Abstract
Circulating levels of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) are increased during acute lung injury; however, combined effects of HGF and VEGF on pulmonary endothelial cell (EC) permeability remain to be elucidated. We have previously shown differential remodeling of focal adhesions (FA) caused by barrier-protective and barrier-disruptive mechanical and chemical stimuli. This study examined a role of FA protein paxillin in the pulmonary EC barrier responses induced by HGF and VEGF. VEGF increased, but HGF decreased, pulmonary EC permeability. These effects were accompanied by differential patterns of site-specific phosphorylation of focal adhesion kinase (FAK) and paxillin and FA redistribution. HGF antagonized random FA formation caused by VEGF challenge and promoted FA accumulation at the cell periphery. HGF attenuated VEGF-induced paxillin redistribution, FA remodeling, and endothelial permeability. SiRNA-based paxillin knockdown attenuated VEGF-induced EC permeability, myosin light chain phosphorylation, and stress fiber and paracellular gap formation. Paxillin knockdown also decreased HGF-induced EC barrier enhancement and suppressed activation of Rac and its effector PAK1. Expression of paxillin-S273 deficient on PAK1 phosphorylation site prevented HGF-induced cytoskeletal remodeling. These data show a dual role of paxillin in the HGF- and VEGF-mediated endothelial barrier regulation and suggest essential paxillin role in the modulation of Rac-Rho crosstalk. Our results also support a model of pulmonary EC barrier recovery during resolution of ALI via switch from VEGF to HGF signaling.
Keywords: paxillin, small GTPase, pulmonary endothelium, permeability, growth factors
CLINICAL RELEVANCE
We show that paxillin may serve as integrator of hepatocyte growth factor (HGF)/vascular endothelial growth factor (VEGF) signaling to small GTPases regulating endothelial cell (EC) permeability. Our data support a model of pulmonary EC barrier recovery during resolution of acute lung injury via switch from VEGF to HGF signaling.
Vascular endothelial growth factor (VEGF), initially named “vascular permeability factor,” causes pronounced permeability in vivo and exhibits potent pro-angiogenic effects (1, 2). Increased VEGF levels in pulmonary circulation detected in animal models (3) and in patients with acute lung injury (4) were associated with persistent inflammatory stimulation and pathologic mechanical stretch (5). VEGF is a potent angiogenic factor, but also exhibits barrier-disruptive effects on the vascular endothelium and activation of Rho GTPase–dependent signaling cascade (6–8).
Hepatocyte growth factor (HGF) in the lung is expressed by various cell types, including lung macrophages (9), bronchial and alveolar epithelium (10), and fibroblasts (11). HGF is a potent angiogenic factor (12), and chronic elevation of HGF stimulates angiogenesis in target tissues accompanied by temporal leakiness of the newly formed vessels (13). However, similar to another barrier-protective and angiogenic factor, sphingosine 1-phosphate (14), HGF causes potent barrier-protective responses in cerebral and human pulmonary endothelium (15–18), which are mediated by small GTPase Rac and PI3 kinase–dependent mechanisms (16, 18). Both VEGF and HGF appear elevated during acute lung injury (5, 19). However, relations between HGF and VEGF in the control of lung endothelial barrier function remain to be elucidated.
Focal adhesions (FA) linking pulmonary endothelial cells (ECs) to underlying substrate have been considered as mechanosensors and signaling “hubs” (20), which may define EC remodeling and permeability changes associated with ventilator-induced lung injury (VILI). Specific patterns of FA protein interactions and site-specific phosphorylation have been associated with barrier-protective and barrier-disruptive EC responses to mechanical and chemical stimuli (21–27). Several FA-associated proteins play a key role in FA assembly, interaction with cytoskeleton, and FA-dependent signal transduction. FA-associated adaptor protein paxillin is one of the major focal adhesion kinase (FAK) substrates of focal adhesion–associated tyrosine kinase FAK (28). Paxillin phosphorylation at Tyr31 and Tyr118 by FAK or Src family kinases is important for paxillin redistribution and increased interaction with other FA-associated proteins (29). In addition, paxillin and FAK may locally regulate the activity of the Rho GTPase by FAK-induced phosphorylation and paxillin-mediated recruitment and activation of Rho-specific regulators, such as p190RhoGEF (30) and p190RhoGAP (31). Paxillin may also regulate local Rac activity by recruitment of Rac-specific GEF βPIX (32, 33). Formation of focal adhesion-associated paxillin-GIT1-βPIX-PAK signaling complex and its peripheral localization requires paxillin phosphorylation by PAK1 at Ser273 (34). It is hypothesized that this complex may further regulate Rac GTPase activity and enhance EC monolayer barrier properties. Thus, the mechanisms of Rac and Rho regulation by FA-associated proteins appear to be dependent on physiologic context.
This study investigated differential FA and cytoskeletal remodeling in response to HGF and VEGF associated with differential EC permeability responses. We have also examined a role of paxillin in the VEGF- and HGF-mediated signaling by small GTPases and EC barrier regulation.
MATERIALS AND METHODS
Reagents and Cell Culture
Antibodies to paxillin and Rac were obtained from BD Transduction Laboratories (San Diego, CA); phospho-specific paxillin and FAK antibodies were purchased from Biosource (Invitrogen, Carlsbad, CA); and phospho-PAK1, di-phospho-MLC, and PAK1 antibodies were obtained from Cell Signaling (Beverly, MA). All reagents used for immunofluorescence staining were purchased from Molecular Probes (Eugene, OR). Unless specified, all biochemical reagents were obtained from Sigma (St. Louis, MO). Human pulmonary artery endothelial cells (HPAEC) were obtained from Lonza, Inc. (Allendale, NJ). Bovine pulmonary artery endothelial cells (BPAEC) were obtained from American Type Tissue Culture Collection (Culture line-CCL 209; Rockville, MD) and cultured as described previously (21, 35). All experimental data obtained for HPAEC were reproduced in BPAEC culture. Comparison of these cell types did not reveal any major differences in cellular responses to HGF or VEGF stimulation.
Si-RNA–Based Knockdown of Rac and Paxillin in Pulmonary EC
Depletion of endogenous Rac and paxillin protein content in pulmonary EC was performed using gene-specific siRNA duplexes as previously described (36). In brief, pre-designed Rac1-specific (36) and paxillin-specific (37) siRNAs of standard purity were ordered from Ambion (Austin, TX) in purified, desalted, deprotected, annealed, double-strand form. The following 21–base pair duplexes of siRNA were used: Rac1, 5′-GGAGAUUGGUGCUGUAAAAtt-3′ and 5′-UUUUACAGCACC-AAUCUCCtt-3′; paxillin, 5′-CCCUGACGAAAGAGAAGCCUAUU-3′ and 5′-UAGGCUUCUCUUUCGUCAGGGUU-3′. Nonspecific, nontargeting siRNA duplex #1 from Dharmacon (Lafayette, CO) was used as a control treatment. Cells were grown to 70% confluence, and the transfection of siRNA (final concentration 50 nM) was performed using DharmaFECT1 transfection reagent (Dharmacon) according to manufacturer's protocol. After 48 hours, cells were harvested and used for experiments.
Expression Plasmids and Transfection Protocol
Plasmids encoding wild-type paxillin and S273A paxillin mutant bearing GFP-tag were kindly provided by Dr. C. E. Turner (State University of New York Upstate Medical University) and Dr. A. R. Horwitz (University of Virginia). Transient transfections of pulmonary EC were performed as described previously (36, 38). In brief, EC grown onto gelatin-coated glass coverslips in 12-well plates at 70% confluence were incubated with 1 ml of OPTI-MEM medium containing 1 μg DNA and 3 μl of Fugene 6 (Boehringer Mannheim–Roche, Indianapolis, IN) for 4 hours in a CO2 incubator at 37°C. Medium was replaced with complete medium containing 10% fetal calf seum, and cells were incubated for an additional 24 hours and used for experiments with agonist stimulation.
Rac activation assays were performed using commercially available assay kits purchased from Upstate Biotechnology (Lake Placid, NY), as we have previously described (36). In brief, after stimulation cell lysates were collected and GTP-bound Rac was captured using pulldown assays with immobilized PBD domain according to manufacturer's protocols. The levels of activated Rac as well as total Rac content were evaluated by Western blot analysis and quantified by scanning densitometry of the autoradiography films. The levels of activated Rac were normalized to total Rac level for densitometry evaluations.
Immunofluorescence Staining and Image Analysis
ECs grown on glass coverslips were stimulated with agonist of interest or left untreated. Cells were fixed and subjected to immunofluorescence staining for paxillin of F-actin; image analysis was performed as described elsewhere (25, 39). In brief, ECs grown on glass coverslips were fixed after agonist treatment in 3.7% formaldehyde solution in PBS for 10 minutes at 4°C, washed three times with PBS, permeabilized with 0.2% triton X-100 in PBS-Tween (PBST) for 30 minutes at room temperature, and blocked with 2% bovine serum albumin in PBST for 30 minutes. Incubation with antibodies of interest was performed in blocking solution for 1 hour at room temperature followed by staining with Alexa 488–conjugated secondary antibodies (Molecular Probes, Eugene, OR). Actin filaments were stained with Texas Red–conjugated phalloidin (Molecular Probes) for 1 hour at room temperature. After immunostaining, the glass slides were analyzed using a Nikon video-imaging system (Nikon Instech Co., Tokyo, Japan) consisting of a inverted microscope Nikon Eclipse TE300 with epi-fluorescence module using 60×A/1.40 oil objective connected to SPOT RT monochrome digital camera (temperature of 37°C) and image processor (Diagnostic Instruments, Sterling Heights, MI). The images were acquired using SPOT 3.5 acquisition software (Diagnostic Instruments) and processed with Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) and Adobe Illustrator CS (Adobe Systems) software. Quantitative analysis of paxillin-positive focal adhesions and paracellular gap formation was performed as previously described (25, 39). The 16-bit images were analyzed using Image J software (National Institutes of Health, Washington, DC). Images were differentially segmented between gaps (black) and cells (highest gray value) based on image grayscale levels. The gap formation was expressed as a ratio of the gap area to the area of the whole image. At least 20 microscopic fields for each experimental condition were analyzed. A similar technique was used to monitor focal adhesion remodeling or actin peripheral rim formation. The values were statistically processed using Sigma Plot 7.1 (SPSS Science, Chicago, IL) software.
Measurement of Transendothelial Electrical Resistance
The cellular barrier properties were measured using the highly sensitive biophysical assay with an electrical cell-substrate impedance sensing system (Applied Biophysics, Troy, NY) as described previously (35). In brief, cells were grown on small gold microelectrodes (10−4 · cm2) in complete culture medium containing 10% fetal bovine serum and growth factor supplement. Two hours before transendothelial electrical resistance (TER) measurements, the culture medium was changed to a medium containing 2% fetal calf serum. The total electrical resistance was measured dynamically across the monolayer, and the effects of stimulation with HGF or VEGF were monitored over the time. Increased TER was noted as cells adhered and spread over the microelectrode and was maximal at full confluence, whereas cell retraction, paracellular gap formation, rounding, or loss of adhesion were reflected by a decrease in TER. Resistance was normalized to the initial voltage and expressed as a fraction of the normalized resistance value. TER values from at least six microelectrodes corresponding to each experimental condition were pooled at discrete time points using custom-designed Epool software and plotted versus time as the mean ± SD.
Statistical Analysis
Results are expressed as means ± SD of three to eight independent experiments. Stimulated samples were compared with controls by unpaired Student's t test. For multiple-group comparisons, one-way ANOVA followed by the post hoc Fisher's test were used. P < 0.05 was considered statistically significant.
RESULTS
Effects of VEGF and HGF on Focal Adhesion Redistribution and EC Barrier Properties
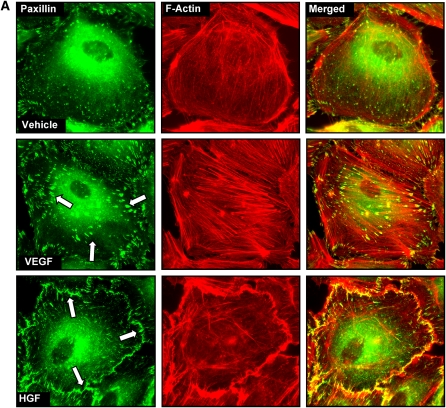
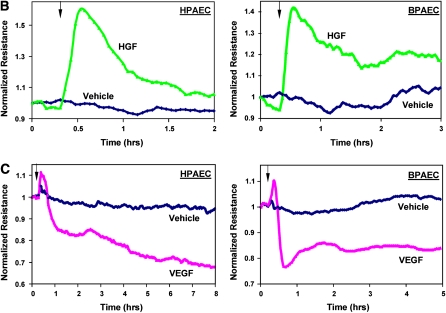
Consistent with differential effects on EC permeability described in our and other previous studies (6, 18, 40), VEGF and HGF induced differential patterns of focal adhesion distribution (Figure 1). VEGF treatment induced reduction in a number of smaller size paxillin-containing focal adhesions and random formation of enlarged focal adhesions (0.46 ± 0.23 μm2 in VEGF-stimulated cells versus 0.13 ± 0.05 μm2 in nonstimulated EC, P < 0.01) that serve as anchoring sites for actomyosin filaments (Figure 1A, middle panels). In turn, HGF stimulation enhanced peripheral F-actin rim in the human pulmonary EC and caused redistribution of paxillin-containing focal adhesions, which formed an almost continuous line at the cell periphery (Figure 1A, lower panels). In the following experiments a comparison of VEGF and HGF responses were performed in human and bovine pulmonary artery EC (HPAEC and BPAEC, respectively). Cells were stimulated with growth factors followed by measurements of transendothelial permeability. In both cell types HGF induced rapid TER increases, reflecting enhancement of EC barrier (Figure 1B, upper panels). In contrast, VEGF stimulation caused drop in TER, which reflects EC barrier compromise (Figure 1B, lower panels).
Figure 1.
Effects of hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) on the focal adhesion remodeling and endothelial cell (EC) barrier properties. (A) Human pulmonary artery ECs (HPAEC) grown on glass coverslips were stimulated with VEGF (200 ng/ml, 20 min) or HGF (20 ng/ml, 20 min), and redistribution of focal adhesions and cytoskeletal remodeling were examined by double immunofluorescence staining for paxillin (left panels) and F-actin (middle panels). Yellow staining in merged images (right panels) represents the areas of paxillin colocalization with actin filaments. Shown are representative results of three independent experiments. (B and C) Measurements permeability responses to HGF (20 ng/ml) (B) or VEGF (200 ng/ml) (C) stimulation were performed in the HPAEC and BPAEC cultures by monitoring TER changes, as described in Materials and Methods. Shown are representative results of three to five independent experiments.
HGF Affects VEGF-Induced FA Remodeling
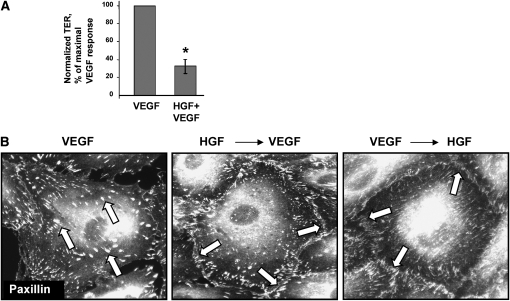
To test whether HGF is capable of inhibiting the VEGF-induced EC barrier dysfunction, pulmonary ECs were treated with HGF before VEGF challenge and compared with cells stimulated with VEGF alone. HGF markedly attenuated VEGF-induced hyperpermeability judged by measurements of TER across EC monolayers (Figure 2A). Immunofluorescence staining of paxillin showed that pretreatment as well as post-treatment of pulmonary EC with HGF prevented formation of randomly distributed enlarged focal adhesions caused by VEGF and promoted accumulation of focal adhesions at the cell periphery (Figure 2B). These data show protective effects of HGF against VEGF-induced EC permeability and suggest a critical role for paxillin in the regulation of endothelial barrier.
Figure 2.
Effects of HGF on VEGF-induced EC barrier dysfunction. (A) Bovine pulmonary artery ECs (BPAEC) plated on microelectrodes were stimulated with VEGF (200 ng/ml) with or without HGF (20 ng/ml, 20 min) pretreatment, and EC permeability response to VEGF or VEGF + HGF was monitored by measurements of transendothelial electrical resistance (TER). (B) Human pulmonary EC were treated with VEGF alone, or pretreated with HGF followed by VEGF stimulation, or treated with VEGF followed by HGF addition. Immunofluorescence staining was performed using paxillin antibody. Shown are representative results of three to five independent experiments.
Effects of VEGF and HGF on Paxillin Phosphorylation
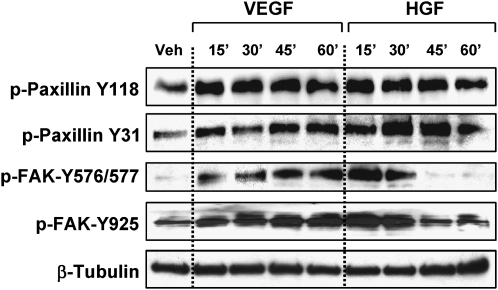
To evaluate effects of VEGF and HGF on site-specific phosphorylation of focal adhesion proteins paxillin and FAK, pulmonary EC monolayers were stimulated with growth factors for various periods of time, and protein tyrosine phosphorylation profiles were determined in cell lysates using site-specific antibodies. VEGF and HGF induced similar patterns of time-dependent paxillin phosphorylation at FAK-specific sites Y118 and Y31 (41, 42). However, VEGF caused more sustained phosphorylation of FAK at Src-dependent sites Y576/577 and Y925 compared with HGF (Figure 3). Sustained FAK phosphorylation at Y576/577 and Y925 was also observed upon EC stimulation with another barrier-disruptive agonist, thrombin (23).
Figure 3.
Effects of HGF and VEGF on paxillin phosphorylation. HPAEC were treated with VEGF (200 ng/ml) or HGF (20 ng/ml) for the indicated periods of time. Phosphorylation of paxillin and FAK was analyzed by immunoblotting of cell lysates with a panel of phospho-specific antibodies. Equal protein loadings were verified by membrane reprobing with β-tubulin antibodies. Shown are representative results of three independent experiments.
Effects of Paxillin Knockdown on Agonist-Mediated EC Barrier Regulation
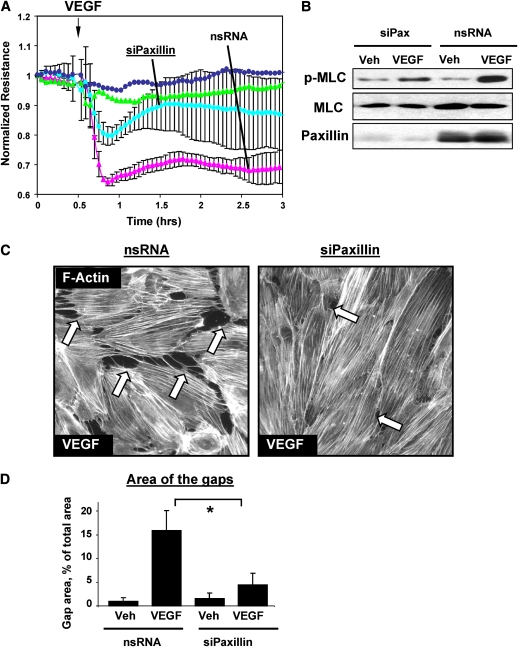
Because paxillin serves as a molecular scaffold and facilitates protein networking and signal transmission in focal adhesions (32), in the following experiments we tested a hypothesis that paxillin plays a role in the mediation of EC permeability responses to barrier-protective and barrier-disruptive stimuli. A role of paxillin was tested using siRNA-based paxillin knockdown. Paxillin depletion partially attenuated VEGF-induced increase in EC permeability (Figure 4A). These effects were accompanied by reduced myosin light chain (MLC) phosphorylation in VEGF-stimulated cells (Figure 4B), indicating attenuation of VEGF-induced activation of Rho pathway in endothelial cells (7, 8). F-actin staining of EC monolayers revealed significant reduction of VEGF-induced paracellular gap formation in paxillin-depleted cells (Figures 4C and 4D).
Figure 4.
Effect of paxillin de-pletion on VEGF-induced EC permeability, signaling, and cytoskeletal remodeling. Pulmonary EC were transfected with paxillin-specific siRNA followed by VEGF (200 ng/ml, 20 min) stimulation. Control transfections were performed using nonspecific RNA. Depletion of target proteins induced by specific siRNA duplexes was confirmed by immunoblotting with appropriate antibodies, as compared with treatment with nonspecific RNA. Immunoblotting with β-tubulin antibodies was used as a normalization control. (A) TER measurements were performed in HPAEC transfected with paxillin-specific or nonspecific RNA duplexes followed by VEGF treatment. (B) VEGF-induced myosin light chain (MLC) phosphorylation was determined in the total BPAEC lysates using di-phospho-MLC–specific antibodies. (C) Immunofluorescence staining of HPAEC for F-actin was performed using Texas Red phalloidin. VEGF-induced paracellular gaps are marked by arrows. (D) Quantitative analysis of VEGF-induced gap formation in EC transfected with paxillin-specific or nonspecific siRNAs. Results are representative of three to five independent experiments, *P < 0.01.
Paxillin knockdown also attenuated HGF-induced endothelial barrier-protective responses. EC transfection with paxillin-specific siRNA decreased HGF-induced increases in TER (Figure 5A), accompanied by suppression of protein tyrosine phosphorylation induced by HGF, decreased activation of Rac and its downstream target PAK1 (Figures 5B–5D). As a result, paxillin depletion reduced the formation of peripheral cytoskeletal rim in response to HGF (Figure 5E), as compared with EC transfected with nonspecific RNA. These results strongly suggest a key role for paxillin in bidirectional regulation of EC cytoskeleton, barrier properties, and small GTPase activities in HGF- and VEGF-stimulated pulmonary EC.
Figure 5.
Effect of paxillin depletion on HGF-induced barrier enhancement, cell signaling, and cytoskeletal remodeling. Pulmonary EC were transfected with paxillin-specific siRNA followed by HGF (20 ng/ml, 20 min) stimulation. Control transfections were performed using nonspecific RNA. (A) TER measurements were performed in HPAEC transfected with paxillin-specific or nonspecific RNAs followed by HGF treatment. (B) Rac activation was determined in control and HGF-stimulated HPAEC using pull-down assay. Lower panel shows total Rac content in EC lysates. (C) HGF-induced PAK1 phosphorylation was determined in the total BPAEC lysates using phospho-PAK1–specific antibody. (D) HGF-induced cytoskeletal remodeling was analyzed in HPAEC by immunofluorescence staining for F-actin using Texas Red phalloidin. Areas of peripheral actin accumulation are marked by arrows. Shown results are representative of three to five independent experiments.
Role of Paxillin Phosphorylation at S273 in HGF- and VEGF-Mediated Endothelial Barrier Regulation
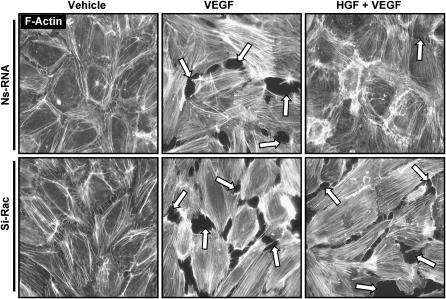
Paxillin phosphorylation at S273 is essential for peripheral localization of the GIT1-PIX-PAK complex and may be critical for regulation of Rac by focal adhesions (34). To further test an involvement of Rac pathway in the HGF-protective effects against VEGF-induced EC barrier disruption, pulmonary EC transfected with Rac-specific siRNA were preincubated with HGF followed by VEGF challenge. VEGF treatment caused similar levels of EC monolayer disruption in control (nonspecific RNA) and Rac siRNA-treated cells (Figure 6, middle panels). In contrast, Rac knockdown significantly suppressed protective effect of HGF against VEGF-induced stress fiber and gap formation, as compared with cells transfected with nonspecific RNA (Figure 6, right panels).
Figure 6.
Effect of Rac knockdown on HGF-mediated endothelial barrier protection. HPAEC grown on glass coverslips were transfected with nonspecific RNA or treated with Rac-specific siRNA. After incubation with either vehicle or HGF (20 ng/ml, 20 min), cells were stimulated with VEGF (200 ng/ml, 20 min), followed by immunofluorescence staining using Texas Red phalloidin to visualize F-actin filaments. Paracellular gaps are shown by arrows. Shown are representative results of three independent experiments.
In the following experiments we examined involvement of Rac/PAK-dependent paxillin phosphorylation (S273) in the mechanisms of HGF-induced pulmonary EC barrier protection. EC were transfected with GFP-tagged phosphorylation-deficient paxillin-S273A mutant, and cytoskeletal remodeling after HGF or VEGF challenge was analyzed by immunofluorescence staining for F-actin (Figure 7A). Transfected cells were visualized by GFP tag. HPAEC transfected with GFP-tagged wild-type paxillin (Figure 7B) served as controls. HGF-induced formation of lamellipodia-like structures, which contribute to EC barrier enhancement, as well as reduction of central stress fibers was observed in nontransfected EC and cells expressing wild-type paxillin (Figures 7A and 7B), but was abolished in the cluster of adjacent EC expressing paxillin S273A mutant. High-magnification insets from these experiments (Figure 7C) show diffuse lamellipodia-like F-actin structures (branched F-actin meshwork) in nontransfected and wild-type paxillin-transfected cells, which were not found in paxillin S273A–expressing EC (only structured F-actin filaments can be seen). In agreement with the notion that paxillin phosphorylation at S273 mediates Rac-mediated, but not Rho-mediated, signaling (34), EC transfection with paxillin-S273A did not affect Rho-dependent stress fiber formation or cell retraction in response to VEGF (Figure 7A, right panels). These data show a key role of paxillin in the Rac-mediated actin remodeling essential for barrier-protective responses to HGF.
Figure 7.
Role of PAK1-dependent paxillin phosphorylation in HGF-induced EC barrier enhancement. HPAEC were transiently transfected with (A) GFP-tagged paxillin-S273A mutant or (B) GFP-tagged wild-type paxillin. After stimulation with VEGF (200 ng/ml, 20 min) or HGF (20 ng/ml, 20 min), cells were fixed and subjected to immunofluorescence staining for F-actin with Texas Red phalloidin. Transfected cells are visualized by GFP tag expression (shown by arrows in A and B). (C) Insets represent HGF-mediated cytoskeletal remodeling in nontransfected EC or cells transfected with paxillin wild-type or paxillin-S273A mutants. Results are representative of three independent experiments.
DISCUSSION
Increases or decreases in endothelial monolayer permeability are associated with profound but strikingly different patterns of pulmonary endothelial cytoskeletal remodeling and rearrangements of cell adhesive complexes (14, 36, 38, 43–45) associated with stimulation of Rac or Rho signaling pathways (23, 38, 43, 46). However, relations between endothelial permeability, focal adhesion remodeling, and local regulation of small GTPases by focal adhesions under physiologic and pathologic conditions remain poorly understood.
This study shows that Rac mediates HGF protective effect against VEGF-induced permeability in pulmonary endothelium. HGF has been implicated in the restoration of lung barrier function and alveolar epithelial integrity during recovery phase of acute lung injury (19, 47). Novel therapeutic strategies using HGF to ameliorate cardiovascular disease have been also suggested (12, 48). However, HGF-protective effects and therapeutic potential toward the restoration of pulmonary vascular EC barrier function in the course of ALI remain to be elucidated. EC from different vascular beds exhibit different permeability responses to HGF. HGF gene transfer increased rat blood–glioma barrier permeability (49). HGF treatment also increased permeability of EC from human umbilical vein (50) and retinal vascular endothelium (51). In turn, elevation of HGF protected against vascular leak in vivo in the cerebral endothelium (15), markedly enhanced basal pulmonary EC barrier properties in a Rac-dependent manner (16, 17), and reduced barrier-disruptive effects of thrombin via Rac-dependent down-regulation of thrombin-induced Rho pathway (18). Available data suggest that rapid and substantial elevation of VEGF may occur early in the development of ALI (5), whereas increased HGF levels were detected at later time points and associated with initiation of lung repair process (19). Thus, attenuation of VEGF-induced EC barrier compromise by HGF described in this study may represent the switch mechanism leading to restoration of lung vascular barrier function and resolution of ALI.
Another major finding of this study is a dual role of paxillin in the regulation of EC barrier and modulation of Rac and Rho signaling triggered by barrier-protective (HGF) and barrier-disruptive (VEGF) growth factors. Previous studies described a Rho-dependent mechanism of VEGF-induced MLC phosphorylation in EC from different vascular beds (8, 52). The reduction of VEGF-induced MLC phosphorylation in paxillin-depleted human pulmonary EC observed in this study (Figure 4B) indicates attenuation of Rho signaling associated with inhibition of endothelial hyperpermeability and gap formation caused by VEGF (Figures 4A, 4C, and 4D). Paxillin on its own may hypothetically contribute to the additional local Rho regulation within the focal adhesions, for example by interaction with microtubules and associated Rho activator GEF-H1, or by binding the Rho-negative regulator p190RhoGAP (see Ref. 29 for review). Thus, in the context of VEGF stimulation, paxillin may further support Rho signaling and promote VEGF-induced barrier dysfunction. These mechanisms require further elucidation.
In contrast to VEGF, HGF induces formation of paxillin containing focal adhesions at the cell periphery (16, 18). Activation of PI3-kinase by HGF results in submembrane generation of phosphoinositides and activation of membrane-associated Rac-specific guanine nucleotide exchange factor (GEF) Tiam1 (16, 17), which activates Rac and Rac effector PAK1 (Figure 5). Agonist-induced site-specific phosphorylation of paxillin may promote its association with focal adhesion–associated GEF βPIX (29, 53) and lead to additional activation of βPIX by focal adhesions. Thus, we speculate that in this locale, Rac activity may be further stimulated by focal adhesion–associated GEF βPIX. In support of this mechanism, our data indicate that HGF induced paxillin phosphorylation at Rac-PAK1–specific site S273 (not shown), and expression of paxillin S273A phosphorylation–deficient mutant abolished HGF-induced enhancement of cortical actin structures, which requires local Rac activity. Taken together, these data suggest a positive feedback mechanism of Rac regulation by focal adhesions in the EC stimulated with HGF.
In conclusion, the results of this study suggest that paxillin may serve as integrator of HGF/VEGF signaling to small GTPases and be involved in the fine tuning of Rac/Rho signaling via specific recruitment of Rac and Rho-specific regulatory molecules to focal adhesions. Our results are consistent with the notion that dynamic changes in the VEGF and HGF circulating levels in the course of acute lung injury may underlie bi-phasic changes in the lung vascular endothelial permeability in the onset and resolution phases of ALI.
This work was supported by National Heart, Lung, and Blood Institute grants HL076259, HL075349, and HL58064, and the American Lung Association Career Investigator Grant for K.G.B.; and by a National Scientist Development Grant from the American Heart Association and an American Lung Association Biomedical Research Grant for A.A.B.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0099OC on July 29, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dvorak HF. Discovery of vascular permeability factor (VPF). Exp Cell Res 2006;312:522–526. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. [DOI] [PubMed] [Google Scholar]
- 3.Choi WI, Quinn DA, Park KM, Moufarrej RK, Jafari B, Syrkina O, Bonventre JV, Hales CA. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med 2003;167:1627–1632. [DOI] [PubMed] [Google Scholar]
- 4.Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax 2006;61:621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol 2004;97:1605–1617. [DOI] [PubMed] [Google Scholar]
- 6.Becker PM, Verin AD, Booth MA, Liu F, Birukova A, Garcia JG. Differential regulation of diverse physiological responses to VEGF in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 2001;281:L1500–L1511. [DOI] [PubMed] [Google Scholar]
- 7.Wojciak-Stothard B, Ridley AJ. Rho GTPases and the regulation of endothelial permeability. Vascul Pharmacol 2002;39:187–199. [DOI] [PubMed] [Google Scholar]
- 8.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and rock signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 2006;13:237–247. [DOI] [PubMed] [Google Scholar]
- 9.Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 2001;24:608–615. [DOI] [PubMed] [Google Scholar]
- 10.Yanagita K, Matsumoto K, Sekiguchi K, Ishibashi H, Niho Y, Nakamura T. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J Biol Chem 1993;268:21212–21217. [PubMed] [Google Scholar]
- 11.Vivekananda J, Awasthi V, Awasthi S, Smith DB, King RJ. Hepatocyte growth factor is elevated in chronic lung injury and inhibits surfactant metabolism. Am J Physiol Lung Cell Mol Physiol 2000;278:L382–L392. [DOI] [PubMed] [Google Scholar]
- 12.Tomita N, Morishita R, Higaki J, Ogihara T. Novel molecular therapeutic approach to cardiovascular disease based on hepatocyte growth factor. J Atheroscler Thromb 2000;7:1–7. [DOI] [PubMed] [Google Scholar]
- 13.Lesko E, Majka M. The biological role of HGF-met axis in tumor growth and development of metastasis. Front Biosci 2008;13:1271–1280. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by edg-dependent cytoskeletal rearrangement. J Clin Invest 2001;108:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Date I, Takagi N, Takagi K, Kago T, Matsumoto K, Nakamura T, Takeo S. Hepatocyte growth factor attenuates cerebral ischemia-induced learning dysfunction. Biochem Biophys Res Commun 2004;319:1152–1158. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JG. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3beta. FASEB J 2002;16:950–962. [DOI] [PubMed] [Google Scholar]
- 17.Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JG. CD44 regulates hepatocyte growth factor-mediated vascular integrity: role of c-met, tiam1/Rac1, dynamin 2, and cortactin. J Biol Chem 2007;282:30643–30657. [DOI] [PubMed] [Google Scholar]
- 18.Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced permeability in the human pulmonary endothelial cells by tiam1-mediated activation of the Rac pathway and by tiam1/Rac-dependent inhibition of the rho pathway. FASEB J 2007;21:2776–2786. [DOI] [PubMed] [Google Scholar]
- 19.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 2002;282:L924–L940. [DOI] [PubMed] [Google Scholar]
- 20.Han B, Lodyga M, Liu M. Ventilator-induced lung injury: role of protein-protein interaction in mechanosensation. Proc Am Thorac Soc 2005;2:181–187. [DOI] [PubMed] [Google Scholar]
- 21.Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol 2002;26:453–464. [DOI] [PubMed] [Google Scholar]
- 22.Shikata Y, Birukov KG, Garcia JG. S1p induces FA remodeling in human pulmonary endothelial cells: Role of Rac, git1, fak and paxillin. J Appl Physiol 2003;94:1193–1203. [DOI] [PubMed] [Google Scholar]
- 23.Shikata Y, Birukov KG, Birukova AA, Verin AD, Garcia JG. Involvement of site-specific fak phosphorylation in sphingosine-1 phosphate- and thrombin-induced focal adhesion remodeling: role of src and git. FASEB J 2003;17:2240–2249. [DOI] [PubMed] [Google Scholar]
- 24.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res 2005;304:40–49. [DOI] [PubMed] [Google Scholar]
- 25.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 2006;168:1749–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta D, Tiruppathi C, Sandoval R, Minshall RD, Holinstat M, Malik AB. Modulatory role of focal adhesion kinase in regulating human pulmonary arterial endothelial barrier function. J Physiol 2002;539:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holinstat M, Knezevic N, Broman M, Samarel AM, Malik AB, Mehta D. Suppression of rhoa activity by focal adhesion kinase-induced activation of p190rhogap: role in regulation of endothelial permeability. J Biol Chem 2006;281:2296–2305. [DOI] [PubMed] [Google Scholar]
- 28.Turner CE. Paxillin. Int J Biochem Cell Biol 1998;30:955–959. [DOI] [PubMed] [Google Scholar]
- 29.Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev 2004;84:1315–1339. [DOI] [PubMed] [Google Scholar]
- 30.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol 2005;6:56–68. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki T, Nakata A, Mukai M, Shinkai K, Yano H, Sabe H, Schaefer E, Tatsuta M, Tsujimura T, Terada N, et al. Involvement of phosphorylation of tyr-31 and tyr-118 of paxillin in MM1 cancer cell migration. Int J Cancer 2002;97:330–335. [DOI] [PubMed] [Google Scholar]
- 32.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol 2000;2:E231–E236. [DOI] [PubMed] [Google Scholar]
- 33.Turner CE. Paxillin interactions. J Cell Sci 2000;113:4139–4140. [DOI] [PubMed] [Google Scholar]
- 34.Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. Paxillin phosphorylation at ser273 localizes a git1-pix-pak complex and regulates adhesion and protrusion dynamics. J Cell Biol 2006;173:587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol 2001;281:L565–L574. [DOI] [PubMed] [Google Scholar]
- 36.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via CDC42 and Rac. Circ Res 2004;95:892–901. [DOI] [PubMed] [Google Scholar]
- 37.Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol 2004;166:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of rho gtpases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 2004;67:64–77. [DOI] [PubMed] [Google Scholar]
- 39.Birukova AA, Birukov KG, Smurova K, Adyshev DM, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 2004;18:1879–1890. [DOI] [PubMed] [Google Scholar]
- 40.Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW II, Duran WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol 2003;284:H92–H100. [DOI] [PubMed] [Google Scholar]
- 41.Schaller MD, Parsons JT. Pp125fak-dependent tyrosine phosphorylation of paxillin creates a high-affinity binding site for crk. Mol Cell Biol 1995;15:2635–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bellis SL, Miller JT, Turner CE. Characterization of tyrosine phosphorylation of paxillin in vitro by focal adhesion kinase. J Biol Chem 1995;270:17437–17441. [DOI] [PubMed] [Google Scholar]
- 43.Birukova AA, Malyukova I, Poroyko V, Birukov KG. Paxillin-beta-catenin interactions are involved in Rac/CDC42-mediated endothelial barrier-protective response to oxidized phospholipids. Am J Physiol Lung Cell Mol Physiol 2007;293:L199–L211. [DOI] [PubMed] [Google Scholar]
- 44.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem 2004;279:24692–24700. [DOI] [PubMed] [Google Scholar]
- 45.Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and EPAC1/RAP1-dependent Rac activation. Exp Cell Res 2007;313:2504–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betapix mediate rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol 2007;211:608–617. [DOI] [PubMed] [Google Scholar]
- 47.Geiser T. Mechanisms of alveolar epithelial repair in acute lung injury: a translational approach. Swiss Med Wkly 2003;133:586–590. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem (Tokyo) 1996;119:591–600. [DOI] [PubMed] [Google Scholar]
- 49.Book AA, Ranganathan S, Abounader R, Rosen E, Laterra J. Scatter factor/hepatocyte growth factor gene transfer increases rat blood-glioma barrier permeability. Brain Res 1999;833:173–180. [DOI] [PubMed] [Google Scholar]
- 50.Martin TA, Mansel RE, Jiang WG. Antagonistic effect of NK4 on HGF/SF induced changes in the transendothelial resistance (TER) and paracellular permeability of human vascular endothelial cells. J Cell Physiol 2002;192:268–275. [DOI] [PubMed] [Google Scholar]
- 51.Clermont AC, Cahill M, Salti H, Rook SL, Rask-Madsen C, Goddard L, Wong JS, Bursell D, Bursell SE, Aiello LP. Hepatocyte growth factor induces retinal vascular permeability via MAP-kinase and PI-3 kinase without altering retinal hemodynamics. Invest Ophthalmol Vis Sci 2006;47:2701–2708. [DOI] [PubMed] [Google Scholar]
- 52.van Nieuw Amerongen GP, Koolwijk P, Versteilen A, van Hinsbergh VW. Involvement of RhoA/Rho kinase signaling in VEGF-induced endothelial cell migration and angiogenesis in vitro. Arterioscler Thromb Vasc Biol 2003;23:211–217. [DOI] [PubMed] [Google Scholar]
- 53.Brown MC, West KA, Turner CE. Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell 2002;13:1550–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]