Abstract
Tobacco-related diseases are leading causes of death worldwide, and many are associated with expression of matrix metalloproteinase-1 (MMP-1). We have reported extracellular signal–regulated kinase (ERK)1/2-dependent induction of MMP-1 by cigarette smoke in lung epithelial cells. Our objectives were to define regions of the human MMP-1 promoter required for activation by smoke, to identify differences in responses of the 1G/2G −1607 polymorphic promoters to smoke, and to identify relevant transcription factors whose activity in airway epithelial cells is increased by smoke. The responses of deletion and mutant promoter constructs were measured in transfected cells during exposure to cigarette smoke extract (CSE). DNA oligonucleotide arrays were used to identify transcription factors activated after smoke exposure. CSE activated the MMP-1 promoter, and this induction was prevented by PD98059 blockade of ERK1/2 phosphorylation. Deletion studies revealed the distal 1kb promoter region (−4438 to −3280 upstream of the transcription start site) is essential for CSE induction of MMP-1, and confers activation of a minimal promoter. Studies of 1G and 2G MMP-1 polymorphic promoter variants revealed higher 2G allele basal and CSE-responsive activities than the 1G allele. Cotransfection, mithramycin, and electrophoretic mobility shift assay studies identified activating and repressive roles for Sp1 and PEA3 transcription factors, respectively. Oligonucleotide DNA arrays confirmed activation of Sp1 and PEA3 by CSE. These data demonstrate that the MMP-1 promoter is a direct target of cigarette smoke in lung epithelial cells. This characterization of a smoke response region in the distal MMP-1 promoter has implications for smoking-related diseases such as cancer, heart disease, and emphysema.
Keywords: metalloproteinase, emphysema, tobacco, chronic obstructive pulmonary disease, polymorphism
CLINICAL RELEVANCE
Smoke induces matrix metalloproteinase (MMP)-1 in lung epithelial cells and in human emphysema. Here we identify smoke-induced transcription factors, and the MMP-1 promoter as a target of tobacco smoke, improving our understanding of the biology of the smoker's lung.
The gene encoding matrix metalloproteinase 1 (MMP-1), an interstitial collagenase, is tightly regulated during embryonic development and in adult tissues (1). Several chronic adult diseases are characterized by elevated expression of MMP-1, including pulmonary emphysema (2) and lung cancer (3); these diseases are primarily associated with tobacco smoke exposure (4–6). However, the specific involvement of genetic and environmental factors contributing to MMP-1 expression in these diseases is unknown. Polymorphisms in either the coding or noncoding (regulatory) sequences of genes occur with greater than 1% frequency in a population. Polymorphisms in coding regions can affect the amino acid sequence of the protein product, resulting in altered catalytic activity, folding, binding, solubility, or post-translational modifications such as phosphorylation. Polymorphisms in the regulatory regions, particularly promoter elements, can enhance or disrupt gene transcription (7). Previous work has suggested an association between polymorphic MMP-1 promoter regions and lung cancer (3, 8–10), colorectal cancers (11–13), and melanoma invasion (14). Therefore, it is important to identify whether there are differential responses of polymorphic MMP-1 promoter sequences to tobacco smoke.
Studies in our laboratory have demonstrated that cigarette smoke can up-regulate MMP-1 expression in lung epithelial cells through a mitogen-activated-protein kinase (MAPK)-driven pathway (15). Increased expression of MMP-1 is seen in patients with emphysema and increased MMP-1 levels in the lung leads to lung destruction and emphysema in mice (2,16). Thus it is conceivable that polymorphic MMP-1 promoter elements targeted by smoke could modify pulmonary MMP-1 expression and emphysema risk. However, these elements have not been identified and no data exist on the response of the promoter in lung cells exposed to tobacco smoke. The sequence of the full-length human MMP-1 promoter has been reported, and several polymorphisms identified (17). Specifically, insertion of a guanine (G) at position −1607 creates an erythromatosis twenty-six (ETS) binding site adjacent to the activating protein-1 (AP-1) site at position −1602, and this genotype has been shown to correlate with declines in pulmonary function in smokers (18). The present study examined the response of the MMP-1 promoter to cigarette smoke and identified the distal 1-kb region as a region that is activated by smoke, required for induction of MMP-1 by smoke, and is a direct target of extracellular signal–regulated kinase (ERK)-1/2 signaling.
MATERIALS AND METHODS
Plasmids
Full-length and deletion constructs of the 1G and 2G MMP-1 promoters have been previously described (17). Full-length MMP-1 promoters with mutations in the distal PEA3 (polyoma enhancer activator 3) binding sites at −3838 and −3824 and with mutant AP-1 sites at −73 and −1602 were also used (19). The distal 1-kb region of the MMP-1 promoter was excised from a 9,268-bp full-length 2G MMP-1 plasmid as a 1,120-bp SacI/PstI fragment, purified (GeneClean, Carlsbad, CA), and ligated upstream of a truncated proximal −1700 2G MMP-1 promoter reporter. Vector ends were dephosphorylated and a 5:1 molar ratio of insert: vector was used in the ligation reaction. Products were confirmed by restriction digestion and DNA sequencing.
Cell Culture and Transient Transfection
Human small airway epithelial cells (SAECs) were from Clonetics (East Rutherford, NJ). Approximately 105 cells/well were seeded in 12-well culture plates (BD Falcon, San Jose, CA). The next day cells were transfected with 1 μg/well full-length or mutant human MMP-1 1G or 2G promoter constructs, diluted in 100 μl Optimem I (Invitrogen Carlsbad, CA), plus 50 ng of pRLTK Renilla luciferase plasmid. DNA amounts were equalized with pGL3 basic promoterless plasmid. The next day cells were treated with control media or media containing 5% cigarette smoke extract (CSE) for 24 hours. Addition of inhibitors was performed 1 hour before CSE treatment. Cells were lysed in Dual Luciferase Buffer (Promega, Madison, WI) and MMP-1 promoter firefly activity was read in a plate luminometer (Turner Designs, Sunnyvale, CA). Data are fold induction (CSE/Control) of at least three independent experiments read in triplicate, normalized to Renilla luciferase.
Electrophoretic Mobility Shift Assays
SAECs were seeded at 7.5 × 105 cells per T75 flask, and treated with control media or 5% CSE media at 80% confluence for 24 hours. After treatment, the cells were washed once and scraped in ice-cold PBS. Nuclear extracts were prepared according to established protocols (20). Briefly, pelleted cells were resuspended in cold, detergent-free, low salt Buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 0.5 μg/ml pepstatin A, 5 μg/ml trypsin-chemotrypsin inhibitor), followed by membrane lysis using 10% NP40 (IGEPAL). The suspension was pelleted and resuspended in chilled Buffer C (20 mM HEPES, 0.4M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 0.5 μg/ml pepstatin A, 5 μg/ml trypsinchemotrypsin inhibitor). After centrifugation, the protein content of the supernatant (nuclear protein) was determined using the Bradford assay (Bio-Rad, Temecula, CA). Double-stranded consensus (5′-ATT CGA TCG GGG CGG GGC GAG C-3′) and mutant (5′-ATT CGA TCG GTT CGG GGC GAG C-3′) oligonucleotide probes for the Sp1 transcription factor were from Santa Cruz Biotechnologies (Santa Cruz, CA). Probes (20 ng in 1 μl) were end-labeled with γ-32P-ATP using T4 polynucleotide kinase, 1× forward reaction buffer in a 20-μl volume, at 37°C for 1 hour. The reaction was stopped by placing the tubes on ice and raising the volume to 100 μl with H2O (final DNA concentration = 0.2 ng/μl). Unincorporated ATP was removed by centrifugation (750 × g, 2 min) through ProbeQuant G-50 Micro Columns (Catalog # 27–5335–01; Amersham, Piscataway, NJ). One microliter of probe was incubated with 5 to 10 μg nuclear extract in a 10-μl binding reaction for 1 hour at 37°C with 2 μl binding buffer (5% glycerol, 1 mM EDTA, 100 mM KCl, 5 mM MgCl2, 10 mM Tris-HCl pH 7.8, 1 μl sheared herring sperm DNA). Nuclear protein-oligo complexes were loaded in 5% polyacrylamide gels and electrophoresed in TBE buffer at 120 V for 1 hour. Gels were pre-run at 175 V until the current reached 30 mA. Gels were dried for 2 hours at 80°C and exposed to X-ray film.
DNA Array Screening of Transcription Factor Activities Induced by CSE
To identify transcription factors activated in primary human lung epithelial cells during smoke exposure, we analyzed transcription factor expression in SAEC nuclear extracts using transcription factor oligonucleotide arrays (Array I; Panomics, Fremont, CA). The manufacturer's instructions were followed (21). Briefly, nuclear extracts isolated as above were incubated with biotin-labeled DNA binding probes to allow the formation of nuclear protein–DNA complexes. After this incubation, the uncomplexed probes were separated by spin column centrifugation and the DNA–protein complexes eluted and denatured to liberate free probes. The free probes were then hybridized to nitrocellulose membranes (arrays) containing spots of transcription factor–binding sequences. Probes for individual transcription factors that bound to the arrays were revealed using streptavidin binding, washing, and chemiluminescence detection. Two array sets were hybridized with biotinylated probes precipitated with nuclear lysates from two independent smoke exposure experiments. Average hybridization spot intensities were quantified using Kodak 2.9 image analysis software (Kodak, Rochester, NY).
Immunoblotting
Immunoblotting was performed as described (15). Briefly, nuclear extracts prepared as above were separated on 12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad). Membranes were placed in blocking buffer (5% nonfat milk in Tris-Buffered-SalineTween-20 [TBST]) for 1 hour, followed by overnight incubation at 4°C with rabbit polyclonal nucleophosmin antibody (ab15440; Abcam, Cambridge, MA [kind gift of laboratory of Ira Tabas]) or rabbit polyclonal Sp1 antibody (sc-14027; Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:1,000 in blocking buffer. The next day the membranes were washed three times for 20 minutes in TBST, followed by incubation at room temperature for 1 hour with goat anti-rabbit horseradish peroxidase–conjugated secondary antibody (sc-2004; Santa Cruz Biotechnology) at 1:10,000 in blocking buffer. Bands were detected using an enhanced chemiluminescence reagent (Pierce, Rockford, IL).
Bioinformatic Identification of Transcription Factor Binding Sites in the Distal MMP-1 Promoter
The TRANSFAC software program employing Transcription Element Search Software (TESS, http://www.cbil.upenn.edu/tess/) was used to locate transcription factor–binding sites in the promoter of MMP-1 (Genbank Accession Number AF023338). Distal sequences from −4438 bp to −2844 bp upstream of the MMP-1 start site were analyzed in the search string.
Statistical Analysis
Multiple comparisons were made using ANOVA with Dunnett's multiple comparison post tests using Prism software (GraphPad Software, San Diego, CA). All other statistics, including mean determination and SD, were performed using two-tailed Student's t test in Excel. A value of P < 0.05 was considered significant. All data are from at least three independent experiments.
RESULTS
Induction of MMP-1 Promoter Activity by Cigarette Smoke
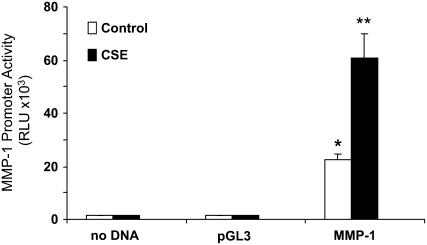
Cigarette smoke increases MMP-1 mRNA levels, and this induction is ERK1/2-dependent (15). We therefore hypothesized that the MMP-1 promoter could be activated directly by cigarette smoke and that this activation would require ERK1/2 activity. To determine promoter activity in SAECs during smoke exposure, cells were transfected with a reporter plasmid containing the luciferase gene driven by the full-length human MMP-1 promoter (17). Primary SAECs demonstrated baseline promoter activity, which was increased on average 3-fold by 5% CSE at 24 hours (P < 0.05; Figure 1). These data suggest that the MMP-1 promoter is a target of cigarette smoke in cultured human airway epithelial cells, and are consistent with our previous finding that CSE increases MMP-1 transcription (15).
Figure 1.
Matrix metalloproteinase (MMP)-1 promoter activity is enhanced by cigarette smoke. Primary human small airway epithelial cells (SAECs) were transfected with 1 μg of luciferase reporter plasmid containing either no promoter (pGL3) or the 4372-bp MMP-1 promoter (MMP-1). Forty-eight hours later, the cells were treated with SAGM (control media) or SAGM containing 5% cigarette smoke extract (CSE). Twenty-four hours later, the cells were lysed and the luciferase activity determined. Transfections and treatments were performed in triplicate. *P < 0.05 versus control pGL3. **P < 0.01 versus control MMP-1.
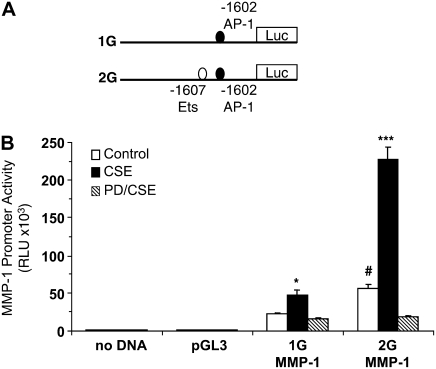
Induction of the 1G and 2G MMP-1 Promoters by CSE Requires ERK1/2 MAPK Activity
Although the 2G promoter has demonstrated higher activity levels than the 1G allele, increased MMP-1 expression has been observed in the absence of the 2G polymorphism (22). We compared the activity of both the 1G and 2G variants in SAECs at baseline and during CSE exposure. The two MMP-1 full-length promoter expression plasmids and proximal ETS and AP1 transcription factor–binding sites are shown in Figure 2A. As shown in Figure 2B, the 2G MMP-1 promoter has 2-fold higher expression at baseline than the 1G construct (open bars, P < 0.05). Both the 1G and 2G promoters were activated by CSE (solid bars). The 2G promoter demonstrated greater sensitivity to induction by CSE (average fold increase over baseline: 2.0-fold for 1G; 3.5-fold for 2G; P < 0.05). We next examined the role of ERK MAPK. Blockade of ERK1/2 kinase activation using PD98059 (10 μM) prevented induction of the both promoters by CSE (Figure 2B, hatched bars). Together these data demonstrate that induction of MMP-1 by smoke is regulated at the level of the promoter and that ERK1/2 MAPK signaling is required. Relevant to our understanding of increased MMP-1 expression in emphysematous lung tissue of chronic smokers (2), these increases take place in small airway epithelial cells. Because both the 1G and 2G promoters fail to be activated in the presence of PD98059, it is likely that there are ERK-targeted transcription factors in addition to AP-1 and ETS that are regulating MMP-1 gene expression.
Figure 2.
The G-1607GG SNP increases the response of the MMP-1 promoter to CSE. (A) Diagram of the MMP-1 promoter portion of the full-length 1G (deletion) and 2G (insertion) MMP-1 promoter constructs (vector backbone not shown). (B) Activation of the MMP-1 promoter is extracellular signal–regulated kinase (ERK)1/2 dependent. Lung epithelial cells were transfected with full-length MMP-1 promoter, and the response was measured as Relative Light Units (RLU) under control (open bars) or CSE (solid bars) conditions after 24 hours. Pretreatment with PD98059 (hatched bars) before CSE prevents the induction of both promoters, suggesting that cigarette smoke activates ERK1/2 transcription factor targets, which bind to regions other than the −1607 polymorphic site. *P < 0.05 and ***P < 0.001 compared with control; #P < 0.05 2G versus 1G control activity.
Proximal AP-1 Elements Are Required for Baseline MMP-1 Promoter Activity in SAEC
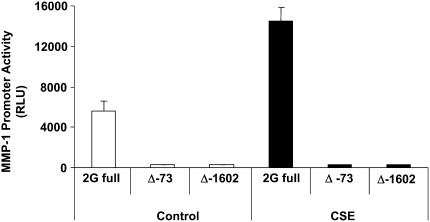
Although the 1G and 2G polymorphic sequences vary by the presence of an ETS oncogene–binding site, both sequences have an AP-1 site at −1602. To better understand the role of proximal AP-1 elements in the response of the MMP-1 promoter to CSE, we used AP-1 mutants to examine the requirement of the −1602 site and a more proximal −73 site for CSE-mediated induction. Construction of these plasmids, in which either the −73 or −1602 AP-1 site is deleted, has been described (17). Both AP-1 mutants lacked activity at baseline compared with the wild-type MMP-1 promoter (Figure 3, open bars). Not surprisingly, neither AP-1 mutant was activated by CSE (Figure 3, solid bars). Together the data indicate that these two proximal AP-1 sites are necessary for activity of the MMP-1 promoter.
Figure 3.
Proximal binding sites for the transcription factor activator protein (AP)-1 are required for baseline and CSE-mediated activities of the MMP-1 promoter. Deletion (Δ) of either the −73 or the 1,602 AP-1 site results in loss of promoter activity, demonstrating that these sites are required for basal expression and CSE-mediated induction of MMP-1. Both mutants fail to be activated by CSE. “2G” indicates full-length 2G MMP-1 promoter.
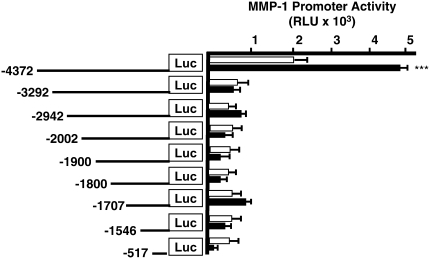
Identifying Cigarette Smoke–Responsive Regions in the MMP-1 Promoter Using Deletion Analysis
To identify the region in the MMP-1 promoter containing a putative cigarette smoke response region, we performed transfection studies using a series of 2G promoter deletion constructs (17, 23). Although several of these constructs had modest activity at baseline, only the full-length 2G construct was consistently and significantly activated by CSE treatment (P < 0.001; Figure 4). These data suggested two potential mechanisms for CSE-mediated MMP-1 expression: (1) induction of MMP-1 by smoke requires the full-length 4.4-kb promoter sequence, or (2) the distal 1 kb of the promoter contains an essential smoke-responsive region, since loss of this region prevents activation by CSE (Figure 4). No difference was detected in the basal or CSE-mediated activation of the −1546 and −1707 constructs, suggesting that the proximal 2G (ETS) element at 1,607 is not sufficient for MMP-1 induction by CSE.
Figure 4.
The distal 1kb of the MMP-1 promoter is required for induction by CSE. MMP1 promoter deletion constructs used to identify regulatory elements involved in the induction by smoke. Activity of the deletion constructs in SAECs before (open bars) and after (solid bars) 24 hours of exposure to 5% CSE. The full-length 2G MMP-1 promoter is activated by CSE treatment. All other proximal deletion constructs are not induced significantly by CSE. These data suggest that induction by smoke requires the distal 1 kb region of the promoter, and that the 2G (ETS) element alone is not sufficient for induction by CSE. ***P < 0.001 versus control activity. Data shown are RLU.
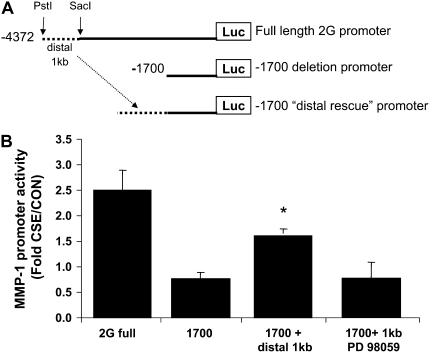
Sufficiency of the Distal 1 kb to Confer Smoke Responsiveness
As a result of the deletion studies, which revealed impaired CSE-responsiveness of the truncated promoters compared with the −4372 full-length promoter, we hypothesized that the distal 1 kb of the MMP-1 promoter (−3.2kb to −4.2kb upstream of the transcription start site) was required for induction by smoke. To determine whether this distal sequence could function in a different location along the promoter, we subcloned this 1.1-kb distal region to just upstream of the −1707 deletion construct (Figure 5A), creating a new construct that we termed “−1707/distal 1 kb”. The −1707 deletion construct was not consistently nor significantly activated by CSE. However, the 1707/distal 1 kb plasmid demonstrated 2.2-fold higher activity than the −1707 construct alone, demonstrating that the distal promoter can confer CSE-responsiveness in a more proximal site. These data also suggest that the distal 1 kb contains a region that is activated by CSE. However, the promoter activity of the distal construct was not as high as that of the full-length 2G wild-type sequence (Figure 5B), suggesting that although the distal region is indeed activated by CSE, this sequence may require additional elements between −3200 bp and −1707 bp for maximal induction. We next examined the role of ERK MAPK. Inhibition of ERK1/2 activity with PD98059 prevented the induction of the −1707 distal 1 kb construct, implying that this distal region is a target of ERK1/2 signaling (Figure 5B, far right bar).
Figure 5.
The distal 1 kb of the MMP-1 promoter is activated by cigarette smoke. (A) Diagram of the cloning strategy used to generate the −1700/distal 1 kb plasmid. The distal 1 kb region (dashed line) of the MMP-1 promoter, containing putative cigarette smoke response elements, was removed from the 2G full-length plasmid using SacI/PstI restriction digestion. (B) Transfection of each plasmid revealed that the distal 1 kb confers CSE-responsiveness to the truncated −1700 promoter. This induction is blocked by PD98059. *P < 0.05 compared with −1700. Data shown are fold induction by CSE normalized to control activity.
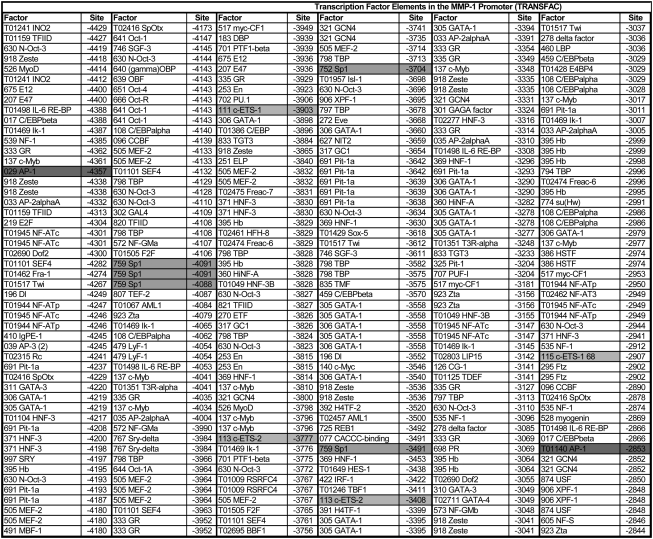
Mapping Transcription Factor–Binding Sites in the MMP-1 Promoter
To identify relevant transcription factors involved in the regulation of MMP-1 expression during cigarette smoke exposure, we mapped putative transcription factor–binding sites in the MMP-1 promoter sequence (Accession Number AF023338) using TESS software (Figure 6). We focused our search to the distal promoter, as this region was activated by CSE and could be activated by CSE even when moved to a more proximal (−1707) region of the promoter. The results of these analyses identified a number of transcription factor–binding sites not previously reported in the MMP-1 promoter, such as GATA-1 and Pit-1. In addition, numerous binding sites for AP-1, AP-2, ETS-1 and -2, Sp1, C/EBP, and Fra-1 were found at several locations in this region (Figure 6).
Figure 6.
Transcription factor–binding sites in the distal MMP-1 promoter. The full-length (4,438-bp) human MMP-1 promoter sequence (AF023338) was analyzed for predicted transcription factor–binding sites using TESS-TRANSFAC software. The table shows only the distal 2 kb. Binding sites of interest are highlighted, in particular sites for those factors found to be up-regulated by CSE using the DNA arrays (AP-1, Ets, Sp1). In the “Factor” column, the number in front of the factor name is a unique factor identifier assigned by the software. In the “Site” column, the number indicates the position upstream of the TSS (transcription start site). Factors are sorted according to their position along the promoter in the 5′ to 3′ direction. Shading indicates transcription factors examined in the current study.
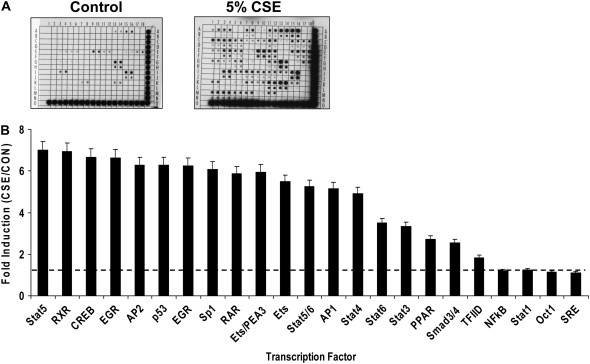
CSE Activates Relevant Transcription Factors in Primary Human Lung Epithelial Cells
To identify transcription factors activated in primary human lung epithelial cells during smoke exposure, we analyzed transcription factor expression in SAEC nuclear extracts using transcription factor oligonucleotide arrays (Panomics). Global transcription factor expression/DNA binding analyses using SAEC nuclear extracts were performed after 24 hours of treatment with media or 5% CSE. Densitometric analyses of the DNA arrays demonstrated that tobacco smoke induced the expression and DNA binding of numerous transcription factors (Figure 7A). The most potently induced factors are graphed in rank order (Figure 7B). Only slight increases were detected in C/EBP (data not shown). Interestingly, although previous reports have suggested a role for NF-κB in the induction of MMP-1 to redox stimuli, we did not detect a change in nuclear NF-κB levels/DNA binding after 24 hours of CSE treatment. However, examination of SAEC lysates after only 2 hours of exposure revealed that NF-κB was detectable at higher levels than cells treated with control media (data not shown).
Figure 7.
Identification of transcription factors induced by CSE. (A) Representative images of a DNA array pair, showing membranes from Control (left) and 5% CSE–treated cells (right). DNA consensus oligonucleotides are spotted by the manufacturer (Panomics) at a minimum of two locations on the array. Each spot represents a transcription factor. The higher number and intensity of spots on the CSE array indicates that CSE induces expression and/or the DNA-binding activity of numerous transcription factors. (B) Histogram of the CSE-mediated fold-induction of selected transcription factors that were induced by CSE, as determined by comparative densitometric analysis of DNA array pairs. The dashed line indicates the level at which no induction occurred (average ratio of spot intensities of CSE arrays/CON arrays = 1).
We next combined the finding of transcription factors having distal MMP-1 promoter–binding sites (Figure 6) with the finding of factors whose DNA binding was significantly activated by CSE (Figures 7 and 7B) to identify Sp1 and Ets/PEA3 as transcription factors most likely to be involved in the activation of MMP-1 expression by CSE. In addition, previous reports demonstrate that ERK MAPK can activate/phosphorylate these factors (24, 25).
Roles of ETS/PEA3 in MMP-1 Promoter Activation
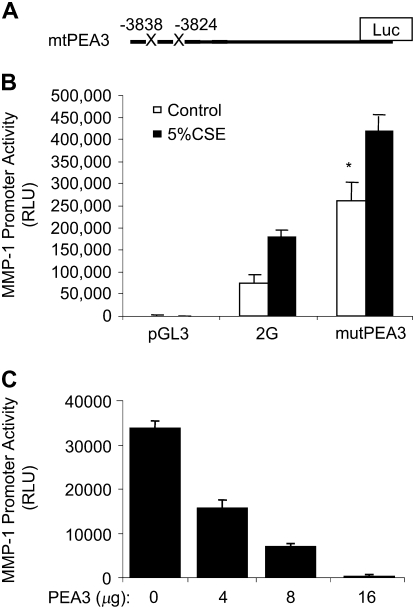
To identify the specific elements within this distal 1-kb region that mediate MMP-1 induction by CSE, we analyzed the activity of PEA3 mutant promoters. There are at least two ETS/PEA3 binding sites in the distal 1.1-kb sequence of the MMP-1 promoter (Figure 6 and Ref. 17). We transfected SAECs with a 2G plasmid containing mutant PEA3-binding sites in the distal region (Figure 8A). Surprisingly, this mutant MMP-1 promoter demonstrated 3.2-fold higher baseline activity than the wild-type promoter (Figure 8A, control [open bars], P < 0.01). In addition, the mutant PEA3 construct was induced by CSE (solid bars). These data suggest that these PEA3 sites may repress MMP-1 basal expression, since their destruction results in higher promoter activity compared with the wild-type promoter at baseline. Comparison of the fold activation of wild-type and mutant promoters by CSE revealed no statistical difference in induction by CSE (2.1-fold induction of the wild-type promoter versus 1.8-fold induction of the mutant promoter), suggesting that these two PEA3 sites do not contribute specifically to CSE-mediated induction. It is conceivable that the high baseline activity of the mutant promoter confounds our ability to detect further activation by CSE. To better define the direct effect of the PEA3 transcription factor on induction of the MMP-1 promoter by CSE, we co-transfected SAECs with the 2G wild-type promoter in the presence of a PEA3 overexpression construct (26). A dose-dependent reduction in CSE-mediated promoter activity was detected with PEA3 expression (Figure 8B). DNA amounts were held constant in all groups. Together the results from the mutation and overexpression studies suggest that PEA3 represses CSE-mediated MMP-1 promoter activation and that PEA3 sites in the distal MMP-1 promoter can repress basal MMP-1 promoter activity.
Figure 8.
Mutation of distal PEA3 sites enhances 2G promoter activity. (A) Diagram of the PEA3 mutant MMP-1 promoter construct (mutPEA3), with the mutated distal PEA3 sites marked with an “X”. The proximal Ets site at −1607 was not changed. (B) Mutation of two of these sites in the distal 1 kb enhances basal and CSE-inducible activity, suggesting that these sites may repress transcription. (C) PEA3 overexpression inhibits MMP-1 promoter induction by CSE. Inhibition is dose dependent. SAECs were co-transfected with the 2G and pcDNA3-PEA3 overexpression plasmids. DNA amounts were kept equal among all groups by the addition of pGL3 DNA. All data are CSE data.*P < 0.05. The amounts of co-transfected DNA are higher than typical transfection studies, but are in the range of the amounts used by the providers of the PEA3 plasmid (Mien-Chie Hung, M.D. Anderson Cancer Center).
Inhibition of Sp1 Transcription Factor Binding Prevents Induction of the MMP-1 Promoter by CSE
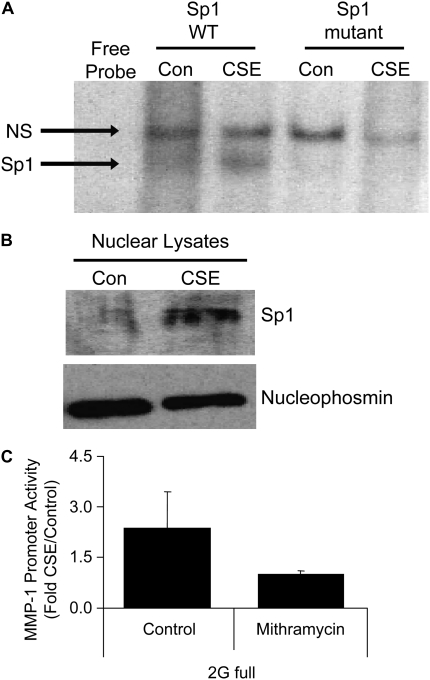
Various transcription factors have been shown to activate the MMP-1 promoter, often in a cell type– and treatment-specific manner (17). Our promoter mapping analysis revealed numerous cis elements present within the distal 1 kb of the MMP-1 promoter. These sites are predicted to mediate the transcription control of MMP-1 during cigarette smoke exposure. However, although recent studies have examined transcription factor activation in lung cancer cells (27), very little is known about the repertoire of MMP-1–inducing transcription factors activated by cigarette smoke in primary lung epithelial cells. Failure of the 1G and 2G promoters to be activated in the presence of PD98059 demonstrates that the ERK pathway is required. The data also reveal that ERK activity is necessary for basal activity of both promoters. Our transcription factor arrays and TRANSFAC studies suggested that Sp1 was a nuclear protein that not only was induced by CSE exposure (Figure 7), but also contained binding sites in the distal 1 kb of the MMP1 promoter (Figure 6); these two characteristics are essential for direct involvement of a transcription factor in MMP-1 induction by CSE. Sp1 is a phosphorylation target of ERK1/2. To determine whether Sp1 DNA–binding activity was increased by CSE, electrophoretic mobility shift assay studies were performed. Nuclear extracts from SAECs treated with 5% CSE for 24 hours demonstrated Sp1 DNA binding that was not present in nuclear extracts from control SAECs (Figure 9A). Sp1-binding activity was not detected in the presence of 100-fold excess unlabeled Sp1 oligonucleotide. Further, nuclear extracts did not bind a mutant Sp1 oligonucleotide, demonstrating the specificity of the binding reaction. In addition, immunoblots of lysates from SAECs treated with 5% CSE revealed increased nuclear expression levels of Sp1 protein, compared with lysates from SAECs treated with media alone (Figure 9B). Mithramycin A is a hypocalcemic antibiotic shown in previous studies to inhibit binding of Sp1 to its G-C–rich DNA sequences (28, 29) To determine the requirement of Sp1 in the induction of MMP-1 promoter by CSE, we treated SAECs that had been transfected with the 2G promoter with 5 μM mithramycin A for 30 minutes before 5% CSE exposure (Figure 9C). Mithramycin A completely prevented induction of the MMP-1 promoter by CSE, supporting a role for the DNA binding activity of Sp1 as a requirement for CSE-mediated activation of the MMP-1 promoter.
Figure 9.
The transcription factor Sp1, an ERK1/2 nuclear target, is induced by CSE and is required for CSE-mediated MMP-1 promoter activation. (A) Electrophoretic mobility shift assay using nuclear lysates from control or CSE-treated lung epithelial cells. No specific band is detected in the Sp1 mutant oligonucleotide. Bottom panel, immunoblot for nucleophosmin. (B) Sp1 immunoblot of nuclear lysates from SAECs treated with 5% CSE (top panel). Nuclear levels of the protein nucleophosmin (bottom panel) is shown as a control. (C) CSE-mediated activation of the full-length 2G MMP-1 promoter under control conditions or after pretreatment with mithramycin, a chemical Sp1 DNA-binding inhibitor.
DISCUSSION
Numerous reports have identified MMP-1 expression in lung tissue of patients with chronic obstructive pulmonary disease (COPD) (2, 30). In addition, our group has demonstrated that MMP-1 leads to lung destruction in an animal model of emphysema and that MMP-1 expression is induced by cigarette smoke in the resident lung epithelial cell. This induction occurs in a MAPK-dependent fashion. In the present report, we extend our previous findings by examining the response of various MMP-1 promoter constructs to CSE in airway epithelial cells to identify the distal 1 kb region of the MMP-1 promoter as responsive to CSE. Deletion and mutant promoter experiments demonstrated that the distal 1 kb region (−4438 to −3280) of the MMP-1 promoter is required for induction of MMP1 by cigarette smoke in lung epithelial cells. Although additional cis elements not yet identified may be important in the regulation of cigarette smoke induction of MMP-1 in vivo, PEA3 elements and Sp1 DNA binding appear to be important factors in the response of MMP-1 to tobacco smoke. The epithelial cells in our study express various transcription factors known to activate the MMP-1 promoter, many of which have been shown to mediate MMP-1 expression in response to growth factors in vitro (31, 32). With regard to CSE-mediated induction, several important conclusions about MMP-1 transcription may be made. In particular, the two MMP-1 alleles share several regulatory features. First, blockade of ERK1/2 MAPK using PD98059 (10 μM) prevented induction of the 1G and 2G promoters by CSE (Figure 2), indicating that classical MAPK signaling controls transcription of both alleles. Second, Sp1 blockade prevented promoter activation by CSE (Figure 9C). Although we used mithramycin as an inhibitor of Sp1, it is conceivable that secondary events are involved. Future studies using mutant Sp1 promoter constructs will confirm the role of Sp1 in MMP-1 induction by tobacco smoke.
Classical DNA–protein precipitation studies have suggested that it is the dynamic formation of protein complexes at specific promoter sequences that mediate discrete differences to stimuli (33). These studies focus on the transcription factors, coactivators, and enzymes that bind and modify the chromatin, leading to enhanced RNA polymerase II access. Our identification of Sp1 and ETS factors as likely components of the MMP-1 transcriptional activation machinery will no doubt lead to additional studies of accessory nuclear factors mediating these polymorphic effects. For example, a recent study examined the role for chromatin-modifying enzymes in MMP-1 expression (34). Specifically, HDAC inhibitors blocked MMP-1 and MMP-13 induction by proinflammatory cytokines at the mRNA level.
Previous reports have identified a role for NF-κB in the induction of MMP-1 to various stimuli (35–40). Indeed, NF-κB has been shown to be activated in the lungs of mice exposed to cigarette smoke (41). Further, a recent study revealed that early activation of NF-κB by tobacco smoke can be abrogated by treatment with an ERK inhibitor (27). However, several recent reports detected decreased nuclear-associated NF-κB subunit p65 in alveolar macrophages isolated from smoke-exposed mice (42–44). While we did not detect an increase in NF-κB DNA binding after 24 hours of exposure to CSE, this does not exclude a role for NF-κB in the induction of remodeling-associated genes after cigarette exposure. Previous studies looked at earlier/transient changes in NF-κB after tobacco smoke exposure, and thus we may have been unable to detect NF-κB changes at 24 hours. The detection of early (but not later) increases in levels of NF-κB does not exclude a role for NF-κB in the response of lung epithelia to smoke. The issue is complicated by reports that certain components of tobacco smoke can inhibit NF-κB binding to DNA (45), NF-κB signaling in primate lungs (46), and NF-κB activation (44). Several studies detected decreased nuclear NF-κB subunit p65 in alveolar macrophages isolated from smoke-exposed mice (42–44). Interestingly, the recent study revealing that activation of NF-κB by tobacco smoke can be abrogated by treatment with an ERK inhibitor (27) suggests that any potential regulation of the MMP-1 promoter by NF-κB is downstream of MAPK activation. The role of NF-κB in the short-term and long-term response of the lung to tobacco smoke injury, and its role in MMP-1 regulation, is certainly complex and requires further study. These results reveal the complexity of the signaling pathways activated by tobacco smoke.
Elevated MMP-1 promoter activity has been detected in cells and clinical samples that possess at least one allele containing the 2G insertion sequence (7, 19, 23, 47–51). Studies examining these MMP-1 promoter polymorphisms in disease have looked at human cancers and found an association between the presence of the 2G allele and increased risk for lung cancer (52), colorectal cancer (11–13), and metastatic melanoma (19). Relevant to our data on MMP-1 and CSE, cigarette smoking is the cause of numerous cancers (53), is estimated to be the cause of 30% of all cancer-related deaths in the United States (54), and directly reduces pulmonary function (55).
In addition, a population study examined the association of the 2G promoter polymorphism to lung function in a cohort of smokers (18), and another study looked at patients with COPD (56). The former study concluded that the presence of the 2G allele in smokers was associated with a more rapid decline in lung function. Interestingly, the association was still valid after adjusting for smoking and age. Our study suggests that the presence of the 2G allele contributes to increased basal MMP-1 expression in cells and tissues, and that tobacco smoke exposure augments this expression. Future clinical studies will need to collectively compare promoter polymorphisms, lung function, MMP-1 expression, and smoking status to better understand these relationships.
The present data support the hypothesis that the regulatory factors ETS and Sp1 in lung epithelial cells modify the expression of MMP-1 during smoke exposure and that persistent activation of the distal MMP-1 promoter by chronic smoking may contribute to diseases such as emphysema. Here for the first time we identify the transcription factors activated by CSE in human airway epithelial cells. We have shown that in addition to the known site of transcriptional divergence (−1607), distal elements of the promoter and specific transcription factors are specifically involved in controlling MMP-1 transcription. We would predict that persistent activation of the distal MMP-1 promoter element by decades of smoking contributes to elevated MMP-1 expression detected in emphysematous lung tissue (2, 30). Although it is possible that additional cis elements, transcription factors, and accessory proteins may be important in the regulation of MMP-1 in vivo, ETS/PEA3 and Sp1 factors are likely involved in the response of MMP-1 to tobacco smoke. In addition, the identification of a cigarette smoke–responsive promoter region suggests a mechanism by which other MMP proteases can be up-regulated in smoke-associated pathologies and provides a unifying link to the destructive processes observed in these diseases.
Acknowledgments
The PEA3 overexpression plasmid was a generous gift from Dr. Mien-Chie Hung (The University of Texas M.D. Anderson Cancer Center, Houston, TX). Nucleophosmin antibody was a kind gift of the laboratory of Ira Tabas (Columbia University College of Physicians and Surgeons).
This work was supported by the Alpha-1 Foundation, the Flight Attendant Medical Research Institute, and NIH RO1 HL086936 (all to J.M.D.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0310OC on July 10, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol 2005;37:283–288. [DOI] [PubMed] [Google Scholar]
- 2.Imai K, Dalal S, Chen E, Downey R, Schulman L, Ginsburg M, D'Armiento J. Human collagenase (Matrix Metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med 2001;163:786–791. [DOI] [PubMed] [Google Scholar]
- 3.Biondi ML, Turri O, Leviti S, Seminati R, Cecchini F, Bernini M, Ghilardi G, Guagnellini E. MMP1 and MMP3 polymorphisms in promoter regions and cancer. Clin Chem 2000;46:2023–2024. [PubMed] [Google Scholar]
- 4.Osann, KE. Lung cancer in women: the importance of smoking, family history of cancer, and medical history of respiratory disease. Cancer Res 1991;51:4893–4897. [PubMed] [Google Scholar]
- 5.Bronnum-Hansen H, Juel K. Estimating mortality due to cigarette smoking: two methods, same result. Epidemiology 2000;11:422–426. [DOI] [PubMed] [Google Scholar]
- 6.Ezzati M, Lopez AD. Regional, disease specific patterns of smoking-attributable mortality in 2000. Tob Control 2004;13:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol 2000;19:623–629. [DOI] [PubMed] [Google Scholar]
- 8.Su L, Zhou W, Park S, Wain JC, Lynch TJ, Liu G, Christiani DC. Matrix metalloproteinase-1 promoter polymorphism and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2005;14:567–570. [DOI] [PubMed] [Google Scholar]
- 9.Su L, Zhou W, Asomaning K, Lin X, Wain JC, Lynch TJ, Liu G, Christiani DC. Genotypes and haplotypes of matrix metalloproteinase 1, 3 and 12 genes and the risk of lung cancer. Carcinogenesis 2006;27:1024–1029. [DOI] [PubMed] [Google Scholar]
- 10.Fang S, Jin X, Wang R, Li Y, Guo W, Wang N, Wang Y, Wen D, Wei L, Zhang J. Polymorphisms in the MMP1 and MMP3 promoter and non-small cell lung carcinoma in North China. Carcinogenesis 2005;26:481–486. [DOI] [PubMed] [Google Scholar]
- 11.Hinoda Y, Okayama N, Takano N, Fujimura K, Suehiro Y, Hamanaka Y, Hazama S, Kitamura Y, Kamatani N, Oka M. Association of functional polymorphisms of matrix metalloproteinase (MMP)-1 and MMP-3 genes with colorectal cancer. Int J Cancer 2002;102:526–529. [DOI] [PubMed] [Google Scholar]
- 12.Zinzindohoue F, Lecomte T, Ferraz JM, Houllier AM, Cugnenc PH, Berger A, Blons H, Laurent-Puig P. Prognostic significance of MMP-1 and MMP-3 functional promoter polymorphisms in colorectal cancer. Clin Cancer Res 2005;11:594–599. [PubMed] [Google Scholar]
- 13.Ghilardi G, Biondi ML, Mangoni J, Leviti S, DeMonti M, Guagnellini E, Scorza R. Matrix metalloproteinase-1 promoter polymorphism 1G/2G is correlated with colorectal cancer invasiveness. Clin Cancer Res 2001;7:2344–2346. [PubMed] [Google Scholar]
- 14.Noll WW, Belloni DR, Rutter JL, Storm CA, Schned AR, Titus-Ernstoff L, Ernstoff MS, Brinckerhoff CE. Loss of heterozygosity on chromosome 11q22–23 in melanoma is associated with retention of the insertion polymorphism in the matrix metalloproteinase-1 promoter. Am J Pathol 2001;158:691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer B, Kolesnikova N, Sonett J, D'Armiento J. Extracellular regulated kinase/mitogen activated protein kinase is up-regulated in pulmonary emphysema and mediates matrix metalloproteinase-1 induction by cigarette smoke. J Biol Chem 2004;279:17690–17696. [DOI] [PubMed] [Google Scholar]
- 16.D'Armiento J, Dalal SS, Okada Y, Berg RA, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell 1992;71:955–961. [DOI] [PubMed] [Google Scholar]
- 17.Rutter JL, Mitchell TI, Buttice G, Meyers J, Gusella JF, Ozelius LJ, Brinckerhoff CE. A single nucleotide polymorphism in the matrix metalloproteinase-1 promoter creates an Ets binding site and augments transcription. Cancer Res 1998;58:5321–5325. [PubMed] [Google Scholar]
- 18.Joos L, He JQ, Shepherdson MB, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Hum Mol Genet 2002;11:569–576. [DOI] [PubMed] [Google Scholar]
- 19.Tower GB, Coon CC, Benbow U, Vincenti MP, Brinckerhoff CE. Erk 1/2 differentially regulates the expression from the 1G/2G single nucleotide polymorphism in the MMP-1 promoter in melanoma cells. Biochim Biophys Acta 2002;1586:265–274. [DOI] [PubMed] [Google Scholar]
- 20.Mochida-Nishimura K, Surewicz K, Cross JV, Hejal R, Templeton D, Rich EA, Toossi Z. Differential activation of MAP kinase signaling pathways and nuclear factor-kappaB in bronchoalveolar cells of smokers and nonsmokers. Mol Med 2001;7:177–185. [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X, Norman M, Li X. Use of an array technology for profiling and comparing transcription factors activated by TNFalpha and PMA in HeLa cells. Biochim Biophys Acta 2003;1642:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Benbow U, Tower GB, Wyatt CA, Buttice G, Brinckerhoff CE. High levels of MMP-1 expression in the absence of the 2G single nucleotide polymorphism is mediated by p38 and ERK1/2 mitogen-activated protein kinases in VMM5 melanoma cells. J Cell Biochem 2002;86:307–319. [DOI] [PubMed] [Google Scholar]
- 23.Tower GB, Coon CI, Brinckerhoff CE. The 2G single nucleotide polymorphism (SNP) in the MMP-1 promoter contributes to high levels of MMP-1 transcription in MCF-7/ADR breast cancer cells. Breast Cancer Res Treat 2003;82:75–82. [DOI] [PubMed] [Google Scholar]
- 24.Chupreta S, Du M, Todisco A, Merchant JL. EGF stimulates gastrin promoter through activation of Sp1 kinase activity. Am J Physiol Cell Physiol 2000;278:C697–C708. [DOI] [PubMed] [Google Scholar]
- 25.Baker DA, Mille-Baker B, Wainwright SM, Ish-Horowicz D, Dibb NJ. Mae mediates MAP kinase phosphorylation of Ets transcription factors in Drosophila. Nature 2001;411:330–334. [DOI] [PubMed] [Google Scholar]
- 26.Xing X, Wang SC, Xia W, Zou Y, Shao R, Kwong KY, Yu Z, Zhang S, Miller S, Huang L, Hung MC. The ets protein PEA3 suppresses HER-2/neu overexpression and inhibits tumorigenesis. Nat Med 2000;6:189–195. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Harper R, Barchowsky A, Di YP. Identification of multiple MAPK-mediated transcription factors regulated by tobacco smoke in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2007;293:L480–L490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller DM, Polansky DA, Thomas SD, Ray R, Campbell VW, Sanchez J, Koller CA. Mithramycin selectively inhibits transcription of G-C containing DNA. Am J Med Sci 1987;294:388–394. [DOI] [PubMed] [Google Scholar]
- 29.Ray R, Snyder RC, Thomas S, Koller CA, Miller DM. Mithramycin blocks protein binding and function of the SV40 early promoter. J Clin Invest 1989;83:2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segura-Valdez L, Pardo A, Gaxiola M, Uhal B, Becerril C, Selman M. Upregulation of Gelatinase A and B, Collagenases 1 and 2, and Increased Parenchymal Cell Death in COPD. Chest 2000;117:684–694. [DOI] [PubMed] [Google Scholar]
- 31.Brauchle M, Gluck D, Di Padova F, Han J, Gram H. Independent role of p38 and ERK1/2 mitogen-activated kinases in the upregulation of matrix metalloproteinase-1. Exp Cell Res 2000;258:135–144. [DOI] [PubMed] [Google Scholar]
- 32.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res 2002;4:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ptashne M, Gann A. Transcription initiation: imposing specificity by localization. Essays Biochem 2001;37:1–15. [DOI] [PubMed] [Google Scholar]
- 34.Young DA, Lakey RL, Pennington CJ, Jones D, Kevorkian L, Edwards DR, Cawston TE, Clark IM. Histone deacetylase inhibitors modulate metalloproteinase gene expression in chondrocytes and block cartilage resorption. Arthritis Res Ther 2005;7:R503–R512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, Barnes JL, Chandrasekar B. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NFkappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol 2007;293:H3356–H3365. [DOI] [PubMed] [Google Scholar]
- 36.Fan Z, Yang H, Bau B, Soder S, Aigner T. Role of mitogen-activated protein kinases and NFkappaB on IL1beta-induced effects on collagen type II, MMP-1 and 13 mRNA expression in normal articular human chondrocytes. Rheumatol Int 2006;26:900–903. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Wahl LM. Oxidative stress augments the production of matrix metalloproteinase-1, cyclooxygenase-2, and prostaglandin E2 through enhancement of NF-kappa B activity in lipopolysaccharide-activated human primary monocytes. J Immunol 2005;175:5423–5429. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed S, Wang N, Lalonde M, Goldberg VM, Haqqi TM. Green tea polyphenol epigallocatechin-3-gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J Pharmacol Exp Ther 2004;308:767–773. [DOI] [PubMed] [Google Scholar]
- 39.Faour WH, He Q, Mancini A, Jovanovic D, Antoniou J, Di Battista JA. Prostaglandin E2 stimulates p53 transactivational activity through specific serine 15 phosphorylation in human synovial fibroblasts. Role in suppression of c/EBP/NF-kappaB-mediated MEKK1-induced MMP-1 expression. J Biol Chem 2006;281:19849–19860. [DOI] [PubMed] [Google Scholar]
- 40.Raymond L, Eck S, Hays E, Tomek I, Kantor S, Vincenti M. RelA is required for IL-1beta stimulation of Matrix Metalloproteinase-1 expression in chondrocytes. Osteoarthritis Cartilage 2007;15:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valenca SS, Castro P, Pimenta WA, Lanzetti M, Silva SV, Barja-Fidalgo C, Koatz VL, Porto LC. Light cigarette smoke-induced emphysema and NFkappaB activation in mouse lung. Int J Exp Pathol 2006;87:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaschler GJ, Zavitz CC, Bauer CM, Skrtic M, Lindahl M, Robbins CS, Chen B, Stampfli MR. Cigarette smoke exposure attenuates cytokine production by mouse alveolar macrophages. Am J Respir Cell Mol Biol 2008;38:218. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Cowan MJ, Hasday JD, Vogel SN, Medvedev AE. Tobacco smoking inhibits expression of proinflammatory cytokines and activation of IL-1R-associated kinase, p38, and NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4 agonists. J Immunol 2007;179:6097–6106. [DOI] [PubMed] [Google Scholar]
- 44.Birrell MA, Wong S, Catley MC, Belvisi MG. Impact of tobacco-smoke on key signaling pathways in the innate immune response in lung macrophages. J Cell Physiol 2008;214:27–37. [DOI] [PubMed] [Google Scholar]
- 45.Lambert C, Li J, Jonscher K, Yang TC, Reigan P, Quintana M, Harvey J, Freed BM. Acrolein inhibits cytokine gene expression by alkylating cysteine and arginine residues in the NF-kappaB1 DNA binding domain. J Biol Chem 2007;282:19666–19675. [DOI] [PubMed] [Google Scholar]
- 46.Zhong CY, Zhou YM, Joad JP, Pinkerton KE. Environmental tobacco smoke suppresses nuclear factorkappaB signaling to increase apoptosis in infant monkey lungs. Am J Respir Crit Care Med 2006;174:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishioka Y, Sagae S, Nishikawa A, Ishioka S, Kudo R. A relationship between matrix metalloproteinase-1 (MMP-1) promoter polymorphism and cervical cancer progression. Cancer Lett 2003;200:49–55. [DOI] [PubMed] [Google Scholar]
- 48.Dickey ID, Scully SP. Identification of a single nucleotide polymorphism in the MMP-1 promoter in chondrosarcoma. J Surg Oncol 2004;87:130–133. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto T, Uchida K, Okayama N, Imate Y, Suehiro Y, Hamanaka Y, Ueyama Y, Shinozaki F, Yamashita H, Hinoda Y. Association of matrix metalloproteinase (MMP)-1 promoter polymorphism with head and neck squamous cell carcinoma. Cancer Lett 2004;211:19–24. [DOI] [PubMed] [Google Scholar]
- 50.Matsumura S, Oue N, Kitadai Y, Chayama K, Yoshida K, Yamaguchi Y, Toge T, Imai K, Nakachi K, Yasui W. A single nucleotide polymorphism in the MMP-1 promoter is correlated with histological differentiation of gastric cancer. J Cancer Res Clin Oncol 2004;130:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCready J, Broaddus WC, Sykes V, Fillmore HL. Association of a single nucleotide polymorphism in the matrix metalloproteinase-1 promoter with glioblastoma. Int J Cancer 2005;117:781–785. [DOI] [PubMed] [Google Scholar]
- 52.Zhu Y, Spitz MR, Lei L, Mills GB, Wu X. A single nucleotide polymorphism in the matrix metalloproteinase1 promoter enhances lung cancer susceptibility. Cancer Res 2001;61:7825–7829. [PubMed] [Google Scholar]
- 53.Gorlova OY, Zhang Y, Schabath MB, Lei L, Zhang Q, Amos CI, Spitz MR. Never smokers and lung cancer risk: a case-control study of epidemiological factors. Int J Cancer 2006;118:1798–1804. [DOI] [PubMed] [Google Scholar]
- 54.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev 2005;14:2287–2293. [DOI] [PubMed] [Google Scholar]
- 55.Watson L, Vonk JM, Lofdahl CG, Pride NB, Pauwels RA, Laitinen LA, Schouten JP, Postma DS. Predictors of lung function and its decline in mild to moderate COPD in association with gender: results from the Euroscop study. Respir Med 2006;100:746–753. [DOI] [PubMed] [Google Scholar]
- 56.Ianbaeva DG, Korytina GF, Viktorova TV. Mol Biol (Mosk) 2004;38:973–979. (Complex search for antiprotease-protease enzyme gene polymorphisms in patients with chronic obstructive pulmonary diseases). [PubMed] [Google Scholar]