Abstract
The genes encoding Rev1 and DNA polymerase ζ (Rev3/Rev7) are together required for the vast majority of DNA damage-induced mutations in eukaryotes from yeast to humans. Here, we provide insight into the critical role that the Saccharomyces cerevisiae Rev1 C-terminus plays in the process of mutagenic DNA damage tolerance. The Rev1 C-terminus was previously thought to be poorly conserved and therefore not likely to be important for mediating protein-protein interactions. However, through comprehensive alignments of the Rev1 C-terminus, we have identified novel and hitherto unrecognized conserved motifs that we show play an essential role in REV1-dependent survival and mutagenesis in S. cerevisiae, likely in its post-replicative gap filling mode. We further show that the minimal C-terminal fragment of Rev1 containing these highly conserved motifs is sufficient to interact with Rev7.
1. Introduction
In addition to the variety of processes which repair damage to the genome, cells also possess mechanisms to promote DNA replication past replication forks stalled by lesions in the DNA, termed DNA damage tolerance [1]. One of these pathways is translesion synthesis (TLS) which, in the yeast Saccharomyces cerevisiae, employs the specialized DNA polymerases Pol η (encoded by RAD30), Pol ζ (encoded by the REV3 catalytic subunit and the REV7 accessory subunit) and Rev1. These non-replicative DNA polymerases are able to catalyze nucleotide insertion opposite to missing or damaged bases that the highly stringent, replicative DNA polymerases are unable to use as templates for replication [1–3].
In order to perform lesion bypass, TLS polymerases sacrifice replication accuracy on undamaged DNA, exhibiting error rates of 10−5 to 10−1, compared with ~10−7 for replicative polymerases [4,5]. As a consequence, the cell employs a variety of different regulatory strategies to control the action of these potentially mutagenic polymerases. Remarkably, recent studies have shown that certain groups of TLS polymerases are specialized to bypass particular classes of DNA lesions, termed cognate lesions, with remarkable accuracy [5–8]. In contrast, translesion synthesis by Rev1 and Pol ζ is frequently mutagenic due to the particular catalytic properties of these enzymes [9]. Indeed, the REV genes were first identified by virtue of their reversionless, or non-mutable, phenotypes and are responsible for introducing the majority of mutations induced by a variety of DNA damaging agents in the genomes of eukaryotes, from yeast to humans [10–14].
The REV1 gene encodes a deoxycytidyl transferase that predominantly incorporates dCMPs across template Gs and abasic sites [15,16]. Intriguingly, this unique polymerase activity does not appear to be critically required for Rev1 function in TLS, as inactivation of the catalytic activity does not substantially decrease overall levels of mutagenesis [17,18], although the mutation spectrum is altered in strains bearing catalytically dead rev1 alleles [19,20]. Rather, Rev1-dependent mutagenesis requires the N-terminal BRCT domain (BRCA1 C-Terminus) [21], as well as the C-terminal Ubiquitin-Binding Motifs (UBMs) [22]. Mutations in these domains abrogate Rev1 function in survival and mutagenesis after DNA damage [10,18,21,23–25]. Since BRCT domains and UBMs typically function as protein-protein interaction modules [22,26,27], current models postulate that the primary function of Rev1 in lesion bypass may be structural rather than catalytic [17,28,29]. Rev1 is thought to recruit other polymerases, such as Pol ζ, to sites of damage to facilitate lesion bypass [30]. We have presented evidence suggesting that Rev1 functions primarily during G2/M after the bulk of replication has been completed, and have advanced the idea that Rev1 functions to recognize and/or mark aberrant DNA structures, such as gaps remaining opposite lesions. Through its protein-protein interactions, Rev1 may then recruit factors to promote post-replicative gap filling [31].
The Pol ζ catalytic subunit REV3 encodes a replicative B family DNA polymerase [32]; however, it requires an accessory subunit, Rev7, for robust polymerase activity [33,34]. Recent evidence suggests that Rev7 also participates in processes outside TLS, including the spindle checkpoint and regulation of gene expression [35–37]. Unlike other B-family DNA polymerases, Pol ζ is poorly processive and lacks a 3’–5’ proofreading activity [33]. Although Pol ζ can bypass certain DNA lesions, it appears to function primarily by catalyzing nucleotide insertion and extension at mismatched primer termini [9]. This catalytic activity therefore promotes the introduction of mutations in undamaged DNA [9].
While most DNA-damage-induced mutations in S. cerevisiae are attributed to the action of Rev1 and Pol ζ, a physical interaction between these two polymerases in yeast has only been demonstrated relatively recently [38–41]. In vertebrates, the interaction between Rev1 and Rev7 occurs in the C-terminal ~100 amino acids of Rev1 [18,28,29,42,43]. This C-terminal region in yeast and other lower organisms was previously thought not to be relevant for interaction with Rev7 due to poor conservation at the primary sequence level among various eukaryotes [29,42,44]. However, a recent study has shown that the interaction between the Rev1 C-terminus and Rev7 is retained in yeast, flies and the nematode C. elegans [45]. Additionally, previous studies have shown that truncations of the yeast C-terminus impair survival and mutagenesis after DNA damage [21,39,46,47] and eliminate Rev1-dependent stimulation of Pol ζ TLS activity [39,48]. Futhermore, our previous results have suggested that, like its mammalian Rev1 counterparts, the yeast Rev1 C-terminus plays a crucial role in coordinating interactions with proteins in the process of damage tolerance [40]. Therefore, we undertook studies to determine whether the vertebrate C-terminal interaction domain was conserved in lower eukaryotes and to characterize the role of the Rev1 C-terminus in S. cerevisiae.
We have performed a comprehensive analysis of a minimal C-terminal region of S. cerevisiae Rev1 by studying the effect of point mutations in conserved residues and overexpression of truncation constructs on REV1-dependent survival and mutagenesis after DNA damage. We have uncovered novel motifs that, when disrupted, lead to a complete loss of function of the REV1 gene in vivo. Several of these motifs are predicted to comprise the hydrophobic core of an α-helical bundle formed by the C-terminal ~100 amino acids. We show that overproduction of a region of the Rev1 C-terminus containing these motifs confers a dominant-negative effect on survival and mutagenesis after DNA damage. Mutation of the motifs partially relieves this dominant-negative activity. Importantly, the minimal C-terminal region of Rev1 containing the conserved motifs is sufficient for a physical interaction with Rev7 in vivo.
2. Materials and Methods
2.1 Yeast Strains
The S. cerevisiae strains used in this study (Table 1) are derivatives of W1588-4C and W1588-4A which are W303 strains corrected for RAD5 [49]. The rev1Δ strain was generated by moving the rev1::kanMX4 cassette from the deletion library into a W15488-4C strain containing a bar1::LEU2 disruption. The integrated REV1 and mutated rev1 alleles were tagged with a C-terminal –TEV-ProA-His7 epitope tag using pYM10 [50] as described previously [31].
TABLE 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| W1588-4C | MATa leu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1-1 RAD5 | [49] |
| W1588-4A | MATα leu2-3,112 ade2-1 can1-100 his3-11,15 ura3-1 trp1-1 RAD5 | [49] |
| rev1Δ bar1Δ | W1588-4C rev1::kanMX4, bar1::LEU2 | [31] |
| YLW20 | W1588-4A rev1::KANMX4 | [40] |
| YLW70 | W1588-4C bar1::LEU2 | [40] |
| YSD5 | YLW70 REV7-13MYC::HIS3MX6 | [40] |
| YLW155 | W1588-4C rev1::kanMX4::pRS306-rev1-105-TEV-ProA-His7, bar1::LEU2 | this study |
| YLW156 | W1588-4C rev1::kanMX4::pRS306-rev1-107-TEV-ProA-His7, bar1::LEU2 | this study |
| YLW157 | W1588-4C rev1::kanMX4::pRS306-rev1-108-TEV-ProA-His7, bar1::LEU2 | this study |
| YLW158 | W1588-4C rev1::kanMX4::pRS306-rev1-110-TEV-ProA-His7, bar1::LEU2 | this study |
| YLW159 | W1588-4C rev1::kanMX4::pRS306-rev1-111-TEV-ProA-His7, bar1::LEU2 | this study |
| YLW160 | W1588-4C rev1::kanMX4::pRS306-rev1-1-TEV-ProA-His7, bar1::LEU2 | this study |
| YLW161 | W1588-4C rev1::kanMX4::pRS306-rev1-AA-TEV-ProA-His7, bar1::LEU2 | this study |
| YLW162 | W1588-4C rev1::kanMX4::pRS306-REV1-TEV-ProA-His7, bar1::LEU2 | this study |
2.2 Plasmids
The pRS416-REV1 plasmid was generated using primers pRS-Rev1-fwd and pRS-Rev1-rev to amplify the REV1 gene by PCR from the genome. The PCR fragment was digested with BclI and KpnI and cloned into the pRS416 vector digested with BamHI and KpnI. This produced a 3.4 kb region containing 210 bp of the REV1 promoter and 217 bp of the 3′ UTR. It has previously been shown using deletion analysis that REV1 constructs containing 210 bp of the REV1 promoter or 27 bp of the 3′ UTR are able to complement a rev1-1 strain [21]. The pRS306-REV1 plasmid was created by cloning the 3.4 kb REV1 fragment released by digestion of pRS416-REV1 with SpeI and KpnI into the pRS306 backbone digested with SpeI and KpnI. The pRS306-REV1 plasmid and site-directed mutant derivatives were linearized at the SexAI site in the 210 bp REV1 promoter, and transformed into the rev1Δ bar1Δ strain. Integrated constructs were selected on SC−Ura media and verified by PCR using the primers pRS-Rev1-fwd and pRS-Rev1-rev. The –TEV-ProA-His7 epitope tag was fused to the C-terminus of the WT REV1 gene or various rev1 mutants in these strains using the plasmid pYM10 [50] as described previously [31].
For overexpression studies, the PCR fragments encoding the Rev1 amino acids from 155–268 (BRCT), 747–935 (CTΔ50), 747–885 (CTΔ100), 567–767 (PAD), 780–985 (ΔUBM1), 837–985 (ΔUBM1+2), 751–836 (UBM1+2), 886–985 (CT100), 936–985 (CT50) and 886–936 (CT886-936) were subcloned into the 2µM pAS311 plasmid containing the HA-HIS tags and driven by the GAL promoter [40].
2.3 DNA Damage Sensitivity and Mutagenesis Assays
For MMS sensitivity assays (Fig. 6, Fig. 7, Fig. S1 and Fig. S2), yeast strains (W1588-4A or rev1Δ) were grown for 2 days in Synthetic Complete (SC) media lacking tryptophan and supplemented with 2% raffinose (SC−Trp+Raf) for two days. The strains were then subcultured into SC−Trp supplemented with 2% galactose (SC−Trp+Gal) and grown at 30 °C to ~1×107 cells/ml. Ten-fold serial dilutions were plated on SC−Trp+Gal media plates and containing the indicated amounts of MMS. Colonies were counted after 4 days at 30°C.
Fig. 6.
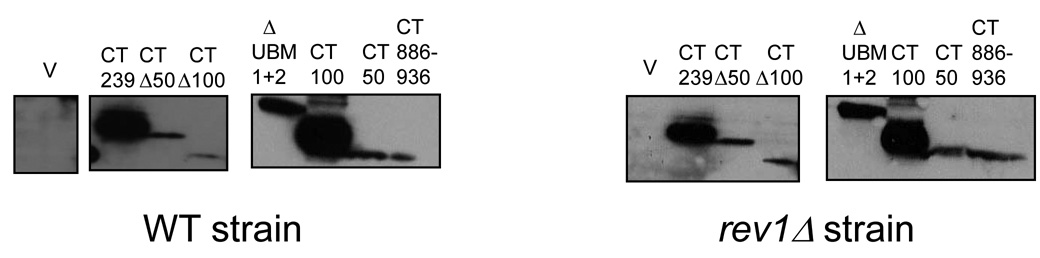
Delineation of the minimal region of Rev1 required to manifest the dominant-negative phenotype of decreased survival upon overproduction after DNA damage. (A) Survival of WT and rev1Δ strains transformed with the indicated Rev1 truncation constructs after growth on plates containing the indicated amounts of MMS. Error bars represent the standard deviation of the results derived from three independent colonies. (B) Immunoblot analysis from WT and rev1Δ strains expressing the indicated Rev1 fragment using an anti-HA antibody.
Fig. 7.
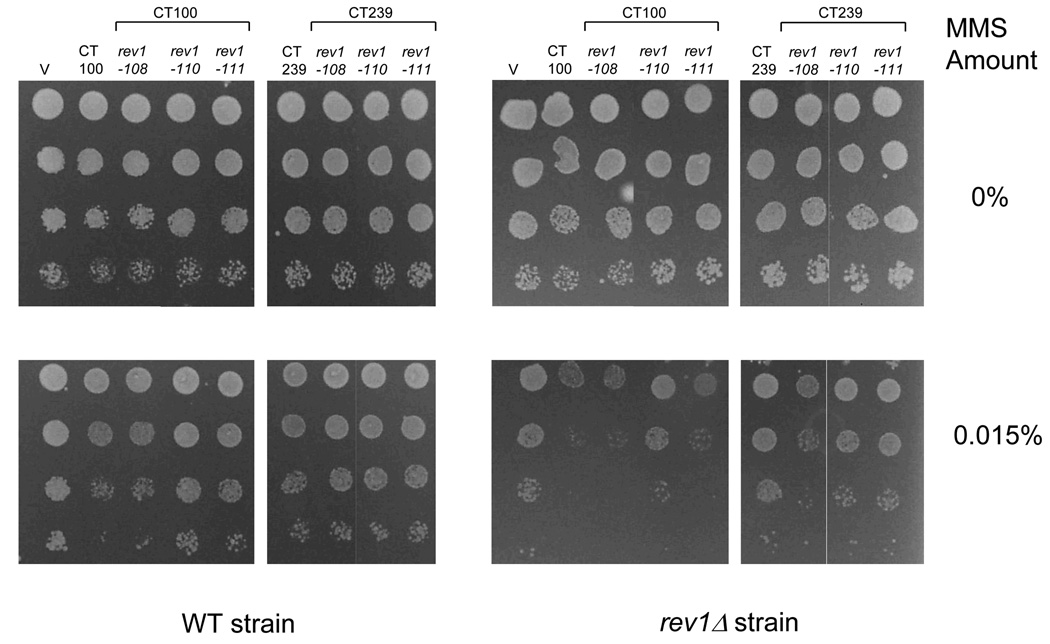
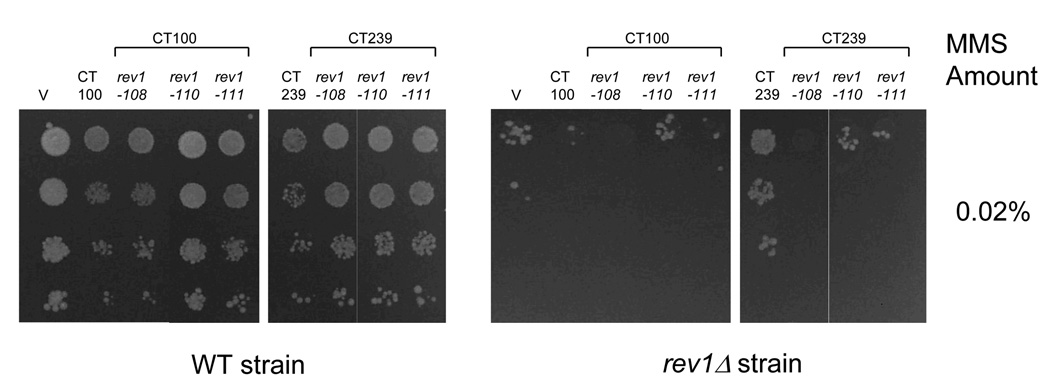
Effect of mutations in conserved C-terminal motifs on survival of strains after DNA damage. (A) WT and rev1Δ strains transformed with the empty vector (V) or WT and mutant derivatives of the CT100 and CT239 fragments were grown on plates containing the indicated amounts of MMS. (B) Immunoblot analysis from the WT and rev1Δ strains transformed with the Rev1 constructs as in (A).
For UV assays, three independent colonies of each strain were grown for two days to ~1×108 cell/ml at 30 °C in SC−Trp+Gal (Fig. 8) or SC−Ura (Fig. 2 and Fig. 3). Appropriate dilutions were plated on SC−Trp+Gal (Fig. 8) or SC (Fig. 2 and Fig. 3) to monitor survival. Mutation frequencies were analyzed by plating undiluted aliquots on SC−Ade+Gal (Fig. 8) or SC−Trp (Fig. 2 and Fig. 3) to score for reversion of the ade2-1 or trp1-1 allele respectively. Plates were irradiated at 1 J/m2/sec using a G15T8 UV lamp (General Electric) at 254 nm and grown for 3–4 or 6–7 days at 30 °C in the dark for survival and mutagenesis assays respectively. The reversion frequencies were calculated by subtracting the spontaneous value from the frequency obtained at the 10 J/m2 UV dose.
Fig. 8.
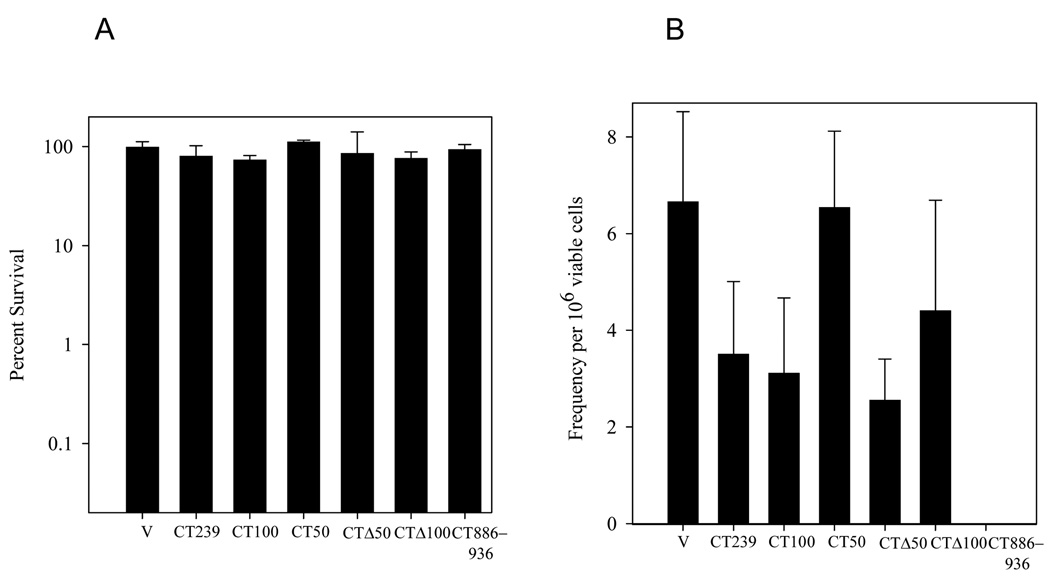
Effect of overproduction of Rev1 C-terminal deletion constructs on UV-survival and UV-induced mutagenesis. (A) Survival of WT yeast strains transformed with either the empty vector (V) or expressing the indicated Rev1 C-terminal constructs after irradiation with 10 J/m2 UV. Error bars represent the standard deviation of the results derived from three independent colonies. (B) Mutation frequency of ade2-1 for the same strains as in (A). Note that due to the growth defect of WT cells overproducing the CT886-936 fragment, no colonies were obtained on the SC−Ade plates. Error bars represent the standard deviation of the results derived from three independent colonies.
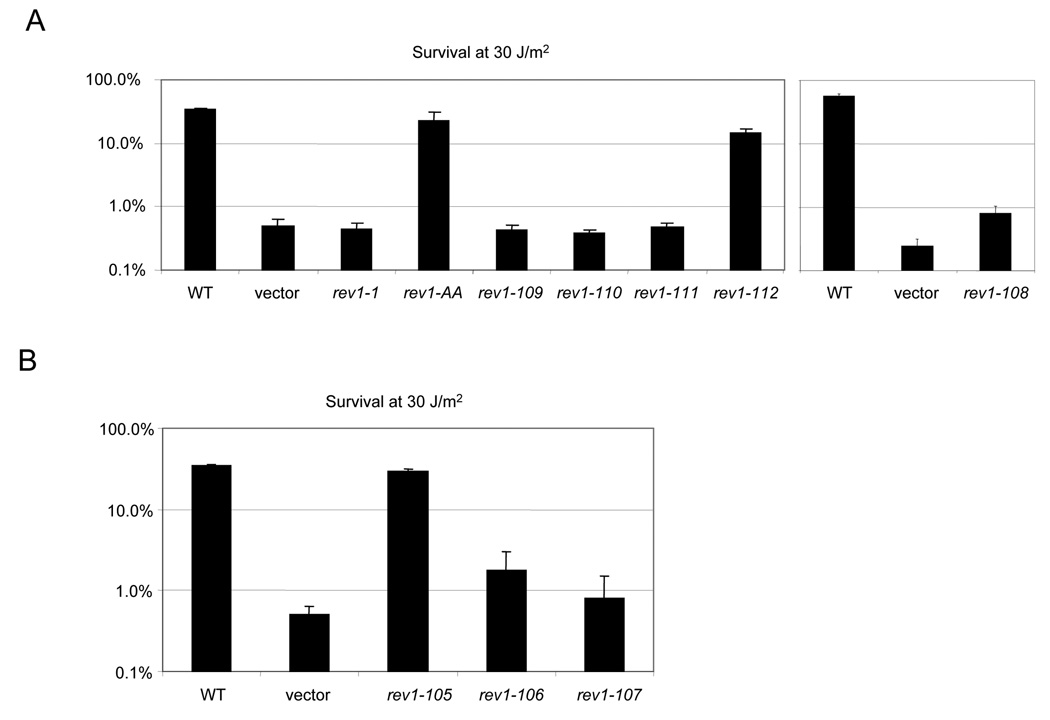
Fig. 2.
Mutations in predicted polymerase interaction motifs and UBM2 disrupt REV1-mediated survival. (A) Survival after a dose of 30 J/m2 UV irradiation of the rev1Δ strain bearing a low-copy plasmid expressing WT REV1 or the rev1-1, rev1-AA, or rev1 C-terminal mutants under the native REV1 promoter (B). Survival after a dose of 30 J/m2 UV irradiation of the rev1Δ strain transformed with plasmids expressing the WT or rev1 UBM mutants.
Fig. 3.
Chromosomal mutations disrupting C-terminal motifs and UBM2 lead to impaired REV1-mediated survival and mutagenesis. (A) Survival of the rev1-1, rev1-AA, and indicated C-terminal mutants after UV irradiation with 10 J/m2. (B) Reversion frequency for the rev1-1, rev1-AA, and indicated C-terminal mutants, monitored by reversion of the trp1-1 allele, after 10 J/m2 UV irradiation. Error bars represent the standard deviation of the results derived from three independent colonies. Note that as no Trp+ colonies were recovered for the rev1-108 strain, the mutation frequency was calculated to be at or below the limit of detection for this strain of 22.2 revertants per 107 survivors. (C) Survival of the indicated UBM mutants after UV irradiation with 10 J/m2. (D) Reversion frequency for the indicated UBM mutants after UV irradiation with 10 J/m2.
The cell-cycle UV survival assays in Fig. 4 were performed as previously described [31]. At least three independent cultures of each strain were arrested with 50 ng/ml α-factor or 15 µg/ml nocodazole for 3 h at 30 °C and washed with water or 1% DMSO in YEP media to remove α-factor or nocodazole, respectively. Microscopic analysis of cells confirmed arrest. Cells were diluted appropriately in water, plated on SC media, immediately UV irradiated at 1 J/m2/sec by using a G15T8 UV lamp (General Electric) at 254 nm, and incubated for 3 days at 30 °C in the dark.
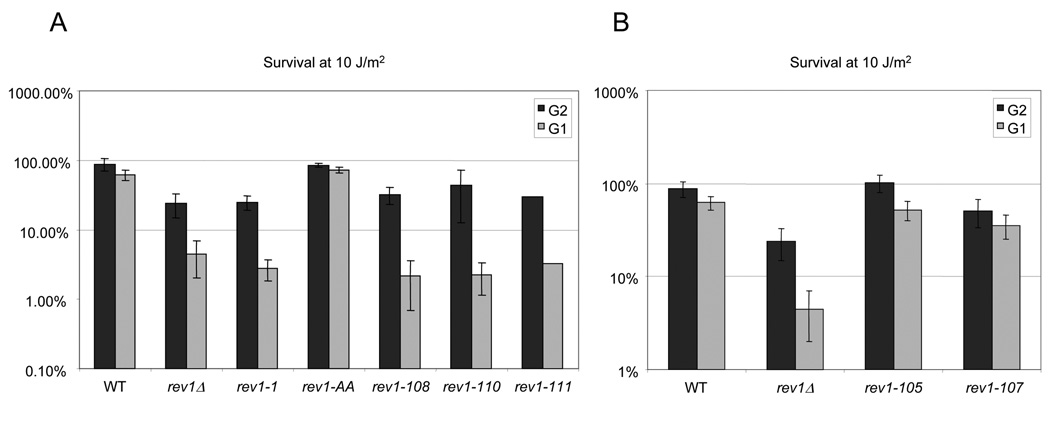
Fig. 4.
Differential survival of the chromosomal rev1 mutants after UV irradiation throughout the cell cycle. (A) Survival after a UV dose of 10 J/m2 of the rev1-1, rev1-AA, or indicated C-terminal rev1 mutant strains, after release from G1 or G2 arrest. The values for rev1-111 are the average of two replicates. (B) Survival after a UV dose of 10 J/m2 of rev1 strains mutated in the UBMs, showing no hypersensitivity to UV irradiation after release from G1 or G2 arrest. Error bars represent the standard deviation of the results derived from three independent colonies.
2.4 Cell Cycle Arrest
The cell cycle arrest experiments in Fig. 5 were performed as described previously [31]. Logarithmically growing yeast were arrested with 50 ng/ml α-factor for 4 h at 25 °C, washed to remove α-factor, and were resuspended in 25 °C media to initiate the timecourse.
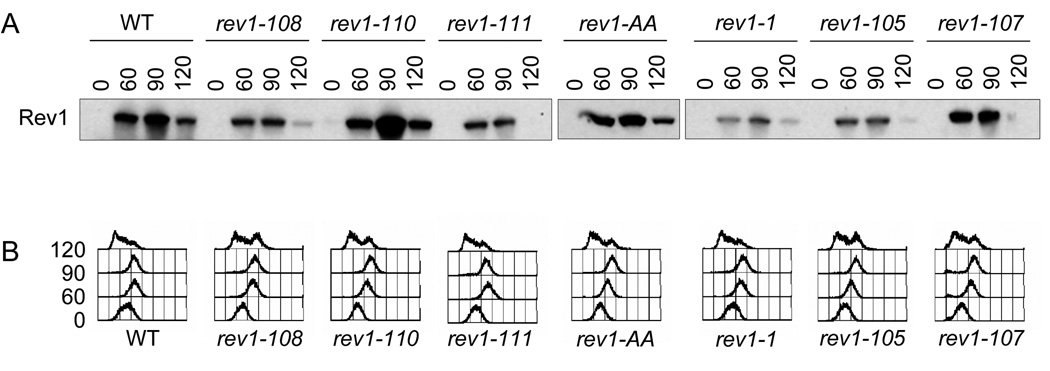
Fig. 5.
Rev1 protein levels expressed by the chromosomal rev1 mutants during the cell-cycle. (A) Immunoblot analysis using the protein A epitope tag to visualize the WT and indicated mutant Rev1 protein levels throughout the cell cycle. The membrane was stained with Ponceau-S prior to immunoblotting to confirm equal loading. (B) FACS profiles of time points after α-factor release showing progression through the cell cycle.
2.5 Immunoprecipitation and Immunoblot Assays
The W1588-4C strain transformed with the plasmids containing the Rev1 constructs described in Fig. 9 and Fig. 10 was initially grown for 2 days in SC−Trp+Raf at 30 °C. The cells were then subcultured in a total volume of ~100 ml and grown overnight at 30 °C in SC−Trp+Gal to ~1×107 cells/ml. The cells were centrifuged (4,000×g, 5 min at 4 °C) and washed once in water. All further steps were carried out at 4 °C. Lysis and immunoprecipitations were carried out as described [40]. For the experiment described in Fig. 10C and D, immunoprecipitations were carried out in the presence of 100 µg each of the peptides rev1-108 (NH2-ICQLVKQWVAETLGD-COOH) or rev1-110-111 (NH2-FVKVLIKLCDSNRVHLVL-COOH). However, only the peptide rev1-108 was soluble in the buffer used to dissolve the peptides.
Fig. 9.
Interaction between Rev1 C-terminal fragments and Rev7. (A, B) Co-immunoprecipitations of Rev7-13Myc and the indicated HA-tagged Rev1 C-terminal truncation constructs. Immunoprecipitated proteins were subjected to immunoblot analysis using an anti-HA antibody that detects Rev1 (A) or an anti-Myc antibody that detects Rev7 (B). (C) Co-immunoprecipitations from WT strain (untagged Rev7) overproducing the indicated HA-tagged Rev1 fragment. Immunoprecipitated proteins were immunoblotted using an anti-HA antibody.
Fig. 10.
Effect of mutations in conserved C-terminal motifs on the Rev1-Rev7 interaction. (A) Lysates from the Rev7-13Myc strain transformed with the empty vector (V) or expressing WT and the indicated mutant derivatives of CT100 were immunoprecipitated using an anti-Myc antibody to pull down Rev7 and immunoblotted with an anti-HA antibody to detect Rev1. A portion of the lysate was run as the input. (B) Lysates from the Rev7-13Myc strain transformed with the empty vector (V) or expressing WT and the indicated mutant derivatives of CT100 were immunoprecipitated using an anti-HA antibody to pull down the Rev1 C-terminal fragments and immunoblotted with an anti-Myc antibody to detect Rev7. A portion of the lysate was run as the input. (C) Immunoprecipitations performed as above with an anti-Myc antibody (C) or an anti-HA antibody (D) were carried out in the presence or absence of the Rev1 peptide described in the materials and methods. Immunoprecipitated proteins were immunoblotted using an anti-HA antibody (C) or an anti-Myc antibody (D).
For immunoblotting, whole cell extracts were prepared by TCA precipitation [50], separated on SDS-PAGE, and transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore) using a Mini-PROTEAN II transfer apparatus (Bio-Rad). Antibodies used were rabbit PAP antibody (Sigma) against the protein A tag, anti-HA.11 (Covance), anti-Myc (Upstate), and anti-phosphoglycerate kinase (Molecular Probes).
2.6 Site-Directed Mutagenesis
Site-directed mutations were generated according to the protocol of the Quikchange Mutagenesis kit (Stratagene), except using an annealing temperature of 50 °C and extension time of 2 min/kb. Mutations were verified by sequencing. Primers are listed in Table 2 and a summary of the mutations can be found in Table 3.
TABLE 2.
Primers used in this study
| Primer Name | Primer Sequence | Mutation |
|---|---|---|
| pRS-Rev1-fwd | GCTTTGAGTTGGGGTAGATTATCGC | none |
| pRS-Rev1-rev | GTGTTGGTACCAAAGGAGGAGTCGGCCATTCC | none |
| rev1-1-fwd2 | TACACGAGATGATAGTTTTACATGGCaGAAAATTTT TACACTATTTGTCTTC |
rev1-1 |
| rev1-1-rev2 | GAAGACAAATAGTGTAAAAATTTTCtGCCATGTAAA ACTATCATCTCGTGTA |
rev1-1 |
| Rev1-D467A | GATTTTACCTATATCTATTGcTGcAGCTGTTTGTGTG | rev1-AA |
| E468A | AGGATAATCCC | |
| Rev1-D467A | GGGATTATCCTCACACAAACAGCTgCAgCAATAGAT | rev1-AA |
| E468A-r | ATAGGTAAAATC | |
| SC1 | GTGACGAACAGAGCTTTCGAAGCCGCCGCGGCAGC TGTAAAAAATGACATTAACAACG |
rev1-105 |
| SC1-r | CGTTGTTAATGTCATTTTTTACAGCTGCCGCGGCGG CTTCGAAAGCTCTGTTCGTCAC |
rev1-105 |
| SC2 | CTATGGAAGAACAGTTTATGAATGCAGCCGCGGCC GCAATTCGAGCAGAAGTAAGGCACG |
rev1-106 |
| SC2-r | CGTGCCTTACTTCTGCTCGAATTGCGGCCGCGGCTG CATTCATAAACTGTTCTTCCATAG |
rev1-106 |
| SC3 | TATGAATGAACTACCAACCCAAGCTGCCGCGGCAG CAAGGCACGACTTGAGAATTCAG |
rev1-107 |
| SC3-r | CTGAATTCTCAAGTCGTGCCTTGCTGCCGCGGCAGC TTGGGTTGGTAGTTCATTCATA |
rev1-107 |
| SC4 | CGGTTCAAAAAAATTTGTCAAGCCGCGGCACAAGC GGCTGCCGAAACTTTAGGTGATGGA |
rev1-108 |
| SC4-r | TCCATCACCTAAAGTTTCGGCAGCCGCTTGTGCCGC GGCTTGACAAATTTTTTTGAACCG |
rev1-108 |
| SC5 | CGAAACTTTAGGTGATGGAGGGGCGCATGCAGCAG CTGCTAAATTATTCGTGAAATATTT |
rev1-109 |
| SC5-r | AAATATTTCACGAATAATTTAGCAGCTGCTGCATGC GCCCCTCCATCACCTAAAGTTTCG |
rev1-109 |
| SC6 | GCCGCATGAAAAAGATGTTAAATTATTCGCGGCCG CGGCGGCTAAACTTTGCGATTCTAATAGAGTCCAT |
rev1-110 |
| SC6-r | ATGGACTCTATTAGAATCGCAAAGTTTAGCCGCCGC GGCCGCGAATAATTTAACATCTTTTTCATGCGGC |
rev1-110 |
| SC7 | TATTTGATTAAACTTTGCGATTCTAATGCAGCGCAT GCAGCTGCTCATTTATCAAACCTAATATCAAGGG |
rev1-111 |
| SC7-r | CCCTTGATATTAGGTTTGATAAATGAGCAGCTGCAT GCGCTGCATTAGAATCGCAAAGTTTAATCAAATA |
rev1-111 |
| SC8 | CCACTTTTAAACAGAAATAAACATGCTGCCCAGGC CGCGGCTAAACTTGACATGGACTTTGAAG |
rev1-112 |
| SC8-r | CTTCAAAGTCCATGTCAAGTTTAGCCGCGGCCTGGG CAGCATGTTTATTTCTGTTTAAAAGTGG |
rev1-112 |
TABLE 3.
Site-directed mutants used in this study
| Allele | Amino acid changes | Location | Reference |
|---|---|---|---|
| rev1-1 | G193R | BRCT domain | [10,21] |
| rev1-AA | D467A E468A | pol domain | [17] |
| rev1-105 | L763A P764A E765A D766A | UBM1 | this study |
| rev1-106 | E820A L821A P822A T823A Q824A | UBM2 | this study |
| rev1-107 | I825A R826A E828A V829A | UBM2 | this study |
| rev1-108 | L889A V890A K891A W893A V894A | C-terminus | this study |
| rev1-109 | P903A E905A K906A D907A V908A | C-terminus | this study |
| rev1-110 | V912A K913A Y914A L915A I916A | C-terminus | this study |
| rev1-111 | R923A V924A L926A V927A L928A | C-terminus | this study |
| rev1-112 | T972A Y973A T975A V976A R977A | C-terminus | this study |
2.7 Computational Analysis
Manual alignments were generated using the Lasergene suite of sequence analysis programs. Helical wheels were adapted from http://cti.itc.virginia.edu/~cmg/Demo/wheel/wheelApp.html. Secondary structure predictions were made using the 3D-PSSM program at http://www.sbg.bio.ic.ac.uk/~3dpssm/.
3. RESULTS
3.1 Conservation of the sequence of the Rev1 C-terminus
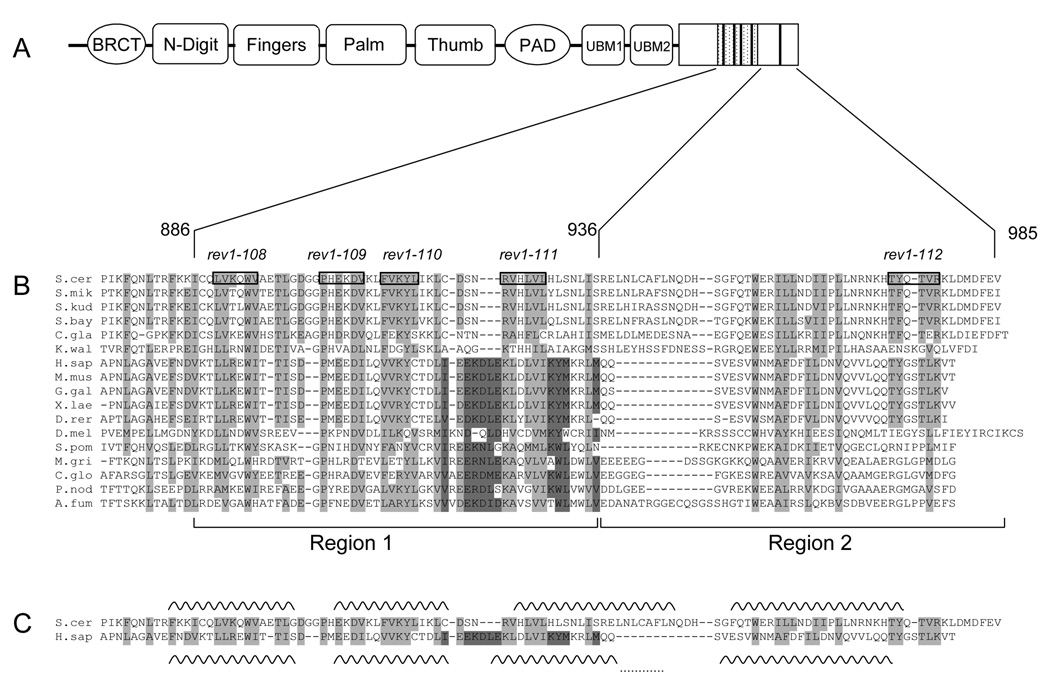
To investigate the conservation of the sequence and function of the C-terminus of Rev1, we began by determining whether the region characterized in vertebrates was present in other organisms. Using multiple sequence alignments of sequences from diverse eukaryotic phyla, we uncovered regions of homology in the last ~100 amino acids that are conserved from yeast to humans (Fig. 1A, B). This conservation indicates that the region of Rev1 which interacts with other TLS polymerases in vertebrate systems is present in lower eukaryotes as well, at least at the sequence level. Notably, however, this region appears to be completely lacking from Rev1 sequences from plants (A. thaliana, O. sativa), as well as nematodes (C. elegans, C. briggsae).
Fig. 1.
The C-terminal ~100 amino acids of Rev1 are conserved across a wide range of organisms. (A) Schematic of the domain structure of S. cerevisiae Rev1 with the minimal Rev7-interacting 50 amino acid Region 1 indicated by the hatched box and the conserved motifs indicated by dark bars. (B) Multiple sequence alignment of Rev1 sequences from selected species. Boxed residues and the text above indicate the alanine-patch mutations. (See Table 3 for more details). Amino acids highlighted in light grey show conservation across all species, while amino acids highlighted in dark grey indicate a conserved region not found in S. cerevisiae and closely related yeasts. Species abbreviation: S. cer, S. cerevisiae; S. mik, S. mikatae; S. kud, S. kudriavzevii; S. bay, S. bayanus; C. gla, C. glabrata; K. wal, K. waltii; H. sap, H. sapiens; M. mus, M. musculus; G. gal, Gallus gallus ; X. lae, X. laevis; D. rer, D. rerio; D. mel, D. melanogaster; S. pom, S. pombe; M. gri, M. grisea; C. glo, C. globosum; P. nod, P. nodorum; A. fum, A. fumigatus. Rev1 sequences from over 50 organisms were used to generate the alignment, but only 17 are shown due to space considerations. (C) Predicted secondary structure of the C-terminus of Rev1. Alignment of yeast and human Rev1 excerpted from (B) for clarity with the predicted helices for each sequence indicated by wavy lines. The dots connecting the last two helices of human Rev1 indicate that this helix is predicted to be continuous. (D) Helical wheel projections of the first predicted helix. Dark grey indicates hydrophobic residues, red indicates positively charged residues, blue indicates negatively charged residues and yellow indicates polar residues.
The first ~50 amino acids of the C-terminal ~100 amino acids (Region 1, see Fig. 1B) are more highly conserved than the last ~50 amino acids (Region 2). Region 1 is predicted to have a conserved secondary structure composed of two α-helices (Fig. 1C). Consistent with this observation, the conserved amino acids, which tend to be hydrophobic in nature, occur in clusters of one to three residues separated by one or two non-conserved charged or polar residues. This pattern of hydrophobic amino acids suggests that the predicted α-helical stretches, in particular the first helix, may form amphipathic helices (Fig. 1D). Like Region 1, Region 2 is predicted to be primarily α-helical, however the location of the putative helices is not as conserved throughout Rev1 sequences from various species (Fig. 1C).
We also observed other regions between the end of the polymerase domain (residue 746) and the last ~100 amino acids of the C-terminus (starting with residue 874) which display homology among Rev1 sequences from all species. These motifs are characterized by an invariant Leu-Pro sequence and, during the course of these studies, were reported to be Ubiquitin-Binding Motifs (UBMs) critical in mediating REV1 function [24,25,51].
3.2 Conserved motifs mediate REV1 function in vivo
To examine whether the conserved Regions 1 and 2 perform an important role in REV1 function in yeast as they do in vertebrates, we mutated patches of five amino acids to alanines and assayed the resulting rev1 mutants for their ability to function in DNA damage tolerance. We chose five particularly conserved motifs (108, 109, 110, 111, and 112) distributed throughout the C-terminal ~100 amino acids (Fig. 1A, B) and also mutated five amino acid patches in the UBMs (alleles rev1-105, -106, and -107) to generate a panel of eight novel mutations (Table 3). We also included the well-characterized rev1-1 BRCT mutation [10] and the rev1-AA catalytic dead allele [17,18,47]. To gain an initial understanding of the function of the mutated residues, we complemented a rev1Δ strain with low-copy plasmids bearing the wild-type (WT) or mutant alleles under the native REV1 promoter (Fig. 2).
Strikingly, we observe that the mutations of the motifs in Region 1 completely inactivate REV1 function in survival after UV irradiation (Fig. 2A), as well as on methyl methanesulfonate (MMS) plates (data not shown). In contrast, the rev1-112 strain, mutated in the less well conserved Region 2 (Fig. 1B), displays only a moderate reduction in survival (Fig. 2A). This suggests that Region 1 may predominantly mediate the function of the Rev1 C-terminus, which is likely to be promoting protein-protein interactions.
As expected, the rev1-1 strain displays a significant decrease in survival after DNA damage (Fig. 2A). The rev1-AA catalytic dead mutant exhibits essentially WT survival (Fig. 2A), confirming that the dCMP transferase activity of Rev1 is not required for its function following UV irradiation.
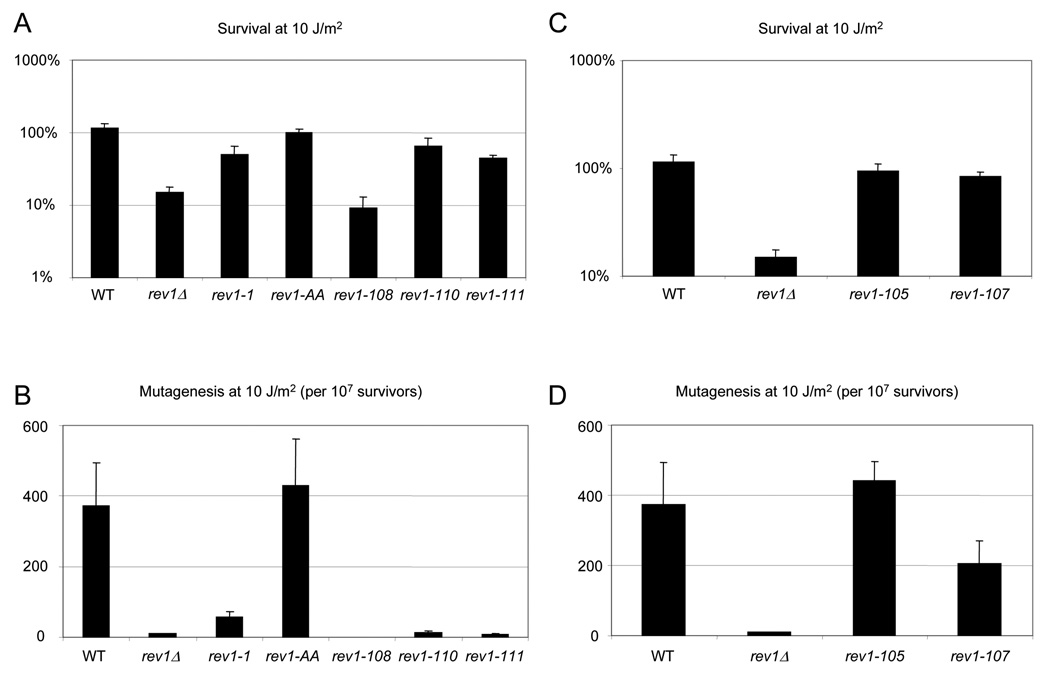
We then introduced the WT REV1 gene and a subset of the mutant rev1 alleles back into the rev1Δ strain in single-copy on the chromosome under the native REV1 promoter (Fig. 3). In the more physiologically relevant chromosomal context, we again noted that the C-terminal motifs are required for REV1-mediated survival and mutagenesis after UV irradiation. After irradiation with low doses of UV (10 J/m2) such that the WT strain exhibits no decrease in survival, the rev1-110 and rev1-111 strains show a moderate defect in survival (Fig. 3A) and a total loss of REV1-dependent mutagenesis, similar to the rev1-1 and rev1Δ strains (Fig. 3B). The rev1-108 strain displays a profound defect in survival and mutagenesis even at this low dose of UV (10 J/m2) (Fig. 3A, B). In fact, we recovered no revertant colonies for the rev1-108 strain, indicating that the mutation frequency for this strain is less than the limit of detection of 22.2 mutations per 107 survivors. In contrast, the rev1-AA catalytic dead mutant strain displayed WT survival and mutagenesis after UV irradiation (Fig. 3A, B).
These results show that the novel, conserved motifs we identified are essential for REV1-mediated survival following UV irradiation and exposure to MMS, as well as UV-induced mutagenesis. The homology of this region of yeast Rev1 with the polymerase-interaction region of vertebrate Rev1, suggests that these motifs may promote the interaction with the error-prone Pol ζ.
We also monitored survival and mutagenesis after DNA damage of strains bearing mutations in the UBMs. Consistent with published data [24,25,51], we observe that mutation of UBM1 (rev1-105) has no effect on REV1-mediated survival or mutagenesis after UV irradiation (Fig. 2B, Fig. 3C, and 3D) or MMS treatment (data not shown). However, mutations of UBM2 (rev1-106 and rev1-107) perturb REV1 function. The plasmid-borne UBM2 mutants rev1-106 and rev1-107 show a substantial sensitivity to a dose of 30 J/m2 UV and MMS treatment (Fig. 2B, data not shown), although not as severe as the sensitivity conferred by mutation of the motifs in Region 1 of the Rev1 C-terminus or by the rev1Δ strain (Fig. 2A, data not shown). After irradiation with a low dose of 10 J/m2 UV, the chromosomal UBM2 mutant rev1-107 shows no substantial decrease in survival, but exhibits a reduced frequency of trp1-1 reversion corresponding to ~50% of the WT value (Fig. 3C, D), again displaying a lesser defect than that of the mutations in Region 1 (Fig. 3A, B). Therefore, our results indicate that the UBM2, although essential for full REV1 activity, may perform a less important function than the extreme C-terminal ~100 amino acids.
3.3 Cell-cycle expression and function of motif mutants
Having established that the short peptide motifs in the conserved Region 1 of the Rev1 C-terminus, as well as UBM2, are required for REV1-mediated survival and mutagenesis, we examined whether these motifs contribute to REV1 function differentially through the cell cycle. We sought to determine whether the motifs provide a primarily post-replicative function or whether they perform a role that is required throughout the cell cycle. As we have shown previously, the rev1Δ strain, as well as the rev3Δ and rev7Δ strains, exhibits enhanced UV killing when irradiated after release from the G1 phase of the cell cycle relative to cells irradiated after release from G2 [31]. We expected that if the motifs mediate protein-protein interactions required for the post-replicative gap-filling function of Rev1, mutations in these motifs would show a specific defect in survival following UV irradiation after release from G1, rather than a generalized sensitivity at all phases of the cell cycle. Indeed, we observe that the C-terminal mutations show a hypersensitivity to UV irradiation after release from G1 phase indistinguishable from that of the rev1Δ strain (Fig. 4A). The rev1-1 BRCT mutant strain also displays a similar hypersensitivity to UV irradiation after release from G1 (Fig. 4A). In contrast, we do not observe a decrease in survival after UV irradiation of the rev1-AA catalytically inactive mutant strain (Fig. 4A) or the UBM1 (rev1-105) mutant strain (Fig. 4B) at any stage of the cell cycle. Interestingly and unexpectedly, however, the UBM2 mutant strain (rev1-107) shows a slight increase in UV killing after release from both G1 and G2 arrests (Fig. 4B).
We confirmed that the mutant Rev1 proteins are expressed at approximately WT levels (Fig 5A), indicating that mutations in the UBMs or C-terminal ~100 amino acids do not drastically destabilize the protein or cause aberrant accumulation due to decreased proteolysis. Importantly, all of the mutant Rev1 proteins also show essentially normal regulation during the cell cycle, with very low levels during G1 and maximal expression after replication (Fig. 5A, B). Ponceau-S staining of the membrane confirmed equal protein loading. Therefore, the function disrupted in the C-terminal mutants is most likely not due to misregulation of protein levels, but rather presumably to a lack of protein-protein interactions.
3.4 Conserved peptide motifs within the Rev1 C-terminus are required for the dominant-negative effect on survival after DNA damage
Our results above indicate a role for the C-terminal motifs present in Region 1 in mediating Rev1 protein-protein interactions in vivo. Previously, we have suggested that the dominant-negative phenotype for DNA damage-induced survival and mutagenesis, which is observed upon ectopic overproduction of the Rev1 C-terminus, may result from titrating interacting proteins away from the full-length Rev1 protein or other protein complexes important in DNA damage tolerance [40]. We reasoned that if the conserved function of the Rev1 C-terminus is to mediate critical protein-protein interactions and if the C-terminal motifs are required for this function, overexpression of a C-terminal construct lacking Region 1 would no longer confer the dominant-negative effect on the survival of strains exposed to MMS. Therefore, we constructed a series of Rev1 C-terminal fragments depicted in the summary in Fig. 11, overexpressed these Rev1 fragments in both WT and rev1Δ strains, and assayed the survival of the cells after MMS treatment.
Fig. 11.
Schematic representation of the various Rev1 C-terminal deletion fragments. The 985 amino acid Rev1 protein consists of the N-terminal BRCT region, the polymerase domain (amino acids 297–746) comprising the N-digit that interacts with the incoming dCTP and the fingers, palm, thumb and PAD domains conserved among Y-family polymerases. The Rev1 C-terminal region is composed of two copies of the Ubiquitin-Binding Motif (UBM1 and UBM2). The hatched region in the figure represents Region 1 of the Rev1 C-terminus and the bars within this region, the short peptide motifs. On the right is a summary of the regions of Rev1 that confer the dominant–negative phenotype on survival after DNA damage and that interact with Rev7. ND: Not determined.
As shown in Fig. 6A, overexpression of the Rev1 fragments CT50 and CTΔ100, both lacking Region 1, do not confer sensitivity of the WT strains to MMS relative to cells transformed with the empty vector. In contrast, overproduction of the Rev1 C-terminal fragments CT239, ΔUBM1+2, CT100 and CTΔ50, all of which possess the conserved peptide motifs in Region 1, causes a marked sensitivity of WT cells to MMS exposure (Fig. 6A). Immunoblot analysis of lysates derived from strains overproducing each of these Rev1 fragments using an anti-HA antibody confirms their expression in WT and rev1Δ strains (Fig. 6B). This data suggests that Region 1, which contains the conserved motifs, is required for the dominant-negative phenotype, indicating that this region mediates protein-protein interactions.
Almost all of the observed sensitization to MMS of the WT strain by these plasmids appears to result from a dominant-negative effect, since only a moderate sensitization is observed when they were introduced into the rev1Δ strain (Fig. 6A and Fig. S1A). We note that CTΔ50 is overproduced at a very modest level, yet this fragment confers a substantial dominant-negative effect (Fig. 6). Moreover, the Rev1 BRCT region, though overproduced to a greater extent than the CT239 fragment, does not exhibit this phenotype, while the CT239 fragment does (Fig. S2). Therefore, the dominant-negative effect on survival after MMS treatment is not a consequence of vast overproduction of each C-terminal Rev1 fragment, as the level of overexpression does not correlate with the severity of the dominant-negative phenotype.
Interestingly, overproduction of a fragment containing Region 1 alone (CT886-936) does not confer the dominant-negative effect on survival after MMS treatment (Fig. 6A). Rather, this Rev1 fragment displays a strong growth defect in both WT and rev1Δ strains in the absence of exogenous DNA damage (data not shown). We conclude that overproduction of Region 1 of the Rev1 C-terminus consisting solely of the short peptide motifs is necessary, but not sufficient, for the dominant-negative effect on survival after DNA damage. Some additional portion of Rev1 either N-terminal (CT239 or CTΔ50) or C-terminal (CT100) to Region 1 needs to be present to confer the dominant-negative effect.
We next asked if alanine-patch mutations of the conserved motifs in Region 1 of the CT100 fragment would alleviate the dominant-negative effect after DNA damage. As shown in Fig. 7A, mutation of motif 110 in the CT100 construct significantly abrogates the dominant-negative effect on survival after MMS treatment in the WT strain, while mutation of motif 111 moderately reduces the sensitivity to MMS. In the CT239 constructs, the effects of these mutations are more subtle, however all three motif mutants modestly alter the dominant-negative effect on survival after MMS treatment (Fig. 7A). We note that mutation of motif 108 in both the CT100 and the CT239 fragments shows a dominant-negative effect that is more pronounced in the rev1Δ strain than in the WT strain, indicating that this effect is independent of the WT REV1 allele. Immunoblot analysis confirms that the CT100 and CT239 constructs containing the alanine-patch mutations are expressed to an extent similar to their WT counterparts in both the WT and the rev1Δ strains (Fig. 7B). These results show that, while the conserved peptide motifs in the Rev1 C-terminus are required for native Rev1 function, mutation of each motif individually only partially abrogates the dominant-negative phenotype upon overproduction after DNA damage.
3.5 Effect of overproduction of the Rev1 C-terminus containing conserved peptide motifs on mutagenesis after DNA damage
To determine if the Rev1 fragments lacking Region 1, which contains the short peptide motifs, are also defective for the dominant-negative phenotype on mutagenesis, we exposed WT strains overproducing these truncation constructs to a low, 10 J/m2 UV dose and assayed reversion of the ade2-101 allele. The survival of WT cells overproducing each of the Rev1 truncation constructs is not significantly altered at this low UV dose (Fig. 8A). However, overexpression of the fragments containing Region 1 (CT239, CT100 and CTΔ50) confers a decrease in UV-induced mutagenesis (Fig. 8B). In contrast, overproduction of fragments lacking Region 1 (CT50 and CTΔ100) does not appreciably alter the reversion frequency of WT cells after DNA damage (Fig. 8B). Therefore, Region 1 is important for the dominant-negative phenotype affecting mutagenesis as well as survival.
Overproduction of Region 1 alone (CT886-936) does not alter the survival of WT cells after UV irradiation (Fig. 8A). However, the severe growth defect exhibited by overproduction of the Region 1 fragment (CT886-936) in the absence of exogenous DNA damage precludes the detection of revertants in this strain (Fig. 8B). Thus, we have shown that Region 1 is necessary, but not sufficient, to confer a defect in survival and mutagenesis upon overproduction of the Rev1 C-terminus after DNA damage, which we suggest results from improper protein-protein interactions with Rev7 and other proteins [40].
3.6 Delineation of the minimal region of the Rev1 C-terminus required for interaction with Rev7
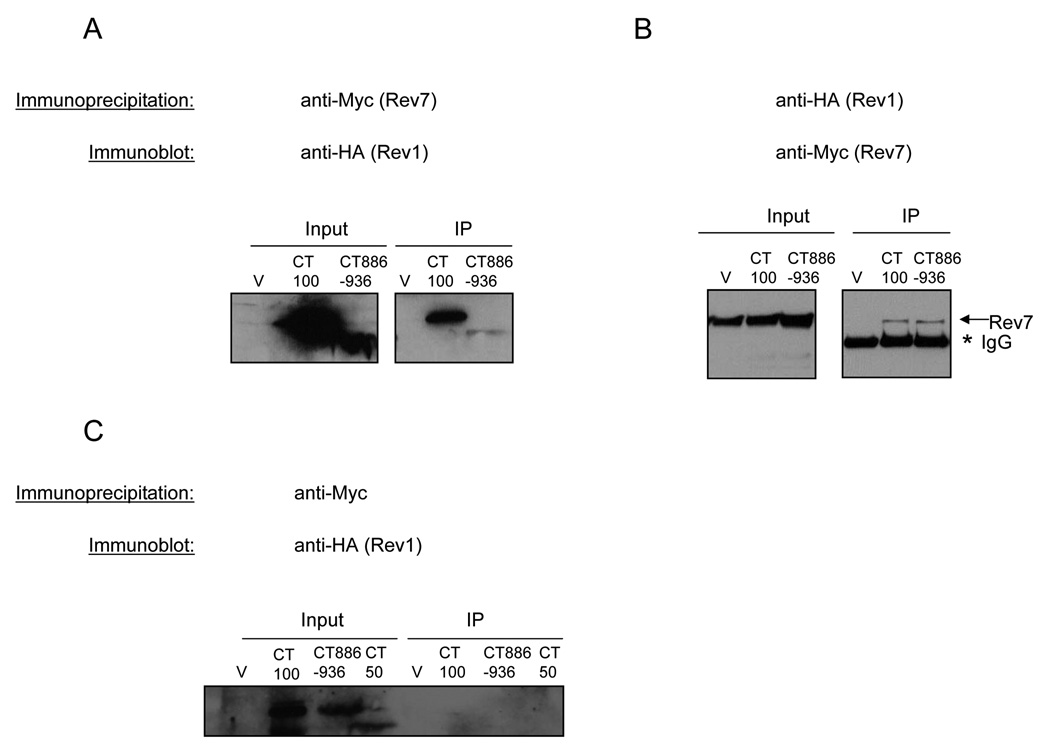
In an effort to investigate whether the short peptide motifs in Region 1 might be involved in an interaction with the Rev7 accessory subunit of Pol ζ, we transformed the HA-tagged Rev1 C-terminal fragments into a strain containing Rev7-13Myc at the endogenous locus and performed co-immunoprecipitations from these strains. Rev7 pulls down the Rev1 C-terminal fragment CT100, which possesses Regions 1 and 2, (Fig. 9A) and vice versa (Fig. 9B), whereas Rev7 does not immunoprecipitate CT50, which lacks Region 1 (data not shown). To determine if Region 1 by itself, which contains the most highly conserved and required peptide motifs, is sufficient to pull down Rev7, we performed co-immunoprecipitations from Rev7-13Myc strains expressing the C-terminal fragment CT886-936. Rev7 pulls down the CT886-936 fragment containing the conserved peptide motifs alone in Region 1 (Fig. 9A) and vice versa (Fig. 9B), in support of our previous results implicating these conserved amino acids in an interaction with Rev7. Although the CT100 fragment is expressed to a greater extent than the CT886-936 region (Fig. 6B), equivalent amounts of Rev7 are immunoprecipitated with each Rev1 fragment (Fig. 9A and B). We surmise that the differential sensitivities of the anti-HA and anti-Myc antibodies contribute to this effect. The interaction between the proteins is not due to non-specific binding of the HA-tagged Rev1 to the Protein-G-sepharose matrix as Rev1 is not pulled down in immunoprecipitations carried out from cells with untagged Rev7 (Fig. 9C). Our results show that Region 1 containing the conserved peptide motifs in the Rev1 C-terminus can interact with Rev7.
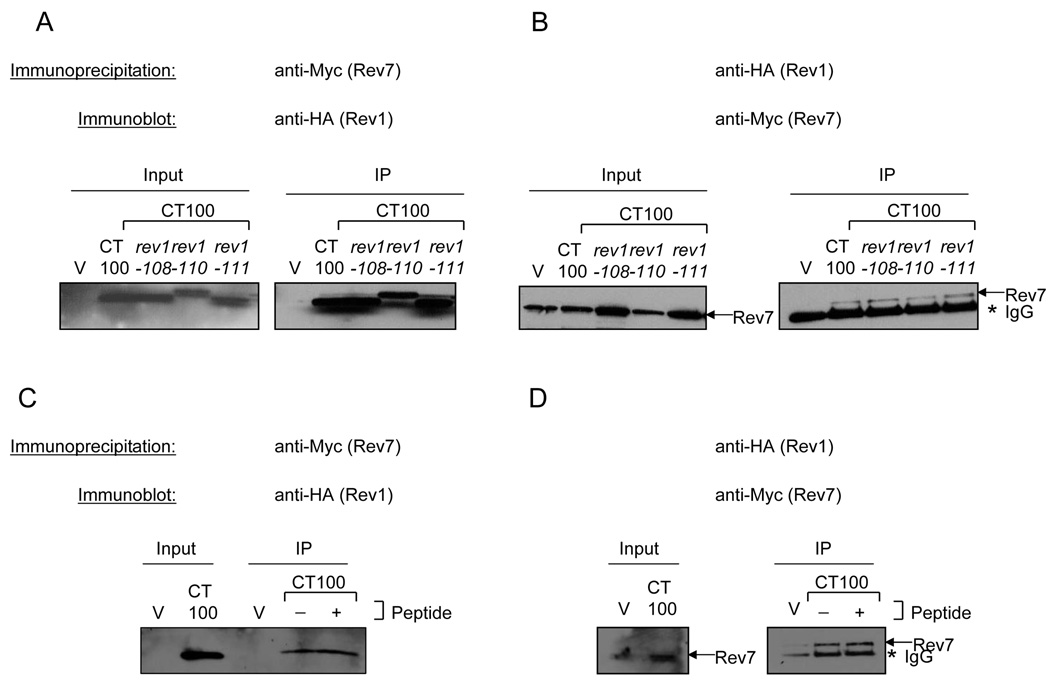
Due to the poor solubility and expression of the CT886-936 fragment that contains Region 1, we chose to examine if disruption of the conserved motifs within the well expressed CT100 fragment affected the interaction with Rev7. Therefore, to gain insight into the roles of the Rev1 motifs in mediating REV1 function (Fig. 2 and Fig. 3), we created alanine-patch mutations in a subset of these motifs (rev1-108, rev1-110, and rev1-111) in the CT100 fragment and performed co-immunoprecipitations as above. Rev7 pulls down the WT CT100 fragment and the mutant derivatives (Fig. 10A) and vice versa (Fig. 10B), suggesting that mutations in each of these motifs individually are insufficient to abrogate the interaction with Rev7 in vivo. We note that the rev1-110 mutant of the CT100 protein migrates slightly slower than the other Rev1 CT100 fragments. The reason for this is unclear; we have sequenced this plasmid and confirmed that it expresses the correct protein. Similarly, mutations in the conserved motifs within the larger CT239 fragment also do not disrupt the interaction with Rev7 (data not shown). The simplest interpretation of our results is that there is an extended interface between Region 1 of Rev1 and Rev7 such that the various motif mutations disrupt the functionality of the interface without completely abrogating the physical interaction between the two proteins.
To further probe the nature of the interaction between Rev7 and the Rev1 C-terminus we synthesized 2 peptides: one spanning motif 108 and the other spanning motifs 110–111 of Region 1 of the Rev1 C-terminus. The peptide spanning motif 108 was soluble but the peptide spanning motifs 110–111 was poorly soluble in the buffer used to dissolve the peptides. Adding an excess amount of the peptide spanning motif 108 does not interfere with the immunoprecipitation reaction between Rev7 and either CT100 (Fig. 10C and D) or CT886-936 (data not shown). Although it is formally possible that motif 108 has a role besides interacting with Rev7, this result is consistent with there being an extended interaction interface between Rev1 and Rev7. These results nevertheless demonstrate that Region 1 containing the peptide motifs is both necessary and sufficient for the interaction with Rev7.
4. Discussion
The region C-terminal to the polymerase domain of Rev1 plays a critical role in REV1-dependent DNA damage tolerance, by mediating interactions with ubiquitinated proteins as well as other TLS polymerases, and by enabling the monoubiquitination of Rev1 itself [24,25,51,52]. Here we present a detailed structure-function study of the Rev1 C-terminus and show that it is conserved, both at the sequence and functional levels, from yeast to mammals. However, the structural conservation of Rev1 reflected in the amino acid sequence of multiple lower eukaryotes is not as extensive as that between vertebrates as a group [45]. Secondary structure predictions indicate that the C-terminus is highly α-helical. We note that the helices appear predominantly amphipathic, allowing potential folding of the C-terminus into an α-helical bundle with the hydrophobic regions in the core. It is these hydrophobic residues that are most conserved in the Rev1 sequences, while the charged and polar residues, which are likely to face outwards into solution, show unique patterns of charge distribution among different Rev1 sequences (Fig. 1D). This may allow species-specific binding interfaces to interact with different effector proteins in various organisms. This also may explain why a Rev1-binding consensus sequence has been difficult to observe, even though defined regions of several TLS polymerases have been shown to interact with the Rev1 C-terminus. Additionally, we observe a particularly conserved region (highlighted in dark gray in Fig. 1B) which is present only in organisms that possess either DNA polymerase κ or ι. We hypothesize that this region may mediate an interaction with these polymerases and has diverged in organisms, like S. cerevisiae, which do not possess Pol κ or ι. Moreover, the last 50 amino acids of the Rev1 C-terminus (Region 2) are relatively poorly conserved from yeast to mammals and may serve as a unique interaction region for other species-specific interactions.
The C-terminal ~100 amino acids of Rev1 interacts with DNA polymerase η in vertebrate cells [28,29,43]. Yet, despite the fact that this region of Rev1 is conserved at the sequence level (Fig. 1B), an interaction with Pol η through the Rev1 C-terminus cannot be detected in yeast [45,46]. Rather, using purified proteins, it has been shown that the yeast Rev1 PAD can pull down Pol η [46]. Perhaps in other organisms, Pol η interacts primarily with the poorly conserved Region 2 of the Rev1 C-terminus. Or perhaps a species-specific charged interface on the predicted α-helical bundle is not optimized for binding between yeast Rev1 and yeast Pol η.
It is interesting to note that the C-terminal ~100 amino acids, which comprise the polymerase-interaction region, appear to be very poorly conserved, or even completely lacking, in plants and worms as has been previously reported [18,45]. Moreover, plants possess only a single UBM and worms none at all. This may reflect a different role for Rev1 in these species or perhaps the separation of function of the polymerase and recruitment domains into two separate polypeptides.
Consistent with other reports, we have demonstrated that mutations in UBM2, but not UBM1, have deleterious effects on Rev1 function in vivo (Fig. 2 and Fig. 3) [24,51]. Additionally, it has been shown that UBM2, but not UBM1, is required for an interaction with ubiquitinated PCNA and hyperstimulation of Rev1 catalytic activity in vitro. However, this study found that deletion of 120 amino acids from the C-terminus of Rev1 does not affect stimulation of Rev1 catalytic activity or hyperstimulation by ubiquitinated PCNA in vitro [51].
Although we observed subtle differences in the cell cycle expression patterns of the mutants, they do not correlate with the striking phenotypes of the mutants nor, given the slight variability in the timing of release from α-factor observed even with replicates of the same strain, could we confidently conclude that they represented a real distinction between strains. Rather, the essentially normal expression of the mutant Rev1 proteins during the cell cycle indicates that neither the UBM repeats nor the C-terminus is essential for an interaction with protein partners that may promote cell-cycle-dependent accumulation or degradation of Rev1. Combined with the fact that we observe no significant change in the cell-cycle regulation of Rev1 in rad6Δ, rad18Δ, mms2Δ, or ubc13Δ backgrounds (data not shown), the normal expression of the UBM mutants indicates that the factors which mono- or poly-ubiquitinate PCNA to regulate the DNA damage tolerance response are not involved in controlling Rev1 protein expression. Rather, we expect that this might be due to a combinatorial series of regulation steps at the transcriptional, translational, and post-translational levels [31]. Since Rev1 protein levels were similar among the various mutant strains, we hypothesize that the mutations in the C-terminal motifs and UBMs disrupt interactions with factors important for Rev1 function. These could be proteins that Rev1 recruits to gaps opposite to lesions (eg. Pol ζ) or which are responsible for maintaining Rev1 at sites of DNA damage (eg. ubiquitinated PCNA).
We have shown previously that rev1Δ, rev3Δ, and rev7Δ strains display increased sensitivity to UV irradiation after release from the G1 phase of the cell cycle relative to irradiation after release from G2 arrest [31]. We have proposed that this cell-cycle dependence in DNA-damage-induced cell death reflects a function of Rev1 and DNA polymerase ζ in the post-replicative resumption of DNA synthesis at sites of ssDNA gaps opposite to DNA lesions. Since we predict that the C-terminal motifs are important for an interaction with Pol ζ, we expected to see that the strains bearing mutations in the C-terminal ~100 amino acids would likewise show a hypersensitivity to UV irradiation after release from G1 arrest. Indeed, we observe that strains carrying C-terminal mutations exhibit a hypersensitivity to UV irradiation after release from G1 phase indistinguishable from the rev1Δ strain (Fig. 4). This is consistent with a role for the motifs in Rev1-mediated recruitment of TLS polymerases to sites of remaining DNA damage after replication.
Interestingly, the rev1-1 BRCT mutant strain also shows a hypersensitivity to UV irradiation after release from G1, similar to the rev1Δ and the other rev1 C-terminal mutants, indicating that the BRCT interaction with PCNA [24], Rev7 [40], or possibly aberrant DNA structures [53] is most relevant to REV1 function after replication has generated gaps opposite to DNA lesions. In contrast, the UV sensitivity of the rev1-AA catalytically inactive mutant strain is indistinguishable from the WT strain throughout the cell cycle, suggesting that the polymerase activity of Rev1 is not required for survival after UV damage at any point in the cell cycle. The UBM2 mutant strain shows a slight sensitization to UV killing after release from both G1 and G2 arrests. This lack of differential sensitivity throughout the cell cycle may indicate that interaction with ubiquitinated proteins, in particular monoubiquitinated PCNA, is important for Rev1 function at all phases of the cell cycle. Alternatively, the lack of UV sensitivity observed for the UBM2 mutant in Fig. 3 and Fig. 4 may be due to the very low dose of UV used (10 J/m2) which may have been insufficient to elicit significant monoubiquitination of PCNA.
We overproduced various deletions of the Rev1 C-terminus and determined that Region 1, containing the most highly conserved peptide motifs, is required to manifest the dominant-negative effect on survival and mutagenesis after DNA damage (Fig. 6, Fig. 8, Fig. 11, and Fig. S1). Mutation of the conserved amino acids within the motifs 110 and 111, which are critical for native Rev1 function (Fig. 2 and Fig. 3), partially relieves the dominant-negative effect on survival after MMS treatment (Fig. 7A) and, further, a complete deletion of Region 1 eliminates this phenotype (Fig. 6A and Fig. 11).
Although individual mutations in the conserved motifs in Region 1 of the Rev1 C-terminus do not abrogate the interaction between Rev1 and Rev7 (Fig. 10A and B), a complete deletion of Region 1 eliminates the Rev7 interaction (Fig. 9 and Fig. 11). The simplest interpretation of these results is that Region 1 of the Rev1 C-terminus, which is sufficient for the Rev7 interaction, forms an extended interface with Rev7 that is important for function. Mutation of the individual peptide motifs would interfere with the functionality of the interface but not eliminate the interaction between the two proteins. Our finding that a peptide spanning the conserved motif 108 was unable to compete away the binding of Rev1 to Rev7 is consistent with this interpretation. Further, we observe that the alanine-patch mutations of the motifs in the full-length Rev1 protein decrease survival and mutagenesis after DNA damage to an extent equivalent to the rev1Δ strain (Fig. 2 and Fig. 3). Taken together, these results suggest that the conserved motifs (108–111) together mediate an interaction with Rev7 such that mutation of each motif individually does not prevent Rev1’s ability to interact physically with Rev7, even though it profoundly affects whether the resulting Rev1-Rev7 interaction is functional.
Additionally, we have proposed that the dominant-negative phenotype likely results from a titration of other proteins, in addition to Rev7, away from the full-length Rev1 and/or other protein complexes, for example Rev3 [39]. Therefore, the partial abrogation of this phenotype that we observe in individual motif mutants may derive from a disruption of interactions with these other proteins. The identity of the proteins recruited by the Rev1 C-terminus after DNA damage is currently under investigation.
In summary, our results show that Region 1 of the Rev1 C-terminus encompasses short peptide motifs that are conserved at the sequence level throughout the different eukaryotic phyla and define the minimal region for interaction with the TLS polymerase Pol ζ. Given the crucial role of the mammalian Rev1 C-terminus in coordinating interactions with other potentially mutagenic TLS polymerases, these results may make it possible to extend our observations from yeast to mammalian systems to identify small molecules that disrupt these interactions and hence mutagenesis brought about by chemotherapeutic drugs in vivo [54].
Supplementary Material
Acknowledgements
We thank the members of the laboratories of Drs. G.C. Walker and S. P. Bell for helpful discussions, particularly Brenda Minesinger and Rachel Woodruff. We also thank Allison Mo and Meisha Bynoe for technical assistance. This work was supported by an American Cancer Society Research Professorship (to G.C.W), a National Cancer Institute (NCI) grant CA21615 (to G.C.W) and a National Institute of Environmental Health Sciences (NIEHS) grant P30 ES002109 from the Center of Environmental Health Sciences (MIT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. DNA Repair and Mutagenesis. Washington, D. C.: ASM Press; 2005. [Google Scholar]
- 2.Goodman MF. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu Rev Biochem. 2002;71:17–50. doi: 10.1146/annurev.biochem.71.083101.124707. [DOI] [PubMed] [Google Scholar]
- 3.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 4.Kunkel TA. DNA replication fidelity. J Biol Chem. 2004;279:16895–16898. doi: 10.1074/jbc.R400006200. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg EC, Wagner R, Radman M. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science. 2002;296:1627–1630. doi: 10.1126/science.1070236. [DOI] [PubMed] [Google Scholar]
- 6.Jarosz DF, Godoy VG, Delaney JC, Essigmann JM, Walker GC. A single amino acid governs enhanced activity of DinB DNA polymerases on damaged templates. Nature. 2006;439:225–228. doi: 10.1038/nature04318. [DOI] [PubMed] [Google Scholar]
- 7.Johnson RE, Prakash S, Prakash L. Efficient bypass of a thymine-thymine dimer by yeast DNA polymerase, Poleta. Science. 1999;283:1001–1004. doi: 10.1126/science.283.5404.1001. [DOI] [PubMed] [Google Scholar]
- 8.Masutani C, Kusumoto R, Yamada A, Dohmae N, Yokoi M, Yuasa M, Araki M, Iwai S, Takio K, Hanaoka F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence CW. Cellular functions of DNA polymerase zeta and Rev1 protein. Adv Protein Chem. 2004;69:167–203. doi: 10.1016/S0065-3233(04)69006-1. [DOI] [PubMed] [Google Scholar]
- 10.Lemontt JF. Mutants of yeast defective in mutation induced by ultraviolet light. Genetics. 1971;68:21–33. doi: 10.1093/genetics/68.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawrence CW, Das G, Christensen RB. REV7, a new gene concerned with UV mutagenesis in yeast. Mol Gen Genet. 1985;200:80–85. doi: 10.1007/BF00383316. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs PE, Wang XD, Li Z, McManus TP, McGregor WG, Lawrence CW, Maher VM. The function of the human homolog of Saccharomyces cerevisiae REV1 is required for mutagenesis induced by UV light. Proc Natl Acad Sci U S A. 2000;97:4186–4191. doi: 10.1073/pnas.97.8.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibbs PE, McGregor WG, Maher VM, Nisson P, Lawrence CW. A human homolog of the Saccharomyces cerevisiae REV3 gene, which encodes the catalytic subunit of DNA polymerase zeta. Proc Natl Acad Sci U S A. 1998;95:6876–6880. doi: 10.1073/pnas.95.12.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung HW, Chun AC, Wang Q, Deng W, Hu L, Guan XY, Nicholls JM, Ling MT, Chuan Wong Y, Tsao SW, Jin DY, Wang X. Inactivation of human MAD2B in nasopharyngeal carcinoma cells leads to chemosensitization to DNA-damaging agents. Cancer Res. 2006;66:4357–4367. doi: 10.1158/0008-5472.CAN-05-3602. [DOI] [PubMed] [Google Scholar]
- 15.Haracska L, Prakash S, Prakash L. Yeast Rev1 protein is a G template-specific DNA polymerase. J Biol Chem. 2002;277:15546–15551. doi: 10.1074/jbc.M112146200. [DOI] [PubMed] [Google Scholar]
- 16.Nelson JR, Lawrence CW, Hinkle DC. Deoxycytidyl transferase activity of yeast REV1 protein. Nature. 1996;382:729–731. doi: 10.1038/382729a0. [DOI] [PubMed] [Google Scholar]
- 17.Haracska L, Unk I, Johnson RE, Johansson E, Burgers PM, Prakash S, Prakash L. Roles of yeast DNA polymerases delta and zeta and of Rev1 in the bypass of abasic sites. Genes Dev. 2001;15:945–954. doi: 10.1101/gad.882301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross AL, Simpson LJ, Sale JE. Vertebrate DNA damage tolerance requires the C-terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 2005;33:1280–1289. doi: 10.1093/nar/gki279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otsuka C, Loakes D, Negishi K. The role of deoxycytidyl transferase activity of yeast Rev1 protein in the bypass of abasic sites. Nucleic Acids Res Suppl. 2002:87–88. doi: 10.1093/nass/2.1.87. [DOI] [PubMed] [Google Scholar]
- 20.Ross AL, Sale JE. The catalytic activity of REV1 is employed during immunoglobulin gene diversification in DT40. Mol Immunol. 2006;43:1587–1594. doi: 10.1016/j.molimm.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Larimer FW, Perry JR, Hardigree AA. The REV1 gene of Saccharomyces cerevisiae: isolation, sequence, and functional analysis. J Bacteriol. 1989;171:230–237. doi: 10.1128/jb.171.1.230-237.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bienko M, Green CM, Crosetto N, Rudolf F, Zapart G, Coull B, Kannouche P, Wider G, Peter M, Lehmann AR, Hofmann K, Dikic I. Ubiquitin-binding domains in Y-family polymerases regulate translesion synthesis. Science. 2005;310:1821–1824. doi: 10.1126/science.1120615. [DOI] [PubMed] [Google Scholar]
- 23.Jansen JG, Tsaalbi-Shtylik A, Langerak P, Calleja F, Meijers CM, Jacobs H, de Wind N. The BRCT domain of mammalian Rev1 is involved in regulating DNA translesion synthesis. Nucleic Acids Res. 2005;33:356–365. doi: 10.1093/nar/gki189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo C, Sonoda E, Tang TS, Parker JL, Bielen AB, Takeda S, Ulrich HD, Friedberg EC. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol Cell. 2006;23:265–271. doi: 10.1016/j.molcel.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 25.Guo C, Tang TS, Bienko M, Parker JL, Bielen AB, Sonoda E, Takeda S, Ulrich HD, Dikic I, Friedberg EC. Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol Cell Biol. 2006;26:8892–8900. doi: 10.1128/MCB.01118-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Callebaut I, Mornon JP. From BRCA1 to RAP1: a widespread BRCT module closely associated with DNA repair. FEBS Lett. 1997;400:25–30. doi: 10.1016/s0014-5793(96)01312-9. [DOI] [PubMed] [Google Scholar]
- 27.Glover JN, Williams RS, Lee MS. Interactions between BRCT repeats and phosphoproteins: tangled up in two. Trends Biochem Sci. 2004;29:579–585. doi: 10.1016/j.tibs.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Guo C, Fischhaber PL, Luk-Paszyc MJ, Masuda Y, Zhou J, Kamiya K, Kisker C, Friedberg EC. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. Embo J. 2003;22:6621–6630. doi: 10.1093/emboj/cdg626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tissier A, Kannouche P, Reck MP, Lehmann AR, Fuchs RP, Cordonnier A. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase pol eta and REVl protein. DNA Repair (Amst) 2004;3:1503–1514. doi: 10.1016/j.dnarep.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Nelson JR, Gibbs PE, Nowicka AM, Hinkle DC, Lawrence CW. Evidence for a second function for Saccharomyces cerevisiae Rev1p. Mol Microbiol. 2000;37:549–554. doi: 10.1046/j.1365-2958.2000.01997.x. [DOI] [PubMed] [Google Scholar]
- 31.Waters LS, Walker GC. The critical mutagenic translesion DNA polymerase Rev1 is highly expressed during G(2)/M phase rather than S phase. Proc Natl Acad Sci U S A. 2006;103:8971–8976. doi: 10.1073/pnas.0510167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison A, Christensen RB, Alley J, Beck AK, Bernstine EG, Lemontt JF, Lawrence CW. REV3, a Saccharomyces cerevisiae gene whose function is required for induced mutagenesis, is predicted to encode a nonessential DNA polymerase. J Bacteriol. 1989;171:5659–5667. doi: 10.1128/jb.171.10.5659-5667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson JR, Lawrence CW, Hinkle DC. Thymine-thymine dimer bypass by yeast DNA polymerase zeta. Science. 1996;272:1646–1649. doi: 10.1126/science.272.5268.1646. [DOI] [PubMed] [Google Scholar]
- 34.Torpey LE, Gibbs PE, Nelson J, Lawrence CW. Cloning and sequence of REV7, a gene whose function is required for DNA damage-induced mutagenesis in Saccharomyces cerevisiae. Yeast. 1994;10:1503–1509. doi: 10.1002/yea.320101115. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Fang G. MAD2B is an inhibitor of the anaphase-promoting complex. Genes Dev. 2001;15:1765–1770. doi: 10.1101/gad.898701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfleger CM, Salic A, Lee E, Kirschner MW. Inhibition of Cdh1-APC by the MAD2-related protein MAD2L2: a novel mechanism for regulating Cdh1. Genes Dev. 2001;15:1759–1764. doi: 10.1101/gad.897901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Yang SH, Sharrocks AD. Rev7/MAD2B links c-Jun N-terminal protein kinase pathway signaling to activation of the transcription factor Elk-1. Mol Cell Biol. 2007;27:2861–2869. doi: 10.1128/MCB.02276-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acharya N, Haracska L, Johnson RE, Unk I, Prakash S, Prakash L. Complex formation of yeast rev1 and rev7 proteins: a novel role for the polymerase-associated domain. Mol Cell Biol. 2005;25:9734–9740. doi: 10.1128/MCB.25.21.9734-9740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acharya N, Johnson RE, Prakash S, Prakash L. Complex formation with Rev1 enhances the proficiency of Saccharomyces cerevisiae DNA polymerase zeta for mismatch extension and for extension opposite from DNA lesions. Mol Cell Biol. 2006;26:9555–9563. doi: 10.1128/MCB.01671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Souza S, Walker GC. Novel role for the C terminus of Saccharomyces cerevisiae Rev1 in mediating protein-protein interactions. Mol Cell Biol. 2006;26:8173–8182. doi: 10.1128/MCB.00202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirano Y, Sugimoto K. ATR homolog Mec1 controls association of DNA polymerase zeta-Rev1 complex with regions near a double-strand break. Curr Biol. 2006;16:586–590. doi: 10.1016/j.cub.2006.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakumo Y, Ogura Y, Ishii H, Numata S, Ichihara M, Croce CM, Fishel R, Takahashi M. Interactions in the error-prone postreplication repair proteins hREV1, hREV3, and hREV7. J Biol Chem. 2001;276:35644–35651. doi: 10.1074/jbc.M102051200. [DOI] [PubMed] [Google Scholar]
- 43.Ohashi E, Murakumo Y, Kanjo N, Akagi J, Masutani C, Hanaoka F, Ohmori H. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells. 2004;9:523–531. doi: 10.1111/j.1356-9597.2004.00747.x. [DOI] [PubMed] [Google Scholar]
- 44.Kannouche PL, Lehmann AR. Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle. 2004;3:1011–1013. [PubMed] [Google Scholar]
- 45.Kosarek JN, Woodruff RV, Rivera-Begeman A, Guo C, D'Souza S, Koonin EV, Walker GC, Friedberg EC. Comparative analysis of in vivo interactions between Rev1 protein and other Y-family DNA polymerases in animals and yeasts. DNA Repair (Amst) 2008;7:439–451. doi: 10.1016/j.dnarep.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acharya N, Haracska L, Prakash S, Prakash L. Complex formation of yeast Rev1 with DNA polymerase eta. Mol Cell Biol. 2007;27:8401–8408. doi: 10.1128/MCB.01478-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otsuka C, Kunitomi N, Iwai S, Loakes D, Negishi K. Roles of the polymerase and BRCT domains of Rev1 protein in translesion DNA synthesis in yeast in vivo. Mutat Res. 2005;578:79–87. doi: 10.1016/j.mrfmmm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Guo D, Xie Z, Shen H, Zhao B, Wang Z. Translesion synthesis of acetylaminofluorene-dG adducts by DNA polymerase zeta is stimulated by yeast Rev1 protein. Nucleic Acids Res. 2004;32:1122–1130. doi: 10.1093/nar/gkh279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao X, Muller EG, Rothstein R. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol Cell. 1998;2:329–340. doi: 10.1016/s1097-2765(00)80277-4. [DOI] [PubMed] [Google Scholar]
- 50.Knop M, Siegers K, Pereira G, Zachariae W, Winsor B, Nasmyth K, Schiebel E. Epitope tagging of yeast genes using a PCR-based strategy: more tags and improved practical routines. Yeast. 1999;15:963–972. doi: 10.1002/(SICI)1097-0061(199907)15:10B<963::AID-YEA399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 51.Wood A, Garg P, Burgers PM. A ubiquitin-binding motif in the translesion DNA polymerase Rev1 mediates its essential functional interaction with ubiquitinated proliferating cell nuclear antigen in response to DNA damage. J Biol Chem. 2007;282:20256–20263. doi: 10.1074/jbc.M702366200. [DOI] [PubMed] [Google Scholar]
- 52.Friedberg EC, Lehmann AR, Fuchs RP. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol Cell. 2005;18:499–505. doi: 10.1016/j.molcel.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi M, Figaroa F, Meeuwenoord N, Jansen LE, Siegal G. Characterization of the DNA binding and structural properties of the BRCT region of human replication factor C p140 subunit. J Biol Chem. 2006;281:4308–4317. doi: 10.1074/jbc.M511090200. [DOI] [PubMed] [Google Scholar]
- 54.Wells JA, McClendon CL. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.