eating behavior and pursuit of drugs and other rewards, through mechanisms that have been suggested to include local accumbens inhibition and disinhibition of ventral pallidum [48,50,113,115–122].
In an early study addressing chemical mechanisms of reward enhancement within the ventral pallidum, microinjection of a GABAA receptor antagonist (bicuculline) in ventral pallidum was shown to dramatically increase eating behavior and food intake [52], which has also been observed subsequently [78,123] (Figure 1). In order to test whether this stimulation of the motivation to eat involved enhancement of hedonic impact of food, we and others have conducted taste reactivity tests of ‘liking’ changes induced by bicuculline in the ventral pallidum [123] (Figure 1). Taste reactivity results indicated that GABA blockade in the ventral pallidum completely fails to elevate hedonic reactions to taste rewards. Neither in the posterior area where lesions produce aversion nor in any other area did bicuculline microinjection cause any detectable elevation of normal hedonic reactions to sucrose [78,123]. This has led us to conclude that ventral pallidal GABA disinhibition appears to be a mechanism of enhancing ‘wanting’-without enhancing ‘liking’-for food reward [50,54].
Such a pattern of ‘wanting’ without ‘liking’ augmentation is similar to the effect previously shown to result from activating mesolimbic dopamine systems by several manipulations: electrical stimulation of the medial forebrain bundle, systemic amphetamine or cocaine administration, microinjection of amphetamine into nucleus accumbens, neural sensitization induced by repeated psychostimulant exposure, or elevation of synaptic dopamine levels by genetic knockdown of the dopamine transporter [124–128]. In all of these cases of pure ‘wanting’ enhancement, dopaminergic stimulation enhanced motivated behavior to obtain or consume reward but failed to enhance hedonic reactions to tastes. Similarly in humans psychostimulant exposure that elevates dopamine levels and subjective ratings of drug or food reward ‘wanting’ has been reported in several studies to fail to also enhance subjective ratings of pleasure liking or euphoria [129,130]. It is not yet known how ventral pallidum GABA and mesolimbic dopamine interact in such cases, but there are several possibilities. For example, dopamine neurons also innervate ventral pallidum directly [25,26] and appear to have an important, but as yet unspecified, role in reward. For example, psychostimulant microinjection into the ventral pallidum is sufficient to condition a place preference [131] and increase eating behavior [132], while D1 receptor antagonist microinjection reduces intake [78] and dopaminergic terminal lesions block the development of a preference for cocaine-paired environments [133]. Local dopamine release can suppress the inhibitory influence of GABA transmission on ventral pallidum firing [134], indicating a modulatory role in GABAergic disinhibition of neural activity that may be important for motivation enhancements. Additionally, direct ventral pallidal modulation of midbrain dopamine activity via projections to ventral tegmental area [135–138] is another possible mechanism by which ventral pallidal GABAergic disinhibition might stimulate ‘wanting’ without ‘liking’.
However, while GABAergic disinhibition of ventral pallidum neurons does not increase hedonic impact, GABAA receptor activation by the agonist muscimol into the central ventral pallidum reduces normal hedonic reactions to sucrose and elevates aversive reactions [78,139], which may parallel the aversion consequence of ventral pallidum lesions [60]. Additionally, normal avoidance and aversion to a taste previously paired with lithium-chloride sickness can be reduced by ventral pallidal GABAA activation with bicuculline [140]. One possibility is that baseline neuronal activity in ventral pallidum has a necessary role in normal hedonic valuation, although depolarization induced by GABAergic disinhibition is not sufficient to cause enhancement of food’s hedonic valuation (despite enhancing the motivation to eat and reducing the expression of learned aversions).
Ventral pallidum opioids: ‘liking’ and ‘wanting’ stimulation in a posterior hotspot
Opioid neurotransmission is a more potent substrate for hedonic reward in ventral pallidum. Mu opioid stimulation is capable of enhancing hedonic ‘liking’ as well as motivational ‘wanting’, at least in a cubic millimeter hotspot of posterior ventral pallidum. Brain opioids have long been linked to hedonic and motivational properties of food, drugs and other incentives [141–147]. The ventral pallidum has abundant mu opioid receptors [148–151], and ventral pallidum opioid transmission is centrally involved in conditioned place preference and drug self-administration [30,152–156].
In contrast to GABA blockade, it turns out that increase in opioid transmission in the ventral pallidum is sufficient to enhance hedonic ‘liking’ reactions to sucrose as well as motivational ‘wanting’ to eat, but only in a restricted subregion of posterior ventral pallidum (Figure 1). In a recent study, we identified this posterior subregion as an opioid hedonic hotspot where microinjections of the mu opioid agonist DAMGO more than doubled taste ‘liking’ (hedonic reactions to sucrose) and quadrupled food ‘wanting’ (eating behavior) [123]. Fos plume mapping of drug functional spread helped reveal that the opioid hedonic hotspot was contained in the posterior half of the ventral pallidum and was roughly a cubic millimeter in size. Interestingly, the hedonic hotspot overlaps with the area where lesions abolish food reward and cause sucrose aversion [60], although an explicit comparison mapping of these opposite manipulations has yet to be made.
The special reward features of the posterior hotspot are underscored by observations that mu opioid stimulation of a more central and anterior coldspot location in ventral pallidum actually suppresses taste ‘liking’ reactions and eating behavior below normal levels, instead of enhancing them [123]. This difference reveals that opioid ‘liking’ and ‘wanting’ enhancement mechanisms are both highly localized to the hotspot in the posterior ventral pallidum. By contrast, the same reward functions are suppressed by the opioid coldspot in the anterior ventral pallidum.
An intriguingly similar segregation of positive affect to the posterior half ventral pallidum has recently been observed in human brain activations. In functional MRI studies, posterior ventral pallidum was reported to become more active during the presentation of images depicting appetizing food like chocolate cake, perhaps corresponding to the rat posterior hotspot above [157,158]. By contrast, negative pictures of disgusting and rotten food were reported to stimulate activity in more anterior regions of the ventral pallidum, perhaps corresponding to the rat anterior coldspot [158].
The posterior opioid hotspot additionally may be the best location for stimulating instrumental seeking for brain stimulation reward. In perhaps the first foreshadowing of the hedonic hotspot, microinjection of an opioid agonist in the posterior ventral pallidum was reported to increase the amount a rat will work to self-stimulate through electrodes implanted in the medial forebrain bundle, while the same microinjection in anterior ventral pallidum suppressed self-stimulation below normal levels [46]. That bivalent pattern seems quite similar to the more precisely mapped effects on taste ‘liking’ reactions and spontaneous food intake [123]. Also, the posterior ventral pallidum has an advantage for brain stimulation facilitation by microinjections of a delta opioid agonist [159]. Further, self-stimulation of an electrode in the ventral pallidum itself also appears to have a anterior-posterior gradient, as electrical current thresholds required for maintaining self-stimulation decline from the anterior to posterior ends, indicating that stimulation in the posterior hotspot is a more potent reward to animals even at low intensities [106].
Why would the hotspot in posterior ventral pallidum have more positive reward functions than the anterior ventral pallidum? There are a few known neurobiological features of the hotspot in the posterior ventral pallidum that might be involved. The posterior ventral pallidum appears to have higher enkephalin levels than anterior ventral pallidum [160,161], a higher ratio of noncholinergic to cholinergic cells [162], and a less dense concentration of presynaptic mu opioid receptors [149] compared to anterior ventral pallidum. Lastly, marked differences in anatomy have been noted for dorsal and lateral versus ventral and medial subregions of ventral pallidum in a plane at the anteroposterior level of the anterior commissure [14–16,37], which may be relevant as posterior ventral pallidum is more laterally placed in the brain (though the functional role of medial-lateral or dorsal-ventral subdivisions in reward is not yet clear).
Accumbens-pallidum opioid interaction: asymmetric paths for ‘liking’ and ‘wanting’
The ventral pallidum hotspot forms a functional circuit with another cubic-millimeter hedonic hotspot in medial shell of nucleus accumbens that similarly uses mu opioid signals to control ‘liking’ and ‘wanting’ [50,54,163]. To evaluate interactions between hotspots and structures, we pitted opioid activation of the ventral pallidum hotspot against simultaneous opioid suppression of the accumbens hotspot, and vice versa [164]. We found that opioid inactivation in the nucleus accumbens (via microinjection of naloxone in nucleus accumbens hotspot) blocked the elevation of ‘liking’ normally caused by opioid activation in the posterior ventral pallidum (via microinjection of DAMGO in ventral pallidum hotspot). Similarly, ‘liking’ elevation from opioid stimulation of the accumbens hotspot was blocked by opioid inactivation of the ventral pallidum hotspot. In addition, opioid activation in either hotspot also increased distant Fos protein expression in the other hotspot (and naloxone suppressed distant Fos), showing that opioid activation in one site recruits the other into activation as a neurobiological circuit. Thus, ‘liking’ elevation appears to require simultaneous participation of both opioid hotspots in ventral pallidum and accumbens (Figure 4).
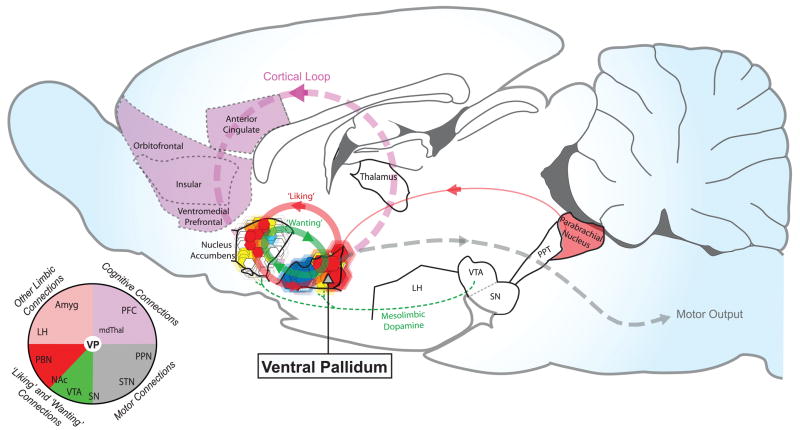
Figure 4.
Sagittal rodent brain diagram highlighting the ventral pallidum as a final pathway for limbic ‘liking’ and ‘wanting’ signals. ‘Liking’ systems (shown in red) link together opioid hedonic hotspots in the posterior ventral pallidum and dorsal-rostral accumbens shell, and potential link with a GABAergic hedonic signal in the parabrachial nucleus. ‘Wanting’ systems (green) link together mesolimbic dopamine, and opioid motivational signals in the accumbens and ventral pallidum, and larger circuits. The ventral pallidum is also connected with mesolimbic-thalamocortical loops (pink) and basal ganglia or brainstem motor output (gray) to influence cognition and action. Pie chart schematic shows ventral pallidum at an intersection of limbic connections with cognitive, motor, and reward structures. VTA ventral tegmental area; SN substantia nigra; PPT pedunculopontine tegmentum; LH lateral hypothalamus; PBN parabrachial nucleus; PFC prefrontal cortex; STN subthalamic nucleus; NAc nucleus accumbens; VTA ventral tegmental area; SN substantia nigra; mdThal mediodorsal thalamus; PBN parabrachial nucleus; Amyg amygdala.
These results imply that a traditional anatomical view of the ventral pallidum as purely a serial output for ventral striatal signals is only partially true. The ventral pallidum can equally influence the functional reward output of upstream accumbens as be influenced as a downstream consequence of accumbens activation, indicating a bidirectional interaction in which opioid-related information flows both ways. This possibility is also consistent with known bidirectional physiological interactions and reciprocal anatomical connections [1,39,44,165–168].
The circuit dynamics for controlling ‘wanting’ turn out to be a bit different from those controlling ‘liking’. Elevation of food ‘wanting’ appears to be asymmetrically dominated by opioid activation of the accumbens hotspot [164]. We found that eating elevation after opioid activation in the accumbens hotspot was not blocked by opioid blockade of the ventral pallidum hotspot. However, accumbens opioid blockade prevented any eating stimulation by opioid activation of the ventral pallidum hotpot. For motivation to eat (food ‘wanting’), opioid activation in the nucleus accumbens seems able to stimulate food intake in the absence of endogenous opioid recruitment in ventral pallidum (perhaps through connections with other motivation sites like the lateral hypothalamus[164,169–174]).
Neuronal activity in Ventral Pallidum Hotspot Codes Reward
How are reward ‘liking’ and ‘wanting’ encoded by neuronal firing within the ventral pallidum hotspot? A neural reward code must represent features of a sensory reward in profiles of neural activity, and the information in this neural representation is available for communication to efferent targets [175]. We have found evidence in recent behavioral electrophysiology experiments that the firing patterns of neurons in the ventral pallidum hotspot code reward and associated stimuli, and may discriminate learning, ‘liking’ and ‘wanting’ components of reward.
Ventral pallidal neurons fire with a phasic burst of excitation to a sucrose pellet reward [176]. If that sucrose pellet is paired associatively as an unconditioned stimulus (UCS) with a tone conditioned stimulus (CS+), ventral pallidum neurons develop an anticipatory excitation response to the auditory Pavlovian CS+ that predicts future sucrose reward as rats learn [176]. When multiple serial CS+ cues are presented, ventral pallidum neurons fire to each cue, and with extended training develop a more prominent phasic excitation to the first predictive cue (CS+1) compared to a subsequent cue (CS+2) that occurs closest to reward [127,176] (Figure 3). This pattern is similar to mesolimbic dopamine neurons [177](but see: [178]), and maximal firing to the first predictor has been taken to imply representation of cue predictive value [127,177,179]. Unlike dopamine neurons, however, neurons in the ventral pallidum hotpot continue to be strongly activated by sucrose rewards even long after they are predicted (when the Pavlovian association is well established), suggesting that the firing can represent hedonic information in addition to the predictive value of cues.
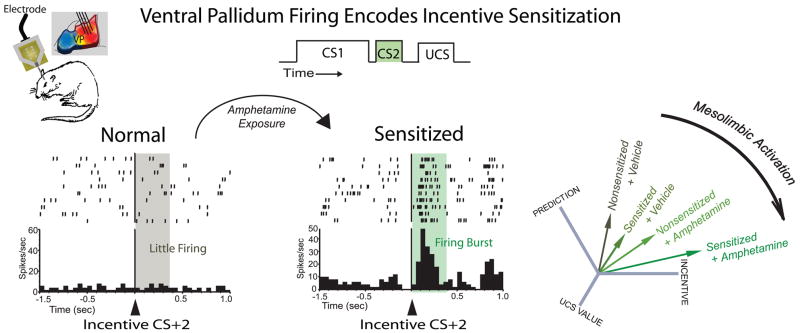
Figure 3.
Ventral pallidum neurons encode incentive sensitization. Mesolimbic dopamine stimulation after acute or repeated (sensitized) amphetamine injection increases incentive salience coding in magnified phasic burst of firing of to an incentive cue (CS+2), but not maximally predictive cue (CS+1), in a cue series. When the cue sequence is learned, ventral pallidum neurons fire little to the fully predicted CS+2 (shown at left is an example neuron). After acute or repeated amphetamine exposure to increase ‘wanting’, ventral pallidum neurons fire vigorous bursts of excitation to the same CS+2 incentive cue in an extinction session (shown in center is an example neuron recorded after amphetamine sensitization) [127]. Profile analysis (Right) on responses to multiple stimuli shows that dopaminergic ‘wanting’ increases by sensitization or acute amphetamine injection shift ventral pallidum firing away from a more predictive cue (CS+1) toward a incentive cue (CS+2), and fail to increase firing to the predicted UCS reward. Similar magnification of incentive firing over prediction firing in ventral pallidum neurons occurs with dopamine or opioid stimulation directly in the nucleus accumbens [195].
However, firing to reward properties of stimuli is confounded with sensory stimulus identity (e.g. sweetness of reward), arousal, and motor reactions, making their functional interpretation difficult. More focused experiments are required to pinpoint the coded neuronal representation for the hedonic value of reward and incentive value of a cue. Recently, we have conducted a series of studies designed explicitly to isolate hedonic, motivation, and learning features in excitatory bursts of ventral pallidum firing.
Ventral pallidum neurons encode reward hedonic ‘liking’ and incentive salience ‘wanting’
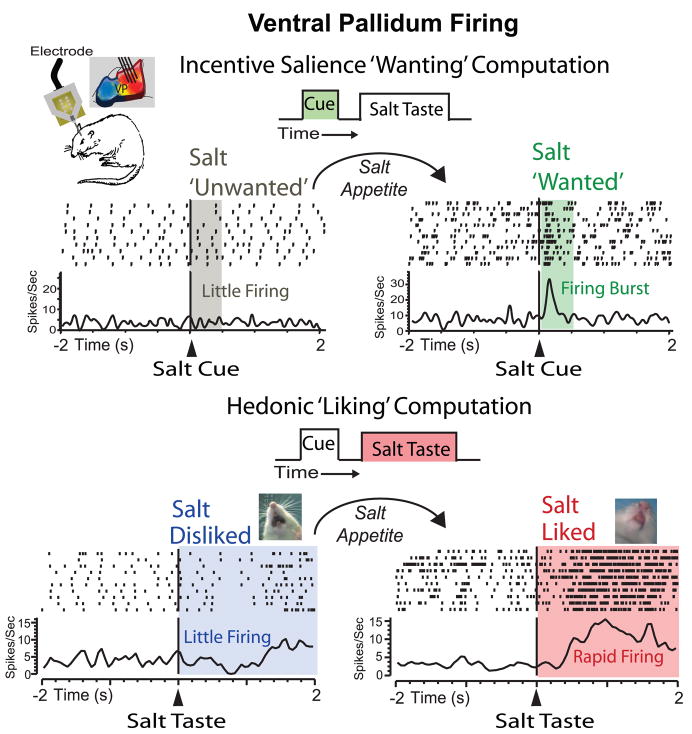
To isolate hedonic signals in ventral pallidum activity, we challenged ventral pallidum neurons with a shift in hedonic value of reward while keeping sensory identify stable [180] (Figure 2). When rats were in a normal physiological state, ventral pallidal neurons were observed to fire vigorously in response to an orally infused sucrose taste, which evoked hedonic ‘liking’ reactions in a behavioral taste reactivity test, but firing was less to an intensely salty taste (3X seawater concentration) that evoked aversive reactions. However, after animals were given diuretic injections to deplete them of bodily sodium, a sodium appetite developed and the intense salt taste evoked hedonic ‘liking’ reactions similar to sucrose. Ventral pallidal neurons then responded with an increased firing rate to the intense salt taste, equal in magnitude to the response evoked by the sucrose taste [180] (Figure 2) Additional analyses of firing during oral, grooming, and locomotion movements indicated that ventral pallidum firing did not simply encode movements. Ventral pallidum neurons thus pass the most stringent test for hedonic coding that is dis-confounded from sensory coding: being able to track hedonic shifts of a single sensory stimulus from nasty to pleasant [116,175,180,181].
Figure 2.
Ventral pallidum hotspot neurons encode the incentive salience of cues and hedonic value of rewards. (Top) Neurons fire little to a learned cue predicting an unpalatable salt taste, but fire in a phasic burst when the cue gains incentive value after sodium depletion and salt-appetite (raster and histogram traces show an example neuron recorded during baseline homeostasis and another neuron recorded after salt depletion firing in response to the salt cue at time zero) [182]. This dynamic computation integrates prior learned associations and physiological state to update incentive salience ‘on-the-fly’ and prior to ever re-experiencing the predicted taste. (Bottom) Normally, the unpalatable salt taste evokes ‘disliking’ reactions (e.g. oral gaping) and evokes little ventral pallidum firing, but after sodium depletion the same taste evokes ‘liking’ reactions (e.g. tongue protrusions) and bursts of ventral pallidal excitatory firing to compute magnified hedonic impact [180].
A separate but related study asked whether ventral pallidum neurons could additionally code the incentive salience of Pavlovian cues predicting salt or sucrose, in a way that could differentiate incentive ‘wanting’ from learned prediction of reward outcome [182]. Incentive salience theory posits that Pavlovian reward cues take on motivation features, which are similar in some ways to the motivation features of the unconditioned rewards they predict [183–185]. For example, reward cues become attractive and ‘wanted’ themselves. Although the incentive value of a conditioned reward cue is confounded with its predictive value, there is a way to disentangle these features in studies of neuronal coding by exploiting natural appetites. ‘Wanting’ for cues that predict sweet or salty rewards can be modulated directly by the same hunger, and sodium appetite physiological states that modulate the value of sucrose or salt UCS rewards, even if the unconditioned rewards have never been experienced in those appetite states [184,186–189]. For example, rats that have learned that a sour or bitter flavor is associated with salt, will later seek out and drink just the sour or bitter flavor by itself when they are in a physiological salt appetite state, and will show positive hedonic facial reactions to the conditioned flavor that previously was aversive [187,188,190]. In reverse, cues paired with normally pleasant sweet tastes can come to evoke aversive reactions and avoidance if the associated taste is devalued in a separate context (e.g., through pairing with lithium-chloride sickness) [190,191]. We harnessed this feature of cue incentive modulation to ask if ventral pallidum neurons code the motivation value of a learned cue, independently of what hedonic consequence the cue predicts based on its prior associative pairings with an unconditioned stimulus [182]. We paired auditory tone CS+s with either sucrose taste or intense sodium chloride taste UCSs that were infused into a rat’s mouth. Initially after training, an auditory CS+ tone predicting oral infusion of an aversive salt taste evoked virtually no firing response from posterior ventral pallidal neurons, whereas a tone predicting sucrose evoked a large firing response. After sodium depletion was induced by hormone injections, the salt and sucrose tones equally evoked intense firing from ventral pallidal neurons, even in extinction and prior to ever re-experiencing the salt taste in its new ‘liked’ state (salt ‘liking’ was confirmed in a subsequent taste reactivity test) (Figure 2). This indicates that ventral pallidum firing tracks the incentive salience of reward-predictive cues, and can integrate physiological needs to engage incentive motivation in an ‘on the fly’ manner without time spent learning new cue-reward contingencies.
The ventral pallidum may code for incentive motivation in humans as well. In one intriguing recent functional MRI study, ventral pallidal activity was elevated during the presentation of images signaling that a relatively large amount of money would be earned, which also evoked a motivated behavioral response (lever squeeze) to obtain the money [192]. Remarkably, ventral pallidal activity was even increased when the presentation of a relatively large money reward was too fast to be consciously detected, even though it still evoked a motivated behavioral response. Motivation-related activations of ventral pallidum in neuroimaging studies have similarly been observed with consciously detectable stimuli, including smells predicting tasty food or pictures depicting food [193,194].
Ventral pallidum neurons encode incentive sensitization and distinguish ‘wanting’ versus ‘liking’ enhancements
In the neural recording studies above, incentive salience ‘wanting’ and hedonic ‘liking’ were enhanced together by natural appetite, but ‘wanting’ can be separately enhanced alone by other manipulations, such as mesolimbic dopamine activation by amphetamine or psychostimulant drug-induced sensitization [105]. Can hedonic versus motivational features of reward codes in neuronal firing be separately tracked or told apart by ventral pallidum circuits? The answer seems to be yes.
A recent study in our group found that sensitization of motivation ‘wanting’, but not ‘liking’, caused by repeated drug exposure is encoded in profiles of ventral pallidum neural activity [127] (Figure 3). First, rats were trained to associate a series of two cues (CS+1 followed by CS+2) with a sucrose reward. They were then given acute amphetamine injections, chronic intermittent amphetamine injections to cause sensitization, or both, and examined for ventral pallidum neuronal responses to reward cues in the context of elevated dopamine transmission. After sensitization, the response profile of ventral pallidum neurons shifted away from prediction coding (maximal response to initial predictor CS+1) and towards incentive coding (maximal response to a CS+2 cue that occurred subsequently just before reward when motivation was highest), even though the sensitized rats had been drug-free for two weeks prior to testing (Figure 3). This incentive magnification change was not attributable to new learning as it occurred when the animals had no opportunity to experience new cue-reward pairings after sensitization was induced. Acute administration of amphetamine on the day of test similarly shifted neural responding toward the incentive cue, and combining acute amphetamine with prior sensitization biased the profile towards incentive responding most of all.
In a further study, we separated ‘liking’ and ‘wanting’ firing codes in ventral pallidum more distinctly by enhancing them individually with different pharmacological manipulations [195]. We used neurochemically and neuroanatomically focused manipulations of the nucleus accumbens to enhance ‘wanting’ only (with dopaminergic amphetamine microinjection) or to enhance ‘liking’ as well as ‘wanting’ (with opioid DAMGO microinjection). At the same time, we assessed phasic excitations in ventral pallidal firing that encode cue incentive salience and reward hedonics. Accumbens stimulation with dopamine or opioid agonists led to a striking magnification of ventral pallidum phasic excitation peaks to an incentive CS+2 cue, without affecting excitation to the first and maximally predictive CS+1 cue (nor to a CS-minus that lacked any incentive value), and did so on the very first cue presentations in extinction prior to re-learning or reward revaluation. Both DAMGO and amphetamine also increased consumption of a tasty chocolate candy as a behavioral consequence of heightened ‘wanting’. By contrast, only opioid stimulation also enhanced hedonic ‘liking’ reactions to sucrose and caused ventral pallidum neurons to also magnify their firing response to a sucrose reward. Amphetamine by comparison completely failed to enhance either behavioral ‘liking’ reactions or ventral pallidal excitatory firing to sucrose. Our results suggested that the ventral pallidum may use separate population and firing rate activity patterns to distinguish ‘wanting’ from ‘liking’ enhancements. This would mean that ventral pallidum neurons may convey distinctly-coded signals for ‘liking’ versus ‘wanting’ features of reward enhancement to downstream structures, which might modulate decision making and behavioral reactions appropriate to the particular reward component being enhanced.
Reward cues also activate ventral pallidum in humans, and we speculate that the incentive-coding patterns we have observed in rats may underlie ventral pallidum blood flow activation to drug reward cues in drug addicts that trigger motivation to take drug again. Cue-triggered ‘wanting’ for drugs may be a very basic response that does not need elaborate cognition. For example, Childress et al. [196] presented cocaine addicted subjects with pictures of drug-associated cues (e.g. images of drug taking) that could not be consciously detected. Yet the images not only triggered ventral pallidum activation (along with other limbic/cortex sites), but the intensity of ventral pallidal (and amygdala) activation predicted later positive affective reactions to the same cues when they were consciously seen (specifically, facilitated correct labeling of positive affective words). This shows that even subconsciously detected drug cues can engage ventral pallidum activation in addicts to promote motivational reactions, which may contribute to exacerbation of drug taking in the presence of incentive cues. Indeed, there is now a wealth of evidence that the ventral pallidum is an important structure for drug seeking, taking, and relapse in animal models of addiction [81,83,90,91,111,131,154,197–200], and a key site of neuroadaptations following repeated drug exposure that may contribute to sensitization, addiction, and relapse vulnerabilities [201–207]. How incentive processing of reward cues by ventral pallidum circuits contribute to drug taking and addiction will be an important issue for future research.
Ventral Pallidum Roles in Affiliation and Sex
Another important natural reward for evolution and behavior is sex and social affiliation, and several studies have revealed a ventral pallidal involvement in those processes too. For example, in humans, ventral pallidum/ventral globus pallidus activity is reported to increase during male sexual arousal [208], and in response to subliminally presented pictures of happy human faces or sexual images [196,209]. In the Callicebus Titi monkey, a monogamous species of South American primate, males that are co-habitating and pair bonded with their female mate show elevated ventral pallidal glucose metabolism, which was interpreted as implying a link between ventral pallidum neural activity and the maintenance of male/female affiliation [210].
In rodents, an exciting body of work on the monogamous prairie vole has demonstrated that transmission of the hormone vasopressin in the ventral pallidum is especially critical in regulating social affiliation and pair bonding [211–214]. After cohabitating and mating with a female, monogamous male voles form a preference for that particular female over another, and vasopressin in the ventral pallidum appears to play a critical role. Monogamous male prairie voles have greater vasopressin V1a receptor binding in the ventral pallidum compared to a non-monogamous species of meadow vole in which a male may have multiple female mates but not form any pair bonds [215]. Male prairie voles contain a greater density of V1a receptors in the ventral pallidum compared to females [216], and active mating behavior by the male vole engages vasopressin-dependant mechanism that increase Fos expression in the ventral pallidum [217]. Pair bonding can even be enhanced in some prairie voles by directly stimulating vasopressin V1a receptors in the ventral pallidum via viral vector gene delivery of V1a receptors, which also causes male voles to increase their affiliative behavior towards other males [215]. Further, V1a receptor upregulation in the ventral pallidum can cause the sexually promiscuous meadow vole to behave like a prairie vole and form a preference for a single familiar female [218].
However, at present it is too early to say if the ventral pallidum mediates ‘liking’ and/or ‘wanting’, or learning, of affiliation and sex. Still, it is worth highlighting at this early stage that the ventral pallidum is a common site for enhancing at least two critical natural rewards, both sex and food, as well as drug rewards.
The Ventral Pallidum as a Limbic Final Common Pathway
To summarize, a growing body of work has demonstrated major roles for the ventral pallidum in food reward, sex, social affiliation, electrical brain stimulation reward, drugs of abuse, winning money, and other rewards. The ventral pallidum is a convergent point for limbic reward signals and an intermediate stage to diverse cognitive, affective and motor processes. It is a central site for coding and causing enhancements of reward learning, hedonics, and motivation (Figure 4).
Its centrality in reward anatomy and function might be captured most comprehensively by describing the ventral pallidum as a ‘limbic final common pathway’ for reward signals in the brain. This concept borrows from Charles Sherrington’s early formulation of “final common pathway” to describe spinal motor nuclei as the last-stage through which signals must travel to influence movement [219]. Applied to limbic reward functions, the ventral pallidum might analogously be viewed as an essential convergent point for hedonic and motivational signaling pathways in the brain, and thus a final pathway for reward. This does not mean that reward functions are all contained within the ventral pallidum, nor that the ventral pallidum is necessarily the only pathway for reward to influence behavior or cognition. It simply suggests that reward pathways converge upon ventral pallidum, and that this funneling of signals plays a special role in generating major reward components. As with motor nuclei for movement, the ventral pallidum as a limbic final pathway passes the tests of being necessary for reward (without it, hedonics and motivation cannot be enhanced), sufficient to enhance reward (contains mechanisms that actively enhance hedonic impact and motivation), and a site of neural reward representation (represents hedonics and motivation in patterns of activity that can be sent to efferent target systems). These roles distinguish the ventral pallidum as among the most crucial sites for reward and motivation in the brain.
Acknowledgments
Research leading to this review was supported by grants from the NIH to KCB (MH63649 and DA015188) and JWA (DA017752).
Footnotes
Author list: Kyle S. Smith, Ph.D,. Massachusetts Institute of Technology, McGovern Institute for Brain Research, Room 46-6133, Cambridge, MA 02139, Email: kyless@mit.edu, Amy J. Tindell, Ph.D., University of Michigan, Department of Psychology, 525 E. University, Ann Arbor, MI 48109, Email: amy.j.tindell@gmail.com, J. Wayne Aldridge, Ph.D., University of Michigan, Department of Neurology, 1150 W. Medical Center Dr. Medical Science Building 1 – 3317, Ann Arbor, MI 48109, Email: jwaynea@umich.edu, Kent C. Berridge, Ph.D., University of Michigan, Department of Psychology, 525 E. University, Ann Arbor, MI 48109, Email: berridge@umich.edu
The only other neural lesion known to cause active aversion to sweet tastes is the classic ‘thalamic preparation’, in which the entire telencephalon composed of all structures anterior to the thalamus is removed by suction ablation or similar surgery, leaving intact thalamus, hypothalamus, midbrain and brainstem [67,68]. Importantly, the ‘thalamic preparation’ may damage the ventral pallidum, which is part of the telencephalon, raising the possibility that ventral pallidum damage might similarly be responsible for the thalamic animal’s aversion to sucrose. The importance to positive hedonic reactions of a ventral telencephalic structure such as ventral pallidum is emphasized by the consideration that basic hedonic reactions to taste are preserved in decerebrate animals transected above the superior colliculus but below most of the hypothalamus (with only the brainstem functioning) [68–70]. This presents a rather curious scenario: removing the ventral pallidum by itself or with the rest of the telencephalon, while leaving the diencephalic hypothalamus and thalamus as well as the brainstem, dramatically reduces hedonic reactions. But removing the ventral pallidum and telencephalon, plus the diencephalic hypothalamus and thalamus, fails to have much of an effect on hedonics. How can this be? For many behavioral functions, the brain contains a hierarchical organization such that brainstem signals are regulated by forebrain structures [71–74]. The taste pathway traverses through brainstem structures, such as nucleus of the solitary tract and parabrachial nucleus in rodents, then through the forebrain in bifurcating gustatory sensory paths (e.g. to gustatory thalamus then gustatory cortex) and limbic paths (e.g. to ventral pallidum) [75,76]. We have argued that basic affective and emotional reactions can be generated by the brainstem (e.g. in parabrachial nucleus: [77]), but in the normal intact brain these signals are under inhibitory control by forebrain hedonic structures like the ventral pallidum. This may be why we can observe relatively normal hedonics in decerebrated animals but impaired hedonics in animals with localized ventral pallidal lesions. See [74] and [54] for more detail.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heimer L, Wilson RD. The subcortical projections of allocortex: similarities in the neural associations of the hippocampus, the periform cortex and the neocortex. In: Santini M, editor. Golgi centennial symposium proceedings. New York: Raven Press; 1975. pp. 173–93. [Google Scholar]
- 2.Switzer RC, 3rd, Hill J, Heimer L. The globus pallidus and its rostroventral extension into the olfactory tubercle of the rat: a cyto- and chemoarchitectural study. Neuroscience. 1982;7:1891–904. doi: 10.1016/0306-4522(82)90005-7. [DOI] [PubMed] [Google Scholar]
- 3.de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- 4.Heimer L, Harlan RE, Alheid GF, Garcia MM, de Olmos J. Substantia innominata: a notion which impedes clinical-anatomical correlations in neuropsychiatric disorders. Neuroscience. 1997;76:957–1006. doi: 10.1016/s0306-4522(96)00405-8. [DOI] [PubMed] [Google Scholar]
- 5.Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- 6.Churchill L, Zahm DS, Kalivas PW. The mediodorsal nucleus of the thalamus in rats--I. forebrain gabaergic innervation. Neuroscience. 1996;70:93–102. doi: 10.1016/0306-4522(95)00351-i. [DOI] [PubMed] [Google Scholar]
- 7.Haber SN, Nauta WJ. Ramifications of the globus pallidus in the rat as indicated by patterns of immunohistochemistry. Neuroscience. 1983;9:245–60. doi: 10.1016/0306-4522(83)90291-9. [DOI] [PubMed] [Google Scholar]
- 8.Haber SN, Groenewegen HJ, Grove EA, Nauta WJ. Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol. 1985;235:322–35. doi: 10.1002/cne.902350304. [DOI] [PubMed] [Google Scholar]
- 9.Bengtson CP, Osborne PB. Electrophysiological properties of anatomically identified ventral pallidal neurons in rat brain slices. Ann N Y Acad Sci. 1999;877:691–4. doi: 10.1111/j.1749-6632.1999.tb09303.x. [DOI] [PubMed] [Google Scholar]
- 10.Zahm DS, Zaborszky L, Alheid GF, Heimer L. The ventral striatopallidothalamic projection: II. The ventral pallidothalamic link. J Comp Neurol. 1987;255:592–605. doi: 10.1002/cne.902550410. [DOI] [PubMed] [Google Scholar]
- 11.Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982;8:727–49. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- 12.Lavin A, Grace AA. Physiological properties of rat ventral pallidal neurons recorded intracellularly in vivo. J Neurophysiol. 1996;75:1432–43. doi: 10.1152/jn.1996.75.4.1432. [DOI] [PubMed] [Google Scholar]
- 13.Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- 14.Zahm DS, Williams E, Wohltmann C. Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. J Comp Neurol. 1996;364:340–62. doi: 10.1002/(SICI)1096-9861(19960108)364:2<340::AID-CNE11>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Zahm DS, Heimer L. Two transpallidal pathways originating in the rat nucleus accumbens. J Comp Neurol. 1990;302:437–46. doi: 10.1002/cne.903020302. [DOI] [PubMed] [Google Scholar]
- 16.Zahm DS, Heimer L. Ventral striatopallidal parts of the basal ganglia in the rat: I. Neurochemical compartmentation as reflected by the distributions of neurotensin and substance P immunoreactivity. J Comp Neurol. 1988;272:516–35. doi: 10.1002/cne.902720406. [DOI] [PubMed] [Google Scholar]
- 17.Heimer LSRD, Van Hoesen GW. Ventral striatum and ventral pallidum: Components of the motor system? Trends Neurosci. 1982;5:83–7. [Google Scholar]
- 18.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 19.Mogenson GJ, Yang CR. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–90. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- 20.Saper SB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 21.Reep RL, Winans SS. Efferent connections of dorsal and ventral agranular insular cortex in the hamster, Mesocricetus auratus. Neuroscience. 1982;7:2609–35. doi: 10.1016/0306-4522(82)90087-2. [DOI] [PubMed] [Google Scholar]
- 22.Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol. 1987;258:317–38. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- 23.Grove EA. Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol. 1988;277:315–46. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- 24.Carnes KM, Fuller TA, Price JL. Sources of presumptive glutamatergic/aspartatergic afferents to the magnocellular basal forebrain in the rat. J Comp Neurol. 1990;302:824–52. doi: 10.1002/cne.903020413. [DOI] [PubMed] [Google Scholar]
- 25.Napier TC, Muench MB, Maslowski RJ, Battaglia G. Is dopamine a neurotransmitter within the ventral pallidum/substantia innominata? Adv Exp Med Biol. 1991;295:183–95. doi: 10.1007/978-1-4757-0145-6_9. [DOI] [PubMed] [Google Scholar]
- 26.Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50:371–86. doi: 10.1016/0306-4522(92)90430-a. [DOI] [PubMed] [Google Scholar]
- 27.Chrobak JJ, Napier TC. Opioid and GABA modulation of accumbens-evoked ventral pallidal activity. J Neural Transm Gen Sect. 1993;93:123–43. doi: 10.1007/BF01245342. [DOI] [PubMed] [Google Scholar]
- 28.Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–78. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 29.Mitrovic I, Napier TC. Substance P attenuates and DAMGO potentiates amygdala glutamatergic neurotransmission within the ventral pallidum. Brain Res. 1998;792:193–206. doi: 10.1016/s0006-8993(98)00130-9. [DOI] [PubMed] [Google Scholar]
- 30.Olive MF, Maidment NT. Opioid regulation of pallidal enkephalin release: bimodal effects of locally administered mu and delta opioid agonists in freely moving rats. J Pharmacol Exp Ther. 1998;285:1310–6. [PubMed] [Google Scholar]
- 31.Turner MS, Lavin A, Grace AA, Napier TC. Regulation of limbic information outflow by the subthalamic nucleus: excitatory amino acid projections to the ventral pallidum. J Neurosci. 2001;21:2820–32. doi: 10.1523/JNEUROSCI.21-08-02820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zahm DS, Zaborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutamate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Res. 1985;325:317–21. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]
- 33.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 1998;797:73–93. doi: 10.1016/s0006-8993(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 34.Groenewegen HJ, Berendse HW. Connections of the subthalamic nucleus with ventral striatopallidal parts of the basal ganglia in the rat. J Comp Neurol. 1990;294:607–22. doi: 10.1002/cne.902940408. [DOI] [PubMed] [Google Scholar]
- 35.Maurice N, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Position of the ventral pallidum in the rat prefrontal cortex-basal ganglia circuit. Neuroscience. 1997;80:523–34. doi: 10.1016/s0306-4522(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 36.Grove EA. Efferent connections of the substantia innominata in the rat. J Comp Neurol. 1988;277:347–64. doi: 10.1002/cne.902770303. [DOI] [PubMed] [Google Scholar]
- 37.Zahm DS. The ventral striatopallidal parts of the basal ganglia in the rat--II. Compartmentation of ventral pallidal efferents. Neuroscience. 1989;30:33–50. doi: 10.1016/0306-4522(89)90351-5. [DOI] [PubMed] [Google Scholar]
- 38.Groenewegen HJ, Berendse HW, Haber SN. Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience. 1993;57:113–42. doi: 10.1016/0306-4522(93)90115-v. [DOI] [PubMed] [Google Scholar]
- 39.Churchill L, Kalivas PW. A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. J Comp Neurol. 1994;345:579–95. doi: 10.1002/cne.903450408. [DOI] [PubMed] [Google Scholar]
- 40.Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–7. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- 41.Phillipson OT, Griffiths AC. The topographic order of inputs to nucleus accumbens in the rat. Neuroscience. 1985;16:275–96. doi: 10.1016/0306-4522(85)90002-8. [DOI] [PubMed] [Google Scholar]
- 42.Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–77. [PubMed] [Google Scholar]
- 43.Pirot S, Jay TM, Glowinski J, Thierry A. Anatomical and electrophysiological evidence of an excitatory amino acid projection fromt the thalamic mediodorsal nucleus to the prefrontal cortex in the rat. Eur J Neurosci. 1994;6:1225–34. doi: 10.1111/j.1460-9568.1994.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 44.Napier TC, Mitrovic I. Opioid modulation of ventral pallidal inputs. Ann N Y Acad Sci. 1999;877:176–201. doi: 10.1111/j.1749-6632.1999.tb09268.x. [DOI] [PubMed] [Google Scholar]
- 45.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 46.Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32:1305–14. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]
- 47.Waraczynski MA. The central extended amygdala network as a proposed circuit underlying reward valuation. Neurosci Biobehav Rev. 2005 doi: 10.1016/j.neubiorev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Berridge KC. Brain reward systems for food incentives and hedonics in normal appetite and eating disorders. In: Kirkham TC, Cooper SJ, editors. Appetite and Body Weight. Academic Press; 2007. pp. 191–216. [Google Scholar]
- 49.Heimer L, Van Hoesen GW. The limbic lobe and its output channels: Implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2005 doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Peciña S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–11. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- 51.Zahm DS. The evolving theory of basal forebrain functional-anatomical ‘macrosystems’. Neurosci Biobehav Rev. 2006;30:148–72. doi: 10.1016/j.neubiorev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res. 1999;825:199–203. doi: 10.1016/s0006-8993(99)01239-1. [DOI] [PubMed] [Google Scholar]
- 53.Panagis G, Nomikos GG, Miliaressis E, Chergui K, Kastellakis A, Svensson TH, Spyraki C. Ventral pallidum self-stimulation induces stimulus dependent increase in c-fos expression in reward-related brain regions. Neuroscience. 1997;77:175–86. doi: 10.1016/s0306-4522(96)00471-x. [DOI] [PubMed] [Google Scholar]
- 54.Smith KS, Mahler SM, Peciña S, Berridge KC. Hedonic Hotspots: Generating Sensory Pleasure in the Brain. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford, U.K: Oxford University Press; in press. [Google Scholar]
- 55.Morgane PJ. Alterations in feeding and drinking behavior of rats with lesions in globi pallidi. Am J Physiol. 1961;201:420–8. doi: 10.1152/ajplegacy.1961.201.3.420. [DOI] [PubMed] [Google Scholar]
- 56.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951;24:123–40. [PMC free article] [PubMed] [Google Scholar]
- 57.Teitelbaum P, Stellar E. Recovery from the failure to eat produced by hypothalamic lesions. Science. 1954;120:894–5. doi: 10.1126/science.120.3126.894. [DOI] [PubMed] [Google Scholar]
- 58.Teitelbaum P, Epstein AN. The lateral hypothalamic syndrome: recovery of feeding and drinking after lateral hypothalamic lesions. Psychol Rev. 1962;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- 59.Schallert T, Whishaw IQ. Two types of aphagia and two types of sensorimotor impairment after lateral hypothalamic lesions: observations in normal weight, dieted, and fattened rats. J Comp Physiol Psychol. 1978;92:720–41. doi: 10.1037/h0077504. [DOI] [PubMed] [Google Scholar]
- 60.Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- 61.Stellar JR, Brooks FH, Mills LE. Approach and withdrawal analysis of the effects of hypothalamic stimulation and lesions in rats. J Comp Physiol Psychol. 1979;93:446–66. doi: 10.1037/h0077590. [DOI] [PubMed] [Google Scholar]
- 62.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 63.Grill HJ, Norgren R. The taste reactivity test. I Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–79. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- 64.Grill HJ, Berridge KC. Taste reactivity as a measure of the neural control of palatability. In: Epstein AN, Sprague J, editors. Progress in Psychobiology and Physiological Psychology. New York: Academic Press; 1985. pp. 1–6. [Google Scholar]
- 65.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 66.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–98. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 67.Bard P. On emotional expression after decortication with some remarks on certain theoretical views. Part II The Psych Review. 1934;41:424–49. [Google Scholar]
- 68.Grill HJ, Norgren R. The taste reactivity test. II Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–97. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- 69.Miller FR, Sherrington CS. Some Observations on the Bucco-Pharyngeal Stage of Reflex Deglutition in the Cat. Q J Exp Physiol. 1915;9:147–86. [Google Scholar]
- 70.Berridge KC. Brainstem systems mediate the enhancement of palatability by chlordiazepoxide. Brain Res. 1988;447:262–8. doi: 10.1016/0006-8993(88)91128-6. [DOI] [PubMed] [Google Scholar]
- 71.Panksepp J. Affective Neuroscience: A Conceptual Framework for the Study of Emotions. In: Strongman K, editor. International reviews of studies in emotions. Chichester: Wiley; 1991. pp. 59–99. [Google Scholar]
- 72.Damasio AR. The feeling of what happens: body and emotion in the making of consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- 73.Hughlings Jackson J. In: Selected writings of John Hughlings Jackson Vol 1 and 2. Taylor J, editor. London: Staples Press; 1958. [Google Scholar]
- 74.Berridge KC. Comparing the emotional brain of humans and other animals. In: Davidson RJ, Goldsmith HH, Scherer K, editors. Handbook of Affective Sciences. Oxford: Oxford University Press; 2003. pp. 25–51. [Google Scholar]
- 75.Pfaffmann C, Norgren R, Grill HJ. Sensory affect and motivation. Ann N Y Acad Sci. 1977;290:18–34. doi: 10.1111/j.1749-6632.1977.tb39713.x. [DOI] [PubMed] [Google Scholar]
- 76.Pritchard TC, Hamilton RB, Norgren R. Projections of the parabrachial nucleus in the old world monkey. Exp Neurol. 2000;165:101–17. doi: 10.1006/exnr.2000.7450. [DOI] [PubMed] [Google Scholar]
- 77.Soderpalm AH, Berridge KC. The hedonic impact and intake of food are increased by midazolam microinjection in the parabrachial nucleus. Brain Res. 2000;877:288–97. doi: 10.1016/s0006-8993(00)02691-3. [DOI] [PubMed] [Google Scholar]
- 78.Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- 79.Miller JM, Vorel SR, Tranguch AJ, Kenny ET, Mazzoni P, van Gorp WG, Kleber HD. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry. 2006;163:786–8. doi: 10.1176/ajp.2006.163.5.786. [DOI] [PubMed] [Google Scholar]
- 80.Vijayaraghavan L, Vaidya JG, Humphreys CT, Beglinger LJ, Paradiso S. Emotional and motivational changes after bilateral lesions of the globus pallidus. Neuropsychology. 2008;22:412–8. doi: 10.1037/0894-4105.22.3.412. [DOI] [PubMed] [Google Scholar]
- 81.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–60. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robledo P, Koob GF. Two discrete nucleus accumbens projection areas differentially mediate cocaine self-administration in the rat. Behav Brain Res. 1993;55:159–66. doi: 10.1016/0166-4328(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 84.Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22:3765–75. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–37. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- 86.Arvanitogiannis A, Waraczynski M, Shizgal P. Effects of excitotoxic lesions of the basal forebrain on MFB self-stimulation. Physiol Behav. 1996;59:795–806. doi: 10.1016/0031-9384(95)02157-4. [DOI] [PubMed] [Google Scholar]
- 87.Waraczynski M, Demco C. Lidocaine inactivation of the ventral pallidum affects responding for brain stimulation reward more than it affects the stimulation’s reward value. Behav Brain Res. 2006;173:288–98. doi: 10.1016/j.bbr.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 88.Bourdelais A, Kalivas PW. Amphetamine lowers extracellular GABA concentration in the ventral pallidum. Brain Res. 1990;516:132–6. doi: 10.1016/0006-8993(90)90907-s. [DOI] [PubMed] [Google Scholar]
- 89.Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Chrobak JJ, Salamone JD. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience. 2008;152:321–30. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McAlonan GM, Robbins TW, Everitt BJ. Effects of medial dorsal thalamic and ventral pallidal lesions on the acquisition of a conditioned place preference: further evidence for the involvement of the ventral striatopallidal system in reward-related processes. Neuroscience. 1993;52:605–20. doi: 10.1016/0306-4522(93)90410-h. [DOI] [PubMed] [Google Scholar]
- 91.Hiroi N, White NM. The ventral pallidum area is involved in the acquisition but not expression of the amphetamine conditioned place preference. Neurosci Lett. 1993;156:9–12. doi: 10.1016/0304-3940(93)90426-l. [DOI] [PubMed] [Google Scholar]
- 92.Dallimore JE, Mickiewicz AL, Napier TC. Intra-ventral pallidal glutamate antagonists block expression of morphine-induced place preference. Behav Neurosci. 2006;120:1103–14. doi: 10.1037/0735-7044.120.5.1103. [DOI] [PubMed] [Google Scholar]
- 93.Robbins TW, Everitt BJ. Comparative functions of the central noradrenergic, dopaminergic and cholinergic systems. Neuropharmacology. 1987;26:893–901. doi: 10.1016/0028-3908(87)90067-0. [DOI] [PubMed] [Google Scholar]
- 94.Everitt BJ, Robbins TW, Evenden JL, Marston HM, Jones GH, Sirkia TE. The effects of excitotoxic lesions of the substantia innominata, ventral and dorsal globus pallidus on the acquisition and retention of a conditional visual discrimination: implications for cholinergic hypotheses of learning and memory. Neuroscience. 1987;22:441–69. doi: 10.1016/0306-4522(87)90346-0. [DOI] [PubMed] [Google Scholar]
- 95.Evenden JL, Marston HM, Jones GH, Giardini V, Lenard L, Everitt BJ, Robbins TW. Effects of excitotoxic lesions of the substantia innominata, ventral and dorsal globus pallidus on visual discrimination acquisition, performance and reversal in the rat. Behav Brain Res. 1989;32:129–49. doi: 10.1016/s0166-4328(89)80080-4. [DOI] [PubMed] [Google Scholar]
- 96.Dunnett SB, Everitt BJ, Robbins TW. The basal forebrain-cortical cholinergic system: interpreting the functional consequences of excitotoxic lesions. Trends Neurosci. 1991;14:494–501. doi: 10.1016/0166-2236(91)90061-x. [DOI] [PubMed] [Google Scholar]
- 97.Muir JL, Page KJ, Sirinathsinghji DJ, Robbins TW, Everitt BJ. Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav Brain Res. 1993;57:123–31. doi: 10.1016/0166-4328(93)90128-d. [DOI] [PubMed] [Google Scholar]
- 98.Dudchenko P, Sarter M. GABAergic control of basal forebrain cholinergic neurons and memory. Behav Brain Res. 1991;42:33–41. doi: 10.1016/s0166-4328(05)80037-3. [DOI] [PubMed] [Google Scholar]
- 99.Chiba AA, Bucci DJ, Holland PC, Gallagher M. Basal forebrain cholinergic lesions disrupt increments but not decrements in conditioned stimulus processing. J Neurosci. 1995;15:7315–22. doi: 10.1523/JNEUROSCI.15-11-07315.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise–induced enhancement of learning. J Neurosci. 2006;26:3791–7. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann N Y Acad Sci. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- 102.Kalivas PW, Jackson D, Romanidies A, Wyndham L, Duffy P. Involvement of pallidothalamic circuitry in working memory. Neuroscience. 2001;104:129–36. doi: 10.1016/s0306-4522(01)00054-9. [DOI] [PubMed] [Google Scholar]
- 103.Floresco SB, Braaksma DN, Phillips AG. Involvement of the ventral pallidum in working memory tasks with or without a delay. Ann N Y Acad Sci. 1999;877:711–6. doi: 10.1111/j.1749-6632.1999.tb09308.x. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y, Bailey KR, Toupin MM, Mair RG. Involvement of ventral pallidum in prefrontal cortex-dependent aspects of spatial working memory. Behav Neurosci. 2005;119:399–409. doi: 10.1037/0735-7044.119.2.399. [DOI] [PubMed] [Google Scholar]
- 105.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 106.Panagis G, Miliaressis E, Anagnostakis Y, Spyraki C. Ventral pallidum self-stimulation: a moveable electrode mapping study. Behav Brain Res. 1995;68:165–72. doi: 10.1016/0166-4328(94)00169-g. [DOI] [PubMed] [Google Scholar]
- 107.Murray B, Shizgal P. Physiological measures of conduction velocity and refractory period for putative reward-relevant MFB axons arising in the rostral MFB. Physiol Behav. 1996;59:427–37. doi: 10.1016/0031-9384(95)02077-2. [DOI] [PubMed] [Google Scholar]
- 108.Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 109.Murray B, Shizgal P. Attenuation of medical forebrain bundle reward by anterior lateral hypothalamic lesions. Behav Brain Res. 1996;75:33–47. doi: 10.1016/0166-4328(96)00155-6. [DOI] [PubMed] [Google Scholar]
- 110.Burgdorf J, Wood PL, Kroes RA, Moskal JR, Panksepp J. Neurobiology of 50-kHz ultrasonic vocalizations in rats: electrode mapping, lesion, and pharmacology studies. Behav Brain Res. 2007;182:274–83. doi: 10.1016/j.bbr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 111.Caille S, Parsons LH. Intravenous heroin self-administration decreases GABA efflux in the ventral pallidum: an in vivo microdialysis study in rats. Eur J Neurosci. 2004;20:593–6. doi: 10.1111/j.1460-9568.2004.03497.x. [DOI] [PubMed] [Google Scholar]
- 112.Caille S, Parsons LH. Cannabinoid Modulation of Opiate Reinforcement through the Ventral Striatopallidal Pathway. Neuropsychopharmacology. 2005 doi: 10.1038/sj.npp.1300848. [DOI] [PubMed] [Google Scholar]
- 113.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–9. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 114.Kretschmer BD. Functional aspects of the ventral pallidum. Amino Acids. 2000;19:201–10. doi: 10.1007/s007260070050. [DOI] [PubMed] [Google Scholar]
- 115.Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993;265:1253–60. [PubMed] [Google Scholar]
- 116.Wheeler RA, Carelli RM. The neuroscience of pleasure. Focus on “Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2175–6. doi: 10.1152/jn.00727.2006. [DOI] [PubMed] [Google Scholar]
- 117.Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–70. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–20. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mahler SM, Smith KS, Berridge KC. Endocannabinoid Hedonic Hotspot for Sensory Pleasure: Anandamide in Nucleus Accumbens Shell Enhances ‘Liking’ of Sweet Reward. Neuropsychopharmacology. 2007;32:2267–78. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 120.Stratford TR, Kelley AE. GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci. 1997;17:4434–40. doi: 10.1523/JNEUROSCI.17-11-04434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kelley AE, Schiltz CA, Landry CF. Neural systems recruited by drug- and food-related cues: studies of gene activation in corticolimbic regions. Physiol Behav. 2005;86:11–4. doi: 10.1016/j.physbeh.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 122.Torregrossa MM, Tang XC, Kalivas PW. The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA. Neurosci Lett. 2008;438:142–5. doi: 10.1016/j.neulet.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–49. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peciña S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J Neurosci. 2003;23:9395–402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–30. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue–triggered “wanting” for sucrose reward. J Neurosci. 2001;21:7831–40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci. 2005;22:2617–34. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- 128.Berridge KC, Valenstein ES. What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behav Neurosci. 1991;105:3–14. doi: 10.1037//0735-7044.105.1.3. [DOI] [PubMed] [Google Scholar]
- 129.Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–35. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- 130.Volkow ND, Wang GJ, Fowler JS, Logan J, Jayne M, Franceschi D, Wong C, Gatley SJ, Gifford AN, Ding YS, Pappas N. Nonhedonic” food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse. 2002;44:175–80. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 131.Gong W, Neill D, Justice JB., Jr Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707:64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- 132.Jones JL, Smith KS, Berridge KC. Food reward and the ventral pallidum: dopamine activation of eating and locomotion. Soc Neurosci Abstr. 2005 [Google Scholar]
- 133.Gong W, Neill D, Justice JB., Jr 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 1997;754:103–12. doi: 10.1016/s0006-8993(97)00059-0. [DOI] [PubMed] [Google Scholar]
- 134.Johnson PI, Napier TC. GABA- and glutamate-evoked responses in the rat ventral pallidum are modulated by dopamine. Eur J Neurosci. 1997;9:1397–406. doi: 10.1111/j.1460-9568.1997.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 135.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 136.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 137.Geisler S, Marinelli M, Degarmo B, Becker ML, Freiman AJ, Beales M, Meredith GE, Zahm DS. Prominent Activation of Brainstem and Pallidal Afferents of the Ventral Tegmental Area by Cocaine. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kalivas PW, Churchill L, Klitenick MA. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience. 1993;57:1047–60. doi: 10.1016/0306-4522(93)90048-k. [DOI] [PubMed] [Google Scholar]
- 139.Ho CY, Berridge KC. personal observations. [Google Scholar]
- 140.Inui T, Shimura T, Yamamoto T. The role of the ventral pallidum GABAergic system in conditioned taste aversion: effects of microinjections of a GABAA receptor antagonist on taste palatability of a conditioned stimulus. Brain Res. 2007;1164:117–24. doi: 10.1016/j.brainres.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 141.Cooper SJ. Effects of opiate agonists and antagonists on fluid intake and saccharine choice in the rat. Neuropharmacology. 1983;22:323–8. doi: 10.1016/0028-3908(83)90247-2. [DOI] [PubMed] [Google Scholar]
- 142.Morley JE, Levine AS, Yim GK, Lowy MT. Opioid modulation of appetite. Neurosci Biobehav Rev. 1983;7:281–305. doi: 10.1016/0149-7634(83)90020-9. [DOI] [PubMed] [Google Scholar]
- 143.Doyle TG, Berridge KC, Gosnell BA. Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav. 1993;46:745–9. doi: 10.1016/0091-3057(93)90572-b. [DOI] [PubMed] [Google Scholar]
- 144.Berridge KC. Pleasures of the brain. Brain Cogn. 2003;52:106–28. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 145.Rideout HJ, Parker LA. Morphine enhancement of sucrose palatability: analysis by the taste reactivity test. Pharmacol Biochem Behav. 1996;53:731–4. doi: 10.1016/0091-3057(95)02077-2. [DOI] [PubMed] [Google Scholar]
- 146.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–77. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 147.Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 148.Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- 149.Olive MF, Anton B, Micevych P, Evans CJ, Maidment NT. Presynaptic versus postsynaptic localization of mu and delta opioid receptors in dorsal and ventral striatopallidal pathways. J Neurosci. 1997;17:7471–9. doi: 10.1523/JNEUROSCI.17-19-07471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moskowitz AS, Goodman RR. Light microscopic autoradiographic localization of mu and delta opioid binding sites in the mouse central nervous system. J Neurosci. 1984;4:1331–42. doi: 10.1523/JNEUROSCI.04-05-01331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Johnson PI, Napier TC. Ventral pallidal injections of a mu antagonist block the development of behavioral sensitization to systemic morphine. Synapse. 2000;38:61–70. doi: 10.1002/1098-2396(200010)38:1<61::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 152.Olive MF, Bertolucci M, Evans CJ, Maidment NT. Microdialysis reveals a morphine-induced increase in pallidal opioid peptide release. Neuroreport. 1995;6:1093–6. doi: 10.1097/00001756-199505300-00005. [DOI] [PubMed] [Google Scholar]
- 153.Zarrindast MR, Ebrahimi-Ghiri M, Rostami P, Rezayof A. Repeated pre-exposure to morphine into the ventral pallidum enhances morphine-induced place preference: involvement of dopaminergic and opioidergic mechanisms. Behav Brain Res. 2007;181:35–41. doi: 10.1016/j.bbr.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 154.Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25:4512–20. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Martin TJ, Coller M, Co C, Smith JE. Micro-opioid receptor alkylation in the ventral pallidum and ventral tegmental area, but not in the nucleus accumbens, attenuates the effects of heroin on cocaine self-administration in rats. Neuropsychopharmacology. 2008;33:1171–8. doi: 10.1038/sj.npp.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Skoubis PD, Maidment NT. Blockade of ventral pallidal opioid receptors induces a conditioned place aversion and attenuates acquisition of cocaine place preference in the rat. Neuroscience. 2003;119:241–149. doi: 10.1016/s0306-4522(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 157.Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Calder AJ, Beaver JD, Davis MH, van Ditzhuijzen J, Keane J, Lawrence AD. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur J Neurosci. 2007;25:3422–8. doi: 10.1111/j.1460-9568.2007.05604.x. [DOI] [PubMed] [Google Scholar]
- 159.Johnson PI, Stellar JR. Comparison of delta opiate receptor agonist induced reward and motor effects between the ventral pallidum and dorsal striatum. Neuropharmacology. 1994;33:1171–82. doi: 10.1016/s0028-3908(05)80007-3. [DOI] [PubMed] [Google Scholar]
- 160.Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans CJ. Microdialysis of extracellular endogenous opioid peptides from rat brain in vivo. Neuroscience. 1989;33:549–57. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- 161.Holt AG, Newman SW. Distribution of methionine and leucine enkephalin neurons within the social behavior circuitry of the male Syrian hamster brain. Brain Res. 2004;1030:28–48. doi: 10.1016/j.brainres.2004.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bengtson CP, Osborne PB. Electrophysiological properties of cholinergic and noncholinergic neurons in the ventral pallidal region of the nucleus basalis in rat brain slices. J Neurophysiol. 2000;83:2649–60. doi: 10.1152/jn.2000.83.5.2649. [DOI] [PubMed] [Google Scholar]
- 163.Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–86. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mogenson GJ, Swanson LW, Wu M. Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J Neurosci. 1983;3:189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hakan RL, Berg GI, Henriksen SJ. Electrophysiological evidence for reciprocal connectivity between the nucleus accumbens septi and ventral pallidal region. Brain Res. 1992;581:344–50. doi: 10.1016/0006-8993(92)90730-w. [DOI] [PubMed] [Google Scholar]
- 167.Hakan RL, Eyl C, Henriksen SJ. Neuropharmacology of the nucleus accumbens: systemic morphine effects on single-unit responses evoked by ventral pallidum stimulation. Neuroscience. 1994;63:85–93. doi: 10.1016/0306-4522(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 168.Hakan RL, Eyl C. Neuropharmacology of the nucleus accumbens: iontophoretic applications of morphine and nicotine have contrasting effects on single-unit responses evoked by ventral pallidal and fimbria stimulation. Synapse. 1995;20:175–84. doi: 10.1002/syn.890200212. [DOI] [PubMed] [Google Scholar]
- 169.Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–8. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- 171.Kim EM, Quinn JG, Levine AS, O’Hare E. A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Res. 2004;1029:135–9. doi: 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 172.Will MJ, Franzblau EB, Kelley AE. The amygdala is critical for opioid-mediated binge eating of fat. Neuroreport. 2004;15:1857–60. doi: 10.1097/00001756-200408260-00004. [DOI] [PubMed] [Google Scholar]
- 173.Bodnar RJ, Lamonte N, Israel Y, Kandov Y, Ackerman TF, Khaimova E. Reciprocal opioid-opioid interactions between the ventral tegmental area and nucleus accumbens regions in mediating mu agonist-induced feeding in rats. Peptides. 2005;26:621–9. doi: 10.1016/j.peptides.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 174.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 175.Aldridge JW, Berridge KC. Neural Coding of Pleasure: “Rose-Tinted Glasses” of the Ventral Pallidum. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford, U.K: Oxford University Press; in press. [Google Scholar]
- 176.Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24:1058–69. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 178.Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005;25:6235–42. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 180.Tindell AJ, Smith KS, Peciña S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- 181.Berridge KC, Flynn FW, Schulkin J, Grill HJ. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98:652–60. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- 182.Tindell AJ, Smith KS, Berridge KC, Aldridge JW. VP neurons integrate learning and physiological signals to code incentive salience of conditioned cues. Soc Neurosci Abstr. 2005 [Google Scholar]
- 183.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 184.Toates FM. Motivational Systems. Cambridge: Cambridge University Press; 1986. [Google Scholar]
- 185.Bindra D. How adaptive behavior is produced: a perceptual-motivation alternative to response reinforcement. Behav Brain Sci. 1978;1:41–91. [Google Scholar]
- 186.Kriekhaus EE, Wolf G. Acquisition of sodium by rats: Interaction of innate mechanisms and latent learning. J Comp Physiol Psychol. 1968;65:197–201. doi: 10.1037/h0025547. [DOI] [PubMed] [Google Scholar]
- 187.Fudim OK. Sensory preconditioning of flavors with a formalin-produced sodium need. J Exp Psychol Anim Behav Process. 1978;4:276–85. doi: 10.1037//0097-7403.4.3.276. [DOI] [PubMed] [Google Scholar]
- 188.Berridge KC, Schulkin J. Palatability shift of a salt-associated incentive during sodium depletion. Q J Exp Psychol B. 1989;41:121–38. [PubMed] [Google Scholar]
- 189.Berridge KC. Reward learning: Reinforcement, incentives, and expectations. In: Medin DL, editor. The Psychology of Learning and Motivation. New York: Academic Press; 2001. pp. 223–78. [Google Scholar]
- 190.Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learn Mem. 2007;14:581–9. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. J Exp Psychol Anim Behav Process. 1979;5:65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- 192.Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316:904–6. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F. Separable substrates for anticipatory and consummatory food chemosensation. Neuron. 2008;57:786–97. doi: 10.1016/j.neuron.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41:636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 195.Smith KS, Berridge KC, Aldridge JW. Ventral pallidal neurons distinguish ‘liking’ and ‘wanting’ elevations caused by opioids versus dopamine in nucleus accumbens. Soc Neurosci Abstr. 2007 [Google Scholar]
- 196.Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rademacher DJ, Kovacs B, Shen F, Napier TC, Meredith GE. The neural substrates of amphetamine conditioned place preference: implications for the formation of conditioned stimulus-reward associations. Eur J Neurosci. 2006;24:2089–97. doi: 10.1111/j.1460-9568.2006.05066.x. [DOI] [PubMed] [Google Scholar]
- 198.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 199.Kolodziejska-Akiyama KM, Cha YM, Jiang Y, Loh HH, Chang SL. Ethanol-induced FOS immunoreactivity in the brain of mu-opioid receptor knockout mice. Drug Alcohol Depend. 2005;80:161–8. doi: 10.1016/j.drugalcdep.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 200.Strother WN, McBride WJ, Lumeng L, Li TK. Effects of acute administration of ethanol on cerebral glucose utilization in adult alcohol-preferring and alcohol-nonpreferring rats. Alcohol. 2005;35:119–28. doi: 10.1016/j.alcohol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 201.McDaid J, Dallimore JE, Mackie AR, Mickiewicz AL, Napier TC. Cross-sensitization to morphine in cocaine-sensitized rats: behavioral assessments correlate with enhanced responding of ventral pallidal neurons to morphine and glutamate, with diminished effects of GABA. J Pharmacol Exp Ther. 2005;313:1182–93. doi: 10.1124/jpet.105.084038. [DOI] [PubMed] [Google Scholar]
- 202.McDaid J, Dallimore JE, Mackie AR, Napier TC. Changes in accumbal and pallidal pCREB and deltaFosB in morphine-sensitized rats: correlations with receptor-evoked electrophysiological measures in the ventral pallidum. Neuropsychopharmacology. 2006;31:1212–26. doi: 10.1038/sj.npp.1300854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McDaid J, Tedford CE, Mackie AR, Dallimore JE, Mickiewicz AL, Shen F, Angle JM, Napier TC. Nullifying drug-induced sensitization: behavioral and electrophysiological evaluations of dopaminergic and serotonergic ligands in methamphetamine-sensitized rats. Drug Alcohol Depend. 2007;86:55–66. doi: 10.1016/j.drugalcdep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 204.Napier TC, Istre ED. Methamphetamine-induced sensitization includes a functional upregulation of ventral pallidal 5-HT2A/2C receptors. Synapse. 2008;62:14–21. doi: 10.1002/syn.20460. [DOI] [PubMed] [Google Scholar]
- 205.Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther. 1994;270:1387–96. [PubMed] [Google Scholar]
- 206.Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–6. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Smith DG, Learn JE, McBride WJ, Lumeng L, Li TK, Murphy JM. Long-term effects of alcohol drinking on cerebral glucose utilization in alcohol-preferring rats. Pharmacol Biochem Behav. 2001;69:543–53. doi: 10.1016/s0091-3057(01)00553-6. [DOI] [PubMed] [Google Scholar]
- 208.Rauch SL, Shin LM, Dougherty DD, Alpert NM, Orr SP, Lasko M, Macklin ML, Fischman AJ, Pitman RK. Neural activation during sexual and competitive arousal in healthy men. Psychiatry Res. 1999;91:1–10. doi: 10.1016/s0925-4927(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 209.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Res. 2007;1184:245–53. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–8. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 212.Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: implications for autism. Philos Trans R Soc Lond B Biol Sci. 2006;361:2187–98. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 214.Wang Z, Aragona BJ. Neurochemical regulation of pair bonding in male prairie voles. Physiol Behav. 2004;83:319–28. doi: 10.1016/j.physbeh.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 215.Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–6. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lim MM, Murphy AZ, Young LJ. Ventral striatopallidal oxytocin and vasopressin V1a receptors in the monogamous prairie vole (Microtus ochrogaster) J Comp Neurol. 2004;468:555–70. doi: 10.1002/cne.10973. [DOI] [PubMed] [Google Scholar]
- 217.Lim MM, Young LJ. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience. 2004;125:35–45. doi: 10.1016/j.neuroscience.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 218.Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429:754–7. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 219.Sherrington CS. The integrative action of the nervous system. New York: C. Scribner’s sons; 1906. [Google Scholar]