Abstract
Several lines of evidence indicate that perturbations in the extracellular thiol/disulfide redox environment correlate with the progression and severity of acute lung injury (ALI). Cysteine (Cys) and its disulfide Cystine (CySS) constitute the most abundant, low-molecular-weight thiol/disulfide redox couple in the plasma, and Cys homeostasis is adversely affected during the inflammatory response to infection and injury. While much emphasis has been placed on glutathione (GSH) and glutathione disulfide (GSSG), little is known about the regulation of the Cys/CySS couple in ALI. The purpose of the present study was to determine whether endotoxin administration causes a decrease in Cys and/or an oxidation of the plasma Cys/CySS redox state (Eh Cys/CySS), and to determine whether these changes were associated with changes in plasma Eh GSH/GSSG. Mice received endotoxin intraperitoneally, and GSH and Cys redox states were measured at time points known to correlate with the progression of endotoxin-induced lung injury. Eh in mV was calculated using Cys, CySS, GSH, and GSSG values by high-performance liquid chromatography and the Nernst equation. We observed distinct effects of endotoxin on the GSH and Cys redox systems during the acute phase; plasma Eh Cys/CySS was selectively oxidized early in response to endotoxin, while Eh GSH/GSSG remained unchanged. Unexpectedly, subsequent oxidation of Eh GSH/GSSG and Eh Cys/CySS occurred as a consequence of endotoxin-induced anorexia. Taken together, the results indicate that enhanced oxidation of Cys, altered transport of Cys and CySS, and decreased food intake each contribute to the oxidation of plasma Cys/CySS redox state in endotoxemia.
Keywords: lipopolysaccharide, oxidative stress, thiol/disulfide redox state, anorexia, acute lung injury
CLINICAL RELEVANCE
Our findings suggest a distinct role for oxidation of Cys/CySS redox state in acute lung injury (ALI). Understanding how oxidized Cys/CySS can potentiate the inflammatory response to injury may help in the optimization of strategies for controlling ALI.
Acute lung injury (ALI) is initiated by an unregulated inflammatory response to a physical trauma or infection (1), most commonly sepsis (2), and often leads to severe respiratory failure termed the acute respiratory distress syndrome (ARDS) (3, 4). Several lines of evidence indicate that perturbations in thiol status is related to the pathogenesis of ALI and ARDS (5, 6). In particular, levels of glutathione (GSH), a major cellular thiol antioxidant, are markedly decreased in the epithelial lining fluid (ELF) of patients with sepsis and ARDS (7), and impaired homeostasis of lung and plasma GSH predisposes to ARDS (8). Numerous animal studies have addressed perturbations in the GSH system during endotoxemia (9, 10). However, GSH synthesis depends on the amino acid cysteine (Cys), and relatively little is known about the response of this precursor pool.
While GSH, and its oxidized form glutathione disulfide (GSSG), constitute the most abundant cellular redox couple (11), the cysteine/cystine (Cys/CySS) couple predominates in the plasma (12). Furthermore, the steady-state redox potential (Eh) of Cys/CySS is regulated independently of Eh GSH/GSSG (13–15), and plasma Eh Cys/CySS is oxidized in association with oxidative stress (16, 17). Oxidized extracellular Eh Cys/CySS induces up-regulation of surface adhesion markers on endothelial cells (18), and conditions epithelial cells to apoptosis (19). These cellular processes can contribute to the pathogenesis in ALI, and activation of these processes by oxidized Eh Cys/CySS occurs in the absence of changes to the GSH pool. In addition, the inconclusive outcomes from the therapeutic use of N-acetyl cysteine (NAC), a Cys precursor, in patients with ALI may be related to unknown variations in Cys/CySS redox state (20–22).
Experimental administration of bacterial lipopolysaccharide (endotoxin/LPS) to animals produces pathophysiologic changes similar to those seen in ALI in humans (23) and allows for the dissection of regulatory events critical in the pathogenesis of ALI. The purpose of this study was to test whether endotoxin causes a decrease in Cys and/or an oxidation of the Cys/CySS redox state and to determine whether Cys/CySS redox state changes are associated with changes in GSH/GSSG redox state under these pathophysiologic conditions. Mice were given endotoxin intraperitoneally and GSH/GSSG and Cys/CySS redox states were measured at time points known to correlate with the progression of lung injury (23). Because food intake affects blood Cys concentrations (24), PBS-treated mice were pair-fed to intake of mice receiving LPS. Ad libitum PBS-treated mice served as a separate set of controls. Here we showed that endotoxin had distinct effects on the GSH/GSSG and Cys/CySS redox systems during the acute phase of injury, and that sustained oxidation of Cys/CySS and GSH/GSSG redox states occurred as a result of LPS induced-anorexia.
MATERIALS AND METHODS
Materials
Except as indicated, all chemicals were purchased from Sigma Chemical Corporation (St. Louis, MO). Distilled, deionized water was used for analytical purposes. High-performance liquid chromatography (HPLC) quality solvents were used for HPLC.
Experimental Animals
Experiments were conducted using 10- to 14-week-old, female C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME). While some strains, such as the C3H/HeJ (25), are refractory to endotoxin, the C57BL/6 strain is a responsive strain (26, 27). The inflammatory response to endotoxin in the C57BL/6 strain is greater in older mice (28). We used 10- to 14-week-old mice because the pathophysiology of lung injury in this model has been previously characterized by our group (23, 26, 27). For the current experiments, mice were housed individually for pair feeding and maintained on a 12-hour light:12-hour dark cycle at the Division of Animal Resources at Emory University. All animals were fed pelleted rodent food (Test Diet 5015; Lab Diet, Inc., Richmond, IN) and had free access to water. Nestlets were presented daily to each mouse to compensate for the absence of other animals (29). All experiments were initiated during the light cycle. Measurement of food intake in LPS-treated mice was performed by providing mice with a weighed food pellet at the initiation of the experiment and manually recording weight of the remaining food at timed intervals. The amount of food eaten by LPS-treated mice was averaged, and this amount was provided to pair-fed PBS-treated controls. All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee.
LPS Administration
Escherichia coli O111:B6 LPS, dissolved in sterile PBS (100 μg/ml) was administered intraperitoneally at a dose of 1 mg LPS/kg body. Animals were killed at 2, 6, 24, and 48 hours after LPS administration. Pair-fed PBS animals received intraperitoneal injection of PBS and were killed at corresponding time points. Ad libitum–fed PBS controls were treated similarly.
Sample Collection and Analysis of Cys, CySS, GSH, and GSSG
Mice were anesthetized by isofluorane inhalation (Baxter Pharmaceuticals, Deerfield, IL), and blood was collected by submandibular bleeding using a 4-mm mouse bleeding lancet (Medipoint, Inc., Mineola, NY). A quantity of 0.18 ml of collected blood was immediately transferred to 0.02 ml of preservation solution containing γ-glutamyl-glutamate (γ-Glu-Glu) as an internal standard (30). Samples were centrifuged at 16,000 × g for 60 seconds to remove precipitated protein, and 0.1 ml of the supernatant was immediately transferred to an equal volume of ice-cold 10% (wt/vol) perchloric acid. Samples were immediately stored at −80°C.
Bronchoalveolar lavage fluid (BALF) was obtained after mice were killed. Briefly, 0.6 ml of sterile PBS was instilled into the lung via a tracheal incision and withdrawn with gentle suction. This procedure took approximately 10 minutes to complete. The collected BALF was centrifuged at 200 × g for 6 to 7 minutes. A quantity of 0.15 ml of the cell-free supernatant was mixed with an equal volume of ice-cold 10% (wt/vol) perchloric acid containing 20 μM γ-Glu-Glu and stored at −80°C until derivatization with iodoacetic acid and dansyl chloride.
For HPLC analysis (Gilson Medical Electronics, Middleton, WI), derivatized samples were centrifuged, and 50 μl (plasma) or 65 μl (BALF) of the aqueous layer was applied to the Supercosil LC-NH2 column (25 cm × 4.6 mm; Supelco, Bellefunk, PA). Derivatives were separated with a sodium acetate gradient in methanol/water and detected by fluorescence (31). Concentrations of thiols and disulfides were determined by integration relative to the internal standard. Redox states (Eh) of the GSH/GSSG and Cys/CySS pools were calculated from concentrations of GSH, GSSG and Cys, CySS in molar units with the following forms of the Nernst equation for pH 7.4: GSH/GSSG, Eh = −264 + 30 log ([GSSG]/[GSH]2), Cys/CySS, Eh = −250 + 30 log ([CySS]/[Cys]2) (32).
Quantitative Real-Time PCR Analysis
Lung and liver samples were excised, snap-frozen in liquid N2, and stored at −80°C. Total RNA was extracted from tissue using an RNeasy Midi Kit (Qiagen, Inc., Valencia, CA) according to manufacturer's instructions. DNase treatment was performed to remove contaminating genomic DNA. RNA concentration was spectrophotometrically determined at 260 nm, and 0.5 μg of total RNA was used to synthesize 20 μl of cDNA (Invitrogen, Carlsbad, CA). Quantitative real-time PCR was performed on cDNA using gene-specific primers on an iCycler IQ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA). Primers were designed using Beacon Designer Software 4.00 (PREMIER; Biosoft International, Palo Alto, CA) (Table 1). Samples containing serial dilutions of known concentrations of cDNA, encoding the gene of interest, were amplified in parallel. Data were analyzed using the iCycler Software, and starting quantities of message levels of each gene were determined from constructed standard curves. Melt curves were examined to ensure amplification of a single PCR product. Expression levels were normalized to GAPDH, and data are presented as the fold change in LPS/PBS-treated animals compared with untreated controls.
TABLE 1.
DETAILS OF PCR PRIMER SEQUENCES USED IN THE ANALYSES OF EXTRACTED RNA FOR QUANTITATIVE RT-PCR
| Target | Genbank Accession Number | Forward Primer | Reverse Primer | Product Size (bp) |
|---|---|---|---|---|
| IL-1β | NM_008361 | ATCTCGCAGCAGCACATC | CAGCAGGTTATCATCATCATC | 192 |
| TNF-α | NM_013693 | CGTGGAACTGGCAGAAGAG | ACAAGCAGGAATGAGAAGAGG | 96 |
| iNOS2 | NM_010927 | CAGAAGCAGAATGTGACC | GTAGTAGTAGAATGGAGATAGG | 195 |
| xCT | NM_011990 | ACCATCAGTGCGGAGGAG | GAGCCGAAGCAGGAGAGG | 119 |
Definition of abbreviations: iNOS2, inducible nitric oxide synthase 2; xCT, subunit of the CySS transporter.
Histopathology
Lungs were fixed by intratracheal instillation of neutral buffered formalin (10%). After further fixation overnight at room temperature, tissue was embedded in paraffin, sectioned, and stained by hematoxylin and eosin (H&E). All sections were studied by light microscopy.
Cell Culture
Human pulmonary endothelial cells (HULEC-5A, SV-40 large T antigen-transformed line derived from human lung microvasculature) were maintained in endothelial growth medium (EGM) (Lonza, Walkersville, MD), and were transferred to serum-free media 8 to 12 hours before experimental manipulations. Human monocytic cells (U937; ATCC, Rockville, MD) and murine neutrophils were maintained in RPMI-1640 supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA) and 10 U/ml penicillin and streptomycin sulfate. Neutrophils were isolated from heparin-anticoagulated peripheral blood using the Ly-6G isolation kit (Miltenyi Biotec, Auburn, CA). Cells were maintained in a humidified 5% CO2 incubator at 37°C.
To generate the desired range of extracellular redox states, the extracellular thiol/disulfide pool was altered by varying concentrations of Cys and CySS, added to cyst(e)ine-free Dulbecco's modified Eagle's medium as previously described (33). In these experiments, the total extracellular pool size of Cys + CySS was set at 200 μM, while concentrations of Cys and CySS were varied to obtain initial Eh values from −80 mV (physiological) to −46 mV (oxidized). Because human endothelial cells were tested in this experiment, Cys/CySS redox states representative of physiological (−80 mV) and oxidized (−46 mV) values in human plasma were used.
To examine the effect of oxidized Cys/CySS on adherence of neutrophils and monocytes; endothelial cells were exposed to −80 and −46 mV redox media for 24 to 36 hours, and media were changed every 12 hours. Calcein-AM–labeled neutrophils or monocytes were incubated with endothelial cells at a cell density of 1 × 105 cells per well of a 96-well plate. After 30 minutes, medium was aspirated to remove unbound cells. Adherent cells were quantified by measuring fluorescent calcein on a microplate reader (excitation, 470 nm; emission, 530 nm).
Statistical Methods
Data are presented as means (n = 3–5) ± SEM. Statistical analysis was done using SAS v 9.1 (SAS Institute Inc., Cary, NC). Analyses were performed using a Generalized Linear Model, using two-way ANOVA with time and treatment specified as the main effects and (time)·(treatment) as the interaction term. Data from in vitro experiments were analyzed using an unpaired t test. Significance was set at a P value < 0.05 for all tests.
RESULTS
Cytokine Expression and Immunohistochemistry
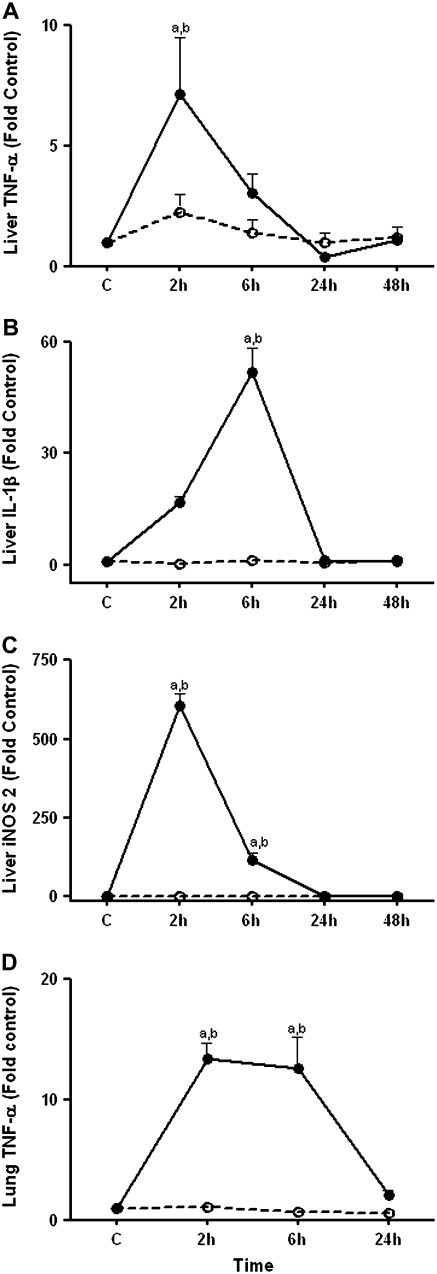
Because bacterial endotoxin is cleared by the liver and early responses to intraperitoneal endotoxin within the lung depends, in part, on hepatic release of inflammatory cytokines (34), we determined mRNA levels of TNF-α (Figure 1A), and IL-1β (Figure 1B) in the liver. TNF-α mRNA increased at 2 hours (P < 0.001) in the liver and subsequently declined while message levels of IL-1β gradually increased, reaching significance at 6 hours (P < 0.05), followed by a sharp decline at 24 hours. In addition to the systemic inflammatory response, clearance of endotoxin by liver Kupffer cells triggers increased production of reactive oxygen and reactive nitrogen species. The inducible nitric oxide synthase (iNOS) system catalyzes the formation of nitric oxide (NO) from arginine, and in contrast to its constitutive isoforms, iNOS is synthesized de novo during inflammation (35). We observed up-regulation in iNOS2 mRNA levels at 2 hours and at 6 hours (P < 0.001) (Figure 1C).
Figure 1.
Effect of LPS on liver TNF-α, IL-1β, inducible nitric oxide synthase (iNOS)2 mRNA, and lung TNF-α mRNA. C57BL/6 mice were treated with 1 mg/kg intraperitoneal LPS (solid circles) or with PBS (open circles). At 2 hours, 6 hours, and 24 hours, mice were killed and lung and liver samples were obtained. Liver samples were also obtained at the 48-hour time point. RNA was extracted from whole tissue and transcript levels of (A) liver TNF-α, (B) liver IL-1β, (C) liver iNOS2, and (D) lung TNF-α were quantified using quantitative RT-PCR. Data are expressed as means ± SEM. aValues significantly different from untreated controls, bvalues significantly different from corresponding pair-fed PBS control.
To evaluate the inflammatory response in the lung we conducted real-time PCR analysis of RNA to determine expression of TNF-α. TNF-α expression was up-regulated 13-fold at 2 hours (P < 0.001) and remained elevated up to 6 hours, after which message levels dropped (Figure 1D). Because message levels of TNF-α, IL-1β, and iNOS2 were not up-regulated in pair-fed PBS controls, we conclude that these responses were induced specifically by LPS and not due to LPS-induced decrease in food intake.
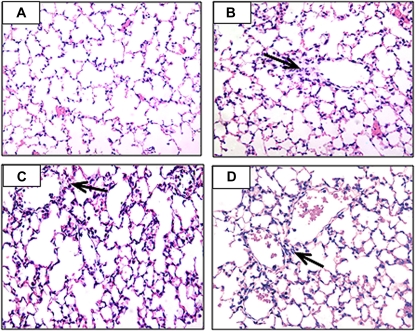
Figure 2 shows photomicrographs of H&E-stained sections of the lung after LPS treatment. The pathophysiology of the lung in the endotoxin model has been previously characterized by our group; endotoxin causes lung edema and altered respiratory function at 2 hours, and structural alterations in the lung between 6 hours and 48 hours (23). To better define the time course of pathologic changes in this model, we determined if there was evidence for lung injury at 2 hours and whether injury persisted at 48 hours. Results showed evidence for neutrophil influx in the lung at 2 hours (Figure 2B, arrow). As previously described, a diffuse inflammatory pattern was seen in the lung at 6 hours and at 24 hours (Figures 2C and 2D, arrows). Injury largely resolved by 48 hours, but some cellularity and alveolar congestion was still evident (not shown).
Figure 2.
Hematoxylin and eosin–stained sections of lung tissue from (A) untreated controls and from endotoxin-treated animals at (B) 2 hours, (C) 6 hours, and (D) 24 hours. Evidence for influx of neutrophils is observed near the airway (arrow) at 2 hours. Diffuse inflammation is observed at 6 hours and at 24 hours (arrows).
Endotoxin-Induced Weight Loss Is a Result of Decreased Food Intake
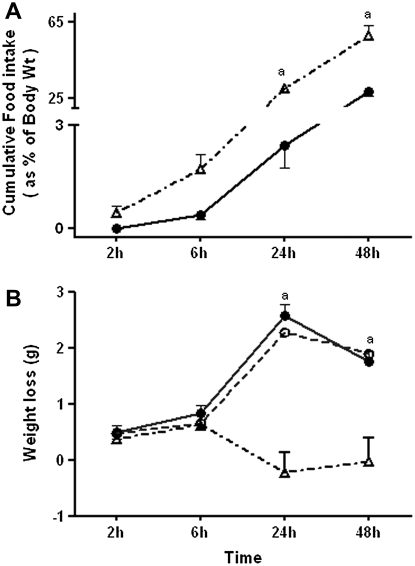
Because food intake affects blood Cys concentrations (24), we designed the study to control for food intake by pair-feeding the control group to the LPS-treated group. LPS administration profoundly decreased food intake in the first 24 hours of treatment. Food intake at 24 hours was 3% of baseline body weight in LPS-treated animals compared with 30% of body weight in ad libitum–fed PBS controls (AF PBS) (P < 0.001) (Figure 3A). Food intake increased 24 hours after LPS treatment, and at 48 hours, food intake in LPS-treated mice was 28% of body weight (measured at 24 h).
Figure 3.
Effect of LPS on food intake and body weight. C57BL/6 mice were treated with 1 mg/kg intraperitoneal LPS or with PBS. PBS-treated animals were either pair-fed (PF PBS) to intake of LPS-treated animals or were fed ad libitum (AF PBS). (A) At 2, 6, 24, and 48 hours after treatment, food intake was measured. Solid circles, LPS; open triangles, AF PBS. (B) Weight loss in LPS-treated animals (solid circles), time-matched PF PBS (open circles), and AF PBS (open triangles) controls is shown. Data are expressed as means + SEM. aValues significantly different from AF PBS controls.
LPS-treated mice lost 13% of body weight within the first 24 hours and regained 6% of the lost weight in the next 24 hours (Figure 3B). Weight loss in pair-fed PBS controls (PF PBS) was comparable to that of LPS-treated animals at all time points. AF PBS controls did not lose weight at 24 and 48 hours. The comparable weight loss observed in LPS and PF PBS animals supports the conclusion that weight loss in LPS-treated animals was predominantly due to decreased food intake rather than changes in metabolic rate due to endotoxemia.
Endotoxin-Induced Oxidation of Plasma Cys/CySS Redox State Correlates with the Initiation of Lung Injury
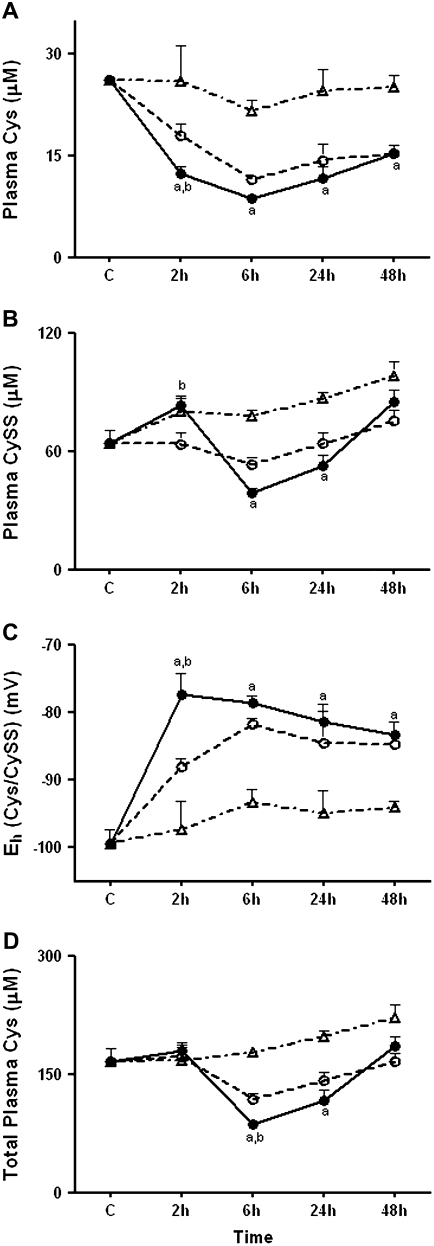
Because oxidative stress has previously been found to affect the Cys/CySS system and LPS induces oxidative stress, we hypothesized that LPS would cause an oxidation of the plasma Cys/CySS couple. We measured Cys and CySS concentrations in the plasma after administration of LPS. In these analyses, blood samples were collected into a preservation solution designed to prevent oxidation after collection (31). LPS significantly decreased plasma Cys (Figure 4A) levels at 2 hours (P < 0.05 compared with PF PBS and AF PBS controls). Cys levels also decreased in PBS-treated mice at 2 hours (P < 0.05 compared with AF PBS controls). The latter effect may be due to cortisol-mediated clearance of plasma Cys in response to food removal or due to the small difference in food intake compared with AF PBS animals (0.4 g in AF PBS controls versus 0 g in PF PBS controls). In contrast to the decrease in Cys, plasma levels of the disulfide, CySS (Figure 4B) increased by an average of 20 μM in LPS-treated animals compared with PF PBS controls (P < 0.05), showing that a substantial shift in the balance of plasma Cys to CySS occurred at 2 hours. The calculated value for plasma Eh Cys/CySS (Figure 4C) showed that LPS-treated animals were oxidized by an average of 10 mV compared with PF PBS controls (P < 0.01), and by an average of 20 mV compared with AF PBS controls (P < 0.001). Under these conditions, the total Cys pool present as Cys + 2*CySS did not change in LPS-treated animals or PBS controls, indicating that LPS-induced oxidation of Cys/CySS occurred without significant effects on the total plasma pool of Cys + CySS (Figure 4D). Thus, the data show that LPS treatment caused a significant oxidation of the Cys/CySS couple in the plasma at 2 hours.
Figure 4.
Effect of LPS on plasma Cys, CySS, Eh Cys/CySS, and total Cys. C57BL/6 mice were treated with 1 mg/kg intraperitoneal LPS or with PBS. At 2, 6, 24, and 48 hours, mice were killed and plasma was collected for high-performance liquid chromatography (HPLC) analysis of (A) Cys and (B) CySS. In C, Eh Cys/CySS was calculated from the Cys and CySS concentrations using the Nernst equation. (D) Total Cys equivalents were obtained by adding together Cys + 2*CySS. Data are expressed as means ± SEM. aValues significantly different from ad libitum–fed PBS controls; bvalues significantly different from corresponding pair-fed PBS control. Solid circles, LPS; open circles, PF PBS; open triangles, AF PBS.
Plasma Cys levels in LPS and PF PBS-treated animals remained below AF PBS controls at all subsequent time points studied (P < 0.01). Interestingly, at 6 hours and at 24 hours, plasma CySS levels significantly decreased in LPS-treated animals compared with AF PBS controls (P < 0.001). The decrease in CySS, in addition to the prevailing low concentrations of Cys, contributed to a decline in the total plasma Cys pool at 6 hours and at 24 hours (P < 0.001, compared with AF PBS controls). Despite the decrease in CySS, calculations of the redox state of Cys/CySS showed that it was considerably oxidized up to 48 hours in LPS-treated animals. This change suggests that after the oxidation of plasma Cys/CySS at 2 hours, induction of CySS uptake may protect against more extensive oxidation and also increase cellular supply of precursors for GSH synthesis.
Thus, the combination of data show that oxidation of plasma Cys/CySS at 2 hours occurs without a change in the total pool size, and precedes maximal pathologic changes in the lung. In contrast, oxidation of Cys/CySS redox state between 6 hours and 48 hours occurred with a decrease in total pool size, suggesting that the sustained oxidation occurred as a consequence of decreased precursor availability relative to tissue demand.
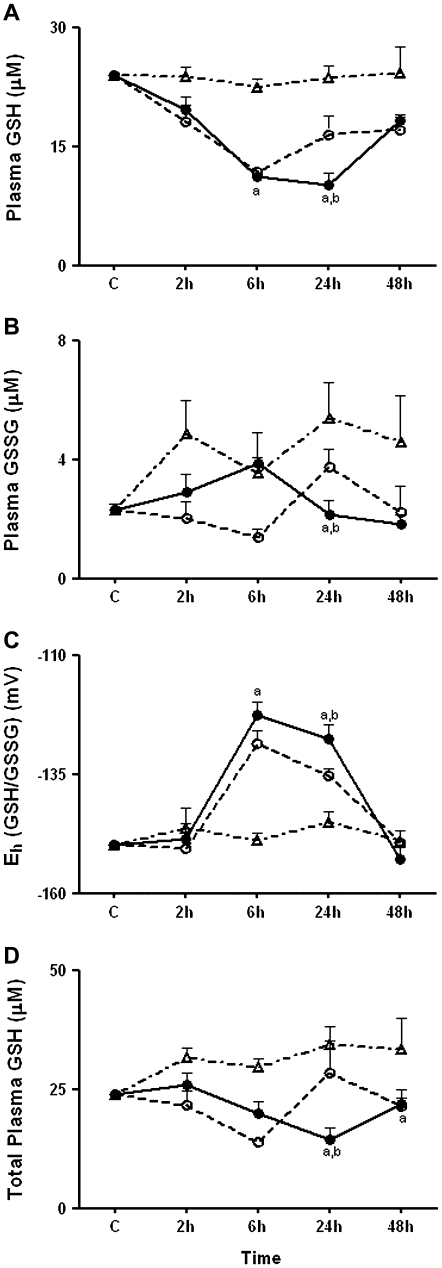
Oxidation of Plasma GSH/GSSG Redox State Followed Oxidation of Plasma Cys/CySS Redox State
Previous research shows that the effects of LPS on plasma GSH are time dependent. LPS induces an acute increase in plasma GSH, due to hepatic GSH efflux, followed by a decrease in GSH due to oxidation (9, 10). In the current study, analysis of GSH and GSSG showed that no significant changes were apparent at 2 hours, whereas a significant decrease in GSH without a corresponding increase in GSSG was present at 6 hours (Figures 5A and 5B). The calculated Eh (GSH/GSSG) was significantly oxidized only at 6 and 24 hours; no oxidation of GSH/GSSG redox state occurred at 2 hours (Figure 5C). Compared with simultaneous measurements of Cys/CySS redox state in Figure 4, these results show that oxidation of GSH/GSSG redox state in plasma follows oxidation of plasma Cys/CySS.
Figure 5.
Effect of LPS on plasma GSH, GSSG, Eh GSH/GSSG, and total GSH. C57BL/6 mice were treated with 1 mg/kg intraperitoneal LPS or with PBS. At 2, 6, 24, and 48 hours, mice were killed and plasma was collected for HPLC analysis of (A) GSH and (B) GSSG. In C, Eh GSH/GSSG was calculated from the GSH and GSSG concentrations using the Nernst equation. (D) Total GSH equivalents were obtained by adding together GSH + 2*GSSG. Data are expressed as means ± SEM. aValues significantly different from ad libitum–fed PBS controls; bvalues significantly different from corresponding pair-fed PBS control.
Oxidation of GSH/GSSG occurred in pair-fed controls as well as in LPS-treated mice. At 6 hours, the decrease in plasma GSH resulted in a 26 mV oxidation of Eh (GSH/GSSG) in LPS-treated animals compared with AF PBS controls (P < 0.001) (Figure 5A). Unexpectedly, a similar decline in plasma GSH was observed in PF PBS controls, indicating that the decrease in plasma GSH at 6 hours was related to the relative decrease in food intake rather than a direct consequence of LPS-induced oxidative stress. At 24 hours, GSH levels remained significantly decreased in LPS-treated animals (10.1 ± 1.5 μM) leading to a significant decrease in the total plasma GSH pool (GSH+2*GSSG) in LPS-treated animals (P < 0.01, compared with PF PBS and AF PBS controls) (Figure 5D). Thus, the results show that oxidation of plasma Eh (GSH/GSSG) follows the early oxidation of Cys/CySS and is largely explained by a decline in food consumption rather than a direct oxidative process activated by LPS.
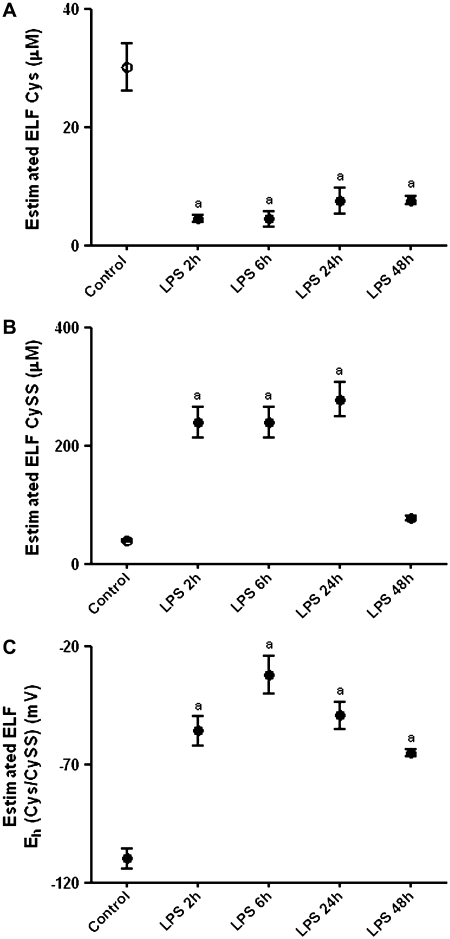
LPS Induced Oxidation of Cys/CySS Redox State in Lung Epithelial Lining Fluid
Because GSH levels in the lung lining fluid are documented to be decreased after endotoxin (36), we hypothesized that levels of the precursor Cys pool would also be compromised. Concentrations of Cys and CySS in the epithelial lining fluid (ELF), obtained immediately after killing, were estimated using the urea dilution factor. The dynamics of Cys in lung lining fluid closely mirrored that of plasma Cys (Figure 6A). At 2 hours, Cys levels in LPS-treated animals were 7-fold lower than in controls, and remained significantly decreased up to 48 hours (P < 0.001). CySS levels markedly increased at 2 hours, remained elevated up to 24 hours (P < 0.001), and dropped to control values at 48 hours (Figure 6B). Eh Cys/CySS was oxidized by 50 mV at 2 hours after LPS (P < 0.001; compared with untreated controls) and remained significantly oxidized up to 48 hours (Figure 6C).
Figure 6.
Effect of LPS on Cys and CySS levels in epithelial lining fluid. Bronchoalveolar lavage was sampled from C57BL/6 mice treated with 1 mg/kg intreaperitoneal LPS and from untreated controls. Levels of (A) Cys, (B) CySS, and (C) Eh Cys/CySS in epithelial lining fluid are shown after correction using a urea dilution factor. Data are expressed as means ± SEM. aValues significantly different from untreated controls.
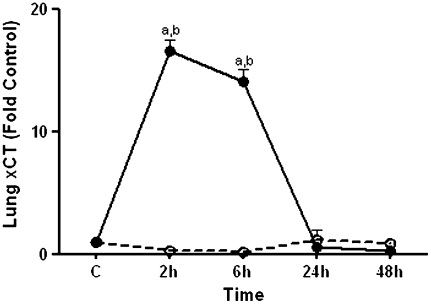
Endotoxin Induced Transcriptional Up-Regulation of xCT in the Lung
LPS is known to increase the expression of the CySS transporter, system xc− in vitro (37). Because the decline in plasma CySS at 6 hours and at 24 hours could be a result of increased transport, we determined message levels of xCT, the subunit of system xc− responsible for transport activity, in the lung. RT-PCR analyses revealed that xCT was increased at 2 hours and at 6 hours (Figure 7). Thus, the decline in plasma pools for Cys + CySS may result from increased CySS uptake due to induction of the xCT system.
Figure 7.
Effect of LPS on xCT mRNA in the lung. C57BL/6 mice were treated with 1 mg/kg intraperitoneal LPS (solid circles) or with PBS (open circles). At 2, 6, 24, and 48 hours, mice were killed and lung samples were obtained. RNA was extracted from whole lung and transcript levels of xCT were quantified using quantitative RT-PCR. Data are expressed as means ± SEM. aValues significantly different from untreated controls; bvalues significantly different from corresponding pair-fed PBS control.
Oxidized Extracellular Cys/CySS Redox State Enhances Adhesion of Leukocytes to Pulmonary Endothelial Cells
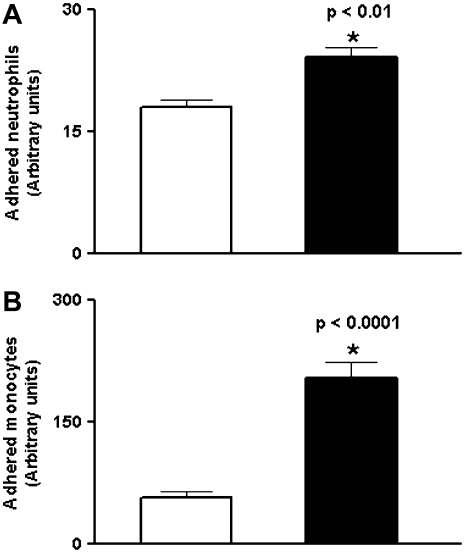
Previous studies have shown that oxidized extracellular Cys/CySS redox state stimulates the adhesion of monocytes to the vascular endothelium (18). Here, we determined whether adherence of leukocytes to pulmonary endothelial cells was augmented under oxidized Cys/CySS redox states. Endothelial cells were exposed to −80 mV and −46 mV redox medium for 24 to 36 hours. Subsequently, labeled leukocytes were incubated with endothelial cells for 30 minutes and the media was aspirated to remove unbound cells. Oxidized Eh Cys/CySS enhanced the adherence of neutrophils (Figure 8A), and monocytes (Figure 8B) to pulmonary endothelial cells (P < 0.01). Thus, the data suggest that early oxidation of Cys/CySS during endotoxemia may contribute to leukocyte influx into the lung.
Figure 8.
Effect of oxidized Eh Cys/CySS on leukocyte adherence to pulmonary endothelial cells. Oxidized Eh Cys/CySS increases adherence of (A) neutrophils by 1.3-fold (P < 0.01), and (B) monocytes by 2-fold (P < 0.0001) to endothelial cells. The values shown are mean ± SEM from three replicates of a representative experiment. Open bars, physiologic Eh Cys/CySS (−80 mV); solid bars, oxidized Eh Cys/CySS (−46 mV).
DISCUSSION
In the present study, we describe the dynamics of plasma GSH/GSSG and Cys/CySS systems during endotoxemia. The findings on Cys/CySS redox state add to the understanding of thiol changes in sepsis. There is substantial evidence that LPS impairs GSH homeostasis. Jaeschke reported that hepatic efflux of GSH occurred during the first hour after endotoxin exposure, and that the magnitude of efflux depended on endotoxin dose (9). More recently, Payabvash and colleagues reported that plasma (total) GSH significantly increased 1 hour after endotoxin (5 mg/kg) and returned to baseline values by 3 hours (10). In contrast, Minamiyama and coworkers found a 2-fold reduction in plasma GSH in rats as early as 2 hours after endotoxin (20 mg/kg) (38). We found that plasma GSH and GSSG levels were comparable to baseline values 2 h after LPS. Therefore, the dynamics of plasma GSH during endotoxemia appear to depend on the dose of endotoxin and the time after administration when the measurement is made. Unexpectedly, we found that oxidation of GSH/GSSG redox state at 6 hours and at 24 hours was largely a consequence of decreased food intake. Thus, the present data do not support a direct role for LPS in oxidation of GSH/GSSG redox state between 6 and 24 hours but rather a role for LPS-induced anorexia in contributing substantially to impaired GSH homeostasis. Since we did not make the measurements at earlier time points, a direct oxidative effect of LPS on GSH/GSSG redox state before 2 hours cannot be excluded.
In contrast to GSH, we observed a significant decline in plasma Cys levels and a resulting oxidation of Eh Cys/CySS at 2 hours after endotoxin. This is consistent with the greater relative reactivity of Cys, compared with GSH, to hydrogen peroxide (39), which is produced early during endotoxin-induced lung injury (40). The pronounced decline in plasma Cys could be solely due to LPS-induced oxidative stress, an interpretation which is supported by an increase in plasma CySS at 2 hours. However, LPS increases activity of the hepatic Cys transporters (41), and stimulated transport could contribute to a decrease in plasma Cys at 2 hours. We found that the initial difference in plasma Cys between LPS-treated mice and pair-fed controls was not sustained over time, indicating a direct effect of reduced food intake on plasma Cys levels between 6 hours and 48 hours.
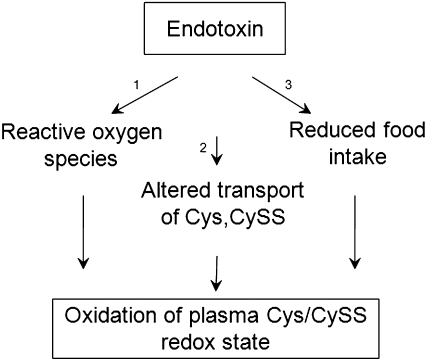
Interestingly, at 6 hours and at 24 hours, plasma CySS levels in LPS-treated mice decreased significantly. The increase in message levels of xCT suggests that this decline is due to increased CySS transport via the CySS/glutamate exchange transport system, system xc−. Increased CySS uptake is known to enhance extracellular Cys and GSH delivery, which can support the transfer S-nitrosothiols across the cell membrane (42). Thus, the balance of plasma Cys/CySS during endotoxemia appears to depend on mechanisms that include oxidation of Cys to CySS, transport of Cys and CySS, and dietary availability of Cys and Cys precursors (Figure 9).
Figure 9.
Endotoxin/LPS can induce oxidation of plasma Cys/CySS redox state by three mechanisms. Endotoxin-induced production of reactive oxygen species (1) is an early event that can contribute to the oxidation of Cys to CySS (2). Increased transport of Cys can further potentiate the early decrease in Cys levels. CySS transport is also induced by endotoxin-mediated effects. In the present study we see that reduced food intake (3) contributes to LPS-induced decrease in Cys, and concominant oxidation of plasma Cys/CySS redox state at later time points.
The data show that Eh Cys/CySS in the plasma is more sensitive than Eh GSH/GSSG to the acute effects of endotoxin, and that Cys/CySS redox state rebounds more slowly than GSH/GSSG redox state. While a precursor–product relationship exists between Cys and GSH, the redox potentials of the couples are not in equilibrium (12). The redox state of plasma Cys/CySS is more oxidized compared with GSH/GSSG redox state in humans (−80 mV for Cys/CySS versus −137 mV for GSH/GSSG) (12) as well as in mice (−100 mV for Cys/CySS versus −150 mV for GSH/GSSG). The more oxidized redox state of Cys/CySS in the plasma is attributed to Cys being proximal to oxidative events in the extracellular fluid compared with GSH (12). For instance, in humans, dietary restriction of sulfur amino acids leads to oxidation of plasma Cys/CySS redox state at 3 days, with no observable oxidation in the GSH/GSSG redox state (43). Thus, the 10-mV oxidation of Eh Cys/CySS at 2 hours after LPS, when Eh GSH/GSSG remains unchanged, is consistent with other studies of the dynamics of the redox state of the plasma Cys pool. Furthermore, the distinct effects of LPS on transport systems for GSH and Cys (Table 2) contributes to the disequilibrium in the redox potentials of the couples at 2 hours. By 48 hours, there is clearance of cytokines in the circulation and the concentrations of plasma GSH reflects re-feeding. Because a rapid increase in GSH synthesis occurs upon re-feeding (44), the disequilibrium in the redox potentials of Cys and GSH at 48 hours is attributed to the use of Cys for GSH (and protein) synthesis.
TABLE 2.
EFFECT OF LPS ON GSH, CySS, CyS TRANSPORT (SYSTEMS)
| Thiol/Transport System | Effects | Model | Mechanism | Putative Function | Ref. |
|---|---|---|---|---|---|
| GSH/transporter unknown | 300% increase in GSH efflux, 15 min after LPS* (5 mg/kg body wt; intravenously) | In vivo, hepatic sinusoids | Increase activity of GSH transporter by LPS-mediated induction of complement | Protect against extracellular oxidant stress | (9) |
| Cystine/xc− | ∼3-fold increase in CySS influx, 6 h after LPS (1 ng/ml) | In vitro, murine primary peritoneal macrophages | Transcriptional upregulation of xCT | Increase cellular GSH | (37) |
| Cysteine/A, ASC | ∼2.7-fold increase in Cys influx 4 h after LPS (7.5mg/kg body wt; intraperitoneally) | Ex vivo, hepatic membrane vesicles | Increase in Vmax of System A and ASC by LPS-mediated induction of cortisol, TNF-α, and cyclooxygenase- derived products | Support gluconeogenesis and synthesis of acute phase proteins | (41) |
Definition of abbreviations: ASC, Na+-dependent neutral amino acid transporter; CyS, cysteine; CySS, disulfide cysteine; GSH, glutathione; xCT, subunit of the CySS transporter.
Salmonella enteritidis LPS.
The observation that adherence of leukocytes to pulmonary endothelial cells is increased under oxidized Eh Cys/CySS suggests that oxidized Cys/CySS may potentiate lung injury by increasing leukocyte influx into the lung. Cys/CySS redox state is now recognized as an independent extracellular redox control system (13–15), and variations in extracellular Eh Cys/CySS can alter biological responses to growth factors such as transforming growth factor (TGF)-α and TGF-β (33, 45). Of significance to ALI, oxidized Eh Cys/CySS activates NF-κB and smad3, critical players in lung injury and in repair (18, 33). Because plasma Cys/CySS is equilibrated with Cys/CySS in the pulmonary lymph (K.L. Brigham and D.P. Jones, unpublished observations), alterations in plasma Cys/CySS redox state can impact cellular processes in the lung. The early oxidation of ELF and plasma Eh Cys/CySS can potentiate the inflammatory response to injury, while the sustained oxidation of Eh Cys/CySS could play a role in modulating the repair process. Furthermore, because Cys is a rate limiting precursor in the synthesis of GSH, a decrease in Cys levels can compromise cellular GSH pools and thereby sensitize cells to acute stress (46). Mechanistic studies investigating the role of oxidized Cys/CySS redox state in lung injury will provide more direct answers on how perturbations in thiol status can contribute to the pathogenesis of ALI, and in doing so may help resolve the discrepancies associated with antioxidant therapies such as NAC.
In summary, we find that early oxidation of plasma Cys/CySS redox state occurred due to LPS treatment. Plasma GSH/GSSG showed no oxidation at 2 hours. Subsequent oxidation of Cys/CySS and GSH/GSSG systems appeared to be due to anorexia because pair-fed controls showed equivalent oxidation. Consequently, the combined observations indicate that enhanced oxidation of Cys, altered transport of Cys and CySS, and decreased food intake each contribute to oxidized Cys/CySS redox state in endotoxemia.
Acknowledgments
The authors thank Dr. Lou Ann Brown and Dr. Tom Ziegler for their critical review of the manuscript. They thank Kristi Porter and Tamara Murphy for providing the endothelial cells and Jeffrey Ritzenthaler for the U937 cells.
This work was supported by NIH Grants ES009047 and ES011195 (D.P.J.) and the McKelvey Center for Lung Transplantation (K.L.B. and M.R.).
Originally Published in Press as DOI: 10.1165/rcmb.2007-0447OC on July 29, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–1349. [DOI] [PubMed] [Google Scholar]
- 2.Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury: predictors of mortality. Am J Respir Crit Care Med 1995;152:1818–1824. [DOI] [PubMed] [Google Scholar]
- 3.Pittet JF, Mackersie RC, Martin TR, Matthay MA. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med 1997;155:1187–1205. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med 2006;27:337–349. [DOI] [PubMed] [Google Scholar]
- 5.Quinlan GJ, Lamb NJ, Tilley R, Evans TW, Gutteridge JM. Plasma hypoxanthine levels in ARDS: implications for oxidative stress, morbidity, and mortality. Am J Respir Crit Care Med 1997;155:479–484. [DOI] [PubMed] [Google Scholar]
- 6.Quinlan GJ, Evans TW, Gutteridge JM. Oxidative damage to plasma proteins in adult respiratory distress syndrome. Free Radic Res 1994;20:289–298. [DOI] [PubMed] [Google Scholar]
- 7.Pacht ER, Timerman AP, Lykens MG, Merola AJ. Deficiency of alveolar fluid glutathione in patients with sepsis and the adult respiratory distress syndrome. Chest 1991;100:1397–1403. [DOI] [PubMed] [Google Scholar]
- 8.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 1998;101:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaeschke H. Enhanced sinusoidal glutathione efflux during endotoxin-induced oxidant stress in vivo. Am J Physiol 1992;263:G60–G68. [DOI] [PubMed] [Google Scholar]
- 10.Payabvash S, Ghahremani MH, Goliaei A, Mandegary A, Shafaroodi H, Amanlou M, Dehpour AR. Nitric oxide modulates glutathione synthesis during endotoxemia. Free Radic Biol Med 2006;41:1817–1828. [DOI] [PubMed] [Google Scholar]
- 11.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 2001;30:1191–1212. [DOI] [PubMed] [Google Scholar]
- 12.Jones DP, Mody VC Jr, Carlson JL, Lynn MJ, Sternberg P Jr. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med 2002;33:1290–1300. [DOI] [PubMed] [Google Scholar]
- 13.Anderson CL, Iyer SS, Ziegler TR, Jones DP. Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol 2007;293:R1069–R1075. [DOI] [PubMed] [Google Scholar]
- 14.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM Jr, Kirlin WG. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J 2004;18:1246–1248. [DOI] [PubMed] [Google Scholar]
- 15.Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, Kolle P, Tschoep K, Issels RD, Daniel PT, et al. The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene 2008;27:1618–1628. [DOI] [PubMed] [Google Scholar]
- 16.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med 2003;35:1582–1588. [DOI] [PubMed] [Google Scholar]
- 17.Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med 2007;176:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation 2005;111:2973–2980. [DOI] [PubMed] [Google Scholar]
- 19.Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P Jr, Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci 2005;46:1054–1061. [DOI] [PubMed] [Google Scholar]
- 20.Suter PM, Domenighetti G, Schaller MD, Laverriere MC, Ritz R, Perret C. N-acetylcysteine enhances recovery from acute lung injury in man: a randomized, double-blind, placebo-controlled clinical study. Chest 1994;105:190–194. [DOI] [PubMed] [Google Scholar]
- 21.Domenighetti G, Suter PM, Schaller MD, Ritz R, Perret C. Treatment with N-acetylcysteine during acute respiratory distress syndrome: a randomized, double-blind, placebo-controlled clinical study. J Crit Care 1997;12:177–182. [DOI] [PubMed] [Google Scholar]
- 22.Bernard GR. N-acetylcysteine in experimental and clinical acute lung injury. Am J Med 1991;91:54S–59S. [DOI] [PubMed] [Google Scholar]
- 23.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol 2005;288:L333–L341. [DOI] [PubMed] [Google Scholar]
- 24.Nkabyo YS, Gu LH, Jones DP, Ziegler TR. Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. J Nutr 2006;136:1242–1248. [DOI] [PubMed] [Google Scholar]
- 25.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–2088. [DOI] [PubMed] [Google Scholar]
- 26.Kabir K, Gelinas JP, Chen M, Chen D, Zhang D, Luo X, Yang JH, Carter D, Rabinovici R. Characterization of a murine model of endotoxin-induced acute lung injury. Shock 2002;17:300–303. [DOI] [PubMed] [Google Scholar]
- 27.Johnston CJ, Finkelstein JN, Gelein R, Oberdorster G. Pulmonary cytokine and chemokine mRNA levels after inhalation of lipopolysaccharide in C57BL/6 mice. Toxicol Sci 1998;46:300–307. [DOI] [PubMed] [Google Scholar]
- 28.Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp Lung Res 2007;33:375–384. [DOI] [PubMed] [Google Scholar]
- 29.Belz EE, Kennell JS, Czambel RK, Rubin RT, Rhodes ME. Environmental enrichment lowers stress-responsive hormones in singly housed male and female rats. Pharmacol Biochem Behav 2003;76:481–486. [DOI] [PubMed] [Google Scholar]
- 30.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 2002;348:93–112. [DOI] [PubMed] [Google Scholar]
- 31.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Glutathione measurement in human plasma: evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta 1998;275:175–184. [DOI] [PubMed] [Google Scholar]
- 32.Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med 2000;28:625–635. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez A, Ramadan B, Ritzenthaler JD, Rivera HN, Jones DP, Roman J. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol 2007;293:L972–L981. [DOI] [PubMed] [Google Scholar]
- 34.Siore AM, Parker RE, Stecenko AA, Cuppels C, McKean M, Christman BW, Cruz-Gervis R, Brigham KL. Endotoxin-induced acute lung injury requires interaction with the liver. Am J Physiol Lung Cell Mol Physiol 2005;289:L769–L776. [DOI] [PubMed] [Google Scholar]
- 35.Bultinck J, Sips P, Vakaet L, Brouckaert P, Cauwels A. Systemic NO production during (septic) shock depends on parenchymal and not on hematopoietic cells: in vivo iNOS expression pattern in (septic) shock. FASEB J 2006;20:2363–2365. [DOI] [PubMed] [Google Scholar]
- 36.Jacob BA, Porter KM, Elms SC, Cheng PY, Jones DP, Sutliff RL. HIV-1-induced pulmonary oxidative and nitrosative stress: exacerbated response to endotoxin administration in HIV-1 transgenic mouse model. Am J Physiol Lung Cell Mol Physiol 2006;291:L811–L819. [DOI] [PubMed] [Google Scholar]
- 37.Sato H, Fujiwara K, Sagara J, Bannai S. Induction of cystine transport activity in mouse peritoneal macrophages by bacterial lipopolysaccharide. Biochem J 1995;310:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minamiyama Y, Takemura S, Koyama K, Yu H, Miyamoto M, Inoue M. Dynamic aspects of glutathione and nitric oxide metabolism in endotoxemic rats. Am J Physiol 1996;271:G575–G581. [DOI] [PubMed] [Google Scholar]
- 39.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med 1999;27:322–328. [DOI] [PubMed] [Google Scholar]
- 40.Minamiya Y, Abo S, Kitamura M, Izumi K, Kimura Y, Tozawa K, Saito S. Endotoxin-induced hydrogen peroxide production in intact pulmonary circulation of rat. Am J Respir Crit Care Med 1995;152:348–354. [DOI] [PubMed] [Google Scholar]
- 41.Inoue Y, Bode BP, Abcouwer S, Souba WW. Attenuation of the endotoxin-stimulated increase in hepatic amino acid transport with a glucocorticoid receptor antagonist. J Surg Res 1995;58:693–701. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc Natl Acad Sci USA 2004;101:7891–7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mannery Y, Ziegler TR, Jones DP. A chemically defined diet with insufficient sulfur amino acids induces oxidation of plasma cysteine/cystine and glutathione/glutathione disulfide redox state in humans [abstract]. FASEB J 2007:A697.
- 44.Leaf G, Neuberger A. The effect of diet on the glutathione content of the liver. Biochem J 1947;41:280–287. [PMC free article] [PubMed] [Google Scholar]
- 45.Nkabyo YS, Go YM, Ziegler TR, Jones DP. Extracellular cysteine/cystine redox regulates the p44/p42 MAPK pathway by metalloproteinase-dependent epidermal growth factor receptor signaling. Am J Physiol Gastrointest Liver Physiol 2005;289:G70–G78. [DOI] [PubMed] [Google Scholar]
- 46.Brown LA, Harris FL, Bechara R, Guidot DM. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alcohol Clin Exp Res 2001;25:1078–1085. [PubMed] [Google Scholar]