Abstract
Dysregulated inflammation has been implicated in cystic fibrosis (CF) airway pathophysiology. The expression of inflammatory genes, like interleukin 8 (IL8), involves chromatin remodeling through histone acetylation. Inflammatory gene hyperacetylation could explain inflammatory mediator dysregulation seen in CF airways. CF airways are exposed to high levels of oxidative stress, and oxidative stress increases histone acetylation and inflammatory gene transcription. Loss of cystic fibrosis transmembrane conductance regulator (CFTR) may even reduce protection against oxidative stress. Consequently, increasing oxidative stress would likely lead to an imbalance of histone acetyl-transferase (HAT) and deacetylase (HDAC) stoichiometry and contribute to the heightened inflammatory response seen in the CF airway. We hypothesize that oxidative stress in CF airways causes increased acetylation of inflammatory gene promoters, contributing to transcriptional activity of these loci. Messenger RNA levels of IL8, IL6, CXCL1, CXCL2, CXCL3, and IL1 are significantly elevated in CF epithelial cell models. Histone H4 acetylation is lower at the IL8 promoter of the non-CF cell lines than the CF models. The reducing agent N-acetyl-cysteine decreases IL8 message and promoter H4 acetylation to non-CF levels, suggesting that oxidative stress contributes to IL8 expression in these models. H2O2 treatment causes increased IL-8 acetylation and mRNA in all cells, but less in the CF-model cells. Together these data suggest a model in which cells without functional CFTR are under increased oxidative stress. Our data suggest intrinsic alterations in the HAT/HDAC balance in CFTR-deficient cells, and that oxidative stress contributes to this alteration.
Keywords: chromatin, oxidative stress, histone acetylation
CLINICAL RELEVANCE
Hyperacetylation is an emerging theme in inflammatory airway diseases. As there are potential pharmacologic modulators of acetylation and deacetylation, these processes are promising therapeutic targets for cystic fibrosis, chronic obstructive pulmonary disease, and asthma.
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene, which encodes a cAMP-regulated chloride channel. Inflammation is believed to be a primary cause of CF pulmonary pathology. Lung inflammation leads to tissue destruction and ultimately respiratory failure, which is the main cause of death among patients with CF. The relationship between a CFTR mutation and CF lung disease, however, is not fully understood. The inflammatory cytokine responses, particularly that of the chemoattractant IL-8, are excessive in bronchoalveolar lavage fluid from patients with CF compared with control subjects (1–10). It is clear that the production of inflammatory mediators is increased from CF lung epithelial cells; a question that still remains is how a mutation in a chloride channel results in an exaggerated innate inflammatory response.
Inflammatory genes are regulated in part through remodeling of chromatin structure. The expression of many inflammatory genes involves the remodeling of chromatin through changes in the level of acetylation. Acetylation of histone tail lysine residues can be directly related to the level of gene transcription (11), and acetylation of a particular combination of residues may influence transcription factor access to a distinct set of genes. To date, studies regarding changes in chromatin structure at inflammatory loci in the context of CF lung disease are lacking. The only published data regarding histone acetyl-transferase (HAT)/histone deacetylase (HDAC) balance in CF is from a study on chronic obstructive pulmonary disease (COPD), and the authors found that neither HAT activity nor HDAC activity was changed in five lung tissue samples from patients with CF (12). Even if there are no global alterations in HAT or HDAC activity in CF, there may still be alterations in specific HATs or HDACs in the absence of CFTR. Small or specific changes in the HAT/HDAC balance could affect transcription of many inflammatory genes, potentially having a profound effect on the initiation, duration, and resolution of an inflammatory response. Loss of tight regulation of inflammatory genes through hyperacetylation could explain some of the dysregulation of inflammatory mediators seen in the CF lung. This dysregulation includes elevated cytokine levels early in life (8, 13), exaggerated levels relative to the bacterial burden present (7), and a prolonged increase in inflammatory mediators after an infection (8).
Oxidative stress increases histone acetylation and activates inflammatory gene transcription (14, 15). The airways of patients with CF are exposed to increased levels of oxidative stress (16). High levels of oxidative stress are generated, at least in part, from the massive infiltration of neutrophills, which dominate the inflammatory response in the CF lung. Patients with CF may have inadequate antioxidant defenses to cope with the elevated oxidative stress that they regularly experience. Oxidative stress contributes to the decline in pulmonary function in these patients (17), and patients with CF have increased levels of oxidative stress markers in their plasma (18, 19). Oxidative stress may lead to an imbalance in the levels of HATs and HDACs, and this may amplify lung inflammation. This imbalance could lead to a chronic and exaggerated inflammatory response, as seen in the CF lung.
It is not clear if loss of CFTR directly affects the redox state in the CF lung. An alteration in lung glutathione levels, however, is a factor in several inflammatory lung diseases, including cystic fibrosis. Glutathione is a protective antioxidant in the lungs, and plays a key role in regulating oxidant-induced lung injury. A reduction in glutathione levels would result in loss of antioxidant protection and increased oxidative burden to the lung. Bronchoalveolar lavage levels of the antioxidant glutathione are diminished in patients with cystic fibrosis (20), and Cftr-null mice lack a significant increase in epithelial lining fluid glutathione when challenged with Pseudomonas aeruginosa (21). These studies indicate an intrinsic change that contributes to a high level of oxidative stress in the CF lung. The CF airways may represent an environment that results in hyperacetylation of inflammatory loci, and a primed situation for an exaggerated immune response.
This delicate balance between acetylation and deacetylation is under investigation in other inflammatory lung diseases. COPD and asthma are also characterized by localized chronic inflammation, increased expression of multiple inflammatory genes, and high levels of oxidative stress. Lung biopsies from patients with asthma revealed reduced HDAC activity, reduced HDAC1 and HDAC2 protein expression, and increased HAT activity (22). The major cause of chronic COPD is smoking. Lung biopsies from smokers revealed reduced HDAC activity and protein expression of HDAC2 (23). To date, there are no reported studies on HAT/HDAC levels or activity in the CF lung. It is likely that with the high level of oxidative stress in the CF lung, there may be an alteration in the HAT/HDAC balance. This alteration may affect the regulation of inflammatory genes and lead to a chronic cycle of high levels of reactive oxygen species, hyperacetylation, and increased expression from inflammatory genes.
Here we report characterization of the acetylation status of the IL8 gene in three paired cell models for CF. In each of the pairs, the cells with reduced or absent CFTR function were derived in different ways, including naturally occurring mutations, dominant-negative inhibition, and antisense inhibition. The three cell line pairs showed consistent changes in IL8 acetylation and expression that could be modulated to a non-CF state by agents known to reduce the redox state of the cells. Conversely, the lines expressing functional CFTR could be induced to a CF state of IL8 acetylation by treatment with agents that increase oxidative load.
MATERIALS AND METHODS
Cell Culture
9/HTEo- pCEP and pCEP-R cell lines.
Human tracheal epithelial cell lines (HTE) were created with 9/HTEo- cells overexpressing the CFTR regulatory (R) domain (pCEPR; CF phenotype) and mock-transfected 9/HTEo- cells (pCEP, non-CF phenotype). pCEPR cells in which CFTR function has been impaired are referred to here as CFTR−, and pCEP cells are referred to as CFTR+. These cell lines were a generous gift from the lab of Dr. Pamela B. Davis (Case Western Reserve University, Cleveland, OH). Cells were grown at 37°C in 95% O2−5% CO2 on Falcon 10-cm-diameter tissue culture dishes (BD, Franklin Lakes, NJ) and were cared for as previously described (24).
16HBEo- antisense and sense cell lines.
Human bronchial epithelial cells (16HBEo-) were stably transfected with plasmids containing the first 131 nucleotides of human CFTR in the sense (16HBE-S) or antisense (16HBE-AS) orientations. The 16HBE-AS cells have a CF-like physiology. 16HBE-AS cells in which CFTR function has been impaired will be referred to as CFTR−, and 16HBE-S cells will be referred to as CFTR+. Cells were grown at 37°C in 95% O2−5% CO2 on Falcon 10-cm-diameter tissue culture dishes and were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, and 200 μg/ml of G-418 as previously described (25). 16HBE-S and 16HBE-AS cells were a generous gift from the lab of Dr. Pamela B. Davis.
IB3–1 and S9 cells.
IB3 bronchial epithelial cells (ΔF508/W1282X) and S9 cells (IB3–1 cells stably transfected with the full-length wild-type CFTR as controls) were a gift from Pamela L. Zeitlin (Johns Hopkins University, Baltimore, MD). IB3 cells are referred to as CFTR− and S9 cells, transduced to express wild-type CFTR, are referred to as CFTR+. These cells were grown at 37°C in 95% O2−5% CO2 on Falcon 10-cm-diameter tissue culture dishes in LHC-8 Basal Medium (Biofluids; Biosource International, Rockville, MD) with 5% fetal bovine serum.
HTE cells.
HTE cells were recovered from a necropsy specimen, as previously described (26–28). Cells were grown in an air–liquid interface (ALI) on collagen-coated, semipermeable membranes as previously described (29). Cells were allowed to differentiate and verified for high transepithelial resistance. Cells were treated with either DMSO 1:1,000 (vehicle control) or 20 μM CFTRinh-172 (gift from Alan Verkman [30]) to establish a CF phenotype. CFTRinh-172 specifically and reversibly inhibits CFTR, but not MDR or other K+ or Cl− channels at the concentrations used for our studies (28). Drugs were added to both the apical and basolateral sides and refreshed every 24 hours. After 72 hours, RNA was harvested and used for QPCR. The HTE cells were all from the same donor specimen. Two filters were treated with DMSO and two with CFTRinh-172. Data are reported as an average RNA value (normalized to GAPDH RNA) for each treatment.
Cell treatments.
Experimental and control cells were placed in serum-free medium 24 hours before any treatment. For antioxidant treatment, cells were exposed to 5 mM N-acetyl-cysteine (NAC) (Sigma, St. Louis, MO) in serum-free medium for 2 hours before RNA and chromatin isolation. For oxidative stress treatment, cells were exposed 200 μM hydrogen peroxide (Sigma) in serum-free medium for 3 hours before RNA and chromatin isolation.
Real-Time Quantitative PCR
RNA quantification.
Total RNA was extracted from cells using Qiagen RNAeasy columns (Qiagen, Valencia, CA) with DNase treatment. cDNA was synthesized using 1 μg of RNA and random primers. RT-PCR samples excluding M-MLV-RT served as negative controls in quantitative PCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used to normalize all QPCR mRNA data. Real-time QPCR was performed using the DyNAmo HS SYBR Green qPCR Kit and the DNA Engine Opticon 2 System (MJ Research, Waltham, MA). PCR conditions were as follows: 95°C for 15 minutes; 95°C for 10 seconds, 60°C for 30 seconds, 72°C for 30 seconds, repeated for 50 cycles; 72°C for 10 minutes. For quantification, eight 10-fold dilutions of purified PCR product were used to make a standard curve.
The following QPCR primers were used. GAPDH: sense, 5′-ACAACTTTGGTATCGTGGAAGGAC-3′; antisense, 5′-AGGCAGGGATGATGTTCTGGAG-3′. IL6: sense, 5′-GAAGATTCCAAAGATGTAGCCGC-3′; antisense, 5′-AGGTTGTTTTCTGCCAGTGCC-3′. IL1: sense, 5′-ACAGTGGCAATGAGGATGACTTG-3′; antisense, 5′-CAAAGATGAAGGGAAAGAAGGTGC-3′. IL8: sense, 5′-TCTCTTGGCAGCCTTCCTGATTTC-3′; antisense, 5′-TCCAGACAGAGCTCTCTTCCATCA-3′. CXCL2: sense, 5′-ACTGAACTGCGCTGCCAGTGCTT-3′; antisense, 5′-TTTCTTAACCATGGGCGATGCGG-3′. CXCL3: sense, 5′-TGCTGCTCCTGCTCCTGGT-3′; antisense, 5′-GGGTTGAGACAAGCTTTCTTCCCA-3′. CXCL1: sense, 5′-CACTGCTGCTCCTGCTCCTGGTA-3′; antisense, 5′-GCAAGCTTTCCGCCCATTCTTGA-3′.
H4 Chromatin Immunoprecipitation
Cells were lysed and chromatin was sheared using Active Motif's Enzymatic Shearing Kit (53006; Carlsbad, CA). Enzymatic shearing for 4 minutes resulted in chromatin fragments of 100–1,000 bp in size. Chromatin immunoprecipitation (ChIP) was performed using Upstate's ChIP kit with an acetyl-histone H4 antibody (17-295). ChIP samples and input DNA were purified using a phenol/chloroform extraction followed by ethanol precipitation. QPCR was used with promoter specific primers to quantify ChIP samples and input DNA. PCR conditions were as follows: 95°C for 15 minutes; 95°C for 10 seconds, 60°C for 30 seconds, 72°C for 30 seconds, repeated for 50 cycles; 72°C for 10 minutes. For quantification, eight 10-fold dilutions of purified PCR product was used to make a standard curve. The following promoter-specific primers were used for QPCR. IL8: sense, 5′-GTTGTAGTATGCCCCTAAGAG-3′; antisense, 5′-CTCAGGGCAAACCTGAGTCATC-3′.
Data Analysis
All RNA data were normalized to GAPDH mRNA levels. ChIP samples were all normalized to the amount of input DNA for each sample. This normalization was performed after the QPCR and before any statistical analysis. All data are presented as the sample average, with standard error bars. All data is presented with the sample average of the untreated CFTR+ sample of each pair set to 1. All other averages were adjusted accordingly. For statistical analysis, a logarithmic transformation was applied to all groups of data. A two-sample equal variance t test was used to compare the means of groups with equal variance. If the variances were unequal (significance determined using an F-test) a two-sample unequal variance t test was performed.
RESULTS
Loss of CFTR Alone Results in Alterations in Cytokine Gene Transcription
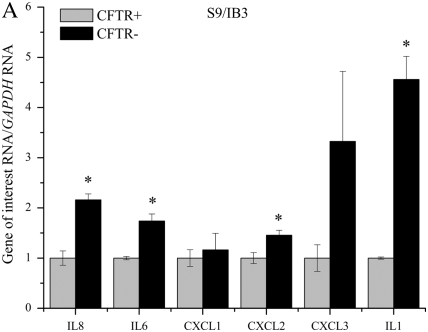
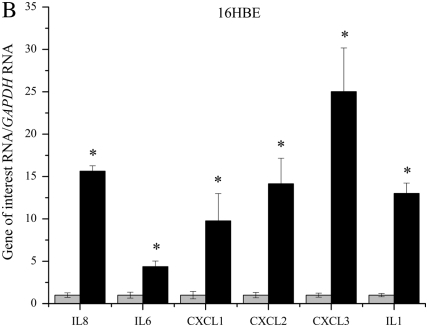
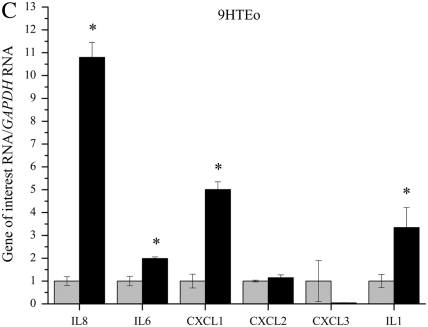
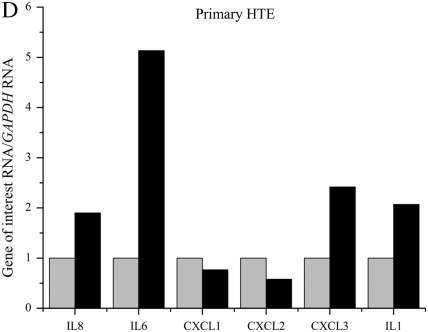
QPCR was used to determine if lack of functional CFTR in our cell models leads to an elevation in mRNA levels of several proinflammatory cytokines. Several cytokines were found to be elevated in three immortalized cell lines with reduced or absent CFTR function (Figures 1A–1C). IL8, IL6, CXCL1, CXCL2, CXCL3, and IL1 mRNA levels were assayed using real-time QPCR in three CFTR− and CFTR+ matched cell lines grown in serum-free medium with no additional inflammatory stimulation. Levels of IL8 mRNA were significantly elevated in all three CF models. Levels of IL6 and IL1 mRNA were also consistently elevated in all three CF models. Levels of CXCL1 and CXCL2 were significantly elevated in two of the three CF models.
Figure 1.
RNA levels of several proinflammatory cytokines are elevated in four cystic fribrossi transmembrane conductance regulator (CFTR)− airway epithelial cell lines compared with CFTR+ controls. All cytokine mRNA levels are normalized to GAPDH mRNA levels. The CFTR+ cell line averages have been set at 1 for each cell pair, and CFTR− averages are normalized to that average. Shaded bars represent CFTR+ control cell data, and solid bars represent CFTR− cell data. Significance was determined by t test. Error bars represent SEM. (A) Cytokine RNA levels in S9 (CFTR+) and IB3 (CFTR−) cells. *P < 0.05 comparing CFTR+ and CFTR− means using a two-tailed t test. IL8: P < 0.007, IL6: P < 0.003, CXCL2: P < 0.04, IL1: P < 0.003; n = 3. (B) Cytokine RNA levels in 16HBE sense (CFTR+) and anti-sense (CFTR−) cells. *P < 0.05 comparing CFTR+ and CFTR− means using a two-tailed t test. IL8: P < 0.001, IL6: P < 0.02, CXCL1: P < 0.02, CXCL2: P < 0.002, CXCL3: P < 0.0006, IL1: P < 0.0004; n = 3. (C) Cytokine RNA levels in 9HTEo PCEP2 (CFTR+) and PCEPR (CFTR−) cells. *P < 0.05 comparing CFTR+ and CFTR− means using a two-tailed t test. IL8: P < 0.000008, IL6: P < 0.0006, CXCL1: P < 0.004, IL1: P < 0.04; n = 3. (D) Cytokine RNA levels in HTE cells (CFTR+) and HTE cells treated with 20 μM CFTRinh-172 (CFTR−) cells. Values are an average of two separate treatments of cells obtained from the same donor (n = 2).
Primary HTE cells treated with 20 μM CFTRinh-172 also showed an elevation in IL8, IL6, CLCL3, and IL1 message levels (Figure 1D). Pharmacologic inhibition of CFTR in primary HTE cells appear to show the same trend of basal elevation in proinflammatory cytokine message levels as we see in the immortalized cell lines, the most consistent elevation between the four CFTR− cells being that of IL8, IL6, and IL1 levels.
Hyperacetylation at the IL8 Promoter Is Observed in Cells Lacking CFTR
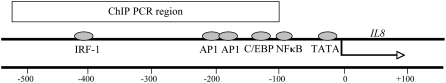
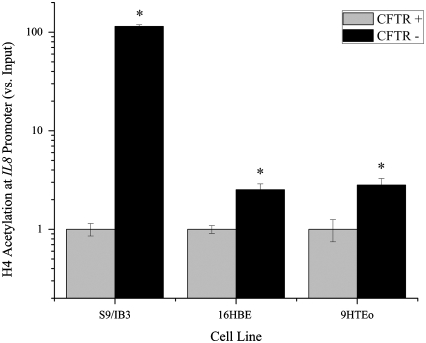
H4 acetylation is fairly well characterized surrounding the NF-κB–binding site of the IL8 promoter, and primer pairs have been established for detection of H4 acetylation (31–35). Because of this characterization, IL8 was chosen as a model gene to investigate promoter acetylation surrounding the NF-κB–binding site in the three CF model cell lines (Figure 2). Enrichment of H4 acetylation at the IL8 promoter was assayed using chromatin immunoprecipitation followed by real-time QPCR. Acetylated H4 levels at the IL8 promoter were elevated in all three CFTR− cell lines grown in serum-free medium (Figure 3). The differences in H4 acetylation between the CFTR− and CFTR+ cells varied, and were the greatest between the IB3 and S9 cell lines.
Figure 2.
Schematic of the IL8 promoter. Previously reported PCR conditions (26, 27) were used to investigate H4 acetylation in the vicinity of the NF-κB–binding site in the IL8 promoter using ChIP followed by QPCR. The IL8 promoter-specific primers amplify a 408-bp region (denoted ChIP PCR region) including the known NF-κB and other transcription factor–binding sites in the upstream sequence located in the PCR amplicon. The scale at the bottom shows position in base pairs, with the transcriptional start site set at 0. This position is also indicated by the arrow.
Figure 3.
Chromatin immunoprecipitation assay shows that H4 acetylation levels at the IL8 promoter are elevated in three CFTR− airway epithelial cell lines compared with CFTR+ controls. Cells were untreated and grown in serum-free medium. All IL8 promoter H4 acetylation levels are normalized to amount of input DNA. The CFTR+ cell line average has been set at 1, and CFTR− average is normalized to that average. Shaded bars represent CFTR+ control cell data, and solid bars represent CFTR− cell data. Significance was determined by t test. Error bars represent SEM. *P < 0.05 comparing CFTR+ and CFTR− means using a two-tailed t test. S9/IB3: P < 0.000008, 16HBE: P < 0.007, 9HTEo: P < 0.02; n = 3.
Treatment with NAC Reduces mRNA Levels and IL8 Promoter H4 Acetylation in Cells Lacking CFTR
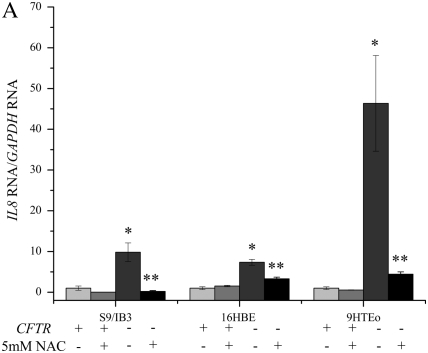
IL8 mRNA levels.
NAC is an antioxidant that is capable of stimulating glutathione (GSH) synthesis, and acting directly as free radical scavengers (36). Levels of IL8 mRNA, normalized to the amount of GAPDH mRNA, were higher in the three CFTR− cell lines before NAC treatment (Figure 4A). After a 2-hour treatment with 5 mM NAC, the levels of IL8 mRNA are reduced in the CFTR− cell lines to nearly CFTR+ control levels. In the IB3 cells, the level of IL8 mRNA after NAC treatment actually fell to below S9 levels. Treatment of the CFTR+ cells with NAC resulted in reduction of IL8 mRNA in the S9 and PCEP2 cells.
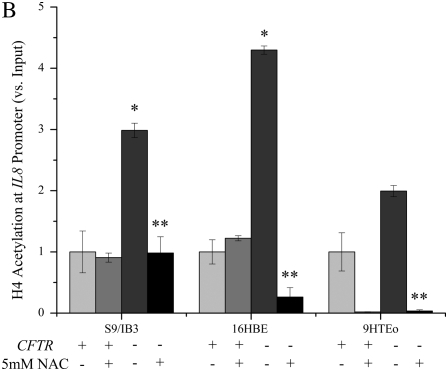
Figure 4.
Treatment with N-acetyl-cysteine (NAC) significantly reduces the IL8 mRNA levels and H4 acetylation at the IL8 promoter in CFTR− cells. Cells were exposed to 5 mM NAC for 2 hour in serum-free medium. The untreated CFTR+ cell line average has been set at 1, and all other averages are normalized to that average. Significance was determined by t test. Error bars represent SEM. (A) All IL8 mRNA levels are normalized to GAPDH mRNA levels. *P < 0.05 comparing untreated CFTR+ and untreated CFTR− means using a one-tailed t test. S9/IB3: P < 0.02, 16HBE: P < 0.007, 9HTEo: P < 0.0004. **P < 0.05 for comparison of untreated CFTR− to NAC-treated CFTR− means using a one-tailed t test. S9/IB3: P < 0.05, 16HBE: P < 0.003, 9HTEo: P = 0.067; n = 3. (B) All IL8 promoter H4 acetylation levels are normalized to amount of input DNA. *P < 0.05 comparing untreated CFTR+ and untreated CFTR− means using a one-tailed t test. S9/IB3: P < 0.04, 16HBE: P < 0.0002, 9HTEo: P < 0.0002. **P < 0.05 for comparison of untreated CFTR− to NAC-treated CFTR− means using a one-tailed t test. S9/IB3: P < 0.03, 16HBE: P < 0.003, 9HTEo: P < 0.007; n = 3.
IL8 promoter H4 acetylation.
Levels of IL8 promoter H4 acetylation at the NF-κB sites are higher in three CFTR− cell lines before NAC treatment (Figure 4B). After a 2-hour treatment with 5 mM NAC, the levels of IL8 promoter H4 acetylation are reduced in the CFTR− cell lines to nearly CFTR+ control levels. In the 16HBE antisense (CFTR−) cells, the level of IL8 promoter acetylation after NAC treatment actually fell to below 16HBE sense (CFTR+) levels. Treatment of the CFTR+ S9 and PCEP2 cells with NAC resulted in reduction of IL8 promoter H4 acetylation.
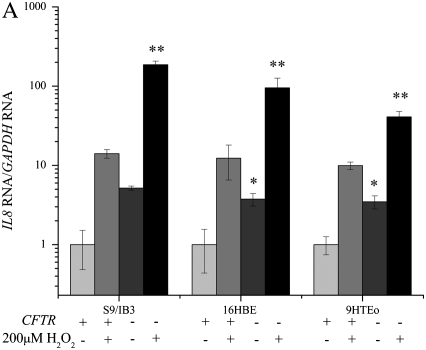
Cells Lacking CFTR Have a Larger IL8 mRNA Response to Hydrogen Peroxide
Hydrogen peroxide is a commonly used inducer of oxidative stress in cell culture systems and is known to induce IL-8 expression (15, 32). Levels of IL8 mRNA, normalized to the amount of GAPDH mRNA, were higher in the CFTR− cell lines before hydrogen peroxide treatment (Figure 5). Hydrogen peroxide treatment results in increased IL8 mRNA in the CFTR+ cells as well. The amount of increase in IL8 mRNA after a 3-hour treatment with 200 μM hydrogen peroxide is greater in the CFTR− cell lines compared with CFTR+ controls.
Figure 5.
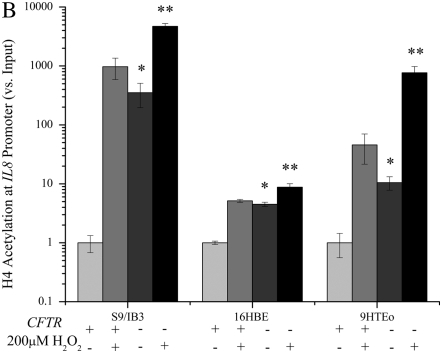
Treatment with hydrogen peroxide significantly increases the IL8 mRNA levels and the level of H4 acetylation at the IL8 promoter in CFTR− cells. Cells were exposed to 200 μM H2O2 for 4 hours in serum-free medium. The untreated CFTR+ cell line average has been set at 1, and all other averages are normalized to that average. Significance was determined by t test. Error bars represent SEM. (A) All IL8 RNA levels are normalized to GAPDH mRNA levels. *P < 0.05 comparing untreated CFTR+ and untreated CFTR− means using a one-tailed t test. S9/IB3: P = 0.1 16HBE: P < 0.03, 9HTEo: P < 0.007. **P < 0.05 for comparison of untreated CFTR− to H2O2-treated CFTR− means using a one-tailed t test. S9/IB3: P < 0.000005, 16HBE: P < 0.002, 9HTEo: P < 0.0003; n = 3. (B) All IL8 promoter H4 acetylation levels are normalized to amount of input DNA. *P < 0.05 comparing untreated CFTR+ and untreated CFTR− means using a one-tailed t test. S9/IB3: P < 0.006 16HBE: P < 0.00008, 9HTEo: P < 0.006. **P < 0.05 for comparison of untreated CFTR− to H2O2-treated CFTR− means using a one-tailed t test. S9/IB3: P < 0.005, 16HBE: P < 0.008, 9HTEo: P < 0.0003; n = 3.
Hydrogen Peroxide Results in a Greater Fold Increase in IL8 Promoter Acetylation in Cells with CFTR
Hydrogen peroxide is known to induce H4 acetylation at the NF-κB site in the IL8 promoter (15, 37). Levels of IL8 promoter acetylation were higher in the CFTR− cell lines before hydrogen peroxide treatment (Figure 5). Hydrogen peroxide treatment results in increased IL8 promoter acetylation in the CFTR+ cells as well. While the level of H4 acetylation at the IL8 promoter is always higher in the CFTR− cells, the relative increase after a 3-hour treatment with 200 μM hydrogen peroxide is greater in the CFTR+ cell lines. While the IL8 promoter is not maximally acetylated in the CFTR− cells before hydrogen peroxide treatment, the CFTR+ cells do show a greater induction in acetylation with an increase in oxidative stress. This suggests that the CFTR− cells are already exposed to high oxidative stress; therefore, applying additional oxidative stress does not increase the level of acetylation to as great an extent as in CFTR+ cells.
DISCUSSION
We have found that messenger RNA levels of IL8, IL6, CXCL1, CXCL2, CXCL3, and IL1 are significantly elevated in CF epithelial cell models. Consistent with the IL8 RNA levels, H4 acetylation was elevated at the IL8 promoter even in the absence of inflammatory stimulation in these CF models. Treatment of these CF cell models with the reducing agent NAC results in a reduction in the amount of IL8 message and promoter H4 acetylation to CFTR+ levels. In reciprocal experiments, hydrogen peroxide treatment caused a substantial and significant increase in IL8 mRNA levels and acetylation, bringing CFTR+ cells to levels comparable to untreated CFTR− cells. These data suggest a model in which oxidative stress is responsible for the differences between CF and non-CF cells in gene expression and acetylation at the IL8 locus, as CF cells exposed to antioxidant (NAC) display non-CF levels of IL8 mRNA and H4 acetylation, while non-CF cells exposed to oxidants (H2O2) display CF levels of these phenotypes. The results presented here imply there is an intrinsic alteration in the HAT/HDAC balance in cells lacking CFTR function in vitro, and that oxidative stress is likely contributing to this alteration.
While lung inflammation leads to tissue destruction and ultimately respiratory failure, the direct relationship between loss of CFTR and CF lung disease is not understood. The inflammatory response in the CF lung occurs early in life (8, 13), is prolonged (13), and may be exaggerated relative to the bacterial burden present (7). IL-8 levels are elevated in the sputum, bronchoalveolar lavage fluid (BALF), and serum of patients with CF, even during stable clinical conditions (4, 5). Studies even suggest that CFTR dysfunction may be an initial cause of increased IL-8 levels in CF airways (1, 7, 8). Since IL-8 secretion appears to be regulated primarily at the level of gene transcription (38), elucidating the factors involved in transcriptional activation of the IL8 gene is essential in understanding CF airway inflammation. Inflammatory genes are regulated in part through remodel of chromatin structure. The expression of many inflammatory genes involves the remodeling of chromatin structure through changes in the level of acetylation. Small alterations in the HAT/HDAC balance could affect the transcription level of many inflammatory genes. This could have a profound effect on the initiation, duration, and resolution of an inflammatory response. Loss of tight regulation of inflammatory genes through hyperacetylation could explain some of the dysregulation of inflammatory mediators seen in the CF lung. Histone H4 acetylation at the IL8 promoter is needed for maximal transcription from this gene and has been implicated in IL8 transcriptional regulation (15, 31, 32, 39).
We hypothesize that loss of tight regulation of inflammatory genes through hyperacetylation could explain some of the dysregulation of inflammatory mediators seen in the CF airway. We surveyed mRNA levels, as a measure of transcriptional regulation, of several inflammatory genes of interest in CF lung disease in three CF cell models. IL-8, IL-6, and IL-1 are proinflammatory mediators that are elevated in the CF airway and promote the destructive inflammatory process in the lung. CXCL1, 2, and 3 are the human orthologs of murine KC and MIP-2. KC and MIP-2 are neutrophil chemoattractants, and have been referred to as the murine functional homologs of human IL-8. KC and MIP-2 have been shown to be elevated in CF mouse models exposed to P. aeruginosa–laden agarose beads (40, 41) and aerosolized Pseudomonas LPS (42). The IL8, IL6, IL1, CXCL1, CXCl2, and CXCL3 promoters all contain putative NF-κB sites and may be regulated in a similar manner.
In cells grown in serum-free medium, the mRNA levels for all six cytokines were elevated in CFTR− cells. Even in the absence of inflammatory mediators, CFTR− cells show a dysregulation of several proinflammatory mediators. While it is difficult to make comparisons between mRNA and protein levels, the differences in mRNA levels between CFTR− and CFTR+ cells appear to be within a reasonable range when compared with protein levels from clinical data (1) and other studies using these cell models (25). The fold difference in IL8 mRNA levels from these CF cell models is also comparable with the difference in IL8 mRNA seen in COPD lung tissue and in the lung tissue of control subjects (12). Even though these data are from immortalized cell models, it does not appear to be overly exaggerated, and may be physiologically relevant.
At the level of mRNA, these data support the hypothesis that lack of functional CFTR in three cell models results in loss of tight regulation of inflammatory genes. These data also support other studies (25, 28, 43, 44) that loss of CFTR may cause an inherent dysregualtion of proinflammatory mediators. To determine if this increase in cytokine mRNA corresponds with promoter hyperacetylation, we assayed H4 acetylation at the IL8 promoter around the NF-κB site. The IL8 promoter showed a consistent elevation in H4 acetylation in the CFTR− cell lines. Even in the absence of inflammatory mediators, cells CFTR− showed hyperacetylation at the IL8 promoter. The magnitude of H4 acetylation between the three cell models varied. IB3 cells had over a 100-fold elevation in H4 enrichment at the IL8 promoter. It is difficult to determine if this is due to extremely low acetylation in the S9 cells or extremely high levels in the IB3 cells, compared with the other two cell models. Acetylation was detectable at the IL8 promoter compared with background in the S9 cells, so it is likely that the level of acetylation was significantly higher in the IB3 cells compared with the PCEPR and antisense cell lines. The IL8 promoter was chosen for further investigation because it the most well characterized of the six genes assayed for mRNA levels; however, it would be interesting to investigate acetylation at other proinflammatory gene promoters to determine if they are also hyperacetylated in CFTR− cells. It is likely that many inflammatory genes would be affected by a shift in the HAT/HDAC balance.
To identify the cause of the hyperacetylation in CFTR− airway epithelial cells, the same three CF cell models and controls were treated with the antioxidant NAC. We hypothesize that IL8 hyperacetylation can be explained, at least in part, by oxidative stress. The airways of patients with CFare exposed to increased levels of oxidative stress (16). Patients with CF may have antioxidant defenses inadequate to cope with the elevated oxidative stress that they regularly experience (17). Glutathione levels are diminished in BAL from patients with CF (20). CFTR knockout mice lack a significant increase in epithelial lining fluid glutathione when challenged with P. aeruginosa (21). There may be an intrinsic lack of antioxidant defenses in cells lacking CFTR. Treatment of CFTR− cells with NAC reduced IL8 promoter H4 acetylation to levels comparable with those of CFTR+ cells. This suggests that if the level of oxidative stress is reduced in these CF cell models, IL8 hyperacetylation can be corrected. These data implicate oxidative stress as a key factor in IL8 hyperacetylation in airway epithelial cells lacking CFTR. Correction in IL8 hyperacetylation corresponds with a decrease in IL8 message levels after NAC treatment. These data suggest that by reducing oxidative stress, IL8 promoter acetylation and message levels in CFTR− cells can be reduced to those of CFTR+ cells.
These data are consistent with the hypothesis that epithelial airway cells lacking CFTR are exposed to higher levels of oxidative stress. When the cell models were exposed to increased exogenous levels of oxidative stress by treatment with hydrogen peroxide, IL8 promoter H4 acetylation increases in all cells. This peroxide-induced increase in promoter acetylation corresponds to a large increase in IL8 message as well. While the level of H4 acetylation at the IL8 promoter is always higher in the CFTR− cells, the amount of increase after hydrogen peroxide is greater in the CFTR+ cell lines. While the IL8 promoter is not maximally acetylated in the CFTR− cells before hydrogen peroxide treatment, the CFTR+ cells do show a greater induction in acetylation with an increase in oxidative stress. This indicates that the CFTR− cells are already exposed to high oxidative stress; therefore, applying additional oxidative stress cannot increase the level of acetylation to as great an extent as in CFTR+ cells. This indicates that airway epithelial cells lacking CFTR do have impairment in their antioxidant defenses. These data indicate that while the CFTR− cells have high amounts of basal IL8 acetylation and message, this is not the maximum level possible in these cells. If the CFTR+ cells had responded to the oxidative stress to the level CFTR− cells, this would have suggested maximum hyperacetylation in the CFTR− cells.
These studies were all performed with immortalized, clonal cell lines and it is therefore difficult to make conclusions about CF pathogenesis. However, since the three CF cell models were all created in different ways, it is unlikely that this hyperacetylation phenotype is simply an artifact. One is a clinical sample that is genetically CF, one uses antisense technology to eliminate CFTR transcription, and the third one uses a dominant-negative form of CFTR to interfere with CFTR function at the cell membrane. Therefore it is unlikely that they would all show the same artifacts when compared with the matched control line. These are cell lines grown in culture, and are not exposed to the same sources of oxidative stress as epithelial cells in the CF lung. However, if loss of CFTR function results is a defect in antioxidant defenses, it is possible that the oxidative stress caused by growing cells in culture is inducing a response similar to that which would be seen in CF lung tissue. CF markers in these cell lines have been consistent with data from CF mouse models and human samples, making them useful models to study IL8 transcriptional regulation.
It is not clear how loss of CFTR can result in a defect in antioxidant defense in immortalized cell lines, or in the CF lung. There are several reports that suggest there may be an intrinsic defect (17, 20, 21). Oxidative stress has been implicated in inducing IL8 expression and promoter acetylation in other inflammatory lung diseases, such as COPD (12), and in lung cell exposure to environmental particles (32). Oxidative stress can nitrate HDAC2, which leads to IL8 promoter hyperacetylation and increased expression that is seem in COPD (45). A hallmark of CF is the large infiltration of neutrophils in the airway. Neutrophil-derived oxidants, along with a possible intrinsic defect in antioxidant defense due to lack of CFTR, could affect the HAT/HDAC balance in the CF airway. Treatment with the currently available antioxidant N-acetyl-cysteine may be able to aid in counteracting this high level of oxidative stress in the CF airway. High-dose oral NAC given to individuals with CF has shown some encouraging results. NAC treatment can markedly decrease sputum elastase activity, which is the strongest predictor of CF pulmonary function (46). IN addition, NAC treatment decreased the neutrophil burden and sputum IL-8 levels. However, lung function measures were not improved with short-term treatment. More potent antioxidants with long-term treatment may have the ability to have a positive impact on CF lung function.
Understanding how loss of CFTR function leads to increased oxidative stress in cell culture systems and in the CF airway will require further investigation. Data presented here show that there appears to be intrinsic IL8 promoter hyperacetylation in airway epithelial cells that lack CFTR function, and that this is likely the result of a reduction in the cells' antioxidant defense. This detection of an IL8 hyperacetylation phenotype will hopefully be useful understanding the pathogenic mechanisms inflammation in CF and suggest therapeutic, more specific, antiinflammatory strategies.
Acknowledgments
The authors thank Dr. Assem Ziady for assistance with primary tracheal epithelial cells and Dr. Mark Schluchter for statistical assistance.
The studies presented here were supported by National Institutes of Health HL68883 and Core Center grant P30 DK027651 to M.L.D.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0464OC on July 17, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 1995;310:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Invest 1999;103:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger M. Lung inflammation early in cystic fibrosis: bugs are indicted, but the defense is guilty. Am J Respir Crit Care Med 2002;165:857. [DOI] [PubMed] [Google Scholar]
- 4.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med 1995;152:2111. [DOI] [PubMed] [Google Scholar]
- 5.Dean TP, Dai Y, Shute JK, Church MK, Warner JO. Interleukin-8 concentrations are elevated in bronchoalveolar lavage, sputum, and sera of children with cystic fibrosis. Pediatr Res 1993;34:159. [DOI] [PubMed] [Google Scholar]
- 6.Hubeau C, Puchelle E, Gaillard D. Distinct pattern of immune cell population in the lung of human fetuses with cystic fibrosis. J Allergy Clin Immunol 2001;108:524. [DOI] [PubMed] [Google Scholar]
- 7.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 1995;151:1075. [DOI] [PubMed] [Google Scholar]
- 8.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med 1999;160:186. [DOI] [PubMed] [Google Scholar]
- 9.Tirouvanziam R, de Bentzmann S, Hubeau C, Hinnrasky J, Jacquot J, Peault B, Puchelle E. Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol 2000;23:121. [DOI] [PubMed] [Google Scholar]
- 10.Zahm JM, Gaillard D, Dupuit F, Hinnrasky J, Porteous D, Dorin JR, Puchelle E. Early alterations in airway mucociliary clearance and inflammation of the lamina propria in CF mice. Am J Physiol 1997;272:C853. [DOI] [PubMed] [Google Scholar]
- 11.Mizuguchi G, Vassilev A, Tsukiyama T, Nakatani Y, Wu C. ATP-dependent nucleosome remodeling and histone hyperacetylation synergistically facilitate transcription of chromatin. J Biol Chem 2001;276:14773. [DOI] [PubMed] [Google Scholar]
- 12.Ito K, Ito M, Elliott WM, Cosio B, Caramori G, Kon OM, Barczyk A, Hayashi S, Adcock IM, Hogg JC, et al. Decreased histone deacetylase activity in chronic obstructive pulmonary disease. N Engl J Med 2005;352:1967. [DOI] [PubMed] [Google Scholar]
- 13.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis 1997;175:638. [DOI] [PubMed] [Google Scholar]
- 14.Rahman I. Oxidative stress, chromatin remodeling and gene transcription in inflammation and chronic lung diseases. J Biochem Mol Biol 2003;36:95. [DOI] [PubMed] [Google Scholar]
- 15.Tomita K, Barnes PJ, Adcock IM. The effect of oxidative stress on histone acetylation and IL-8 release. Biochem Biophys Res Commun 2003;301:572. [DOI] [PubMed] [Google Scholar]
- 16.Hull J, Vervaart P, Grimwood K, Phelan P. Pulmonary oxidative stress response in young children with cystic fibrosis. Thorax 1997;52:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown RK, Wyatt H, Price JF, Kelly FJ. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur Respir J 1996;9:334. [DOI] [PubMed] [Google Scholar]
- 18.Brown RK, Kelly FJ. Evidence for increased oxidative damage in patients with cystic fibrosis. Pediatr Res 1994;36:487. [DOI] [PubMed] [Google Scholar]
- 19.Brown RK, McBurney A, Lunec J, Kelly FJ. Oxidative damage to DNA in patients with cystic fibrosis. Free Radic Biol Med 1995;18:801. [DOI] [PubMed] [Google Scholar]
- 20.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol 1993;75:2419. [DOI] [PubMed] [Google Scholar]
- 21.Day BJ, van Heeckeren AM, Min E, Velsor LW. Role for cystic fibrosis transmembrane conductance regulator protein in a glutathione response to bronchopulmonary pseudomonas infection. Infect Immun 2004;72:2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito K, Caramori G, Lim S, Oates T, Chung KF, Barnes PJ, Adcock IM. Expression and activity of histone deacetylases in human asthmatic airways. Am J Respir Crit Care Med 2002;166:392. [DOI] [PubMed] [Google Scholar]
- 23.Ito K, Lim S, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J 2001;15:1110. [PubMed] [Google Scholar]
- 24.Perez A, Risma KA, Eckman EA, Davis PB. Overexpression of R domain eliminates cAMP-stimulated Cl− secretion in 9/HTEo- cells in culture. Am J Physiol 1996;271:L85. [DOI] [PubMed] [Google Scholar]
- 25.Kube D, Sontich U, Fletcher D, Davis PB. Proinflammatory cytokine responses to P. aeruginosa infection in human airway epithelial cell lines. Am J Physiol Lung Cell Mol Physiol 2001;280:L493. [DOI] [PubMed] [Google Scholar]
- 26.Davis PB, Silski CL, Kercsmar CM, Infeld M. Beta-adrenergic receptors on human tracheal epithelial cells in primary culture. Am J Physiol 1990;258:C71. [DOI] [PubMed] [Google Scholar]
- 27.Kercsmar CM, Infeld MD, Silski CL, Davis PB. Adenosine 3:5′ cyclic monophosphate synthesis by human tracheal epithelial cells. Am J Respir Cell Mol Biol 1990;2:33. [DOI] [PubMed] [Google Scholar]
- 28.Perez A, Issler AC, Cotton CU, Kelley TJ, Verkman AS, Davis PB. CFTR inhibition mimics the cystic fibrosis inflammatory profile. Am J Physiol Lung Cell Mol Physiol 2007;292:L383. [DOI] [PubMed] [Google Scholar]
- 29.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia: methods for establishing primary cultures. Methods Mol Biol 2002;188:115. [DOI] [PubMed] [Google Scholar]
- 30.Ma T, Vetrivel L, Yang H, Pedemonte N, Zegarra-Moran O, Galietta LJ, Verkman AS. High-affinity activators of cystic fibrosis transmembrane conductance regulator (CFTR) chloride conductance identified by high-throughput screening. J Biol Chem 2002;277:37235. [DOI] [PubMed] [Google Scholar]
- 31.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol 2002;3:69. [DOI] [PubMed] [Google Scholar]
- 32.Gilmour PS, Rahman I, Donaldson K, MacNee W. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol 2003;284:L533. [DOI] [PubMed] [Google Scholar]
- 33.Schmeck B, Beermann W, van Laak V, Zahlten J, Opitz B, Witzenrath M, Hocke AC, Chakraborty T, Kracht M, Rosseau S, et al. Intracellular bacteria differentially regulated endothelial cytokine release by MAPK-dependent histone modification. J Immunol 2005;175:2843. [DOI] [PubMed] [Google Scholar]
- 34.Slevogt H, Schmeck B, Jonatat C, Zahlten J, Beermann W, van Laak V, Opitz B, Dietel S, N'Guessan PD, Hippenstiel S, et al. Moraxella catarrhalis induces inflammatory response of bronchial epithelial cells via MAPK and NF-kappaB activation and histone deacetylase activity reduction. Am J Physiol Lung Cell Mol Physiol 2006;290:L818. [DOI] [PubMed] [Google Scholar]
- 35.Schmeck B, Beermann W, van Laak V, Opitz B, Hocke AC, Meixenberger K, Eitel J, Chakraborty T, Schmidt G, Barth H, et al. Listeria monocytogenes induced Rac1-dependent signal transduction in endothelial cells. Biochem Pharmacol 2006;72:1367. [DOI] [PubMed] [Google Scholar]
- 36.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev 1998;3:114. [PubMed] [Google Scholar]
- 37.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J 2004;18:1897. [DOI] [PubMed] [Google Scholar]
- 38.Roebuck KA. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res 1999;19:429. [DOI] [PubMed] [Google Scholar]
- 39.Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem 2002;234–235:239. [PubMed] [Google Scholar]
- 40.van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 2004;287:L944. [DOI] [PubMed] [Google Scholar]
- 41.van Heeckeren AM, Tscheikuna J, Walenga RW, Konstan MW, Davis PB, Erokwu B, Haxhiu MA, Ferkol TW. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med 2000;161:271. [DOI] [PubMed] [Google Scholar]
- 42.Freedman SD, Weinstein D, Blanco PG, Martinez-Clark P, Urman S, Zaman M, Morrow JD, Alvarez JG. Characterization of LPS-induced lung inflammation in cftr−/− mice and the effect of docosahexaenoic acid. J Appl Physiol 2002;92:2169. [DOI] [PubMed] [Google Scholar]
- 43.Perkett EA, Ornatowski W, Poschet JF, Deretic V. Chloroquine normalizes aberrant transforming growth factor beta activity in cystic fibrosis bronchial epithelial cells. Pediatr Pulmonol 2006;41:771. [DOI] [PubMed] [Google Scholar]
- 44.Virella-Lowell I, Herlihy JD, Liu B, Lopez C, Cruz P, Muller C, Baker HV, Flotte TR. Effects of CFTR, interleukin-10, and Pseudomonas aeruginosa on gene expression profiles in a CF bronchial epithelial cell Line. Mol Ther 2004;10:562. [DOI] [PubMed] [Google Scholar]
- 45.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun 2004;315:240. [DOI] [PubMed] [Google Scholar]
- 46.Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc Natl Acad Sci USA 2006;103:4628. [DOI] [PMC free article] [PubMed] [Google Scholar]