Abstract
Previous studies from our lab have demonstrated that upon exposure to physiologic levels of cyclic stretch, alveolar epithelial cells demonstrate a significant decrease in the amount of polymerized tubulin (Geiger et al., Gene Therapy 2006;13:725–731). However, not all microtubules are disassembled, although the mechanisms or implications of this were unknown. Using immunofluorescence microscopy, Western blotting, and immunohistochemistry approaches, we have compared the levels of acetylated tubulin in stretched and unstretched A549 cells and in murine lungs. In cultured cells exposed to cyclic stretch (10% change in basement membrane surface area at 0.25 Hz), nearly all of the remaining microtubules were acetylated, as demonstrated using immunofluorescence microscopy. In murine lungs ventilated for 20 minutes at 12 to 20 ml/kg followed by 48 hours of spontaneous breathing or for 3 hours at 16 to 40 ml/kg, levels of acetylated tubulin were increased in the peripheral lung. In both our in vitro and in vivo studies, we have found that mild to moderate levels of cyclic stretch significantly increases tubulin acetylation in a magnitude- and duration-dependent manner. This appears to be due to a decrease in histone deacetylase 6 activity (HDAC6), the major tubulin deacetylase. Since it has been previously shown that acetylated microtubules are positively correlated to a more stable population of microtubules, this result suggests that microtubule stability may be increased by cyclic stretch, and that tubulin acetylation is one way in which cells respond to changes in exogenous mechanical forces.
Keywords: microtubule, histone deacetylase 6, acetylation, alveolar epithelium
CLINICAL RELEVANCE
Prolonged and even short-term mechanical ventilation can cause profound changes in the alveolar epithelium. We show that stretch in cells and lungs increases in the numbers of stable, acetylated microtubules via inhibition of the HDAC6.
While positive-pressure mechanical ventilation is now considered routine and often saves the lives of patients with acute respiratory distress syndrome (ARDS), mechanical ventilation can cause or worsen lung injury (1–3). Many investigators have looked at the effects of stretch in alveolar epithelial cells (AECs) and have seen a number of structural changes consistent with the injury seen in ventilated lungs (4–6). Consequently, researchers have become interested in understanding what role mechanotransduction plays in the development of lung injury (7–10). To date, most research in the lung as it relates to mechanical loading has focused on damaging loads, and on how cells respond to these damaging loads. However, the lung is a highly dynamic organ, and its constituents are exposed to mechanical loading on a daily basis, including both shearing and normal (tension and compressive) forces (11). While overdistention of a cell can cause highly undesirable outcomes, including apoptosis or necrosis (4, 12), smaller levels of mechanical stretch could prove to be beneficial to cells, and is certainly required for fetal lung development (13). Both types of mechanical load cause changes in the cell; however, it is possible that different pathways are stimulated as the result of the amount of stretch a cell senses.
Numerous changes occur in a cell when it is mechanically stimulated. Signaling cascades and transcription factors are up-regulated and down-regulated, reactive oxygen species are produced, and under extreme stresses, cells enter apoptosis. Furthermore, pronounced alterations of the cytoskeleton occur, including actin reorientation (14), microtubule polymerization/depolymerization (15, 16), intermediate filament organization (17), reorganization of focal adhesion sites (18), and “fluidization” of the cytoskeleton to expedite the reorganization process (19). It is thought that this reorganization is done in part to minimize internal stresses resulting from the external loads applied to these cells (20, 21). Although minimizing internal stresses may not fully explain cytoskeletal reorganization, it is apparent that reorganization is dependent on the amount, type, and duration of the external stimuli applied.
Independent of mechanical loading, the cytoskeleton continuously modifies its composition and shape, both to aid in cellular processes and in response to its outward environment. For example, it has been demonstrated that microtubules undergo multiple types of post-translational modifications, including acetylation of the α-tubulin subunit, which is thought to impart diverse functionality in lieu of having diverse monomeric proteins for assembly (22). This functionality is important since microtubules play an important role in many diverse cellular processes, including cell division, cell motility, and cytoplasmic trafficking. Modifications of α-tubulin can lead to microtubule stability, changes in motility, increased interactions with microtubule-associated proteins, and associations with other cytoskeletal elements (22).
While microtubule acetylation was originally hypothesized to impart structural stability to the filament (23), recent experiments by Haggarty, Yoshida and others have resulted in an unclear definition as to what functional role acetylated microtubules play in the cell (24–28). Some reports suggest that microtuble acetylation leads to microtubule stabilization, preventing microtubule depolymerization (26, 27). Others have suggested that acetylation itself does not increase microtubule stability; however, acetylation is a marker for a pool of stable microtubules as these types of post-translational modifications will accumulate in filaments that have a long half-life (28). More recently, acetylation has been shown to play a pronounced role in cytoplasmic trafficking. Studies from multiple groups have demonstrated that microtubule acetylation not only increases the recruitment of both kinesin and dynein to microtubules, but also enhances the anterograde flux of vesicular traffic (29–31).
Here we have investigated the relationship between acetylated microtubules and cyclic stretch. We demonstrate that cyclic stretch, at parameters that mimic physiologic respiration, increases the amount of acetylated microtubules both in vitro and in vivo. To our knowledge, this is the first such demonstration that mechanical stimulation can alter tubulin post-translational modifications both in vitro and in vivo.
MATERIALS AND METHODS
Cell Culture
All experiments, except where noted, were conducted on A549 cells (ATCC, Manassas, VA), a human lung adenocarcinoma cell line. Stably transfected A549 cells expressing shRNA against HDAC6 were a generous gift from T. P. Yao (Duke University) (26), and were developed using a retrovirus system expressing an RNAi for HDAC6, causing a significant decrease in HDAC6 levels (32). Cells were grown in high-glucose Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, kanamyacin, and antibiotic/antimycotic solution (Invitrogen, Carlsbad, CA). Cells were passaged every 3 to 5 days and maintained at 37°C with 5% CO2. All extractions, washes, fixations, and incubations were conducted with buffers warmed to 37°C unless otherwise noted.
Equibiaxial Cyclic Stretch
For all stretch-related experiments, A549 cells were plated on Pronectin-treated BioFlex culture plates (Flexcell International Corp., Hillsborough, NC) as previously described (15). Cells were stretched using 25 mm BioFlex loading stations with a 10% membrane surface area change (ΔSA) at 15 cycles per minute and a 50% duty cycle, unless otherwise noted.
Protein Extraction and Quantitation
Protein extracts for tubulin that were separated into total tubulin and polymerized tubulin pools were prepared and stored as previously described (33) after a single wash in phosphate-buffered saline (PBS). Briefly, two 15-minute incubations with microtubule stabilization buffer (MTSB; 0.1 M PIPES, pH 6.75, 1 mM EGTA, l mM MgSO4, 2 M glycerol, and protease inhibitors) with or without 0.1% Triton X-100 separated cells into pools containing both polymerized and depolymerized tubulin (those washed in MTSB lacking Triton X-100) and pools containing only polymerized tubulin (MTSB with Triton X-100). The cells were then incubated in lysis buffer (25 mM Tris-HCI, pH 7.4, 0.4 M NaC1, 0.5% SDS) for 5 minutes before scraping. Cell lysates were boiled for 3 minutes, centrifuged at 12,000 × g for 2 minutes, and the resulting supernatant was transferred to a new tube. β-mercaptoethanol was added to 0.1% of the total volume, and the lysates were then boiled for an additional 3 minutes and stored at −70°C. Protein extracts from murine lungs were made from snap-frozen samples that were pulverized using a BioPulverizer (Biospec Products, Bartlesville, OK). Powdered tissue was suspended in 750 μl of lysis buffer (Promega Corporation, Madison, WI), thawed, and vortexed. Three rounds of freeze/thaw cycles were done in liquid nitrogen and a room temperature water bath. Supernatant was separated from debris by centrifugation, and 10–12 μg of total protein was added to SDS sample buffer for Western blot. For tubulin extracts that were not separated, cells were lysed in SDS-PAGE sample buffer (112.5 mM Tris-HCl, pH 6.8, β-mercaptoethanol, 3.6% SDS, 1.8% glycerol, 0.001% bromophenol blue) and stored at −20°C. After separation by SDS-PAGE, proteins were transferred to nitrocellulose and probed using anti–α-tubulin (1:1,000, Catalog #T-9026; Sigma, St. Louis, MO) or anti-acetylated tubulin (1:2,000, Catalog #T-6793; Sigma) antibodies in PBS with 5% fat-free powdered milk. All samples were run in duplicate and were from at least three independent experiments.
Immunofluorescence Imaging
After all treatment regimes, cells were washed with PBS followed by a 10-minute incubation in a 1× fixation/permeabilization buffer (60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 3 mM MgCl2, 0.2% Triton X-100, and 3.7% paraformaldehyde). Cells were then washed in PBS and blocked for 1 hour in PBS containing 1 mg/ml bovine serum albumin (BSA). After blocking, the cells were incubated for 1 hour with the appropriate primary antibody in PBS with 1 mg/ml BSA, washed in PBS, and reacted for 30 minutes with Alexa 488– or Alexa 555–conjugated secondary antibody (1:200; Molecular Probes, Eugene, OR) in PBS with 0.1% BSA. After a second set of washes in PBS, the silastic membranes were excised from the culture plates, placed face up on a microscope slide, and covered with a coverslip. All images were acquired in OpenLab (Improvision, Lexington, MA) with a Hamamatsu Orca II-ER camera (Hamamatsu, Bridgewater, NJ) attached to Leica DRMX-2 upright fluorescent microscope (Leica Microsystems, Bannockburn, IL) using a ×100 oil-immersion objective.
Animal Ventilation
Female Balb/C mice (18–22 g) were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight) and a tracheostomy was performed using a shortened 20-guage angiocath. Two ventilation protocols were used: one to investigate persistence of cytoskeletal changes after short periods of ventilation and recovery and one to look at immediate cytoskeletal changes after longer periods of ventilation that mirror times seen to induce changes in acetylated tubulin levels in cultured cells. In the first, mice were mechanically ventilated through the angiocath with a Harvard Small Animal Ventilator (Model 683) at tidal volumes between 10 and 40 ml/kg body weight (20–80% TLC [34]) for 20 minutes. After ventilation, the animals were allowed to recover and were returned to the vivarium. Forty-eight hours later, the animals were killed by a sodium pentobarbital overdose. The lungs were removed and portions were formalin-fixed for paraffin embedding or snap-frozen in liquid nitrogen for preparation of lung lysates. In the second ventilation strategy, the anesthetized animals were connected to the ventilator (Flexivent; SCIREQ Scientific Respiratory Equipment, Inc., Montreal, PQ, Canada) through the angiocath, and pancuronium (0.25 mg, intraperitoneally) was administered. The animal was ventilated at a tidal volume of 12 ml/kg with a respiratory rate of 150 for 10 minutes, after which baseline measurements of lung mechanics were made using the flexivent protocols. The tidal volume was then increased to 20, 30, 40, or 50 ml/kg and the respiratory rate adjusted to maintain the minute ventilation at 3.5 ml/minute. This ventilator strategy was continued for 2 hours except for measurements of lung mechanics using the Flexivent protocols every 30 minutes (∼ 3 min per measurement). An additional dose of pentobarbital (80 mg/kg, intraperitoneally) was administered after 1 hour and an additional dose of pancuronium was administered if spontaneous movement was noticed. At the end of 2 hours, a thoracotomy was performed with removal of the heart and lungs en bloc and processed as above. All experiments were conducted in accordance with institutional guidelines in compliance with the recommendations of the Guide for Care and Use of Laboratory Animals.
Immunohistochemistry
Paraffin-embedded mouse lungs were sectioned at 6-μm thickness. Sections were dried, deparaffinized, and hydrated into 1× PBS and probed for either acetylated or total α-tubulin using the M.O.M. kit from Vector Labs (Burlingame, CA) following the manufacturer's directions. Primary antibodies were used at a 1:200 dilution. The secondary antibody was a 1:250 dilution of the M.O.M. biotinylated anti-mouse IgG. Detection and visualization was with Vector Labs Vectastain ABC reagent and DAB substrate solution. Sections were counterstained in hematoxylin.
RNA Extraction and Real-Time Quantitative PCR
Total RNA was extracted from stretched cells using QIAshredder and RNeasy kits (Qiagen, Chatworth, CA). Extracted RNA was converted to cDNA by performing reverse transcription using 1 μg total RNA with MuLV reverse transcriptase (Applied Biosystems, Foster City, CA). Quantitative PCR was performed in a 20-μl reaction volume, using the DyNAmo SYBR Green qPCR Kit as described by the manufacturer (Finnzymes, Espoo, Finland) with an Opticon 2 DNA Engine (MJ Research, Watertown, MA). Annealing temperatures were optimized for each set of primers. The threshold was set manually. A melting curve analysis was preformed to ensure reaction specificity. Results were normalized to GAPDH and expressed relative to unstretched data.
HDAC6 Activity Measurements
Cytoplasmic lysates were prepared as previously described to separate HDAC6 from nuclear HDAC activity (35). Cells were then probed for HDAC activity using a fluorescent HDAC activity kit (BioMol International LP, Plymouth Meeting, PA). At various times during the development of the assay, the reagents were quenched, allowing for a determination of the relative activity of the cytoplasmic HDACs.
Statistical Analysis
All statistical analysis was performed with Prism software (GraphPad Software, San Diego, CA), using the Mann-Whitey Wilcoxon test for paired, nonparametric samples. Statistically significant results were denoted at a P value of less than or equal to 0.05.
RESULTS
Stretch Increases Acetylated Tubulin in Cultured Cells
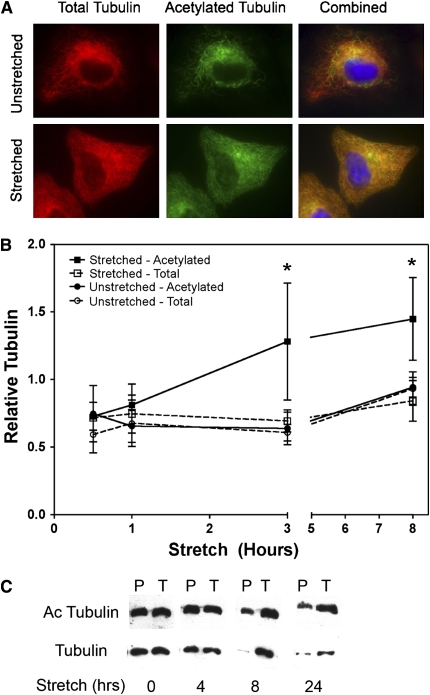
Previous work in our lab demonstrated that cyclic stretch caused reorganization of the microtubule network, including a significant reduction in the amount of polymerized tubulin (15). What was not understood was why some microtubules depolymerized under mechanical loading while others did not. To address this, A549 cells were grown under static conditions or stretched for 24 hours at 0.25 Hz (15 cycles/min) to give a 10% change in basement memrance surface area (ΔSA). When stretched cells were stained for acetylated tubulin, there was an increase in the amount of acetylated tubulin when compared with unstretched controls as demonstrated both by immunofluorescence microscopy (Figure 1A) as well as by Western blot analysis (Figure 1B). When the amounts of tubulin were measured relative to time 0, there was a statistically significant increase in the amount of acetylated tubulin under stretch conditions over time versus unstretched controls (Figure 1B). On average, whole cell lysates from stretched cells showed a 44% increase in acetylated tubulin compared with time-matched unstretched controls, ranging from a 12% increase within 30 minutes of stretching to a 71% increase at 3 hours of stretch. By separating total from polymerized tubulin (i.e., microtubules), the absolute decrease in polymerized microtubules in response to cyclic stretch becomes evident, as does the relative increase in the amounts of acetylated microtubules compared with total tubulin (Figure 1C). To ensure that equal loading of total protein occurred, total tubulin values were also measured for both stretch and unstretched cells, and they remained constant throughout the experiment. As previously reported (15), no changes in cell proliferation or cell death were noted under these stretch conditions. Taken together, these results demonstrate that mild cyclic stretch increases the levels of acetylated microtubules.
Figure 1.
Cyclic stretch alters microtubule network and increases acetylated tubulin in a time-dependent fashion. A549 cells were stretched (10% area strain at 0.25 Hz) or grown under static conditions for up to 24 hours. (A) After 24 hours, A549 cells were fixed and visualized via immunofluorescence microscopy. Antibodies against β-tubulin (red) or acetylated tubulin (green) were used to visualize the microtubule network. (B) A549 cells were harvested at the indicated times (n = 6). Cell extracts were prepared and the relative levels of acetylated (Ac) and total tubulin were determined by Western blotting. Tubulin levels were normalized to time 0 (i.e., unstretched cells). Densitomitry measurements demonstrate an increase in acetylated tubulin with stretch in whole cell lysates that is not seen in cells in static culture across multiple experiments. (C) Representative Western blot comparing acetylated and nonacetylated polymeric (P) or total (T) tubulin from whole-cell lysates of cells stretched for the indicated times. Tubulin pools were separated into polymeric fractions to emphasize the significant changes seen in the composition of the microtubule (polymerized tubulin) pool with stretch. *P < 0.05 versus unstretched total tubulin levels at time = 0.25 hours as determined by a two-tailed Mann-Whitney test.
Stretch Increases Acetylated Tubulin In Vivo
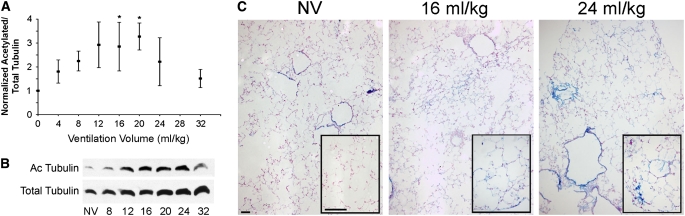
To determine whether or not the same type of acetylation events seen in vitro translated to an in vivo setting, animals were either not mechanically ventilated (“NV”), ventilated for 20 minutes followed by 48 hours of recovery without mechanical ventilation, or ventilated with increasing tidal volumes for 2 hours and analyzed immediately. As shown in Figure 2, mice that were ventilated for 20 minutes demonstrate a magnitude-dependent increase in acetylated tubulin that persists for at least 48 hours after the stretch is applied. We used this short period of ventilation followed by a recovery phase based on our previous findings that brief application of cyclic stretch to cultured cells could result in profound changes in cytoskeletal organization that were immediate and persisted over time (Figure 1 and Ref. 15). Although there was significant variation in the absolute degree of acetylation from animal to animal, accounting for the large standard error in the data points, the same trend was seen for each animal examined (n = 4 per condition). Thus, levels of acetylated microtubules increase and remain elevated for at least 48 hours after cessation of ventilation. This increase in acetylated tubulin appears to be primarily in the peripheral lung as assessed by immunohistochemistry. In animals that received no mechanical ventilation, the majority of detected acetylated tubulin is in the airway epithelium, but with increasing tidal volume ventilation, acetylated tubulin appeared in multiple cell types throughout the periphery of lung (Figure 2C).
Figure 2.
Cyclic stretch increases acetylated tubulin in a time and magnitude dependent fashion. Female Balb/c mice (18–22 g) were anesthetized and ventilated at the indicated tidal volumes and the respiratory rate adjusted to maintain the minute ventilation at 3.5 ml/minute for 20 minutes (n = 4 per condition). The animals were allowed to recover, and 2 days later, total lung lysates were prepared and analyzed for total and acetylated tubulin by Western blot. (A) The ratio of acetylated to total tubulin was normalized to unventilated animals to show that levels of acetylated tubulin increases with ventilation up to 12 to 24 ml/kg and then decreases at higher tidal volumes. (B) Representative Western blot data from nonventilated (NV) and ventilated animals. (C) Representative immunohistochemistry for acetylated tubulin (blue) of paraffin thin sections from non-ventilated (NV) and ventilated animals. Sections were counterstained with eosin. *P < 0.05 versus nonventilated controls as determined by a two-tailed Mann-Whitney test.
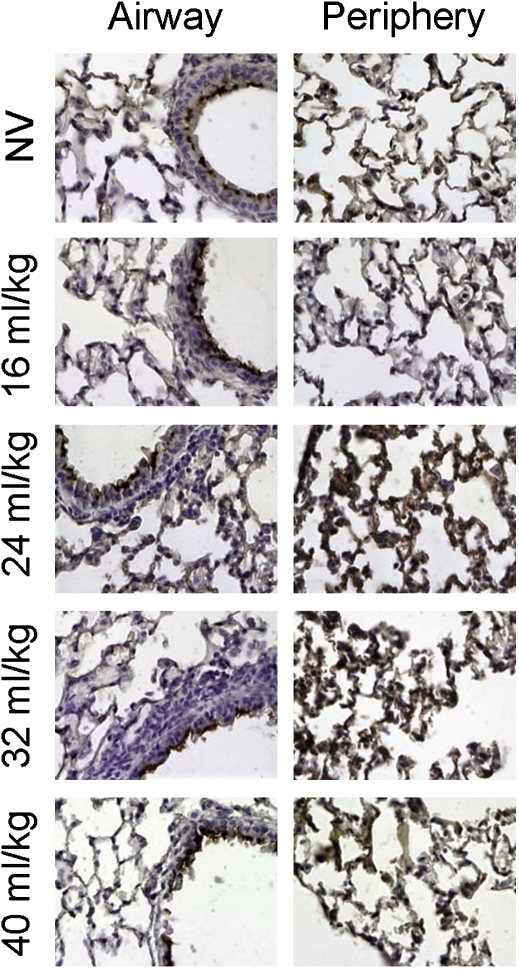
To determine whether the levels of acetylated tubulin increase immediately after ventilation or come up slowly over time, animals were ventilated and their lungs removed immediately for analysis by immunohistochemistry (Figure 3). As seen 48 hours after brief ventilation (Figure 2C), in lungs that received no mechanical ventilation (NV), the levels of acetylated tubulin are low in the periphery and parenchyma as detected by faint staining of acetylated tubulin throughout the lung section, whereas strong staining is seen in the airway epithelium, primarily in the cilia. However, with 2 hours of stretch, increases in acetylated tubulin are seen immediately, primarily in the parenchyma in alveolar epithelial cells. Qualitatively, the increased immunohistochemical staining under both ventilation strategies (Figures 2C and 3) correlates well with the increase in acetylated tubulin measured by Western blot (Figures 2A and 2B), with both methods demonstrating a maximal signal at a ventilation volume of 40 to 60% of total lung capacity (TLC). Taken together, these results demonstrate that cyclic stretch and ventilation cause increases in the levels of acetylated tubulin immediately after ventilation and that these increases persist over time.
Figure 3.
Cyclic stretch in vivo results in increased peripheral acetylated tubulin. Female Balb/c mice (18–22 g) were anesthetized and a tracheostomy was performed. Animals were either not mechanically ventilated (NV) or ventilated (Flexivent; SCIREQ Scientific Respiratory Equipment, Inc., Montreal, PQ, Canada) at a tidal volume of 16, 24, 32, and 40 ml/kg and the respiratory rate adjusted to maintain the minute ventilation at 3.5 ml/minute for 2 hours, after which lungs were removed and processed for paraffin embedding. Six-micron sections were cut and probed for acetylated-tubulin by immunohistochemistry. At the periphery, an increase in brown staining of the airways with increasing tidal volumes indicates increasing amounts of acetylated tubulin.
HDAC6 shRNA Increases Acetylated Tubulin Levels In Vitro
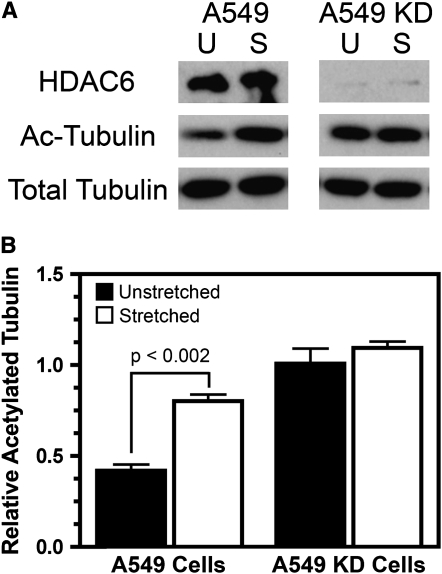
Having established that stretch causes an increase in acetylated tubulin both in vitro and in vivo, we wanted to explore the mechanisms leading to increased tubulin acetylation. The activities of two classes of enzymes can contribute to increased protein acetylation: either an increase in activity of the enzyme that causes acetylation, or a decrease in the activity of the enzyme that causes deacetlyation. Since the enzyme responsible for tubulin acetylation is unknown, but it is known that HDAC6 deacetylates tubulin (26), we wanted to see if A549 cells treated with a shRNA against HDAC6 had modulated microtubule acetylation when stretched. This would determine if a mechanism other than HDAC6 modulation was responsible for microtubule acetylation in stretched cells. For this experiment, stably transfected A549 cells expressing shRNA against HDAC6 were stretched along with non–shRNA-expressing A549 cells. The shRNA-expressing cells show greater than 90% knock-down of HDAC6 protein levels, and show approximately 2.5-fold higher levels of acetylated tubulin even in the absence of cyclic stretch, demonstrating that the level of tubulin acetylation is controlled, at least in part, by HDAC6 (Figure 4) (26). When stretched (10% ΔSA, 0.25 Hz) for 24 hours, the control A549 cells exhibit a 2-fold increase in acetylated tubulin compared with unstretched cells. However, cells expressing HDAC6 shRNA showed no increase in acetylated tubulin over their hyperacetylated state when stretched, suggesting that HDAC6 activity, and not some other unknown process resulting from cyclic stretch, plays an important role in tubulin acetylation in stretched cells.
Figure 4.
Cyclic stretch does not increase acetylated tubulin levels in A549 cells with HDAC6 levels knocked down by shRNA. A549 cells or cells stably transfected to express a shRNA against HDAC6 (A549 KD) were stretched for 24 hours (10% area strain at 0.25 Hz). (A) Western blots of whole cell lysates confirm silencing of HDAC6 in the A549 KD cells and show that acetylated tubulin levels (Ac-Tubulin) were increased in stretched A549 cells (S). However, stretch did not increase acetylated tubulin levels in A549 KD cells with reduced levels of HDAC6. (B) The levels of acetylated relative to total tubulin were quantified by densitometry from Western blots in A (n = 3 samples).
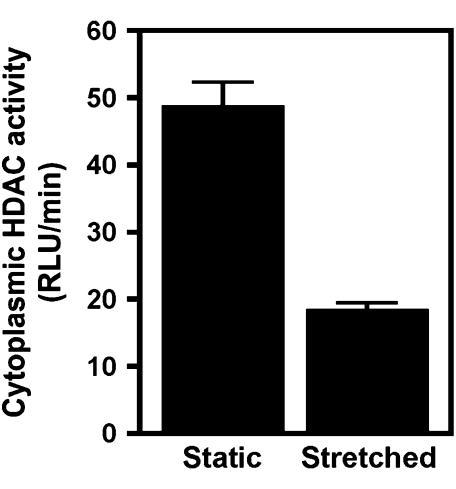
Stretch Decreases Cytoplasmic HDAC Activity
Having established that stretch causes an increase in acetylated tubulin and that stretching cells that have decreased HDAC6 activity does not alter the level of microtubule acetylation in vitro, we wanted to determine if stretch caused a decrease in HDAC6 activity. When A549 cells were stretched for 24 hours (10% ΔSA, 0.25 Hz), cytoplasmic HDAC activity, as measured in cytoplasmic extracts, was decreased over 2.5-fold compared with cells that were not stretched (Figure 5). HDAC6 is the only HDAC that resides almost exclusively in the cytoplasm, and thus a decrease in cytoplasmic HDAC activity should primarily result from a decrease in HDAC6 activity. Therefore it is highly likely that decreased HDAC6 activity is the main mechanism for increased microtubule acetylation in stretched alveolar epithelial cells.
Figure 5.
Cyclic stretch decreases the activity of cytoplasmic HDACs (HDAC6). A549 cells were stretched for 24 hours (10% area strain at 0.25 Hz). Cytoplasmic extracts were prepared and HDAC activity was measured using a fluorimetric assay as described in Materials and Methods. Stretched samples demonstrated a 2.5-fold decrease in cytoplasmic HDAC activity (HDAC6) over unstretched controls.
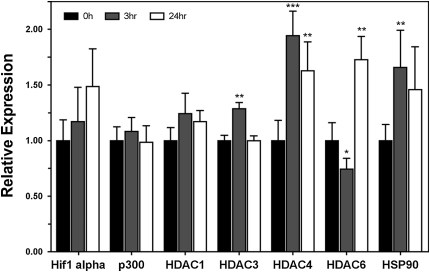
Cyclic Stretch Does Not Alter Transcription of HDAC6 Targets
Knowing that HDAC6 activity was altered through mechanical stimulation, we next wanted to determine if the levels of HDAC6, other targets of HDAC6 (e.g., hsp90, hif-1α, and p300), or other HDACs in general (HDAC1, HDAC3, and HDAC4) were affected at the transcriptional level by stretch. To determine this, mRNA levels of these targets were examined using quantitative polymerase chain reaction (qPCR). In cells that had been stretched for either 3 or 24 hours, none of the examined mRNA levels were increased or decreased by more than a factor of 2, suggesting that any changes in transcription of these genes in response to cyclic stretch is minor (Figure 6).
Figure 6.
Cyclic stretch causes relative changes in mRNA levels as determined by real-time PCR. A549 cells were stretched for either 3 or 24 hours (10% ΔS.A., 0.25 Hz., 50% duty cycle), at which time cell lysates were collected for PCR analysis. Relative expression levels were compared with GAPDH. *P < 0.05, **P < 0.01, ***P < 0.001 versus GAPDH expression level at same time point as determined by a two-tailed Mann-Whitney test.
DISCUSSION
It has been well established that cells undergo numerous changes resulting from mechanical changes in their environment, a phenomenon that has been extensively studied in the lung. Increases in the “stretching” of cells, or changes in the basement membrane surface area of the lung, as seen in ARDS, has been shown to cause decreased endothelial cell barrier function (36), which is dependent on microtubule disassembly and ERK 1/2 and p38 MAPK activation (37). Previous studies from our lab have shown that physiologic levels of cyclic stretch cause significant microtubule reorganization and disassembly (15). In the current study, we show that while this is the case, there is a population of microtubules that remains in these stretched cells that is characterized by their post-translational modifications, namely acetylation. Moreover, mild cyclic stretch appears to increase the amount of acetylated microtubules in cultured cells through the inhibition of the cytoplasmic tubulin deacetylase HDAC6. These results are corroborated by our in vivo measurements, since mice ventilated at low to moderate tidal volumes for 20 minutes showed increased levels of acetylated microtubules in their lungs 48 hours later. This suggests that in vivo, this type of cytoskeletal reorganization is maintained for an extended period of time when compared with the amount of time the exogenous force was applied. To our knowledge, this is the first example in the literature of exogenous mechanical forces resulting in increased microtubule acetylation, and may be an example of how cells reorganize their cytoskeleton in response to changing mechanical environments.
According to a report from Tschumperlin and Margulies (38), a ventilation volume of 40 to 60% of TLC corresponds to a 4 to 10% change in basement membrane surface area, based on morphometric analysis in a rat model. For the current study, all of our in vitro experiments were conducted using a 10% change in basement membrane surface area, and other experiments from our labs have shown that this level of strain induces the largest changes in cell reactivity (e.g., increased transfection efficiency, cytoskeletal reorganization, etc.) (15, 39). From our in vivo data, the maximal response for microtubule acetylation was ventilation volumes between 16 and 24 ml/kg, which corresponds to approximately 40 to 60% of TLC, based on measurements from Lai and Chou (34). Taken together, our results support the previous findings from Tschumperlin and Margulies (38), and demonstrate that in vivo results with animals ventilated at 40 to 60% of TLC correlates nicely with in vitro studies of cell monolayers stretched equibiaxially with a 10% change in basement membrane surface area.
It is unclear to us why microtubule acetylation is magnitude dependent, with a maximal response seen at ventilation volumes between 40 and 60% of TLC. Although the animals remain viable after ventilation at 80% TLC or higher, we have observed that prolonged ventilation (e.g., ≥ 2 h) at higher volumes (80–100% TLC) results in hemorrhage and damage of the lung epithelium (data not shown), as has previously been shown by a number of labs (40–43). However, at lower volumes, even for these extended periods of time, no damage was seen. This suggests that the cytoskeletal reorganization demonstrated here, namely microtubule acetylation, may only occur in cells that are not under extreme modulating forces.
In Brangwynne and colleagues' model of cellular tensegrity, microtubules are the major compressive-bearing structure, thus partially opposing the cellular prestress built up in the tension carrying actin and intermediate filaments (44). By some measures, only about 13% of the tensile forces within a cell are supported by microtubules (45). It has been demonstrated by Trepat and coworkers that a single transient stretch of similar magnitude to our studies causes cytoskeletal “fluidization” in numerous cell types (19), resulting in a decrease in cellular prestress. This mechanism, in part, may help explain our previous findings that cyclic stretch results in a significant increase in microtubule depolymerization (15). However, localized changes in other cellular components that oppose prestress, namely the focal adhesions, could require the cells to alter the microtubules to withstand an increased localized load. This is compounded by the fact that the aforementioned cytoskeletal fluidization may in fact be altering or reducing the cytoskeletal reinforcement that allows for larger compressive loads to be carried by the microtubules (44). In all, these results suggest that the possible reason for such changes in microtubules could be related to the fact that as a major constituent of resisting the cellular compressive load, microtubules may need to alter their chemical composition to a more stable conformation to resist such loads. Although this has not been specifically looked at for microtubule acetylation, other methods of stabilizing microtubules, including treatment with taxol and gluteraldehyde, have been shown to alter the ridigity of microtubules (46, 47), which is a direct measure of the microtubule's ability to not deform (buckle) under compressive loads, suggesting that alterations in the chemical composition (resulting in a structural change) of the microtubule can change the fiber's ability to resist load. In addition to chemical modification, microtubule-associated proteins (MAPs) have also been shown to alter microtubule rigidity (46), and several MAP or MAP-like proteins have been identified to coincide with increased microtubule acetylation (24, 48), suggesting that changes in microtubule stiffness might be a reason for the cytoskeletal reorganization seen when exogenous forces are applied. Regardless of these alterations, if the loads are too great (i.e., higher ventilation volumes), even stiffened microtubules will buckle, resulting in a drastically altered cytoskeleton, and hence, lung injury.
The observation of cytoskeletal “fluidization” due to transient stretching by Trepat and colleagues also has additional consequences (19). Given that microtubules facilitate cytoplasmic trafficking and acetylation of microtubules enhances trafficking (29–31), perhaps the microtubule acetylation in stretched cells preserves a cytoplasmic scaffolding for essential intracellular transport, and/or serves to accelerate trafficking to stressed regions of cells in the presence of an otherwise fluidized cytoskeleton. This would be an altogether different functional role than the tensegrity compression-bearing role, and one that fits with our emerging understanding of the dynamics of the cytoskeleton under dynamic loading.
Although it is still unclear whether acetylation is responsible for increased stability or if it is simply a marker for stable microtubules (24–28), no methodology short of pharmaceutical inhibitors of HDACs, and in particular HDAC6, have been shown in the literature to cause increased microtubule acetylation. As a result, the function of microtubule acetylation has remained a relative mystery. However, based on our experiments, it would appear that microtubule acetylation preserves cytoskeletal structure in cells undergoing cyclic stretch. Both Western blot and immunofluorescence data demonstrate that polymerized microtubules are highly acetylated in stretched cells. We have shown that HDAC6 activity is decreased under stretch conditions; however, HDAC6 mRNA levels are not appreciably changed under these same stretch conditions (Figures 5 and 6). Similarly, when we have looked at cells treated with HDAC inhibitors (including those specific for only HDAC6), we (and others) have found levels of tubulin acetylation similar to those shown in stretched cells (Figure 4). These results demonstrate that moderate, physiologic levels of mechanical stretch can result in significant changes in cellular architecture via modulation of HDAC6. However, how cyclic stretch modulates HDAC6 enzymatic activity remains to be determined. These changes appear to be quite different compared with nonphysiologic levels of mechanical stretch, and as a result should be closely examined to understand what significance, if any, they may play in the pathogenesis of lung injury and repair.
Acknowledgments
The authors thank Josh Gasiorowski, Erin Vaughan, Rui Zhou, Phoebe Loh-Marley, Göhkan Mutlu, and Patty Chess for their helpful discussions, technical expertise, and advice.
This work was supported by National Institutes of Health grants HL71643 (to D.A.D), HL76139 (to R.C.G.), and ES013995 (to G.R.S.B.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0307OC on July 17, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Corbridge TC, Wood LD, Crawford GP, Chudoba MJ, Yanos J, Sznajder JI. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. Am Rev Respir Dis 1990;142:311–315. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 3.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end- expiratory pressure. Am Rev Respir Dis 1974;110:556–565. [DOI] [PubMed] [Google Scholar]
- 4.Hammerschmidt S, Kuhn H, Grasenack T, Gessner C, Wirtz H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Am J Respir Cell Mol Biol 2003;30:396–402. [DOI] [PubMed] [Google Scholar]
- 5.Trepat X, Puig F, Gavara J, Fredberg JJ, Farre R, Navajas DJ. Effect of stretch on structural integrity and micromechanics of human alveolar epithelial cell monolayers exposed to thrombin. Am J Physiol Lung Cell Mol Physiol 2006;290:L1104–L1110. [DOI] [PubMed] [Google Scholar]
- 6.Vlahakis N, Schroeder M, Pagano R, Hubmayr RD. Deformation-induced lipid trafficking in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 2001;208:L938–L946. [DOI] [PubMed] [Google Scholar]
- 7.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol 2005;289:L834–L841. [DOI] [PubMed] [Google Scholar]
- 8.Papaiahgari S, Yerrapureddy A, Hassoun P, Garcia J, Birukov K, Reddy S. EGFR-activated signaling and actin remodeling regulate cyclic stretch-induced NRF2-ARE activation. Am J Respir Cell Mol Biol 2006;36:304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugin J. Molecular mechanisms of lung cell activation induced by cyclic stretch. Crit Care Med 2003; 31(4, Supplement)S200–S206. [DOI] [PubMed] [Google Scholar]
- 10.Wirtz D, Dobbs L. The effects of mechanical forces on lung functions. Respir Physiol 2000;119:1–17. [DOI] [PubMed] [Google Scholar]
- 11.Schumacker PT. Straining to understand mechanotransduction in the lung. Am J Physiol Lung Cell Mol Physiol 2002;282:L881–L882. [DOI] [PubMed] [Google Scholar]
- 12.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells: effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 2000;162:357–362. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Post M. Mechanochemical signal transduction in the fetal lung. J Appl Physiol 2000;89:2078–2084. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa K, Sato N, Obinata T. Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp Cell Res 2001;268:104–114. [DOI] [PubMed] [Google Scholar]
- 15.Geiger RC, Taylor W, Glucksberg MR, Dean DA. Cyclic stretch-induced reorganization of the cytoskeleton and its role in enhanced gene transfer. Gene Ther 2006;13:725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putnam AJ, Schultz K, Mooney DJ. Control of microtubule assembly by extracellular matrix and externally applied strain. Am J Physiol Cell Physiol 2001;280:C556–C564. [DOI] [PubMed] [Google Scholar]
- 17.Ridge K, Linz L, Flitney F, Kuczmarski E, Chou Y, Omary M, Sznajder JI, Goldman RD. Keratin 8 phosphorylation by protein kinase C delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J Biol Chem 2005;280:30400–30405. [DOI] [PubMed] [Google Scholar]
- 18.Smith P, Garcia R, Kogerman L. Strain reorganizes focal adhesions and cytoskeleton in cultured airway smooth muscle cells. Exp Cell Res 1997;232:127–136. [DOI] [PubMed] [Google Scholar]
- 19.Trepat X, Deng L, An SS, Navajas DJ, Tschumperlin DJ, Gerthoffer WT, Butler JP, Fredberg JJ. Universal pysical responses to stretch in the living cell. Nature 2007;447:592–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Apodaca GL. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol 2002;282:F179–F190. [DOI] [PubMed] [Google Scholar]
- 21.Lehoux S, Tedgui A. Cellular mechanics and gene expression in blood vessels. J Biomech 2003;36:631–643. [DOI] [PubMed] [Google Scholar]
- 22.Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol 2003;4:938–947. [DOI] [PubMed] [Google Scholar]
- 23.L'Hernault S, Rosenbaum J. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry 1985;24:473–478. [DOI] [PubMed] [Google Scholar]
- 24.Billger M, Strömberg E, Wallin M. Microtubule-associated proteins-dependent colchicine stability of acetylated cold-labile brain microtubules from the Atlantic cod, Gadus morhua. J Cell Biol 1991;113:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haggarty S, Koeller K, Wong J, Grozinger C, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci USA 2003;100:4389–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature 2002;417:455–458. [DOI] [PubMed] [Google Scholar]
- 27.Matsuyama A, Shimazu T, Sumida Y, Saito A, Yoshimatsu Y, Seigneurin-Berny D, Osada H, Komatsu Y, Nishino N, Khochbin S, et al. In vivo destabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J 2002;21:6820–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palazzo A, Ackerman B, Gundersen GG. Cell biology: tubulin acetylation and cell motility. Nature 2003;421:230. [DOI] [PubMed] [Google Scholar]
- 29.Reed N, Cai D, Blasius T, Jih G, Meyhofer E, Gaertig J, Verhey K. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol 2006;16:2166–2172. [DOI] [PubMed] [Google Scholar]
- 30.Bulinski JC. Microtubule modification: acetylation speeds anterograde traffic flow. Curr Biol 2007;17:R18–R20. [DOI] [PubMed] [Google Scholar]
- 31.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci 2007;27:3571–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, Yao TP. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 2003;115:727–738. [DOI] [PubMed] [Google Scholar]
- 33.Caron JM, Jones AL, Kirschner MW. Autoregulation of tubulin synthesis in hepatocytes and fibroblasts. J Cell Biol 1985;101:1763–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai Y-L, Chou H-C. Respiratory mechanics and maximal expiratory flow in the anesthetized mouse. J Appl Physiol 2000;88:939–943. [DOI] [PubMed] [Google Scholar]
- 35.Adam SA, Marr RS, Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J Cell Biol 1990;111:807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 2003;285:L785–L797. [DOI] [PubMed] [Google Scholar]
- 37.Birukova AA, Birukov K, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 2005;289:L75–L84. [DOI] [PubMed] [Google Scholar]
- 38.Tschumperlin DJ, Margulies AS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 1998;275:L1173–L1183. [DOI] [PubMed] [Google Scholar]
- 39.Taylor W, Gokay KE, Capaccio C, Davis E, Glucksberg MR, Dean DA. Effects of cyclic stretch on gene transfer in alveolar epithelial cells. Mol Ther 2003;7:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belperio JA, Keane MP, Burdick MD, Londhe V, Xue YY, Li K, Phillips RJ, Strieter RM. Critical role for CXCR2 and CXCR2 ligands during the pathogenesis of ventilator-induced lung injury. J Clin Invest 2002;110:1703–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaynar AM, Houghton AM, Lum EH, Pitt BR, Shapiro SD. Neutrophil elastase is needed for neutrophil emigration into lungs in ventilator-induced lung injury. Am J Respir Cell Mol Biol 2008;39:53–60. [DOI] [PMC free article] [PubMed]
- 42.Simon BA, Easley RB, Grigoryev DN, Ma S-F, Ye SQ, Lavoie T, Tuder RM, Garcia JGN. Microarray analysis of regional cellular responses to local mechanical stress in acute lung injury. Am J Physiol Lung Cell Mol Physiol 2006;291:L851–L861. [DOI] [PubMed] [Google Scholar]
- 43.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 1997;99:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brangwynne CP, MacKintosh FC, Kumar S, Geisse NA, Talbot J, Mahadevan L, Parker KK, Ingber DE, Weitz DA. Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. J Cell Biol 2006;173:733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamenovic D, Mijailovich SM, Tolic-Norrelykke IM, Chen J, Wang N. Cell prestress: II. Contribution of microtubules. Am J Physiol Cell Physiol 2002;282:C617–C624. [DOI] [PubMed] [Google Scholar]
- 46.Felgner H, Frank R, Schliwa M. Flexural rigidity of microtubules measured with the use of optical tweezers. J Cell Sci 1996;109:509–516. [DOI] [PubMed] [Google Scholar]
- 47.Kikumoto M, Kurachi M, Tosa V, Tashiro H. Flexural rigidity of individual microtubules measured by a buckling force with optical traps. Biophys J 2006;90:1687–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2, or tau. J Cell Sci 1992;103:953–964. [DOI] [PubMed] [Google Scholar]