Abstract
Pseudomonas aeruginosa is an opportunistic pathogen that can cause severe pneumonia associated with airspace flooding with protein-rich edema in critically ill patients. The type III secretion system is a major virulence factor and contributes to dissemination of P. aeruginosa. However, it is still unknown which particular bacterial toxin and which cellular pathways are responsible for the increase in lung endothelial permeability induced by P. aeruginosa. Thus, the first objective of this study was to determine the mechanisms by which this species causes an increase in lung endothelial permeability. The results showed that ExoS and ExoT, two of the four known P. aeruginosa type III cytotoxins, were primarily responsible for bacterium-induced increases in protein permeability across the lung endothelium via an inhibition of Rac1 and an activation of the RhoA signaling pathway. In addition, inhibition of the αvβ5 integrin, a central regulator of lung vascular permeability, prevented these P. aeruginosa–mediated increases in albumin flux due to endothelial permeability. Finally, prior activation of the stress protein response or adenoviral gene transfer of the inducible heat shock protein Hsp72 also inhibited the damaging effects of P. aeruginosa on the barrier function of lung endothelium. Taken together, these results demonstrate the critical role of the RhoA/αvβ5 integrin pathway in mediating P. aeruginosa–induced lung vascular permeability. In addition, activation of the stress protein response with pharmacologic inhibitors of Hsp90 may protect lungs against P. aeruginosa–induced permeability changes.
Keywords: lung, Pseudomonas aeruginosa, endothelial cells, integrin, heat shock response
CLINICAL RELEVANCE
We demonstrate for the first time that ExoS and ExoT, type III cytotoxins of Pseudomonas aeruginosa, increase lung vascular permeability via a RhoA- and αvβ5 integrin–dependent mechanism.
Pseudomonas aeruginosa is an opportunistic pathogen that causes lethal pneumonia in immunocompromised individuals and in critically ill patients (1). The high mortality of patients who develop P. aeruginosa pneumonia is associated with the development of acute lung injury, characterized by the flooding of the airspaces with a protein-rich edema. P. aeruginosa can cause lung damage by multiple mechanisms. Flagella, pili, and lipopolysaccharide are the initial tethers that facilitate bacterial cell contact by binding the cell surface glycolipid asialo-GM1 (2). Upon cell contact, the type III secretion system allows P. aeruginosa to inject toxins into the cells. Four of these effector proteins, ExoY, ExoS, ExoT, and ExoU, are known to be key determinants of virulence in this bacterium and can lead to host cell destruction and dissemination of P. aeruginosa (3, 4). Other virulence factors associated with P. aeruginosa include elastase, alkaline phosphatase, exotoxin A, and phospholipase, secreted by the type II secretion system, which also participate in host cell invasion by this bacterium (5). In addition, pyoverdin, pyochelin, and pyocyanin, secreted metabolites associated with generation of reactive oxygen species, also are involved in P. aeruginosa–induced host cell injury (5).
Multiple in vivo studies have shown that P. aeruginosa causes the development of severe alveolar pulmonary edema in rodents and that the type III secretion system plays a major role in the epithelial component of lung injury caused by this bacterium (6, 7). However, it is still unknown which particular bacterial toxin and which cellular pathways are responsible for P. aeruginosa–induced increase in lung endothelial permeability.
Activation of the stress protein response (SPR) protects host cells and organs from otherwise lethal insults such as oxidative stress or ischemia-reperfusion injury (8). Besides the classical activation of the SPR with heat or hyperthermia (also called heat shock), SPR can also be induced by various stimuli including corticosteroids (9), catecholamines (10), oxidative stress (11), and a group of compounds called anisomycins, which includes the specific pharmacologic activator 17-allylamino-17-demethoxy-geldanamycin (17-AAG [12]). Prior activation of the stress response has been shown to protect the lungs from damage caused by multiple noxious stimuli (reviewed in Ref. (13). However, whether SPR activation can attenuate the increase in the lung vascular permeability induced by a bacterial infection is unknown.
Thus, the first objective of this study was to determine the mechanisms by which P. aeruginosa causes an increase in lung endothelial permeability. We found that ExoS and ExoT, two type III cytotoxins, were responsible for the increase in protein permeability across the lung endothelium induced by P. aeruginosa. ExoS and ExoT previously have been shown to act as bifunctional toxins that contain an N-terminal RhoGAP domain and a C-terminal ADP-ribosylation domain (14, 15). Thus, our second objective was to determine the role of small GTPases Rac1 and RhoA in mediating P. aeruginosa effects on the lung endothelium. We found that P. aeruginosa increased paracellular permeability across endothelial cell monolayers via an inhibition of Rac1 and a subsequent activation of RhoA. In addition, we demonstrated that inhibition of the αvβ5 integrin, which we previously have shown to be a central regulator of lung endothelial permeability (16), also prevented the P. aeruginosa–mediated increase in lung endothelial permeability. Finally, based on previously reported data (17), the final objective of our studies was to determine whether prior SPR activation could prevent the RhoA-dependent increase in transendothelial albumin flux caused by P. aeruginosa. We found that prior SPR activation or the adenoviral-mediated expression of Hsp72 completely inhibited the paracellular permeability effect of P. aeruginosa on lung endothelial cell monolayers. Taken together, these results demonstrate the critical role of the RhoA/αvβ5 integrin pathway in mediating P. aeruginosa–induced lung vascular permeability.
MATERIALS AND METHODS
Reagents and Antibodies
All cell culture media were prepared by the University of California San Francisco Cell Culture Facility using deionized water and analytical grade reagents. Blocking studies were performed with RhoA kinase (ROCK) inhibitor (Y-27632; Calbiochem, San Diego, CA), anti-αvβ5 blocking (ALULA) and type-specific control antibodies (a generous gift from Dean Sheppard, University of California San Francisco, CA [16]). Primary antibody for β-catenin was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody to phosphotyrosine was obtained from Millipore (Billerica, MA). Goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) was obtained from MP Biomedicals (Solon, OH). The compound 17-allylamino-17-demethoxygeldanamycin (17-AAG) was obtained from Sigma (St. Louis, MO). Texas Red-X phalloidin was obtained from Invitrogen Life Technologies (Carlsbad, CA). Lysis buffer (5× concentrate) and luciferase substrates were purchased from Promega (Madison, WI). The protein concentration of cell lysates was determined using either a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) or a BCA kit (Pierce, Rockford, IL). G-LISA Rac1 and RhoA activation assay biochemistry kit was obtained from Cytoskeleton, Inc. (Denver, CO). Collagen-coated PFTE membrane Costar Transwells were obtained from Fisher Scientific (Santa Clara, CA). The chemiluminescence ECL Plus kit was obtained from Amersham Biosciences (Hercules, CA). 125I-labeled human serum albumin (Jeanatope ISO-TEX Diagnostics, Friendswood, TX) was used as radioactive tracer. Finally, 1-Palmitoyl-2-[1–14C]palmitoyl-phosphatidylcholine, 1-[1–14C]palmitoyl-2-[1–14C]palmitoyl-phosphatidylcholine, and 1-[1–14C]palmitoyl-2-hydroxyl phosphatidylcholine (lysophosphatidylcholine) were purchased from Amersham–Pharmacia Biotech (Piscataway, NJ). 1,2-Dipalmitoyl phosphatidylcholine (PtPC) and 1-palmitoyl-2-hydroxyl-phosphatidylcholine (lysoPC) were from Avanti Polar Lipids (Alabaster, AL).
Cell Culture
Bovine pulmonary arterial endothelial cells (BPAEC) (ATCC, CCL-209; passages all < 8) were used for the majority of experiments, as we have previously described (16). Bovine macrovascular lung pulmonary arterial endothelial cells were cultured on Transwells for 4 days until they formed confluent monolayers. Confluent monolayers were exposed to P. aeruginosa or vehicle as described in the specific protocols. Cells were kept in Dulbecco's modified Eagle's medium/H21 medium containing 10% low endotoxin fetal bovine serum and 1% penicillin/streptomycin/amphotericin in a humidified 95% air and 5% CO2 environment at 37°C. In some experiments, A549 cells, a human alveolar epithelial cell line, were used to compare the relative cytotoxic role of ExoU for the alveolar epithelium and the lung endothelium. A549 cells were maintained in a room air/5% CO2 incubator at 37°C using DMEM-H21 medium containing 10% fetal calf serum and penicillin/streptomycin (GIBCO BRL).
Recombinant Hsp72 Adenovirus
The recombinant adenovirus expressing human Hsp72 was a generous gift from Dr. C. Deutschman (University of Pennsylvania, Philadelphia, PA) and Dr. Y. Weiss (Hadassah-Hebrew University, Jerusalem, Israel [18]). Briefly, the porcine Hsp72 cDNA was subcloned into a p73 shuttle vector with a human CMV promoter and recombined into an E1–E3 deleted type 5 adenoviral vector. The donated replication-deficient vector was then commercially amplified and purified by cesium chloride gradient centrifugation and PD-10 Sephadex chromatography, plaque tittered on 293 cells, and checked for wild-type contamination (ViraQuest Inc., North Liberty, IA). The control vector, Ad-Empty, had no heterologous gene recombined in the deleted E1–E3 region.
Preparation of P. aeruginosa
P. aeruginosa strains used in this study are outlined in Table 1. All PAK strains were a kind gift from Dr. Stephen Lory at Harvard University (Cambridge, MA). PA103 strains were generously provided by Dr. Dara Frank at the Medical College of Wisconsin. Single and combined deletion strains of PAK and PA103 permitted analysis of the contribution of each of the four known P. aeruginosa type III exotoxins to endothelial permeability. As previously described, the ability of wild-type and mutant bacterial strains to produce exotoxins was confirmed by Western blot using antibodies against ExoU, ExoS, and ExoT (19). For endothelial permeability studies, strains were cultured overnight in 5 ml of LB medium before dilution with sterile PBS to a final cell count of 1 × 109 cells ml−1 (determined by OD600 readings). Counts were confirmed by serial dilution and plating on LB agar.
TABLE 1.
Pseudomonas aeruginosa STRAINS USED IN THIS STUDY
| Strain | Genotype | Reference |
|---|---|---|
| PAK | Wild type P. aeruginosa encoding ExoS, ExoT and ExoY | (42) |
| PAKΔexoS | Non-polar ExoS deletion in PAK background | (42) |
| PAKΔexoT | Non-polar ExoT deletion in PAK background | (42) |
| PAKΔexoY | Non-polar ExoY deletion in PAK background | (42) |
| PAKΔexoS/T | Non-polar ExoS and ExoT deletions in PAK background | (42) |
| PAKΔexoS/T/Y | Non-polar ExoS, ExoT and ExoY deletions in PAK background | (42) |
| PA103 | P. aeruginosa laboratory strain encoding ExoU, ExoT and a non-functional ExoY | (6) |
| PA103ΔexoU | Non-polar ExoU deletion in PA103 background | (43) |
| PA103ΔexoT | Non-polar ExoT deletion in PA103 background | (4) |
| PA103ΔexoU/T | Non-polar ExoU and ExoT deletions in PA103 background | (44) |
| PAO1 | Wild type P. aeruginosa encoding ExoS, ExoT and ExoY | (6, 40) |
Measurement of Transendothelial Albumin Flux
Transendothelial albumin flux was measured as previously described (16). Briefly, cells were seeded onto 6.5-mm collagen-coated PFTE membrane Costar Transwells at 1 × 105 cells per well and cultured for 3 days. Cells were exposed to P. aeruginosa for 3 hours (bacterial to bovine cell ratio: PA103, 1:25; PAK, 1:5; PAO1, 1:5). In some experiments, cells were pretreated with Y-27632 (10 μM) or its vehicle for 1 hour. Some cell monolayers were pretreated with an anti-αvβ5 blocking or an isotype specific control antibody (10 μg/ml) for 1 hour before exposure to P. aeruginosa. During the last hour of incubation with P. aeruginosa strains, 125I-albumin (0.05 μCi) was applied to each upper compartment at 37°C. After 1 hour, the media from the lower compartment were collected and counted in a Wallac Wizard γ-counter (Perkin Elmer, Shelton, CT). Only monolayers retaining more than 90% of tracer at baseline were studied.
Rac1 and RhoA Activation Assays
Rac1 and RhoA activity of endothelial cells was determined using a luminescence-based G-LISA Rac1 and RhoA activation assay biochemistry kit according to the manufacturer's instructions. Briefly, the endothelial cells were grown on 35-mm cell culture dishes to 50% confluence. After serum starvation for 6 hours, the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle was placed on the cells for 10 minutes before harvesting of cell lysates. Lysates were clarified by centrifugation at 4°C (14,000 × g, 2 min), the protein concentration determined, and final protein concentrations adjusted to 1.0 mg/ml. The lysates were added to plates coated with a Rac or Rho-GTP binding protein before incubation for 30 minutes at 4°C. Next a primary antibody specific for Rac or Rho was added and incubated for 45 minutes at room temperature. Finally, an HRP-conjugated secondary antibody is added and incubated for 45 minutes at room temperature. Luminescence was determined using the Wallac Victor 1420 (Perkin Elmer).
Immunofluorescence for Visualization of β-Catenin and Actin Stress Fibers
Endothelial cells were grown on collagen-coated glass coverslips to confluence. Cells were serum starved for 6 hours and pretreated with either Y-27362 (10 μM), anti-αvβ5 blocking Ab (10 μg/ml), control Ab (10 μg/ml), or vehicle (saline) for 1 hour, then exposed to the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or the vehicle for 10 minutes to detect actin stress fibers or for 3 hours for β-catenin staining. Cells were then fixed with 4% paraformaldehyde for 20 minutes, permeabilized with 0.5% Triton X-100, then stained with Texas Red-X phalloidin for actin stress fibers. For β-catenin or αvβ5 integrin staining, cells were incubated with primary antibody for β-catenin or αvβ5 integrin for 2 hours (1:50 dilution) at room temperature, before incubation with fluorescein isothiocyanate–conjugated antibody for 1 hour (1:50 dilution) at room temperature, mounted, and imaged using a Leica DM5000B microscope (Leica, Wetzlar, Germany) equipped for epifluorescence.
Immunoprecipitation for Detecting Phospho–β-Catenin
Endothelial cells were grown on collagen-coated 35-mm cell culture plates to confluence. Cells were pretreated with either Y-27362 (10 μM), anti-αvβ5 blocking antibody (10 μg/ml), control antibody (10 μg/ml), or vehicle (saline) for 1 hour and then exposed to the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 10 minutes. Cells were washed twice in PBS, and then solubilized in lysis buffer (150 mM NaCl, 5 mM MgCl2, 10 mM Tris HCl, pH 7.5, 1% Triton X-100) supplemented with a cocktail of phosphatase and protease inhibitors. Lysates were precleared with protein G–Sepharose beads and incubated with 2 μg of a rabbit polyclonal antibody to β-catenin overnight at 4°C. Cell lysates were then incubated with protein G–Sepharose beads under continuous mixing for 2 hours at 4°C. The Sepharose-bound immune complexes were washed with lysis buffer and boiled in 2× Laemmli sample buffer. Proteins were separated by 7.5% SDS-PAGE, transferred onto nitrocellulose membranes, blocked with 5% milk in TBS containing 0.1% Tween-20, and incubated with the appropriate antibodies (1:1,000 dilution of rabbit polyclonal antibody to phosphotyrosine overnight at 4°C; 1:2,000 dilution of goat anti-rabbit IgG conjugated with HRP for 1 h at room temperature). Immunoreactive bands were visualized using enhanced chemiluminescence.
Cell Viability Assay
Cell viability was measured by the Alamar Blue assay after exposure to the various experimental conditions. Cell media were replaced with medium containing 10% Alamar Blue and placed at 37°C in a cell incubator for 2 hours. The media were collected and read on a spectrophotometric plate reader at 530 nm.
Lysophospholipase Assay
For the lysoPLA assay, lysophosphatidylcholine (lysoPC; 1-palmitoyl-2-hydroxyl-PC; 50 nmol/reaction) containing radiolabeled 1-[1 14C]palmitoyl-2-hydroxyl-phosphoryl choline (105 cpm/reaction) was used to make lipid micelle assay substrate. Cell lysates from the various cell lines (2 × 105 cells in 50 mM Tris–HCl, pH 7.4, with 0.5% Triton X-100) were added either alone (control) or with 25 pmol of recombinant ExoU purified in J.P.W.-K.'s laboratory (20) to 50 μl of radiolabeled micelles and incubated for 1 hour at 37°C in a reaction buffer (50 mM Tris, pH 7.4, 10 mM EDTA, 30% glycerol). The enzymatic reaction was then quenched by mixing with 2.5 ml Dole's reagent (32% isopropyl alcohol/67% heptane/1% of 1 N H2SO4, 20:5:1) and vortexed. The samples were then centrifuged for 2 minutes and the upper phase was transferred to a new tube containing 100 mg silica gel. After vortexing and allowing the silica gel to settle, radioactivity of 200 μl of supernatant was counted by a liquid scintillation counter. The ratio of lysophospholipase activity on cell-line lysates with ExoU to the activity without ExoU was calculated.
Superoxide Dismutase Measurement
Basal superoxide dismutase (SOD) activity was measured using a SOD assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions. SOD activity (Cu/Zn, Mn, and Fe-SOD) was assessed by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine and quantified by an SOD standard curve. One SOD unit is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
Cytotoxicity Assay
In vitro cytotoxicity tests were performed as previously described (21). Briefly, 2 × 104 cells in culture medium were transferred to 96-well tissue culture plates and incubated for 18 hours. After reaching confluence, the culture medium was exchanged for a medium containing either the ExoU-producing P. aeruginosa strain PA103 or the ExoU-deficient PA103ΔU and applied to the cells for 4 hours. In negative control wells, the medium was exchanged for fresh culture medium containing no bacteria. In positive control wells, 10× Trizol was added to the medium 45 minutes before the end of the experiment to induce maximal cell lysis. Cytotoxicity was assessed by the release of lactate dehydrogenase (LDH) from lysed cells using a cytotoxicity assay kit (Cytotox 96; Promega). Cytotoxicity was expressed as a ratio of observed LDH release from lysed cells in experimental wells to the LDH release by maximally lysed cells incubated with Trizol. ExoU-specific cytotoxicity was expressed as the difference between the cytotoxicity due to wild-type PA103 and the cytotoxicity due to the ExoU-deficient PA103ΔU.
Statistical Analysis
All data are summarized as mean ± SEM. One-way ANOVA and the Fisher's exact t test were used to compare experimental with control groups. A P value of < 0.05 was considered statistically significant.
RESULTS
P. aeruginosa–Mediated Increase in Protein Permeability across Bovine Lung Endothelial Cell Monolayers Is ExoS and ExoT Dependent
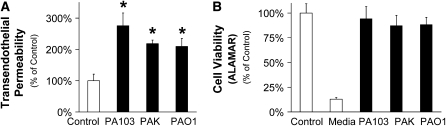
Exposure to one of three different strains of P. aeruginosa—wild-type PA103 (secretes ExoU, ExoT, and a nonfunctional ExoY); wild-type PAK (secretes ExoS, ExoT, and ExoY); or wild-type PAO1 (secretes ExoS, ExoT, and ExoY)—caused a significant increase in protein permeability across confluent lung endothelial cell monolayers without affecting cell viability (Figures 1A and 1B).
Figure 1.
Pseudomonas aeruginosa increases protein permeability across bovine lung endothelial cell monolayers without affecting cell viability. (A) Bovine pulmonary arterial endothelial cells (BPAEC) monolayers were treated with the following P. aeruginosa strains: PA103 (bacterial to bovine cell ratio: 1:25), PAK (bacterial to bovine cell ratio: 1:5), or PAO1 (bacterial to bovine cell ratio: 1:5) or their vehicle for 3 hours. Paracellular protein permeability was measured with 125I-albumin. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls. (B) BPAEC monolayers were treated with the following P. aeruginosa strains: PA103 (bacterial to bovine cell ratio: 1:25), PAK (bacterial to bovine cell ratio: 1:5), or PAO1 (bacterial to bovine cell ratio: 1:5) or their vehicle for 3 hours. Cell viability was measured with the Alamar Blue assay. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM.
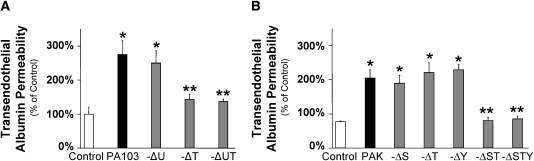
The next series of experiments were designed to determine which of the known type III toxins played a role in P. aeruginosa–mediated increased protein permeability across confluent lung endothelial cell monolayers. Exposure to the wild-type P. aeruginosa strain PA103 or its isogenic ExoU mutant caused a significant increase in protein permeability across confluent lung endothelial cell monolayers (Figures 1A and 2A). In contrast, deletion of ExoT or the combined deletion of ExoU and ExoT in a PA103 background inhibited the increase in protein permeability compared with that caused by wild-type PA103 (Figure 2A). We next examined the effect of a deletion of ExoS, ExoT, or ExoY from wild-type PAK on the protein permeability of lung endothelial cell monolayers. A single deletion of ExoS, ExoT, or ExoY did not affect the bacterium-induced increase in permeability. However, the combined deletion of ExoS and ExoT or the triple deletion of ExoS, ExoT, and ExoY inhibited the increase in protein permeability caused by PAK (Figure 2B). Taken together, these results indicate that the closely related ExoS and ExoT, but not ExoY or ExoU, in part mediate the increase in lung endothelial permeability caused by P. aeruginosa.
Figure 2.
P. aeruginosa–mediated increase in protein permeability across bovine lung endothelial cell monolayers is ExoS and ExoT dependent. (A) BPAEC monolayers were treated with the wild-type P. aeruginosa strain PA103, its isogenic mutants with single or combined deletion for ExoU, ExoT, or ExoU and ExoT (bacterial to bovine cell ratio: 1:25) or their vehicle for 3 hours. Paracellular protein permeability was measured with 125I-albumin. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from wild-type PA103. (B) BPAEC monolayers were treated with the wild-type P. aeruginosa strain PAK, its isogenic mutants with single or combined deletion for ExoS; ExoT; ExoY or ExoS and ExoT; or ExoS, ExoT, and ExoY (bacterial to bovine cell ratio: 1:5) or their vehicle for 3 hours. Paracellular protein permeability was measured with 125I-albumin. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from wild-type PAK.
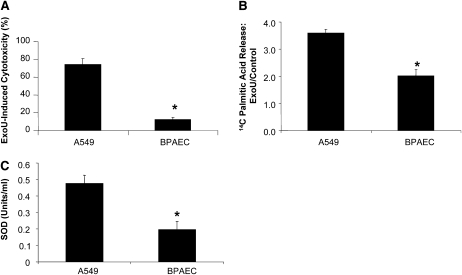
To understand why ExoU deletion had no effect on P. aeruginosa–induced protein permeability across confluent lung endothelial cell monolayers, we compared the cytotoxic effects of ExoU-producing P. aeruginosa strain PA103 to that of the ExoU-deficient PA103ΔU mutant on the two cell types, A549 alveolar epithelial and lung endothelial cell monolayers. These results show that ExoU was significantly less cytotoxic for lung endothelial cells compared with the alveolar epithelial cells (Figure 3A). These results are likely due to a significantly lower lysophospholipase activity induced by ExoU exposure to lung endothelial cells compared with the activity induced after exposure to A549 alveolar epithelial cells (Figure 3B). Furthermore, as a recent study demonstrated that superoxide dismutase is an obligatory co-factor of ExoU (22), we measured the basal SOD activity in lung endothelial and in the A549 alveolar epithelial cells. The results indicate that there was significantly less SOD activity in lung endothelial cells compared with that activity measured in alveolar epithelial cells (Figure 3C). These results provide an explanation for the observed decreased toxicity of ExoU in lung endothelial cells.
Figure 3.
The distal lung epithelium is more susceptible than the lung endothelium to ExoU-mediated cytotoxicity. (A) A549 human alveolar epithelial and BPAEC cell monolayers were exposed to either ExoU-producing P. aeruginosa strain PA103 or the ExoU-deficient PA103ΔU for 4 hours. Cytotoxicity was assessed by the release of lactate dehydrogenase (LDH) from lysed cells. Cytotoxicity was expressed as a ratio of observed LDH release from lysed cells in experimental wells to the LDH release by maximally lysed cells incubated with Trizol. ExoU-specific cytotoxicity was expressed as the difference between the cytotoxicity caused by wild-type PA103 and ExoU-deficient PA103ΔU. (B) A549 human alveolar epithelial and BPAEC cell monolayers were exposed to 25 pmol of recombinant ExoU or its vehicle for 1 hour. Lysophospholipase activity was measured as described in Materials and Methods. ExoU-mediated lysophospholipase activity was expressed as the ratio between the baseline lysophospholipase activity and that measured in the presence of ExoU. (C) Basal SOD activity was measured in confluent A549 human alveolar epithelial and BPAEC cell monolayers. SOD activity was assessed by measuring the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine and quantified by an SOD standard curve. One SOD unit is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. For all panels, results are shown as means ± SEM; *P ≤ 0.05 from values measured in A549 cells.
P. aeruginosa–Mediated Increase in Protein Permeability across Bovine Lung Endothelial Cell Monolayers Is Mediated via the RhoA/αvβ5 Integrin Pathway
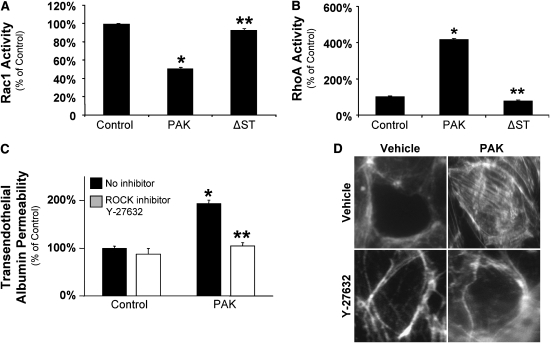
ExoS and ExoT from the type III secretion system of P. aeruginosa are bifunctional toxins that contain an N-terminal RhoGAP domain and a C-terminal ADP-ribosylation domain (14, 15). Thus, in the next series of experiments, we evaluated the role of the small Rho GTPases, Rac1 and RhoA, in modulating the lung endothelial permeability induced by P. aeruginosa. We tested the hypothesis that the cytotoxins ExoS and ExoT inhibit baseline Rac1 activity required for the maintenance of the barrier function in lung endothelial cells, thus causing a corresponding increase in RhoA activity, as Rac1 has been shown to be upstream of RhoA in regulating their reciprocal activities (23). Exposure of confluent lung endothelial cell monolayers to wild-type P. aeruginosa strain PAK caused a significant decrease in Rac1 activity and a corresponding increase in RhoA activity within 10 minutes. This effect was not observed when monolayers were exposed to the P. aeruginosa strain PAK with a combined deletion of ExoS and ExoT (Figures 4A and 4B). Furthermore, the increase in transendothelial albumin flux induced by PAK was blocked by the inhibition of ROCK, the immediate downstream effector of RhoA (Figure 4C). In addition, P. aeruginosa exposure induced (F)-actin to polymerize and form stress fibers, a cellular mechanism that precedes cell contraction and subsequent paracellular permeability. The formation of actin stress fibers also was blocked by exposure to a ROCK inhibitor before PAK stimulation (Figure 4D).
Figure 4.
P. aeruginosa–mediated increase in protein permeability across bovine lung endothelial cell monolayers is RhoA dependent. (A) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5), its isogenic mutant with a combined deletion for ExoS and ExoT, or its vehicle for 10 minutes. Rac1 activity was measured as described in Materials and Methods. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from wild-type PAK. (B) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5), its isogenic mutant with a combined deletion for ExoS and ExoT, or its vehicle for 10 minutes. RhoA activity was measured as described in Materials and Methods. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from wild-type PAK. (C) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 3 hours. Some cell monolayers were pretreated with a RhoA kinase inhibitor (Y-27632) (10 μM) or its vehicle before exposure to PAK or its vehicle. Paracellular protein permeability was measured with 125I-albumin. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from cell monolayers treated with PAK alone. (D) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 10 minutes. Some cell monolayers were pretreated with a RhoA kinase inhibitor (Y-27632) (10 μM) or its vehicle before exposure to PAK or its vehicle. Cells were then fixed, permeabilized, and stained with rhodamin-phalloidin. One representative blot of four experiments is shown.
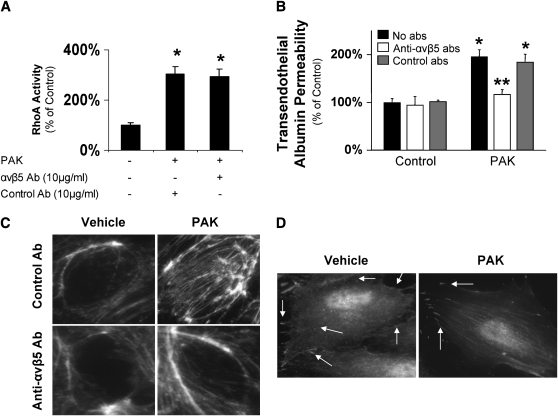
Previously, we identified the αvβ5 integrin as one of the central regulators of the lung endothelial paracellular permeability and reported that this integrin is downstream of RhoA signaling and critical for the formation of actin stress fibers (16). Interestingly, P. aeruginosa–induced activation of RhoA was not inhibited by blocking the αvβ5 integrin, suggesting that RhoA signaling was upstream of the αvβ5 integrin in P. aeruginosa–mediated signaling that leads to increased permeability in lung endothelial cells (Figure 5A). In contrast, we found that blocking the αvβ5 integrin function inhibited the PAK-mediated increased permeability across lung endothelial cell monolayers (Figure 5B) and actin polymerization (Figure 5C). To further understand the function of the αvβ5 integrin in the endothelial barrier, cells treated or untreated with P. aeruginosa were washed, fixed, and both the actin filaments and αvβ5 were labeled using fluorescent-labeled phalloidin or immunofluorescent-labeled antibodies, respectively (Figure 5D). Endothelial cells that were not exposed to P. aeruginosa showed a diffuse staining of the αvβ5 integrin that masks the fluorescent staining of the actin filaments. In contrast, endothelial cells that were exposed to P. aeruginosa showed concentrations of the αvβ5 integrin at distinct points in the cell membrane that coincided with the tip of the stress fibers. These data suggest that the function of the αvβ5 integrin is to serve as an anchor point for the actin stress fibers that are formed after P. aeruginosa–induced activation of the RhoA pathway (Figure 5D).
Figure 5.
P. aeruginosa–mediated increase in protein permeability across bovine lung endothelial cell monolayers is αvβ5 integrin dependent. (A) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 10 minutes. Some cell monolayers were pretreated with blocking Ab to the αvβ5 integrin or isotype control Ab before exposure to PAK or its vehicle. RhoA activity was measured as described in Materials and Methods. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls. (B) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 3 hours. Some cell monolayers were pretreated with a blocking antibody to αvβ5 integrin or its isotype control antibody before exposure to PAK or its vehicle. Paracellular protein permeability was measured with 125I-albumin. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from cell monolayers treated with PAK alone. (C) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 10 minutes. Some cell monolayers were pretreated with a blocking antibody to αvβ5 integrin or its isotype control antibody before exposure to PAK or its vehicle. Cells were then fixed, permeabilized, and stained with rhodamin-phalloidin. One representative blot of four experiments is shown.
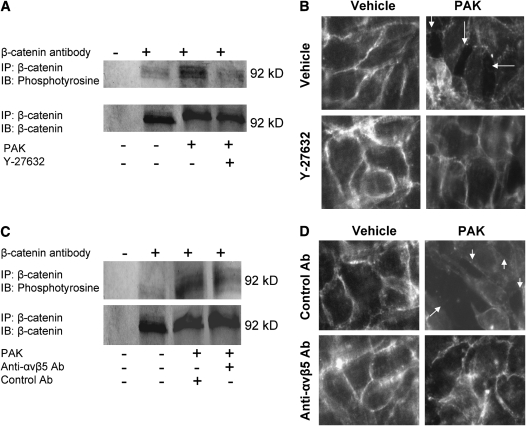
To further determine the mechanisms of P. aeruginosa–mediated increase in protein permeability, we examined whether exposure of lung endothelial cells to PAK would affect the phosphorylation of β-catenin, one of the critical components of the adherens junction protein complex. Previous studies have identified that inflammatory mediators such as thrombin, VEGF, and TGF-β cause myosin phosphorylation, actin polymerization, and disruption of the adherens junctions by phosphorylation of components such as β-catenin, with subsequent degradation by the ubiquitin–proteasome system (16). Our data indicate that wild-type P. aeruginosa strain PAK caused phosphorylation of β-catenin in lung endothelial cell monolayers, and that this phosphorylation was prevented by pretreating the cells with a ROCK inhibitor or an antibody to the αvβ5 integrin (Figures 6A and 6C). Furthermore, P. aeruginosa strain PAK caused the formation of paracellular gaps between lung endothelial cells that was largely prevented by a pretreatment with a ROCK inhibitor or an antibody to the αvβ5 integrin (Figures 6B and 6D). Finally, the formation of paracellular gaps was associated with a decrease in the expression of β-catenin at the cell membrane, indicating a disassembly of the adherens junction complex (Figures 6B and 6D). Overall, our results indicate that P. aeruginosa disrupted the adherens junctions and caused the contraction of endothelial cells via a RhoA/αvβ5 integrin-dependent mechanism leading to increased paracellular permeability.
Figure 6.
P. aeruginosa causes adherens junction disassembly and formation of paracellular gaps in bovine pulmonary arterial endothelial cell monolayers. (A) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 10 minutes. Some cell monolayers were pretreated with a RhoA kinase inhibitor (Y-27632) (10 μM) or its vehicle before exposure to PAK or its vehicle. Cells were then harvested and cell extracts were subjected to immunoprecipitation with an antibody against β-catenin and immunoblotted with an antibody to phosphotyrosine. The same blots were then reprobed with an antibody to β-catenin. One representative blot of four experiments is shown. (B) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 3 hours. Some cell monolayers were pretreated with a RhoA kinase inhibitor (Y-27632) (10 μM) or its vehicle before exposure to PAK or its vehicle. Cells were then fixed, permeabilized, and incubated with a primary Ab against β-catenin and a fluorescein isothiocyanate (FITC)-conjugated secondary antibody. (C) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 10 minutes. Some cell monolayers were pretreated with a blocking antibody to αvβ5 integrin or its isotype control antibody before exposure to PAK or its vehicle. Cells were then harvested and cell extracts were subjected to immunoprecipitation with an antibody against β-catenin and immunoblotted with an antibody to phosphotyrosine. The same blots were then reprobed with an antibody to β-catenin. (D) BPAEC cell monolayers were treated with the wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 3 hours. Some cell monolayers were pretreated with blocking Ab to the αvβ5 integrin or isotype control Ab before exposure to PAK or its vehicle. Cells were then fixed, permeabilized, and incubated with a primary Ab against β-catenin and an FITC-conjugated secondary antibody. For all experiments, one representative blot is shown. Three additional experiments gave comparable results.
SPR Activation Prevents P. aeruginosa–Mediated Increase in Protein Permeability across Bovine Lung Endothelial Cell Monolayers
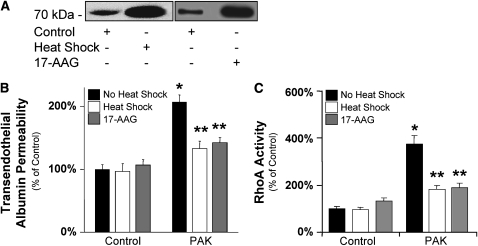
Because we previously have reported that SPR activation prevents the activation of the RhoA-dependent VEGF-mediated increase in lung endothelial permeability (17), the final objective of our studies was to determine whether a prior SPR activation also would prevent the RhoA-dependent increase in transendothelial albumin flux caused by P. aeruginosa. Cell monolayers were exposed to wild-type P. aeruginosa strain PAK for 3 hours and protein permeability across these monolayers was measured with 125I-albumin. In some experiments, cells were stress preconditioned with heat (60 min at 43°C, then recovered overnight at 37°C) or pretreated with the Hsp90 inhibitor 17-AAG (10 ng/ml) for 8 hours before the exposure to PAK. Both of these treatments are known to increase the expression of Hsp 72, consistent with SPR activation. Both heat- and 17-AAG–induced increases in Hsp72 expression (Figure 7A) and were associated with attenuation of the PAK-dependent increase in protein permeability across these lung endothelial cell monolayers (Figure 7B). In addition, stress preconditioning also reduced the PAK-mediated increase in cell RhoA activity (Figure 7C).
Figure 7.
P. aeruginosa–mediated increase in protein permeability across bovine lung endothelial cell monolayers is prevented by the activation of the heat shock response. (A) BPAEC cell monolayers were either treated with 17-AAG (10 ng/ml) or its vehicle for 8 hours or were pretreated with heat (43°C for 60 min), then recovered overnight at 37°C before being harvested. Control cell monolayers were maintained at 37°C. The expression of Hsp72 protein was determined by Western blotting. One representative blot is shown. Three additional experiments gave comparable results. (B) BPAEC cell monolayers were either treated with 17-AAG (10 ng/ml) or its vehicle for 8 hours or were pretreated with heat (43°C for 60 min), then recovered overnight at 37°C before exposure to wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 3 hours. Control cell monolayers were maintained at 37°C. Paracellular protein permeability was measured with 125I-albumin. All experiments were performed at least in triplicates and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from cell monolayers treated with PAK alone. (C) BPAEC cell monolayers were either treated with 17-AAG (10 ng/ml) or its vehicle for 8 hours or were pretreated with heat (43°C for 60 min), then recovered overnight at 37°C before exposure to wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 10 minutes. Control cell monolayers were maintained at 37°C. RhoA activity was measured as described in Materials and Methods. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from cell monolayers treated with PAK alone.
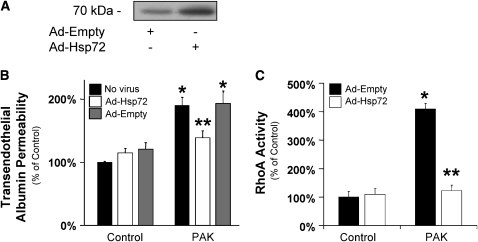
Previous studies have reported that overexpression of the recombinant Hsp72 can inhibit several cell transduction pathways such as NF-κB (24) or iNOS (25–28). Thus, in the last series of experiments, we determined whether pretreatment of lung endothelial cells with an adenovirus encoding the Hsp72 (AdHSP) protein would prevent the increase in transendothelial albumin flux caused by P. aeruginosa. Transfection of lung endothelial cells with AdHSP significantly increased the expression of Hsp72 protein (Figure 8A) and inhibited the PAK-dependent increase in protein permeability across these lung endothelial cell monolayers (Figure 8B). This protective effect was absent in cell lines transfected with the control virus (Figure 8B). Furthermore, transfection of lung endothelial cells with AdHSP also reduced the PAK-mediated increase in cell RhoA activity (Figure 8C). In summary, these results indicate that SPR activation or adenoviral gene transfer of Hsp72 interfered with the P. aeruginosa–activated cell signaling pathway mediated through the RhoA/αvβ5 integrin that results in protein permeability across the lung endothelium.
Figure 8.
P. aeruginosa–mediated increase in protein permeability across bovine lung endothelial cell monolayers is prevented by the adenoviral gene transfer of Hsp72. (A) BPAEC cell monolayers were infected with a recombinant adenovirus encoding Hsp72 (multiplicity of infection [MOI] = 50) or an empty adenovirus and harvested 48 hours after infection. The expression of Hsp72 protein was determined by Western blotting. One representative blot is shown. Three additional experiments gave comparable results. (B) BPAEC cell monolayers were infected with a recombinant adenovirus encoding Hsp72 (MOI = 100) or an empty adenovirus for 48 hours and were then exposed to wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 3 hours. Paracellular protein permeability was measured with 125I-albumin. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from cell monolayers treated with PAK alone. (C) BPAEC cell monolayers were infected with a recombinant adenovirus encoding Hsp72 (MOI = 100) or an empty adenovirus for 48 hours and were then exposed to wild-type P. aeruginosa strain PAK (bacterial to bovine cell ratio: 1:5) or its vehicle for 10 minutes. RhoA activity was measured as described in Materials and Methods. All experiments were performed at least in triplicate and repeated three times. Data are shown as percentage of controls; results are shown as means ± SEM; *P ≤ 0.05 from controls; **P ≤ 0.05 from cell monolayers treated with PAK alone.
DISCUSSION
P. aeruginosa is an opportunistic pathogen that causes lethal pneumonia in immunocompromised individuals and critically ill patients (1). The high mortality of patients who develop P. aeruginosa pneumonia is associated with the occurrence of acute lung injury, characterized by flooding of the airpaces with a protein-rich edema. However, the exact mechanism by which P. aeruginosa causes an increase in lung vascular permeability is unknown. Thus, the overall objective of this study was to determine the molecular mechanisms involved in the increase in lung vascular permeability by this bacterium. For the first time, this study shows that (1) ExoS and ExoT, bi-functional type III cytotoxins of P. aeruginosa, are responsible for the increase in protein permeability across the lung endothelium via a RhoA-dependent mechanism; (2) inhibition of the αvβ5 integrin, a central regulator of lung vascular permeability, completely prevented P. aeruginosa–mediated increase in protein permeability; and (3) prior activation of the stress protein response attenuated the effect of P. aeruginosa on the barrier function of the lung endothelium by limiting the activation of the small GTPase RhoA. These results demonstrate a critical role for the RhoA/αvβ5 integrin pathway in mediating P. aeruginosa–induced increase in lung vascular permeability.
The first objective of the present study was to determine the molecular mechanisms by which P. aeruginosa caused an increase in the albumin flux across lung endothelial cell monolayers. We found that ExoS and ExoT, but not ExoU or ExoY, are responsible for P. aeruginosa–mediated decrease in lung endothelium barrier function. To our knowledge, this is the first study to report which toxin(s) from the type III secretion system are responsible for increased lung endothelial barrier permeability after exposure to P. aeruginosa. Sayner and coworkers reported that a P. aeruginosa strain genetically modified to only produce large amounts of ExoY, a cytoplasmic adenylate cyclase, caused disruption of the lung endothelial monolayers (29). That study demonstrated that soluble bacterial adenylate cyclases directly affected the barrier function of lung endothelial cells because these enzymes are not compartmentalized and not under the control of the phosphodiesterases (30). However, because the amount of ExoY produced by the engineered strain of P. aeruginosa was much larger than usually produced by clinical isolates, these findings may not be relevant to clinical P. aeruginosa infections. In fact, in the present study, deleting ExoY did not affect the ability of P. aeruginosa to increase protein permeability across the lung endothelium.
The deletion of ExoU from wild-type P. aeruginosa strain PA103 did not alter transendothelial albumin flux. This is surprising, as ExoU, a cytotoxin with phospholipase activity, has been shown in multiple experimental and clinical studies to be the most virulent toxin of P. aeruginosa (6, 7, 31). However, ExoU requires cytosolic superoxide dismutase as a cofactor to activate its phospholipase activity (22). Indeed, the abundance of superoxide dismutase in the lung epithelium and in the distal airspaces may render this barrier particularly susceptible to ExoU injury (32). Our results support this hypothesis by demonstrating that significantly greater ExoU-mediated cytotoxicity and lysophospholipase activity was evident in A549 epithelial cells that was associated with higher levels of SOD activity. In contrast, lung endothelial cells exhibited lower levels of cytotoxicity and lysophospholipase activity and lower levels of cytosolic SOD activity. Therefore, our results indicate that the lung endothelium is less vulnerable to ExoU than the lung epithelium and that ExoS and ExoT were more important in increasing endothelial permeability.
What are the cellular pathways by which P. aeruginosa affects the lung vascular protein permeability? Previous experimental work has shown that ExoS and ExoT are bi-functional toxins that contain both an N-terminal RhoGAP domain and a C-terminal ADP-ribosylation domain (14, 15). Thus, we tested the hypothesis that the cytotoxins ExoS and ExoT inhibit baseline Rac1 activity required for the maintenance of the barrier function in lung endothelial cells, thus causing a corresponding increase in RhoA activity, as Rac1 has been shown to be upstream of RhoA in regulating their reciprocal activities (23). Indeed, the increase in lung endothelial permeability caused by this bacterium was RhoA-, but not src-dependent (data not shown). This is similar to our previously reported results in studies examining the effect of other important mediators, such as thrombin, VEGF, and TGF-β, on the lung endothelium (16).
Passage of solutes through the endothelial barrier is thought to occur via a transcellular pathway or via receptor-activated transcytosis (33). The relative contribution of these pathways remains incompletely understood. However, it has been suggested that the formation of actin stress fibers in endothelial cells is important for the formation of gaps between cells. This is believed to result from imbalanced competition between cytoskeletal, adhesive cell–cell, and cell–matrix forces that leads to an increased flux of solutes and protein between and across endothelial cells (33). In our experimental model, P. aeruginosa induced the formation of actin stress fibers via polymerization of (F)-actin. Furthermore, the next series of experiments showed a critical role for the αvβ5 integrin in controlling the formation of actin stress fiber and the subsequent increase in lung endothelial permeability mediated by P. aeruginosa. Although our studies did not directly distinguish between paracellular and transcellular pathways, the ability of αvβ5 integrin to regulate both stress fiber formation and transendothelial flux suggests that the paracellular pathway is more relevant for the P. aeruginosa–mediated increase in lung vascular permeability.
We have previously reported that SPR activation prevents the activation of RhoA-dependent VEGF-mediated increase in lung endothelial permeability (17). Thus, the final objective of these studies was to determine whether a prior SPR activation would also prevent the RhoA-dependent increase in transendothelial albumin flux caused by P. aeruginosa. SPR activation was induced either with heat or with 17-AAG, a benzoquinone ansamycin that we have previously shown to activate stress protein expression (12). The results showed that SPR activation significantly decreased endothelial cell RhoA activity and transendothelial albumin flux induced by P. aeruginosa. In addition, the overexpression of Hsp72 mediated by adenoviral transfection of lung endothelial cells inhibited the P. aeruginosa–mediated increase in lung endothelial permeability in the absence of SPR activation. Hsp72 has been shown to bind and inhibit expression and function of some inflammatory mediators, such as NF-κB (24) or iNOS (25–28). Thus, in the present study, the protective effect of SPR activation was at least in part related to the expression of the inducible Hsp72, as both heat stress and 17-AAG treatment caused the expression of Hsp72 in lung endothelial cells. The present investigation adds to a growing number of studies that show that SPR activation mediates cytoprotection in cell and animal models of acute lung injury (34–39). However, to our knowledge, the present work is the first study to show that prior SPR activation inhibits the effect of live bacteria on the lung endothelial barrier in part via the expression of the inducible Hsp72.
A limitation of this series of studies is that we used cells derived from proximal pulmonary macrovascular endothelium. Indeed, microvascular lung endothelial cells would be more relevant to study pulmonary capillary leak. However, we have previously reported that blocking the αvβ5 integrin in vivo protects against the increase in lung vascular permeability induced by ischemia-reperfusion or ventilator-induced lung injury in rodents (16, 17). Given these results, we suggest that the protective mechanism of blocking the αvβ5 integrin in macrovascular endothelium is relevant to microvascular endothelium such that blocking the αvβ5 integrin in microvascular cells would inhibit protein permeability mediated by P. aeruginosa. Thus, taken together, these results demonstrate a critical role for this integrin in mediating the increase in lung endothelial paracellular permeability induced by exposure to P. aeruginosa.
Clinical Relevance
What is the clinical relevance of our findings? With an understanding of the mechanisms for lung cellular injury by P. aeruginosa, new therapies can be proposed. If the type III secretory toxins are involved in both endothelial and epithelial cell injury, therapies that block this virulence mechanism would be useful in preventing both types of injuries. Further, the integrity of the two cellular barriers affect each other (40). We have previously investigated the damaging effect of P. aeruginosa on the distal lung epithelium. In particular, we have shown that a large amount of the P. aeruginosa bacteria instilled into the distal airspaces go through the distal lung epithelium come in contact with the lung endothelium before reaching the bloodstream and cause the development of pulmonary edema (41). We have also reported that the intravenous administration of P. aeruginosa resulted in an increase of the vulnerability of the alveolar capillary barrier to an exposure to a nontoxic inoculum of the same bacterium instilled into the distal airspace of the lung (40). This previously published study demonstrated how P. aeruginosa in the circulation could amplify the minor lung epithelial injury caused by bacteria that colonize the distal airways.
In summary, these studies demonstrate for the first time the mechanisms by which P. aeruginosa increases lung vascular permeability. We specifically found that ExoS and ExoT, type III cytotoxins of P. aeruginosa, are responsible for the increase in protein permeability across the lung endothelium induced by this bacterium via RhoA- and αvβ5 integrin–dependent mechanisms (Figure 9). Furthermore, prior activation of the stress protein response blocked the effect of P. aeruginosa on the barrier function of lung endothelium by preventing the activation of the small GTPase RhoA. The findings reported here have potential clinical relevance. Indeed, transient blockade of the αvβ5 integrin by a humanized antibody or small RGD peptides and activation of the heat shock response using pharmacologic inhibitors of Hsp90 have previously been shown to be safe in humans (12). Therefore, these therapies may provide new potential drugs to treat lung injury induced by P. aeruginosa, an infection that is associated with high mortality in critically ill patients.
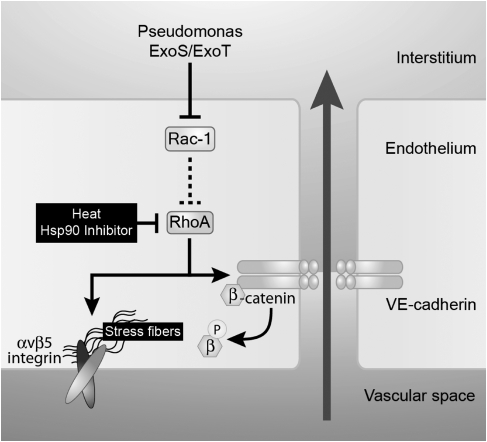
Figure 9.
Schematics of the effect of Pseudomonas aeruginosa on the lung endothelial barrier. Our model diagrams the P. aeruginosa–induced signaling pathway that leads to an increase in lung endothelial permeability. ExoS and ExoT, two cytotoxins from the type III secretion system of P. aeruginosa, increase lung endothelial permeability via the inhibition of Rac1 and the activation of the RhoA/αvβ5 signaling.
This work was supported in part by grant HL074005 (SCCOR project 4: J.-F.P. and project 2: J.P.W.-K.) from the National Institutes of Health.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0454OC on August 14, 2008
Conflict of Interest Statement: M.T.G. has received honoraria or travel support in the past 5 years for consulting or lecturing from CSL Behring GmbH, Hattersheim am Main, Germany. Y.G.W. has a pending US patent application “A Method of Preventing Acute Pulmonary Cell Injury” No. 10/150, 054 submitted on May 16, 2002. J.P.W.-K. receives money from “Critical Care Secrets” and “Anesthesiology and Miller's Text Book.” C.S.D. has a pending US patent application “A Method of Preventing Acute Pulmonary Cell Injury” No. 11/283, 151. This is for the adenoviral expression vector for Hsp70 used in this manuscript, and does not at this time constitute a financial interest in the subject of this manuscript. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rello J, Rue M, Jubert P, Muses G, Sonora R, Valles J, Niederman MS. Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med 1997;25:1862–1867. [DOI] [PubMed] [Google Scholar]
- 2.Gupta SK, Berk RS, Masinick S, Hazlett LD. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect Immun 1994;62:4572–4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun 2004;72:6969–6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol 1997;25:547–557. [DOI] [PubMed] [Google Scholar]
- 5.Kipnis E, Sawa T, Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Mal Infect 2006;36:78–91. [DOI] [PubMed] [Google Scholar]
- 6.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 2001;183:1767–1774. [DOI] [PubMed] [Google Scholar]
- 7.Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 2002;30:521–528. [DOI] [PubMed] [Google Scholar]
- 8.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 2002;295:1852–1858. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Chang J, Kirchhoff SR, Knowlton AA. Activation of HSF and selective increase in heat-shock proteins by acute dexamethasone treatment. Am J Physiol Heart Circ Physiol 2000;278:H1091–H1097. [DOI] [PubMed] [Google Scholar]
- 10.Johnson JD, Fleshner M. Releasing signals, secretory pathways, and immune function of endogenous extracellular heat shock protein 72. J Leukoc Biol 2006;79:425–434. [DOI] [PubMed] [Google Scholar]
- 11.Marini M, Frabetti F, Musiani D, Franceschi C. Oxygen radicals induce stress proteins and tolerance to oxidative stress in human lymphocytes. Int J Radiat Biol 1996;70:337–350. [DOI] [PubMed] [Google Scholar]
- 12.Soti C, Nagy E, Giricz Z, Vigh L, Csermely P, Ferdinandy P. Heat shock proteins as emerging therapeutic targets. Br J Pharmacol 2005;146:769–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler DS, Wong HR. Heat shock response and acute lung injury. Free Radic Biol Med 2007;42:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goehring UM, Schmidt G, Pederson KJ, Aktories K, Barbieri JT. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. J Biol Chem 1999;274:36369–36372. [DOI] [PubMed] [Google Scholar]
- 15.Kazmierczak BI, Engel JN. Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42. Infect Immun 2002;70:2198–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su G, Hodnett M, Wu N, Atakilit A, Kosinski C, Godzich M, Huang XZ, Kim JK, Frank JA, Matthay MA, et al. Integrin alphavbeta5 regulates lung vascular permeability and pulmonary endothelial barrier function. Am J Respir Cell Mol Biol 2007;36:377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godzich M, Hodnett M, Frank JA, Su G, Pespeni M, Angel A, Howard MB, Matthay MA, Pittet JF. Activation of the stress protein response prevents the development of pulmonary edema by inhibiting VEGF cell signaling in a model of lung ischemia-reperfusion injury in rats. FASEB J 2006;20:1519–1521. [DOI] [PubMed] [Google Scholar]
- 18.Weiss YG, Maloyan A, Tazelaar J, Raj N, Deutschman CS. Adenoviral transfer of HSP-70 into pulmonary epithelium ameliorates experimental acute respiratory distress syndrome. J Clin Invest 2002;110:801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Augustin DK, Song Y, Baek MS, Sawa Y, Singh G, Taylor B, Rubio-Mills A, Flanagan JL, Wiener-Kronish JP, Lynch SV. Presence or absence of lipopolysaccharide O antigens affects type III secretion by Pseudomonas aeruginosa. J Bacteriol 2007;189:2203–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pankhaniya RR, Tamura M, Allmond LR, Moriyama K, Ajayi T, Wiener-Kronish JP, Sawa T. Pseudomonas aeruginosa causes acute lung injury via the catalytic activity of the patatin-like phospholipase domain of ExoU. Crit Care Med 2004;32:2293–2299. [DOI] [PubMed] [Google Scholar]
- 21.Sawa T, Ohara M, Kurahashi K, Twining SS, Frank DW, Doroques DB, Long T, Gropper MA, Wiener-Kronish JP. In vitro cellular toxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect Immun 1998;66:3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato H, Feix JB, Frank DW. Identification of superoxide dismutase as a cofactor for the pseudomonas type III toxin, ExoU. Biochemistry 2006;45:10368–10375. [DOI] [PubMed] [Google Scholar]
- 23.Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 2005;288:L749–L760. [DOI] [PubMed] [Google Scholar]
- 24.Weiss YG, Bromberg Z, Raj N, Raphael J, Goloubinoff P, Ben-Neriah Y, Deutschman CS. Enhanced heat shock protein 70 expression alters proteasomal degradation of IkappaB kinase in experimental acute respiratory distress syndrome. Crit Care Med 2007;35:2128–2138. [DOI] [PubMed] [Google Scholar]
- 25.Kiang JG, Bowman PD, Wu BW, Hampton N, Kiang AG, Zhao B, Juang YT, Atkins JL, Tsokos GC. Geldanamycin treatment inhibits hemorrhage-induced increases in KLF6 and iNOS expression in unresuscitated mouse organs: role of inducible HSP70. J Appl Physiol 2004;97:564–569. [DOI] [PubMed] [Google Scholar]
- 26.Lau SS, Griffin TM, Mestril R. Protection against endotoxemia by HSP70 in rodent cardiomyocytes. Am J Physiol Heart Circ Physiol 2000;278:H1439–H1445. [DOI] [PubMed] [Google Scholar]
- 27.Lee KH, Hwang YH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. The heat-shock-induced suppression of the IkappaB/NF-kappaB cascade is due to inactivation of upstream regulators of IkappaBalpha through insolubilization. Exp Cell Res 2004;299:49–56. [DOI] [PubMed] [Google Scholar]
- 28.Lee KH, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Heat shock protein 70 negatively regulates the heat-shock-induced suppression of the IkappaB/NF-kappaB cascade by facilitating IkappaB kinase renaturation and blocking its further denaturation. Exp Cell Res 2005;307:276–284. [DOI] [PubMed] [Google Scholar]
- 29.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 2004;95:196–203. [DOI] [PubMed] [Google Scholar]
- 30.Sayner S, Stevens T. Soluble adenylate cyclase reveals the significance of compartmentalized cAMP on endothelial cell barrier function. Biochem Soc Trans 2006;34:492–494. [DOI] [PubMed] [Google Scholar]
- 31.Tamura M, Ajayi T, Allmond LR, Moriyama K, Wiener-Kronish JP, Sawa T. Lysophospholipase A activity of Pseudomonas aeruginosa type III secretory toxin ExoU. Biochem Biophys Res Commun 2004;316:323–331. [DOI] [PubMed] [Google Scholar]
- 32.Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, Frank DW, Martin TR, Wiener-Kronish JP. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest 1999;104:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 2006;86:279–367. [DOI] [PubMed] [Google Scholar]
- 34.Pittet JF, Lee H, Pespeni M, O'Mahony A, Roux J, Welch WJ. Stress-induced inhibition of the NF-kappaB signaling pathway results from the insolubilization of the IkappaB kinase complex following its dissociation from heat shock protein 90. J Immunol 2005;174:384–394. [DOI] [PubMed] [Google Scholar]
- 35.Hiratsuka M, Yano M, Mora BN, Nagahiro I, Cooper JD, Patterson GA. Heat shock pretreatment protects pulmonary isografts from subsequent ischemia-reperfusion injury. J Heart Lung Transplant 1998;17:1238–1246. [PubMed] [Google Scholar]
- 36.Hiratsuka M, Mora BN, Yano M, Mohanakumar T, Patterson GA. Gene transfer of heat shock protein 70 protects lung grafts from ischemia-reperfusion injury. Ann Thorac Surg 1999;67:1421–1427. [DOI] [PubMed] [Google Scholar]
- 37.Ito K, Ozasa H, Kojima N, Miura M, Iwa T, Senoo H, Horikawa S. Pharmacological preconditioning protects lung injury induced by intestinal ischemia/reperfusion in rat. Shock 2003;19:462–468. [DOI] [PubMed] [Google Scholar]
- 38.McCormick PH, Chen G, Tlerney S, Kelly CJ, Bouchier-Hayes DJ. Clinically relevant thermal preconditioning attenuates ischemia-reperfusion injury. J Surg Res 2003;109:24–30. [DOI] [PubMed] [Google Scholar]
- 39.Javadpour M, Kelly CJ, Chen G, Stokes K, Leahy A, Bouchier-Hayes DJ. Thermotolerance induces heat shock protein 72 expression and protects against ischaemia-reperfusion-induced lung injury. Br J Surg 1998;85:943–946. [DOI] [PubMed] [Google Scholar]
- 40.Pittet JF, Kudoh I, Wiener-Kronish JP. Endothelial exposure to Pseudomonas aeruginosa proteases increases the vulnerability of the alveolar epithelium to a second injury. Am J Respir Cell Mol Biol 1998;18:129–135. [DOI] [PubMed] [Google Scholar]
- 41.Kudoh I, Wiener-Kronish JP, Hashimoto S, Pittet JF, Frank D. Exoproduct secretions of Pseudomonas aeruginosa strains influence severity of alveolar epithelial injury. Am J Physiol 1994;267:L551–L556. [DOI] [PubMed] [Google Scholar]
- 42.Lee VT, Smith RS, Tummler B, Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection. Infect Immun 2005;73:1695–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finck-Barbancon V, Yahr TL, Frank DW. Identification and characterization of SpcU, a chaperone required for efficient secretion of the ExoU cytotoxin. J Bacteriol 1998;180:6224–6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 1998;95:13899–13904. [DOI] [PMC free article] [PubMed] [Google Scholar]